Abstract
Introduction
Complications associated with the application of the Reamer–irrigator–Aspirator (RIA) system are described in the literature. However, to date a systematic review and meta-analysis to assess prevalence of complications associated with the use of the RIA system have not been conducted.
Materials and methods
The review is registered with PROSPERO (CRD42021269982). MEDLINE, the Web of Science Core Collection, and Embase were searched from the inception to 10 August 2021. The primary objective was to assess complications and blood loss associated with the use of the RIA system.
Results
Forty-seven studies involving 1834 procedures performed with the RIA system were finally included. A total of 105 complications were reported, with a pooled estimated overall prevalence of 1.7% with a 95% confidence interval (CI) of 0.40 to 3.60, with cortex perforation being the largest reported complication with a total of 34 incidences. A significant subgroup difference was observed (p = 0.02). In subgroup 1 (bone graft harvesting), complication prevalence was 1.4% (95% CI 0.2–3.4); in subgroup 2 (clearance intramedullary canal) it was 0.7% (95% CI 0.00–6.30) and in subgroup 3 (reaming with RIA system prior to nail fixation) 11.9% (95% CI 1.80–26.40). No statistically significant difference for tibia and femur as RIA system application site was observed (CI 0.69–4.19). In studies reporting blood loss, a mean volume of 803.29 ml, a mean drop of hemoglobin of 3.74 g/dl and a necessity of blood transfusion in 9.72% of the patients were observed.
Conclusions
The systematic review and meta-analysis demonstrate a low overall prevalence rate of complications associated with the RIA system. However, especially the risk of cortical perforation and the frequently reported relevant intraoperative blood loss are complications that should be anticipated in perioperative management and ultimately considered when using the RIA system.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00402-022-04621-z.
Keywords: Reamer–Irrigator–Aspirator, Complications, Reaming, Blood loss, Review
Introduction
The Reamer–Irrigator–Aspirator (RIA) system (Synthes, Inc., West Chester, PA), which provides continuous irrigation and suction during the reaming of long bones, was first developed to reduce the incidence of fat embolism (FE) and thermal necrosis [1, 2]. Although often times neglected in the current literature, it were Dankwardt–Lillieström and colleagues, who more than 50 years ago originated the suction irrigation reaming method and demonstrated in a preclinical rabbit model that clearance of the medullary canal of long bones prior to reaming or nail fixation might result in improved bone healing and less FE by reducing the expulsion of bone marrow into the blood vessels of the cortical bone [3–6]. Rooted in their concept and subsequent (technical) device advancements, the RIA system was attributed a potentially important role in the prevention of FE and a favorable local effect on the microstructure of the bone and, thus, on fracture healing [1, 6–9]. Therefore, clearing the medullary canal of bone marrow-rich reaming debris prior to intramedullary nailing was the primary indication for the RIA system, for which it was approved by the FDA in 2000 [10].
The osteogenic potential of reaming debris, particularly originating from the endocortex, was first described more than 30 years ago; however, its extraction from the reamer head was tedious [11, 12]. Indeed, the literature indicates no differences in union rate comparing autologous bone graft (ABG) harvested from the iliac crest (IC) as the gold standard compared to RIA graft [13, 14]. With the RIA system, clinicians soon recognized the promising capacity to harvest large quantities of endosteal ABG from the intramedullary cavity of lower leg long bones [15, 16]. Therefore, due to the ability to harvest large amounts of ABG with a filter system, the RIA system was repurposed as an ABG collector. In addition, less donor site pain, fewer infections, and a lower rate of adverse events have been observed with the use of the RIA system, and the cost of its use may be offset by the time saved in the operating room, especially when compared to harvesting posterior ICBG [13, 14]. Thus, the RIA system was approved by the FDA in 2005 for obtaining ABG associated with a large harvesting capacity [10].
However, application of the RIA system can also cause serious complications. The RIA system is considered an one-time aggressive reamer with continuous irrigation at the reamer head that can cause eccentric reaming and blood loss, causing iatrogenic fractures and cardiopulmonary disturbances, respectively [17, 18]. However, precisely these features of powerful evacuation on medullary canal content furthered the application of the RIA device as a multipurpose tool to lower the risk of dissemination into the soft tissues and systemic circulation as well as clearing the femoral/tibial canal of cement debris. Indeed, preclinical large animal studies showed that application of the RIA system was associated with a reduced amount of FE [19, 20] and lower systemic effects compared to conventional reaming [21, 22]. Based on these findings, the RIA system has been applied to reduce intravasation of intramedullary content, such as malignancies [23], infections [24], and cement removal [25]. However, systematic reporting of the associated complications is essential; yet, surprisingly, no systematic review of the complications associated with the use of the RIA system has been conducted.
The aim of this systematic review and meta-analysis was to assess the prevalence of intra- and postoperative complications in patients treated with the RIA system.
Materials and methods
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement (PRISMA 2020 checklist available in Supplementary Material 1) [26] in conjunction with the associated literature search extension PRISMA-S [27]. This review has been registered with PROSPERO: CRD42021269982. Search strategy development and documentation were improved by close collaboration with a librarian at the Queensland University of Technology.
Eligibility criteria
A framework for the identification of studies eligible for inclusion was applied as per the components of population, exposure of interest, and outcome in accordance with Moola et al. 2020 [28]. Accordingly, studies were included if they were conducted in patients (no age constraints) who were treated with the RIA system irrespective of specific clinical/surgical indications (population). The original RIA system (original RIA/RIA 1) has recently been updated, and a new version—termed RIA 2 system—is now available [29]. Therefore, all clinical studies reporting complications associated with the use of both systems were included (exposure of interest). Eligible studies provided data on any intra- or postoperative complications or sequalae during follow-up related to the RIA system application (outcome). The exclusion criteria entailed studies that did not use the RIA system or did not assess complications related to the RIA system; studies published in languages other than English, Dutch or German; case reports with less than two cases; review articles; in vitro studies and conference abstracts.
Search strategy
MEDLINE (via PubMed), Web of Science Core Collection (Clarivate Analytics), and Embase (via Elsevier) were the databases used for the computerized search strategy. The systematic search was performed on 10 August 2021 (PRISMA-S checklist available in Supplementary Material 2). The strategy was modified and adjusted for each database searched, with no restrictions on language or time period. Detailed search strategies used are available in Supplementary Material 3. Following the literature search, all identified research reports were collated and uploaded to EndNote 20.0.1 (Clarivate Analytics, PA, USA) and duplicates removed. Subsequently, titles and abstracts were screened for inclusion by two independent reviewers (ML and LPW). Full texts were obtained for the eligible studies and screened for inclusion. Reference lists of the included studies were manually screened to identify additional studies for inclusion. All study details were imported into the Joanna Briggs Institute System for the Unified Management, Assessment and Review of Information (JBI SUMARI, Adelaide, Australia), and reasons for exclusion of full-text studies were recorded.
Data collection
Relevant data was retrieved manually and included the following: study characteristics (authors, year of publication, level of evidence), study design (prospective/retrospective), RIA system procedure details (total number per study, indication), surgical approach including anatomical site of reamed bone (femur/tibia) and bone access (antegrade/retrograde). In addition, the outcome measures were documented, including intraoperative cortex perforations requiring internal fixation or not requiring internal fixation, low energy bone fracture during follow-up, RIA device failure including reamer assembly disengagement, broken tip of the RIA drive shaft or metallic debris, cardiopulmonary complications and systemic infection, nerve injuries, hematoma/hemarthrosis and wound or local infection. In case of missing data, the respective authors were contacted.
Data synthesis and analysis
In the first step, the extracted data on the study characteristics and outcome measures are presented descriptively. As per the clinical indication to apply the RIA system three subgroups were formed and complication prevalence assessed accordingly. The first subgroup included all studies that reported on the application for ABG harvesting, the second subgroup on clearance of the intramedullary canal (treatment of osseous infections or bone malignancy and removing intramedullary cement) and the third subgroup for reamed nail fixation. Further, the number of total complications per surgical site (femur/tibia) were compared. If ten or more complications per outcome measure or surgical site (tibia/femur) were present, a meta-analysis was performed using a random effects model.
Statistical analyses of the data required for the meta-analyses, as well as creation of the forest plots, were performed using R programming software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria) with RStudio version 1.3.1073 (RStudio Inc., Boston, MA). The R software packages used to conduct the meta-analyses of proportions using a random effects model were ‘metafor’ [30] and ‘meta’ [31, 32]. The forest plot showing the odds ratios (OR) of the application sites of femur and tibia was created with the R software package ‘metafor’ [30]. The preferred heterogeneity variance estimator of restricted maximum likelihood (REML) [33] was applied. To transform prevalence estimates for proportional meta-analysis, the recommended method of the Freeman–Tukey double arcsine transformation was used [34, 35]. The meta-analyses are reported grouped by a random effects model [36] and presented with 95% confidence intervals (CI) [35]. Values of p < 0.05 were considered statistically significant. Heterogeneity between studies was assessed visually by means of forest plots and quantitatively measured by the index of heterogeneity squared (I2) statistics with 95% CIs and are considered low, moderate and high where I2 values are below 25%, between 25 and 75% and above 75%, respectively [37].
Risk of bias assessment
Two authors (ML and LPW) independently assigned the level of evidence according to the recommendations of the Centre for Evidence-Based Medicine (Oxford, United Kingdom) [38] and assessed the methodologic quality and risk of bias using the JBI critical appraisal tools applicable for the different study types [28, 39, 40]. Any disagreements were resolved through discussion or, if necessary, the senior author's (DWH) judgment was decisive. The appraisal tools are listed in Supplementary Material 4. Funnel plots were used to illustrate the risk of publication bias. The funnel plot for the analyses using proportional data was created with the R package ‘berryFunctions’, and for the subgroup analysis of comparing the tibia and femur as RIA system application site the funnel plot was created with the R package ‘metafor’.
