Abstract
Introduction
The aim of the present study was to develop a technical process to reproducibly generate terrible triad injuries (TTI) in fresh-frozen human cadaveric specimens, while leaving the skin intact. Such “pre-fractured” specimens, used for scientific analysis and for surgical education, might help to improve current treatment, which is complex and prone to complications.
Materials and methods
To induce the desired fractures, a custom-made fracturing unit was used to apply an axial force on the extended cadaveric elbow specimens, with the forearm pronated and under valgus load. To simulate the valgus load, a pneumatic cylinder was developed to apply valgus stress to the joint by an additional force vector from the lateral side of the joint.
Results
The success rate of TTI induction was 92.3% (12/13). Of the 12 radial head fractures, 3 (25%) were classified Mason type II and 9 (75%) Mason type III. The coronoid fractures were grouped in tip subtype 2 (5 fractures, 41.7%), anteromedial facet (AMF) subtype 2 (4 fractures, 33.3%), AMF subtype 3 (1 fracture, 8.3%) and basal subtype 1 (2 fractures, 16.7%).
Conclusions
The present study provides an instrument for successful and reproducible production of dislocation fracture patterns with their typical accompanying soft tissue lesions. The methodology might be applied on a broad basis to be able to perform biomechanical studies regarding primary stability of fixation concepts for TTI and to educate surgeons in a fairly realistic scenario with the surgical treatment of TTI.
Keywords: Fracture simulation, Terrible triad injury, Elbow, Surgical education, Fresh-frozen cadaveric specimens, Intact soft tissue
Introduction
Teaching of surgeons is primarily performed in the clinical setting. Increasing public scrutiny, regulations on working hours, high economic pressure and the steadily rising number of surgical techniques call for new, more efficient training opportunities [1]. Virtual reality simulations, synthetic bone preparations and formalin-fixed or fresh-frozen human cadaveric specimens provide opportunities for surgical training [1]. Dislocation fractures of the elbow are highly demanding to the surgeon and are frequently associated with complications such as stiffness, recurrent joint instabilities, neuropathies of the ulnar nerve, and implant-associated issues [2, 3]. Improved surgical training beyond the operating room might be of help to counteract this. Prerequisite is the availability of corresponding fracture morphologies in cadaveric specimens with an intact skin envelope. Such “pre-fractured” fresh-frozen cadaveric specimens with interindividual anatomy variability would allow a realistic training situation, as recently published by our study group [4]. The biomechanical induction of dislocation fracture patterns is the subject of this work, focusing on terrible triad injuries (TTI).
TTI were first described by Hotchkiss and denote dislocations of the elbow joint in the sense of osteoligamentous injuries [5]. They are composed of the triad of a posterior elbow joint dislocation, a radial head fracture and a fracture of the coronoid process.
An in vivo video analysis of the accident mechanism by Schreiber et al. suggests the elbow joint to commonly dislocate in extension with the forearm pronated, resulting in an axial compression and an initial valgus moment, caused by the inward rotation of the body [6]. In accordance with this, an MRI study by Rhyou et al. suggests a medial injury initiation in posterolateral dislocations of the elbow joint [7]. Fitzpatrick et al. demonstrated that the forearm rotation (pronation) is the primary determinant of the resulting dislocation fracture pattern (TTI) during low-energy axial fracturing without intact soft tissue envelope in a biomechanical study [8]. Fern et al. highlighted the biomechanical effects on joint stability of TTI. [9]
The hypothesis of the study is that it is possible to reproducibly generate TTI based on objective parameters.
Materials and methods
Specimens
For the present study, 13 fresh-frozen human cadaveric upper extremities (5 right and 8 left) were available. The specimens were obtained from body donors; written consent was available. Institutional ethics committee approval was given prior to this study (VT 20–1399). The mean age at the time of death was 80 ± 9 (66–94) years. Six of the 13 specimens (46.2%) originated from female donors.
