Abstract
Introduction
Pediatric hydrocephalus is highly prevalent and therefore a major neurosurgical problem in Africa. In addition to ventriculoperitoneal shunts, which have high cost and potential complications, endoscopic third ventriculostomy is becoming an increasingly popular technique especially in this part of the world. However, performing this procedure requires trained neurosurgeons with an optimal learning curve. For this reason, we have developed a 3D printed training model of hydrocephalus so that neurosurgeons without previous experience with endoscopic techniques can acquire these skills, especially in low-income countries, where specific techniques training as this, are relatively absent.
Research Question
Our research question was about the possibility to develop and produce a low-cost endoscopic training model and to evaluate the usefulness and the skills acquired after training with it.
Material and Methods
A neuroendoscopy simulation model was developed. A sample of last year medical students and junior neurosurgery residents without prior experience in neuroendoscopy were involved in the study. The model was evaluated by measuring several parameters, as procedure time, number of fenestration attempts, diameter of the fenestration, and number of contacts with critical structures.
Results
An improvement of the average score on the ETV-Training-Scale was noticed between the first and last attempt (11.6, compared to 27.5 points; p<0.0001). A statistically significant improvement in all parameters, was observed.
Discussion and Conclusion
This 3D printed simulator facilitates acquiring surgical skills with the neuroendoscope to treat hydrocephalus by performing an endoscopic third ventriculostomy. Furthermore, it has been shown to be useful to understand the intraventricular anatomical relationships.
Keywords: Neuroendoscopy, Low- and middle-income countries, Neurosurgical training, Hydrocephalus, Global neurosurgery, Health equity
Highlights
-
•
Hydrocephalus is one of the most prevalent neurosurgical diseases in low-income countries.
-
•
Endoscopic third ventriculostomy may lead to restore the cerebrospinal fluid circulation in a more physiologic way.
-
•
Implementing training simulators might facilitate the acquisition of the necessary skills in a safe and practical way.
1. Introduction
Obstructive hydrocephalus involves an obstruction of normal cerebrospinal fluid (CSF) circulation between the ventricular system and the subarachnoid space, finally leading to an increased intracranial pressure. The incidence of newborn hydrocephalus in certain African countries has been reported to be as much as ten times higher than the incidence in developed countries (Dewan et al., 2018; Warf, 2010).
The classic treatment of hydrocephalus consists of placing a ventriculoperitoneal (VP) shunt, which allows to alleviate the CSF pressure, releasing the intracranial hypertension and facilitating normal kids’ neurological development. However, in low-income countries (LIC) this procedure has two main problems: its high cost, and the high percentage of complications related to infections and malfunctioning (Mwachaka et al., 2010; Zhan et al., 2014; Cheng et al., 2017).
Thanks to the advent of neuroendoscopic techniques, instruments and devices, hydrocephalus may be solved in a relevant percentage of patients by performing an endoscopic third ventriculostomy (ETV), therefore avoiding the gaps in these LIC. Endoscopic techniques produce minimal lesion to the healthy cerebral tissue with less complications and provides excellent clinical results in terms of hydrocephalus resolution (Jiménez et al., 2007; Shim et al., 2017; Lu et al., 2019; Jiang et al., 2018; Jimenez et al., 2017; Uche et al., 2018; Linares et al., 2021). However, the neuroendoscopy learning curve requires observing, as well as laboratory practice with neuroimage systems or even with virtual reality models before any attempt to perform an ETV (Baby et al., 2020). Acquiring this learning curve remains a challenge in LIC due to the absence of this technology and training opportunities.
The main goal of our study was to develop and evaluate a low cost, reproducible and easily transportable ETV 3D printed model to help neurosurgeons from LIC acquiring the needed skills to perform such a useful technique in a safe and efficient way.
2. Material and methods
2.1. 3D printed neuroendoscopy model
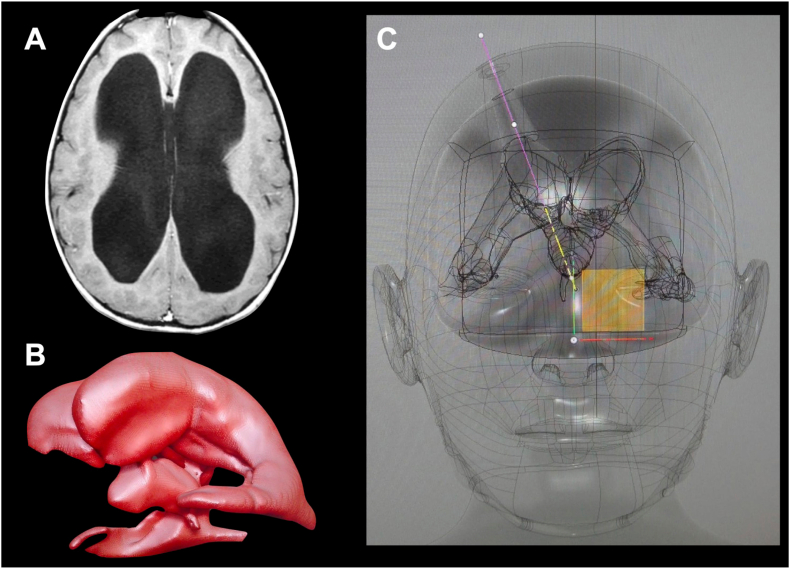
A magnetic resonance (MR) and computed tomography (CT) from an ideal patient (from an anatomical perspective) with obstructive hydrocephalus were selected. Patient's family gave permission to use his anonymized image records from our Hospital image recording system. The DICOM files were extracted, and a 3D reconstruction was performed using Horos™ (Horos project, Geneva Switzerland). The 3D surface rendering tool was used to create a 3D reconstruction of the skin and skull from the CT scan, while the brain and ventricular system were rendered from the MR. Both 3D models were exported as stereolithographic (STL) files, which were final imported into Meshmixer (http://www.meshmixer.com; Autodesk, Inc. San Rafael, California, USA), a free-to-use 3D modeling software (Fig. 1).
