Abstract
Stress and depression have been reported in gestational diabetes mellitus (GDM). Though inflammation and oxidative stress are associated with depression, there are no reports of link of cardiometabolic risks (CMR) to stress and depression in GDM. Normal pregnant women (control group, n = 164) and women with GDM (study group, n = 176) at 36th week of gestation were recruited for the study. Blood pressure (BP), body composition, heart rate variability (HRV), glycated hemoglobin (HbA1C), markers of insulin resistance, oxidative stress, inflammation and endothelial dysfunction, were assessed. Perceived stress score (PSS), quality of life (QoL) scale, Indian diabetic risk score (IDRS) and Edinburg postnatal depression score (EPDS) were assessed. Association of potential contributors to PSS and EDPS were assessed by correlation and regression analyses. There was significant increase in PSS, EPDS, IDRS scores, HbA1C, malondialdehyde (MDA) (oxidative stress marker) and high-sensitive C-reactive protein and interleukin-6 (inflammatory markers), and significant decrease in total power (TP) of HRV (marker of cardiovagal modulation), QoL and nitric oxide (endothelial dysfunction marker) in study group compared to control group. Though many cardiometabolic risk parameters were correlated with PSS and EPDS, the significant independent association was observed for TP, HbA1C, MDA and interleukin-6. However, interleukin-6 had maximum contribution to PSS (β = 0.550, p < 0.001) and EPDS (β = 0.393, p < 0.001) as demonstrated by multiple regression analysis. Inflammation, oxidative stress, glycation status and decreased cardiovagal modulation are associated with stress and depression at 36th week of gestation in GDM.
Subject terms: Physiology, Psychology, Biomarkers, Endocrinology, Health care
Introduction
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with the onset or first recognition during the present pregnancy at 24–28 weeks of gestation1. In the recent past, there is an increased incidence of diabetes during pregnancy with almost 21 million births (16.2%) affected due to hyperglycemia2. Prevalence of GDM in India is 18.9%, ranging between 3.8% to 41% in various parts of the country3. The major maternal and neonatal adverse effects of GDM include increased risk of preterm delivery, pre-eclampsia, caesarean section delivery, development of type 2 diabetes mellitus (T2DM) post-delivery, fetal macrosomia, neonatal hypoglycemia, neonatal respiratory distress, and childhood obesity and insulin resistance, followed by impaired glucose tolerance and T2DM later in life4,5. Women with history of GDM have a sevenfold increased risk of developing T2DM, with 20–70% risk in the first decade after delivery and GDM increases the future cardiovascular disease (CVD) risk by twofold6. It is important to note that studies have consistently shown that women with a history of GDM have a higher prevalence of cardiometabolic risk factors, such as dyslipidemia, hypertension, obesity, and metabolic syndrome, compared to their peers. Additionally, by three months postpartum, this adverse cardiovascular risk factor profile is evident6.
Depression during pregnancy has been reported to adversely affect women and their children7,8. Anxiety, psychological stress and depression are associated with GDM9–12. Psychological stress and depression in GDM severely affect the maternal and fetal outcomes13,14. Also, it has been reported that the prenatal depression in GDM is linked to post-partum depression for a longer duration and adverse cardiovascular (CV) consequences15,16. Recently we have reported the decreased heart rate variability (HRV) and cardiovagal modulation associated with depression in women during antenatal period, which exposes them to CV risks17. However, till date the mechanism that causes CV risks in GDM have not been well studied and the association of CV risks to mental illness in GDM has not been reported. It is important for healthcare workers to know the relationship between stress and depression with fetomaternal outcomes, and if the depression-associated problems can be prevented in the perinatal period. In a recently conducted pilot study at 36th week of gestation in 15 women having GDM, we observed that cardiometabolic risks, adverse fetomaternal outcomes and poor psychophysical health were improved by practice of short course of yoga18. But, in this study due to less sample size, we could not assess the enormity of cardiometabolic risks, the mechanisms that could lead to development of these risks and the link of depression to the risks and the maternal–fetal outcomes.
Inflammation has long been proposed as a pathophysiologic mechanism of depression19. There is accumulating evidence that anti-inflammatory interventions could be promising antidepressants, particularly in patients with increased peripheral inflammatory biomarkers19,20. Elevated C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) are the major molecular inflammatory signature associated with depression in general population21,22. Among the chemical mediators, high-sensitive C-reactive protein (hsCRP) and IL-6 are mainly reported to be associated with GDM23–25. However, till date the association of CRP and IL-6 with depression and cardiometabolic risks in GDM has not been studied. Oxidative stress and endothelial dysfunction have also been implicated in the pathophysiology of GDM26,27. But the association of oxidative stress and endothelial dysfunction with stress and depression-mediated increased cardiometabolic risks in GDM has not been reported yet.
We have reported the decreased HRV as an indicator of reduced cardiovagal modulation and sympathovagal imbalance as a marker of CV risk in various disorders including diabetes28–34. In a recent study, we have demonstrated the link of decreased level of nitric oxide as marker of endothelial dysfunction with reduced HRV in gestational hypertension35. However, the extent of HRV and cardiovagal modulation and their link to depression in GDM has not been reported till date. In GDM, a cardiometabolic risk intensifies in later part of pregnancy. We have investigated the link of retrograde inflammation, oxidative stress, endothelial dysfunction and decreased cardiovagal modulation to stress and depression at 36th week of pregnancy in gestational diabetes mellitus.
Methods
Study design
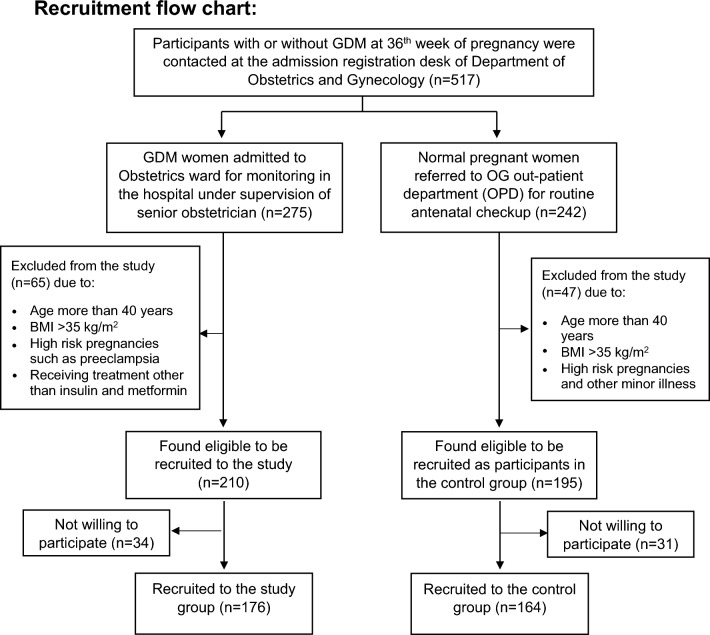
The present descriptive-analytical study was conducted in the Department of Physiology in collaboration with the Department of Obstetrics and Gynaecology (OG), and Biochemistry, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Puducherry, India from October, 2020–April, 2022 after obtaining approval from Scientific Advisory Committee and Ethics Committee of JIPMER, Puducherry. All the methods were performed in accordance with the institutional guidelines and regulations. A total of 517 pregnant women registered at admission counter of OG department of JIPMER hospital were screened. Following inclusion and exclusion criteria, 176 GDM women and 164 normal pregnant women at 36th week of gestation were recruited (as depicted in the recruitment flow-chart below).
Written informed consent was obtained from all the participants prior to the initiation of the study. All the investigations were done at the bedside of the subjects in the OG Ward. GDM was diagnosed based on the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria in which 75-gm oral glucose tolerance test (OGTT) was done at 24–28 weeks in fasting and GDM was diagnosed if any one of the following cut-off is met i.e. ≥ 92 mg/dl (≥ 5.2 mmol/l) or 1-h ≥ 180 mg/dl (≥ 10 mmol/l) or 2-h ≥ 153 mg/dl (≥ 8.5 mmol/l)36.
Sample size calculation
Sample size was calculated using G*Power 3.1.9.7. Based on the previous report of PSS in GDM37, sample size was calculated with 90% power to detect the difference between means of PSS in GDM and normal pregnant women with a significance level of (alpha) 0.01. The minimum sample size was calculated to be 106 in each group. However, since stress levels are highly subjective and HRV parameters have wider variations in pregnancy, the sample size was kept at minimum 160 in each group, in the present study.
Grouping of subjects
Study group (n = 176)
Subjects of study group were pregnant women diagnosed with gestational diabetes and admitted to OG ward of JIPMER hospital at 36th week of gestation.
Inclusion criteria
GDM women at 36th gestational weeks between the age of 18 and 40 years, and receiving insulin and/or oral anti-diabetic agents as part of anti-diabetic treatment protocol practiced in JIPMER hospital (Table 1) and followed by others38, in addition to the routine diabetic diet for GDM management (Table 2) and those willing to participate in the study, were included.
