Abstract
Introduction
It was hypothesized that pelvic retroversion in Adult Spinal Deformity (ASD) can be related to an increased hip loading explaining the occurrence of hip-spine syndrome.
Research question
How pelvic retroversion can modify acetabular orientation in ASD during walking?
Methods
89 primary ASD and 37 controls underwent 3D gait analysis and full-body biplanar X-rays. Classic spinopelvic parameters were calculated from 3D skeletal reconstructions in addition to acetabular anteversion, abduction, tilt, and coverage. Then, 3D bones were registered on each gait frame to compute the dynamic value of the radiographic parameters during walking. ASD patients having a high PT were grouped as ASD-highPT, otherwise as ASD-normPT. Control group was divided in: C-aged and C-young, age matched to ASD-hightPT and ASD-normPT respectively.
Results
25/89 patients were classified as ASD-highPT having a radiographic PT of 31° (vs 12° in other groups, p < 0.001). On static radiograph, ASD-highPT showed more severe postural malalignment than the other groups: ODHA = 5°, L1L5 = 17°, SVA = 57.4 mm (vs 2°, 48° and 5 mm resp. in other groups,all p < 0.001). During gait, ASD-highPT presented a higher dynamic pelvic retroversion of 30° (vs 15° in C-aged), along with a higher acetabular anteversion of 24° (vs 20°), external coverage of 38° (vs 29°) and a lower anterior coverage of 52° (vs 58°,all p < 0.05).
Conclusion
ASD patients with severe pelvic retroversion showed an increased acetabular anteversion, external coverage and lower anterior coverage during gait. These changes in acetabular orientation, computed during walking, were shown to be related to hip osteoarthritis.
Keywords: Adult spinal deformity, Gait analysis, Hip, Spine, Biomechanics
Highlights
-
•
Pelvic retroversion in ASD can modify acetabular orientation during walking.
-
•
89 ASD & 37 controls had their hip orientation parameters calculated during gait.
-
•
ASD with high PT had more external and posterior acetabular coverage during walking.
-
•
These dynamic hip alterations are in favor of developing femoro-acetabular impingement.
1. Introduction
As the population growths older, the number of degenerative pathologies multiplies and occur in conjunction with wear and tear of the tissues, leading to musculoskeletal problems, particularly in the spine (Fehlings et al., 2015). Adult Spinal Deformity (ASD) consists of a variety of postural and spino-pelvic alterations of the lumbar or thoracolumbar spine, involving one or more of the three planes (Schwab et al., 2005; Acaroğlu et al., 2016). It is defined as the presence of pain or loss of function with an increase in one of the following radiographic parameters: Pelvic Tilt (PT), Sagittal Vertical Axis (SVA), Pelvic incidence minus lumbar lordosis (PI-LL) mismatch, coronal Cobb angle, and Thoracic Kyphosis (TK) (Lafage et al., 2016; Schwab et al., 2012). ASD has a physical and mental impact on the individual. In fact, it can alter daily function, such as walking (Kawkabani et al., 2021; Semaan et al., 2022), sitting and standing (Saad et al., 2022), and can be associated to anxiety and depression in extreme cases (Schwab et al., 2003; Bess et al., 2016). The severity of the deformity is quantified radiologically and can often guide surgical decision (Fujishiro et al., 2018, 2020).
ASD patients are known to present sagittal malalignment leading to the recruitment of compensatory mechanisms in order to maintain a horizontal gaze, and to keep their head and center of gravity above their feet (Dubousset, 1994). Pelvic retroversion is the first compensatory mechanism adopted by the patients in order to maintain postural balance (Lafage et al., 2009a). Knee flexion develops in later stages in order to palliate for the exhaustion of pelvic retroversion (Lafage et al., 2008; Obeid et al., 2011a; Hovorka et al., 2008), and is generally considered as the final stage of compensation (Obeid et al., 2011b).