Fig. 1.
(A) Axial MR slice at the level of the lateral ventricles body. An enlarged ventricular system was chosen as the ideal case to create the training model. (B) 3D render of the lateral and third ventricles. (C) 3D design of the simulator, in which the ETV ideal trajectory shows the connection of three relevant points: floor of the anterior third ventricle, geometric center of foramen of Monro and finally coronal burr-hole. The burr-holes and trajectory were designed to fit perfectly on the ideal endoscopic trajectory. The orthogonal orientation cube is shown in yellow on its coronal perspective. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
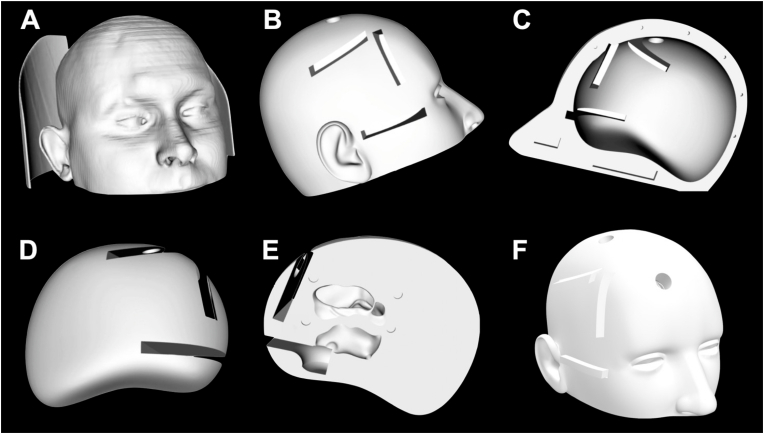
The STL skin and skull parts were fused and treated as a single object, while the brain and ventricles were treated as an independent one. Two ideal entry points were chosen: one was placed 2 cm lateral to the midline at the level of the coronal suture, and a second one 3.5 cm lateral to the midline and 5 cm anterior to the coronal suture. Two 14 mm-diameter holes were designed on the skin-skull object at the previously mentioned points. A couple of tubular trajectories directed to the ventricle were created in the brain object from the previously designed burr holes. These trajectories were 20 mm wide. The brain surface was smoothed to fit perfectly into the skull inner side limiting its movement. The septum pellucidum as well as the tuber cinereum were erased from the model itself, as these are planned to be the targets for the endoscopic exercises (septostomy and third ventricle floor fenestration). Both objects, skin-skull, and brain, were divided in the sagittal midline to be easily opened. Three 5 mm high and 30 mm wide cassettes were designed from the skin surface to the coronal and precoronal burr-holes and underlying trajectories, and to the tuber cinereum to fit perfectly into the model. The necessary opening was designed in both skin-skull and brain objects. These cassettes contained a space where a paraffin membrane could be easily fixed to simulate the cerebral pia for the first two cassettes and the third ventricle floor for the third one. These cassettes allowed to repeat the exercises as many times as desired just replacing the paraffin membrane (Fig. 2, Fig. 3).
Fig. 2.
Different views of the model 3D design. (A) Skin 3D rendering extracted from the CT scan. (B) Lateral view of the skin-skull object after 3D render and cleaning process. The burr-holes and entry points for the pial and tuber cinereum cassettes are shown. (C) Medial view of the skin-skull object after 3D render and cleaning process. The burr-holes and entry points for the pial and tuber cinereum cassettes are shown. (D) Lateral view of the brain-ventricles object after 3D render and cleaning process. The endoscopic trajectories and location of the pial and tuber cinereum cassettes are shown. (E) Medial view of the brain-ventricles object after 3D render and cleaning process. The frontal horn and body of right lateral ventricle, as well as the right half of the third ventricle are shown from the medial aspect.