Results
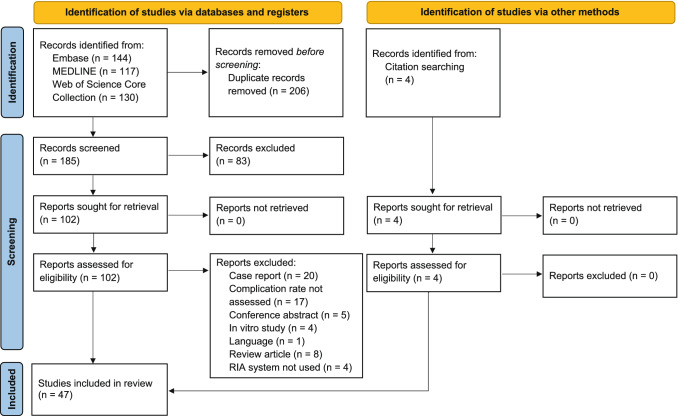
The database search identified 185 publications after duplicates were removed. A total of 83 articles did not match the inclusion criteria and were excluded after title and abstract screening. Four additional studies were identified via citation searching, resulting in 106 articles eligible for full-text assessment. Full-text assessment resulted in the exclusion of 59 studies, and reasons for exclusion of these articles are listed in Supplementary Material 5. Thus, 47 studies were included in the qualitative and quantitative synthesis. Figure 1 depicts the PRISMA 2020 flowchart with the study selection procedure.
Fig. 1.
PRISMA 2020 flowchart [26]
Study characteristics and findings
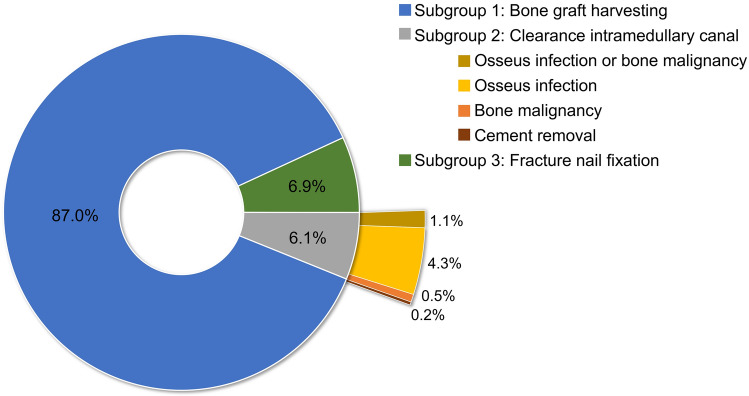
Characteristics of the included studies are shown in Table 1. The total number of included RIA system procedures was 1834, of which 1677 (91.4%) were performed on the surgical site of femur and 157 (8.6%) on the tibia. In 87.0% (1,596/1834) of the cases, the RIA device was applied for ABG harvesting (subgroup 1); 6.1% (112/1834) for clearance of the intramedullary canal, which included osseous infection, bone malignancy and cement removal (subgroup 2) and 6.9% (126/1834) for intramedullary reaming prior to nail fixation (subgroup 3) (Fig. 2). In total, 105 complications were reported in 1834 procedures performed with the RIA device. Intraoperative cortex perforations account for the most complications (34/1834), followed by cardiopulmonary complications/systemic infection (29/1834). All findings are listed in Table 2.
Table 1.
Study characteristics
| Authors/Year of publication | Study design | Level of evidence | Prospective/retrospective | Total number of RIA system procedures | RIA system indication (number of procedures, if applicable for multiple groups) | Anatomical site (if applicable number of procedures) | Surgical access (number of procedures, if applicable for multiple groups) |
|---|---|---|---|---|---|---|---|
| Barlow and Kuhn (2014) [55] | Case series | 4 | Retrospective | 3 | ABG harvesting | Femur | Retrograde |
| Belthur et al. (2008) [41] | Cohort study | 3 | Retrospective | 41 | ABG harvesting |
Femur (37) Tibia (4) |
Antegrade |
| Calori et al. (2014) [56] | Cohort study | 3 | Retrospective | 35 | ABG harvesting | Femur | Antegrade |
| Cipriano et al. (2012) [23] | Case series | 4 | Retrospective | 21 | Treatment of osseous infections or bone malignancy | Femur | Antegrade |
| Conway et al. (2014) [77] | Case series | 4 | Retrospective | 15 | ABG harvesting | Femur |
Antegrade (9) Retrograde (6) |
| Davis et al. (2015) [78] | Cohort study | 3 | Retrospective | 94 | ABG harvesting | Femur |
Antegrade (62) Retrograde (32) |
| Dawson et al. (2014) [14] | Randomized controlled trial | 1 | Prospective | 56 | ABG harvesting | Femur | Antegrade |
| Donders et al. (2016) [18] | Case series | 4 | Retrospective | 2 | ABG harvesting | Femur |
Antegrade (1) Retrograde (1) |
| Eisenstein et al. (2016) [79] | Case series | 4 | Retrospective | 6 | ABG harvesting | Femur | Retrograde |
| Grote et al. (2015) [57] | Case series | 4 | Prospective | 5 | ABG harvesting | Femur | Antegrade |
| Hall et al. (2017) [69] | Randomized controlled trial | 1 | Prospective | 11 | Fracture stabilization | Femur | Antegrade |
| Han et al. (2015) [42] | Case series | 4 | Retrospective | 57 | ABG harvesting |
Femur: (43) Tibia (14) |
Antegrade |
| Haubruck et al. (2018) [43] | Case series | 4 | Retrospective | 341 | ABG harvesting |
Femur (336) Tibia (5) |
Antegrade (Femur: 336; Tibia: 1) Retrograde (Tibia: 4) |
| Herscovici and Scaduto (2012) [80] | Case series | 4 | Retrospective | 30 | ABG harvesting | Tibia |
Antegrade (3) Retrograde (27) |
| Jakma et al. (2014) [81] | Case series | 4 | Retrospective | 32 | ABG harvesting | Femur |
Antegrade (31) Retrograde (1) |
| Kanakaris et al. (2011) [67] | Case series | 4 | Prospective | 42 | ABG harvesting (18) | Femur | Antegrade |
| Fracture stabilization (7) | |||||||
| Treatment of osseous infections (8) or bone malignancy (9) | |||||||
| Kanakaris et al. (2014) [44] | Case series | 4 | Prospective | 24 | Treatment of osseous infections |
Femur (14) Tibia (10) |
Antegrade |
| Krappinger et al. (2015) [82] | Case series | 4 | Retrospective | 4 | ABG harvesting | Femur | Antegrade |
| Kusnezov et al. (2017) [83] | Case series | 4 | Retrospective | 15 | ABG harvesting | Femur | Antegrade |
| Le Baron et al. (2019) [45] | Cohort study | 3 | Prospective | 30 | ABG harvesting |
Femur (16) Tibia (14) |
Antegrade |
| Lehman et al. (2012) [84] | Case series | 5 | Retrospective | 3 | ABG harvesting | Tibia | Retrograde |
| Lowe et al. (2010) [46] | Case series | 4 | Retrospective | 97 | ABG harvesting |
Femur (93) Tibia (4) |
Antegrade |
| Lowe et al. (2011) [25] | Case series | 5 | Retrospective | 3 | Removing intramedullary cement |
Femur (1) Tibia (2) |
Antegrade |
| Mansour and Conway (2015) [85] | Case series | 5 | Retrospective | 2 | ABG harvesting | Femur | Retrograde |
| Marchand et al. (2017) [47] | Cohort study | 3 | Retrospective | 62 | ABG harvesting |
Femur (49) Tibia (13) |
Antegrade for harvesting in tibia; Retrograde and antegrade in femur (depending on non-union location) |
| Marko et al. (2016) [58] | Case series | 5 | Retrospective | 2 | ABG harvesting | Femur | Antegrade |
| Martella et al. (2021) [48] | Cohort study | 3 | Retrospective | 65 | ABG harvesting |
Femur (60) Tibia (5) |
Antegrade |
| McCall et al. (2010) [49] | Case series | 4 | Prospective | 24 | ABG harvesting |
Femur (22) Tibia (2) |
Antegrade |
| Metsemakers et al. (2019) [86] | Case series | 4 | Retrospective | 72 | ABG harvesting | Femur | Antegrade |
| Moghaddam et al. (2017) [87] | Case series | 4 | Retrospective | 76 | ABG harvesting | Femur | Antegrade |
| Moghaddam et al. (2015) [74] | Cohort study | 2 | Prospective | 48 | ABG harvesting | Femur | Antegrade |
| Naruka et al. (2019) [88] | Case series | 4 | Retrospective | 3 | ABG harvesting | Femur | Antegrade |
| Newman et al. (2008) [89] | Case series | 4 | Retrospective | 10 | ABG harvesting | Femur | Antegrade |
| Niikura et al. (2021) [50] | Case series | 4 | Retrospective | 42 | ABG harvesting (32) |
Femur (40) Tibia (2) |
Antegrade |
| Fracture stabilization (1) | |||||||
| Treatment of osseous infections (9) | |||||||
| Nodzo et al. (2014) [90] | Cohort study | 3 | Retrospective | 29 | ABG harvesting | Femur | Antegrade |
| O’Callaghan et al. (2019) [59] | Case series | 5 | Retrospective | 2 | ABG harvesting | Femur | Antegrade |
| Onsea et al. (2021) [51] | Cohort study | 3 | Retrospective | 24 | Treatment of osseous infections |
Femur (12) Tibia (12) |
Antegrade |
| Quintero et al. (2010) [60] | Case series | 5 | Retrospective | 20 | ABG harvesting | Femur |
Antegrade (19) Retrograde (1) |
| Qvick et al. (2013) [52] | Case series | 4 | Retrospective | 204 | ABG harvesting (201) |
Femur (179) Tibia (25) |
Antegrade (Femur: 175; Tibia: 7) Retrograde (Femur: 4; Tibia: 18) |
| Treatment of osseous infections (3) | |||||||
| Rauck et al. (2016) [91] | Case series | 5 | Retrospective | 2 | ABG harvesting | Femur | Antegrade |
| Seagrave et al. (2014) [92] | Case series | 4 | Retrospective | 3 | ABG harvesting | Femur | Retrograde |
| Stafford and Norris (2010) [93] | Case series | 4 | Retrospective | 27 | ABG harvesting | Femur | Antegrade |
| Streubel et al. (2010) [70] | Case series | 4 | Retrospective | 97 | Fracture stabilization | Femur | Antegrade (30) Retrograde: (53); Greater trochanter (11) Lateral femur (6) |
| Volgas et al. (2010) [75] | Randomized controlled trial | 1 | Prospective | 10 | Fracture stabilization | Femur | Antegrade |
| Walker et al. (2019) [94] | Case series | 4 | Retrospective | 8 | ABG harvesting | Femur | Antegrade |
| Waterman et al. (2017) [53] | Case series | 4 | Prospective | 24 | ABG harvesting |
Femur (20) Tibia (4) |
Antegrade |
| Zalavras et al. (2007) [54] | Case series | 4 | Retrospective | 11 | Treatment of osseous infections |
Femur (3) Tibia (8) |
Antegrade |
Fig. 2.
