Abstract
In areas of moderate to intense Plasmodium falciparum transmission, malaria in pregnancy remains a significant cause of low birth weight, stillbirth, and severe anaemia. Previously, fetal sex has been identified to modify the risks of maternal asthma, pre-eclampsia, and gestational diabetes. One study demonstrated increased risk of placental malaria in women carrying a female fetus. We investigated the association between fetal sex and malaria in pregnancy in 11 pregnancy studies conducted in sub-Saharan African countries and Papua New Guinea through meta-analysis using log binomial regression fitted to a random-effects model. Malaria infection during pregnancy and delivery was assessed using light microscopy, polymerase chain reaction, and histology. Five studies were observational studies and six were randomised controlled trials. Studies varied in terms of gravidity, gestational age at antenatal enrolment and bed net use. Presence of a female fetus was associated with malaria infection at enrolment by light microscopy (risk ratio 1.14 [95% confidence interval 1.04, 1.24]; P = 0.003; n = 11,729). Fetal sex did not associate with malaria infection when other time points or diagnostic methods were used. There is limited evidence that fetal sex influences the risk of malaria infection in pregnancy.
Subject terms: Epidemiology, Parasitology
Introduction
Malaria is an infectious disease caused by parasites of the genus Plasmodium and transmitted by infected female Anopheles mosquitoes1. In 2020, about 241 million people had malaria and 627,000 died. The same year, 11.6 million pregnant women were exposed to malaria in sub-Saharan Africa, and malaria infection resulted in 819,000 neonates with low birth weight (LBW, < 2500 g)2. Most malarial infections worldwide are attributed to Plasmodium falciparum and Plasmodium vivax, with P. falciparum accounting for most of the morbidity and mortality, especially in sub-Saharan Africa. In Africa, it is estimated that a quarter of cases of severe anaemia in pregnant women and a fifth of LBW and stillbirth cases can be linked to malaria3–5. Of note, P. falciparum-infected erythrocytes can sequester in the placental intervillous space, and placental malaria is associated with LBW and stillbirth3.
The risk of malaria in pregnancy is modulated by different factors. Transmission intensity drives the development of acquired immunity, and this leads to differences in disease risk and severity between areas of high and low transmission3. Furthermore, parity-specific immunity acquired through pregnancies seems to contribute to a lower risk of placental malaria and the poor birth outcomes associated with it 3. Young maternal age may be an independent risk factor, as studies have shown that younger women are more susceptible to placental malaria and its poor outcomes, compared to older women with the same gravidity6.
There have been several studies exploring if and how fetoplacental sex might impact maternal health7,8. Male fetal sex has been associated with pregnancy complications such as term pre-eclampsia and gestational diabetes in meta-analysis of studies including over 12.5 million women8. Mothers of male fetuses are at increased risk of pre-eclampsia, but male fetuses may be more likely to maintain their growth trajectory compared to female fetuses. The mechanism could involve redirecting maternal blood flow to the placenta through vasoconstriction of maternal microvasculature, which is enhanced in pre-eclamptic compared normotensive women with a male fetus9,10. In contrast, there was no difference in the microvasculature of normotensive and pre-eclamptic women carrying female fetuses9. A study of asthma in pregnancy also demonstrated reduced growth in female compared to male fetuses. In the same study, the use of glucocorticoids as treatment for mild asthma was shown to improve growth in female fetuses, suggesting that the sex-specific growth impairment is associated with inflammation pathways11. It was demonstrated that placentas of asthmatic pregnant women with female (but not male) fetuses had an increased expression of pro-inflammatory cytokines, namely tumor necrosis factor α (TNFα) and interleukin 6 (IL-6), compared to healthy women12. Similarly, lipopolysaccharide-stimulated peripheral blood mononuclear cells obtained from women pregnant with females released more IL-6, TNFα and IL-1β than cells from women pregnant with males12,13. These findings suggest that the fetus and/or its placenta could influence maternal physiology in a sex-specific manner.
Little is known about the effect of fetal sex on the risk of infection in the mother (the ‘host’), with most studies focusing on whether associations between maternal infection (e.g., malaria) and infant health outcomes (e.g., malaria incidence) differ by sex14. In women infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), maternal SARS-CoV-2-specific antibody titers were lower when the mother carried a male fetus15. So far, only one study has focused on maternal malaria risk. Adam et al. studied the relationship between placental malaria and fetal sex in women in eastern Sudan. Placental malaria was detected on placental histology and 88% of infections were past infections. The odds of placental malaria were 2.55 times higher when the fetus was female16. To complement the findings of this study, we conducted a meta-analysis of data collected from cohort studies and clinical trials that followed pregnant women in P. falciparum-endemic areas 17, to determine the association between fetal sex and malaria infection during pregnancy.
Results
Characteristics of study population
Of the 13 studies in the original pooled dataset, two studies were excluded. One was unavailable for this meta-analysis, and one did not record maternal malarial infection by LM, PCR or histology18,19. Of the remaining 11 studies included, five were observational and six were clinical trials measuring the effect of chemoprevention or insecticide-treated nets20–31. Nine studies were conducted in sub-Saharan African countries and two in PNG. Among 12,830 women enrolled in these studies, 12,821 singleton pregnancies with data on fetal sex were included in this analysis.
