Abstract
Background
Traditional systemic immunosuppressants and advanced therapies improve signs and symptoms of moderate-to-severe atopic dermatitis (AD). However, data are limited in severe and/or difficult-to-treat AD. In the phase 3 JADE COMPARE trial of patients with moderate-to-severe AD receiving background topical therapy, once-daily abrocitinib 200 mg and 100 mg showed significantly greater reductions in the symptoms of AD than placebo and significantly greater improvement in itch response (with abrocitinib 200 mg) than dupilumab at week 2.
Objective
This study assessed the efficacy and safety of abrocitinib and dupilumab in a subset of patients with severe and/or difficult-to-treat AD in a post hoc analysis of the JADE COMPARE trial.
Methods
Adults with moderate-to-severe AD received once-daily oral abrocitinib 200 mg or 100 mg, dupilumab 300 mg subcutaneous injection every 2 weeks, or placebo with concomitant medicated topical therapy. Severe and/or difficult-to-treat AD subgroups were classified by baseline characteristics [Investigator’s Global Assessment (IGA) 4, Eczema Area and Severity Index (EASI) > 21, failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids), percentage of body surface area (%BSA) > 50, upper quartiles of EASI (EASI > 38) and %BSA (%BSA > 65), and combined subgroup of IGA 4, EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)]. Assessments included IGA score of 0 (clear) or 1 (almost clear) and a ≥ 2-point improvement from baseline, ≥ 75% and ≥ 90% improvement from baseline in EASI (EASI-75 and EASI-90), ≥ 4-point improvement from baseline in Peak Pruritus-Numerical Rating Scale (PP-NRS4), time to PP-NRS4, least squares mean (LSM) change from baseline in 14-day PP-NRS (days 2–15), Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI) up to week 16.
Results
The proportion of patients achieving IGA 0/1, EASI-75, and EASI-90 responses was significantly greater with abrocitinib 200 mg than placebo (nominal p < 0.05) across all subgroups with severe and/or difficult-to-treat AD. Across most subgroups, PP-NRS4 response was significantly greater with abrocitinib 200 mg than placebo (nominal p < 0.01); the time to achieve this response was shorter with abrocitinib 200 mg (range 4.5–6.0 days) than abrocitinib 100 mg (range 5.0–17.0 days), dupilumab (range 8.0–11.0 days), and placebo (range 3.0–11.5 days). LSM change from baseline in POEM and DLQI was significantly greater with abrocitinib 200 mg than placebo (nominal p < 0.001) across all subgroups. Clinically meaningful differences were observed between abrocitinib and dupilumab for most evaluated endpoints across several subgroups, including in patients who failed or were intolerant to prior systemic therapy.
Conclusions
Abrocitinib provided rapid and substantially greater improvements in skin clearance and quality of life compared with placebo and dupilumab in subgroups of patients with severe and/or difficult-to-treat AD. These findings support the use of abrocitinib for severe and/or difficult-to-treat AD.
Trial registration:
ClinicalTrials.gov, NCT03720470.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-023-00785-5.
Plain Language Summary
Atopic dermatitis (AD), also known as atopic eczema, is a skin disease that causes itchy and red skin patches. People can be diagnosed with severe and/or difficult-to-treat AD if their signs and symptoms of AD are extremely severe and their AD cannot be adequately treated by common medicines. Abrocitinib is a treatment that has been shown in clinical trials to improve the symptoms of AD. We analyzed data from the JADE COMPARE study, which included 837 people who were treated with abrocitinib, dupilumab (another treatment for AD), or placebo. Many of these people had severe symptoms when they entered the study. Some had AD signs and symptoms that did not improve after they took common medicines for AD. We studied how well abrocitinib worked in these people with severe and/or difficult-to-treat AD. We found that these people achieved clear skin and itch relief at week 16 after treatment with abrocitinib 200 mg compared with placebo (no drug control). Additionally, they achieved significant relief from itch faster with abrocitinib 200 mg compared with abrocitinib 100 mg, dupilumab, or placebo. People reported less severe AD and better quality of life after treatment with abrocitinib compared with placebo. Together, the findings of our study provide important evidence for healthcare providers as they determine a treatment plan for people with severe and/or difficult-to-treat AD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-023-00785-5.