Bar of pie chart showing the subgroup distribution of the RIA system indication
Table 2.
Incidence of donor site morbidity following use of the RIA system
| Authors/Year of publication | Intra-operative cortex perforations | Low energy bone fracture during follow-up | RIA device failure (reamer assembly disengagement, broken tip of the RIA drive shaft or metallic debris) |
Cardio-pulmonary complications/systemic infection | Nerve injuries | Hematoma/hemarthrosis | Wound or local infection | Total complications | |
|---|---|---|---|---|---|---|---|---|---|
| No internal fixation performed | Internal fixation was performed | ||||||||
| Barlow and Kuhn (2014) [55] | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Belthur et al. (2008) [41] | 1/41 | 1/41 | 0/41 | 2/41 | 0/41 | 0/41 | 0/41 | 0/41 | 4/41 |
| Calori et al. (2014) [56] | 0/35 | 0/35 | 0/35 | 2/35 | 0/35 | 0/35 | 0/35 | 0/35 | 2/35 |
| Cipriano et al. (2012) [23] | 0/21 | 0/21 | 0/21 | 2/21 | 0/21 | 0/21 | 0/21 | 0/21 | 2/21 |
| Conway et al. (2014) [77] | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 |
| Davis et al. (2015) [78] | 0/94 | 4/94 | 0/94 | 0/94 | 0/94 | 0/94 | 0/94 | 0/94 | 4/94 |
| Dawson et al. (2014) [14] | 0/56 | 0/56 | 1/56 | 0/56 | 0/56 | 0/56 | 0/56 | 0/56 | 1/56 |
| Donders et al. (2016) [18] | 0/2 | 0/2 | 0/2 | 0/2 | 2/2 | 0/2 | 0/2 | 0/2 | 2/2 |
| Eisenstein et al. (2016) [79] | 0/6 | 0/6 | 0/6 | 1/6 | 0/6 | 0/6 | 0/6 | 0/6 | 1/6 |
| Grote et al. (2015) [57] | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Hall et al. (2017) [69] | 0/11 | 1/11 | 0/11 | 0/11 | 1/11 | 0/11 | 0/11 | 0/11 | 2/11 |
| Han et al. (2015) [42] | 0/57 | 1/57 | 0/57 | 0/57 | 0/57 | 0/57 | 0/57 | 2/57 | 3/57 |
| Haubruck et al. (2018) [43] | 0/341 | 1/341 | 2/341 | 1/341 | 2/341 | 0/341 | 1/341 | 1/341 | 8/341 |
| Herscovici and Scaduto (2012) [80] | 1/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 1/30 |
| Jakma et al. (2014) [81] | 6/32 | 1/32 | 0/32 | 0/32 | 3/32 | 0/32 | 0/32 | 0/32 | 10/32 |
| Kanakaris et al. (2011) [67] | 0/42 | 0/42 | 0/42 | 1/42 | 0/42 | 0/42 | 2/42 | 0/42 | 3/42 |
| Kanakaris et al. (2014) [44] | 1/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 1/24 |
| Krappinger et al. (2015) [82] | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Kusnezov et al. (2017) [83] | 0/15 | 0/15 | 1/15 | 0/15 | 0/15 | 0/15 | 0/15 | 0/15 | 1/15 |
| Le Baron et al. (2019) [45] | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 0/30 | 1/30 | 0/30 | 1/30 |
| Lehman et al. (2012) [84] | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Lowe et al. (2010) [46] | 1/97 | 1/97 | 4/97 | 0/97 | 0/97 | 0/97 | 0/97 | 0/97 | 6/97 |
| Lowe et al. (2011) [25] | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Mansour and Conway (2015) [85] | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Marchand et al. (2017) [47] | 0/62 | 0/62 | 0/62 | 0/62 | 0/62 | 0/62 | 0/62 | 1/62 | 1/62 |
| Marko et al. (2016) [58] | 0/2 | 0/2 | 0/2 | 0/2 | 1/2 | 0/2 | 0/2 | 0/2 | 1/2 |
| Martella et al. (2021) [48] | 0/65 | 0/65 | 0/65 | 0/65 | 2/65 | 0/65 | 0/65 | 0/65 | 2/65 |
| McCall et al. (2010) [49] | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 |
| Metsemakers et al. (2019) [86] | 3/72 | 2/72 | 0/72 | 0/72 | 0/72 | 0/72 | 0/72 | 0/72 | 5/72 |
| Moghaddam et al. (2017) [87] | 0/76 | 0/76 | 0/76 | 0/76 | 0/76 | 0/76 | 0/76 | 0/76 | 0/76 |
| Moghaddam et al. (2015) [74] | 0/48 | 0/48 | 0/48 | 0/48 | 0/48 | 0/48 | 0/48 | 0/48 | 0/48 |
| Naruka et al. (2019) [88] | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Newman et al. (2008) [89] | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
| Niikura et al. (2021) [50] | 5/42 | 0/42 | 1/42 | 0/42 | 0/42 | 0/42 | 0/42 | 0/42 | 6/42 |
| Nodzo et al. (2014) [90] | 0/29 | 0/29 | 0/29 | 0/29 | 0/29 | 0/29 | 0/29 | 2/29 | 2/29 |
| O’Callaghan et al. (2019) [59] | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Onsea et al. (2021) [51] | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/24 |
| Quintero et al. (2010) [60] | 2/20 | 0/20 | 0/20 | 0/20 | 1/20 | 0/20 | 0/20 | 0/20 | 3/20 |
| Qvick et al. (2013) [52] | 1/204 | 1/204 | 2/204 | 0/204 | 0/204 | 0/204 | 0/204 | 0/204 | 4/204 |
| Rauck et al. (2016) [91] | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 | 0/2 |
| Seagrave et al. (2014) [92] | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 |
| Stafford and Norris (2010) [93] | 0/27 | 0/27 | 0/27 | 0/27 | 0/27 | 0/27 | 0/27 | 0/27 | 0/27 |
| Streubel et al. (2010) [70] | 0/97 | 0/97 | 0/97 | 0/97 | 16/97 | 0/97 | 0/97 | 9/97 | 25/97 |
| Volgas et al. (2010) [75] | 0/10 | 0/10 | 0/10 | 0/10 | 1/10 | 0/10 | 0/10 | 0/10 | 1/10 |
| Walker et al. (2019) [94] | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 |
| Waterman et al. (2017) [53] | 0/24 | 0/24 | 2/24 | 1/24 | 0/24 | 0/24 | 0/24 | 0/24 | 3/24 |
| Zalavras et al. (2007) [54] | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 | 0/11 |
| Total (no/total no) | 21/1834 | 13/1834 | 13/1834 | 10/1834 | 29/1834 | 0/1834 | 4/1834 | 15/1834 | 105/1834 |
| 34/1834 | |||||||||
Quality assessment
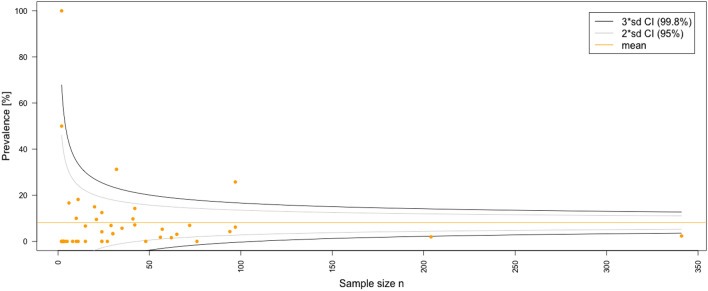
The results of the critical appraisal of the methodologic quality of all included articles can be found in Table 3. Using JBI’s critical appraisal tools, the average score for the case series (n = 35) was 4.4/10 (44%), for the cohort studies (n = 9) 7.2/11 (66%) and for the randomized controlled trials (RCT) (n = 3) 9.7/13 (75%). The quality assessment using JBI critical appraisal tools revealed that, the overall 62% of the criteria were met. The funnel plot for the overall prevalence of complications detected good symmetrical distribution of the referral points. The majority of the values are close to the no-effect line and very few are outside the CI range of 95%. Overall, poor data dispersion was apparent, indicating a rather low risk of publication bias (Fig. 3). Similarly, no asymmetry in the funnel plot of intervention effect estimates from individual studies versus a measure of individual study size was observed for the comparison of complications in the tibia and femur and, therefore, potential publication bias of the studies can be considered low (Fig. 4).