To exclude possible confounding variables for fracture simulation, the specimens were radiographically checked for degenerative changes and implants. In addition, the range of motion was examined under fluoroscopic control, especially to diagnose possible extension and forearm rotation deficits.
CT imaging of each specimen was performed (Phillips iCT 256, Phillips, Amsterdam, Netherlands) and our Institutions Picture Archiving and Communication System (IMPAX) was available to calculate Hounsfield Units (HU) as a reference for bone mineral density in 8 specimens prior to fracturing. Based on the study of Wagner et al. measurements were performed on 3 sequential coronal computed tomography scan slices, however, at the level of the proximal radial–ulnar joint [10]. The region of interest was defined via a circle to best fit the radial head without involving the cortical surface. The mean of 3 measurements was calculated for each specimen.
Preparation
Humerus
After thawing at room temperature, the cadaveric arms were freed from their soft tissue envelope at the mid-humerus and then osteotomized by an oscillating bone saw. The guiding principle for the exact osteotomy localization was that the remaining joints of the upper extremity (shoulder and wrist) could still be used for further scientific purposes. After osteotomy of the humerus, a soft tissue dissection was performed over a length of 5 cm distal to the release point. A raspatory was used to remove remaining tissue from the humerus. Afterwards, the remaining soft tissue was protected by a skin suture.
Forearm
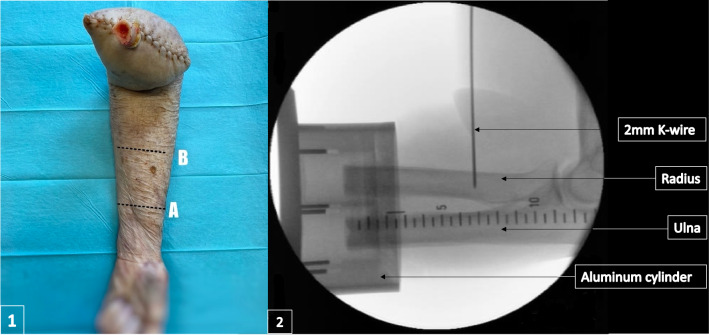
The specimens’ arm was flexed 90° at the elbow joint and the forearm was rotated into 60° of pronation by use of a standard goniometer. In this position, a 2 mm K wire was drilled parallel to the longitudinal axis of the humerus, 7 cm proximal to the planned site of forearm osteotomy (Fig. 1). This method was developed to ensure the correct rotation of the forearm (60°), even after the subsequent osteotomy: If the elbow joint was now 90° flexed and the K wire aligned parallel to the humerus, the forearm was 60° pronated. The correct position of the K wire was verified under radiographic control. Now, the forearm osteotomy was be performed. The osteotomy level was 7 cm distal to the drilling site of the K wire. The forearm was subsequently freed from its soft tissue envelope over a length of 5 cm proximal to the release point with the assistance of a raspatory. Only the interosseous membrane of the forearm was left intact to maintain its stabilizing influence. The soft tissue of the forearm was also sutured.
Fig. 1.
1, A marks the planned location of osteotomy, B marks the planned drilling location of the K wire 7 cm proximal to (A). The specimens’ arm was supinated for this photograph. However, the K wire drilling took place in 60° pronation; 2, Radiological verification of the correct K wire position in the radius
Specimen fixation
To clamp the cadaveric specimens into the fracturing unit, the humerus as well as radius and ulna were fixed into custom-made aluminum cylinders with a 4 mm-thick foundation. To embed the specimens, we used polymethyl methacrylate (PMMA). During the potting process of both, radius and ulna, into a shared aluminum cylinder, care was taken to ensure that the elbow joint was flexed 90° and the K wire remained parallel to the humerus, thus maintaining 60° of pronation. The foundation of the aluminum cylinder was aligned perpendicular to the longitudinal axes of the humerus and radius and ulna, respectively, to allow precise axial force induction.
Fracturing unit and fracture simulation
Fracturing of the cadaveric specimens was performed in a custom-made fracturing unit as used in earlier work by the study group (Fig. 2) [11–13].
Fig. 2.
