Abstract
Introduction
and Research Question: Invasive growth of meningiomas into CNS tissue is rare but of prognostic significance. While it has entered the WHO classification as a stand-alone criterion for atypia, its true prognostic impact remains controversial. Retrospective analyses, on which the current evidence is based, show conflicting results. Discordant findings might be explained by different intraoperative sampling methodologies.
Material and methods
To assess the applied sampling methods in the light of the novel prognostic impact of CNS invasion, an anonymous survey was designed and distributed via the EANS website and newsletter. The survey was open from June 5th until July 15th, 2022.
Results
After exclusion of 13 incomplete responses, 142 (91.6%) datasets were used for statistical analysis. Only 47.2% of participants’ institutions utilize a standardized sampling method, and 54.9% pursue a complete sampling of the area of contact between the meningioma surface and CNS tissue. Most respondents (77.5%) did not change their sampling practice after introduction of the new grading criteria to the WHO classification of 2016. Intraoperative suspicion of CNS invasion changes the sampling for half of the participants (49.3%). Additional sampling of suspicious areas of interest is reported in 53.5%. Dural attachment and adjacent bone are more readily sampled separately if tumor invasion is suspected (72.5% and 74.6%, respectively), compared to meningioma tissue with signs of CNS invasion (59.9%).
Discussion and conclusions
Intraoperative sampling methods during meningioma resection vary among neurosurgical departments. There is need for a structured sampling to optimize the diagnostic yield of CNS invasion.
Keywords: Meningioma, Intraoperative sampling, CNS invasion, Brain invasion, Survey, EANS
Highlights
-
•
CNS invasion is a stand-alone criterion for atypia and higher grading in meningioma.
-
•
Incomplete sampling can impede with the detection of CNS invasion in meningioma.
-
•
Current intraoperative sampling methods during meningioma resection vary among neurosurgical departments.
-
•
There is lack of awareness of the importance of complete sampling.
-
•
A standardized sampling recommendation is needed.
1. Introduction
Meningiomas arise from arachnoid cap cells and represent the most common primary brain tumor, making up approximately one third of all encountered intracranial tumor entities (Ostrom et al., 2022). Microsurgical resection is the treatment of choice for the majority of meningiomas, while radiation can be an effective method to achieve tumor control in selected cases (Goldbrunner et al., 2016). However, despite our best efforts, tumor recurrences are quite common and have even been reported to be as high as 40% in a series with prolonged follow-up of 25 years (Pettersson-Segerlind et al., 2011). Great efforts have been made to detect tumor prone to recurrence as early as possible and improve the risk stratification in order to decide on the need of adjuvant care. Several immunohistochemical and molecular markers have been identified thus far and include tumor characteristics such as the loss of the trimethylation H3K27me3, the homozygous deletion of CDKN2A/B and the detection of the TERT promotor mutation, which are all associated with a higher risk of tumor recurrence (Behling et al., 2020a; Sahm et al., 2016; Sievers et al., 2020; Katz et al., 2018). The histopathological detection of invasive growth of meningioma cells into adjacent CNS tissue has been integrated into the WHO Classification of CNS Tumors since 2016 (Louis et al., 2016, 2021). However, its prognostic significance is frequently questioned due to conflicting data from retrospective studies (Baumgarten et al., 2016; Behling et al., 2020b, 2021a; Biczok et al., 2019; Banan et al., 2021; Ryba et al., 2022). One source of uncertainty that is frequently discussed is the absence of a standardized intraoperative tumor sampling method, which is believed to influence the diagnostic accuracy of histopathologically detecting CNS invasion (Behling et al., 2021b; Pizem et al., 2014). This may be one of the main reasons for the conflicting data in the literature regarding the prognostic impact of invasive growth in meningioma.
To shed light on this issue, we report the results of an EANS-wide survey that assessed differences in intraoperative sampling methods during meningioma resection as well as the perception of the current role of CNS invasion.