Table 3.
| Case series | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors/Year of publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Total yes (%) | ||
| Conway et al. (2014) [77] | Y | Y | U | U | N | Y | N | Y | N | N/A | 4/10 (40) | ||
| Eisenstein et al. (2016) [79] | Y | Y | N | Y | Y | N | N | N | N | N/A | 4/10 (40) | ||
| Stafford and Norris (2010) [93] | Y | Y | U | Y | Y | Y | N | Y | N/A | N | 6/10 (60) | ||
| Waterman et al. (2017) [53] | Y | N | U | Y | Y | Y | N | N | U | N | 4/10 (40) | ||
| Walker et al. (2019) [94] | Y | Y | U | Y | Y | Y | Y | Y | N | N/A | 7/10 (70) | ||
| Streubel et al. (2010) [70] | Y | Y | Y | Y | Y | Y | N | Y | N | Y | 7/10 (70) | ||
| Seagrave et al. (2014) [92] | N | N | N | N | N | Y | N | Y | N | N/A | 2/10 (20) | ||
| Donders et al. (2016) [18] | N | N | N | N | N/A | Y | N | Y | N | N/A | 2/10 (20) | ||
| Han et al. (2015) [42] | Y | U | U | Y | Y | N | Y | Y | N | N | 5/10 (50) | ||
| Herscovici and Scaduto (2012) [80] | N | U | U | N | N | Y | N | U | N | N | 1/10 (10) | ||
| Jakma et al. (2014) [81] | Y | Y | U | Y | Y | Y | N | Y | U | N | 6/10 (60) | ||
| Kanakaris et al. (2011) [67] | Y | Y | Y | Y | Y | Y | N | N | N | N/A | 6/10 (60) | ||
| Kanakaris et al. (2014) [44] | Y | Y | Y | Y | Y | Y | N | Y | N | N/A | 7/10 (70) | ||
| Qvick et al. (2013) [52] | Y | N | N | Y | Y | Y | N | Y | U | N/A | 5/10 (50) | ||
| O’Callaghan et al. (2019) [59] | N | U | Y | U | N | Y | Y | Y | N | N/A | 4/10 (40) | ||
| Niikura et al. (2021) [50] | Y | Y | U | Y | Y | Y | N | N | U | N | 5/10 (50) | ||
| Newman et al. (2008) [89] | Y | N | N | N | N | Y | N | Y | U | N/A | 3/10 (30) | ||
| Naruka et al. (2019) [88] | N | Y | Y | U | N/A | Y | Y | Y | U | N/A | 5/10 (50) | ||
| Moghaddam et al. (2017) [87] | Y | N | Y | Y | Y | Y | Y | Y | U | Y | 8/10 (80) | ||
| Metsemakers et al. (2019) [86] | Y | N | U | Y | N | Y | N | Y | N | Y | 5/10 (50) | ||
| McCall et al. (2010) [49] | N | N | Y | Y | U | N | N | Y | N | N | 3/10 (30) | ||
| Lowe et al. (2011) [25] | N/A | Y | Y | U | U | Y | N | N | N | N/A | 3/10 (30) | ||
| Lehman et al. (2012) [84] | N/A | Y | N | U | U | Y | N | Y | N/A | N/A | 3/10 (30) | ||
| Kusnezov et al. (2017) [83] | Y | U | U | Y | Y | Y | N | Y | U | N | 5/10 (50) | ||
| Krappinger et al. (2015) [82] | N | Y | Y | U | U | N | N | Y | U | N/A | 3/10 (30) | ||
| Grote et al. (2015) [57] | Y | N | Y | Y | U | N | N | N | N | N | 3/10 (30) | ||
| Cipriano et al. (2012) [23] | Y | Y | Y | Y | U | N | N | Y | U | N | 5/10 (50) | ||
| Barlow and Kuhn (2014) [55] | N | Y | Y | U | U | N | N | Y | N | N | 3/10 (30) | ||
| Lowe et al. (2010) [46] | N | Y | Y | U | U | Y | N | Y | N | N/A | 4/10 (40) | ||
| Mansour and Conway (2015) [85] | N | Y | Y | U | U | Y | N | Y | U | N/A | 4/10 (40) | ||
| Quintero et al. (2010) [60] | N | Y | N | U | U | Y | N | Y | N | N | 3/10 (30) | ||
| Rauck et al. (2016) [91] | N | Y | N | U | U | Y | N | Y | N | N/A | 3/10 (30) | ||
| Haubruck et al. (2018) [43] | Y | Y | Y | Y | Y | Y | N | Y | N | Y | 8/10 (80) | ||
| Zalavras et al. (2007) [54] | Y | Y | N | Y | N | Y | N | Y | U | N | 5/10 (50) | ||
| Marko et al. (2016) [58] | N | Y | N | U | U | Y | Y | Y | N | N/A | 4/10 (40) | ||
| Subtotal (%) | 4.4/10 (44) | ||||||||||||
| Cohort studies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors/Year of publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Total yes (%) | |
| Belthur et al. (2008) [41] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | 9/11 (82) | |
| Calori et al. (2014) [56] | Y | U | Y | N | N | Y | Y | Y | Y | N/A | N | 6/11 (55) | |
| Davis et al. (2015) [78] | Y | Y | Y | Y | Y | Y | Y | Y | U | N/A | Y | 9/11 (82) | |
| Onsea et al. (2021) [51] | N | Y | Y | U | U | N/A | Y | Y | U | N/A | Y | 5/11 (45) | |
| Nodzo et al. (2014) [90] | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10/11 (91) | |
| Moghaddam et al. (2015) [74] | Y | N | Y | N | U | Y | Y | Y | Y | N | Y | 7/11 (64) | |
| Le Baron et al. (2019) [45] | N | Y | Y | Y | N | Y | N | U | N | N | Y | 5/11 (45) | |
| Marchand et al. (2017) [47] | U | Y | Y | N | N/A | Y | Y | Y | Y | N/A | Y | 7/11 (64) | |
| Martella et al. (2021) [48] | Y | Y | Y | N | N/A | U | Y | Y | Y | N/A | Y | 7/11 (64) | |
| Subtotal (%) | 7.2/11 (66) | ||||||||||||
| Randomized controlled trials | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors/Year of publication | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Total yes (%) | ||||||||||
| Dawson et al. (2014) [14] | U | U | Y | N | Y | N | Y | N | Y | Y | Y | Y | Y | 8/13 (62) | ||||||||||
| Volgas et al. (2010) [75] | Y | Y | Y | U | N | Y | Y | Y | Y | Y | Y | Y | Y | 11/13 (85) | ||||||||||
| Hall et al. (2017) [69] | Y | Y | Y | N | N | Y | Y | U | Y | Y | Y | Y | Y | 10/13 (77) | ||||||||||
| Subtotal (%) | 9.7/13 (75) | |||||||||||||||||||||||
| Total | 62% | |||||||||||||||||||||||
Y yes, N no, N/A not applicable, U unknown
Fig. 3.
Funnel plot for the overall prevalence of complications
Fig. 4.
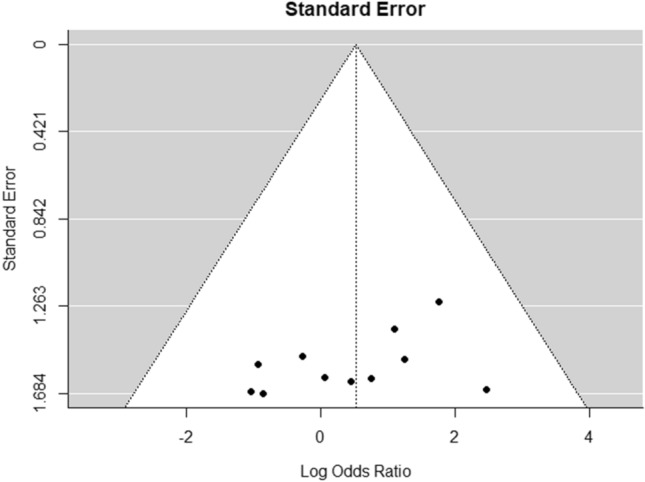
Funnel plot for complications reported in both the tibia and the femur
Pooled estimates complication rate
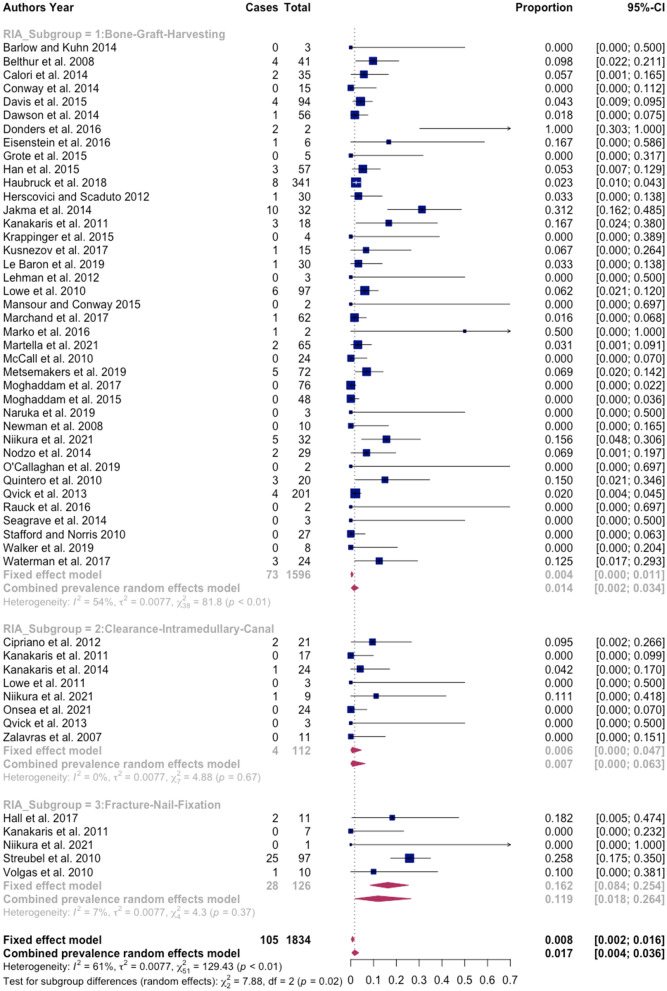
Studies included in the proportional meta-analysis for the total complication prevalence were of moderate heterogeneity, as determined by the I2 statistics (61%). Unexplained heterogeneity within subgroups was moderate in the first subgroup (I2 = 54%) and low in the second (I2 = 0%) and third (I2 = 7%) subgroups. Therefore, the validity of the effect estimates for subgroup 1 is uncertain, as individual trial results are inconsistent. However, overall, sufficient evenly distributed trials for the subgroup analysis to produce meaningful results were observed. Applying meta-analyses of (weighted) average proportion using a random effects model, the pooled estimate of the overall rate of complications associated with RIA system application was 1.7% (95% CI 0.40–3.60). Using the random effects model for subgroup 1, the pooled estimate of the total complication rate was 1.4% (95% CI 0.20–3.40). Among those procedures performed in subgroup 2, the pooled estimate of the rate of total complications was 0.7% (95% CI 0.00–6.30). The pooled estimate of the overall complication rate of RIA system procedures in subgroup 3 was 11.9% (95% CI 1.80–26.40). The test for subgroup differences suggests that there is a statistically significant subgroup effect (p = 0.02). Comparing the three subgroups, a higher combined prevalence rate of complications in subgroup 3 (11.9%) compared with subgroup 1 (1.4%) and subgroup 2 (0.7%) was observed. Very low complication rates of the individual outcome measures impaired reliability of the subgroup analyses using the random effects model. A forest plot for the total number of complications was constructed to visualize the estimates with their CIs (Fig. 5). Additional forest plots for the individual outcome measures can be found in Supplementary Material 6.