Hip osteoarthritis can occur in some patients under the hip-spine syndrome, a very well-known concept in the literature representing cases where spine deformity is concurrent with hip osteoarthritis (Rivière et al., 2017, 2018; Day et al., 2018). A recent study showed that ASD subjects compensating with an excessive pelvic tilt and knee flexion presented an altered hip orientation, showing a more tilted acetabulum, with decreased anterior coverage and increased posterior coverage and anteversion (Mekhael et al., 2021). These alterations, investigated in the static standing position, and indicative of femoro-acetabular impingement, might be at the origin of the development of hip osteoarthritis (Reid et al., 2010).
While it is known that daily life activities might increase forces on the spine, hips and knees compared to static position, we hypothesized that the modification seen in hip orientation in standing position persists during walking in ASD patients with increased pelvic retroversion.
Thus, the aim of this study was to investigate the relationship between pelvic retroversion and changes in acetabular orientation and global alignment in ASD during walking.
2. Methods
This is an IRB-approved (CEHDF 1259) cross sectional study where control subjects and patients with ASD consulting a center for radiographic assessment were enrolled.
2.1. Population
In total, 89 primary ASD (52 ± 20 years, 70F) and 37 asymptomatic subjects (46 ± 14 years, 25F) were enrolled. ASD were included if they presented back pain and at least, one of the radiographic diagnostic criteria as defined by the International Spine Study Group (Kim et al., 2017): pelvic tilt (PT) > 25°, a mismatch between pelvic incidence and lumbar lordosis PI-LL > 10°, frontal Cobb angle >20°, sagittal vertical axis (SVA) > 50 mm, or thoracic kyphosis (TK) > 60°. The exclusion criteria of the control group were any orthopedic history and/or back pain.
2.2. Data acquisition
All subjects filled the following health related quality of life (HRQOL) questionnaires: Oswestry Disability Index (ODI), Short Form - 36 (SF-36) item survey assessing general quality of life with both its physical (PCS) and mental (MCS) components, and the Visual Analog Scale for pain (VAS). Subjects were equipped with reflective markers on the head, spine, trunk and lower limbs according to the modified Davis protocol (Plug In Gait model) and the Leardini protocol (Fig. 1a) (Leardini et al., 2011; Davis and Ounpuu, 1991). They all underwent 3D gait analysis at self-selected speed using 8 infrared cameras (Vicon Motion Systems, Oxford, UK). A static trial was recorded at first. Then, each subject walked several trials that were compared for kinematic consistency using Polygon (Vicon Motion Systems, Oxford, UK). One representative trial was considered for the calculation of kinematics (of the trunk, pelvis and lower limbs), the gait deviation index (GDI) and spatio-temporal parameters (Semaan et al., 2022; Schwartz and Rozumalski, 2008).
Fig. 1.
Radiographic and 3D gait acquisition with reflective markers placement and registration technique between the two imaging modalities.
Additionally, subjects underwent full-body bi-planar X-rays (EOS Imaging®, Paris, France) in the free-standing position with gait reflective markers still in place. Then, 3D reconstructions of the spine, pelvis, lower limbs were performed by well-trained operators using a specific software (Fig. 1b).
The following spinopelvic and global alignment parameters were calculated using 3D reconstructions from static radiographs: Pelvic Tilt (rPT, in °), Sagittal Vertical Axis (rSVA, in mm), the Odontoid Hip Axis Angle (rODHA, in °): angle between the vertical reference line the line joining the top of the odontoid (OD) and the center of the hip axis (HA), L1S1 (°), T1T12 (thoracic kyphosis in °), frontal Cobb angle (in °), pelvic incidence (PI in °) and PI-LL mismatch (°) (Fig. 2a).
Fig. 2.
Radiographic parameters calculated on 3D skeletal reconstructions: a) postural and spino-pelvic parameters, b) acetabular parameters.
The following hip parameters were calculated using the 3D pelvic and lower limb reconstructions from static radiographs (Ghostine et al., 2016): acetabular anteversion (in °), tilt (in °), external coverage (known also as the vertical center edge angle, in °), anterior and posterior coverage over the femoral head (in °) Fig. 2b). The right limb was analyzed in this study.
