Abstract
Introduction
Cranioplasty is required after decompressive craniectomy (DC) to restore brain protection and cosmetic appearance, as well as to optimize rehabilitation potential from underlying disease. Although the procedure is straightforward, complications either caused by bone flap resorption (BFR) or graft infection (GI), contribute to relevant comorbidity and increasing health care cost. Synthetic calvarial implants (allogenic cranioplasty) are not susceptible to resorption and cumulative failure rates (BFR and GI) tend therefore to be lower in comparison with autologous bone. The aim of this review and meta-analysis is to pool existing evidence of infection-related cranioplasty failure in autologous versus allogenic cranioplasty, when bone resorption is removed from the equation.
Materials and methods
A systematic literature search in PubMed, EMBASE, and ISI Web of Science medical databases was performed on three time points (2018, 2020 and 2022). All clinical studies published between January 2010 and December 2022, in which autologous and allogenic cranioplasty was performed after DC, were considered for inclusion. Studies including non-DC cranioplasty and cranioplasty in children were excluded. The cranioplasty failure rate based on GI in both autologous and allogenic groups was noted. Data were extracted by means of standardized tables and all included studies were subjected to a risk of bias (RoB) assessment using the Newcastle-Ottawa assessment tool.
Results
A total of 411 articles were identified and screened. After duplicate removal, 106 full-texts were analyzed. Eventually, 14 studies fulfilled the defined inclusion criteria including one randomized controlled trial, one prospective and 12 retrospective cohort studies. All but one study were rated as of poor quality based on the RoB analysis, mainly due to lacking disclosure why which material (autologous vs. allogenic) was chosen and how GI was defined. The infection-related cranioplasty failure rate was 6.9% (125/1808) for autologous and 8.3% (63/761) for allogenic implants resulting in an OR 0.81, 95% CI 0.58 to 1.13 (Z = 1.24; p = 0.22).
Conclusion
In respect to infection-related cranioplasty failure, autologous cranioplasty after decompressive craniectomy does not underperform compared to synthetic implants. This result must be interpreted in light of limitations of existing studies. Risk of graft infection does not seem a valid argument to prefer one implant material over the other. Offering an economically superior, biocompatible and perfect fitting cranioplasty implant, autologous cranioplasty can still have a role as the first option in patients with low risk of developing osteolysis or for whom BFR might not be of major concern.
Trial registration
This systematic review was registered in the international prospective register of systematic reviews. PROSPERO: CRD42018081720.
Keywords: Cranioplasty, Allogenic cranioplasty, Alloplastic cranioplasty, Infection, Resorption, Osteolysis
Highlights
-
•
Cranioplasty is required after decompressive craniectomy to restore brain protection and cosmetic appearance.
-
•
Although cranioplasty is a straightforward procedure, complications are common.
-
•
Complications contribute to relevant comorbidity and increasing health care cost.
-
•
Autologous cranioplasty after decompressive craniectomy does not underperform compared to synthetic implants.
-
•
Risk of graft infection does not seem a valid argument to prefer one implant material over the other.
1. Introduction
1.1. Rationale
Decompressive craniectomy (DC) is a life-saving procedure that allows outward expansion of brain tissue by removing a large portion of the skull and opening or enlarging the dura mater. The procedure is typically indicated for intractable elevation of intracranial pressure (ICP) after traumatic brain injury (TBI) (Hawryluk et al., 2020; Hutchinson et al., 2019). In recent years, there has been a resurgence of interest in DC with a broadening of clinical indications including middle cerebral artery ischemia (MMI) (Lin and Frontera, 2021), intracerebral (ICH) (Fung et al., 2016) and subarachnoid hemorrhage (SAH) (Veldeman et al., 2022).
The resulting calvarial defect requires reconstruction once brain swelling has subsided, in a procedure called cranioplasty. Despite being technically straightforward, this procedure is associated with a high degree of complications and failure. After initial removal, the autologous bone flap can either be temporarily stored or be discarded. If re-implantation of autologous bone (autologous cranioplasty) is planned, the flap can be conserved in a subcutaneous abdominal pouch or be cryopreserved (−80 °C). Bone flap preservation within the abdominal wall's fat tissue offers the theoretical benefit of osteocyte/osteoblast survival and improved bone flap-skull incorporation after cranioplasty (Ishikawa et al., 2022). Nonetheless, cryopreservation is often preferred as abdominal storage can result in, in situ osteolysis or infection (Shafiei et al., 2021).
The two major sources of failure after autologous cranioplasty, are bone flap resorption (BFR) and graft infection (GI). BFR occurs in around 11.3% of cases (van de Vijfeijken et al., 2018), is mostly seen in younger patients and after traumatic injury associated with fragmentation of the bone flap (Signorelli et al., 2022). Osteolysis compromises skull integrity and might pose aesthetical challenges. GI occurred in 5.6% of cranioplasty procedures as observed in a 2018 systemic review, and warrants implant removal with the need for secondary allogenic cranioplasty after antibiotic treatment (van de Vijfeijken et al., 2018).
BFR compromising brain protection is rare and revision is more often indicated based on patient initiative to correct compromised esthetics. In recent years, the documented lower failure rates of allogenic cranioplasty have led many to conclude that primary allogenic cranioplasty is preferable (van de Vijfeijken et al., 2018; Gerstl et al., 2022). However, these conclusions are based on the composite failure rate caused by both GI and BFR of which the latter does not occur in alloplastic implants.
Artificial skull implants can be made from a variety of synthetic materials each with their strengths and weaknesses in terms of price, biocompatibility, radiolucency, and anticipated complications. In current neurosurgical practice, titanium (Ti), polymethyl methacrylate (PMMA), polyether ether ketone (PEEK), polyethylene (PE) and hydroxyapatite (HA) are the most commonly used materials provided by manufacturers of calvarial implants (Gerstl et al., 2022; Alkhaibary et al., 2020; Henry et al., 2021a). Maximal biocompatibility and osteoconductivity are achieved by optimizing the implant's surface properties, and porosity during production. Based on thin-sliced CT images, a 3D personalized skull implant (PSI) can be manufactured. These custom implants are expensive, production takes time and no allograft materials appears to outperform the other in terms of overall degree of complications (Gerstl et al., 2022; Henry et al., 2021a). Conversely, autologous bone flaps are readily available, preservation costs lay far below the cost of a PSI's, and they might protect against infection due to osteogenic incorporation and revascularization.