Table 1.
Treatment received by pregnant women in control group and study group.
| Sl. no | Control group (n = 164) (normal pregnant women) | Study group (n = 176) (women with GDM) |
|---|---|---|
| 1 |
Standard antenatal medications for healthy pregnant women (i) Tab. Folate—5 mg, once daily (ii) Tab. FST—200 mg, twice daily (iii) Tab. Calcium—500 mg, once daily (iv) Tab. Vitamin C—100 mg, once daily |
Standard antenatal medications for women with gestational diabetes admitted to JIPMER hospital (i) Tab. Folate—5 mg, once daily (ii) Tab. FST—200 mg, twice daily (iii) Tab. Calcium—500, mg once daily (iv) Tab. Vitamin C—100 mg, once daily |
| 2 | No medication |
(i) Only insulina—104 patients (ii) Both insulina and OADb—47 patients (iii) Only OADb—25 patients |
| 3 | No diet restriction | Diabetic diet (composition of diabetic diet was given in the table below) |
| 4 | Routine antenatal counseling | Routine antenatal counseling |
aRI: 4U–10 U, NPH: 4U–12U, Actrapid: 6U–20U, Insulatard: 14U–18U, Mixtard: 6U–14U.
bTab. Metformin 500 mg.
GDM gestational diabetes mellitus, FST ferrous sulphate tablets, OAD oral anti-diabetic drug.
Table 2.
Diet given to GDM women admitted to obstetrics ward of JIPMER hospital. Three different calories of food items were given according to their individual requirements, as prescribed by the hospital nutritionist monitored on daily basis.
| Quantity | 1500 calorie | 1800 calorie | 2000 calorie | |
|---|---|---|---|---|
| Morning 6.30 AM | ||||
| Tea/milk without sugar | 1 | 100 ml | 150 ml | 150 ml |
| Breakfast | ||||
| Rice/wheat/raggi (idly/dosai/upma) | 1 | 50 g raggi | 60 g raggi | 60 g raggi |
| Sambar/chutney (tomato/onion/coriander/mint/curry leaves) | 2 table spoon |
15 g dal 50 g vegetable |
20 g dal 50 g vegetable |
25 g dal 50 g vegetable |
| Morning | ||||
| Buttermilk/coconut water/soup (vegetable, mushroom, corn, dal) | 1 glass | 200 ml | 200 ml | 200 ml |
| Lunch | ||||
| Rice/chappathi | 225 g | 75 g | 75 g | 80 g |
| Rasam/sambar | ¾ cup | 5 g dal | 10 g dal | 10 g dal |
| Chicken/fish/egg/dal | 1 piece/1 katori |
40 g 13 g |
60 g 15 g |
75 g 15 g |
| Greens | ½ cup | 100 g | 100 g | 100 g |
| Vegetable curry | 2 katoris | 100 g | 100 g | 100 g |
| Buttermilk | 1 glass | 150 ml | 150 ml | 150 ml |
| Evening | ||||
| Tea/milk (without sugar) | 1 glass | 100 ml | 150 ml | 150 ml |
| Vegetable sandwich/sundal | ¼ cup | 30 g | 45 g | 50 g |
| Dinner | ||||
| Chappathi/wheat rice | 2 | 50 g | 70 g | 75 g |
| Dal/sambar | 1 katori | 10 g | 10 g | 10 g |
| Vegetable curry | 2 katoris | 150 g | 150 g | 150 g |
| Night | ||||
| Milk | ½ glass | 100 ml | 100 ml | 150 ml |
| Fruit | 1 | 50 g | 75 g | 100 g |
| Total oil quantity | 20 g | 20 g | 25 g | |
| Total butter quantity | 10 g | |||
| Total salt intake | Less than 6 gm | |||
Exclusion criteria
GDM women with severe anemia, polyhydramnios, multiple pregnancy, pregnancy-induced hypertension, cardiac problems, history of depression, GDM women with history of PCOS on metformin treatment and body mass index (BMI) > 35 kg/m2 were excluded from the study39.
Control group (n = 164)
Normal pregnant women at 36th week of gestation, attending OG out-patient department (OPD) for 3rd trimester antenatal check-up, were recruited as control group participants. Subjects having BMI > 35 kg/m2, age more than 40 years, previous history of depression and not willing to participate were excluded from the study.
Procedure
Anthropometric measurements
All the subjects’ body weight (BW) in kg and height in cm were measured and body mass index (BMI) was calculated by Quetelet's index formula.
Blood pressure-related parameters
Following 10 min of supine rest in their bed, heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were recorded using automated blood pressure monitor (Omron automatic blood pressure monitor HEM-8712, Omron Healthcare Company Ltd, Tokyo, Japan). Mean arterial pressure (MAP) and rate pressure product (RPP) were calculated. RPP, an index of myocardial work stress40 was calculated using the formula, RPP = (BHR × SBP) × 10−2.
Body composition parameters
Body composition was assessed Bodystat® (Model QuadScan 4000®, Isle of Man, United Kingdom) which works based on the bioelectrical impedance analysis (BIA). Body composition was measured following the procedure as described earlier30. Subjects were rested in supine position. The recording electrodes were positioned on the dorsal surface of hand proximal to metacarpal–phalangeal and on the foot proximal to metatarsal–phalangeal joints. Body composition parameters included total body fat, visceral fat, resting metabolism (RM), subcutaneous fat (whole body) and skeletal muscle (whole body), lean body mass, body fat mass index (BFMI), fat-free mass index (FFMI), and ratio of RM to body weight.
Gestational weight gain observed during pregnancy is associated with an increase in the maternal, foetal and placental tissue, changes in the amniotic and extracellular fluid, and blood volume expansion. It seems that BIA has a better prognostic potential for gestational and post-partum outcomes than body mass index41. It has been observed that the BIA method can be successfully used to study the effect of excessive gestational weight gain in pregnancy on the development of obstetric complications, including gestational diabetes mellitus42.
HRV recording
Following 10 min of rest in supine position, short-term HRV was recorded based on the recommendation of Task Force on HRV43 and as described earlier35. ECG electrodes were connected and Lead II ECG was acquired for 15 min at a rate of 20,000 samples/sec using BIOPAC MP 46 data acquisition system (BIOPAC Inc., USA). The data were transferred from BIOPAC to a laptop with Biopac Student Lab (BSL) software. With due care, ectopics and artifacts were meticulously removed from the recorded ECG. By using the R wave detector in the BSL software, the extraction of RR tachogram was done from the edited 900 s ECG. HRV analysis was done by using software (Kubios HRV standard, version 3.5.0) that analyzes the frequency spectral components and time-domain components using Fast Fourier transformation in the RR trend. The frequency-domain indices of HRV recorded were total power (TP), component of low frequency expressed as normalized unit (LFnu), component of high frequency expressed as normalized unit (HFnu), and LF–HF ratio. The time-domain indices of HRV included square root of the mean of the sum of the squares of differences between adjacent RR interval (RMSSD), mean and standard deviation of RR intervals (SDNN), adjacent RR interval differing more than 50 ms (NN50), and NN50 counts divided by all RR intervals (pNN50).
Perceived stress scale
Perceived Stress Scale (PSS) was used to determine the stress level of the subjects17,44. The questionnaire consists of 10 questions with a score of 0–4 each. The score ranges from 0 to 40. A score of 0–13 is low stress, 14–26 is moderate stress and 27–40 is considered as high stress.
Edinburgh postnatal depression scale
Edinburgh Postnatal Depression Scale (EPDS) was used to assess the participant mood in the past 1 week17,45. It entails ten questions with a score of 0–3 each question. The score of EPDS ranges from 0 to 30. The higher the scores, risk of depression is high and a score of 10 or greater indicates possible depression.
Flanagan’s quality of life
In the present study, Flanagan Quality of Life (QoL) scale was used to assess the quality of life of the participants46. The questionnaire comprises of 16 items covering five aspects including physical and Material well-being; relations with other people; social, community and civic activities; personal development and fulfilment; and recreation. The score ranges from 16 to 112.
Participants were not asked to fill the questionnaires on their own. However, to avoid biased information, an independent researcher was assigned for the task. Time taken to fill the above-mentioned three set of questionnaires was about 15 to 20 min.