Skeletal segments were extracted as 3D points and meshes expressed in the X-ray booth coordinate system. The 3D locations of reflective markers were also extracted. An image registration technique was applied using the computation of rigid body transformation matrix between the X-ray booth and the gait analysis environment for each segment at each time of the gait analysis.
The global alignment, spinopelvic and hip parameters were then computed on the moving skeletal segments in 3D during the gait cycle using Matlab (Mathworks, Nattwik, USA) (Rebeyrat et al., 2022), while reducing soft tissue artefacts using a novel technique (Skalli et al., 2018; Lahkar et al., 2021). Mean values and range of motion (ROM) of each radiographic parameters were computed during the gait cycle. The suffix d was added to each parameter in order to differentiate the dynamic value calculated during walking from the radiographic value calculated on the static X-ray designated by the suffix r.
2.3. Statistical analysis
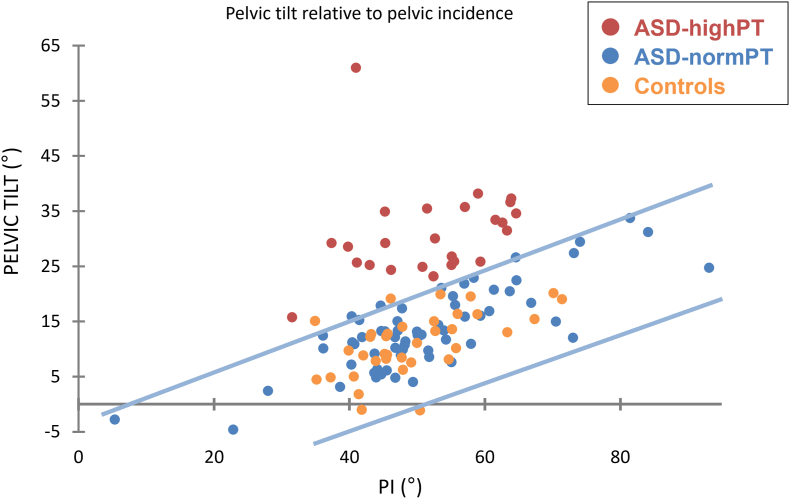
Pelvic retroversion was analyzed according to PI for all subjects to check for abnormal pelvic tilt, using the equation and reference corridor assessed in a previous study (PT = 0.37∗PI-7°, Amabile et al., 2018). Patients with ASD having a PT related to PI that was superior to 2 standard deviations (SD) of controls, were grouped as ASD-highPT, otherwise as ASD-normalPT (Fig. 3).
Fig. 3.
Pelvic tilt distribution of patients according to their pelvic incidence: High-PT assessed from Amabile et al., 2018). Blue lines represent the 95% confidence interval (corridor of normality) in controls. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Demographics, HRQOL outcomes, radiographic parameters in static position computed from biplanar X-Ray 3D reconstruction, and during walking computed from gait data, in addition to classical kinematic parameters, were compared between ASD-highPT, ASD-normalPT and controls using Kruskal-Wallis test with Conover-Iman pairwise comparisons to search for group effect. The level of significance was set at 0.05 and adjusted with Bonferroni correction to accommodate for multiple comparisons. In the case of a significant difference in age between groups, the split of the control group in C-young and C-aged was considered.
The variation of postural, spinopelvic and hip parameters during walking were displayed for each group during the normalized gait cycle as kinematic waveforms and compared between each other using the statistical parametric mapping (SPM) method (Pataky, 2012).
The relationship between dynamic and static parameters as well as HRQOL outcomes were evaluated through a univariate analysis, using Pearson’s r correlation coefficient.
Statistical tests were run under Xlstat® (Addinsoft, Paris, France; version 2020.1.3.65336) and Matlab (MathWorks, Inc., Natick, USA).
