Abstract
Background
Molecular diagnostics has greatly refined sinonasal tumor pathology over the past decade. While much of the attention has focused on carcinomas, it is becoming clear that there are emerging mesenchymal neoplasms which have previously defied classification.
Methods
Here, we present a 33-year-old woman with a multiply recurrent sinonasal spindle cell tumor exhibiting distinctive features, and not easily classifiable into a specific category.
Results
The hypercellular tumor was composed of plump spindled cells, with uniform vesicular chromatin arranged as vague fascicles around a prominent hemangiopericytoma-like vasculature. The mitotic rate was brisk at 10 per 10 high power fields. By immunohistochemistry, it was only positive for EMA (focal) and SATB2 (diffuse, weak). Fusion analysis uncovered EWSR1::BEND2, a fusion which is best known for being seen in astroblastoma, but which has not yet been reported in sarcomas.
Conclusion
This case underscores the utility of fusion analysis when confronted with a sinonasal spindle cell neoplasm which does not neatly fit into any specific category. It remains to be seen if EWSR1::BEND2 sinonasal sarcoma represents a distinct entity.
Keywords: Sinonasal tract, Sarcoma, EWSR1:BEND2, Molecular diagnostics
Introduction
Sinonasal tumor classification has been revolutionized in the past decade, largely due to molecular discoveries. There are many new entities which are either defined or are highly associated with a particular fusion or mutation [1]. While the most refinement has occurred in the group of tumors known as sinonasal “small round cell tumors,” a molecular approach is also beginning to uncover novel spindle cell entities which had previously eluded classification. The best example of this phenomenon is biphenotypic sinonasal sarcoma, a tumor defined by PAX3 rearrangements which was first described only a decade ago, but which now is well-recognized and not even particularly rare [2, 3]. Molecular analysis on difficult-to-classify sinonasal spindle cell tumors will undoubtedly continue to identify tumors which had previously been unrecognized in this site or are novel altogether [4–6]. Here, we describe a young woman with a distinctive, multiply recurrent sinonasal sarcoma harboring an EWSR1::BEND2 fusion. This expands the differential diagnosis of sinonasal spindle cell tumors and also the role for molecular analysis in characterizing this group of neoplasms.
Clinical History and Follow-up
The patient was a 33-year-old woman who presented with epistaxis. On physical examination, a red, vascular mass was seen, and imaging revealed a 4.3 cm enhancing left nasal cavity mass centered on the middle turbinate. The tumor was removed endoscopically, and an initial diagnosis of glomangiopericytoma was made. The mass recurred 6 months later, and was again removed endoscopically, with a resulting diagnosis of recurrent glomangiopericytoma. Nine months later, the patient had recurrent epistaxis and facial pressure, and a recurrent mass was visualized. The tumor was resected once again. This time, a diagnosis of low-grade unclassified spindle cell sarcoma was made, and fusion analysis was sought in an attempt to better classify it.
Histologic Findings
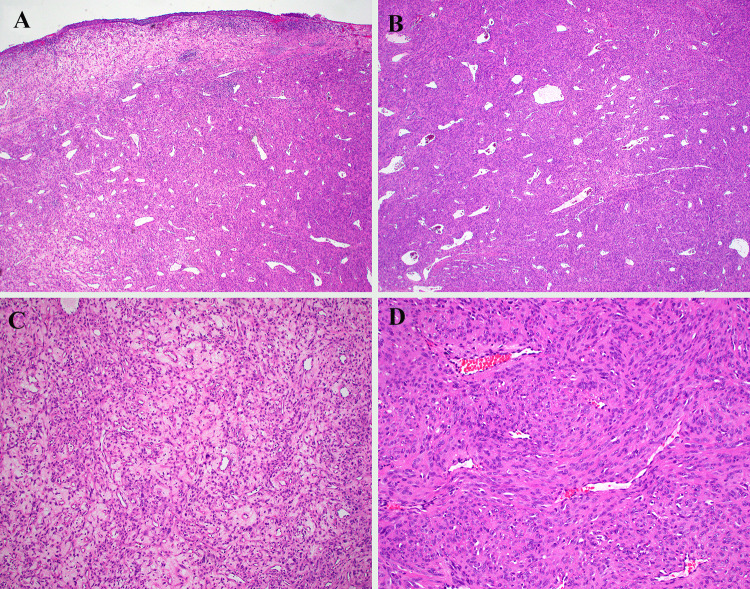
The tumor had an identical appearance initially and with each recurrence. It was variably hypercellular, arranged in vague fascicles around a very prominent hemangiopericytoma (HPC)-like vasculature (Fig. 1A–D). The tumor cells were plump and spindled, with eosinophilic cytoplasm with ill-defined cell borders. The tumor cell nuclei were oval with vesicular chromatin and prominent nucleoli (Fig. 2A–B). The nuclei were mostly uniform, although there were some scattered cells with giant nuclei. The mitotic rate was brisk (10 per 10 high power fields) with occasional atypical forms. Osteoid was not identified. Necrosis was absent. The surface epithelium was partially ulcerated but otherwise normal (Fig. 1A).