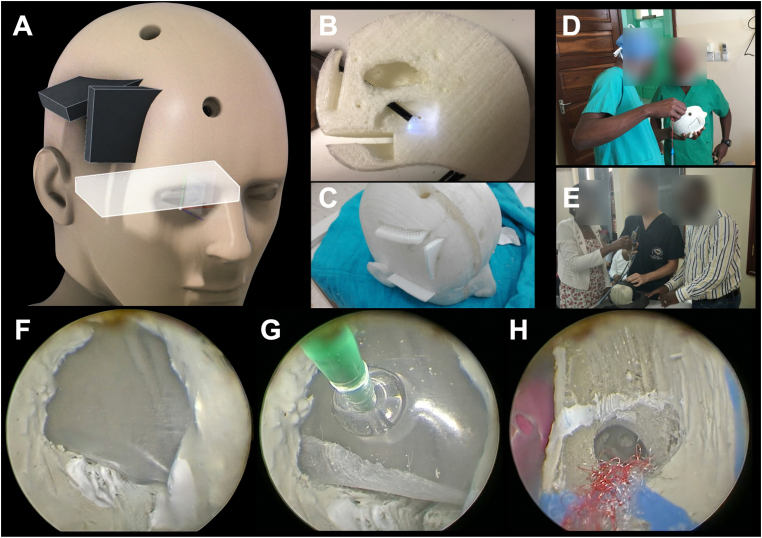
Fig. 3.
(A) 3D reconstruction of the model showing the location of the coronal and precoronal burr-holes, as well as the frontal and basal cassettes. (B) Midsagittal view of the 3D printed brain/ventricles model showing the trajectory of an endoscope to the posterior third ventricle through the precoronal burr-hole. (C) Superior view of the whole model with the cassettes on site. (D & E) Different views of the 3D simulator during a hands-on ETV course in Tanzania. (F) The floor of the third ventricle is simulated by the paraffin membrane including the tuber cinereum. The mammillary bodies are painted in white, and the hypothalamus is represented in the anterior lateral wall of the third ventricle. (G) Third ventricle floor fenestration. This view shows the paraffin being fenestrated with the Fogarty balloon. (H) Superior view of the right foramen of Monro with the artistic representation of the septal and thalamostriate veins, as well as the choroid plexus. The fornix relative location is represented by a white line. A septostomy can be appreciated giving access to the left frontal horn where a gelatin-based pink object was placed simulating a tumor for endoscopic biopsy. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
All the objects and cassettes were 3D printed. Finally, anatomical details such as the choroid plexus, infundibulum and mamillary bodies were manually added (Fig. 3).
2.2. Study protocol
2.2.1. Theoretical training
Before performing the surgical ETV simulator evaluation, all individuals included in the study received a theoretical training. The training included the pathophysiology of obstructive hydrocephalus, anatomy of the ventricular system, as well as the traditional approach (shunting) and the endoscopic techniques. Images and videos of real ETV procedures and pictures of our model were showed to the subjects.
2.2.2. Model evaluation and measured variables
To evaluate the usefulness of the simulation model, subjects without previous experience in neuroendoscopy have been selected. Half of them were last year medical students from the Miguel Hernández University in Alicante, Spain. The other half was composed by junior neurosurgery residents with short or no experience using the neuroendoscope.
After the theoretical explanation, each subject performed a minimum of five ETV attempts, with the aim of observing if there was any improvement throughout the development of the different performances.
For the evaluation itself we chose five parameters to be measured during any attempt of ETV performance: surgical time, number of attempts necessary to perform the fenestration, diameter of the fenestration, number of contacts to critical structures as fornix at the level of the foramen of Monro and hypothalamus in the third ventricle anterolateral walls. We introduced all these parameters into a table and assigned a score to each of them. The total score was analyzed as a single variable to understand the global results, as well as individual scores. We named this as the ETV-training-scale (Table 1).
Table 1.
ETV-Training-scale. Correspondence of the parameters with the score awarded for each of them.
| Points | Time | Fenestration attempts | Fenestration diameter | Hypothalamus contacts | Fornix contacts |
|---|---|---|---|---|---|
| 1 | <2,5 min | 1 | >100% Fogarty® diameter | 0 | 0 |
| 2 | 2,6–3,5 min | 2 | 1 | 1 | |
| 3 | 3,6–4,5 min | 3 | <100% Fogarty® open | 2 | 2 |
| 4 | 4,6–5,5 min | 4 | 3 | 3 | |
| 5 | >5,5 min | 5 or more | Fogarty® diameter closed or less | 4 or more | 4 or more |
The main hypothesis of our study was that our neuroendoscopy training model will allow junior surgeons with limited or no experience in endoscopic procedures to perform the ETV in a faster and safer way. This hypothesis will be checked as an improvement higher than 50% in the ETV-training-scale scores between the first and last attempts (Table 2, Table 3).
Table 2.
First attempt results: length of the procedure (in minutes), number of contacts to the fornix and hypothalamus, fenestration size, fenestration attempts, together with individual and total scores.