3. Results
Patients with ASD were grouped as follow: 25 in the ASD-highPT group (65 ± 14: (mean ± SD) y.o., 21F) and 64 in the ASD-normalPT group (46 ± 18y.o., 49F). Control subjects were divided in C-aged (>60 y.o.) and C-young (<60 y.o.) in order to match with the age of ASD-highPT and ASD-normalPT groups respectively. There were 12 subjects in the C-aged group (60 ± 8 y.o., 5F) and 25 in the C-young group (39 ± 10 y.o., 20F).
3.1. HRQOL scores and static radiographic parameters
Both ASD-highPT and ASD-normalPT had a significantly decreased physical component summary on the SF-36 questionnaire when compared to C-aged and C-young groups (38, 41, 51, 47, respectively, all p < 0.05). ASD-highPT had a significantly higher score for ODI and VAS for pain when compared to ASD-normalPT, C-aged and C-young (Fig. 4).
Fig. 4.
Comparison of HRQOL scores between groups.
ASD having a high PT relatively to their PI had a higher radiographic PT of 31° (vs 11.5° in C-aged, p < 0.001) for a similar PI between groups (average of 50°), ODHA (5.1 ± 3.5° vs 2.2 ± 1.2° in C-aged), SVA (57.4 ± 52.6 mm vs 7.7 ± 20 mm in C-aged), PI-LL mismatch (20.8 ± 19.2° vs −10.7 ± 5.7° in C-aged) and knee flexion (11.9 ± 14.1° vs 1.4 ± 4.5° in C-aged, all p < 0.05) when compared to ASD-normalPT, C-aged and C-young. They also had a decreased LL (30.1 ± 20.7° vs 61.5 ± 8.4°; Fig. 5).
Fig. 5.
Between-group comparisons of postural and spino-pelvic parameters calculated on static radiographs.
Patients in the ASD-highPT group had a significantly increased acetabular tilt (36.7 ± 8.6° vs 26.3 ± 4.7° in C-aged), acetabular anteversion (25.9 ± 4.2° vs 20.6 ± 3.0° in C-aged), and a significantly decreased anterior coverage over the femoral head (50.8 ± 6.8° vs 59.7 ± 4.1°) when compared to patients in the ASD-normalPT group as well as to controls in C-aged and C-young (Fig. 6).
Fig. 6.
Between-group comparisons of acetabular parameters calculated on static radiographs.
3.2. Walking pattern
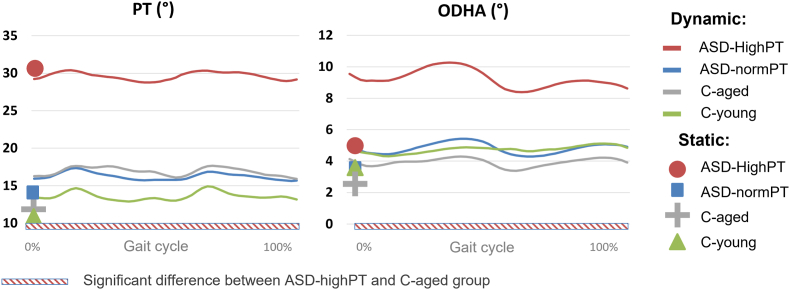
During walking, the ASD-highPT group had a significantly increased (average) dynamic pelvic retroversion, identical to their rPT, when compared to ASD-normPT, C-aged and C-young (29.4 ± 7.9° vs 16.9 ± 9.5° in C-aged, p < 0.001; Fig. 7). They also had an increased dODHA during the whole gait cycle (9.1 ± 5.3° vs 4.7 ± 2.6° in C-aged, p < 0.01), with an increase of its ROM (3.7 ± 1.5° vs 2.3 ± 0.8° in C-aged, p < 0.05), when compared to the other groups.
Fig. 7.
Pelvic tilt and ODHA computed during walking.
The ASD-highPT group presented an increased acetabular anteversion (23.8 ± 4.7° vs 21.4 ± 3.8° in C-aged group, p < 0.05) with a higher external coverage of the acetabulum (38.3 ± 6.0° resp. vs 30.2 ± 6.7° in C-aged, p < 0.001). They also presented a significantly lower anterior coverage of the acetabulum (52.9 ± 5.1° vs 57.4 ± 6.9° in C-aged) and a significantly higher acetabular tilt (33.0 ± 8.4° vs 28.2 ± 5.2°) during the gait cycle (Fig. 8).