Fig. 5.
Forest plot for total complication prevalence
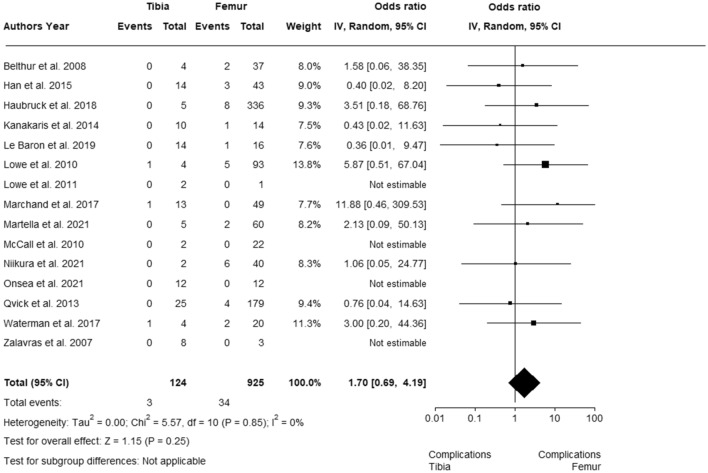
In 15 studies [25, 41–54], the results were described for applications in which the RIA system was used in either femur or tibia in individual patients. The studies included in the meta-analysis comparing the estimated complication rate when the RIA system was applied to the tibia compared with the femur had low heterogeneity, as determined by the I2 statistic (0%). Using the random effects model, the total OR of 1.7 (95% CI 0.69–4.19) was nonsignificant in favor of the femur associated with more overall complications (Fig. 6).
Fig. 6.
Forest plot for odds ratio comparing tibia and femur using the RIA system
Blood loss
Thirteen studies [18, 42, 43, 45, 47, 48, 50, 55–60] reported blood loss in a total number of 336 performed procedures with the RIA system. Of these 13 studies, five studies [47, 48, 50, 56, 58] reported the volume of blood lost, which resulted in a mean of 803.29 ml per case. Furthermore, five out of the 13 studies reported a drop in hemoglobin, resulting in a mean of 3.74 g/dl. In total, 60 out of 583 patients (9.72%) included in the studies [18, 42, 43, 45, 47, 48, 57, 59, 60] required blood transfusion. The findings on blood loss are summarized in Table 4.
Table 4.
Findings on blood loss associated with using the RIA system
| Authors/Year of publication | Total number of procedures assessed | Blood loss | Blood transfusion (no/total no) | |
|---|---|---|---|---|
| Volume (ml) | Drop in hemoglobin (g/dl) | |||
| Barlow and Kuhn (2014) [55] | 3 | ‘No excessive blood loss’ | ||
| Calori et al. (2014) [56] | 35 | 572.85 | 1.66 | – |
| Donders et al. (2016) [18] | 2 | – | 4.40 | 2/2 |
| Grote et al. (2015) [57] | 5 | – | 3.30 | 0/5 |
| Han et al. (2015) [42] | 57 | – | 3.15 | 7/57 |
| Haubruck et al. (2018) [43] | 11 | – | 6.20 | 11/341 |
| Le Baron et al. (2019) [45] | 30 | – | – | 3/30 |
| Marchand et al. (2017) [47] | 62 | 647.00 | – | 27/62 |
| Marko et al. (2016) [58] | 2 | 1250.00 | – | – |
| Martella et al. (2021) [48] | 65 | 388.00 | – | 9/64 |
| Niikura et al. (2021) [50] | 42 | 1158.60 | – | – |
| O’Callaghan et al. (2019) [59] | 2 | – | – | 0/2 |
| Quintero et al. (2010) [60] | 20 | – | – | 1/20 |
| Total | 336 | 803.29 | 3.74 | 60/583 (9.72%) |
Discussion
To date, neither the complications of the RIA system in ABG harvesting nor in the alternative applications of clearance of the intramedullary canal and nail fixation of long bone fractures have been systematically investigated. Yet, it is imperative to have knowledge of the complication prevalence associated with the RIA system in order to achieve optimal informed patient consent and to optimize perioperative management and intraoperative use of the device.
The meta-analysis with weighted average proportions for RIA system application revealed an overall low prevalence of complications (1.7%), which is in line with a recent narrative review [61]. A considerable aspect of the RIA system is the possibility of harvesting large quantities of bone graft from the femur, whereby especially an extra-articular approach via the greater trochanter is possible, while the semi-elastic plastic nitinol unit is aligned with the mid-axis of the femur [52]. However, particularly in the tibia, application of the original RIA 1 device (smallest available reamer size of 12 mm diameter) can be technically challenging due to an eccentric starting point with a relative rigid tip and semi-flexible shaft-tubing system. Although for the femur cadaveric studies have shown no significant interference with the biomechanical properties of the harvested femur [62, 63], no such in vitro biomechanical data are available for the tibia as harvesting site. Therefore, the observed tendency of higher complication rates in the femur described in the present meta-analysis (OR 1.7; 95% CI 0.69–4.19) appears, at first glance, to be counterintuitive. However, this result could be influenced by the design of primary research studies, such as the largest study [52], in which the RIA system was applied to the same side of the lower extremity for tibial index surgeries and to the contralateral side in femoral index surgeries. Therefore, the increased complication rate observed when using the RIA system in the femur could be associated with the postoperative weight bearing of the harvested femur, whereas in the harvested tibia, the weight bearing of the extremity was restricted.
Noteworthy, the RIA 2 system, launched in 2020, includes exchangeable reamer heads with the smallest diameter of 10 mm. Early data for RIA 2 system applied in cadaveric femur and tibia, however, indicates that it should be used with caution in the tibia because of increased perforation risk [64]. Clinical data of RIA 2 system applications have not yet been published. Nonetheless, taken together, technical or surgical error such as eccentric reaming and cortical thinning are of importance as they seem to be one of the major reasons for intra- and postoperative complications associated with using the RIA system [52]. Therefore, while intraoperative diligence and fluoroscopic monitoring are paramount to decrease the risk of technical errors and complications, it is important to note that surgeons who frequently use the RIA system describe a steep learning curve in the use of the system [43, 65]. To shorten the learning curve and reduce complications, special training at a ‘center of excellence’ is recommended [43].
In cases of osteomyelitis or peri-implant infection in the long bones of the lower extremity, the entire length of the medullary canal must be debrided, and the RIA system can be used instead of conventional reamers to take advantage of its additional irrigation and aspiration function [66]. Aggressive (over)reaming is not required compared to those cases in which harvesting large bone graft volumes is the goal. The treatment of osteomyelitis or peri-implant infection are scheduled as elective procedures. Thus, very low prevalence of complications of 0.7% (95% CI 0.0–6.3) is expected and makes the application of the RIA system for this indication appealing. The advantage of minimizing intramedullary pressure and potential intravasation of, for example, tumor cells, as well as obtaining multiple samples for further histological analysis, argue for the use in bone malignancy cases [23, 67]. Further, next to debulking of intramedullary neoplasm, in a pilot study with three cases illustrated by Lowe et al. (2011) [25], by profiting from the sharp, front-end cutting reamer heads, the RIA system showed the capacity to remove debonded cement fragments when traditional removal methods such as intramedullary hooks, reverse curettes, flexible osteotomes or stacked guide rods [68] failed.
For intramedullary reaming during treatment of femoral shaft fractures no clinically relevant protective effects of the RIA system are reported in nonpolytrauma patients [69], while there might be a potential advantage in polytrauma patients [67]. In line with the results of the present meta-analysis of increased complication rate for the subgroup of RIA system reaming before nail fixation (complication prevalence of 11.9%; 95% CI 1.8–26.4), its application for this purpose should be chosen with caution. However, we note that a potential confounding factor causing differences in treatment effect between the subgroups might have been that this group consists of acute fracture treatments. In particular, one large study (n = 97 cases) in subgroup 3 reported cardiopulmonary complications/systemic infections or wound infections/local infections in 25 cases [70], all of which may have been related to the index injury rather than the use of the RIA system. Thus, in particular, fracture treatment itself is a study characteristic that may be confounded with the covariate of interest; namely, RIA system-associated complications.