Key points
| After treatment with abrocitinib 200 mg, more people who had severe and/or difficult-to-treat AD achieved clear skin and itch relief at week 16 compared with placebo. |
| Significant relief from itch was achieved faster with abrocitinib 200 mg compared with abrocitinib 100 mg, dupilumab, or placebo. |
| After treatment with abrocitinib, people with severe and/or difficult-to-treat AD reported less severe AD and better quality of life compared with those who took placebo. |
Introduction
Atopic dermatitis (AD) is a heterogeneous disease with overall prevalence rates in adults of 2–5% globally [1]. Based on various patient-reported outcome measures of disease severity such as the Patient-Oriented Eczema Measure (POEM), Patient-Oriented Scoring of Atopic Dermatitis (PO-SCORAD), and Patient Global Assessment (PGA), an estimated 2–21% of the overall AD population can be classified as having severe disease (POEM score > 16, PO-SCORAD score ≥ 50, or self-reported PGA as severe) [1]. Current guidelines for moderate-to-severe AD recommend treatment with systemic immunosuppressants such as cyclosporine and methotrexate, the interleukin (IL)-4 receptor α antagonist dupilumab, oral Janus kinase (JAK)-selective inhibitors baricitinib, upadacitinib, and abrocitinib, and the IL-13-targeting monoclonal antibody tralokinumab [2].
Abrocitinib demonstrated efficacy in patients with moderate-to-severe AD in multiple randomized clinical trials [3–6]. However, data are limited in patients who belong to a more severe spectrum of AD and have failed or were intolerant to previous systemic therapies, and are therefore deemed difficult to treat. In the phase 3 JADE COMPARE study (NCT03720470) of patients with moderate-to-severe AD receiving background medicated topical therapy, abrocitinib 200 mg and 100 mg once daily resulted in significantly greater reductions in signs and symptoms of AD versus placebo at weeks 12 and 16 of treatment [6]. Abrocitinib 200 mg also provided significantly greater efficacy versus dupilumab in itch response as early as week 2 [6]. In this post hoc analysis, we assessed the efficacy of abrocitinib and dupilumab in subgroups of patients from JADE COMPARE who were considered severe and/or difficult to treat based on various disease characteristics at baseline.
Methods
Study Design
The study design of JADE COMPARE was described previously [6]. Briefly, JADE COMPARE (NCT03720470) was a multicenter, randomized, double-blind, double-dummy, placebo-controlled, phase 3 study that compared the efficacy and safety of abrocitinib (200 mg and 100 mg) and dupilumab versus placebo in adult patients with moderate-to-severe AD who received concomitant topical therapy [6]. The trial was not designed as a direct comparison of efficacy endpoints between abrocitinib and dupilumab (except for the key secondary endpoint of itch response from day 2 to day 15) [6].
Patients
Patients in JADE COMPARE were ≥ 18 years of age and had moderate-to-severe AD for ≥ 1 year at baseline, with an Investigator’s Global Assessment (IGA) score ≥ 3, percentage of body surface area (%BSA) involvement ≥ 10, Eczema Area and Severity Index (EASI) score ≥ 16, Peak Pruritus Numerical Rating Scale (PP-NRS; used with permission from Regeneron Pharmaceuticals, Inc., and Sanofi) score ≥ 4, and documented recent history (≤ 6 months prior to screening) of inadequate response to topical treatment or requirement for systemic therapy to control AD [6]. Prior use of systemic JAK inhibitors or dupilumab was not permitted. Patients were randomly assigned 2:2:2:1 to receive once-daily oral abrocitinib (200 mg or 100 mg), placebo, or subcutaneous dupilumab 300 mg every 2 weeks (following a 600-mg loading dose) for 16 weeks. Patients used nonmedicated topical treatments for ≥ 7 days prior to study baseline, and when required by the protocol guidelines, medicated topical treatment once daily during the study duration.
In this post hoc analysis, subgroups of severe and/or difficult-to-treat AD were classified according to baseline characteristics as follows: IGA 4; EASI > 21; failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids); %BSA > 50; and combined subgroup of IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids). Patients were also classified by upper quartile scores of EASI > 38 and %BSA > 65.
This study was conducted in accordance with the Declaration of Helsinki and the International Council for Harmonization and Good Clinical Practice and approved by the institutional review board or ethics committee at each study site. All patients provided written informed consent [6].