Estimation of biochemical parameters
Five ml of fasting blood samples were collected and serum was separated and stored at − 80 °C for further analysis. Fasting blood glucose (FBG) was noted from the patients’ case records. Insulin was measured using enzyme linked immunosorbent assay (ELISA) (Calbiotech, USA). For determination of insulin resistance, homeostatic model assessment (HOMA) of insulin resistance was calculated [HOMA-IR = FBG (mg/dL) × Insulin (μIU/L)/405]. HbA1c was determined from whole blood using commercial kits for turbidimetric immunoassay (Quantia, Tulip diagnostics). Malondialdehyde (MDA), the oxidative stress marker was measured by colorimetric assay kit (Elabscience, USA) and inflammatory markers such as interleukin-6 (IL-6) (Diaclone, France) and high sensitive C-reactive protein (hsCRP) (Calbiotech, USA) were measured by ELISA kits according to the manufacturer instructions. Nitric oxide derivatives (nitrate and nitrite) were estimated by colorimetric method using microplate reader (Elabscience, USA).
Statistical analysis
Statistical Package for the Social Sciences (SPSS) version 13 (SPSS Software Inc., Chicago, IL, USA) was used for statistical analysis. Normality of data was tested by Kolmogorov–Smirnov test and accordingly data were expressed as mean ± SD and median (interquartile range) for parametric and non-parametric data respectively. For comparison of data between control and study groups, the level of significance was tested by unpaired t test for parametric data, Mann–Whitney U test for non-parametric data. The association of PSS and EPDS with various parameters was assessed by Spearman correlation analysis. The independent contribution of various parameters to PSS and EPDS was assessed by multiple regression analysis. The p value less than 0.05 were considered statistically significant.
Ethics approval and consent to participate
Approval was obtained from Institute Ethics Committee (Human studies) of JIPMER (Reference number: JIP/IEC/2020/09). All the methods were performed in accordance with the institutional guidelines and regulations. Individual written informed consent was obtained from the participants before recruiting into the study.
Results
There was no significant difference in age between the subjects of control group and study group. There was a significant increase (p < 0.001) in the body weight and BMI in the study group compared to control group (Table 3).
Table 3.
Comparison of anthropometric, body composition and blood pressure-related parameters in normal pregnant women (control group) and women with GDM (study group) at 36th week of gestation.
| Parameters | Control group (n = 164) | Study group (n = 176) | p value |
|---|---|---|---|
| Age (years) | 27.35 (24.00–30.00) | 27.50 (25.00–30.00) | 0.764 |
| Body weight (kg) | 65.41 ± 8.78 | 71.04 ± 12.37 | < 0.001 |
| BMI (kg/m2) | 25.80 (22.70–28.80) | 28.29 (26.22–31.37) | < 0.001 |
| Body composition parameters | |||
| Total body fat (%) | 27.12 ± 4.38 | 33.10 ± 5.55 | < 0.001 |
| Visceral fat (%) | 5.86 ± 2.10 | 7.91 ± 2.71 | < 0.001 |
| Body lean (%) | 72.85 ± 7.15 | 66.17 ± 6.80 | < 0.001 |
| RM (kcal/day) | 1245.70 ± 136.90 | 1424.03 ± 182.60 | < 0.001 |
| BFMI (kg/m2) | 7.38 ± 0.88 | 9.67 ± 1.08 | < 0.001 |
| FFMI (kg/m2) | 19.75 ± 1.37 | 20.26 ± 1.42 | 0.001 |
| Subcutaneous fat (%) | 27.70 (22.50–33.20) | 31.50 (26.40–35.67) | < 0.001 |
| Skeletal muscle (%) | 26.77 ± 2.95 | 24.49 ± 2.98 | < 0.001 |
| RM/BW (kcal/kg) | 19.03 ± 1.85 | 20.49 ± 2.56 | < 0.001 |
| RM/BF (kcal/kg) | 71.02 ± 7.45 | 60.56 ± 6.10 | < 0.001 |
| Blood pressure related parameters | |||
| HR (per min) | 80.12 ± 7.30 | 90.15 ± 10.07 | < 0.001 |
| SBP (mmHg) | 111.00 (103.00–119.00) | 120.00 (110.00–125.00) | < 0.001 |
| DBP (mmHg) | 71.00 (65.00–79.00) | 78.00 (70.00–83.00) | < 0.001 |
| MAP (mmHg) | 84.95 (77.50–92.80) | 91.67 (83.75–96.33) | < 0.001 |
| RPP (mmHg) | 90.85 (78.85–105.20) | 106.09 (98.28–116.61) | < 0.001 |
The data presented are mean ± SD and median (IQR). Comparison was done by unpaired t test for parametric data and Mann Whitney U test for non-parametric data. P value < 0.05 was considered statistically significant.
BMI body mass index, RM resting metabolism, BFMI body fat mass index, FFMI fat-free mass index, BW body weight, BF body fat, HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, MAP mean arterial pressure, RPP rate pressure product.
Body composition parameters such as total body fat, visceral fat, RM, BFMI, FFMI (p = 0.001), subcutaneous fat and RM/BW were found to be significantly increased (p < 0.001) and body lean, skeletal muscle and RM/BF were found to be significantly decreased (p < 0.001) in study group compared to control group (Table 3). HR, SBP, DBP, MAP, and RPP of study subjects were significantly increased (p < 0.001) in study group compared to the control group subjects (Table 3).
Among the frequency domain indices of HRV, TP and HFnu were significantly reduced (p < 0.001), and LFnu and LF–HF ratio were significantly increased (p < 0.001) in study group compared to control group subjects. Time domain indices such as SDNN, RMSSD, NN50 and pNN50 were significantly reduced (p < 0.001) in subjects of study group compared to control group (Table 4).
Table 4.
Comparison of frequency domain and time domain indices of heart rate variability, perceived stress scale, quality of life and Indian diabetic risk score in normal pregnant women (control group) and women with GDM (study group) at 36th week of gestation.
| Parameters | Control group (n = 164) | Study group (n = 176) | p value |
|---|---|---|---|
| HRV frequency domain indices | |||
| TP (ms2) | 950.74 (610.20–1310.20) | 506.50 (393.00–597.00) | < 0.001 |
| LFnu | 50.50 (38.70–62.36) | 62.04 (51.85–65.56) | < 0.001 |
| HFnu | 46.90 (40.25–61.86) | 37.74 (33.65–47.93) | < 0.001 |
| LF–HF ratio | 1.10 (0.72–1.40) | 1.62 (1.08–1.93) | < 0.001 |
| HRV time domain indices | |||
| SDNN (ms) | 40.10 (27.35–52.10) | 21.20 (17.40–29.70) | < 0.001 |
| RMSSD (ms) | 45.32 (31.20–58.80) | 23.70 (18.40–30.30) | < 0.001 |
| NN50 | 33.15 (25.20–43.62) | 28.00 (22.00–32.00) | < 0.001 |
| pNN50 (%) | 10.27 (7.10–13.20) | 6.05 (4.59–6.88) | < 0.001 |
| Stress and risk scores | |||
| PSS score | 14.30 (11.50–17.80) | 24.00 (21.00–27.75) | < 0.001 |
| EPDS score | 7.60 ± 1.80 | 10.57 ± 2.21 | < 0.001 |
| Flanagan QoL score | 105.00 (93.00–113.50) | 90.00 (82.25–95.00) | < 0.001 |
| IDRS | 40.00 (35.20–45.80) | 60.00 (50.00–70.00) | < 0.001 |
The data presented are mean ± SD and median (IQR). Comparison was done by unpaired t test for parametric data and Mann Whitney U test for non-parametric data. P value < 0.05 was considered statistically significant.
TP total power of HRV, LFnu low-frequency power normalized, HFnu high-frequency power normalized, LF–HF ratio ratio of LF to HF, SDNN standard deviation of normal to normal (NN) interval, RMSSD square root of the mean of the sum of the squares of the differences between adjacent NN intervals, NN50 number of interval differences of successive NN intervals greater than 50, pNN50 NN50 counts divided by all RR intervals, PSS perceived stress scale, EPDS Edinburgh postnatal depression scale, QoL quality of life, IDRS Indian diabetic risk score.
PSS score, EPDS and IDRS were significantly increased in study group (p < 0.001) when compared to compared group, whereas QoL score was significantly reduced (p < 0.001) in study group than control group subjects (Table 4).
Glucose-related parameters such as FBG, insulin, HOMA-IR and HbA1C were significantly increased (p < 0.001) in study group compared to control group. Inflammatory markers such as IL-6 and hsCRP, and the oxidative stress marker, MDA were significantly increased (p < 0.001), while NO was significantly decreased (p < 0.001) in the subjects of study group compared to control group (Table 5).
Table 5.