Clinically significant blood loss during RIA system application, which is associated with continuous irrigation due to the abundant intramedullary blood supply, was first reported in smaller case series [18, 58] and more recently confirmed in multiple larger studies [43, 47, 50, 71]. In the present systematic literature review, a mean blood loss of 803.29 ml, a drop in hemoglobin of 3.74 mg/dl and required blood transfusion in 9.72% of patients were observed. It should be noted that blood loss, particularly in surgically complex patients, is multifactorial and might be affected by perioperative and postoperative fluid administration regimes; the complexity of the nonunion repair, including the extent of recipient site preparation; and the amount of ABG required [71]. Nonetheless, these findings are paramount for every surgeon, as surgical planning includes interdisciplinary communication and preparation for potential intraoperative blood loss. Patients at a high risk for anemia-associated comorbidities [72] may benefit from intraoperative auto-transfusion of the blood lost due to the RIA system. However, cell saver filter cannot handle the large volume of fluid [58] that is produced when using the RIA system. Therefore, transfusion risk should be discussed with patients, and the choice of the RIA system should be carefully considered in patients with anemia or bleeding risk [71]. Furthermore, reduced blood loss with repeated use of the device by the same surgical team is described [50], potentially by avoiding prolonged suction and aspiration using the RIA system in later stages of the learning curve. This is emphasized by the recently reported results of a pilot study of 24 patients with femoral bone graft harvesting and a mean drop of postoperative hemoglobin as high as 4.1 g/dl as well as the necessity for transfusion of packed red blood cells in 33% of cases [73].
Limitations
We note several limitations to this study. The retrospective design of most of the included studies is an important limitation. Only ten studies had a prospective study design [14, 44, 45, 49, 53, 57, 67, 69, 74, 75] of which only three were RCTs [14, 69, 75], which represents an important source of selection bias. Furthermore, trials per subgroup for total complications were not evenly distributed. However, valid interpretation of the findings was conducted following the guidelines for improving the interpretation of subgroup analyses in reviews [76]. A complication with more than ten events out of the total 1834 procedures was considered clinically relevant by the authors and, therefore, an additional meta-analysis was performed only in these cases (Supplementary Material 6).
Conclusions
As demonstrated in this systematic review and meta-analysis, the overall prevalence of complications with the RIA system is low. Yet, in particular, the risk of cortex perforations and commonly reported relevant intraoperative blood loss are complications to be anticipated in the perioperative management and ultimately during the use of the RIA system.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge the Queensland University of Technology liaison librarian, Dr. Brendan Sinnamon, for assistance with the literature search and refinement of the search protocol.
Authors’ contributions
ML, PK, and DWH conceived the idea for this systematic review. ML is the guarantor of this systematic review and wrote the first manuscript draft. ML, LPW, FMB, FH, PK, and DWH developed the methodology for the systematic review. The manuscript was drafted by ML, LPW, and FMB and revised by FH, PK, and DWH. ML developed the search strategy and performed the statistical analysis. ML and LPW screened the studies and assessed the risk of bias. ML conducted the data extraction and data synthesis. DWH acted as a third reviewer and arbitrator, if necessary. All authors read, provided feedback on and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors gratefully acknowledge the support of the Alexander von Humboldt Foundation and the Queensland University of Technology, jointly funding a Feodor Lynen Research Fellowship of the Alexander von Humboldt Foundation awarded to Markus Laubach. In addition, financial support for this project was provided by the Australian Research Council via the ARC Training Centre for Multiscale 3D Imaging, Modelling and Manufacturing (M3D Innovation, project IC 180100008).
Declarations
Conflict of interest
All authors have no conflict of interests to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Markus Laubach, Email: m.laubach@qut.edu.au.
Lucas P. Weimer, Email: l.weimer@student.maastrichtuniversity.nl
Felix M. Bläsius, Email: fblaesius@ukaachen.de
Frank Hildebrand, Email: fhildebrand@ukaachen.de.
Philipp Kobbe, Email: pkobbe@ukaachen.de.
Dietmar W. Hutmacher, Email: dietmar.hutmacher@qut.edu.au
References
- 1.Green J. History and development of suction-irrigation-reaming. Injury. 2010;41:S24–S31. doi: 10.1016/S0020-1383(10)70005-X. [DOI] [PubMed] [Google Scholar]
- 2.Cox G, Jones E, McGonagle D, Giannoudis PV. Reamer-irrigator-aspirator indications and clinical results: a systematic review. Int Orthop. 2011;35:951–956. doi: 10.1007/s00264-010-1189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danckwardt-Lillieström G, Grevsten S, Johansson H, Olerud S. Periosteal bone formation on medullary evacuation. A bone formation model. Ups J Med Sci. 1972;77:57–61. doi: 10.1517/03009734000000010. [DOI] [PubMed] [Google Scholar]
- 4.Danckwardt-Lillieström G, Lorenzi L, Olerud S. Intracortical circulation after intramedullary reaming with reduction of pressure in the medullary cavity: a microangiographic study on the rabbit tibia. JBJS. 1970;52:1390–1394. doi: 10.2106/00004623-197052070-00013. [DOI] [PubMed] [Google Scholar]
- 5.Danckwardt-Lillieström G, Lorenzi GL, Olerud S. Intramedullary nailing after reaming: an investigation on the healing process in osteotomized rabbit tibias. Acta Orthop Scand. 1970;41:1–78. doi: 10.3109/ort.1970.41.suppl-134.01. [DOI] [PubMed] [Google Scholar]
- 6.Danckwardt-Lillieström G. Reaming of the medullary cavity and its effect on diaphyseal bone: a fluorochromic, microangiographic and histologic study on the rabbit tibia and dog femur. Acta Orthop Scand. 1969;40:1–165. doi: 10.3109/ort.1969.40.suppl-128.01. [DOI] [PubMed] [Google Scholar]
- 7.Lempert M, Halvachizadeh S, Ellanti P, Pfeifer R, Hax J, Jensen KO, Pape H-C. Incidence of fat embolism syndrome in femur fractures and its associated risk factors over time—a systematic review. J Clin Med. 2021;10:2733. doi: 10.3390/jcm10122733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stürmer K, Tammen E. Verminderung der corticalen Gefässschädigung durch kontinuierliches Spülen und Absaugen während des Aufbohrens der Markhöhle. Hefte Unfallheilkd. 1986;181:236–240. [Google Scholar]
- 9.Stürmer KM. Measurement of intramedullary pressure in an animal experiment and propositions to reduce the pressure increase. Injury. 1993;24:S7–S21. doi: 10.1016/0020-1383(93)90002-N. [DOI] [PubMed] [Google Scholar]
- 10.Hartsock LA, Barfield WR, Kokko KP, Liles LL, Wind T, Green J, Giannoudis PV. Randomized prospective clinical trial comparing reamer irrigator aspirator (RIA) to standard reaming (SR) in both minimally injured and multiply injured patients with closed femoral shaft fractures treated with reamed intramedullary nailing (IMN) Injury. 2010;41:S94–S98. doi: 10.1016/S0020-1383(10)70018-8. [DOI] [PubMed] [Google Scholar]
- 11.Wenisch S, Trinkaus K, Hild A, Hose D, Herde K, Heiss C, Kilian O, Alt V, Schnettler R. Human reaming debris: a source of multipotent stem cells. Bone. 2005;36:74–83. doi: 10.1016/j.bone.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EE, Marder RA. Open intramedullary nailing and bone-grafting for non-union of tibial diaphyseal fracture. J Bone Jt Surg Am. 1987;69:375–380. doi: 10.2106/00004623-198769030-00008. [DOI] [PubMed] [Google Scholar]
- 13.Oliva F, Migliorini F, Cuozzo F, Torsiello E, Hildebrand F, Maffulli N. Outcomes and complications of the reamer irrigator aspirator versus traditional iliac crest bone graft harvesting: a systematic review and meta-analysis. J Orthop Traumatol. 2021;22:50. doi: 10.1186/s10195-021-00612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawson J, Kiner D, Gardner W, 2nd, Swafford R, Nowotarski PJ. The reamer-irrigator-aspirator as a device for harvesting bone graft compared with iliac crest bone graft: union rates and complications. J Orthop Trauma. 2014;28:584–590. doi: 10.1097/bot.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 15.Kobbe P, Tarkin IS, Pape HC. Use of the 'reamer irrigator aspirator' system for non-infected tibial non-union after failed iliac crest grafting. Injury. 2008;39:796–800. doi: 10.1016/j.injury.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma. 2010;24:S36–S40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 17.Dehghan N, Schemitsch EH. Extended applications of the reamer-irrigator-aspirator (RIA) system. Injury. 2017;48:S47–S51. doi: 10.1016/j.injury.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Donders JC, Baumann HM, Stevens MF, Kloen P. Hemorrhagic-induced cardiovascular complications during reamer-irrigator-aspirator-assisted femoral nonunion treatment. J Orthop Trauma. 2016;30:e294–298. doi: 10.1097/bot.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 19.Husebye EE, Lyberg T, Opdahl H, Laurvik H, Roise O. Cardiopulmonary response to reamed intramedullary nailing of the femur comparing traditional reaming with a one-step reamer-irrigator-aspirator reaming system: an experimental study in pigs. J Trauma. 2010;69:E6–14. doi: 10.1097/TA.0b013e3181d27928. [DOI] [PubMed] [Google Scholar]