| Time (min) | Points | Hypothalamus contacts | Points | Fornix contacts | Points | Fenestration size (%) | Points | Fenestation attempts | Points | Total points | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 6,8 | 1 | 1 | 4 | 4 | 1 | 40 | 5 | 4 | 2 | 11 |
| 2 | 6,2 | 1 | 1 | 4 | 2 | 3 | 40 | 5 | 3 | 3 | 15 |
| 3 | 7,21 | 1 | 1 | 4 | 1 | 4 | 40 | 5 | 3 | 3 | 16 |
| 4 | 9,05 | 1 | 1 | 4 | 1 | 4 | 40 | 5 | 3 | 3 | 17 |
| 5 | 2,65 | 4 | 2 | 3 | 0 | 5 | Fogarty® closed | 1 | 7 | 1 | 14 |
| 6 | 3,96 | 3 | 0 | 5 | 3 | 2 | 40 | 5 | 7 | 1 | 20 |
| 7 | 5,08 | 2 | 1 | 4 | 3 | 2 | 40 | 5 | 4 | 2 | 20 |
| 8 | 4,9 | 2 | 1 | 4 | 1 | 4 | Fogarty® closed | 1 | 4 | 2 | 17 |
| 9 | 3,94 | 3 | 0 | 5 | 1 | 4 | Fogarty® closed | 1 | 3 | 3 | 21 |
| 10 | 3,28 | 4 | 2 | 3 | 1 | 4 | 40 | 5 | 2 | 4 | 26 |
| 11 | 6 | 1 | 2 | 3 | 1 | 4 | Fogarty® closed | 1 | 5 | 1 | 17 |
| 12 | 5,33 | 2 | 3 | 2 | 1 | 4 | Fogarty® closed | 1 | 5 | 1 | 18 |
| 13 | 3,25 | 4 | 0 | 5 | 2 | 3 | Fogarty® closed | 1 | 3 | 3 | 26 |
| 14 | 6,55 | 1 | 2 | 3 | 0 | 5 | Fogarty® closed | 1 | 4 | 2 | 21 |
| 15 | 2,87 | 4 | 1 | 4 | 0 | 5 | 40 | 5 | 1 | 5 | 23 |
| Average | 5,14 | 2,27 | 1,2 | 3,8 | 1,4 | 3,6 | 40 | 3,13 | 3,87 | 2,4 | 11,6 |
Table 3.
Last (fifth) attempt results: length of the procedure (in minutes), number of contacts to the fornix and hypothalamus, fenestration size, fenestration attempts, together with individual and total scores.
| Time (min) | Points | Hypothalamus contacts | Points | Fornix contacts | Points | Fenestration size (%) | Points | Fenestation attempts | Points | Total points | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2,98 | 4 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 29 |
| 2 | 2,08 | 5 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 30 |
| 3 | 2,45 | 5 | 0 | 5 | 1 | 4 | 100 | 10 | 1 | 5 | 29 |
| 4 | 2,49 | 5 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 30 |
| 5 | 1,95 | 5 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 30 |
| 6 | 4,1 | 3 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 28 |
| 7 | 3 | 4 | 0 | 5 | 0 | 5 | 100 | 10 | 2 | 4 | 28 |
| 8 | 2,25 | 5 | 0 | 5 | 0 | 5 | 100 | 10 | 2 | 4 | 29 |
| 9 | 2,66 | 4 | 0 | 5 | 0 | 5 | 100 | 10 | 2 | 4 | 28 |
| 10 | 2,46 | 5 | 1 | 4 | 0 | 5 | 100 | 10 | 1 | 5 | 29 |
| 11 | 3,7 | 3 | 0 | 5 | 0 | 5 | 40 | 5 | 1 | 5 | 23 |
| 12 | 3 | 4 | 0 | 5 | 0 | 5 | 40 | 5 | 1 | 5 | 24 |
| 13 | 4 | 3 | 0 | 5 | 1 | 4 | 40 | 5 | 1 | 5 | 22 |
| 14 | 3,88 | 3 | 0 | 5 | 0 | 5 | 40 | 5 | 1 | 5 | 23 |
| 15 | 2,95 | 4 | 0 | 5 | 0 | 5 | 100 | 10 | 1 | 5 | 29 |
| Average | 2,93 | 4,13 | 0,07 | 4,93 | 0,13 | 4,87 | 84 | 8,67 | 1,2 | 4,8 | 27,4 |
2.3. Statistical analysis
2.3.1. Sample size calculation
To calculate the number of necessary subjects (‘n’) in our study, we used the following formula:
We have chosen the standard errors alpha 0.05 and beta 0.2 (statistical power of 0.8), being zα/2 = 1.96 and zβ = 0.842.
On the other hand, we have assumed a theoretical standard deviation (s) of 5. In the absence of previous studies, we estimated this as an approximate calculation, and a deviation from the level of an entire group would be expected. On the other hand, d = 5, since it is the cut-off point of each interval in the scale that we have used to measure the primary variable, so it will be the differences between the minimum values that we want to detect.
Therefore, a total number of 15 subjects were necessary to be included into the study to reach enough statistical power.
2.3.2. Hypothesis contrast test
The statistical analysis was carried out on the free-source statistical analysis software ‘R' (version 4.0.5 through the RStudio version 1.3.959 interface) on the macOS Catalina version 10.15.5 operative system. The paired sample t-test was used to compare the variables between the first and the last attempts. For multiple comparisons, the ANOVA test was used.
Therefore, a comparison of the different evaluated parameters was carried out.
-
-
A1 to A5: overall score into the ETV-training-scale at first and last attempts.
-
-
T1 to T5: timing of first and last attempts.
-
-
H1 and H5: number of contacts to the hypothalamus at first and last attempts.
-
-
F1 and F5: number of contacts to the fornix at first and last attempts.
-
-
E1 and E5: diameter of the fenestration at the first and last attempts.
-
-
NI1 and NI5: number of fenestrations attempts at first and last performances.