Fig. 8.
Acetabular parameters computed during walking.
The ASD-highPT also had an overall abnormal walking kinematics compared to other groups: they walked with a slower gait speed (0.8 ± 0.3 m s−1 vs 1.2 ± 0.3 m s−1 in C-aged group), shorter step length (0.5 ± 0.1m vs 0.6 ± 0.1m for other groups) and reduced cadence (95.6 ± 13.7 step/min vs 110.2 ± 16.4 step/min in C-aged). The overall gait deviation index showed an altered gait for the ASD-highPT patients (80.2 ± 9.3 vs 94.7 ± 12.9 in C-aged, all p < 0.001).
3.3. Correlation between radiographic, HRQOL outcomes and dynamic parameters
The univariate analysis showed that the dynamic acetabular tilt, anteversion and external coverage were mostly correlated to SVA, PT, PI-LL and knee flexion on static radiographs (r between 0.28 and 0.67, p < 0.05), as well as the physical component of SF36 (PCS), the VAS for pain and ODI (r between 0.23 and 0.34, p < 0.05; Table 1).
Table 1.
Correlations (Pearson’s r) between hip parameters computed during walking and postural and spino-pelvic parameters calculated on static radiographs as well as HRQOL scores.
| Pearson’s r correlation |
Static radiographs |
HRQOL scores |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetabular parameters during walking | ODHA | SVA | PT | PI-LL | T1T12 | Cobb angle | Knee flexion | L1S1 | PCS-SF36 | MCS-SF36 | VAS for pain | ODI | |
| Acetabular Anteversion | Average | 0.30 | 0.44 | 0.42 | −0.24 | ||||||||
| ROM | 0.23 | −0.29 | −0.31 | ||||||||||
| Acetabular Tilt | Average | 0.31 | 0.47 | 0.45 | −0.25 | ||||||||
| ROM | −0.29 | 0.28 | −0.32 | −0.24 | |||||||||
| External Acetabular Coverage | Average | 0.30 | 0.50 | 0.67 | 0.58 | 0.28 | −0.38 | −0.23 | 0.28 | 0.31 | |||
| ROM | 0.43 | 0.36 | |||||||||||
4. Discussion
Spinal deformity in adults is known to deteriorate quality of life and autonomy of patients during daily life activities. To compensate for their spinal deformity, as the alignment of the head upon the pelvis was shown as a quasi-invariant in asymptomatic population, patients increase their pelvic retroversion in standing position. This mechanism was found to be associated with changes in hip orientation. This study investigated hip orientation in ASD during walking. Patients with excessive pelvic retroversion had an increased dynamic acetabular tilt, anteversion, external and posterior coverage with decreased anterior coverage.
In this study, pelvic retroversion was analyzed with regard to pelvic incidence. Indeed, each individual has its own incidence, and manages to keep the head upon the pelvis with proper adjustment of pelvic retroversion and spine curvatures (lumbar lordosis, thoracic kyphosis, cervical lordosis). In asymptomatic controls, the relationship between pelvic retroversion and incidence is well described (Vialle et al., 2005; Amabile et al., 2018). It ranges within a corridor which is considered as a reference. When the spinal curvatures are altered, due to local (tissue alteration) or global (neuromuscular) factors, pelvis retroversion is a major compensation phenomenon. Patients with excessive pelvic retroversion (ASD-highPT) are those who had their radiographic PT above the normality corridor (2SD) of reference pelvic retroversion relatively to their pelvic incidence, defined by controls. As expected, these patients were older than those with normal PT. In order to accommodate for natural changes in pelvic retroversion due to spine ageing, an older control group (C-aged) was considered for further comparisons.
Patients with excessive pelvic retroversion had more deteriorated HRQOL scores when compared to other patients and to controls. This is in accordance with previous studies that showed a positive relationship between the increase of pelvic retroversion and the deteriorated HRQOL scores (Lafage et al., 2009b).