2. Methods
A web-based survey was constructed using the online platform “SurveyMonkey” (SurveyMonkey Inc., San Mateo, California, USA). The survey consisted of 18 questions that evaluated the intraoperative sampling method during meningioma surgery. Furthermore, the responder-specific clinical and scientific experience with the surgical treatment of meningiomas was assessed. Emphasis was placed on any possible sampling adjustments made for certain intraoperative scenarios and the survey inquired about the opinions concerning characteristics of CNS invasion. If applicable, a 5-tiered Likert scale was applied to score the level of agreement of participants with specific statements. The complete set of questions of the survey is provided in Supplementary Table 1. A link to the survey was distributed via the mailing list of the European Association of Neurosurgical Societies (EANS) on June 5th, 2022, and it was additionally posted on the EANS website. The survey was closed on July 15th, 2022. Overall, 155 responses were collected, and after exclusion of 13 incomplete responses, 142 (91.6%) datasets were used for further statistical analysis.
Participation in the survey was voluntary. No personal data was collected for the analysis of this study. The survey is part of the Invader-Study of the first and last author's institution. Approval by the local Ethics Committee was obtained (project number: 191/2021BO).
Data processing was done with the JMP® Statistical Discovery Software (Version 15.1.0, SAS Institute, Cary, North Carolina, US) and Microsoft® Excel (Version 16.66.1, Microsoft, Redmond, Washington, US).
3. Results
Respondent's position, institution and exposure to meningioma surgery
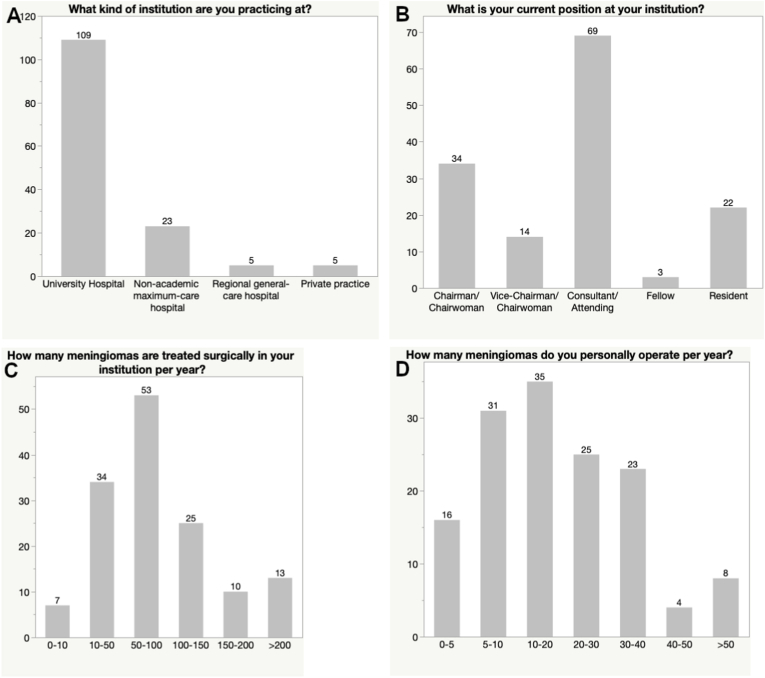
Most respondents were currently practicing at a university hospital (76.8%) or a non-academic maximum-care hospital (16.1%), while a limited number worked in a regional general hospital or in private practice (3.5% each). The majority (82.4%) were experienced surgeons practicing well after initial neurosurgical training (33.8% chair/vice-chair and 48.6% attending/consultant). A smaller portion of responders was still in training (15.5%) or in the middle of fellowship (2.1%) at the time of the survey.
The case load of the participants’ institutions was mostly considered high, with 71.1% of participating respondents practicing in centers treating 50–100 or more meningiomas per year. Two-third of individual respondents stated to operate on 10–20 cases or more personally per year (66.9%) (see Fig. 1).
Fig. 1.
Bar graph showing the responses to questions regarding the participants' institution (A), position (B) and exposure to meningioma surgery (C and D).