1.2. Objectives
By pooling existing data, we wish to reassess cranioplasty failure rates of autologous versus allogenic implants after removing bone flap resorption out of the equation. Herein, we focus exclusively on graft infection and not on other indications leading to cranioplasty revision.
2. Methods
2.1. Protocol and registration
The review protocol was developed in January 2018. Inclusion and exclusion criteria, searched time period and a comprehensive trial evaluation checklist were composed. This review was registered in 2018 at the international prospective register of systematic reviews: PROSPERO: CRD42020144827. The manuscript is written in accordance with the PRISMA statement for reporting systematic reviews (Liberati et al., 2009) and recommendations made by the Cochrane Collaboration (Cumpston et al., 2019).
2.2. Eligibility criteria
All prospective and retrospective trials directly or indirectly evaluating complications of cranioplasty, after decompressive (hemi)craniectomy were considered for inclusion. The search time frame was set to publications published between January 2010 and December 2022.
Studies had to report data on adult patients (age > 18 years), receiving primary autologous or allogenic cranioplasty and report cranioplasty failure as the result of graft infection. Studies had to include both autologous and allogenic cranioplasty. For the latter group, both personalized and intraoperatively hand molded implants as well as tailored Ti meshes, were included.
The main outcome parameter was cranioplasty failure defined as surgical site (and consequently graft) infection prompting removal of the skull implant.
Prospective and retrospective cohort studies were included. Case reports and small (n < 10) cases series were excluded. Further exclusion criteria were pediatric cranioplasty (if more than 10% of cohort), cranioplasty after craniectomy other than decompressive (non-DC, e.g. after bone flap infection for miscellaneous craniotomies), and penetrating head injury (gunshot wounds, warfare related DC).
2.3. Information sources and search procedure
A literature search of PubMed, EMBASE and, ISI Web of Science medical databases, was performed in January 2018, mid-2020 and December 2022. The following search terms were used: cranioplasty OR cranioplasty failure OR cranioplasty complications OR surgical site infection AND decompressive (hemi)craniectomy. All searches were restricted to clinical trials in human adults written in English, German, French or Dutch.
2.4. Data collection
A standardized table based on the PICO (population, intervention, control, and outcomes) format, was used to extract the following information: patient characteristics (age, sex), sample size, indication of craniectomy (TBI, MMI, SAH, or ICH), type of cranioplasty, used allogenic material (Ti, PMMA, PEEK, PE, HA, or other), GI rate, applied definition of GI, and study design.
2.5. Risk of bias analysis
Risk of bias (RoB) was assessed by the Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses (Lo et al., 2014). Included studies are scored on an 8-point scale with a higher score indicating higher quality. Scoring was performed by two independent assessors (TR and MV). Inconsistencies herein were discussed until reaching consensus. The score was converted according to the AHRQ (Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions) standard into good, fair, and poor quality based on the risk of bias analysis.
2.6. Data analysis and summary measures
Data was analyzed using Review Manager 5.4.1 (The Cochrane Collaboration, London, United Kingdom). Odds ratios (ORs) of cranioplasty failure and 95% confidence intervals (CI) were calculated for both the autologous and allogenic group. Odds ratios were pooled using the Mantel-Haenszel method with a fixed-effects model (p > 0.05, I2 < 50%). Publication bias was assessed by means of a funnel plot. Statistical significance was defined as a two-sided p < 0.05.
3. Results
3.1. Study selection
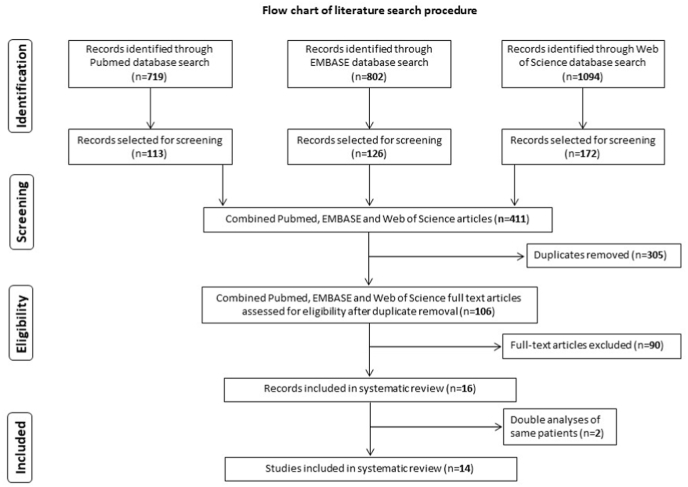
Our search strategy identified 411 studies, from three databases, of which 106 articles remained after duplicate removal. Ninety manuscripts were removed as they did not fulfil the defined inclusion criteria after full-text analysis. Two additional publications were excluded based on the fact they presented post hoc analyses of patient collectives which were already described in two included papers (Honeybul et al., 2018; Morton et al., 2018). Finally, 14 studies met the eligibility criteria and were included in the review and meta-analysis (Brommeland et al., 2015; Chaturvedi et al., 2016; Herteleer et al., 2017; Honeybul and Ho, 2016; Honeybul et al., 2017; Im et al., 2012; Kim et al., 2013, 2015, 2017; Lee et al., 2012; Morton et al., 2016; Quah et al., 2016; Rashidi et al., 2019; Rosseto et al., 2015) An overview of all included studies is provided in Table 1. The literature search procedure is depicted in Fig. 1.
Table 1.
Overview and characteristics of all included studies.