Approximately two-thirds of the women included were aged 24 years or younger. Gravidity, trimester at antenatal enrolment, and bed net ownership varied across studies. Most of the participants lived in rural areas. In the studies where chemoprevention was used, the most commonly used strategy was intermittent preventive treatment with sulfadoxine-pyrimethamine. Details of each study participants’ characteristics are outlined in Table 1.
Table 1.
Characteristics of study participants in the 11 studies which were part of the M3 Initiative (n = 12,821) 17.
| Benin-Stoppam20 | Congo-Landis21 | EMEP-MON 22,23 | ISTp-Malawi 48 | Kenya-Ayisi 25 | Kenya-2 26 | Malawi-LAIS 29 | PNG-IPTp27 | PNG-Sek 28 | STOPMIP-Kenya30 | Burkina Faso 31 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | 791 | 164 | 471 | 1618 | 3388 | 711 | 1190 | 1943 | 293 | 1228 | 1026 |
| Maternal age | 26.4 [6.1] | 27.5 [5.3] | 25.3 [6.4] | 22.5 [5.1] | 21.7 [4.6] | 25.8 [6.7] | 25 [6.4] | 24.5 [5.5] | 25.1 [5.6] | 23.4 [5.7] | 24.4 [6.2) |
| Gravidity | |||||||||||
| 1 (Primigravid) | 147 (19.9) | 43 (26.2) | 94 (20.0) | 551 (34.1) | 1656 (48.9) | 127 (17.9) | 267 (22.4) | 966 (47.5) | 115 (39.2) | 411 (33.4) | 210 (20.5) |
| 2 (Secundigravid) | 173 (21.9) | 22 (13.4) | 77 (16.3) | 450 (27.8) | 748 (22.1) | 118 (16.6) | 213 (17.9) | 494 (24.3) | 54 (18.4) | 242 (19.7) | 216 (21.3) |
| 3+ (Multigravid) | 471 (59.5) | 99 (60.4) | 300 (63.7) | 617 (38.1) | 984 (29.0) | 466 (65.5) | 710 (59.7) | 573 (28.2) | 124 (42.3) | 576 (46.9) | 606 (59.1) |
| Trimester at enrolment* | |||||||||||
| 1 | 174 (22.4) | 6 (3.7) | 69 (14.7) | 0 (0) | 0 (0) | 53 (9.1) | 0 (0) | 103 (7.9) | 2 (0.8) | 23 (1.9) | 388 (37.9) |
| 2 | 616 (77.9) | 158 (96.3) | 260 (55.4) | 1601 (99.0) | 0 (0) | 326 (56.0) | 1190 (100) | 1749 (90.1) | 212 (84.1) | 1011 (84.5) | 596 (58.2) |
| 3 | 1 (0.1) | 0 (0) | 140 (29.9) | 17 (1.1) | 3388 (100) | 203 (34.9) | 0 (0) | 90 (4.6) | 38 (15.1) | 194 (15.8) | 40 (3.9) |
| Bed net use | |||||||||||
| Yes | 254 (32.1) | 164 (100) | N/A | 331 (20.5) | N/A | 348 (48.9) | 877 (73.7 | 1798 (92.5) | 240 (83.0) | 690 (56.1) | N/A |
| No | 537 (67.9) | 0 (0) | N/A | 1287 (79.5) | N/A | 363 (51.1) | 313 (26.3) | 145 (7.5) | 49 (17.0) | 539 (43.9) | N/A |
| Rural residents | 791 (100) | 0 (0) | 471 (100) | 1605 (99.2) | 722 (21.3) | 711 (100) | 1190 (100) | 1185 (61) | 282 (96.20) | 1050 (85.40) | N/A |
| Chemo-prevention | IPTp-SP | IPTp-SP | IPTp-SP | IPTp-SP, ISTp-DP | – | IPTp-SP | ITp-SP, IPTp-SPAZ | SPCQ, IPTp-SPAZ | SPCQ | IPTp-SP, IPTp-DP, ISTp-DP | IPTp-SP |
| No of IPTp doses (median) | 2 | 2 | N/A | 4 | N/A | N/A | 4 | N/A | 2 | 2 | |
| Female infant | 394 (49.1) | 85 (51.8) | 249 (52.9) | 810 (50.1) | 1697 (50.1) | 358 (50.4) | 584 (49.1) | 1078 (55.5) | 141 (48.1) | 605 (49.3) | 514 (50.2) |
Data are n (%) or mean [SD].
ISTp intermittent screen and treat in pregnancy, IPTp intermittent preventive treatment in pregnancy, DP dihydroartemisinin-piperaquine, N/A not available, SP sulphadoxine-pyrimethamine, SPAZ SP plus azithromycin, SPCQ SP plus chloroquine (single course SP plus CQ at enrolment, followed by weekly CQ).