- 20.Schult M, Kuchle R, Hofmann A, Schmidt-Brakling T, Ortmann C, Wassermann E, Schmidhammer R, Redl H, Joist A. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24:1186–1192. doi: 10.1002/jor.20106. [DOI] [PubMed] [Google Scholar]
- 21.Pape HC, Zelle BA, Hildebrand F, Giannoudis PV, Krettek C, van Griensven M. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Jt Surg Am. 2005;87:2515–2522. doi: 10.2106/jbjs.D.02024. [DOI] [PubMed] [Google Scholar]
- 22.Halvachizadeh S, Teuben M, Lempert M, Kalbas Y, Cesarovic N, Lipiski M, Benninger E, Cinelli P, Pfeifer R, Pape H-C. Protective effects of new femoral reaming techniques (Reamer irrigator aspirator, RIA I and II) on pulmonary function and posttraumatic contusion (CT morphology)—results from a standardized large animal model. Injury. 2020 doi: 10.1016/j.injury.2020.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Cipriano CAMD, Arvanitis LDMD, Virkus WWMD. Use of the reamer-irrigator-aspirator may reduce tumor dissemination during intramedullary fixation of malignancies. Orthopedics (Online) 2012;35:e48–e52. doi: 10.3928/01477447-20111122-09. [DOI] [PubMed] [Google Scholar]
- 24.Tosounidis TH, Calori GM, Giannoudis PV. The use of Reamer-irrigator-aspirator in the management of long bone osteomyelitis: an update. Eur J Trauma Emerg Surg. 2016;42:417–423. doi: 10.1007/s00068-016-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowe JA, Vosburg C, Murtha YM, Della Rocca GJ, Crist BD. A new technique for removing intramedullary cement. J Orthop Trauma. 2011;25:762–766. doi: 10.1097/BOT.0b013e318210f218. [DOI] [PubMed] [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB, Blunt H, Brigham T, Chang S, Clark J, Conway A, Couban R, de Kock S, Farrah K, Fehrmann P, Foster M, Fowler SA, Glanville J, Harris E, Hoffecker L, Isojarvi J, Kaunelis D, Ket H, Levay P, Lyon J, McGowan J, Murad MH, Nicholson J, Pannabecker V, Paynter R, Pinotti R, Ross-White A, Sampson M, Shields T, Stevens A, Sutton A, Weinfurter E, Wright K, Young S, Group P-S PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:39. doi: 10.1186/s13643-020-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, Currie M, Lisy K, Qureshi R, Mattis P, Mu P-F. Chapter 7: systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. [Google Scholar]
- 29.Pape H-C. RIA 2 system: next generation reamer-irrigator-aspirator. Davos: AO Technical Commission, AO Foundation; 2019. [Google Scholar]
- 30.Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 31.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R. Springer; 2015. [Google Scholar]
- 32.Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langan D, Higgins JPT, Jackson D, Bowden J, Veroniki AA, Kontopantelis E, Viechtbauer W, Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res Synth Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [DOI] [PubMed] [Google Scholar]
- 34.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 35.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evid Implement. 2015;13:147–153. doi: 10.1097/xeb.0000000000000054. [DOI] [PubMed] [Google Scholar]
- 36.Saha S, Chant D, McGrath J. Meta-analyses of the incidence and prevalence of schizophrenia: conceptual and methodological issues. Int J Methods Psychiatr Res. 2008;17:55–61. doi: 10.1002/mpr.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Marx RG, Wilson SM, Swiontkowski MF. Updating the assignment of levels of evidence. JBJS. 2015;97:1–2. doi: 10.2106/jbjs.N.01112. [DOI] [PubMed] [Google Scholar]
- 39.Tufanaru C, Munn Z, Aromataris E, Campbell J, Hopp L. Chapter 3: systematic reviews of effectiveness. In: Aromataris e, Munn Z., editors. JBI manual for evidence synthesis. JBI; 2019. [Google Scholar]
- 40.Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synth. 2020;18:2127–2133. doi: 10.11124/jbisrir-d-19-00099. [DOI] [PubMed] [Google Scholar]
- 41.Belthur MV, Conway JD, Jindal G, Ranade A, Herzenberg JE. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466:2973–2980. doi: 10.1007/s11999-008-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han F, Peter L, Lau ET, Thambiah J, Murphy D, Kagda FH. Reamer Irrigator Aspirator bone graft harvesting: complications and outcomes in an Asian population. Injury. 2015;46:2042–2051. doi: 10.1016/j.injury.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Haubruck P, Ober J, Heller R, Miska M, Schmidmaier G, Tanner MC. Complications and risk management in the use of the reaming-irrigator-aspirator (RIA) system: RIA is a safe and reliable method in harvesting autologous bone graft. PLoS ONE. 2018;13:e0196051–e0196051. doi: 10.1371/journal.pone.0196051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kanakaris N, Gudipati S, Tosounidis T, Harwood P, Britten S, Giannoudis PV. The treatment of intramedullary osteomyelitis of the femur and tibia using the reamer-irrigator-aspirator system and antibiotic cement rods. Bone Jt J. 2014;96-b:783–788. doi: 10.1302/0301-620x.96b6.32244. [DOI] [PubMed] [Google Scholar]
- 45.Le Baron M, Vivona JP, Maman P, Volpi R, Flecher X. Can the Reamer/Irrigator/Aspirator System replace anterior iliac crest grafting when treating long bone nonunion? Orthop Traumatol Surg Res. 2019;105:529–533. doi: 10.1016/j.otsr.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 46.Lowe JA, Della Rocca GJ, Murtha Y, Liporace FA, Stover MD, Nork SE, Crist BD. Complications associated with negative pressure reaming for harvesting autologous bone graft: a case series. J Orthop Trauma. 2010;24:46–52. doi: 10.1097/BOT.0b013e31819c0ccb. [DOI] [PubMed] [Google Scholar]
- 47.Marchand LS, Rothberg DL, Kubiak EN, Higgins TF. Is this autograft worth it?: the blood loss and transfusion rates associated with reamer irrigator aspirator bone graft harvest. J Orthop Trauma. 2017;31:205–209. doi: 10.1097/bot.0000000000000811. [DOI] [PubMed] [Google Scholar]
- 48.Martella A, Schumaier AP, Sirignano MN, Sagi HC, Wyrick JD, Archdeacon MT. Reamer Irrigator Aspirator Versus Iliac Crest Bone Grafting and Proximal Tibial Curettage: Is There a Difference in Blood Loss and Transfusion Rates? J Orthop Trauma. 2021;36(4):163–166. doi: 10.1097/BOT.0000000000002256. [DOI] [PubMed] [Google Scholar]
- 49.McCall TA, Brokaw DS, Jelen BA, Scheid DK, Scharfenberger AV, Maar DC, Green JM, Shipps MR, Stone MB, Musapatika D, Weber TG. Treatment of large segmental bone defects with reamer-irrigator-aspirator bone graft: technique and case series. Orthop Clin North Am. 2010;41:63–73. doi: 10.1016/j.ocl.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Niikura T, Oe K, Fukui T, Hayashi S, Matsumoto T, Matsushita T, Kuroda R. Clinical experience of the use of reamer irrigator aspirator in Japanese patients: a report of the first 42 cases. J Orthop Sci. 2021;26:459–465. doi: 10.1016/j.jos.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Onsea J, Pallay J, Depypere M, Moriarty TF, Van Lieshout EMM, Obremskey WT, Sermon A, Hoekstra H, Verhofstad MHJ, Nijs S, Metsemakers W-J. Intramedullary tissue cultures from the reamer-irrigator-aspirator system for diagnosing fracture-related infection. J Orthop Res. 2021;39:281–290. doi: 10.1002/jor.24816. [DOI] [PubMed] [Google Scholar]
- 52.Qvick LM, Ritter CA, Mutty CE, Rohrbacher BJ, Buyea CM, Anders MJ. Donor site morbidity with reamer-irrigator-aspirator (RIA) use for autogenous bone graft harvesting in a single centre 204 case series. Injury. 2013;44:1263–1269. doi: 10.1016/j.injury.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Waterman B, Davey S, Kanlic E, Abdelgawad A. Intramedullary bone graft harvest using reamer-irrigator-aspirator system: a case series. J Surg Orthop Adv. 2017;26:233–238. [PubMed] [Google Scholar]
- 54.Zalavras CG, Singh A, Patzakis MJ. Novel technique for medullary canal débridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:31–34. doi: 10.1097/BLO.0b013e318098673f. [DOI] [PubMed] [Google Scholar]
- 55.Barlow BT, Kuhn KM. Technique and indications for the retrograde use of the reamer-irrigator-aspirator. Curr Orthop Pract. 2014;25:183–188. doi: 10.1097/BCO.0000000000000081. [DOI] [Google Scholar]
- 56.Calori GM, Colombo M, Mazza EL, Mazzola S, Malagoli E, Mineo GV. Incidence of donor site morbidity following harvesting from iliac crest or RIA graft. Injury. 2014;45:S116–S120. doi: 10.1016/j.injury.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 57.Grote S, Helfen T, Muck F, Regauer M, Prall WC. Femoral marrow cavity bone harvesting used for arthroscopic refilling of misplaced or enlarged bone tunnels in revision ACL surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23:808–815. doi: 10.1007/s00167-013-2736-0. [DOI] [PubMed] [Google Scholar]
- 58.Marko N, Dimitrios G, Matija K. Massive blood loss during graft harvesting with the reamer irrigator-aspirator. MOJ Orthop Rheumatol. 2016;5:00166. doi: 10.15406/mojor.2016.05.00166. [DOI] [Google Scholar]