3.1. Current sampling methods
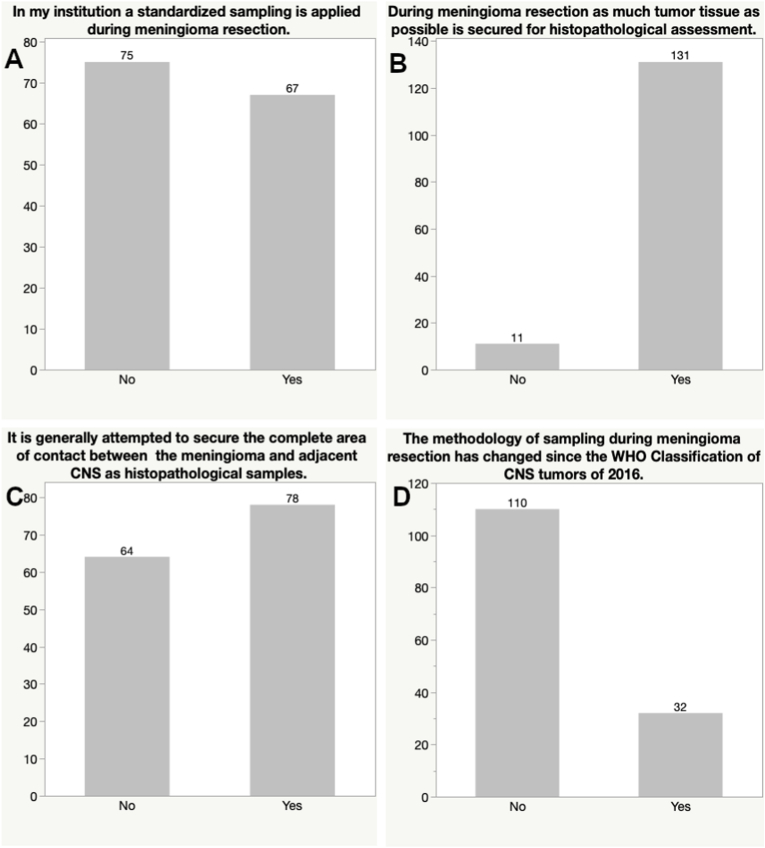
Overall, only 47.2% of the participants’ institutions utilize a standardized sampling during meningioma surgery, but the majority usually attempts to provide as much tissue as possible for histopathology (92.3%). Almost half of the respondents stated that complete sampling of the area of contact between the meningioma surface and adjacent CNS tissue is not attempted (45.1%). Thirty-two replied that the sampling method has been changed after the update of the WHO classification of CNS tumors of 2016 (22.5%), while the majority did not adjust their intraoperative sampling procedures (77.5%) (see Fig. 2).
Fig. 2.
Bar graph depicting the answers to questions focusing on characteristics of current sampling methodologies.
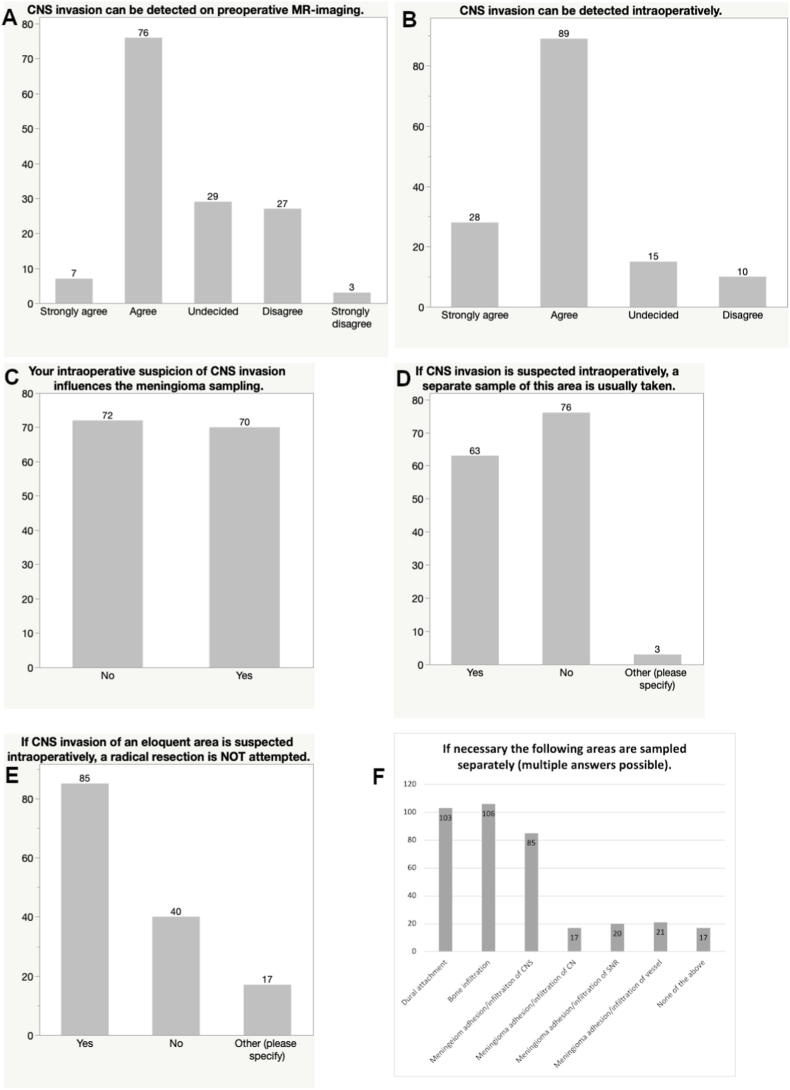
Regarding the detectability of CNS invasion, 58.5% agreed with the statement of the questionnaire that this was possible based on preoperative MR-imaging, while 20.4% were undecided and 21.1% disagreed. Stronger agreement was expressed when asked whether CNS invasion could be detected intraoperatively (82.4%), while only 10.6% were undecided and 7.0% disagreed on the matter (no respondent was in strong disagreement, see Fig. 3).
Fig. 3.
Bar graphs showing the perception of the value of intraoperative findings and preoperative MR-imaging characteristics regarding CNS invasive growth (A and B) and participants' responses regarding intraoperative proceedings in the case of suspicious invasive growth (C, D, E and F).