Fig. 1.
A The tumor grew as an expansive mass in the sinonasal submucosa beneath a partially ulcerated surface epithelium. B Dilated, hemangiopericytoma-like vessels were conspicuous. C While some areas were more modestly cellular with myxohyaline stroma, D most of the tumor was hypercellular, growing as vague fascicles
Fig. 2.
The tumor cells were epithelioid to spindled, with ample eosinophilic cytoplasm and mostly uniform oval, hypochromatic nuclei. Scattered lymphocytes were seen, and the mitotic rate was brisk at 10 per 10 high power fields
Immunohistochemical Findings
The tumor was weakly positive for SATB2 (Fig. 3A), focally positive for EMA (Fig. 3B), and negative for STAT6, SMA, S100, SOX10, CD34, desmin, myogenin, MyoD1, beta-catenin (membranous only), AE1/AE3, SSTR2, PR, ERG, CD21, O13, synaptophysin, CD163, SS18-SSX, and ALK1. In situ hybridization for EBV (EBER) and FGF23 were also negative.
Fig. 3.
Most immunohistochemical assays were negative, with the exception of A SATB2 which was diffuse but weak, and B EMA which was focal
Molecular Findings
The tumor was further evaluated by a previously described custom next-generation sequencing (NGS) test for the identification of fusion events in 93 genes [7]. In brief, total RNA was extracted from the formalin-fixed paraffin embedded block and used in an anchored multiple PCR NGS assay that identified an EWSR1::BEND2 fusion transcript between EWSR1 exon 12 and BEND2 exon 5. This fusion event is predicted to be in-frame and was supported by 1172 reads spanning the breakpoint.
Discussion
The differential diagnosis for a mesenchymal neoplasm in the sinonasal tract is broad, and it is growing larger, largely with the aid of molecular-based diagnostic refinement. The tumor presented resembled many known sinonasal mesenchymal entities, but did not fit perfectly into any category. Meningioma was suggested by epithelioid features, bland cytology, and EMA staining, but PR and SSTR2 were negative. Prominent, staghorn, hemangiopericytoma-like vessels can be seen in many sinonasal tumors including synovial sarcoma, glomangiopericytoma, biphenotypic sinonasal sarcoma, and solitary fibrous tumor, but the current tumor was negative for SMA (for glomangiopericytoma and biphenotypic sinonasal sarcoma), S100 (for biphenotypic sinonasal sarcoma), SS18-SSX (for synovial sarcoma), CD34 and STAT6 (for solitary fibrous tumor), and nuclear beta-catenin (for all four considerations). The presented case did not fit into any known category, but was felt to be a sarcoma based on its degree of cellularity, elevated mitotic activity, and rapid local recurrences. Given the recent emergence of newly recognized, fusion-defined sinonasal tumors, [4–6] fusion analysis was performed to help clarify the diagnosis, and it revealed EWSR1::BEND2. This unusual molecular result underscored the uniqueness of this sinonasal neoplasm.
EWSR1::BEND2 is a fusion between the genes for EWS RNA binding protein (EWSR1) and BEN domain containing 2 (BEND2). This is not a novel fusion, and it has been previously reported in astroblastoma, especially those arising in the spinal cord or brainstem, as well as rare pancreatic neuroendocrine tumors and salivary adenocarcinomas, not otherwise specified [8–10]. Despite harboring the same fusion, the case presented cannot be classified as any of these tumors. It was purely spindled, and negative for epithelial, neuroendocrine, and glial immunohistochemical markers. Interestingly, there is a reported case of an abdominal wall soft tissue sarcoma harboring MN1::BEND2, the most common fusion in astroblastoma [11]. Similarly, that case did not resemble astroblastoma, and had different immunohistochemical and methylation patterns [11]. In our opinion, the published photomicrographs of that tumor are reminiscent of the case presented here. Presumably, MN1 and EWSR1 have similar function when fused to BEND2 in astroblastoma, so it is conceivable that there is a family of soft tissue sarcomas which harbor astroblastoma-related fusions. There are fusions which can be encountered in a variety of tumor types, perhaps best exemplified by the EWSR1::ATF1 fusion which can be seen in clear cell sarcoma, [12] angiomatoid fibrous histiocytoma, [13] hyalinizing clear cell carcinoma, [14] and malignant mesothelioma [15]. Thus, it is reasonable to hypothesize that distinct phenotypes of neoplasms harboring the same fusion event are context-dependent and the result of differentiation programs already present in distinct tumor progenitor cells. Clearly, more cases will be needed to make this determination.