Comparison of insulin resistance markers, oxidative stress marker and inflammatory markers in normal pregnant women (control group) and women with GDM (study group) at 36th week of gestation.
| Parameters | Control group (n = 164) | Study group (n = 176) | p value |
|---|---|---|---|
| Glucose-related parameters | |||
| FBG (mg/dL) | 86.17 ± 12.45 | 116.86 ± 16.62 | < 0.001 |
| Insulin (uIU/ml) | 10.60 (5.10–16.80) | 23.85 (16.38–31.29) | < 0.001 |
| HOMA-IR | 3.20 (1.10–5.20) | 6.33 (4.16–9.03) | < 0.001 |
| HbA1C (gm%) | 5.18 ± 0.86 | 6.81 ± 1.19 | < 0.001 |
| Oxidative stress marker | |||
| MDA (μmol/L) | 15.10 (11.20–19.80) | 22.95 (17.62–25.60) | < 0.001 |
| Inflammatory markers | |||
| IL-6 (pg/mL) | 12.45 ± 3.05 | 20.12 ± 5.34 | < 0.001 |
| hsCRP (μmol/L) | 3.10 (2.15–5.10) | 7.28 (4.71–9.65) | < 0.001 |
| Endothelial dysfunction marker | |||
| NO (µM/L) | 30.80 ± 3.80 | 25.43 ± 2.83 | < 0.001 |
The data presented are mean ± SD and median (IQR). Comparison was done by unpaired t test for parametric data and Mann Whitney U test for non-parametric data. P value < 0.05 was considered statistically significant.
FBG fasting blood glucose, HOMA-IR homeostatic model assessment-insulin resistance, HbA1C glycated hemoglobin, MDA malondialdehyde, IL-6 interleukin-6, hsCRP high sensitive c-reactive protein, NO nitric oxide.
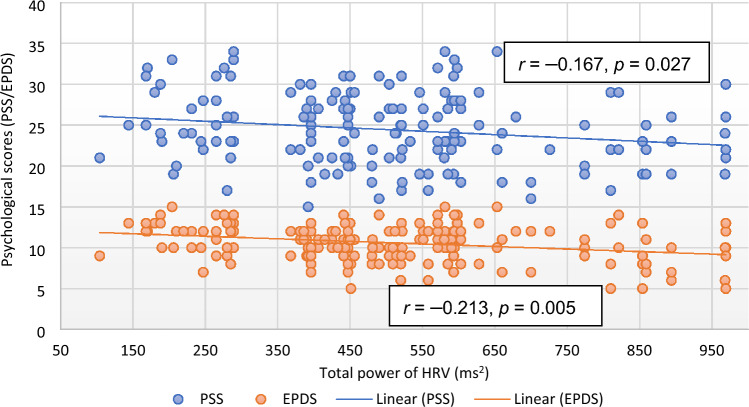
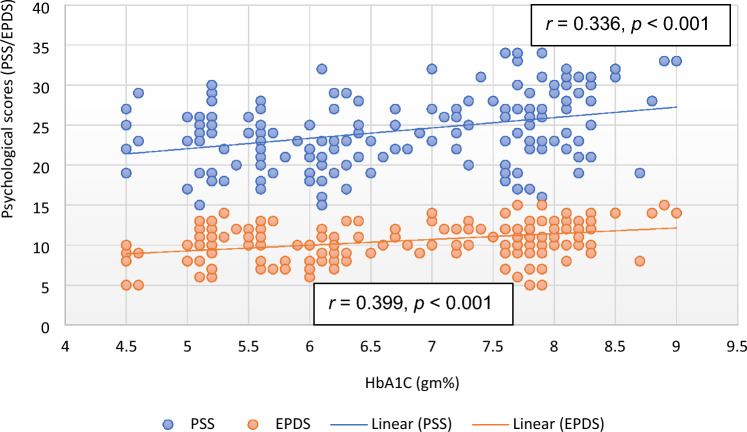
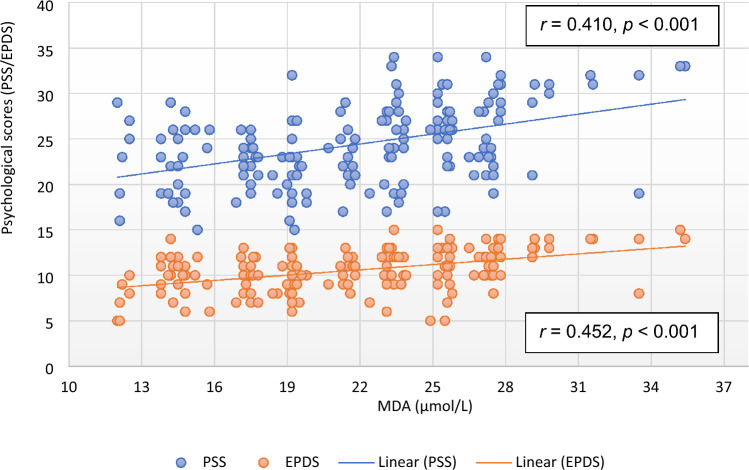
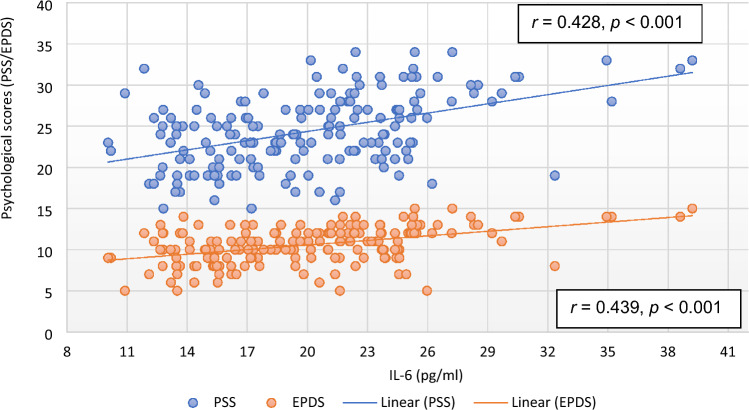
Except EPDS and QoL, there was no significant correlation of PSS with any of the parameter in the subjects of control group. However, the correlation of RPP (r = 0.193, p = 0.010), RM (r = 0.151, p = 0.045), HRV parameters [TP (r = − 0.167, p = 0.027; Fig. 1), LFnu (r = 0.235, p = 0.002), LF–HF ratio (r = 0.184, p = 0.014)], HbA1C (r = 0.336, p < 0.001; Fig. 2), oxidative stress marker [MDA (r = 0.410, p < 0.001; Fig. 3)], inflammatory marker, IL-6 (r = 0.428, p < 0.001; Fig. 4), endothelial dysfunction marker, NO (r = − 0.381, p < 0.001) and depression and risk scores such as EPDS (r = 0.582, p < 0.001), QoL (r = − 0.299, p < 0.001) and IDRS (r = 0.195, p = 0.009) with PSS were significant in the study group (Table 6).
Figure 1.
Correlation of total power of heart rate variability with psychological scores. EPDS Edinburgh postnatal depression scale, HRV heart rate variability, PSS perceived stress scale.
Figure 2.
Correlation of glycated hemoglobin with psychological scores. EPDS Edinburgh postnatal depression scale, HbA1C glycated hemoglobin, PSS perceived stress scale.
Figure 3.
Correlation of malondialdehyde with psychological scores. EPDS Edinburgh postnatal depression scale, MDA malondialdehyde, PSS perceived stress scale.
Figure 4.
Correlation of interleukin-6 with psychological scores. EPDS Edinburgh postnatal depression scale, IL-6 interleukin-6, PSS perceived stress scale.
Table 6.
Spearman correlation of PSS with various parameters in control group (n = 164) and study group (n = 176) at 36th week of gestation.
| Parameters | Control group at 36th week | Study group at 36th week | ||
|---|---|---|---|---|
| r value | p value | r value | p value | |
| BMI | –0.045 | 0.398 | 0.013 | 0.867 |
| HR | 0.109 | 0.185 | 0.120 | 0.114 |
| SBP | –0.145 | 0.095 | 0.090 | 0.235 |
| DBP | –0.102 | 0.197 | 0.077 | 0.310 |
| RPP | –0.152 | 0.085 | 0.193 | 0.010 |
| RM | 0.115 | 0.157 | 0.151 | 0.045 |
| TP | 0.108 | 0.186 | –0.167 | 0.027 |
| LFnu | 0.099 | 0.192 | 0.235 | 0.002 |
| HFnu | 0.053 | 0.286 | –0.140 | 0.064 |
| LF–HF ratio | –0.098 | 0.217 | 0.184 | 0.014 |
| HbA1C | 0.110 | 0.175 | 0.336 | < 0.001 |
| MDA | –0.103 | 0.195 | 0.410 | < 0.001 |
| hsCRP | –0.083 | 0.213 | 0.109 | 0.149 |
| IL-6 | –0.110 | 0.183 | 0.428 | < 0.001 |
| NO | 0.154 | 0.088 | –0.381 | < 0.001 |
| EPDS | 0.198 | 0.024 | 0.582 | < 0.001 |
| QoL | 0.162 | 0.048 | –0.299 | < 0.001 |
| IRDS | –0.095 | 0.220 | 0.195 | 0.009 |
The p value < 0.05 was considered significant; r: correlation coefficient.