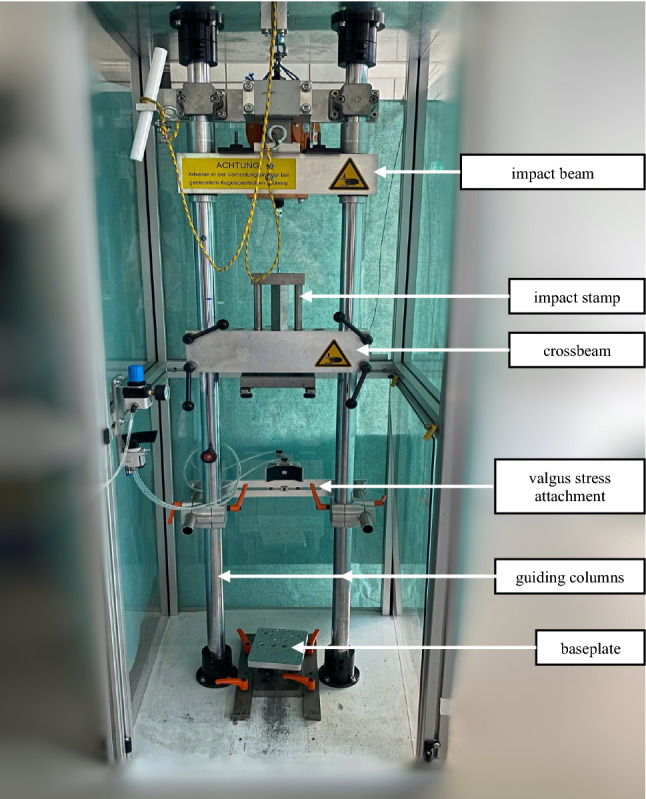
Fracture unit set-up
The unit mainly consists of two vertical guiding columns, which carry an impact beam and a crossbeam, both height adjustable. The impact beam is guided by the guiding columns in a vertical direction and can act on an impact stamp, which itself is led by the crossbeam. The impact stamp features a contact plate at its upper and lower end. The upper contact plate communicates with the impact beam, the lower contact plate can be connected to the foundation of the proximal aluminum cylinder in which the humerus was previously fixed. The impact stamp exhibits a length of more than 120 mm, by that allowing appropriate impaction. The distal aluminum cylinder comes to rest on a base plate at the bottom of the fracturing unit. As the crossbeam is height-adjustable, specimens of different lengths can be brought into the desired position.
The height from which the impact beam is dropped and the weight acting on the impact stamp can be adjusted individually. Dropping the impact beam onto the upper contact plate of the impact stamp pushes the stamp downwards, resulting in compression of the specimen.
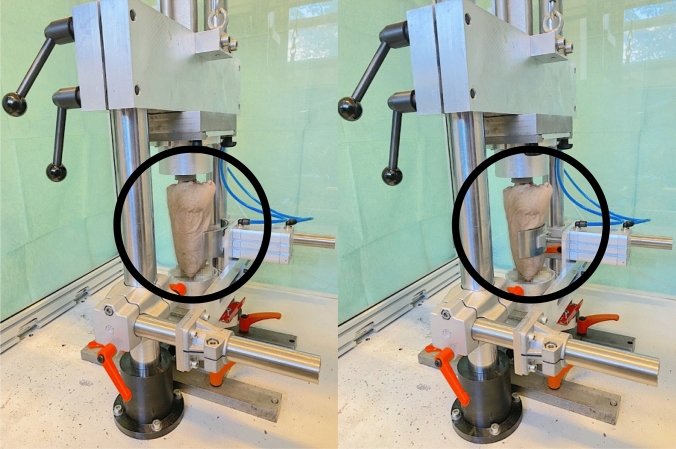
Based on empirical data of elbow fracture induction, kinetic energies of 50 J were applied for fracturing. In the event of the necessity to perform a second hit, the applied energy was increased to 75 J. The drop weight was 15 kg in all cases. To apply the desired energy, a height of 34 cm was set for the first hit and a height of 51 cm in case of a required second hit. The energy to be applied was calculated using the formula of potential energy (; E = potential energy, m = mass, g = gravitational acceleration, h = height). To simulate the postulated initial valgus moment, a valgus stress add-on device was designed to be attached to the two vertical guiding columns (Fig. 3). Thus, valgus stress can be generated in addition to axial compression. The power arm is operated pneumatically. The pneumatic cylinder allows a perpendicular force between 6 Newton (N) and 500 N, calculated by the formula: force = piston area × air pressure.