Both MN1 and EWSR1 have transcriptional activator activity and the fusion of MN1 or EWSR1 to BEND2 has been demonstrated to enhance transcription of BEND2 exons downstream from the breakpoints [16–18]. The EWSR1::BEND2 fusion in the current case retains the carboxy-terminal BEN-domains of BEND2 which mediate sequence-specific DNA binding [19]. Therefore, it is likely that the downstream consequences of the fusion are determined by the aberrant recruitment of the amino-terminus EWSR1 transcriptional activation domain to binding sites determined by the BEND2 binding domains, perhaps together with upregulated expression via the EWSR1 promoter. Although studies characterizing BEND2 gene function are limited, studies of BEND2 and other BEN-domain containing gene family members (BEND3/4/5/6/7, NACC1/2, BANP) have shown that BEN proteins tend to interact with a variety of proteins, most likely in a context-dependent manner, and that most of the interacting proteins are components of transcription-repressive complexes involved in chromatin remodeling and/or modification [20–22].
Among sinonasal spindle cell tumors, there has been an emergence of fusion-associated neoplasms. These include not only biphenotypic sinonasal sarcoma, RREB1::MKL2 sarcoma, and FUS::NACC1 sarcoma, [4–6] but also multiple, as-of-yet unpublished cases the authors have encountered (unpublished observations). Indeed, this group of lesions may be the next to undergo a molecular-based classification upheaval. This case was originally diagnosed as glomangiopericytoma as it was felt to be the closest histologic fit, but with many likely still-unrecognized sinonasal spindle cell tumors, pathologists should resist the temptation to force a tumor into a specific diagnostic category if the histologic and immunophenotypic features do not conform well to those entities. This case illustrates how fusion analysis can affirm a tumor’s uniqueness and identify novel entities in the sinonasal spindle cell neoplasm differential diagnosis.
Conclusion
We identified a unique low-grade sinonasal sarcoma with prominent hemangiopericytoma-like vessels, plump spindled cells with an elevated mitotic rate, which harbored a EWSR1::BEND2 fusion. While this fusion is becoming increasingly recognized in various tumors, it has not been previously seen in a soft tissue sarcoma. This case highlights the utility of fusion analysis when confronted with a sinonasal spindle cell neoplasm which does not neatly fit into any specific category. More widespread fusion analysis will be needed to determine if EWSR1::BEND2 sarcoma represents a distinct sinonasal malignancy, or if it is part of a family of soft tissue sarcomas harboring astroblastoma-related fusions.
Author Contributions
VM and JAB designed the study, performed data collection and interpretation, and prepared the manuscript. JYP performed data collection and interpretation. All authors read and approved the final paper.
Funding
This study was funded by the Jane B. and Edwin P. Jenevein M.D Endowment for Pathology at UT Southwestern Medical Center. No external funding was obtained for this study.
Data Availability
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
Declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (UT Southwestern IRB 112017-073).
Consent to Participate/Publication
The IRB-approved study did not require informed consent.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thompson LDR, Bishop JA. Update from the 5th Edition of the World Health Organization classification of head and neck tumors: nasal cavity, paranasal sinuses and skull base. Head Neck Pathol. 2022;16(1):1–18. doi: 10.1007/s12105-021-01406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis JT, Oliveira AM, Nascimento AG, Schembri-Wismayer D, Moore EA, Olsen KD, et al. Low-grade sinonasal sarcoma with neural and myogenic features: a clinicopathologic analysis of 28 cases. Am J Surg Pathol. 2012;36(4):517–525. doi: 10.1097/PAS.0b013e3182426886. [DOI] [PubMed] [Google Scholar]