Half of the respondents answered that the intraoperative suspicion of CNS invasion influences their sampling method (49.3%) and that they are taking a separate sample from the suspicious area in this setting (53.5%). For comparison, the following areas are usually sampled separately in case of suspicious intraoperative findings: Dural attachment (72.5%), Bone infiltration (74.6%), meningioma adhesion/infiltration of CNS (59.9%), meningioma adhesion/infiltration of cranial nerves (12.0%), meningioma adhesion/infiltration of spinal nerve root (14.1%), meningioma adhesion/infiltration of vessels (14.8%) or none of the above (12.0%).
If CNS invasion is suspected intraoperatively in an eloquent area, 59.9% of respondents will not opt for a radical resection, while 28.2% do and 12.0% base their decision on intraoperative neuromonitoring, tumor location as well as age and comorbidities (see Fig. 3).
3.2. Tissue sampling for scientific use
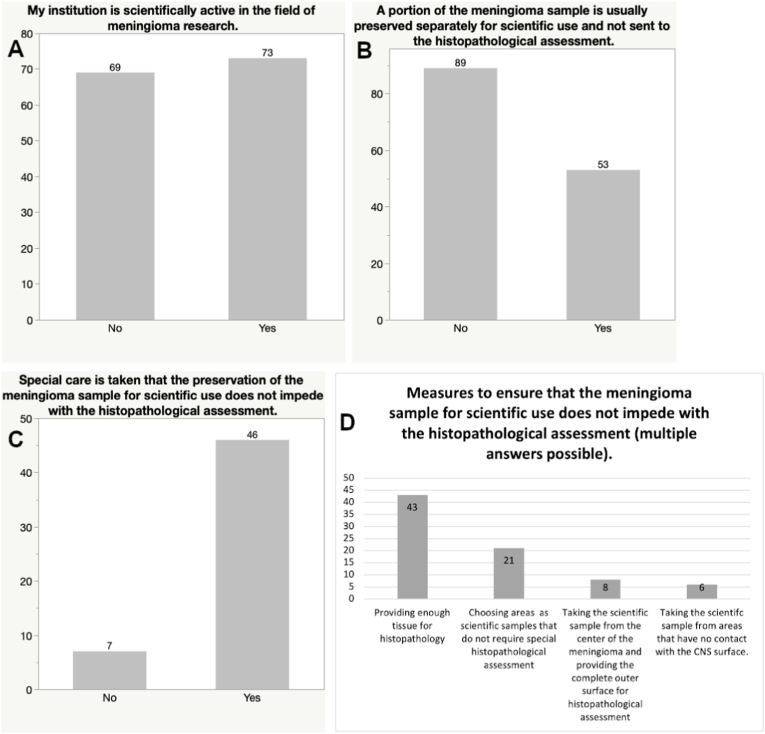
Approximately half of the neurosurgeons participating in the survey stated that they were scientifically involved in meningioma research (51.4%) and one third stated that a tumor tissue sample is frequently taken for scientific use and not sent for histopathological assessment (37.3%). Measures to ensure that scientific sampling does not impede with a comprehensive histopathological evaluation were further evaluated. Eighty-one percent of study participants try to provide enough tissue for histopathological assessment and 39.6% choose areas for scientific sampling that are assumed to be non-critical for histopathological evaluation. Only 15.1% of respondents stated to take the scientific sample from the center of the meningioma and provide the complete outer surface for histopathology, whereas merely 11.3% take the scientific sample from areas that have no contact to the CNS surface (see Fig. 4). A few comments expressed a sequential algorithm permitting the use of remaining tumor tissue for scientific purposes, only after histopathological assessment has been finalized.