| Author | Year | Study type | Sample size | Mean or median age | Female/Male | Length of follow-up (months) | Indication for DC | Definition of SSI | Applied materials | Risk of bias (NOS) | Risk of bias (AHRQ) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brommeland | 2015 | r-co | 87 | 31 (median) | 54/33 | 9.9 (median) | TBI (n = 74); MMI (n = 13) | n/a | autologous (n = 77)/allogenic (n = 10); HA, PMMA, PE | ∗∗∗∗ | poor |
| Chaturvedi | 2016 | r-co | 74 | 32 (median) | 22/52 | 32 (mean) | TBA (all) | Infection requiring removal of implant | autologous (n = 66)/allogenic (n = 8); Ti, PMMA | ∗∗∗∗∗ | poor |
| Herteleer | 2017 | r-co | 74 | 40.2 (mean) | 24/50 | 15 (mean) | TBI (n = 47); MMI (n = 8); | n/a | autologous (n = 70)/allogenic (n = 4); n/a | ∗∗∗∗ | poor |
| Honeybul | 2016 | r-co | 512 | 39 (median) | 151/361 | n/a | TBI (n = 330); MMI(n = 37); SAH (n = 16); ICH (n = 36); infections (n = 81) | Infection requiring removal of implant | autologous (n = 326)/allogenic (n = 186); Ti | ∗∗∗∗ | poor |
| Honeybul | 2017 | rct | 62 | 44 (median) | 17/45 | min. of 12 months | TBI (n = 43); MMI (n = 9); SAH/ICH (n = 11); Tumor (n = 1) | Infection requiring removal of implant and systemic antibiotics | autologous (n = 31)/allogenic (n = 31); Ti | ∗∗∗∗∗∗∗∗ | good |
| Im | 2012 | r-co | 131 | 50.1 (mean) | 49/82 | 15 (median) | TBI (n = 61); vascular? (n = 62); Tumor (n = 8) | Infection requiring removal of implant | autologous (n = 83)/allogenic (n = 48); PMMA, PE | ∗∗∗∗∗ | poor |
| Kim | 2013 | r-co | 85 | 50.3 (mean) | 27/58 | 15.9 (mean) | TBI (n = 42); vascular? (n = 43) | Infection requiring removal of implant | autologous (n = 58)/allogenic (n = 26); PE | ∗∗∗∗∗ | poor |
| Kim | 2015 | r-co | 78 | 53.0 (mean) | 31/47 | 16.4 (mean) | TBI (n = 46); MMI (n = 12); SAH (n = 11); miscellaneous (n = 9) | Infection requiring removal of the graft | autologous (n = 62)/allogenic (n = 16) | ∗∗∗∗∗ | poor |
| Kim | 2017 | r-co | 127 | 53.0 (mean) | 18/109 | 15 (mean) | TBI (n = 79); MMI (n = 3); ICH (n = 15); SAH (n = 17); miscellaneous (n = 13) | Infection requiring removal of implant | autologous (n = 30)/allogenic (n = 97); PMMA | ∗∗∗∗∗ | poor |
| Lee | 2012 | r-co | 140 | 47.5 (median) | 39/101 | n/a | TBI (n = 72); vascular? (n = 54); tumor (n = 13); infection (n = 1) | Infection requiring removal of implant | autologous (n = 118)/allogenic (n = 22); PMMA, PE | ∗∗∗∗ | poor |
| Morton | 2016 | r-co | 754 | 44 (mean) | 299/455 | 7.5 (mean) | TBI (n = 388); ICH (n = 104); SAH (n = 102); MMI (n = 73); miscellaneous (n = 87) | n/a | autologous (n = 532)/allogenic (n = 222); PEEK, PE, other | ∗∗∗ | poor |
| Quah | 2016 | p-co | 25 | 40 (mean) | 21/49 | 23 (mean) | TBI (n = 47); ICH (n = 9); MMI (n = 8); miscellaneous (n = 6) | CDC definition of surgical wound infection | autologous (n = 31)/allogenic; Ti, PEEK, PMMA | ∗∗∗∗∗ | poor |
| Rashidi | 2019 | r-co | 329 | 51.2 (mean) | 134/195 | 13.2 (mean) | TBI (n = 119); MMI (n = 96); ICH (n = 42); SAH (n = 38); miscellaneous (n = 34) | Infection requiring removal of implant | autologous (n = 303)/allogenic (n = 26); PEEK, PMMA | ∗∗∗∗∗ | poor |
| Rosseto | 2015 | r-co | 45 | 31.9 (mean) | 8/37 | 2 - 26 (range) | TBI (n = 38); MMI (n = 4); miscellaneous (n = 3) | Infection (by clearly defined criteria) requiring removal of implant | autologous (n = 16)/allogenic (n = 29); PMMA | ∗∗∗∗∗ | poor |
DC, decompressive craniectomy; HA, hydroxylapatite; ICH, intracranial hemorrhage; MMI, malignant middle cerebral artery ischemia; n/a, not available, not reported; p-co, prospective cohort study; PE, polyethylene; PEEK, polyether ether ketone; PMMA, polymethyl methacrylate; r-co, retrospective cohort study; rct, randomized controlled trial; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; Ti, titanium.
Fig. 1.
Flow-chart of the article-screening and -inclusion process with depiction of the number of articles removed after each stage.
3.2. Study characteristics
The majority of included studies had a retrospective cohort design (n = 12) (Brommeland et al., 2015; Chaturvedi et al., 2016; Herteleer et al., 2017; Honeybul and Ho, 2016; Im et al., 2012; Kim et al., 2013, 2015, 2017; Lee et al., 2012; Morton et al., 2016; Rashidi et al., 2019; Rosseto et al., 2015). One prospective cohort study (Quah et al., 2016) and one randomized controlled study were included (Honeybul et al., 2017). The definition of GI varied slightly between studies, but was in all but three studies in simplified terms equated to a surgical site infection prompting removal of the implant. In three studies, a written definition of GI was not provided and the diagnosis was left to the discretion of the treating physician (Brommeland et al., 2015; Herteleer et al., 2017; Morton et al., 2016). Included studies involved 2.569 patients, of which 1808 (70.4%) received an autologous graft and 761 (29.6%) a synthetic implant. Most commonly used allogenic materials were Ti (n = 288; 38%), followed by PMMA (n = 187; 25%) (sometimes referred to as acrylic) and PEEK (n = 176; 23%).
3.3. Risk of bias within and across studies
All but one study were rated as being of poor quality based on our RoB analysis, mainly because the basis on which the decision to either perform autologous or allogenic cranioplasty was not outlined. This resulted in a lack of comparability and control of confounding factors. Some studies describe autologous cranioplasty was preferred whenever available but failed to specify what factors availability depended on. Herein a potential selection bias might be introduced. Detailed results of our interpretation and implementation of the RoB via the NOS tool is provided as Supplemental Table 1.
3.4. Results of individual studies
The comparison of individual allogenic materials was not the aim of this review/meta-analysis. Included studies are listed based on the main synthetic material which was used in the allogenic groups, merely for structuring purposes.
3.4.1. Autologous bone versus titanium
In a 2020 systemic review including 13 studies encompassing all sorts of cranioplasty procedures (including non-DC), Ti performed similarly compared to non-Ti implants regarding GI (Zhu et al., 2021). We identified four studies in which Ti implants were used in a total of 288 patients, making it the most widely used artificial material in this review.
In a retrospective cohort study involving 512 patients, Honeybul et al. (Honeybul and Ho, 2016) aimed to identify risk factors for post-cranioplasty complications. The incidence of GI were identical between autologous (8.2%) and Ti (8.2%) cranioplasty procedures.