- 3.Le Loarer F, Laffont S, Lesluyes T, Tirode F, Antonescu C, Baglin AC, et al. Clinicopathologic and molecular features of a series of 41 biphenotypic sinonasal sarcomas expanding their molecular spectrum. Am J Surg Pathol. 2019;43(6):747–754. doi: 10.1097/PAS.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens TM, Rooper LM, Bacchi CE, Fernandes IL, Antonescu CR, Gagan J, et al. Teratocarcinosarcoma-like and adamantinoma-like head and neck neoplasms harboring NAB2::STAT6: unusual variants of solitary fibrous tumor or novel tumor entities? Head Neck Pathol. 2022;16(3):746–754. doi: 10.1007/s12105-022-01444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mechtersheimer G, Andrulis M, Delank KW, Volckmar AL, Zhang L, von Winterfeld M, et al. RREB1-MKL2 fusion in a spindle cell sinonasal sarcoma: biphenotypic sinonasal sarcoma or ectomesenchymal chondromyxoid tumor in an unusual site? Genes Chromosom Cancer. 2021;60(8):565–570. doi: 10.1002/gcc.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rooper LM, Gagan J, Bishop JA. A low grade nasopharyngeal sarcoma with FUS::NACC1 fusion and immunohistochemical evidence of epithelial differentiation: expanding the clinicopathologic spectrum of an emerging entity. Head Neck Pathol. 2022 doi: 10.1007/s12105-022-01488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia R, Patel N, Uddin N, Park JY. Development and clinical validation of a multiplex gene fusion assay. Lab Med. 2020;51(5):512–518. doi: 10.1093/labmed/lmz102. [DOI] [PubMed] [Google Scholar]
- 8.Lucas CG, Gupta R, Wu J, Shah K, Ravindranathan A, Barreto J, et al. EWSR1-BEND2 fusion defines an epigenetically distinct subtype of astroblastoma. Acta Neuropathol. 2022;143(1):109–113. doi: 10.1007/s00401-021-02388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todorovic E, Dickson BC, Weinreb I. Salivary gland cancer in the era of routine next-generation sequencing. Head Neck Pathol. 2020;14(2):311–320. doi: 10.1007/s12105-020-01140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65–71. doi: 10.1038/nature21063. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida A, Satomi K, Kobayashi E, Ryo E, Matsushita Y, Narita Y, et al. Soft-tissue sarcoma with MN1-BEND2 fusion: a case report and comparison with astroblastoma. Genes Chromosom Cancer. 2022;61(7):427–431. doi: 10.1002/gcc.23028. [DOI] [PubMed] [Google Scholar]
- 12.Moller E, Praz V, Rajendran S, Dong R, Cauderay A, Xing YH, et al. EWSR1-ATF1 dependent 3D connectivity regulates oncogenic and differentiation programs in clear cell sarcoma. Nat Commun. 2022;13(1):2267. doi: 10.1038/s41467-022-29910-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi S, Szuhai K, Ijszenga M, Tanke HJ, Zanatta L, Sciot R, et al. EWSR1-CREB1 and EWSR1-ATF1 fusion genes in angiomatoid fibrous histiocytoma. Clin Cancer Res. 2007;13(24):7322–7328. doi: 10.1158/1078-0432.CCR-07-1744. [DOI] [PubMed] [Google Scholar]
- 14.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50(7):559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 15.Ke H, Gill AJ, McKenzie C, Kench JG, Chan RCF, Pavlakis N, et al. Malignant peritoneal mesothelioma with EWSR1-ATF1 fusion: a case report. JTO Clin Res Rep. 2021;2(11):100236. doi: 10.1016/j.jtocrr.2021.100236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturm D, Orr BA, Toprak UH, Hovestadt V, Jones DTW, Capper D, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164(5):1060–1072. doi: 10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamasaki K, Nakano Y, Nobusawa S, Okuhiro Y, Fukushima H, Inoue T, et al. Spinal cord astroblastoma with an EWSR1-BEND2 fusion classified as a high-grade neuroepithelial tumour with MN1 alteration. Neuropathol Appl Neurobiol. 2020;46(2):190–193. doi: 10.1111/nan.12593. [DOI] [PubMed] [Google Scholar]
- 18.Burford A, Mackay A, Popov S, Vinci M, Carvalho D, Clarke M, et al. The ten-year evolutionary trajectory of a highly recurrent paediatric high grade neuroepithelial tumour with MN1:BEND2 fusion. Sci Rep. 2018;8(1):1032. doi: 10.1038/s41598-018-19389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng L, Liu J, Niu L, Kamran M, Yang AWH, Jolma A, et al. Distinct structural bases for sequence-specific DNA binding by mammalian BEN domain proteins. Genes Dev. 2022;36(3–4):225–240. doi: 10.1101/gad.348993.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Xie D, Luo M, Lin X, Nie H, Chen J, et al. Identification and characterization of BEND2 as a key regulator of meiosis during mouse spermatogenesis. Sci Adv. 2022;8(21):eabn1606. doi: 10.1126/sciadv.abn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J, Zhang Y, You Q, Huang C, Zhang T, Wang M, et al. Highly enriched BEND3 prevents the premature activation of bivalent genes during differentiation. Science. 2022;375(6584):1053–1058. doi: 10.1126/science.abm0730. [DOI] [PubMed] [Google Scholar]
- 22.Kurniawan F, Prasanth SG. A BEN-domain protein and polycomb complex work coordinately to regulate transcription. Transcription. 2022;13(1–3):82–87. doi: 10.1080/21541264.2022.2105128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Not applicable.