Assessments
The subgroups of severe and/or difficult-to-treat AD were assessed for achievement of an IGA response, defined as IGA score of 0 (clear) or 1 (almost clear) and a ≥ 2-point improvement from baseline, ≥ 75% improvement from baseline in EASI (EASI-75) response, ≥ 90% improvement from baseline in EASI (EASI-90) response, a ≥ 4-point improvement from baseline in Peak Pruritus Numerical Rating Scale (PP-NRS4), time to PP-NRS4 response, least squares mean (LSM) change from baseline in 14-day PP-NRS (days 2–15), Patient-Oriented Eczema Measure (POEM), and Dermatology Life Quality Index (DLQI) scores. All parameters, except the time to PP-NRS4 response and LSM change from baseline in 14-day PP-NRS, were evaluated at week 16.
All-cause treatment-emergent adverse events (TEAEs) and serious TEAEs were assessed in patients who had IGA 4, or EASI > 21, or %BSA > 50, or failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids) at baseline.
Statistical Analysis
For IGA, %BSA, EASI, and PP-NRS, baseline was defined as the last measurement before first dosing (day 1). For POEM and DLQI, baseline was defined as the measurement collected on or prior to day 1. Efficacy and safety analyses were performed in the full analysis set (all randomly assigned patients who received ≥ 1 dose of study drug). All efficacy endpoints were assessed by treatment group in each severe and/or difficult-to-treat AD patient subgroup.
Binary data (responder/non-responder) were summarized using proportion of responders [95% confidence interval (CI)]; 95% CIs were based on the normal approximation of binomial proportions (or the Clopper–Pearson method when there were 0% or 100% responders); missing data after discontinuation were defined as nonresponse. p-Values were used to assess statistical comparisons between both abrocitinib doses versus placebo and versus dupilumab and were calculated using the Cochran–Mantel–Haenszel method. All p-values were nominal and not controlled for multiplicity. Statistical comparisons for proportion of responders with dupilumab versus placebo were based on 95% CIs for the estimated differences. The LSM change from baseline (95% CI) for 14-day PP-NRS, POEM, and DLQI scores was calculated using mixed-model repeated measure using all observed data and containing fixed factors of treatment, visit, treatment by visit interaction, baseline disease severity, baseline value, and an unstructured covariance matrix. Statistical comparisons for the LSM change from baseline values for both doses of abrocitinib versus dupilumab and dupilumab versus placebo were based on 95% CIs for the LSM difference.
Results
Baseline Characteristics