So far only a single randomized controlled trial has been performed allocating patients to either autologous bone or personalized Ti implants. This trial by Honeybul et al. randomized 31 patients in each treatment arm (Honeybul et al., 2017, 2018). After a two-year follow-up, seven patients in the autologous group developed resorption of which five were surgically re-treated by allogenic cranioplasty (Honeybul et al., 2018). The primary objective of cost-effectiveness was confirmed in favor of primary Ti cranioplasty due to cumulative cost associated revision surgery in the autologous group. Graft infections were noted in neither of both groups which could possibly be a sampling effect based on small group sizes. The authors concluded that due to lower costs and number of reoperations needed, the use of primary Ti cranioplasty should be favored. However, this result was critically discussed stating autologous bone still performed well in some patients even demonstrating bony fusion on radiologically follow-up. The cost-effectiveness analysis was shifted in favor of allogenic cranioplasty because implants were manufactured by the hospital and not bought from a medical company, which drastically reduced costs.
3.4.2. Autologous bone versus PMMA
Amongst the 14 selected studies, 8 applied PMMA for the synthetic grafting, involving 187 patients in total (Brommeland et al., 2015; Chaturvedi et al., 2016; Im et al., 2012; Kim et al., 2017; Lee et al., 2012; Quah et al., 2016; Rashidi et al., 2019; Rosseto et al., 2015). Polymethyl methylacrylate (PMMA) in cranial vault reconstruction is used either as a two-component bone cement to hand mold implants intraoperatively, or to produce CT-based patient-specific implant. In none of the included studies, PMMA performed better in preventing GI compared to autologous bone. Brommeland et al. (2015), assessed 87 cranioplasty patients, investigated possible determinants of post-operative complications in both autologous and allogenic cranioplasty. Patients who received their autologous bone flap did not suffer a higher rate of GI than in the allogenic group. However, 23 patients in this study had prior craniotomies (before DC) which lets assume different incision types for the actual DC had to be used with possible reduced scalp perfusion (Veldeman et al., 2020). In a similar setup, Chaturvedi et al. (2016) compared PMMA and Ti grafts with autologous bone in 74 TBI patients. With only eight patients receiving a synthetic graft, this is one of many studies with disproportioned groups and underrepresentation of allogenic cranioplasty. Because the rationale behind material choice was not disclosed, a possible selection bias of unclear origin and effect might have been introduced which is not corrected for. In three comparative series of cranioplasty procedures involving PMMA, by Im et al.‘s (Im et al., 2012), Lee et al.‘s (Lee et al., 2012) and Kim et al. (2017), prior decompressive craniectomy was also performed for tumorous disease and infection. Both indications might either directly (infection) or indirectly (repetitive surgery in tumor patients) predispose to GI and thereby distort results. However, in none of these studies was autologous cranioplasty associated with a higher degree of patient requiring graft removal due to infection.
Rosseto et al. reported a series of 45 cranioplasty patients of which 29 received a PMMA implant (Rosseto et al., 2015). No significant difference regarding the incidence of GI could be observed between autologous bone flaps and PMMA. Based on the reported age range, it can be assumed that pediatric cases were also included in this series and it is not disclosed how many patients were underage. Because this study by Rosseto et al. did not focus on pediatric cranioplasty and the infection-related failure rate was reported, the study was included in our meta-analysis.
3.4.3. Autologous bone versus PEEK
Three eligible studies used PEEK PSIs for cranioplasty, including a total of 176 patients. Morton et al., 2016, 2018 and Rashidi et al. (2019) aimed to assess the influence of graft material, including PEEK, on overall post-cranioplasty complication rates. Morton et al. published the largest cranioplasty cohort to date including 754 patients, of which 532 received their autologous bone flap and 151 a PEEK implant. An overall infection-related failure rate of 6.6% was reported, occurring after a median of 31 days post-operatively. The risk of infection was not affected by the choice of implant material. This study received only three NOS/RoB stars, based on both the lack of a clear description of GI and short variable follow-up times. In a smaller series by Rashidi et al. synthetic graft material (mainly PEEK), was not associated with GI but late cranioplasty (>6 months) was (Rashidi et al., 2019).
In the only identified prospective observational study, Quah et al. (2016) conducted a data collection focusing on cranioplasty timing. As a secondary result they concluded that material choice, among which mainly PEEK was used, did not affect the rate of infection-related cranioplasty failure. This study presented with one of the lowest GI rates (4.2%) but applied the somewhat unspecific definition of surgical site infection by the Center for Disease Control (CDC) (Borchardt and Tzizik, 2018).
3.4.4. Autologous bone versus others (PE, HA, other)
In a study including 85 patients, 26 received customized PE implants (Kim et al., 2013). Resection of the temporalis muscle, subgaleal fluid collection after DC, and duration of surgery >120 min, were associated with GI. Although the absolute infection rate in the autologous group (8.4%) was higher than in the PE group (3.8%), material choice was not independently associated with risk of infection. In this study, also early (<30 d) cranioplasty was performed which might be associated with more cerebrospinal fluid (CSF) leaks and a higher GI risk (Iaccarino et al., 2019).
Hydroxyapatite (HA) implants were only used in a single included trial in an unknown number of patients (Brommeland et al., 2015). Although the material comes closest to the biomimetic characteristics of bone, due to its lack in strength it is prone to fracturing, and might therefore not be the optimal choice for mobile patients (Iaccarino et al., 2021).
Kim et al. (2015) analyzed the infection rate following autologous and allogenic cranioplasty, with subsequent implant removal being a defining criteria of infection. An overall infection rate of 9.0% was noted, with female sex and bilateral cranioplasty as independent risk factors. It was not revealed what artificial graft material was used. Nonetheless, material choice was not associated with the risk of infection.