3. Results
3.1. First attempt (Table 2)
Table 2 shows the results obtained by each subject, together with the average of each of the parameters and individual scores. The color scale visually represents the range of values, with red representing the lower and green the higher values. In the first column, time in minutes is displayed, with an average timing of 5.14 min [2.86–9.05]. Regarding the global score, the 15 subjects average reached 11.6 points on the ETV-training-scale. As for the manipulations of the hypothalamus and fornix, most subjects contacted them at least once during the first performance. Regarding the fenestration diameter, none of them was larger than a fully dilated Fogarty® balloon. Approximately half of the subjects performed a fenestration through which the non-inflated Fogarty® balloon could not be inserted loosely. Fenestration attempts were multiple in most cases, up to a maximum of 7 attempts in two of the subjects.
3.2. Fifth attempt (Table 3)
A clear improvement in all the measured variables is noticed when analyzing the fifth attempt. Regarding time, the average was 2.93 min [1.95–4.1], corresponding to an average score of 4.13. Thirteen out of the fifteen subjects (86.67%) performed the intervention without any contact to the fornix, while only one subject (6.67%) contacted the hypothalamus in the fifth and last attempt. The fenestration diameter was larger than the Fogarty® dilated balloon at the last attempt in all but four subjects (73.33%). Regarding the number of fenestration attempts, it was reduced to one in twelve of the subjects (80%). The average global score for the ETV-training-scale was 27.4, out of a maximum of 30.
3.3. Comparative study
All the analyzed parameters showed a significative improvement among the first and fifth attempts (p < 0.001).
3.3.1. Surgical time
The average reduction in surgical time was 2.20 min (2 min and 12 s) with a p-value <0.0001. Therefore the 95% confidence interval was 2.20 [1.09; 3.32 min].
3.3.2. Contacts to the hypothalamus
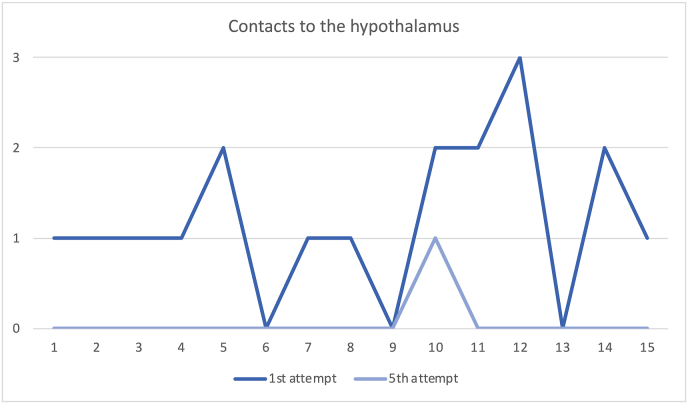
Regarding the number of direct contacts between the surgical instruments and/or even the endoscope itself to the hypothalamus, they were also significantly reduced between the first and last attempts, with a 95% confidence interval of 1.13 [0.67; 1.59] and p-value <0.001 (Fig. 4).
Fig. 4.
Comparison of the contacts to the hypothalamus on each of the 15 subjects between the first and fifth attempts. The marked reduction of the total number of contacts is observed.
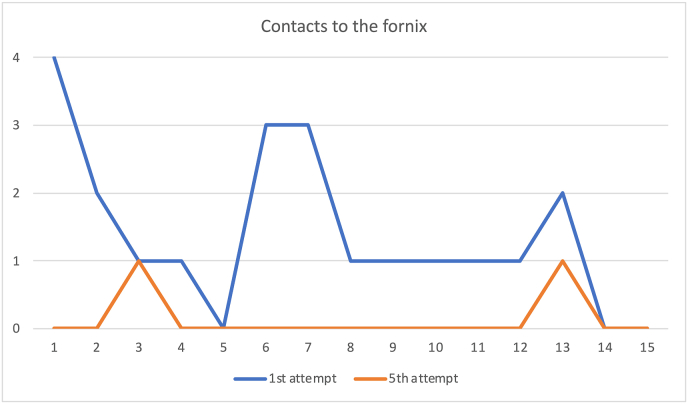
3.3.3. Contacts to the fornix
The number of fornix contacts was also significantly reduced between the first and last attempts, with a 95% confidence interval of 1.27 [0.59, 1.94] and p-value <0.001 (Fig. 5).
Fig. 5.
Comparison of the contacts to the fornix on each of the 15 subjects between the first and fifth attempts. The marked reduction of the total number of contacts is observed.
3.3.4. Fenestration diameter
The difference in the score measuring the fenestration diameter between each subject first and last attempt, was significantly increased, with a 95% confidence interval of 62.67 [50.92; 74.1] and p-value <0.001.
3.3.5. Fenestration attempts
The comparison of the fenestration attempts between the first and the last performance were significantly reduced with a 95% confidence interval of 2.67 [1.72; 3.62] and p-value <0.001.