Patient demographics and baseline disease characteristics were generally comparable across all severe and/or difficult-to-treat AD subgroups (Table 1) and across the treatment arms within each subgroup (Tables S1–S4, Online Resource). Depending on subgroup, between 14.4% and 72.0% of participants met at least one definition of difficult-to-treat AD. Among patients who failed or were intolerant to prior systemic agents (excluding patients who took only corticosteroids), cyclosporine was the most frequently used nonbiologic (Table S5, Online Resource); lack of efficacy was the most common reason for discontinuation of prior therapies (Table S6, Online Resource).
Table 1.
Patient demographics and baseline disease characteristics by severe and/or difficult-to-treat AD subgroup (full analysis set)
| IGA 4 n = 296 |
EASI > 21 n = 603 |
EASI > 38 n = 225 |
Failure or intolerance to prior systemic agentsa n = 121 |
%BSA > 50 n = 360 |
%BSA > 65 n = 214 |
Combined subgroupb n = 48 |
|
|---|---|---|---|---|---|---|---|
| Age, mean ± SD, years | 37.6 ± 14.6 | 36.8 ± 14.3 | 36.5 ± 14.3 | 36.4 ± 13.6 | 36.0 ± 14.2 | 36.2 ± 13.9 | 35.3 ± 12.9 |
| Female, n (%) | 130 (43.9) | 276 (45.8) | 91 (40.4) | 40 (33.1) | 153 (42.5) | 90 (42.1) | 12 (25.0) |
| Race, n (%) | |||||||
| White | 183 (61.8) | 415 (68.8) | 161 (71.6) | 85 (70.2) | 242 (67.2) | 141 (65.9) | 27 (56.3) |
| Black or African American | 18 (6.1) | 26 (4.3) | 10 (4.4) | 1 (0.8) | 14 (3.9) | 10 (4.7) | 1 (2.1) |
| Asian | 86 (29.1) | 145 (24.0) | 49 (21.8) | 31 (25.6) | 96 (26.7) | 56 (26.2) | 17 (35.4) |
| Otherc | 9 (3.0) | 17 (2.8) | 5 (2.2) | 4 (3.3) | 8 (2.2) | 7 (3.3) | 3 (6.2) |
| IGA score, n (%) | |||||||
| 3 (moderate) | 0 (0.0) | 322 (53.4) | 64 (28.4) | 58 (47.9) | 162 (45.0) | 74 (34.6) | 0 (0.0) |
| 4 (severe) | 296 (100) | 281 (46.6) | 161 (71.6) | 63 (52.1) | 198 (55.0) | 140 (65.4) | 48 (100.0) |
| %BSA involvement, mean ± SD | 61.8 ± 22.5 | 57.0 ± 20.9 | 75.8 ± 14.2 | 57.9 ± 22.6 | 71.0 ± 14.0 | 80.4 ± 9.8 | 76.3 ± 13.0 |
| EASI score, mean ± SD | 40.4 ± 12.9 | 35.9 ± 11.8 | 48.8 ± 8.0 | 36.8 ± 13.6 | 41.9 ± 11.3 | 47.2 ± 10.5 | 47.3 ± 10.5 |
| PP-NRS score, mean ± SD | 7.8 ± 1.6 | 7.5 ± 1.6 | 7.9 ± 1.5 | 7.4 ± 1.6 | 7.6 ± 1.6 | 7.7 ± 1.6 | 7.7 ± 1.5d |
| POEM score, mean ± SD | 22.6 ± 4.9 | 21.6 ± 5.2 | 23.0 ± 4.9 | 21.1 ± 5.6 | 22.0 ± 5.2 | 22.4 ± 5.0 | 22.5 ± 5.2 |
| DLQI score, mean ± SD | 17.3 ± 6.4 | 16.0 ± 6.4 | 18.1 ± 6.2 | 16.2 ± 6.1 | 16.6 ± 6.4 | 17.8 ± 6.3 | 17.0 ± 6.0 |
AD atopic dermatitis, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, POEM Patient-Oriented Eczema Measure, PP-NRS Peak Pruritus Numerical Rating Scale
aExcluding patients who took only corticosteroids
bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)
cAmerican Indian or Alaska Native, multiracial, or race not reported
dn = 47 patients
IGA Response at Week 16
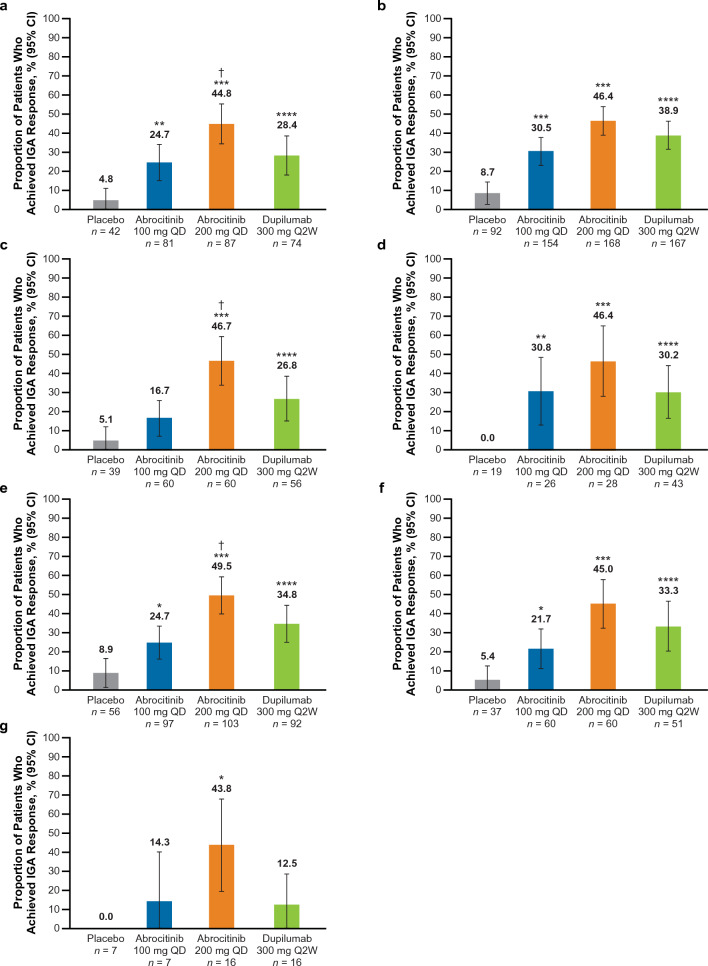
The proportion of patients who achieved an IGA 0/1 response was significantly greater (nominal p < 0.05) with abrocitinib 200 mg than placebo across all subgroups (Fig. 1). A significantly greater proportion of patients achieved IGA 0/1 response with abrocitinib 100 mg versus placebo (nominal p < 0.05) across all subgroups (Fig. 1a–b, d–f) except the EASI > 38 and combined groups (Fig. 1c, g)
Fig. 1.