3.5. Synthesis of results
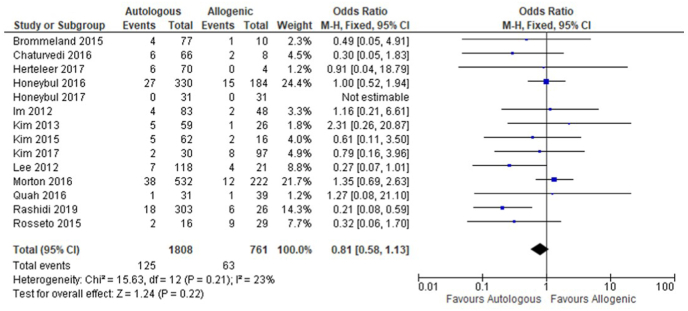
Data from 14 included studies were combined into a meta-analysis of infection-related cranioplasty failure in autologous versus allogenic cranioplasty. The infection-related cranioplasty failure rate was 6.9% (125/1808) for autologous and 8.3% (63/761) for allogenic implants. A weighted average OR of 0.81, 95% CI 0.58 to 1.13 (Z = 1.24; p = 0.22) was calculated. Combined results are presented in a forest plot as Fig. 2. Due to the fact that no infections occurred in both treatment arms of Honeybul et al.‘s trial (Honeybul et al., 2017), these data could not incorporated into the overall OR.
Fig. 2.
Forest plot of all included studies comparing graft infection between patients receiving either autologous bone or allogenic implants.
CI, confidence interval; df, degrees of freedom; I2, I square statistic providing the percentage of variation across studies that is due to heterogeneity. M-H, Mantel-Haenszel method.
A funnel plot was drawn (Supplemental Fig. 1) with a symmetrical distribution of results above and below the mean difference and along the levels of standard error. The risk of publications bias or small study bias is therefore estimated to be limited.
4. Discussion
4.1. Summary of evidence
Based on pooled results, autologous cranioplasty is non-inferior to allogenic materials in preventing infection-related cranioplasty failure. Conversely, the theoretical benefit of how revascularization and incorporation of autologous bone might offer better protection against infection was not confirmed (Veldeman et al., 2020, 2021). Autologous cranioplasty still offers a readily available, anatomically fitting and biocompatible option for cranial vault repair.
While advocating the continued use of autologous bone grafts, it is important to keep in mind that bone resorption as a cause of cranioplasty failure exists. However, its significance from a protective point of view in neurologically compromised or bed-ridden patients, remains questionable. In the 2021 consensus statement from the International Consensus Meeting on Post-traumatic Cranioplasty, 96% of participants agreed that custom-made implants may offer better cosmetic outcomes (Iaccarino et al., 2021). Several studies have corroborated how the risk for BFR differs between individuals and is mainly determined by patient's age, size of the skull defect (with increased risk for larger defects) and the timing of the cranioplasty (with conflicting direction of effect) (Lee et al., 2014; Daou et al., 2016; Fan et al., 2018). Although osteolysis beyond 35 years of age is exceedingly rare, a useful age cut-off cannot be calculated based on existing data (Park et al., 2017; Giese et al., 2021). A personalized osteolysis risk assessment might serve as an indicator when to prioritize allogenic cranioplasty over autologous grafts in patients with high risk of BFR.
It should be pointed out that overall study quality was poor with 12 out of 14 studies being constructed retrospectively with exposure to selection bias. Length of follow-up was highly variable within and between studies, and was even not reported in two studies (Honeybul and Ho, 2016; Lee et al., 2012). The optimal post-cranioplasty length of follow-up is unclear but most infectious complications occurred within 3–6 months after cranioplasty of which the majority happened early (<3 months). In one study, the median interval from cranioplasty to GI was 110 days (39–793) for 13 patients requiring explanation (Lee et al., 2012). Infection of the bone flap more than one year after cranioplasty is rarely seen and mostly the result of skin erosion over the implant or wound dehiscence (Tokoro et al., 1989). Revascularization of autologous bone might provide a theoretically benefit against hematogenous contaminations of the graft, although this mechanism of infection is not proven. In addition, sample sizes remained fairly small and only exceeded 100 patients in less than half (six) studies. Moreover, a standardized definition of post-cranioplasty infection is missing which further reduces study comparability.
With a renewed interest in DC and a growing body of evidence that DC improves outcome in trauma, and ischemic stroke patients, the number of patients requiring post-DC cranioplasty may presumably be rising. The procedure constitutes a necessary step in order to restore brain protection and aesthesis, and potentially improve cognitive functioning (Iaccarino et al., 2019; Honeybul et al., 2013; Shahid et al., 2018). In recent years, multiple systematic review articles devoted to the choice of material, timing and factors associated with failure of cranioplasty after DC, have been published. A 2016 meta-analysis of Ti cranioplasty versus other materials (including autologous bone), documented a lower overall complication rate if Ti was used when pooling 15 studies (Zhu et al., 2021). The risk of implant exposure due to wound dehiscence or skin erosion was higher for Ti implants whereas the risk of CSF leaks was lower, compared to other materials. Although the majority of patients received post-DC cranioplasty, also studies in which non-DC cranioplasty procedures were performed, were included.
In an extensive 2018 systemic review including 228 studies, van de Vijfeijken et al. demonstrated an infection rate of 6.9% in autologous, compared to 5.0% combined for all allogenic materials (van de Vijfeijken et al., 2018). As expected did osteolysis in autologous cranioplasty - which occurred in 11.3% - drive up the cumulative complication rate (35.7%) compared to allogenic materials. Some allograft materials such as PMMA presented with a higher infection rate (7.8%) compared to autologous bone, and the lowest infection rate was observed with HA. Although this latter material was infrequently used and the low incidence might present a sampling error. Gerstl et al. came to similar results when comparing autologous bone with allograft materials in a meta-analysis including 30 studies (Gerstl et al., 2022). Autologous bone presented with a higher complication rate compared to allogenic material but when osteolysis was not considered, this effect evened out. In a systematic review from 2021 including 31 studies, PEEK presented with the lowest infection rate of 5% compared to 8% for autologous bone (Henry et al., 2021a). The included studies in this analysis were, however, also not limited to decompressive craniectomy and also included smaller cranioplasty after e.g. bone flap infection.
Despite extensive recent efforts in better understanding complication development and factors effecting infection and bone flap resorption after cranioplasty, many questions remain unanswered. There exists observational data that early cranioplasty improves neurological recovery (Malcolm et al., 2018) whereas too early cranioplasty is associated with increased risk of CSF fistulas, hydrocephalus and infection (Schuss et al., 2012; Malcolm et al., 2016). The central question regarding material choice should not only be addressed from a surgical complications point of view but also from a health-economic perspective (Lemée et al., 2013). Recent advancements in 3D printing technology create new potential for in-house manufacturing of PSI bypassing medical companies (Kim et al., 2012; Tan et al., 2016; Park et al., 2016). Lower cost of calvarial implants might shift the balance of cost-effectiveness more towards allogenic materials.