Fig. 4.
Bar graphs presenting the scientific activity of participants regarding meningioma research and methodology of scientific tissue sampling during meningioma resection.
4. Discussion
The prognostic impact of CNS invasion in meningioma has led to its integration into the WHO classification of CNS tumors since 2016 as a stand-alone criterion for atypia (Louis et al., 2016, 2021). However, the published data from few retrospective analyses show conflicting results (Baumgarten et al., 2016; Behling et al., 2020b, 2021a; Biczok et al., 2019; Banan et al., 2021). It is quite clear, that a complete sampling of the contact surface of the meningioma with the adjacent CNS parenchyma would be necessary to allow for a maximum detection rate of CNS invasion during histopathological assessment (Behling et al., 2021b). Differences in sampling methodology may have influenced retrospective studies on which the current discussion is based. Therefore, it is of great relevance to assess the current practice of tumor sampling during meningioma resection. This study addresses this issue and is the only one of its kind so far in the field of meningioma research.
Overall, the results of this questionnaire-based survey show a lack of awareness of the implications of CNS invasion for the intraoperative sampling during meningioma resection. For example, less than half of the study participants state to perform a standardized sampling during meningioma resection in their institution and less than a quarter of responding surgeons have changed their practice after the update of the WHO classification in 2016. Furthermore, 45% do not routinely attempt sampling of the complete contact area of the meningioma with the adjacent CNS parenchyma and only half do so if infiltration is suspected intraoperatively. These conceptions suggest that the diagnostic yield of CNS invasion in meningiomas is possibly significantly underestimated. A standardized sampling could improve the validity of future studies (Brokinkel et al., 2017). A difference in the detection rate of brain invasion among different neurosurgical centers has recently been described and varying surgical nuances were proposed as possible influential factor (Timme et al., 2020). Our data now confirms this suspicion and allows for a more detailed view of this issue.
A striking result of our survey was the finding regarding the customs of sampling adjacent structures in the setting of intraoperative suspicion of tumor invasion. While more than 70% of participants sample suspicious areas of dura and bone, meningioma tissue with features of possible infiltrative growth into CNS tissue is only sampled separately by 60% of respondents. The latest WHO guidelines do not consider dural or bone invasion as relevant for tumor grading, despite one study associating bone invasion in atypical meningioma (Gabeau-Lacet et al., 2009). However, CNS invasion in itself is currently relevant for tumor grading. This finding underlines the need to raise awareness of the necessity to provide not just a complete sample, but in particular separate samples of meningioma areas which are suspicious of invasive growth. Regarding the intraoperative assessment, the majority of study participants agreed with the statement that CNS invasion can be detected during resection. So far, no convincing data is available to confirm this perception (Brunasso et al., 2022). One retrospective study suggests a large discordance between intraoperative signs of invasion and true histopathological invasion (Behling et al., 2020b). This issue should be addressed with future studies focusing on a comparative analysis with structured intraoperative and histopathological assessment criteria. On the other hand, detecting invasive growth on preoperative imaging is supported by some retrospective studies (Adeli et al., 2018; Zhang et al., 2022) though fewer respondents seem to agree with this statement when compared to the predictive power of intraoperative assessment (70 vs. 82%, respectively).