When analyzing their static radiographs, patients with excessive pelvic retroversion presented with increased knee flexion, an additional compensatory mechanism that is developed by patients who already exhausted their pelvic retroversion. In fact, these patients had more severe spinal deformity, showing a lack of lordosis (decreased LL and increased PI-LL mismatch), that lead to forward shift of the head and trunk (increased ODHA and SVA).
When analyzing their hips in the standing position, patients with excessive retroversion showed an increased acetabular tilt, anteversion and posterior coverage over the femoral head, all of them showing a more excessive coverage posteriorly, associated with a decreased anterior coverage. These results were concordant with a previous study (Mekhael et al., 2021; Buckland et al., 2015).
When transitioning from the static position to walking, ASD with high PT conserved their excessive pelvic retroversion while increasing their forward shift of the trunk. Indeed, 44% of the ASD-highPT patients increased their ODHA of more than 5° (vs 11% of the normalPT and Control groups). They moved their head forward when walking leading to a less economical dynamic balance.
During walking, patients with excessive pelvic retroversion showed a more excessive acetabular tilt, anteversion during the whole gait cycle, associated with a decreased anterior coverage of the femoral head, when compared to other ASD patients and controls. This result was the same as shown in static standing position. In addition, these patients showed a more excessive exterior coverage of the femoral head when walking.
This altered dynamic hip orientation was in relation with the lack of lumbar lordosis, the PI-LL mismatch and the forward shift of trunk, as shown in the univariate analysis. Moreover, the findings of this study were in relationship with the deterioration in quality of life.
In conclusion, dynamic analysis combined with static radiological 3D analysis provided complementary information that provides better understanding of mechanisms associated to ASD. The study brought to light the importance of considering the pelvic tilt in relation to the pelvic incidence since the pelvic tilt as a stand-alone value cannot reflect the amount of pelvic compensation. Indeed, ASD with abnormally high pelvic tilt appeared the worst in terms of QOL and of static and dynamical balance.
This study revealed for these patients a dynamic hip orientation showing a more excessive acetabular coverage over the femoral head posteriorly (through the acetabular anteversion) and externally, with a decreased coverage anteriorly. While it is not fully confirmed yet, this pattern of hip orientation was suggested to be linked to hip osteoarthritis (Reid et al., 2010; Valera et al., 2018).
Patients’ follow-up is in progress to determine if surgical correction of spinal deformity can improve global alignment and dynamic balance, and therefore reduce alterations of acetabular orientation.
Declaration of competing interest
None.
Acknowledgment
This research was funded by the University of Saint-Joseph (grant FM361), EUROSPINE (TFR 2020#22) and CEDRE (46556SG). The funding sources did not intervene in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Handling Editor: Prof F Kandziora
References
- Acaroğlu R.E., Dede Ö., Pellisé F., et al. Adult spinal deformity: a very heterogeneous population of patients with different needs. Acta Orthop. Traumatol. Turcica. 2016;50:57–62. doi: 10.3944/AOTT.2016.14.0421. [DOI] [PubMed] [Google Scholar]
- Amabile C., Le Huec J.C., Skalli W. Invariance of head-pelvis alignment and compensatory mechanisms for asymptomatic adults older than 49 years. Eur. Spine J. 2018;27:458–466. doi: 10.1007/s00586-016-4830-8. [DOI] [PubMed] [Google Scholar]