Osteolysis can occur but the relevance and need for revision depends on patients’ clinical state and perception of cosmesis. The occurrence of osteolysis is unpredictable but younger age, bone flap fragmentation and a history of traumatic brain injury are clear risk factors (Signorelli et al., 2022; Henry et al., 2021b). Use of an allograft for primary cranioplasty in this subgroup of patients might be beneficial.
4.2. Limitations
The literature research was restricted to three databases and four languages. The majority of included studies are non-randomized or retrospective in design, thus inherently associated with a risk for selection and reporting bias. Furthermore, selected studies showed heterogeneity regarding sample sizes, follow-up and definition of complications. It was decided only the include studies which both reported result for autologous and allogenic cranioplasty to rule out inter-center infection rate variability. This, however, meant many studies still containing valuable data had to be excluded.
It was actively decided neither to focus on cranioplasty timing nor on differences between individual allogenic materials because well designed reviews addressing these issues were published around the time of conceptualization of the review protocol (van de Vijfeijken et al., 2018; Malcolm et al., 2018).
In the initial 2018 draft of the review protocol it was aimed only to include unilateral hemicraniectomies. Mainly because the type of decompressive craniectomy was not specified in many publications, a preliminary search revealed this would only result in a limited number of included studies. Therefore, bifrontal decompressive hemicraniectomy was not an exclusion criterion in the final review protocol. Differences in overlying skin flap vascularization might present with different complication profiles for unilateral versus bifrontal craniectomies which we could not correct for. Both the American and European Association of Tissue Banks have issued guidelines on storing and handling principles of bone flaps which are not specific for bone flaps and interinstitutional practice varies widely (Mirabet et al., 2021). The preservation methods of autologous bone might affect graft longevity and is discarded in this analysis (Mracek et al., 2015; Wui et al., 2016).
4.3. Conclusion
In respect to infection-related cranioplasty failure defined as the need to explant the implanted graft, there was no difference between autologous and any type of allogenic cranioplasty. The level of evidence is limited by the design of included studies with large heterogeneity and a lack standardized definition of graft infection.
Authors’ contributions
TC and MV performed the literature search and all hits were screened and reviewed for eligibility by TC, and MV independently. The Newcastle Ottawa risk of bias tool's grading was performed by TR and MV independently. Conflicting results were discussed until reaching a consensus judgement. The manuscript was drafted by TC and MV and critically reviewed and edited by TR and HC. HC and MV conceptualized and HC supervised the project. All authors read and approved the final manuscript.
Role of the funding source
No funding bodies had any involvement in the preparation of this systematic review, or in the decision to submit the paper for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr W Peul
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bas.2023.101760.
Abbreviations
- BFR
bone flap resorption
- CT
computed tomography
- DC
decompressive craniectomy
- GI
graft infection
- HA
hydroxylapatite
- ICH
intracerebral hemorrhage
- MMI
malignant middle cerebral artery ischemia
- NOS
Newcastle Ottawa scale
- PE
polyethylene
- PMMA
polymethyl methylacrylate
- RoB
risk of bias
- SAH
subarachnoid hemorrhage
- TBI
traumatic brain injury
- Ti
titanium
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- Alkhaibary A., Alharbi A., Alnefaie N., Oqalaa Almubarak A., Aloraidi A., Khairy S. Cranioplasty: a comprehensive review of the history, materials, surgical aspects, and complications. World Neuro. Surg. 2020;139:445–452. doi: 10.1016/j.wneu.2020.04.211. [DOI] [PubMed] [Google Scholar]
- Borchardt R.A., Tzizik D. Update on surgical site infections: the new CDC guidelines. Jaapa. 2018;31(4):52–54. doi: 10.1097/01.JAA.0000531052.82007.42. [DOI] [PubMed] [Google Scholar]
- Brommeland T., Rydning P.N., Pripp A.H., Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand. J. Trauma Resuscitation Emerg. Med. 2015;23:75. doi: 10.1186/s13049-015-0155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi J., Botta R., Prabhuraj A.R., Shukla D., Bhat D.I., Devi B.I. Complications of cranioplasty after decompressive craniectomy for traumatic brain injury. Br. J. Neurosurg. 2016;30(2):264–268. doi: 10.3109/02688697.2015.1054356. [DOI] [PubMed] [Google Scholar]
- Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019;10 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou B., Zanaty M., Chalouhi N., Dalyai R., Jabbour P., Yang S., et al. Low incidence of bone flap resorption after native bone cranioplasty in adults. World Neuro. Surg. 2016;92:89–94. doi: 10.1016/j.wneu.2016.04.115. [DOI] [PubMed] [Google Scholar]
- Fan M.C., Wang Q.L., Sun P., Zhan S.H., Guo P., Deng W.S., et al. Cryopreservation of autologous cranial bone flaps for cranioplasty: a large sample retrospective study. World Neuro. Surg. 2018;109:e853–e859. doi: 10.1016/j.wneu.2017.10.112. [DOI] [PubMed] [Google Scholar]
- Fung C., Murek M., Klinger-Gratz P.P., Fiechter M., Z'Graggen W.J., Gautschi O.P., et al. Effect of decompressive craniectomy on perihematomal edema in patients with intracerebral hemorrhage. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstl J.V.E., Rendon L.F., Burke S.M., Doucette J., Mekary R.A., Smith T.R. Complications and cosmetic outcomes of materials used in cranioplasty following decompressive craniectomy-a systematic review, pairwise meta-analysis, and network meta-analysis. Acta Neurochir. 2022;164(12):3075–3090. doi: 10.1007/s00701-022-05251-5. [DOI] [PubMed] [Google Scholar]