Fig. 3.
Generation of valgus stress at the specimen’s elbow joint by extending the pneumatic cylinder
In a first series of simulations, 7 specimens were fractured merely axially in extension and pronation. Subsequently, the remaining 6 specimens were additionally exposed to 50 N valgus stress via the add-on device.
Artificial damping mechanisms during fracturing were not applied and the internal friction of the pneumatic cylinder neglected in the calculation of the acting forces.
The prepared specimens were clamped into the fracturing unit in full extension and the previously set 60° of pronation, with axial application of the impulse and, in the testing group, the additional valgus load.
X-ray and CT imaging were conducted to analyze the emerging injuries. All fractures were classified independently by two trauma surgeons (TL, KW). Radial head fractures were classified according to a modified Mason classification and fractures of the coronoid process according to O´Driscoll [14, 15]. Mason type I fractures were defined as fractures with a fragment dislocation of < 2 mm or involvement of < 30% of the articular surface, type II fractures as fractures with a 2–5 mm dislocation and involvement ≥ 30% of the articular surface and type III fractures as fractures with a fragment dislocation of > 5 mm or multiple fractures of the radial head.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics Version 27 for macOS (IBM, New York, USA). Means, standard deviations, and ranges were calculated. Associations between the number of radial head fracture fragments (single fragment/multi-fragment) and HU were quantified using Spearman’s rank correlation. Pearson’s correlation was used for the association between specimens’ age at the time of death and HU. The level of significance was defined as p ≤ 0.05 (two-sided tests).
Results
The characteristics of the specimens are listed in Table 1. Multi-fragmented radial head fractures were observed in 9 out of 13 cadaveric specimens (69.2%) and single-fragmentary fractures resulted in 4 cadaveric specimens (30.8%). The measured HU average was 277 ± 42 (208–323). The HU average in the radial head was 261 for multi-fragmentary fractures and 304 for single-fragment fractures. Two specimens (no. 6 and no. 10) required a second hit. The amount of impaction of the impact stamp, the so-called braking distance, averaged 1.8 ± 1.0 (0.8–4.6) cm.
Table 1.
Individual data overview of all specimens
| Specimen | Side | Gender | Age, year | HU | Fragments | Height, cm | Weight, kg | Braking distance, cm | Energy, J | Valgus force, N | TTI | Mason | O’Driscoll |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | R | F | 75 | N/A | Multi | 34 | 15 | 2.0 | 50.0 | – | Yes | Type III | Tip subtype 2 |