*Pregnancies were dated by ultrasound or (when ultrasound was not available) symphysis-fundal height. Studies that determined gestational age at first antenatal visit using symphysis-fundal height exclusively include Kenya-Ayisi, Kenya-2 and PNG-Sek.
Effect of fetal sex on risk of malaria in pregnancy at antenatal enrolment
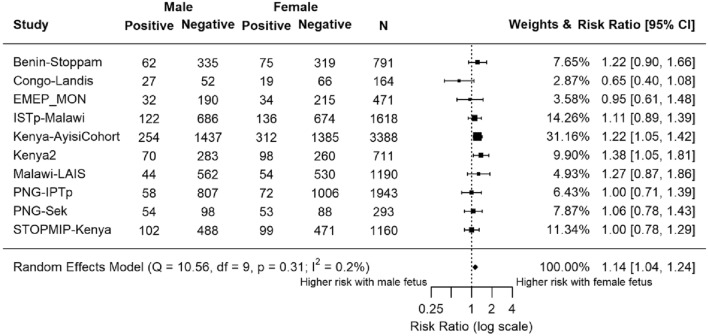
Ten studies (n = 11,729) assessed peripheral malaria status at antenatal enrolment using LM (Fig. 1). Overall, the summary estimate RR was 1.14, suggesting a higher risk of malaria with female fetuses (95% confidence interval [CI] 1.04, 1.24; P = 0.0032). Most studies showed an increased prevalence of peripheral malaria infection at antenatal enrolment in mothers carrying female fetuses (Supplementary Fig. 1). Seven studies found an increased risk of malaria infection associated with carrying a female fetus, although differences were only statistically significant at P < 0.05 for two individual studies, both from Kenya (Fig. 1).
Figure 1.
Forest plot of the association between fetal sex and risk of maternal peripheral malaria infection at antenatal enrolment, by light microscopy. Estimates compared the risk of maternal malaria infection by light microscopy at antenatal clinic enrolment in women carrying female fetuses to those carrying male fetuses. Estimates represented by a small box, where the width of the whisker corresponds to the 95% confidence interval (CI). Size of the box is proportional to the weight of the study. Heterogeneity of studies was not statistically significant (P = 0.3, I2 = 0.2%). N = 11,729.
Four studies (n = 4976) assessed peripheral malaria infection status at enrolment using PCR (Supplementary Fig. 2). The overall change in risk was minimal across all four studies. The summary estimate for this marker was 1.01 (95% CI 0.94, 1.04; P = 0.83).
Effect of fetal sex on risk of peripheral malaria infection at delivery
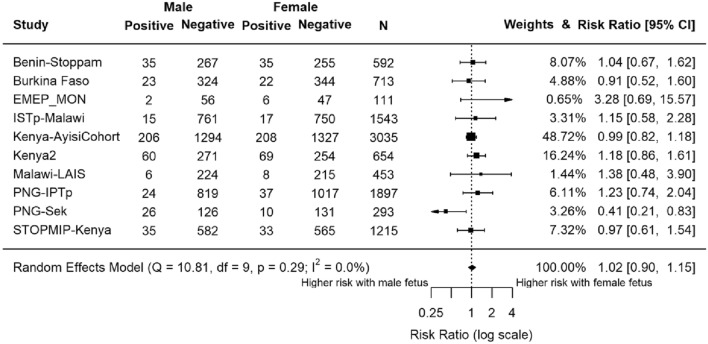
Peripheral malaria infection at delivery was diagnosed by LM in 10 studies (n = 10,506) (Fig. 2). Overall, there was no difference in maternal malaria risk by LM at delivery by fetal sex (RR 1.02, 95% CI 0.90, 1.15; P = 0.78). In six studies, malaria risk was higher with a female fetus and in four it was higher with a male fetus (Fig. 2). The PNG-Sek study showed that women carrying a female fetus had lower risk of malaria at delivery (RR 0.41, 95% CI 0.21, 0.83, P = 0.009).
Figure 2.
Forest plot of the association between fetal sex and risk of maternal peripheral malaria infection at delivery, by light microscopy. Estimates compared the risk of maternal malaria in those carrying female fetuses to those carrying male fetuses. Estimates represented by a small box, where the width of the whisker corresponds to the 95% confidence interval (CI). Size of the box is proportional to the weight of the study. Heterogeneity of studies not statistically significant (P = 0.29, I2 = 0.0%). N = 10,506.
Four studies (n = 2739) assessed peripheral malaria infection at delivery by PCR. Two found a heightened risk with a female fetus, while the other two did not, but none of these differences were statistically significant (Supplementary Fig. 3). The summary estimate for this marker was 1.03 (95% CI 0.80, 1.33; P = 0.80).