PSS perceived stress scale, BMI body mass index, HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, RPP rate pressure product, RM resting metabolism, TP total power of HRV, LFnu low-frequency power normalized, HFnu high-frequency power normalized, LF–HF ratio of LF to HF, HbA1C glycated hemoglobin, MDA malondialdehyde, hsCRP high sensitive c-reactive protein, IL-6 interleukin-6, NO nitric oxide, EPDS Edinburgh postnatal depression scale, QoL quality of life, IRDS Indian diabetic risk score.
Except PSS, none of the parameters were significantly correlated with EPDS in the subjects of control group. In study group subjects, BMI, HR, SBP and DBP were not significantly correlated with EPDS scores, however, RPP (r = 0.168, p = 0.026) and RM (r = 0.153, p = 0.043) showed significant positive correlation to EPDS scores (Table 7).
Table 7.
Spearman correlation of EPDS with various parameters in control group (n = 164) and study group (n = 176) at 36th week of gestation.
| Parameters | Control group at 36th week | Study group at 36th week | ||
|---|---|---|---|---|
| r value | p value | r value | p value | |
| BMI | –0.110 | 0.184 | 0.119 | 0.115 |
| HR | 0.093 | 0.202 | 0.109 | 0.185 |
| SBP | –0.106 | 0.162 | 0.145 | 0.095 |
| DBP | –0.080 | 0.210 | 0.147 | 0.051 |
| RPP | –0.160 | 0.072 | 0.168 | 0.026 |
| RM | 0.102 | 0.195 | 0.153 | 0.043 |
| TP | 0.115 | 0.172 | –0.213 | 0.005 |
| LFnu | 0.090 | 0.192 | 0.287 | < 0.001 |
| HFnu | 0.050 | 0.302 | –0.201 | 0.007 |
| LF–HF ratio | –0.090 | 0.232 | 0.236 | 0.002 |
| HbA1C | 0.112 | 0.181 | 0.399 | < 0.001 |
| MDA | –0.150 | 0.086 | 0.452 | < 0.001 |
| hsCRP | –0.045 | 0.318 | 0.186 | 0.013 |
| IL-6 | –0.142 | 0.112 | 0.439 | < 0.001 |
| NO | 0.158 | 0.078 | –0.419 | < 0.001 |
| PSS | 0.180 | 0.047 | 0.582 | < 0.001 |
| QoL | 0.048 | 0.310 | –0.163 | 0.035 |
| IRDS | –0.175 | 0.052 | 0.178 | 0.018 |
The p value < 0.05 was considered significant; r: correlation coefficient.
EPDS Edinburgh postnatal depression scale, HR heart rate, SBP systolic blood pressure, DBP diastolic blood pressure, RPP rate pressure product, RM resting metabolism, TP total power of HRV, LFnu low-frequency power normalized, HFnu high-frequency power normalized, LF–HF ratio of LF to HF, HbA1C glycated hemoglobin, MDA malondialdehyde, hsCRP high sensitive c-reactive protein, IL-6 interleukin-6, NO nitric oxide, PSS perceived stress scale, QoL quality of life, IRDS Indian diabetic risk score.
IL-6 (r = 0.439, p < 0.001; Fig. 4), MDA (r = 0.452, p < 0.001; Fig. 3), HbA1C (r = 0.399, p < 0.001; Fig. 2), hsCRP (r = 0.186, p < 0.013), were positively correlated and NO (r = − 0.419, p < 0.001) was negatively correlated with EPDS scores in subjects of study group. Among HRV parameters, TP (r = − 0.213, p = 0.005; Fig. 1) and HFnu (r = − 0.201, p = 0.007) were negatively correlated and LFnu (r = 0.287, p < 0.001) and LF–HF ratio (r = 0.236, p < 0.002) were positively correlated with EPDS scores. PSS (r = 0.582, p < 0.001) and IDRS (r = 0.178, p = 0.018) were positively correlated and QoL (r = − 0.163, p = 0.035) was negatively correlated with EPDS (Table 7).
Multiple regression analysis revealed significant individual contribution of RPP (β = 0.154, p = 0.021), TP (β = − 0.182, p = 0.005), HbA1C (β = 0.386, p = 0.016), MDA (β = 0.345, p = 0.004) and IL-6 (β = 0.550, p < 0.001) to PSS in study group. Though RM and NO were correlated with PSS, its independent contribution to PSS was not significant (Table 8). TP (β = − 0.286, p < 0.001), HbA1C (β = 0.237, p = 0.004), MDA (β = 0.337, p < 0.001) and IL-6 (β = 0.393, p < 0.001) significantly contributed to EPDS in study group (Table 9).
Table 8.
Multiple regression analysis of PSS (as dependent variable) with various parameters (as independent variables) in study group at 36th week of gestation, adjusted for plasma insulin.
| Independent variables | Standardized regression coefficient β | 95% C.I. | p value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| RPP | 0.154 | 0.007 | 0.085 | 0.021 |
| TP | –0.182 | –0.007 | –0.001 | 0.005 |
| RM | 0.117 | 0.000 | 0.006 | 0.077 |
| HbA1C | 0.386 | –2.560 | –0.270 | 0.016 |
| MDA | 0.345 | 0.096 | 0.492 | 0.004 |
| IL-6 | 0.550 | 0.221 | 0.681 | < 0.001 |
| NO | –0.035 | –0.415 | 0.524 | 0.819 |
R: 0.578; R2: 0.334; adjusted R2: 0.307.
C.I. confidence interval, PSS perceived stress scale, RPP rate pressure product, TP total power of HRV, RM resting metabolism, HbA1C glycated hemoglobin, MDA malondialdehyde, IL-6 interleukin-6, NO nitric oxide.
Table 9.
Multiple regression analysis of EPDS (as dependent variable) with various parameters (as independent variables) in study group at 36th week of gestation, adjusted for plasma insulin.
| Independent variables | Standardized regression coefficient β | 95% C.I. | p value | |
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| RPP | 0.105 | –0.004 | 0.035 | 0.112 |
| TP | –0.286 | –0.005 | –0.002 | < 0.001 |
| RM | 0.072 | –0.001 | –0.002 | 0.272 |
| HbA1C | 0.237 | –1.009 | 0.133 | 0.004 |
| MDA | 0.337 | 0.046 | 0.243 | < 0.001 |
| IL-6 | 0.393 | 0.048 | 0.277 | < 0.001 |
| NO | –0.010 | –0.242 | 0.227 | 0.948 |
R: 0.591; R2: 0.349; adjusted R2: 0.322.
C.I. confidence interval, EPDS Edinburgh postnatal depression scale, RPP rate pressure product, TP total power of HRV, RM resting metabolism, HbA1C glycated hemoglobin, MDA malondialdehyde, IL-6 interleukin-6, NO nitric oxide.
Discussion
The participants of study group of the present study were GDM patients admitted to obstetrics ward of JIPMER hospital, which is a tertiary healthcare center of central government of India. Usually, the GDM patients admitted to this tertiary care hospital few weeks before delivery are those who have poor glycemic control inspite of treatment or those who are at risk of developing complications during delivery; otherwise they come for delivery just a couple of days before the expected date of delivery or after developing the labour pain. Thus, the GDM women in the study group had relatively less glycemic control in their 3rd trimester of pregnancy inspite of receiving antidiabetic treatment since the diagnosis of GDM. In the present study, the stress and depression scores were significantly increased in study group subjects compared to control group subjects depicting that the perceived psychological stress and depression are more in 36th week of gestation in pregnant women diagnosed with GDM. Earlier report37 suggests that pregnant women with GDM experience higher levels of anxiety and stress compared to non-pregnant women and healthy pregnant women. Diagnosis of GDM in pregnant women increases their anxiety, resulting in 2–4 times susceptibility to have antenatal or postnatal depression than women without GDM during pregnancy47. Pace et al48, reported that when pregnant women with GDM become apprehensive of uncontrolled diabetes resulting in adverse maternal and neonatal outcomes, they are more likely to experience antenatal depression, stress and anxiety49. In these women, the causes of anxiety and depression could be the inability to manage diabetes, serious concerns about the disease, need for dietary restrictions, insulin treatment and the demand for self-assessment of blood glucose50. Also, GDM patients are afraid of treatment and are concerned about the adverse effects of medication on the growth and development of the fetus, resulting in anxiety51. Additionally, women who received insulin treatment experienced significantly higher levels of stress compared to women who only received dietary treatment alone51. In the present study, 85.79% GDM patients (151/176) had received insulin therapy in addition to dietary management (Table 1), which could have been a factor for stress and depression in these patients.