%BSA percentage of body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, QD once daily, Q2W once every 2 weeks. IGA 0/1 response rates at week 16 in patients with baseline a IGA 4, b EASI > 21, c EASI > 38, d failure or intolerance to prior systemic agents,a e %BSA > 50, f %BSA > 65 and g in the combined subgroup.b *p < 0.05, **p < 0.01, and ***p < 0.001 for abrocitinib versus placebo. ****Significant difference between dupilumab versus placebo based on 95% CIs for the estimated difference. †p < 0.05 for abrocitinib versus dupilumab. aExcluding patients who took only corticosteroids. bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)
IGA 0/1 response was significantly greater (based on 95% CIs for estimated differences) with dupilumab versus placebo across all subgroups (Fig. 1a–f) except the combined group (Fig. 1g).
A significant difference was observed between abrocitinib 200 mg and dupilumab in the IGA 4 [estimated difference, 16.4% (95% CI 1.8–31.1), p < 0.0320; Fig. 1a], EASI > 38 [estimated difference, 19.9% (95% CI 2.7–37.0), p < 0.0274; Fig. 1c], and %BSA > 50 [estimated difference, 14.7% (95% CI 1.0–28.4), p < 0.0383; Fig. 1e] subgroups. In the EASI > 21 subgroup, among those who failed or were intolerant to prior systemic therapy, %BSA > 65 subgroup, and the combined subgroup, IGA 0/1 response with abrocitinib 200 mg was numerically greater than dupilumab (Fig. 1b, d, f–g) but there was no significant difference between the two treatment arms.
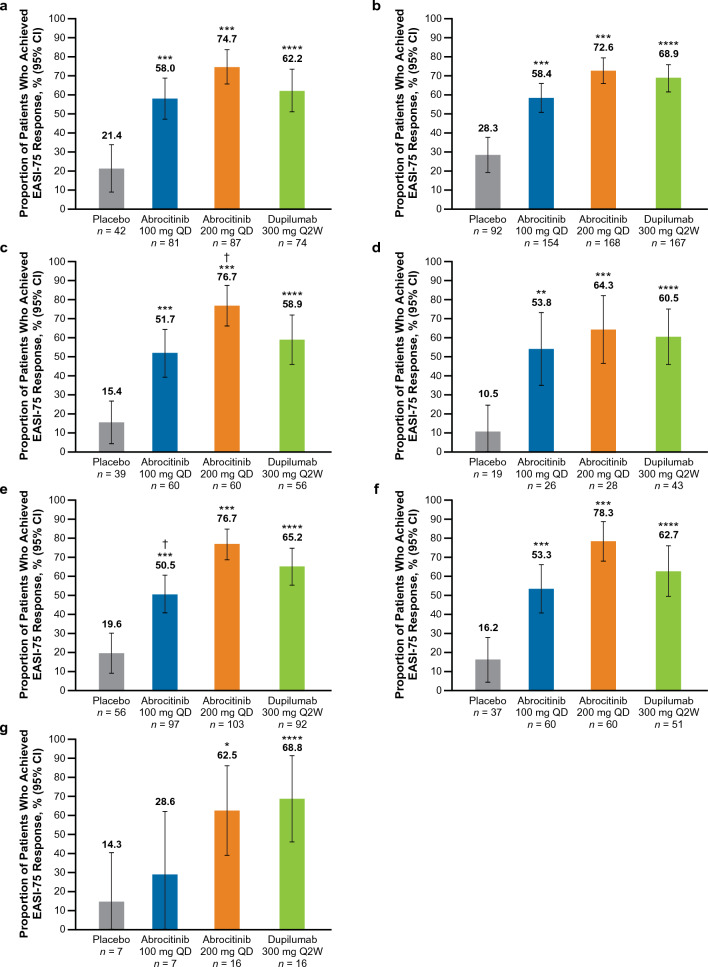
EASI-75 Response at Week 16
The proportion of patients who achieved an EASI-75 response was significantly greater (nominal p < 0.05) with abrocitinib 200 mg than placebo across all subgroups of severe and/or difficult-to-treat AD (Fig. 2a–g). A significantly greater proportion of patients achieved EASI-75 response with abrocitinib 100 mg compared with placebo across all subgroups (nominal p < 0.01; Fig. 2a–f) except the combined group, in which improvements were numerically greater than placebo but did not reach statistical significance (Fig. 2g).