Effect of fetal sex on risk of placental malaria
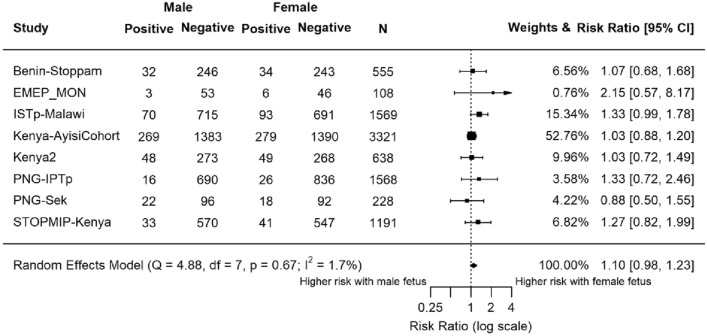
Placental malaria was assessed by LM in eight studies (n = 9178; Fig. 3). Estimates of seven studies indicated heightened risk of malaria in women carrying female fetuses. Overall, the summary estimate was 1.10 (95% CI 0.98, 1.23; P = 0.12).
Figure 3.
Forest plot of the association between fetal sex and risk of placental malaria tested at delivery by light microscopy. Estimates compared the risk of placental malaria infection in women carrying female fetuses to those carrying male fetuses. Estimates represented by a small box, where the width of the whisker corresponds to the 95% CI. Size of the box is proportional to the weight of the study. Heterogeneity of studies not statistically significant (P = 0.67, I2 = 1.7%). N = 9178.
Four studies (n = 4113) assessed for placental malaria infection at delivery using PCR (Supplementary Fig. 4). The summary estimate of this marker was 1.09 (95% CI 0.95, 1.27; P = 0.23).
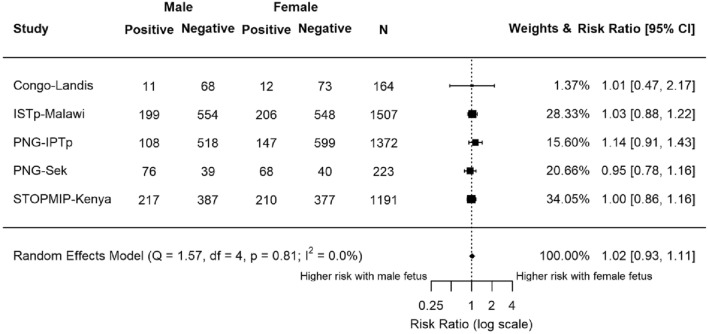
Placental malaria at delivery was assessed by histology in five studies (n = 4457; Fig. 4). We considered both active (n = 468) and past infections (n = 786) as indicative of placental infection. The summary estimate for this marker was 1.02 (95% CI 0.93, 1.11; P = 0.68). Neither the meta-analysis nor any individual study detected a statistically significant association between fetal sex and placental malaria detected by histology.
Figure 4.
Forest plot of the association between fetal sex and risk of placental malaria tested at delivery by histology. Active and past infections were both included. Estimates compared the risk of placental malaria in women carrying female fetuses to those carrying male fetuses. Estimates represented by a small box, where the width of the whisker corresponds to the 95% CI. Size of the box is proportional to the weight of the study. Heterogeneity of studies not statistically significant (P = 0.81, I2 = 0.0%). N = 4457.
Discussion
This study aimed to determine the impact of fetal sex on the risk of malaria in pregnancy. We found that women carrying female fetuses were at higher risk of peripheral malaria infection detected by microscopy at antenatal enrolment (RR 1.14). This was the only one out of seven malaria markers that showed a statistically significant result in meta-analysis, but the directionality of effect was similar when placental infection detected by LM at delivery was considered (RR 1.10). At the individual study level, one small cohort study suggested an association with male sex and increased risk of peripheral infection at delivery, but not other malaria markers28.
Light microscopy at antenatal enrolment was the most frequently measured marker of malaria infection, i.e., many of the included studies used LM to diagnose malaria infection, while fewer used PCR and histology. The large sample size for LM at enrolment contributes to a higher statistical power, reducing the probability of incorrectly accepting the null hypothesis32. Of the ten individual studies where malaria diagnosed by LM at antenatal enrolment was available, fetal sex was statistically significantly associated with malaria infection in two studies, both conducted in western Kenya in the 1990s when malaria transmission was very high, and there was a trend towards increased risk in another three studies (Fig. 1). An association between fetal sex and maternal malaria infection risk may be most discernible at antenatal enrolment, i.e., prior to the provision of various malaria interventions including antimalarial drugs and insecticide-treated nets that were evaluated in participating studies, and that are likely to alter malaria risk.
To date, only one study has explored the interaction between fetal sex and malaria risk in pregnancy. The study followed 339 women living in eastern Sudan, where intermittent preventive treatment in pregnancy (IPTp) is not used, and assessed placental malaria by histology. Risk of placental malaria was reported to be 2.55 times higher in women carrying female fetuses16. We were unable to replicate an association between female sex and placental infection on histology, perhaps because IPTp or intermittent screening and treatment was used in 10 of 11 studies included in this meta-analysis. However, our findings add to the evidence that carrying a female fetus may be associated with a higher risk of malaria parasitaemia in pregnancy. The disparate finding of increased risk of peripheral infection by light microscopy in male babies in a small cohort study from PNG may relate to sample size, and possibly loss-to-follow-up and documentation bias. There may also be sex-specific differences in risk of malaria in infancy. Severe past placental malaria (presence of pigment in > 20% of high power fields by microscopy of placental histology) was associated with an increased incidence of malaria in infancy in male but not female babies14, and IPTp with dihydroartemisinin-piperaquine was found to reduce incidence of malaria in male but not female infants33,34. These studies suggest that fetal sex might play a role in modulating malaria risk in infancy that is associated with placental malaria.