| 2 | L | M | 83 | 317 | Multi | 34 | 15 | 1.2 | 50.0 | – | Yes | Type III | Tip subtype 2 |
| 3 | L | F | 86 | 278 | Single | 34 | 15 | 1.5 | 50.0 | – | Yes | Type II | Tip subtype 2 |
| 4 | L | F | 88 | 232 | Multi | 34 | 15 | 2.5 | 50.0 | – | Yes | Type III | AMF subtype 2 |
| 5 | R | F | 88 | 208 | Multi | 34 | 15 | 4.6 | 50.0 | – | Yes | Type III | Basal subtype 1 |
| 6 | L | M | 69 | N/A | Multi | 51 | 15 | 2.2 | 75.0 | – | Yes | Type III | Basal subtype 1 |
| 7 | R | F | 90 | N/A | Multi | 34 | 15 | 1.7 | 50.0 | – | Yes | Type III | AMF subtype 3 |
| 8 | R | F | 77 | N/A | Single | 34 | 15 | 1.5 | 50.0 | 50 | Yes | Type II | AMF subtype 2 |
| 9 | R | M | 69 | N/A | Multi | 34 | 15 | 1.4 | 50.0 | 50 | Yes | Type III | Tip subtype 2 |
| 10 | L | M | 66 | 294 | Multi | 51 | 15 | 1.2 | 75.0 | 50 | Yes | Type III | AMF subtype 2 |
| 11 | L | M | 83 | 323 | Single | 34 | 15 | 1.9 | 50.0 | 50 | Yes | Type II | AMF subtype 2 |
| 12 | L | M | 71 | 311 | Single | 34 | 15 | 0.8 | 50.0 | 50 | No | Type II | – |
| 13 | L | M | 94 | 252 | Multi | 34 | 15 | 0.9 | 50.0 | 50 | Yes | Type III | Tip subtype 2 |
yr. years, HU hounsfield units, Height height from which the impact beam was released, Weight weight of the impact beam, Braking distance refers to the stamp, Energy potential energy, J joule, N newton, R right, L left, F female, M male, N/A not available, TTI terrible triad, AMF anteromedial facet
The fragments (single fragment/multi-fragment) and HU of the radial head correlated negatively, however, not significant (r = − 0.507; p = 0.2; n = 8). Radial head HU and age of the cadaveric specimens showed a negative, insignificant correlation. (r = − 0.556; p = 0.152, n = 8).
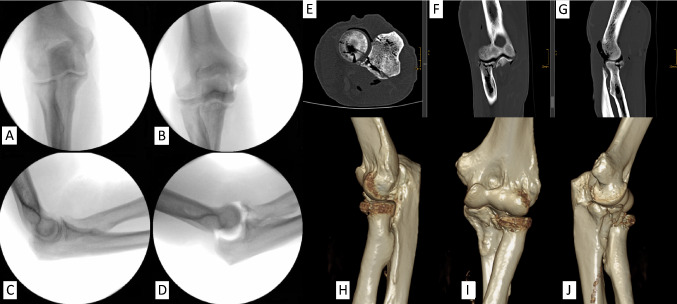
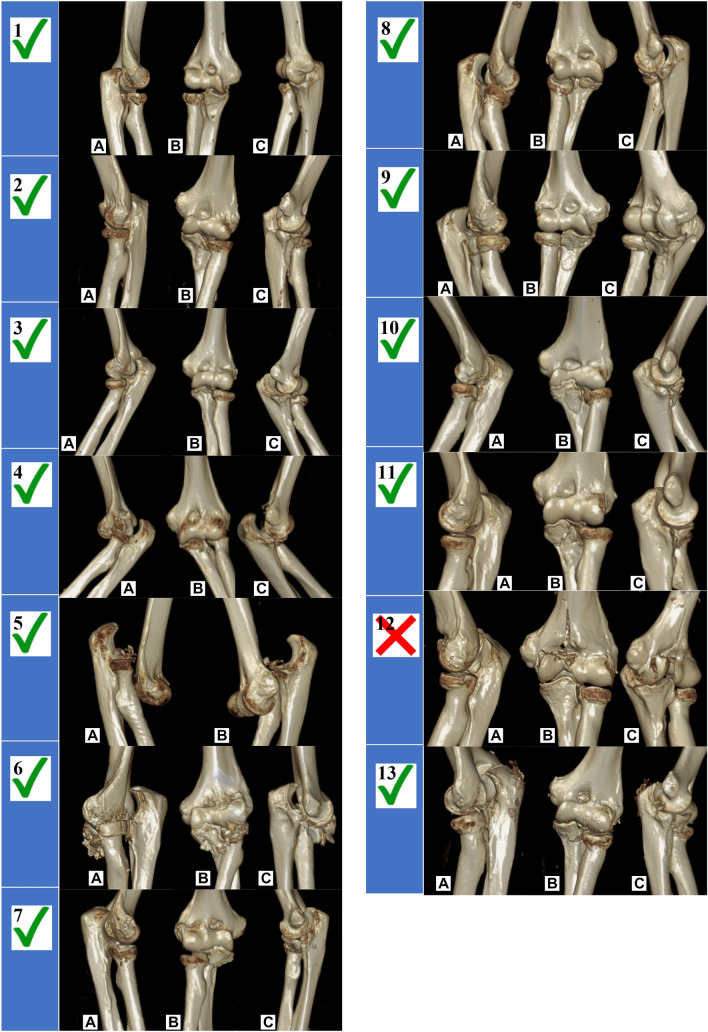
The success rate of TTI induction was 92.3% (12/13). A typical fracture is shown in Figs. 4, 3D reconstructions of all induced fractures are shown in Fig. 5. Regarding the successful simulations, 3 (25%) Mason type II and 9 (75%) Mason type III radial head fractures were induced. Concerning the coronoid, there were 5 (41.7%) tip subtype 2 fractures, 4 (33.3%) AMF subtype 2 fractures, 1 (8.3%) AMF subtype 3 fracture and 2 (16.7%) basal subtype 1 fractures. In one specimen (no. 12), only the radial head was fractured while the coronoid remained intact. Instead of the coronoid, the medial distal humerus was fractured articularly.
Fig. 4.
Typical induced fracture pattern exemplified by the diagnostics of a cadaveric specimen (no. 2). X-ray images before (A and C) and after fracturing (B and D). CT images, E: axial plane, F: coronal plane, G: sagittal plane. 3D-reconstruction, H: lateral view, I: ventral view, J: medial view, preserved integrity of the sublime tubercle and the AMF with simultaneous fracture of the coronoid’s tip. The fracture line runs in the frontal plane