- Bess S., Line B., Fu K.-M., et al. The health impact of symptomatic adult spinal deformity: comparison of deformity types to United States population norms and chronic diseases. Spine. 2016;41:224–233. doi: 10.1097/BRS.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland A.J., Vigdorchik J., Schwab F.J., et al. Acetabular anteversion changes due to spinal deformity correction: bridging the gap between hip and spine surgeons. J. Bone Joint Surg. Am.Pediatr. Spine. 2015;97:1913–1920. doi: 10.2106/JBJS.O.00276. [DOI] [PubMed] [Google Scholar]
- Davis R., Ounpuu S. A gait analysis data collection and reduction technique. Hum. Mov. Sci. 1991;10:575–587. [Google Scholar]
- Day L.M., DelSole E.M., Beaubrun B.M., et al. Radiological severity of hip osteoarthritis in patients with adult spinal deformity: the effect on spinopelvic and lower extremity compensatory mechanisms. Eur. Spine J. 2018;27:2294–2302. doi: 10.1007/s00586-018-5509-0. [DOI] [PubMed] [Google Scholar]
- Dubousset J. Three-dimensional analysis of the scoliotic deformity. Pediatr Spine. 1994;1994:479–496. [Google Scholar]
- Fehlings M.G., Tetreault L., Nater A., et al. The aging of the global population: the changing epidemiology of disease and spinal disorders. Neurosurgery. 2015;77:S1–S5. doi: 10.1227/NEU.0000000000000953. [DOI] [PubMed] [Google Scholar]
- Fujishiro T., Boissière L., Cawley D.T., et al. Decision-making factors in the treatment of adult spinal deformity. Eur. Spine J. 2018 doi: 10.1007/s00586-018-5572-6. [DOI] [PubMed] [Google Scholar]
- Fujishiro T., Boissière L., Cawley D.T., et al. Adult spinal deformity surgical decision-making score. Part 2: development and validation of a scoring system to guide the selection of treatment modalities for patients above 40 years with adult spinal deformity. Eur. Spine J. 2020;29:45–53. doi: 10.1007/s00586-019-06068-0. [DOI] [PubMed] [Google Scholar]
- Ghostine B., Sauret C., Assi A., et al. Influence of patient axial malpositioning on the trueness and precision of pelvic parameters obtained from 3D reconstructions based on biplanar radiographs. Eur. Radiol. 2016;27:1295–1302. doi: 10.1007/s00330-016-4452-x. [DOI] [PubMed] [Google Scholar]
- Hovorka I., Rousseau P., Bronsard N., et al. Mesure de la réserve d’extension de la hanche en relation avec le rachis. Étude comparative de deux méthodes radiologiques. Rev. Chir. Orthop. Reparatrice Appar. Mot. 2008;94:771–776. doi: 10.1016/j.rco.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Kawkabani G., Saliby R.M., Mekhael M., et al. Gait kinematic alterations in subjects with adult spinal deformity and their radiological determinants. Gait Posture. 2021;88:203–209. doi: 10.1016/j.gaitpost.2021.06.003. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Iyer S., Zebala L.P., et al. Perioperative neurologic complications in adult spinal deformity surgery. Spine. 2017;42:420–427. doi: 10.1097/BRS.0000000000001774. [DOI] [PubMed] [Google Scholar]
- Lafage V., Schwab F., Skalli W., et al. Standing balance and sagittal plane spinal deformity: analysis of spinopelvic and gravity line parameters. Spine. 2008;33:1572–1578. doi: 10.1097/BRS.0b013e31817886a2. [DOI] [PubMed] [Google Scholar]
- Lafage V., Schwab F., Patel A., et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine. 2009;34:E599–E606. doi: 10.1097/BRS.0b013e3181aad219. [DOI] [PubMed] [Google Scholar]
- Lafage V., Schwab F., Patel A., et al. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine. 2009;34:E599–E606. doi: 10.1097/BRS.0b013e3181aad219. [DOI] [PubMed] [Google Scholar]
- Lafage R., Schwab F., Challier V., et al. Defining spino-pelvic alignment thresholds. Spine. 2016;41:62–68. doi: 10.1097/BRS.0000000000001171. [DOI] [PubMed] [Google Scholar]
- Lahkar B.K., Rohan P.Y., Assi A., et al. Development and evaluation of a new methodology for Soft Tissue Artifact compensation in the lower limb. J. Biomech. 2021;122 doi: 10.1016/j.jbiomech.2021.110464. [DOI] [PubMed] [Google Scholar]