3.3.6. Global score
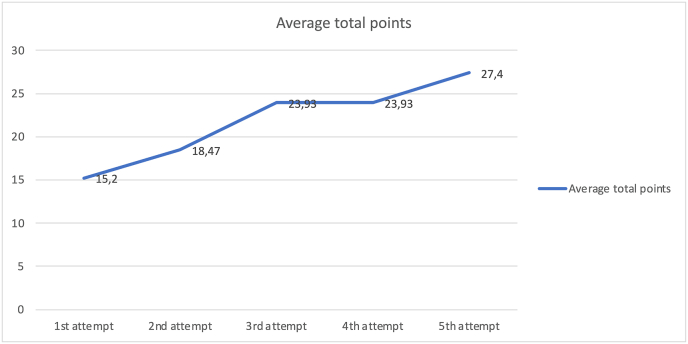
Regarding the total ETV-training-scale scores, there was also a significant improvement: a difference of 12.2 points (p-value <0.0001), with an interval confidence at 95%: 12.2 [10.45; 13.95]. The average score in the first attempt was 15.2, while in the last attempt it was 27.4. An increase of the 80.26% was reached in the global scores (Fig. 6).
Fig. 6.
Comparison of the total scores in each of the attempts. The ascendent curve represents how the individual global scores average increased after each attempt ETV-training-scale.
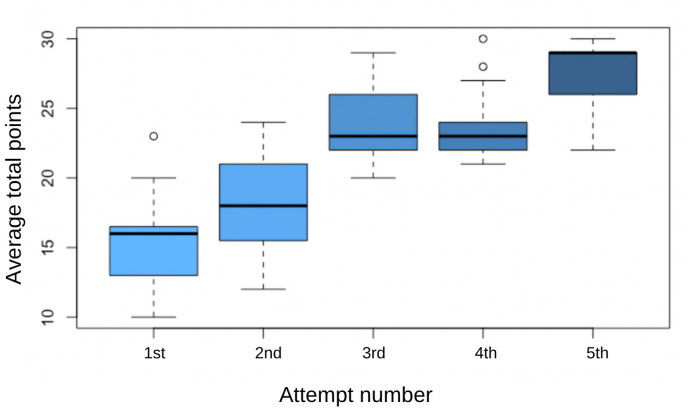
The ANOVA was globally significant, meaning that the attempts were different from each other. As for the one-to-one comparisons, all comparisons were statistically significant except attempt 3 vs attempt 4 (Fig. 7).
Fig. 7.
Boxplot result of ANOVA for the global scores in the ETV-training-scale along all five attempts on every subject.
4. Discussion
Hydrocephalus is one of the most prevalent pediatric brain diseases neurosurgeons deal with. Once detected, its fast treatment is recommended to avoid disastrous consequences that might result on macrocephaly, neurological dysfunction, and children death. Its classic treatment has considered to be inserting a VP shunt. However, in the past two decades, the advent of new endoscopic technologies and techniques has led to a progressive trend, and more and more of these kids are nowadays being treated through an ETV. In addition to the obvious benefits of ETV, some studies have shown lower rates of complications and mortality in favor of ETV, when compared to VP shunts (Lu et al., 2019).
In certain African countries, pediatric hydrocephalus reaches ten times higher incidence than in European countries. Different authors have calculated values of annual incidence as high as more than 225,000 new cases of infant hydrocephalus (Warf, 2010). Therefore, this disease might fit into the definition of a pandemic in terms of numbers, indeed, with potential catastrophic consequences for the young population.
ETV is a safe, effective, and low-cost technique that might solve many cases of childhood hydrocephalus in African countries (Warf, 2010; Jimenez et al., 2017; Filho et al., 2011). However, the acquisition of surgical skills for its practice requires a learning curve since this technique has some peculiarities when compared with classic surgical procedures (Jiang et al., 2018).
Our model was created with the main goal of facilitating the acquisition of the specific endoscopic surgical skills, but we considered mandatory to perform a prior evaluation and validation before including this simulator in already established training programs in Central, East and Southern African countries.
The parameters chosen to create the ETV-training-scale were thought to be key aspects when performing an ETV. A reduced number of attempts to perform the fenestration into the third ventricle floor minimizes the chances of hypothalamic, infundibular, optic apparatus as well as arterial damage. A correct trajectory and endoscopic orientation are gained through the learning curve, and clearly reduce the direct damage to the fornix and hypothalamus. The diameter of the fenestration is key when performing an ETV as a large one opened to the perimesencephalic cisterns will increase the rate of success after the procedure (Zhu et al., 2020). We believe that timing is not a parameter that increases the success by itself, but in conjunction with an improvement of all other variables, would help measuring the skills acquisition.
After analyzing the results obtained, it was proven that the spatial orientation with the neuroendoscope was the first ability the subjects gained, since, after the third attempt, the contacts to the hypothalamus and fornix were drastically reduced in all subjects.
A progressive increasing of the fenestration diameter was statistically significant among the first and last attempts. We have considered that the diameter of the stoma was the most important parameter since, when small, it might close or become obstructed easily (Zhu et al., 2020). On the other hand, the fact of achieving a larger fenestration implies a better control of the surgical technique.
Even though the surgical time was significantly reduced between the first and fifth attempts, we consider that the length of the procedure is not an important parameter by itself when evaluating a surgical technique. However, it must be considered that it could have some relationship with greater manual dexterity, especially when analyzed in conjunction with an improvement of the rest of the parameters, as it was the case in our study. On the other hand, as a general and well-proven rule, shorter surgical length, decreases the risk of infection (Licci et al., 2020; Bodani et al., 2019).