Fig. 5.
Overview of 3D fracture reconstruction; Left side (1–7) without and right side (8–13) with valgus load
Discussion
In contrast to previous studies on the simulation of bony fractures without a dislocation mechanism and publications on the low-energy production of TTI with extensively dissected soft tissue envelope, the present study represents the first work on production of TTI with an intact skin envelope. The success rate of TTI production was 92.3% (12/13). The hypothesis that objective parameters would allow reproducible generation of complex dislocation fractures based on the simulation of the accident mechanism postulated by Schreiber et al. was confirmed [6].
Interestingly, the only unsuccessful attempt (no. 12) also resulted in joint dislocation with concomitant fracture of the radial head, while the coronoid remained intact. Instead of the latter, the medial distal humerus was fractured with involvement of the articular surface.
The methodology of the study provides the first guidance for simulating TTI to practice surgical operation procedures from skin incision to skin suture under realistic conditions. In particular, the preservation of skin, subcutaneous fat, muscle, and nerves makes individual treatment planning and access to the fracture comparable to the actual intraoperative situation. This is equally true for the risks to be considered in fracture care of TTI (e.g., ulnar nerve lesion due to topographical proximity in medial approaches). Thus, the described experimental set-up might be adopted to allow trainee surgeons, as well as experienced surgeons, to treat realistic dislocation fracture patterns in a low-stress environment without endangering patients. As recently evaluated by our study group, such a course format, in which pre-fractured specimens are surgically treated under the guidance of designated experts, would provide an effective surgical trauma care intervention teaching tool [4]. Moreover, the technical process described can be applied to enhance the understanding of elbow pathobiomechanics and to be able to perform biomechanical studies regarding primary stability of fixation concepts for TTI.
In the literature, Mason type II or III radial head fractures are most commonly described in the context of TTI [16]. In the present work, Mason type II fractures were induced in 3 of the 12 successful TTI (25%). Type III fractures were induced in the remaining 9 cadaveric specimens (75%). Regarding the coronoid, fractures of the tip (subtype 1 and 2) are predominantly reported in TTI clinically [16]. This biomechanical study observed 5 (41.7%) tip subtype 2 fractures, 4 (33.3%) AMF subtype 2 fractures, 1 (8.3%) AMF subtype 3 fracture, and 2 (16.7%) basal subtype 1 fractures.