- Leardini A., Biagi F., Merlo A., et al. Multi-segment trunk kinematics during locomotion and elementary exercises. Clin. Biomech. 2011;26:562–571. doi: 10.1016/J.CLINBIOMECH.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Mekhael M., Kawkabani G., Saliby R.M., et al. Toward understanding the underlying mechanisms of pelvic tilt reserve in adult spinal deformity: the role of the 3D hip orientation. Eur. Spine J. 2021;30:2495–2503. doi: 10.1007/S00586-021-06778-4. [DOI] [PubMed] [Google Scholar]
- Obeid I., Hauger O., Aunoble S., et al. Global analysis of sagittal spinal alignment in major deformities: correlation between lack of lumbar lordosis and flexion of the knee. Eur. Spine J. 2011;20:681–685. doi: 10.1007/s00586-011-1936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid I., Hauger O., Aunoble S., et al. Global analysis of sagittal spinal alignment in major deformities: correlation between lack of lumbar lordosis and flexion of the knee. Eur. Spine J. 2011;20(Suppl. 5):681–685. doi: 10.1007/s00586-011-1936-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pataky T.C. One-dimensional statistical parametric mapping in Python. Comput. Methods Biomech. Biomed. Eng. 2012;15:295–301. doi: 10.1080/10255842.2010.527837. [DOI] [PubMed] [Google Scholar]
- Rebeyrat G., Skalli W., Rachkidi R., et al. Assessment of dynamic balance during walking in patients with adult spinal deformity. Eur. Spine J. 2022 doi: 10.1007/S00586-022-07199-7. [DOI] [PubMed] [Google Scholar]
- Reid G.D., Reid C.G., Widmer N., Munk P.L. Femoroacetabular impingement syndrome: an underrecognized cause of hip pain and premature osteoarthritis? J. Rheumatol. 2010;37:1395–1404. doi: 10.3899/jrheum.091186. [DOI] [PubMed] [Google Scholar]
- Rivière C., Hardijzer A., Lazennec J.-Y., et al. Spine-hip relations add understandings to the pathophysiology of femoro-acetabular impingement: a systematic review. Orthop. Traumatol. Surg. Res. 2017;103:549–557. doi: 10.1016/j.otsr.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Rivière C., Lazic S., Dagneaux L., et al. Spine–hip relations in patients with hip osteoarthritis. EFORT Open Rev. 2018;3:39–44. doi: 10.1302/2058-5241.3.170020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad E., Semaan K., Kawkabani G., et al. Alteration of the sitting and standing movement in adult spinal deformity. Front. Bioeng. Biotechnol. 2022;9:1. doi: 10.3389/FBIOE.2021.751193/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab F., Dubey A., Pagala M., et al. Adult scoliosis: a health assessment analysis by SF-36. Spine. 2003;28:602–606. doi: 10.1097/01.BRS.0000049924.94414.BB. [DOI] [PubMed] [Google Scholar]
- Schwab F., Dubey A., Gamez L., et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine. 2005;30:1082–1085. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- Schwab F., Ungar B., Blondel B., et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study. Spine. 2012;37:1077–1082. doi: 10.1097/BRS.0b013e31823e15e2. [DOI] [PubMed] [Google Scholar]
- Schwartz M.H., Rozumalski A. The gait deviation index: a new comprehensive index of gait pathology. Gait Posture. 2008;28:351–357. doi: 10.1016/j.gaitpost.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Semaan K., Rachkidi R., Saad E., et al. Alterations of gait kinematics depend on the deformity type in the setting of adult spinal deformity. Eur. Spine J. 2022;31:3069–3080. doi: 10.1007/s00586-022-07348-y. [DOI] [PubMed] [Google Scholar]
- Valera M., Ibáñez N., Sancho R., et al. Acetabular overcoverage in the horizontal plane: an underdiagnosed trigger of early hip arthritis. A CT scan study in young adults. Arch. Orthop. Trauma. Surg. 2018;138:73–82. doi: 10.1007/S00402-017-2811-Y. [DOI] [PubMed] [Google Scholar]