Regarding the learning curve, it is obvious that the ETV surgical skills and technique are radically different from those used to place a VP shunt. The main difference lies in a fixed channel work compared to an open surgery with total range of movement. Moreover, the neurosurgeon view is directed towards a screen (2D view) instead of to the surgical field (3D vision). Moreover, this technique needs two neurosurgeons to work completely coordinated in a very small space between critical structures such as the fornix and the hypothalamus. Therefore, gaining all these skills to perform an ETV safely is crucial to achieve an optimal surgical result. Surprisingly, we realized that doctors with none or just short experience on endoscopic techniques, were able to perform the ‘in-vitro’ procedure optimally with our model after five attempts. Therefore, the learning curve does not seem flattened, but rather exponential, as we can see in Fig. 6, Fig. 7.
Literature review shows other neuroendoscopy training models (Baby et al., 2020; Filho et al., 2011) all of them showing similar results to ours, especially in the reduction of the mistakes probability (Baby et al., 2020; Filho et al., 2011; Timothy et al., 2022; Marchesini et al., 2022). In our study, this parameter was measured by considering the reduction of contacts to the periforaminal structures and the lateral third ventricle walls. Most of the previously studied surgical models have been developed to deal with intracranial tumors and colloid cysts. [14,21].
Our study draws a learning curve on fifteen young doctors not previously really exposed to endoscopic techniques. Although the technical abilities are way different from the ones used to place a shunt, only five attempts can improve considerably the procedure performed in our model. Therefore, we think that the learning curve is exponential rather than lineal as expected, and models like this can improve the skills and reduce the time to achieve it. Even though a larger study should be carried out to define a realistic learning curve, the ANOVA analysis showed that the curve seems to flatten between the third and fourth attempts, as seen in Fig. 7.
Limitations of our study include, especially the difficulty of finding objective parameters that can validate something such subjective as ‘utility’ of a simulator. To try to objectify this problem, we designed the ETV-training-scale, which assigns a score to the length of surgery, number of contacts to hypothalamus and fornix, number of fenestration of attempts, and finally the diameter of the fenestration. All these parameters were carefully included on the scale as they potentially measure the ability acquisition as well as the reduction of complications. On the other, the realism of the model is in process to be improved, just to create a better scenery of practice.
Paradigms of training programs in neurosurgery are in constant change due to the importance of training manual skills without adding risks on patients’ safety. Simulation models represent a necessary option to achieve this change. Having a simulation center in neurosurgery would help preparing and evaluating neurosurgery trainees during their learning process. Even more, to promote a safe and accurate environment for medical and surgical continuous education. Our aim is to implement these models in African countries. Indeed, our group has already done this in Tanzania, where we have carried out three training courses with theoretical lectures, hands-on workshops based on the model, and live surgeries (Fig. 3).
5. Conclusion
The ETV model shown in this paper can be a useful tool for training young neurosurgeons in endoscopic techniques. Bigger efforts are carried out to improve the anatomical details as well as the tissues of our model. Common efforts must be carried out in High-income countries to motivate a global task to help creating equal medical environments, including training opportunities (Timothy et al., 2022; Marchesini et al., 2022).
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author statement
All authors have contributed to the article. Gonzalez-Lopez P, Gomez-Revuelta C and Fernandez-Jover E contributed to the conception and design of the study. Puchol-Rizo M, Gomez-Revuelta G, Verdu-Martinez I and Gonzalez-Lopez P contributed to the acquisition of data. Puchol-Rizo M and Fernandez Villa de Rey Salgado J contributed to the analysis and interpretation of data. Puchol-Rizo M, Gomez-Revuelta C, Gonzalez-Lopez P and Fernandez-Cornejo V, contributed to drafting the article. Lafuente J, Nieto-Navarro J and Gonzalez-Lopez P contributed to the final approval of the version to be submitted.
Declaration of informed consent
The authors declare that all individuals appearing on Figure 3 gave their consent to appear in this manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: W Peul
Contributor Information
Pablo González-López, Email: gonzalez_pab@gva.es.
Cristina Gómez-Revuelta, Email: crgomezrevuelta@gmail.com.
Martin Puchol Rizo, Email: martinpucholrizo@gmail.com.
Iván Verdú Martínez, Email: ivan.vmtnez@gmail.com.
Jaime Fernández Villa de Rey Salgado, Email: jaimefernandez3a@gmail.com.
Jesús Lafuente, Email: jlbspine@gmail.com.
Eduardo Fernández-Jover, Email: e.fernandez@umh.es.
Víctor Fernández-Cornejo, Email: vjcornejo@hotmail.com.
Juan Nieto-Navarro, Email: nieto_juanav@gva.es.