Undoubtedly, complete sampling of the meningioma surface is not always feasible. Especially in surgery of skull base meningiomas, the technical difficulty of a radical resection whilst preserving the neurovascular structures at risk must have the top priority and extended sampling should not put these goals at risk. However, increasing the awareness of the importance of a complete sampling for histopathological assessment of CNS invasion is necessary, to give the neuropathologist the highest chance of grading the tumor correctly.
It is therefore necessary to gain further insight into the factors that hinder complete sampling and to identify tumor subgroups that are at a higher risk of undergrading. Another important factor influencing the tumor grading via detection of CNS invasion is the fact that especially in large meningiomas often only presumed representative tissue samples are embedded for histology. With a close collaboration of neurosurgeons and neuropathologists marked areas of interest with sutures or dye can facilitate a targeted tissue analysis in such cases (Picart et al., 2022). The alternative to this targeted analysis would be a more complete embedding of the complete tissue that was resected for comprehensive neuropathologic evaluation to improve diagnostic yield. Therefore, the meningioma samples provided for histopathology as well as the application of the most recent criteria relevant to diagnose CNS invasion need to be considered in the analysis as well.
With the advent of CNS invasion as a stand-alone criterion for atypia in the WHO classification since 2016, an unusual increase in cases with histopathological CNS invasion was observed, presumably based on a tendency of some pathologists to assign a higher grade when in doubt (Perry, 2021). On the other hand, a larger retrospective study of 814 meningiomas showed only a slight non-significant increase of WHO 2 meningiomas after the inclusion of CNS invasion in the WHO classification (Rebchuk et al., 2022). Therefore, a varying adherence to the criteria of CNS invasion proclaimed by the recent WHO classification can be suspected, although they have been defined quite clearly, with pial breach and protrusion of meningioma tissue into GFAP-positive tissue as proof of invasive growth. Infiltration of Virchow-Robin spaces on the other hand is not considered as clear invasion (Louis et al., 2016, 2021) but may still be used by some to diagnose CNS invasion, causing cases with a false higher grading. This may have caused some clinicians to question the prognostic value of the detection of CNS invasion, when they see an unremarkable long follow-up in cases with alleged CNS invasive meningiomas. Furthermore, there is a risk of unnecessary overtreatment if adjuvant radiation is applied due to a higher WHO grade based on unsure CNS invasion. Therefore, strict adherence to the histopathological criteria as well as a complete sampling is necessary to produce a larger number of well documented meningioma cases with definite CNS invasion. This will raise the opportunity to conduct high-quality studies regarding the underlying mechanisms for the development of invasive growth, subsequent potential therapeutic targeting and identification of possible biomarkers.
With this study, we have demonstrated the differences of current practice as well as concepts of intraoperative sampling techniques among neurosurgeons and the potential limitation of the detection of CNS invasion in meningiomas. Our results emphasize the need to develop a standardized sampling algorithm, in order to allow for a maximum accuracy of histopathological detection of CNS invasion of meningiomas and to move forward in the understanding of its underlying mechanisms.
5. Conclusions
Current intraoperative sampling methods during meningioma resection vary among neurosurgical departments. Despite the integration of CNS invasion into the WHO classification there is insufficient awareness of the need for a structured and complete sampling to optimize the diagnostic detection of CNS invasion. A standardized sampling recommendation is needed.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and material
The dataset is available upon reasonable request.
Code availability
No applicable.
Ethics approval
The study was approved by the local Ethics Committee (project number: 191/2021BO).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We acknowledge support by the Open Access Publishing Fund of the University of Tübingen.