The mechanisms underpinning the potential association between female fetuses and malaria infection risk in the mother have yet to be elucidated. The placenta acts as an endocrine organ, secreting hormones and cytokines that might affect the physiology of both the mother and the fetus35,36. Several studies found that X chromosome inactivation results in sex-dependent differential gene expression, in both autosomal and sex-linked genes35,37. This includes upregulation of certain immunological mediators, including TNFα, in female placentas38. Previously, high levels of TNFα have been associated with higher parasite density and slower clearance39,40. Perhaps this upregulation of TNFα receptors led to an increased sensitivity to TNFα, resulting in slower clearance of parasites and more detectable infections in mothers carrying female fetuses. Asthma studies have shown that female placentas tend to upregulate cortisol levels in response to changes in maternal cortisol levels, which eventually results in increased expression of TNFα12,41. Possible mechanisms include sex-differential function of placental glucocorticoid receptor isoforms and reductions in placental 11β-hydroxysteroid dehydrogenase type 2 expression41,42. Further studies are required to confirm if a similar mechanism is observed with malaria infection. Upregulation of receptors used by parasites to sequester in the placenta should also be investigated as a possible mechanism in future studies.
The present study, and the preceding study by Adam et al., considered women who had a live born baby only. Male fetuses are at increased risk of stillbirth, and malaria is associated with stillbirth and miscarriage43–45. It is plausible that malaria in early gestation increases the risk of male fetal loss, which may manifest in an increased prevalence of malaria in pregnant women carrying female fetuses.
This study had strengths and limitations. Compared to the study by Adam et al.16, the dataset we used was of substantially larger sample size, including 4457 women for whom placental histology was available. The study population was drawn from a wider geographical area, including Papua New Guinea and multiple sub-Saharan African countries. This allows for more generalisation of the findings to P. falciparum-endemic regions. Furthermore, infection was assessed using LM, PCR and histology. The addition of PCR as a diagnostic tool enabled the detection of sub-microscopic infections. However, only four out of 11 studies tested for malaria infection using PCR, and the meta-analysis lacked power to conclusively evaluate the association between fetal sex and submicroscopic infection. Furthermore, it might be that fetal sex contributes to regulation of parasite density, rather than incidence of infection, and this could manifest in differences in infections detectable by microscopy, but not infections detected by PCR. The M3 initiative did not collate information on parasite densities. Lastly, differences in malaria risk, or the apparent lack of an association between fetal sex and malaria risk at birth, may have been the result of interventions started at antenatal enrolment such intermittent preventive treatment or provision of insecticide treated nets. In order to yield more conclusive results, assessing associations between fetal sex and malaria infection may be strengthened by including more studies that screened for infection, e.g., at enrolment using both LM and PCR, and that include pregnancies that resulted in stillbirth or miscarriage. Moreover, as the study population was diverse, other variables such as the maternal age and gravidity could have been confounders.
In conclusion, carrying a female fetus may be associated with a higher risk of malaria in pregnancy, as previously reported, but the magnitude of any such risk is modest. Women with a female fetus had a higher risk of peripheral malaria infection at antenatal enrolment when determined by LM. This time point included the largest number of observations, and preceded the initiation of interventions such as IPTp or insecticide-treated bed nets that could have potentially masked sex-differential effects. Individual participant data meta-analysis of more recent interventional and cohort studies, adjusting for potential confounders and using ultrasound dating and including fetal losses, could confirm our findings, while efforts should be made to identify the underlying biological mechanisms that could underpin fetal sex-based differences in the risk of malaria in pregnancy.
Methods
Study population
The study population, summarised in detail elsewhere17, was derived from a dataset gathered by the Maternal Malaria and Malnutrition (M3) initiative that originally included data pooled from 13 studies following 13,898 pregnant women in P. falciparum-endemic areas, including Papua New Guinea (PNG) and several countries in sub-Saharan Africa (see cohort profile)17. Studies included were either prospective cohort studies or randomised controlled trials conducted between 1996 and 2015. Variables such as age, gravidity, and body mass index of the participants were recorded. Participants were also assessed for malaria infection at antenatal clinic enrolment and at delivery, using various diagnostic tools including light microscopy (LM) of blood films and polymerase chain reaction (PCR). Most studies used LM to detect parasitaemia, while some also used PCR to detect sub-microscopic infections. At delivery, some studies assessed the presence of placental malaria using LM, PCR or placental histology17, and each was analysed individually.
Where available, placental histology results were classified into three categories. Active infection was characterised by presence of parasites, regardless of presence of pigment in fibrin. Past infection was characterised by absence of parasites and presence of fibrin pigment. No infection was characterised by absence of both parasites and fibrin pigment46.