- Giese H., Meyer J., Unterberg A., Beynon C. Long-term complications and implant survival rates after cranioplastic surgery: a single-center study of 392 patients. Neurosurg. Rev. 2021;44(3):1755–1763. doi: 10.1007/s10143-020-01374-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk G.W.J., Rubiano A.M., Totten A.M., O'Reilly C., Ullman J.S., Bratton S.L., et al. Guidelines for the management of severe traumatic brain injury: 2020 update of the decompressive craniectomy recommendations. Neurosurgery. 2020;87(3):427–434. doi: 10.1093/neuros/nyaa278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J., Amoo M., Taylor J., O'Brien D.P. Complications of cranioplasty in relation to material: systematic review, network meta-analysis and meta-regression. Neurosurgery. 2021;89(3):383–394. doi: 10.1093/neuros/nyab180. [DOI] [PubMed] [Google Scholar]
- Henry J., Amoo M., Murphy A., O'Brien D.P. Complications of cranioplasty following decompressive craniectomy for traumatic brain injury: systematic review and meta-analysis. Acta Neurochir. 2021;163(5):1423–1435. doi: 10.1007/s00701-021-04809-z. [DOI] [PubMed] [Google Scholar]
- Herteleer M., Ectors N., Duflou J., Van Calenbergh F. Complications of skull reconstruction after decompressive craniectomy. Acta Chir. Belg. 2017;117(3):149–156. doi: 10.1080/00015458.2016.1264730. [DOI] [PubMed] [Google Scholar]
- Honeybul S., Ho K.M. Cranioplasty: morbidity and failure. Br. J. Neurosurg. 2016;30(5):523–528. doi: 10.1080/02688697.2016.1187259. [DOI] [PubMed] [Google Scholar]
- Honeybul S., Janzen C., Kruger K., Ho K.M. The impact of cranioplasty on neurological function. Br. J. Neurosurg. 2013;27(5):636–641. doi: 10.3109/02688697.2013.817532. [DOI] [PubMed] [Google Scholar]
- Honeybul S., Morrison D.A., Ho K.M., Lind C.R., Geelhoed E. A randomized controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty. J. Neurosurg. 2017;126(1):81–90. doi: 10.3171/2015.12.JNS152004. [DOI] [PubMed] [Google Scholar]
- Honeybul S., Morrison D.A., Ho K.M., Lind C.R.P., Geelhoed E. A randomised controlled trial comparing autologous cranioplasty with custom-made titanium cranioplasty: long-term follow-up. Acta Neurochir. 2018;160(5):885–891. doi: 10.1007/s00701-018-3514-z. [DOI] [PubMed] [Google Scholar]
- Hutchinson P.J., Kolias A.G., Tajsic T., Adeleye A., Aklilu A.T., Apriawan T., et al. Consensus statement from the international consensus meeting on the role of decompressive craniectomy in the management of traumatic brain injury : consensus statement. Acta Neurochir. 2019;161(7):1261–1274. doi: 10.1007/s00701-019-03936-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino C., Kolias A.G., Roumy L.G., Fountas K., Adeleye A.O. Cranioplasty following decompressive craniectomy. Front. Neurol. 2019;10:1357. doi: 10.3389/fneur.2019.01357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino C., Kolias A., Adelson P.D., Rubiano A.M., Viaroli E., Buki A., et al. Consensus statement from the international consensus meeting on post-traumatic cranioplasty. Acta Neurochir. 2021;163(2):423–440. doi: 10.1007/s00701-020-04663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im S.H., Jang D.K., Han Y.M., Kim J.T., Chung D.S., Park Y.S. Long-term incidence and predicting factors of cranioplasty infection after decompressive craniectomy. J. Korean. Neuro.Surg. Soc. 2012;52(4):396–403. doi: 10.3340/jkns.2012.52.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y., Kamochi H., Ishizaki R., Wataya T. Bone flap preservation in subcutaneous abdominal pocket for decompressive craniectomy. Plast. Reconstr. Surg. Glob. Open. 2022;10(7) doi: 10.1097/GOX.0000000000004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B.J., Hong K.S., Park K.J., Park D.H., Chung Y.G., Kang S.H. Customized cranioplasty implants using three-dimensional printers and polymethyl-methacrylate casting. J. Korean Neuro. Surg. Soc. 2012;52(6):541–546. doi: 10.3340/jkns.2012.52.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Sung S.O., Kim S.J., Kim S.R., Park I.S., Jo K.W. Analysis of the factors affecting graft infection after cranioplasty. Acta Neurochir. 2013;155(11):2171–2176. doi: 10.1007/s00701-013-1877-8. [DOI] [PubMed] [Google Scholar]
- Kim J.S., Park I.S., Kim S.K., Park H., Kang D.H., Lee C.H., et al. Analysis of the risk factors affecting the surgical site infection after cranioplasty following decompressive craniectomy. Korean J. Nutr. 2015;11(2):100–105. doi: 10.13004/kjnt.2015.11.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Kang D.S., Cheong J.H., Kim J.H., Song K.Y., Kong M.H. Comparison of complications following cranioplasty using a sterilized autologous bone flap or polymethyl methacrylate. Korean J. Nutr. 2017;13(1):15–23. doi: 10.13004/kjnt.2017.13.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Chung Y.S., Lee S.H., Yang H.J., Son Y.J. Analysis of the factors influencing bone graft infection after cranioplasty. J. Trauma Acute Care Surg. 2012;73(1):255–260. doi: 10.1097/TA.0b013e318256a150. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Yoo C.J., Lee U., Park C.W., Lee S.G., Kim W.K. Resorption of autogenous bone graft in cranioplasty: resorption and reintegration failure. Korean J. Nutr. 2014;10(1):10–14. doi: 10.13004/kjnt.2014.10.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemée J.M., Petit D., Splingard M., Menei P. Autologous bone flap versus hydroxyapatite prosthesis in first intention in secondary cranioplasty after decompressive craniectomy: a French medico-economical study. Neurochirurgie. 2013;59(2):60–63. doi: 10.1016/j.neuchi.2012.10.138. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Frontera J.A. Decompressive hemicraniectomy for large hemispheric strokes. Stroke. 2021;52(4):1500–1510. doi: 10.1161/STROKEAHA.120.032359. [DOI] [PubMed] [Google Scholar]
- Lo C.K., Mertz D., Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm J.G., Rindler R.S., Chu J.K., Grossberg J.A., Pradilla G., Ahmad F.U. Complications following cranioplasty and relationship to timing: a systematic review and meta-analysis. J. Clin. Neurosci. 2016;33:39–51. doi: 10.1016/j.jocn.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Malcolm J.G., Rindler R.S., Chu J.K., Chokshi F., Grossberg J.A., Pradilla G., et al. Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery. 2018;82(3):278–288. doi: 10.1093/neuros/nyx182. [DOI] [PubMed] [Google Scholar]