Handling Editor: Dr W Peul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2023.101740.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Adeli A., Hess K., Mawrin C., Streckert E.M.S., Stummer W., Paulus W., Kemmling A., Holling M., Heindel W., Schmidt R., Spille D.C., Sporns P.B., Brokinkel B. Prediction of brain invasion in patients with meningiomas using preoperative magnetic resonance imaging. Oncotarget. 2018;9(89):35974–35982. doi: 10.18632/oncotarget.26313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banan R., Abbetmeier-Basse M., Hong B., Dumitru C.A., Sahm F., Nakamura M., Krauss J.K., Hartmann C. Neuropathol Appl Neurobiol; 2021. The Prognostic Significance of Clinicopathological Features in Meningiomas: Microscopic Brain Invasion Can Predict Patient Outcome in Otherwise Benign Meningiomas. [DOI] [PubMed] [Google Scholar]
- Baumgarten P., Gessler F., Schittenhelm J., Skardelly M., Tews D.S., Senft C., Dunst M., Imoehl L., Plate K.H., Wagner M., Steinbach J.P., Seifert V., Mittelbronn M., Harter P.N. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016;132(3):479–481. doi: 10.1007/s00401-016-1598-1. [DOI] [PubMed] [Google Scholar]
- Behling F., Fodi C., Gepfner-Tuma I., Kaltenbach K., Renovanz M., Paulsen F., Skardelly M., Honegger J., Tatagiba M., International Consortium on M., Schittenhelm J., Tabatabai G. Neuro Oncol; 2020. H3K27me3 Loss Indicates an Increased Risk of Recurrence in the Tubingen Meningioma Cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling F., Fodi C., Gepfner-Tuma I., Machetanz K., Renovanz M., Skardelly M., Bornemann A., Honegger J., Tabatabai G., Tatagiba M., Schittenhelm J. CNS invasion in meningioma-how the intraoperative assessment can improve the prognostic evaluation of tumor recurrence. Cancers. 2020;12(12) doi: 10.3390/cancers12123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling F., Fodi C., Wang S., Hempel J.M., Hoffmann E., Tabatabai G., Honegger J., Tatagiba M., Schittenhelm J., Skardelly M. Increased proliferation is associated with CNS invasion in meningiomas. J. Neuro Oncol. 2021;155(3):247–254. doi: 10.1007/s11060-021-03892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behling F., Hempel J.M., Schittenhelm J. Brain invasion in meningioma-A prognostic potential worth exploring. Cancers. 2021;13(13) doi: 10.3390/cancers13133259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biczok A., Jungk C., Egensperger R., von Deimling A., Suchorska B., Tonn J.C., Herold-Mende C., Schichor C. Microscopic brain invasion in meningiomas previously classified as WHO grade I is not associated with patient outcome. J. Neuro Oncol. 2019;145(3):469–477. doi: 10.1007/s11060-019-03312-x. [DOI] [PubMed] [Google Scholar]
- Brokinkel B., Hess K., Mawrin C. Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro Oncol. 2017;19(10):1298–1307. doi: 10.1093/neuonc/nox071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunasso L., Bonosi L., Costanzo R., Buscemi F., Giammalva G.R., Ferini G., Valenti V., Viola A., Umana G.E., Gerardi R.M., Sturiale C.L., Albanese A., Iacopino D.G., Maugeri R. Updated systematic review on the role of brain invasion in intracranial meningiomas: what, when, why? Cancers. 2022;14(17) doi: 10.3390/cancers14174163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabeau-Lacet D., Aghi M., Betensky R.A., Barker F.G., Loeffler J.S., Louis D.N. Bone involvement predicts poor outcome in atypical meningioma. J. Neurosurg. 2009;111(3):464–471. doi: 10.3171/2009.2.JNS08877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbrunner R., Minniti G., Preusser M., Jenkinson M.D., Sallabanda K., Houdart E., von Deimling A., Stavrinou P., Lefranc F., Lund-Johansen M., Moyal E.C., Brandsma D., Henriksson R., Soffietti R., Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. doi: 10.1016/S1470-2045(16)30321-7. [DOI] [PubMed] [Google Scholar]