Data analysis
Univariable meta-analysis was performed using RStudio version 4.0.2. The dataset was reshaped for compatibility with the metafor package in R47. Women without data for fetal sex were excluded, as were women who had not been screened for malaria infection by LM, PCR or histology.
The exposure of interest was fetal sex, defined as male or female, and the outcome measure was malaria infection, assessed using differing techniques at enrolment or delivery. The association between fetal sex and maternal malaria infection was investigated for peripheral malaria infection detected by LM and PCR at enrolment and delivery, and for placental malaria detected by LM, PCR and placental histology. We calculated risk ratios and sampling variances using log-binomial regression analyses (escalc function) and fitted them to a random-effects model to calculate the summary estimate and heterogeneity (rma function). Forest plots were generated using the forest function. Heterogeneity was assessed using the P-value of the Chi-square statistic and I2. A P-value of less than 0.1 and I2 value of more than 40% were considered significant. Rows with missing values for respective malaria infection indicators were omitted. In meta-analyses by time-point and diagnostic modality, studies that had less than 100 women with data for a respective malaria indicator of interest were also excluded, as were studies that had no women with malaria in at least one of the groups (male or female fetus). To account for multiple comparisons (n = 7) a Bonferroni-corrected P-value of < 0.007 was used to denote statistical significance.
All studies received approval by their local ethics board and obtained informed consent from all participants. Details of relevant procedures are highlighted in the published cohort profile 17.
Supplementary Information
Acknowledgements
The authors thank Amaya Ortega Pajares and Isobel Sylvia Walker for their inputs regarding statistical analyses. They acknowledge the contributions of Dr Daniel Westreich and the late Professor Steven Meshnick to the development of the M3 cohort.
Author contributions
S.J.R. and H.W.U. conceived and designed the study. J.C., H.W.U., J.G., V.B., N.F., I.V., H.T., U.A., S.H.L., F.t.K., S.D., P.O., M.O., V.M., L.S., D.T., S.K., J.A., B.N., M.D., M.M., L.K.P., P.A., K.M., A.T.K., I.V., D.S., A.v.E., M.O.K., E.A. and S.J.R. curated the data. A.J.H., S.J.R., H.W.U. and D.W. verified, analysed and interpreted the data. H.W.U., A.J.H., and S.J.R. drafted the original version of the manuscript. All authors read and approved the final manuscript.
Data availability
Data are available from the WWARN data repository (http://www.wwarn.org/working-together/sharing-data/accessing-data) for researchers who meet the criteria for access to confidential data.
Competing interests
The authors of this manuscript have the following competing interests: SHL is a full-time employee of BioMarin Pharmaceutical and holds shares in BioMarin Pharmaceutical. No other competing interests declared. The findings and conclusions presented in this manuscript are those of the authors and do not reflect the official position of the U.S. Centers for Disease Control and Prevention. JC was funded by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (Pre-doctoral Training in Infectious Disease Epidemiology grant #5 T32 AI070114). HWU is funded through a Menzies School of Health Research Fellowship. The STOPPAM project, “Strategies To Prevent Pregnancy-Associated Malaria”, was supported by the European Union’s Seventh Framework Programme (EU FP7); STOPPAM contract number: 200889. The FSP/MISAME study (Burkina Faso) was funded by Nutrition Third World, The Belgium Ministry of Development, Flemish Interuniversity Council, and French Ministry of Development. The ECHO study (Democratic Republic of the Congo) was funded by the Department of Epidemiology, University of North Carolina Chapel Hill, UNC Gillings School of Global Public Health. EMEP was partly supported by the Malaria in Pregnancy (MiP) Consortium, which was funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine, UK and partly by the US Centers for Disease Control and Prevention (CDC), Division of Parasitic Diseases and Malaria through a cooperative agreement with Kenya Medical Research Institute (KEMRI), Center for Global Health Research (CGHR), Kisumu, Kenya. The IPTp-MON study (Kenya) was partly supported by the MiP Consortium, which is funded through agrant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine, UK and partly supported by the CDC. The ITN project (Kenya) was funded by the US Agency for International Development. The Special Health Support Fund from the Royal Netherlands Embassy (Nairobi, Kenya) provided additional support for the study of the impact of ITN in pregnancy. The Kisumu study (Kenya-2) was funded by US Agency for International Development (grants AOT0483-PH1-2171 and HRN-A-00-04-00010-02) and the Netherlands Foundation for the Advancement of Tropical Research. The STOPMIP study (Kenya) was funded by the Malaria in Pregnancy (MiP) Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine, UK. The ISTp study (Malawi) was partly supported by the Malaria in Pregnancy (MiP) Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine, UK and partly funded by the European and Developing Countries Clinical Trials Partnership (EDCTP). The LAIS study was supported by grants from the Academy of Finland (Grants 79787 and 207010), the Foundation for Pediatric Research in Finland, and the Medical Research Fund of Tampere University Hospital. Azithromycin and its placebo were provided free of charge by Pfizer Inc (New York, New York), which also provided funding for the polymerase chain reaction testing of the sexually transmitted infections. The IPTp study (Papua New Guinea [PNG]) was funded by the MiP Consortium, through a grant from the Bill & Melinda Gates Foundation (46099); the Pregvax Consortium, through a grant from the EU FP7-2007-HEALTH (PREGVAX 201588) and the Spanish Government (EUROSALUD 2008 Programme); and Pfizer Inc., through an investigator-initiated research grant (WS394663). The Sek study (PNG) was supported by AusAID (grant to PNG Institute of Medical Research [IMR]), the National Health and Medical Research Council of Australia; Australian Research Council; Wellcome Trust; and Veterans Affairs Research Service. The Walter and Eliza Hall Institute is supported by the NHMRC Infrastructure for Research Institutes Support Scheme and Victorian State Government Operational Infrastructure Support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-37431-3.