In our current study, along with increased stress and depression there was decreased quality of life (QoL) in the study group compared to the control group subjects (Table 4). QoL is the most important indicator for assessing the status of health care in chronic diseases such as diabetes and it has been reported that increased stress is linked to poor QoL in diabetes52. Though decreased QoL has been reported in GDM53,54, the relationship of stress to QoL has not been studied in GDM yet. In our study, PSS (Table 6) and EPDS (Table 7) were highly correlated with decreased QoL in GDM women at 36th week of gestation. It has been observed earlier that depression in diabetes is an important comorbidity that requires careful management because of its severe impact on QoL55. As such, decreased QoL increases the cardiovascular risk56. Therefore, the health care professionals need to consider these findings when treating patients with gestational diabetes. Further, in the present study, IDRS was significantly high in study group compared to control group and IDRS was significantly correlated with PSS (Table 6) and EPDS (Table 7) indicating that GDM women are at the higher risk of developing metabolic syndrome, which is defined as the cluster of conditions consisting of hyperinsulinemia (the upper fourth of the fasting insulin level among nondiabetic subjects) or hyperglycemia (fasting glucose ≥ 110 mg/dl) in addition to at least two of the following: waist girth ≥ 94 cm, dyslipidemia (triglycerides ≥ 150 mg/dl or HDL cholesterol < 40 mg/dl), or BP ≥ 140/90 mmHg or taking BP medication57. Though IDRS is generally used for screening of diabetes in the community58, the higher IDRS is reported to be associated with higher risk of metabolic syndrome and CVD even among people without prediabetes or diabetes59. Thus, decreased QoL and increased IDRS as observed in the study group subjects in the present study could increase the risk of developing CVD in women having GDM.
Though stress and depression have been highly documented in GDM, the pathophysiologic mechanisms of development of these mental illnesses in women with GDM have not been well studied till date. In the present study, the markers of inflammation were significantly increased in study group population (Table 5). Reports of animal studies and clinical trials indicate that among pro-inflammatory cytokines, IL-6 plays a unique role in the pathophysiology and somatic consequences of depression, and also mediate the effects of treatment of depressive disorder60,61. The IL-6 was highly correlated with PSS (r = 0.428, p < 0.001; Table 6) and EPDS (r = 0.439, p < 0.001; Table 7) (Fig. 4) and among all the parameters, IL-6 had highest independent contribution to PSS (β = 0.550, p < 0.001, Table 8) and EPDS (β = 0.393, p < 0.001, Table 9). Thus, findings of our study indicate that inflammation is associated with GDM, and IL-6 could be the potential contributor to the development of stress and depression in GDM. It has been stated earlier that elevated IL-6 activity may lead to depression through stimulation of hypothalamic–pituitary–adrenal axis or by influencing the metabolism of neurotransmitters60,62. Other suggested mechanisms are that IL-6 may increase the activity of indoleamine-2,3-dioxygenase (IDO), which catalyses tryptophan that in turn activate kynurenine pathway and reduce availability of serotonin in the brain63,64. As a result, quinolinic acid, 3-hydroxy kynurenine, and the neurotoxic N-methyl-d-aspartate glutamate agonist are produced, which causes oxidative stress and contributes to the neurodegeneration and depression. IL-6 is also reported to be involved of in neuro-inflammation and brain-derived neurotropic factor (BDNF)-mediated depression in various mental disorders65. Therefore, future studies should focus on the association of IL-6 with BDNF and brain activity of serotonin and IDO to understand the role of neuro-inflammation in the causation of depression in GDM.
Malondialdehyde, the marker of oxidative stress was significantly increased in study group compared to control group. Malondialdehyde was significantly correlated with stress and depression (Fig. 3) and had independent contribution to both PSS (Table 8) and EPDS (Table 9). These findings indicate the possible role of oxidative stress in causation of stress and depression in GDM. Though oxidative stress has been implicated in poor fetomaternal outcomes in GDM66, its role in pathophysiology of stress and depression in GDM has not been reported yet. Nitric oxide, the marker of endothelial dysfunction was significantly increased in study group and was substantially correlated with both stress and depression (Tables 6 and 7). However, nitric oxide had no independent association with PSS (Table 8) and EPDS (Table 9). Though nitric oxide is known to be involved in the pathophysiology of cardiometabolic risks in GDM both in antepartum and postpartum periods67, from the finding of the present study it appears that endothelial dysfunction is unlikely to be linked to stress and depression in GDM.
FBG and HbA1C were significantly high in study group compared to control group, indicating that glycemic control was not effective inspite of antidiabetic treatment given to all GDM patients (Table 1). Glycated hemoglobin was strongly correlated with both stress and depression (Tables 6 and 7; Fig. 2) and had strong independent association with both the parameters. The level of glycated hemoglobin generally reflects the glycemic status in last three months period of a person and a higher glycated hemoglobin represents the poor diabetic control and increased cardiovascular risk68. The higher blood glucose and HbA1C levels has been reported to be associated with higher stress in pregnant women69. Thus, it appears that disease severity (level of glycemic status) could be an indicator of stress and depression in GDM and higher glycated hemoglobin level could herald the onset of depression and cardiovascular disease in GDM. As 151 out of 176 GDM patients received insulin therapy (Table 1), HOMA-IR was not considered for correlation and regression analysis. Also, plasma insulin was adjusted in the multiple regression models (Tables 8 and 9).
Spectral analysis of HRV has been used for assessment of autonomic functions and dysfunctions in health and diseases28–31 and decreased HRV is a known marker of CVD risk33,34,70. In the present study, TP of HRV was considerably decreased in study group and control group (Table 4) and TP had significant correlation (Tables 6 and 7; Fig. 1) and independent association with stress and depression scores (Tables 8 and 9). TP of HRV reflects the overall magnitude of variability of heart rate and the vagal drive of cardiac modulation43,71. Decreased TP is an established marker of CVD risk71. Decrease in time domain indices of HRV also reflects decreased cardiac vagal modulation71. The time-domain indices were significantly decreased in study group subjects (Table 4). Thus, findings of the present study reveal the decreased cardiovagal modulation at 36th week of gestation in women with GDM, which is linked to increased stress and depression in women those who have been receiving antidiabetic treatment for GDM. Increased LF–HF ratio, the marker of sympathovagal imbalance was prominently higher in study group compared to control group, indicating a severe state of autonomic dysregulation in women with GDM. To best of our knowledge, the present study is the first of its kind linking the reduction in HRV and cardiometabolic risks with depression in GDM.
In the present study, RPP was significantly more in study group subjects, and increased RPP was significantly correlated (Tables 6 and 7) and independently associated (Tables 8 and 9) with PSS and EPDS. Increase in RPP physiologically indicates myocardial work stress40. Increase in RPP and heart rate has reported to be associated with CVD risks and sudden cardiac death, especially in individuals having high BP72. In the present study, HR and SBP were significantly more in study group subjects compared to control group subjects. Thus, findings of the present study indicate the presence of increased myocardial work stress in GDM and its possible link to stress and depression in GDM. Though BMI, body fat%, visceral fat%, BFMI, FFMI and resting metabolism were significantly high in the study group compared to control group (Table 3), the body composition and metabolism had no influence on stress and depression (Tables 8 and 9).
All the patients were followed upto delivery and their two important fetomaternal outcomes were noted. In control group, 82.92% (136/164) had normal delivery and 17.08% (28/164) had caesarean delivery, whereas in study group, 55.11% (97/176) had normal delivery and 44.89% (79/176) had caesarean delivery. The birth weight was normal (with reference to Indian standard birth weight of 2.7 kg to 3.1 kg in females, and 2.8 kg to 3.2 kg in males) in 92.68% (152/164) neonates in control group (birth weight 2.94 ± 0.24 kg) and there was no incidence of macrosomia. In study group, 64.77% (114/176) neonates had normal birth weight (2.97 ± 0.27 kg) and 48 neonates had macrosomia, i.e., more than 3.5 kg as per Indian birth weight reference73. Thus, it appears that fetomaternal outcomes were poor in women with GDM.
Persistent increase in IL-6 has been reported in stress reactions and in patients with depression. In the present study, the increased IL-6 were found to be strongly associated with stress and depression in GDM women receiving antidiabetic treatment. All these evidence suggest for future studies to address if restoration of IL-6 level could be the key to treatment of depression associated with inflammation in GDM. In developing countries like India, the glycemic control and fetomaternal outcomes are poor in GDM due to various socio-economic factors that include poor compliance to treatment54,73. Therefore, studies should be designed to assess if non-pharmacological interventions like yoga instituted early in the pregnancy can improve cardiovagal modulation and prevent the development of stress and depression in GDM.
Limitations
The major limitation of the study is that we did not recruit the subjects in 24th to 28th week of gestation to assess the cardiometabolic risks in GDM women when they were diagnosed with the disease. Also, we have not assessed the details of fetomaternal outcomes. However, the present study reports the cardiometabolic risks in GDM women at 36th week of pregnancy, and their potential contributions to stress and depression in GDM.
Acknowledgements
Authors acknowledge the support given by JIPMER Authority to the first author as part of her PhD intramural grant, for conduct of this study.