- Mirabet V., García D., Yagüe N., Larrea L.R., Arbona C., Botella C. The storage of skull bone flaps for autologous cranioplasty: literature review. Cell Tissue Bank. 2021;22(3):355–367. doi: 10.1007/s10561-020-09897-2. [DOI] [PubMed] [Google Scholar]
- Morton R.P., Abecassis I.J., Hanson J.F., Barber J., Nerva J.D., Emerson S.N., et al. Predictors of infection after 754 cranioplasty operations and the value of intraoperative cultures for cryopreserved bone flaps. J. Neurosurg. 2016;125(3):766–770. doi: 10.3171/2015.8.JNS151390. [DOI] [PubMed] [Google Scholar]
- Morton R.P., Abecassis I.J., Hanson J.F., Barber J.K., Chen M., Kelly C.M., et al. Timing of cranioplasty: a 10.75-year single-center analysis of 754 patients. J. Neurosurg. 2018;128(6):1648–1652. doi: 10.3171/2016.11.JNS161917. [DOI] [PubMed] [Google Scholar]
- Mracek J., Hommerova J., Mork J., Richtr P., Priban V. Complications of cranioplasty using a bone flap sterilised by autoclaving following decompressive craniectomy. Acta Neurochir. 2015;157(3):501–506. doi: 10.1007/s00701-014-2333-0. [DOI] [PubMed] [Google Scholar]
- Park E.K., Lim J.Y., Yun I.S., Kim J.S., Woo S.H., Kim D.S., et al. Cranioplasty enhanced by three-dimensional printing: custom-made three-dimensional-printed titanium implants for skull defects. J. Craniofac. Surg. 2016;27(4):943–949. doi: 10.1097/SCS.0000000000002656. [DOI] [PubMed] [Google Scholar]
- Park S.P., Kim J.H., Kang H.I., Kim D.R., Moon B.G., Kim J.S. Bone flap resorption following cranioplasty with autologous bone: quantitative measurement of bone flap resorption and predictive factors. J. Korean. Neuro.Surg. Soc. 2017;60(6):749–754. doi: 10.3340/jkns.2017.0203.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah B.L., Low H.L., Wilson M.H., Bimpis A., Nga V.D.W., Lwin S., et al. Is there an optimal time for performing cranioplasties? Results from a prospective multinational study. World Neuro. Surg. 2016;94:13–17. doi: 10.1016/j.wneu.2016.06.081. [DOI] [PubMed] [Google Scholar]
- Rashidi A., Neumann J., Adolf D., Sandalcioglu I.E., Luchtmann M. An investigation of factors associated with the development of postoperative bone flap infection following decompressive craniectomy and subsequent cranioplasty. Clin. Neurol. Neurosurg. 2019;186 doi: 10.1016/j.clineuro.2019.105509. [DOI] [PubMed] [Google Scholar]
- Rosseto R.S., Giannetti A.V., de Souza Filho L.D., Faleiro R.M. Risk factors for graft infection after cranioplasty in patients with large hemicranial bony defects. World Neuro. Surg. 2015;84(2):431–437. doi: 10.1016/j.wneu.2015.03.045. [DOI] [PubMed] [Google Scholar]
- Schuss P., Vatter H., Marquardt G., Imöhl L., Ulrich C.T., Seifert V., et al. Cranioplasty after decompressive craniectomy: the effect of timing on postoperative complications. J. Neurotrauma. 2012;29(6):1090–1095. doi: 10.1089/neu.2011.2176. [DOI] [PubMed] [Google Scholar]
- Shafiei M., Sourani A., Saboori M., Aminmansour B., Mahram S. Comparison of subcutaneous pocket with cryopreservation method for storing autologous bone flaps in developing surgical wound infection after Cranioplasty: a randomized clinical trial. J. Clin. Neurosci. 2021;91:136–143. doi: 10.1016/j.jocn.2021.06.042. [DOI] [PubMed] [Google Scholar]
- Shahid A.H., Mohanty M., Singla N., Mittal B.R., Gupta S.K. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J. Neurosurg. 2018;128(1):229–235. doi: 10.3171/2016.10.JNS16678. [DOI] [PubMed] [Google Scholar]
- Signorelli F., Giordano M., Caccavella V.M., Ioannoni E., Gelormini C., Caricato A., et al. A systematic review and meta-analysis of factors involved in bone flap resorption after decompressive craniectomy. Neurosurg. Rev. 2022;45(3):1915–1922. doi: 10.1007/s10143-022-01737-z. [DOI] [PubMed] [Google Scholar]
- Tan E.T., Ling J.M., Dinesh S.K. The feasibility of producing patient-specific acrylic cranioplasty implants with a low-cost 3D printer. J. Neurosurg. 2016;124(5):1531–1537. doi: 10.3171/2015.5.JNS15119. [DOI] [PubMed] [Google Scholar]
- Tokoro K., Chiba Y., Tsubone K. Late infection after cranioplasty--review of 14 cases. Neurol. Med.-Chir. 1989;29(3):196–201. doi: 10.2176/nmc.29.196. [DOI] [PubMed] [Google Scholar]
- Veldeman M., Daleiden L., Hamou H., Höllig A., Clusmann H. An altered posterior question-mark incision is associated with a reduced infection rate of cranioplasty after decompressive hemicraniectomy. J. Neurosurg. 2020;134(3):1262–1270. doi: 10.3171/2020.2.JNS193335. [DOI] [PubMed] [Google Scholar]
- Veldeman M., Geiger M., Clusmann H. How I do it-the posterior question mark incision for decompressive hemicraniectomy. Acta Neurochir. 2021;163(5):1447–1450. doi: 10.1007/s00701-021-04812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldeman M., Weiss M., Daleiden L., Albanna W., Schulze-Steinen H., Nikoubashman O., et al. Decompressive hemicraniectomy after aneurysmal subarachnoid hemorrhage-justifiable in light of long-term outcome? Acta Neurochir. 2022;164(7):1815–1826. doi: 10.1007/s00701-022-05250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijfeijken S., Münker T., Spijker R., Karssemakers L.H.E., Vandertop W.P., Becking A.G., et al. Autologous bone is inferior to alloplastic cranioplasties: safety of autograft and allograft materials for cranioplasties, a systematic review. World Neuro. Surg. 2018;117:443. doi: 10.1016/j.wneu.2018.05.193. 52.e8. [DOI] [PubMed] [Google Scholar]
- Wui S.H., Kim K.M., Ryu Y.J., Kim I., Lee S.J., Kim J., et al. The autoclaving of autologous bone is a risk factor for surgical site infection after cranioplasty. World Neuro. Surg. 2016;91:43–49. doi: 10.1016/j.wneu.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Zhu S., Chen Y., Lin F., Chen Z., Jiang X., Zhang J., et al. Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: a systematic review and meta-analysis. J. Clin. Neurosci. 2021;84:66–74. doi: 10.1016/j.jocn.2020.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.