- Katz L.M., Hielscher T., Liechty B., Silverman J., Zagzag D., Sen R., Wu P., Golfinos J.G., Reuss D., Neidert M.C., Wirsching H.G., Baumgarten P., Herold-Mende C., Wick W., Harter P.N., Weller M., von Deimling A., Snuderl M., Sen C., Sahm F. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018;135(6):955–963. doi: 10.1007/s00401-018-1844-9. [DOI] [PubMed] [Google Scholar]
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., Soffietti R., von Deimling A., Ellison D.W. Neuro Oncol; 2021. The 2021 WHO Classification of Tumors of the Central Nervous System: a Summary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrom Q.T., Price M., Neff C., Cioffi G., Waite K.A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015-2019. Neuro Oncol. 2022;24(Suppl. 5):v1–v95. doi: 10.1093/neuonc/noac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. The definition and role of brain invasion in meningioma grading: still controversial after all these years. Free Neuropathology. 2021;2(8):1–6. doi: 10.17879/freeneuropathology-2021-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson-Segerlind J., Orrego A., Lonn S., Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011;76(6):564–571. doi: 10.1016/j.wneu.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Picart T., Dumot C., Guyotat J., Pavlov V., Streichenberger N., Vasiljevic A., Fenouil T., Durand A., Jouanneau E., Ducray F., Jacquesson T., Berhouma M., Meyronet D. Clinical and pathological impact of an optimal assessment of brain invasion for grade 2 meningioma diagnosis: lessons from a series of 291 cases. Neurosurg. Rev. 2022;45(4):2797–2809. doi: 10.1007/s10143-022-01792-6. [DOI] [PubMed] [Google Scholar]
- Pizem J., Velnar T., Prestor B., Mlakar J., Popovic M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin. Neuropathol. 2014;33(5):354–363. doi: 10.5414/NP300750. [DOI] [PubMed] [Google Scholar]
- Rebchuk A.D., Chaharyn B.M., Alam A., Hounjet C.D., Gooderham P.A., Yip S., Makarenko S. The impact of brain invasion criteria on the incidence and distribution of WHO grade 1, 2, and 3 meningiomas. Neuro Oncol. 2022;24(9):1524–1532. doi: 10.1093/neuonc/noac032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryba A., Millesi M., Roetzer T., Marik W., Wolfsberger S. Clinico-pathologic predictors of dismal course in atypical meningiomas: a retrospective single-centre analysis. J. Neurosurg. Sci. 2022 doi: 10.23736/S0390-5616.22.05741-1. [DOI] [PubMed] [Google Scholar]
- Sahm F., Schrimpf D., Olar A., Koelsche C., Reuss D., Bissel J., Kratz A., Capper D., Schefzyk S., Hielscher T., Wang Q., Sulman E.P., Adeberg S., Koch A., Okuducu A.F., Brehmer S., Schittenhelm J., Becker A., Brokinkel B., Schmidt M., Ull T., Gousias K., Kessler A.F., Lamszus K., Debus J., Mawrin C., Kim Y.J., Simon M., Ketter R., Paulus W., Aldape K.D., Herold-Mende C., von Deimling A. TERT promoter mutations and risk of recurrence in meningioma. J. Natl. Cancer Inst. 2016;108(5) doi: 10.1093/jnci/djv377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers P., Hielscher T., Schrimpf D., Stichel D., Reuss D.E., Berghoff A.S., Neidert M.C., Wirsching H.G., Mawrin C., Ketter R., Paulus W., Reifenberger G., Lamszus K., Westphal M., Etminan N., Ratliff M., Herold-Mende C., Pfister S.M., Jones D.T.W., Weller M., Harter P.N., Wick W., Preusser M., von Deimling A., Sahm F. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020;140(3):409–413. doi: 10.1007/s00401-020-02188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timme M., Thomas C., Spille D.C., Stummer W., Ebel H., Ewelt C., Hans F.J., Schick U., Puchner M., Wildforster U., Bruns B., Trost H.A., Holling M., Grauer O., Hess K., Brokinkel B. Brain invasion in meningiomas: does surgical sampling impact specimen characteristics and histology? Neurosurg. Rev. 2020;43(2):793–800. doi: 10.1007/s10143-019-01125-0. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cao Y., Zhang G., Zhao Z., Sun J., Li W., Ren J., Han T., Zhou J., Chen K. Nomogram based on MRI can preoperatively predict brain invasion in meningioma. Neurosurg. Rev. 2022;45(6):3729–3737. doi: 10.1007/s10143-022-01872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset is available upon reasonable request.