- 59.O'Callaghan PK, Matthews JH, Kim PT, Angermeier EW, Kokko KP. bone grafting in total wrist arthrodesis with large bone defects using the reamer-irrigator-aspirator: a case study of 2 patients. J Hand Surg-Am. 2019;44:620. doi: 10.1016/j.jhsa.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Quintero AJ, Tarkin IS, Pape H-C. Technical tricks when using the reamer irrigator aspirator technique for autologous bone graft harvesting. J Orthop Trauma. 2010;24:42–45. doi: 10.1097/BOT.0b013e3181b2b4eb. [DOI] [PubMed] [Google Scholar]
- 61.Billow D, Khlopas A, Chughtai M, Saleh A, Siqueira MB, Marinello P, Mont MA. The reamer-irrigator-aspirator system: a review. Surg Technol Int. 2016;29:287–294. [PubMed] [Google Scholar]
- 62.Silva JA, McCormick JJ, Reed MA, Morse AS, Heffernan MJ, Lavado RD, Billiar K, Wixted JJ. Biomechanical effects of harvesting bone graft with the Reamer/Irrigator/Aspirator on the adult femur: a cadaver study. Injury. 2010;41(Suppl 2):S85–89. doi: 10.1016/s0020-1383(10)70016-4. [DOI] [PubMed] [Google Scholar]
- 63.Finnan RP, Prayson MJ, Goswami T, Miller D. Use of the reamer-irrigator-aspirator for bone graft harvest: a mechanical comparison of three starting points in cadaveric femurs. J Orthop Trauma. 2010;24:36–41. doi: 10.1097/BOT.0b013e3181b04383. [DOI] [PubMed] [Google Scholar]
- 64.Robison JW, Grau-Cruz EE, Bruggers J, Becher S. Bone Graft Volume by Reamer Head Size Using the RIA 2. Tech Orthop. 2022;37(3):149–153. doi: 10.1097/BTO.0000000000000574. [DOI] [Google Scholar]
- 65.Tosounidis TH, Calori GM, Giannoudis PV. The use of Reamer−irrigator-aspirator in the management of long bone osteomyelitis: an update. Eur J Trauma Emerg Surg. 2016;42:417–423. doi: 10.1007/s00068-016-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zalavras CG, Sirkin M. Treatment of long bone intramedullary infection using the RIA for removal of infected tissue: Indications, method and clinical results. Injury. 2010;41:S43–S47. doi: 10.1016/S0020-1383(10)70008-5. [DOI] [PubMed] [Google Scholar]
- 67.Kanakaris NK, Morell D, Gudipati S, Britten S, Giannoudis PV. Reaming Irrigator Aspirator system: early experience of its multipurpose use. Injury. 2011;42(Suppl 4):S28–34. doi: 10.1016/s0020-1383(11)70009-2. [DOI] [PubMed] [Google Scholar]
- 68.Thonse R, Conway J. Antibiotic cement-coated interlocking nail for the treatment of infected nonunions and segmental bone defects. J Orthop Trauma. 2007;21:258–268. doi: 10.1097/BOT.0b013e31803ea9e6. [DOI] [PubMed] [Google Scholar]
- 69.Hall JA, McKee MD, Vicente MR, Morison ZA, Dehghan N, Schemitsch CE, Kreder HJ, Petrisor B, Schemitsch EH. Prospective randomized clinical trial investigating the effect of the reamer-irrigator-aspirator on the volume of embolic load and respiratory function during intramedullary nailing of femoral shaft fractures. J Orthop Trauma. 2017;31:200–204. doi: 10.1097/bot.0000000000000744. [DOI] [PubMed] [Google Scholar]
- 70.Streubel PN, Desai P, Suk M. Comparison of RIA and conventional reamed nailing for treatment of femur shaft fractures. Injury. 2010;41:S51–S56. doi: 10.1016/S0020-1383(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 71.Martella A, Schumaier AP, Sirignano MN, Sagi HC, Wyrick JD, Archdeacon MT. Reamer irrigator aspirator versus iliac crest bone grafting and proximal tibial curettage: is there a difference in blood loss and transfusion rates? J Orthop Trauma. 2022;36:163–166. doi: 10.1097/bot.0000000000002256. [DOI] [PubMed] [Google Scholar]
- 72.Steuber TD, Howard ML, Nisly SA. Strategies for the management of postoperative anemia in elective orthopedic surgery. Ann Pharmacother. 2016;50:578–585. doi: 10.1177/1060028016647977. [DOI] [PubMed] [Google Scholar]
- 73.Bidolegui F, Pereira S, Irigoyen C, Pires RE. Safety and efficacy of a novel retrograde route for femoral bone graft harvesting by reamer-irrigator-aspirator: a pilot study on 24 patients. Patient Saf Surg. 2022;16:2–2. doi: 10.1186/s13037-021-00315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moghaddam A, Zietzschmann S, Bruckner T, Schmidmaier G. Treatment of atrophic tibia non-unions according to 'diamond concept': results of one- and two-step treatment. Injury. 2015;46(Suppl 4):S39–50. doi: 10.1016/s0020-1383(15)30017-6. [DOI] [PubMed] [Google Scholar]
- 75.Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90–93. doi: 10.1016/s0020-1383(10)70017-6. [DOI] [PubMed] [Google Scholar]
- 76.Richardson M, Garner P, Donegan S. Interpretation of subgroup analyses in systematic reviews: a tutorial. Clin Epidemiol Glob Health. 2019;7:192–198. doi: 10.1016/j.cegh.2018.05.005. [DOI] [Google Scholar]
- 77.Conway JD, Shabtai L, Specht SC, Herzenberg JE. Sequential harvesting of bone graft from the intramedullary canal of the femur. Orthopedics. 2014;37:e796–803. doi: 10.3928/01477447-20140825-56. [DOI] [PubMed] [Google Scholar]
- 78.Davis RL, Taylor BC, Johnson N, Ferrel JR, Castaneda J. Retrograde versus antegrade femoral bone graft harvesting using the reamer-irrigator-aspirator. J Orthop Trauma. 2015;29:370–372. doi: 10.1097/bot.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 79.Eisenstein ED, Waterman BR, Kanlic EM, Abdelgawad AA. A New technique for obtaining bone graft in cases of distal femur nonunion: passing a reamer/irrigator/aspirator retrograde through the nonunion site. Am J Orthop (Belle Mead NJ) 2016;45:E493–e496. [PubMed] [Google Scholar]
- 80.Herscovici D, Jr, Scaduto JM. Use of the reamer−irrigator-aspirator technique to obtain autograft for ankle and hindfoot arthrodesis. J Bone Joint Surg Br. 2012;94:75–79. doi: 10.1302/0301-620x.94b1.27690. [DOI] [PubMed] [Google Scholar]
- 81.Jakma TSC, Röling MA, Punt B, Reynders-Frederix P. More adverse events than expected in the outcome after use of the reamer−irrigator-aspirator. Eur J Trauma Emerg Surg. 2014;40:337–341. doi: 10.1007/s00068-013-0345-8. [DOI] [PubMed] [Google Scholar]
- 82.Krappinger D, Lindtner RA, Zegg M, Dal Pont A, Huber B. Masquelet technique for the treatment of large dia- and metaphyseal bone defects. Oper Orthop Traumatol. 2015;27:357–368. doi: 10.1007/s00064-014-0300-9. [DOI] [PubMed] [Google Scholar]
- 83.Kusnezov N, Prabhakar G, Dallo M, Thabet AM, Abdelgawad AA. Bone grafting via reamer-irrigator-aspirator for nonunion of open Gustilo-Anderson type III tibial fractures treated with multiplanar external fixator. Sicot j. 2017;3:30. doi: 10.1051/sicotj/2017002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lehman AAR, Irgit KS, Cush GJ. Harvest of autogenous bone graft using reamer−irrigator-aspirator in tibiotalocalcaneal arthrodesis: Surgical technique and case series. Foot Ankle Int. 2012;33:1133–1138. doi: 10.3113/FAI.2012.1133. [DOI] [PubMed] [Google Scholar]
- 85.Mansour J, Conway JD. Retrograde reamer/irrigator/aspirator technique for autologous bone graft harvesting with the patient in the prone position. Am J Orthop (Belle Mead NJ) 2015;44:202–205. [PubMed] [Google Scholar]
- 86.Metsemakers WJ, Claes G, Terryn PJ, Belmans A, Hoekstra H, Nijs S. Reamer−Irrigator-Aspirator bone graft harvesting for treatment of segmental bone loss: analysis of defect volume as independent risk factor for failure. Eur J Trauma Emerg Surg. 2019;45:21–29. doi: 10.1007/s00068-017-0821-7. [DOI] [PubMed] [Google Scholar]
- 87.Moghaddam A, Ermisch C, Fischer C, Zietzschmann S, Schmidmaier G. Tibial defects and infected non-unions. Treatment results after Masquelet technique. Orthopade. 2017;46:263–273. doi: 10.1007/s00132-016-3305-2. [DOI] [PubMed] [Google Scholar]
- 88.Naruka V, Nardini M, McVie J, Dunning J. The Reamer−Irrigator-Aspirator technique for manubriosternal non-union repair. Interact Cardiovasc Thorac Surg. 2019;29:327–328. doi: 10.1093/icvts/ivz001. [DOI] [PubMed] [Google Scholar]
- 89.Newman JT, Stahel PF, Smith WR, Resende GV, Hak DJ, Morgan SJ. A new minimally invasive technique for large volume bone graft harvest for treatment of fracture nonunions. Orthopedics. 2008;31:257–261. doi: 10.3928/01477447-20080301-29. [DOI] [PubMed] [Google Scholar]
- 90.Nodzo SR, Kaplan NB, Hohman DW, Ritter CA. A radiographic and clinical comparison of reamer−irrigator-aspirator versus iliac crest bone graft in ankle arthrodesis. Int Orthop. 2014;38:1199–1203. doi: 10.1007/s00264-014-2348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rauck RC, Fisk E, Stammen KL, Yu E, Khan SN. Reamer−irrigator-aspirator for autologous bone graft in spinal fusion: An alternative to conventional bone graft substitutes. Curr Orthop Pract. 2016;27:564–569. doi: 10.1097/BCO.0000000000000413. [DOI] [Google Scholar]
- 92.Seagrave RA, Sojka J, Goodyear A, Munns SW. Utilizing reamer irrigator aspirator (RIA) autograft for opening wedge high tibial osteotomy: a new surgical technique and report of three cases. Int J Surg Case Rep. 2014;5:37–42. doi: 10.1016/j.ijscr.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stafford PR, Norris BL. Reamer−irrigator-aspirator bone graft and bi Masquelet technique for segmental bone defect nonunions: a review of 25 cases. Injury. 2010;41(Suppl 2):S72–77. doi: 10.1016/s0020-1383(10)70014-0. [DOI] [PubMed] [Google Scholar]
- 94.Walker M, Sharareh B, Mitchell SA. Masquelet reconstruction for posttraumatic segmental bone defects in the forearm. J Hand Surg Am. 2019;44:342.e341–342.e348. doi: 10.1016/j.jhsa.2018.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.