Abbreviations
- GDM
Gestational diabetes mellitus
- CMR
Cardiometabolic risks
- BP
Blood pressure
- HRV
Heart rate variability
- HbA1C
Glycated hemoglobin
- PSS
Perceived stress score
- QoL
Quality of life
- IDRS
Indian diabetic risk score
- EPDS
Edinburg postnatal depression score
- MDA
Malondialdehyde
- TP
Total power
- CV
Cardiovascular
- TNF-α
Tumor necrosis factor alpha
- IL-6
Interleukin-6
- hsCRP
High-sensitive C-reactive protein
- IADPSG
International association of diabetes and pregnancy study group
- OGTT
Oral glucose tolerance test
- BW
Body weight
- BMI
Body mass index
- HR
Heart rate
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- MAP
Mean arterial pressure
- RPP
Rate pressure product
- BIA
Bioelectrical impedance analysis
- RM
Resting metabolism
- BFMI
Body fat mass index
- FFMI
Fat free mass index
- LFnu
Low frequency power expressed in normalized units
- HFnu
High frequency power expressed in normalized units
- LF–HF ratio
Ratio of low-frequency to high-frequency power
- SDNN
Mean and standard deviation of RR intervals
- RMSSD
Square root of the mean of the sum of the squares of differences between adjacent RR interval
- NN50
Adjacent RR interval differing more than 50 ms
- pNN50
NN50 counts divided by all RR intervals
- HOMA
Homeostatic model assessment
Author contributions
G.K.P., L.C., N.N., K.T.H. and M.R. designed the protocol. M.R. conducted investigations. G.K.P., L.C., N.N. and K.T.H. assisted in conduct of work. G.K.P. supervised the work. M.R. performed biochemical estimations in guidance with N.N. and D.T. G.K.P., M.R., N.N. and K.T.H. did the data analysis. G.K.P. and M.R. prepared manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association Gestational diabetes mellitus. Diab. Care. 2003;26:S103–S105. doi: 10.2337/diacare.26.2007.S103. [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. http://www.idf.org/diabetesatlas.
- 3.Chudasama RK, Kadri AM, Ratnu A, Jain M, Kamariya CP. Magnitude of gestational diabetes mellitus, its influencing factors and diagnostic accuracy of capillary blood testing for its detection at a tertiary care centre, Rajkot, Gujarat. Indian J. Commun. Med. 2019;44:142–146. doi: 10.4103/ijcm.IJCM_283_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulshrestha V, Agarwal N. Maternal complications in pregnancy with diabetes. J. Pak. Med. Assoc. 2016;66:S74–S77. [PubMed] [Google Scholar]
- 5.Chong S, Yu-Mei W, Chen W, Hui-Xia Y. Updates in long-term maternal and fetal adverse effects of gestational diabetes mellitus. Matern. Fetal Med. 2019;1:91–94. doi: 10.1097/FM9.0000000000000019. [DOI] [Google Scholar]
- 6.Wicklow, B., Retnakaran R. Gestational diabetes mellitus and its implications across the life span. Diabetes Metab J. (2023). [DOI] [PMC free article] [PubMed]
- 7.Biaggi A, Conroy S, Pawlby S, Pariante CM. Identifying the women at risk of antenatal anxiety and depression: A systematic review. J. Affect. Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross GP, et al. Relationship between depression and diabetes in pregnancy: A systematic review. World J. Diab. 2016;7:554–571. doi: 10.4239/wjd.v7.i19.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lydon K, et al. Psychological stress associated with diabetes during pregnancy: A pilot study. Ir. Med. J. 2012;105:26–28. [PubMed] [Google Scholar]
- 10.Beka Q, et al. Development of perinatal mental illness in women with gestational diabetes mellitus: A population-based cohort study. Can. J. Diab. 2018;42:350–355.e1. doi: 10.1016/j.jcjd.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Riggin L. Association between gestational diabetes and mental illness. Can. J. Diab. 2020;44:566–571.e3. doi: 10.1016/j.jcjd.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Yang HO, Chen BO, Abdulrahman AM, Li L, Wu N. Associations between gestational diabetes and anxiety or depression: A systematic review. J. Diabetes Res. 2021;2021:9959779. doi: 10.1155/2021/9959779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrn MA, Penckofer S. Antenatal depression and gestational diabetes: A review of maternal and fetal outcomes. Nurs. Womens Health. 2013;17:22–33. doi: 10.1111/1751-486X.12003. [DOI] [PubMed] [Google Scholar]
- 14.Cosson E, et al. Psychosocial deprivation in women with gestational diabetes mellitus is associated with poor fetomaternal prognoses: An observational study. BMJ Open. 2015;5:e007120. doi: 10.1136/bmjopen-2014-007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruohomäki A, et al. The association between gestational diabetes mellitus and postpartum depressive symptomatology: A prospective cohort study. J. Affect. Disord. 2018;241:263–268. doi: 10.1016/j.jad.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 16.Green JB. Cardiovascular consequences of gestational diabetes. Circulation. 2021;143:988–990. doi: 10.1161/CIRCULATIONAHA.120.052995. [DOI] [PubMed] [Google Scholar]
- 17.Shah Z, Pal P, Pal GK, Papa D, Bharadwaj B. Assessment of the association of heart rate variability and baroreflex sensitivity with depressive symptoms and stress experienced by women in pregnancy. J. Affect. Disord. 2020;277:503–509. doi: 10.1016/j.jad.2020.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Renugasundari M, et al. Assessment of a short-course yoga practice on cardiometabolic risks, fetomaternal outcomes and psychophysical health in gestational diabetes mellitus. Biomedicine. 2021;41:337–343. doi: 10.51248/.v41i2.1036. [DOI] [Google Scholar]
- 19.Raison CL, Miller AH. Role of inflammation in depression: implications for phenomenology, pathophysiology and treatment. Mod. Trends Pharmacopsych. 2013;28:33–48. doi: 10.1159/000343966. [DOI] [PubMed] [Google Scholar]
- 20.Frank MS, et al. Serum markers of inflammation mediate the positive association between neuroticism and depression. Front. Psychiatry. 2018;9:609. doi: 10.3389/fpsyt.2018.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 22.Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: A population-based longitudinal study. JAMA Psych. 2014;71:1121–1128. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson AC, Carpenter MW. Inflammatory mediators in gestational diabetes mellitus. Obstet. Gynecol. Clin. N. Am. 2007;34:213–224. doi: 10.1016/j.ogc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Vrachnis N, et al. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int. J. Endocrinol. 2012;2012:549748. doi: 10.1155/2012/549748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robakis TK, Aasly L, Williams KE, Clark C, Rasgon N. Roles of inflammation and depression in the development of gestational diabetes. Curr. Behav. Neurosci. Rep. 2017;4:369–383. doi: 10.1007/s40473-017-0131-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lappas M, et al. The Role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid. Redox Signal. 2011;15:3061–3100. doi: 10.1089/ars.2010.3765. [DOI] [PubMed] [Google Scholar]
- 27.Lan Q, et al. Vascular endothelial dysfunction in gestational diabetes mellitus. Steroids. 2022;184:108993. doi: 10.1016/j.steroids.2022.108993. [DOI] [PubMed] [Google Scholar]
- 28.Jasmine MR, Nanda N, Sahoo J, Velkumary S, Pal GK. Increased osteoprotegerin level is associated with impaired cardiovagal modulation in type-2 diabetic patients treated with oral antidiabetic drugs. BMC Cardiovasc. Disord. 2020;20:453. doi: 10.1186/s12872-020-01729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auroprajna P, et al. Association of sympathovagal imbalance with cognitive impairment in type 2 diabetes in adults. Can. J. Diabetes. 2018;42:44–50. doi: 10.1016/j.jcjd.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Indumathy J, et al. Decreased baroreflex sensitivity is linked to sympathovagal imbalance, body fat mass and altered cardiometabolic profile in pre-obesity and obesity. Metabolism. 2015;64:1704–1714. doi: 10.1016/j.metabol.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Kuppusamy S, Pal GK, Habeebullah S, Ananthanarayanan PH, Pal P. Association of sympathovagal imbalance with cardiovascular risks in patients with polycystic ovary syndrome. Endocr. Res. 2015;40:37–43. doi: 10.3109/07435800.2014.920350. [DOI] [PubMed] [Google Scholar]
- 32.Syamsunder AN, et al. Association of sympathovagal imbalance with cardiovascular risks in overt hypothyroidism. N. Am. J. Med. Sci. 2013;5:554–561. doi: 10.4103/1947-2714.118921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pal GK, Shyma P, Habeebullah S, Shyjus P, Pal P. Spectral analysis of heart rate variability for early prediction of pregnancy-induced hypertension. Clin. Exp. Hypertens. 2009;31:330–341. doi: 10.1080/10641960802621333. [DOI] [PubMed] [Google Scholar]