It is critical to note that although TTI were induced, these typically result from posterolateral rotatory instability, whereby typically only the anterolateral facet of the coronoid is affected in terms of a tip fracture. A classification that respects the fracture morphologies in combined injuries of the radial head and the coronoid process, without simply grouping them together as TTI, is the Writington classification [17]. A common TTI would be classified as a “C” according to this classification. In contrast, a combined injury to the radial head and both facets of the coronoid would be classified as a “B + ” injury. Possibly, even under valgus loading, the test bench does not allow sufficient rotational and dislocation components to prevent distancing and thus shearing of the AMF. This is also shown by the course of the fracture line, which can be traced in Fig. 5: in a clinically typical fracture of the AMF, the fracture line is concave according to the shape of the trochlea. Instead, the fracture lines of the AMF fractures generated in this work tend to extend in a strictly frontal plane into the AMF. The pneumatic cylinder allows a valgus force of up to 500 N. In our test setup, only 50 N were used. It might be necessary to adjust the force to a higher level at this point. However, surgical fracture treatment in practice might benefit from the generated injury patterns, as the involvement of the AMF acquires biomechanical relevance and should be treated accordingly.
The energies applied might be used as a guideline in the future. The cost of the standard HU measurement based on CT scans obtained in advance must be critically evaluated. The initial measurement of HU has not yet been validated for the proximal radioulnar joint. There is currently no correlation with bone density measurements, which are classically assessed by dual x-ray absorptiometry. Wagner et al. correlated HU in the distal radioulnar joint with conventional dual x-ray absorptiometry only [10]. One likely explanation for the fact that the correlation between radial head fragments and HU did not show significance is the small number of available cadaveric specimens. Conventional fluoroscopic scans prior to fracturing were found to be sufficient to exclude possible confounding variables such as degenerative changes, implants, or range of motion deficits.
The mean age of the specimen in this study does not correspond to the incidence peak of the injury (approximately 4th decade of life) [18]. However, this fact is negligible as the specimens are intended for use in surgical training and accordingly do not require representativeness with respect to age, provided the characteristic fracture patterns are present and the individual anatomy is preserved.
Conclusion
TTI of the elbow were successfully induced by applying a high-energy impulse onto human cadaveric specimens while preserving the soft tissue envelope. The described methodology could serve as an approach to broaden knowledge regarding its pathobiomechanics, to perform biomechanical studies regarding its primary stability of fixation concepts and might allow surgeons to train in a low-stress environment beyond the operating theatre without putting patients at risk.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by FL. The first draft of the manuscript was written by FL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
All Authors have received financial research support by Medartis and IBRA. Kilian Wegmann and Lars Peter Müller are paid consultants for Medartis and Arthrex. Michael Hackl has a travelling fellowship sponsored by Wright/Tornier. He is further a speaker and course instructor for Medartis and Arthrex. Fabian Lanzerath, Lars Peter Müller and Kilian Wegmann are inventors of the subject matter of the patent application DE 10 2021 107 222 A1 with University of Cologne being applicant.