References
- 1.Cowman AF, Healer J, Marapana D, Marsh K. Malaria: Biology and disease. Cell. 2016;167:610–624. doi: 10.1016/j.cell.2016.07.055. [DOI] [PubMed] [Google Scholar]
- 2.WHO . World Malaria Report 2021. World Health Organization; 2021. [Google Scholar]
- 3.Rogerson SJ, et al. Burden, pathology, and costs of malaria in pregnancy: New developments for an old problem. Lancet Infect. Dis. 2018;18:e107–e118. doi: 10.1016/S1473-3099(18)30066-5. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin. Microbiol. Rev. 2004;17:760–769. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker PG, Ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: A modelling study. Lancet Glob. Health. 2014;2:e460–467. doi: 10.1016/S2214-109X(14)70256-6. [DOI] [PubMed] [Google Scholar]
- 6.Bihoun B, et al. Age-modified factors associated with placental malaria in rural Burkina Faso. BMC Pregn. Childbirth. 2022;22:248. doi: 10.1186/s12884-022-04568-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Qaraghouli M, Fang YMV. Effect of fetal sex on maternal and obstetric outcomes. Front. Pediatr. 2017;5:144. doi: 10.3389/fped.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broere-Brown ZA, et al. Fetal sex and maternal pregnancy outcomes: A systematic review and meta-analysis. Biol. Sex Differ. 2020;11:26. doi: 10.1186/s13293-020-00299-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark MJ, Dierkx L, Clifton VL, Wright IM. Alterations in the maternal peripheral microvascular response in pregnancies complicated by preeclampsia and the impact of fetal sex. J. Soc. Gynecol. Investig. 2006;13:573–578. doi: 10.1016/j.jsgi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Gong S, et al. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight. 2018;3:120723. doi: 10.1172/jci.insight.120723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy VE, et al. Maternal asthma is associated with reduced female fetal growth. Am. J. Respir. Crit. Care Med. 2003;168:1317–1323. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 12.Scott NM, et al. Placental cytokine expression covaries with maternal asthma severity and fetal sex. J. Immunol. 2009;182:1411–1420. doi: 10.4049/jimmunol.182.3.1411. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell AM, Palettas M, Christian LM. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav. Immun. 2017;60:32–37. doi: 10.1016/j.bbi.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakuru A, et al. Infant sex modifies associations between placental malaria and risk of malaria in infancy. Malar. J. 2020;19:449. doi: 10.1186/s12936-020-03522-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bordt EA, et al. Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses. Sci. Transl. Med. 2021;13:7428. doi: 10.1126/scitranslmed.abi7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adam I, Salih MM, Mohmmed AA, Rayis DA, Elbashir MI. Pregnant women carrying female fetuses are at higher risk of placental malaria infection. PLoS ONE. 2017;12:e0182394. doi: 10.1371/journal.pone.0182394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger HW, et al. Maternal malaria and malnutrition (M3) initiative, a pooled birth cohort of 13 pregnancy studies in Africa and the Western Pacific. BMJ Open. 2016;6:e012697. doi: 10.1136/bmjopen-2016-012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmiegelow C, et al. Malaria and fetal growth alterations in the 3(rd) trimester of pregnancy: A longitudinal ultrasound study. PLoS ONE. 2013;8:e53794. doi: 10.1371/journal.pone.0053794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adu-Afarwuah S, et al. Lipid-based nutrient supplement increases the birth size of infants of primiparous women in Ghana. Am. J. Clin. Nutr. 2015;101:835–846. doi: 10.3945/ajcn.114.091546. [DOI] [PubMed] [Google Scholar]
- 20.Huynh BT, et al. Influence of the timing of malaria infection during pregnancy on birth weight and on maternal anemia in Benin. Am. J. Trop. Med. Hyg. 2010;85:214–220. doi: 10.4269/ajtmh.2011.11-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landis SH, et al. Impact of maternal malaria and under-nutrition on intrauterine growth restriction: A prospective ultrasound study in Democratic Republic of Congo. Epidemiol. Infect. 2009;137:294–304. doi: 10.1017/S0950268808000915. [DOI] [PubMed] [Google Scholar]
- 22.Dellicour S, et al. Risks of miscarriage and inadvertent exposure to artemisinin derivatives in the first trimester of pregnancy: A prospective cohort study in western Kenya. Malar. J. 2015;14:461. doi: 10.1186/s12936-015-0950-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai M, et al. Impact of sulfadoxine-pyrimethamine resistance on effectiveness of intermittent preventive therapy for malaria in pregnancy at clearing infections and preventing low birth weight. Clin. Infect. Dis. 2016;62:323–333. doi: 10.1093/cid/civ881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madanitsa M, et al. Scheduled intermittent screening and treatment with dihydroartemisinin-piperaquine versus intermittent preventive therapy with sulfadoxine-pyrimethamine for malaria in pregnancy in Malawi: An open-label randomized controlled trial. PLoS Med. 2016;13:e31002124. doi: 10.1371/journal.pmed.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.ter Kuile FO, et al. Reduction of malaria during pregnancy by permethrin-treated bed nets in an area of intense perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 2003;68:50–60. doi: 10.4269/ajtmh.2003.68.50. [DOI] [PubMed] [Google Scholar]