- 34.Pal GK, et al. Assessment of sympathovagal imbalance by spectral analysis of heart rate variability in prehypertensive and hypertensive patients in Indian population. Clin. Exp. Hypertens. 2011;33:478–483. doi: 10.3109/10641963.2010.549275. [DOI] [PubMed] [Google Scholar]
- 35.Karthiga K, et al. Attenuation of baroreflex sensitivity and heart rate variability is linked to reduced levels of nitric oxide in pregnant women having risks of developing gestational hypertension. Clin. Exp. Hypertens. 2021;43:356–362. doi: 10.1080/10641963.2021.1883053. [DOI] [PubMed] [Google Scholar]
- 36.Rani PR, Begum J. Screening and diagnosis of gestational diabetes mellitus, Where do we stand. J. Clin. Diagn. Res. 2016;10:QE01–QE04. doi: 10.7860/JCDR/2016/17588.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayase M, Shimada M, Seki H. Sleep quality and stress in women with pregnancy-induced hypertension and gestational diabetes mellitus. Women Birth. 2014;27:190–195. doi: 10.1016/j.wombi.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Seshiah V, et al. Diagnosis and principles of management of gestational diabetes mellitus in the prevailing COVID-19 pandemic. Int. J. Diab. Dev. Ctries. 2020;40:329–334. doi: 10.1007/s13410-020-00860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balaji PA, Varne SR. Physiological effects of yogasanas and pranayama on metabolic parameters, maternal and fetal outcome in gestational diabetes. Natl. J. Physiol. Pharm. Pharmacol. 2017;7:724–728. [Google Scholar]
- 40.Meena R, Pal P, Papa D, Pal GK. Increased rate pressure product is linked to sympathovagal imbalance in Indian obese postmenopausal women. Int. J. Clin. Exp. Physiol. 2018;5:39–43. doi: 10.4103/ijcep.ijcep_15_18. [DOI] [Google Scholar]
- 41.Bosaeus M, Andersson-Hall U, Andersson L, Karlsson T, Ellegård L, Holmäng A. Body composition during pregnancy: Longitudinal changes and method comparisons. Reprod. Sci. 2020;27:1477–1489. doi: 10.1007/s43032-020-00141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obuchowska A, Standyło A, Kimber-Trojnar Ż, Leszczyńska-Gorzelak B. The possibility of using bioelectrical impedance analysis in pregnant and postpartum women. Diagnostics (Basel) 2021;11:1370. doi: 10.3390/diagnostics11081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation93, 1043–1065 (1996). [PubMed]
- 44.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 45.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br. J. Psychiatry J. Ment. Sci. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 46.Flanagan JC. A research approach to improving our quality of life. Am. Psychol. 1978;33:138–147. doi: 10.1037/0003-066X.33.2.138. [DOI] [Google Scholar]
- 47.Hinkle S, et al. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia. 2016;59:2594–2602. doi: 10.1007/s00125-016-4086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pace R, Rahme E, da Costa D, Dasgupta KK. Association between gestational diabetes mellitus and depression in parents: A retrospective cohort study. Clin. Epidemiol. 2018;10:1827–1838. doi: 10.2147/CLEP.S184319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalfrà MG, et al. Quality of life in pregnancy and post-partum: A study in diabetic patients. Qual. Life Res. 2012;21:291–298. doi: 10.1007/s11136-011-9940-5. [DOI] [PubMed] [Google Scholar]
- 50.Haiying L, Jingsi C, Danxi Z. Clinical investigation on mental health status of gestational diabetes patients. Matern. Child Health Care China. 2008;23:3868–3870. [Google Scholar]
- 51.Lee KW, et al. Prevalence and factors associated with depressive, anxiety and stress symptoms among women with gestational diabetes mellitus in tertiary care centres in Malaysia: A cross-sectional study. BMC Preg. Childb. 2019;19:367. doi: 10.1186/s12884-019-2519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dismuke CE, Hernandez-Tejada MA, Egede LE. Relationship of serious psychological distress to quality of life in adults with diabetes. Int. J. Psychiatry Med. 2014;48:135–146. doi: 10.2190/PM.48.2.f. [DOI] [PubMed] [Google Scholar]
- 53.Trutnovsky G, et al. Gestational diabetes: Women's concerns, mood state, quality of life and treatment satisfaction. J. Matern. Fetal Neonatal Med. 2012;25:2464–2466. doi: 10.3109/14767058.2012.683900. [DOI] [PubMed] [Google Scholar]
- 54.Ansarzadeh S, Salehi L, Mahmoodi Z, Mohammadbeigi A. Factors affecting the quality of life in women with gestational diabetes mellitus: A path analysis model. Health Qual. Life Outcomes. 2020;18:31. doi: 10.1186/s12955-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: A population study. Diab. Care. 2004;27:1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 56.Martinelli LM, et al. Quality of life and its association with cardiovascular risk factors in a community health care program population. Clinics (Sao Paulo) 2008;63:783–788. doi: 10.1590/S1807-59322008000600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patil R, Patil MSA. Using Indian diabetes risk score to identify adult women at risk for diabetes. J. Food Nutr. Popul. Health. 2021;5:64. [Google Scholar]
- 59.Mohan V, et al. Diabetes risk score helps identify metabolic syndrome and cardiovascular risk in Indians—The Chennai urban rural epidemiology study (CURES-38) Diab. Obes. Metab. 2007;9:337–343. doi: 10.1111/j.1463-1326.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 60.Ting EY, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int. J. Mol. Sci. 2020;21:2194. doi: 10.3390/ijms21062194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.García-Juárez M, Camacho-Morales A. Defining the role of anti- and pro-inflammatory outcomes of interleukin-6 in mental health. Neuroscience. 2022;492:32–46. doi: 10.1016/j.neuroscience.2022.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Zhao J, Shi W, Lu Y, Gao X, Wang A, Zhang S, et al. Alterations of monoamine neurotransmitters, HPA-axis hormones, and inflammation cytokines in reserpine-induced hyperalgesia and depression comorbidity rat model. BMC Psych. 2022;22:419. doi: 10.1186/s12888-022-04065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson G, et al. Increased IL-6 trans-signaling in depression: Focus on the tryptophan catabolite pathway, melatonin and neuroprogression. Pharmacol. Rep. 2013;65:1647–1654. doi: 10.1016/S1734-1140(13)71526-3. [DOI] [PubMed] [Google Scholar]
- 64.Wang R, Weng Y, Zhao S, Li S, Wen X, Huang G. IL-6 up-regulates indoleamine 2, 3-dioxygenase (IDO) expression in chorionic villi and decidua. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37:158–163. [PubMed] [Google Scholar]
- 65.Giacobbo BL, et al. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019;56:3295–3312. doi: 10.1007/s12035-018-1283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murthy KAS, Bhandiwada A, Chandan SL, Gowda SL, Sindhusree G. Evaluation of oxidative stress and proinflammatory cytokines in gestational diabetes mellitus and their correlation with pregnancy outcome. Indian J. Endocrinol. Metab. 2018;22:79–84. doi: 10.4103/ijem.IJEM_232_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colm JM, Eszter T, Fergus PM, Cathal MM. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: Windows into future cardiometabolic health? Front. Endocrinol. 2020;11:655. doi: 10.3389/fendo.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wareham NJ, Pfister R. Diabetes: Glycated hemoglobin is a marker of diabetes and CVD risk. Nat. Rev. Cardiol. 2010;7:367–368. doi: 10.1038/nrcardio.2010.84. [DOI] [PubMed] [Google Scholar]
- 69.Horsch A, et al. Stress exposure and psychological stress responses are related to glucose concentrations during pregnancy. Br. J. Health Psychol. 2016;21:712–729. doi: 10.1111/bjhp.12197. [DOI] [PubMed] [Google Scholar]
- 70.Pal GK, Pal P, Nanda N, Amudharaj D, Adithan C. Cardiovascular dysfunctions and sympathovagal imbalance in hypertension and prehypertension: Physiological perspectives. Future Cardiol. 2013;9:53–69. doi: 10.2217/fca.12.80. [DOI] [PubMed] [Google Scholar]
- 71.Pal, G. K., Pal, P. & Nanda, N. Autonomic dysfunctions, autonomic function tests and spectral analysis of heart rate variability. in Textbook of Medical Physiology. (eds. Pal, G. K. & Pal, P.). 2nd edn. 214–222 (Elsevier, 2022).
- 72.White WB. Heart rate and the rate-pressure product as determinants of cardiovascular risk in patients with hypertension. Am. J. Hypertens. 1999;12:50S–55S. doi: 10.1016/S0895-7061(98)00280-5. [DOI] [PubMed] [Google Scholar]
- 73.Bhavadharini B, et al. Elevated glycated hemoglobin predicts macrosomia among Asian Indian pregnant women (WINGS-9) Indian J. Endocrinol. Metab. 2018;21:184–189. doi: 10.4103/2230-8210.196003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.