Ethical approval
All procedures performed in our study involving human cadavers were in accordance with the ethical standards of the institutional research committee (Ethical Committee of the Medical Faculty of the University of Cologne-VT 20-1399) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Atesok K, Mabrey JD, Jazrawi LM, Egol KA. Surgical simulation in orthopaedic skills training. J Am Acad Orthop Surg. 2012;20:410–422. doi: 10.5435/jaaos-20-07-410. [DOI] [PubMed] [Google Scholar]
- 2.Ring D, Jupiter JB, Zilberfarb J. Posterior dislocation of the elbow with fractures of the radial head and coronoid. J Bone Joint Surg Am. 2002;84:547–551. doi: 10.2106/00004623-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Siebenlist S, Braun KF. Ellenbogenluxationsfrakturen. Unfallchirurg. 2017;120:595–610. doi: 10.1007/s00113-017-0373-7. [DOI] [PubMed] [Google Scholar]
- 4.Wegmann K, Rausch V, Burkhart KJ, et al. Advanced surgical trauma care course - evaluation of a fracture simulation course concept with intact soft tissue. Z Orthopadie Unfallchirurgie. 2020;158:291–297. doi: 10.1055/a-0983-8322. [DOI] [PubMed] [Google Scholar]
- 5.Hotchkiss RN (1996) Fractures and dislocations of the elbow. In: Rockwood CA, Green DP, Bucholz RW, Heckman JD (eds) Rockwood and Green’s fractures in adults, 4th edn. Philadelphia, Lippincott-Raven, vol 1, pp 929–1024
- 6.Schreiber JJ, Warren RF, Hotchkiss RN, Daluiski A. An online video investigation into the mechanism of elbow dislocation. J Hand Surg. 2013;38:488–494. doi: 10.1016/j.jhsa.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Rhyou IH, Kim YS. New mechanism of the posterior elbow dislocation. Knee Surg Sports Traumatol Arthrosc. 2012;20:2535–2541. doi: 10.1007/s00167-011-1872-7. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick MJ, Diltz M, McGarry MH, Lee TQ. A new fracture model for “Terrible Triad” injuries of the elbow: influence of forearm rotation on injury patterns. J Orthop Trauma. 2012;26:591–596. doi: 10.1097/BOT.0b013e31824135af. [DOI] [PubMed] [Google Scholar]
- 9.Fern SE, Owen JR, Ordyna NJ, et al. Complex varus elbow instability: a terrible triad model. J Shoulder Elbow Surg. 2009;18:269–274. doi: 10.1016/j.jse.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Wagner SC, Dworak TC, Grimm PD, et al. Measurement of distal ulnar hounsfield units accurately predicts bone mineral density of the forearm. J Bone Joint Surg Am. 2017;99:e38. doi: 10.2106/JBJS.15.01244. [DOI] [PubMed] [Google Scholar]
- 11.Ott N, Harbrecht A, Hackl M, et al. Inducing pilon fractures in human cadaveric specimens depending on the injury mechanism: a fracture simulation. Arch Orthop Trauma Surg. 2020 doi: 10.1007/s00402-020-03538-9. [DOI] [PubMed] [Google Scholar]
- 12.Wegmann K, Harbrecht A, Hackl M, et al. Inducing life-like distal radius fractures in human cadaveric specimens: a tool for enhanced surgical training. Arch Orthop Trauma Surg. 2020;140:425–432. doi: 10.1007/s00402-019-03313-5. [DOI] [PubMed] [Google Scholar]
- 13.Wegmann K, Ott N, Hackl M, et al. Simulation of life-like distal humerus and olecranon fractures in fresh frozen human cadaveric specimens. Obere Extrem. 2020;15:137–141. doi: 10.1007/s11678-020-00573-1. [DOI] [Google Scholar]
- 14.Mason ML. Some observations on fractures of the head of the radius with a review of one hundred cases. BJS Br J Surg. 1954;42:123–132. doi: 10.1002/bjs.18004217203. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll SW, Jupiter JB, Cohen MS, et al. Difficult elbow fractures: pearls and pitfalls. Instr Course Lect. 2003;52:113–134. [PubMed] [Google Scholar]
- 16.Rodriguez-Martin J, Pretell-Mazzini J, Andres-Esteban EM, Larrainzar-Garijo R. Outcomes after terrible triads of the elbow treated with the current surgical protocols. A review Int Orthop. 2011;35:851–860. doi: 10.1007/s00264-010-1024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts AC, Singh J, Elvey M, Hamoodi Z. Current concepts in elbow fracture dislocation. Shoulder Elb. 2021;13:451–458. doi: 10.1177/1758573219884010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohn K, Ipaktchi K, Livermore M, et al. Current treatment concepts for “terrible triad” injuries of the elbow. Orthopedics. 2014;37:831–837. doi: 10.3928/01477447-20141124-06. [DOI] [PubMed] [Google Scholar]