- 26.van Eijk AM, et al. Effect of haematinic supplementation and malaria prevention on maternal anaemia and malaria in western Kenya. Trop. Med. Int. Health. 2007;12:342–352. doi: 10.1111/j.1365-3156.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 27.Unger HW, et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: A randomised controlled trial. BMC Med. 2015;13:9. doi: 10.1186/s12916-014-0258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanisic DI, et al. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 2015;109:313–324. doi: 10.1093/trstmh/trv019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luntamo M, et al. Effect of repeated treatment of pregnant women with sulfadoxine-pyrimethamine and azithromycin on preterm delivery in Malawi: A randomized controlled trial. Am. J. Trop. Med. Hyg. 2010;83:1212–1220. doi: 10.4269/ajtmh.2010.10-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai M, et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: An open-label, three-group, randomised controlled superiority trial. Lancet. 2015;386:2507–2519. doi: 10.1016/S0140-6736(15)00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valea I, et al. An analysis of timing and frequency of malaria infection during pregnancy in relation to the risk of low birth weight, anaemia and perinatal mortality in Burkina Faso. Malar. J. 2012;11:71. doi: 10.1186/1475-2875-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg. Med. J. 2003;20:453–458. doi: 10.1136/emj.20.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagannathan P, et al. Dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria during pregnancy and risk of malaria in early childhood: A randomized controlled trial. PLoS Med. 2018;15:e1002606. doi: 10.1371/journal.pmed.1002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakuru A, et al. Impact of intermittent preventive treatment of malaria in pregnancy with dihydroartemisinin-piperaquine versus sulfadoxine-pyrimethamine on the incidence of malaria in infancy: A randomized controlled trial. BMC Med. 2020;18:207. doi: 10.1186/s12916-020-01675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.John R, Hemberger M. A placenta for life. Reprod. Biomed. Online. 2012;25:5–11. doi: 10.1016/j.rbmo.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Fish EN. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc. Natl. Acad. Sci. U.S.A. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasr A, Allam G, Hamid O, Al-Ghamdi A. IFN-gamma and TNF associated with severe falciparum malaria infection in Saudi pregnant women. Malar. J. 2014;13:314. doi: 10.1186/1475-2875-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruizendaal E, et al. Interleukin-10 and soluble tumor necrosis factor receptor II are potential biomarkers of Plasmodium falciparum infections in pregnant women: A case-control study from Nanoro, Burkina Faso. Biomark. Res. 2017;5:34. doi: 10.1186/s40364-017-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saif Z, et al. The human placenta expresses multiple glucocorticoid receptor isoforms that are altered by fetal sex, growth restriction and maternal asthma. Placenta. 2014;35:260–268. doi: 10.1016/j.placenta.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 42.Shearer FJG, Wyrwoll CS, Holmes MC. The role of 11beta-hydroxy steroid dehydrogenase type 2 in glucocorticoid programming of affective and cognitive behaviours. Neuroendocrinology. 2019;109:257–265. doi: 10.1159/000499660. [DOI] [PubMed] [Google Scholar]
- 43.Mondal D, Galloway TS, Bailey TC, Mathews F. Elevated risk of stillbirth in males: Systematic review and meta-analysis of more than 30 million births. BMC Med. 2014;12:220. doi: 10.1186/s12916-014-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore KA, Simpson JA, Scoullar MJL, McGready R, Fowkes FJI. Quantification of the association between malaria in pregnancy and stillbirth: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1101–e1112. doi: 10.1016/S2214-109X(17)30340-6. [DOI] [PubMed] [Google Scholar]
- 45.Moore KA, et al. Safety of artemisinins in first trimester of prospectively followed pregnancies: An observational study. Lancet Infect. Dis. 2016;5:576–583. doi: 10.1016/S1473-3099(15)00547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology. 1993;22:211–218. doi: 10.1111/j.1365-2559.1993.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 47.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010;36:3. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 48.Wylie BJ, et al. Gestational age assessment in malaria pregnancy cohorts: A prospective ultrasound demonstration project in Malawi. Malar. J. 2013;12:183. doi: 10.1186/1475-2875-12-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the WWARN data repository (http://www.wwarn.org/working-together/sharing-data/accessing-data) for researchers who meet the criteria for access to confidential data.