References
- Baby B., Singh R., Singh R., Suri A., Arora C., et al. A review of physical simulators for neuroendoscopy skills training. World Neurosurg. 2020;137:398–407. doi: 10.1016/j.wneu.2020.01.183. [DOI] [PubMed] [Google Scholar]
- Bodani V., Breimer G., Haji F., Looi T., DrakeJ Development and evaluation of a patient-specific surgical simulator for endoscopic colloid cyst resection. J. Neurosurg. 2019;28:1–9. doi: 10.3171/2019.4.JNS183184. [DOI] [PubMed] [Google Scholar]
- Cheng H., Chen B., Soleas I., Ferko N., Cameron C., et al. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg. Infect. 2017;18(6):722–735. doi: 10.1089/sur.2017.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan M., Rattani A., Mekary R., Glancz L., Yunusa I., et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J. Neurosurg. 2018;51(2):1–15. doi: 10.3171/2017.10.JNS17439. [DOI] [PubMed] [Google Scholar]
- Filho F., Coelho G., Cavalheiro S., Lyra M., Zymberg S. Quality assessment of a new surgical simulator for neuroendoscopic training. Neurosurg. Focus. 2011;30(4):17. doi: 10.3171/2011.2.FOCUS10321. [DOI] [PubMed] [Google Scholar]
- Jiang L., Gao G., Zhou Y. Endoscopic third ventriculostomy and ventriculoperitoneal shunt for patients with noncommunicating hydrocephalus: a PRISMA-compliant meta-analysis. Medicine (Baltim.) 2018;97(42):121–139. doi: 10.1097/MD.0000000000012139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez J., Jiménez C., Betancourt Y. Neuroendoscopy. Its usefulness in the hydrocephalus management of children in developing countries. Medicina (B Aires) 2007;67(6):665–673. [PubMed] [Google Scholar]
- Jimenez A., Castillo H., Burckart C., Castillo J. Endoscopic Third Ventriculostomy to address hydrocephalus in Africa: a call for education and community-based rehabilitation. J. Pediatr. Rehabil. Med. 2017;3–4:267–273. doi: 10.3233/PRM-170454. 2017. [DOI] [PubMed] [Google Scholar]
- Licci M., Thieringer F.M., Guzman R., Soleman J. Development and validation of a synthetic 3D-printed simulator for training in neuroendoscopic ventricular lesion removal. Neurosurg. Focus. 2020;1(48) doi: 10.3171/2019.12.FOCUS19841. [DOI] [PubMed] [Google Scholar]
- Linares J., Ros B., Iglesias S., Ros A., Selfa A., et al. Re-Do endoscopic third ventriculostomy. Retrospective analysis of 13 patients. Revista de la Sociedad Española de Neurocirugía. 2021;18(21):26–29. doi: 10.1016/j.neucir.2021.02.001. [DOI] [Google Scholar]
- Lu L., Chen H., Weng S., Xu Y. Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in patients with obstructive hydrocephalus: meta-analysis of randomized controlled trials. World Neurosurg. 2019;129:334–340. doi: 10.1016/j.wneu.2019.04.255. [DOI] [PubMed] [Google Scholar]
- Marchesini N., Ivanov M., Lafuente J., Sala F., Foroglou N., Visocchi M., Olldashi F., Gonzalez-Lopez P., Rzaev J., Tisell M., Paternò V., Rotim K., Timothy J., Rasulic L., Demetriades A.K. Global neurosurgery amongst the EANS community: where are we at? Brain Spine. 2022;2 doi: 10.1016/j.bas.2022.100911. 2022 Jun 28. PMID: 36248142; PMCID: PMC9559959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwachaka P., Obonyo N., Mutiso B., Ranketi S., Mwang'Ombe N. Ventriculoperitoneal shunt complications: a three-year retrospective study in a Kenyan national teaching and referral hospital. Pediatr. Neurosurg. 2010;46(1):1–5. doi: 10.1159/000314050. [DOI] [PubMed] [Google Scholar]
- Shim K., Park E., Kim D., Choi J. Neuroendoscopy: current and future perspectives. J Korean Neurosurg Soc. 2017;60(3):322–326. doi: 10.3340/jkns.2017.0202.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timothy J., Ivanov M., Tisell M., Marchesini N., Lafuente J., Foroglou N., Visocchi M., Olldashi F., Gonzalez-Lopez P., Rzaev J., Whitfield P., Peul W.C., Rasulic L., Demetriades A.K. Working in low- and middle-income countries: learning from each other. Brain and Spine. 2022;2 doi: 10.1016/j.bas.2022.101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uche E., Okorie C., Iloabachie I., Amuta D., Uche N. Endoscopic third ventriculostomy (ETV) and ventriculoperitoneal shunt (VPS) in non-communicating hydrocephalus (NCH): comparison of outcome profiles in Nigerian children. Childs Nerv Syst. 2018;34(9) doi: 10.1007/s00381-018-3848-0. [DOI] [PubMed] [Google Scholar]
- Warf B. Pediatric hydrocephalus in east Africa: prevalence, causes, treatments, and strategies for the future. World Neurosurg. 2010;73(4):296–300. doi: 10.1016/j.wneu.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Zhan R., Zhu Y., Shen Y., Shen J., Tong Y., et al. Post-operative central nervous system infections after cranial surgery in China: incidence, causative agents, and risk factors in 1,470 patients. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33(5):861–866. doi: 10.1007/s10096-013-2026-2. [DOI] [PubMed] [Google Scholar]
- Zhu J., Yang J., Tang C. Design and validation of a 3D-printed simulator for endoscopic third ventriculostomy. Childs Nerv Syst. 2020;36:743–748. doi: 10.1007/s00381-019-04421-8. [DOI] [PubMed] [Google Scholar]