Fig. 2.
%BSA percentage of body surface area, EASI Eczema Area and Severity Index, EASI-75 ≥ 75% improvement from baseline in EASI, IGA Investigator’s Global Assessment, QD once daily, Q2W once every 2 weeks. EASI-75 rates at week 16 in patients with baseline a IGA 4, b EASI > 21, c EASI > 38, d Failure or intolerance to prior systemic agents,a e %BSA > 50, f %BSA > 65 and g in the combined subgroup.b *p < 0.05, **p < 0.01, and ***p < 0.001 for abrocitinib versus placebo. ****Significant difference between dupilumab versus placebo based on 95% CIs for the estimated difference. †p < 0.05 for abrocitinib versus dupilumab. aExcluding patients who took only corticosteroids. bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)
EASI-75 response was significantly greater (based on 95% CIs for estimated differences) with dupilumab versus placebo across all subgroups (Fig. 2a–g).
A significant difference was observed between abrocitinib 200 mg and dupilumab in the EASI > 38 subgroup [estimated difference, 17.7% (95% CI 1.0–34.5), p < 0.0414; Fig. 2c]. EASI-75 response was numerically greater with abrocitinib 200 mg than dupilumab in all subgroups (Fig. 2a–b, d–f), except the combined subgroup (Fig. 2g).
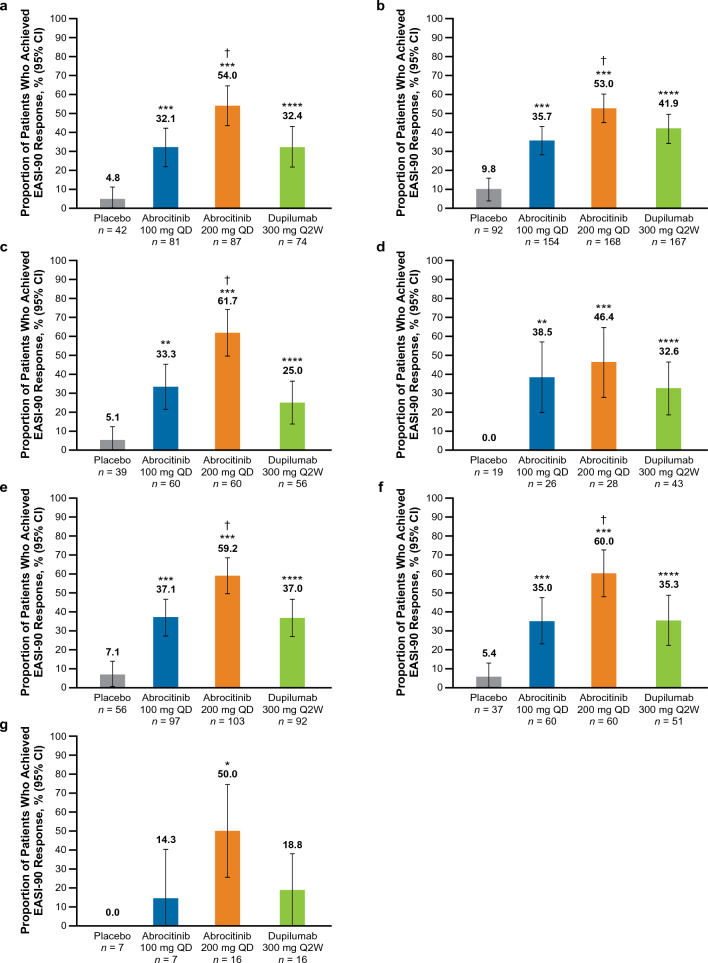
EASI-90 Response at Week 16
The proportion of patients who achieved an EASI-90 response was significantly greater (nominal p < 0.05) with abrocitinib 200 mg than placebo across all subgroups with severe and/or difficult-to-treat AD (Fig. 3). A significantly greater proportion of patients achieved EASI-90 response with abrocitinib 100 mg compared with placebo across all subgroups (nominal p < 0.01; Fig. 3a–f) except the combined group, in which improvements were numerically greater than placebo but did not reach statistical significance (Fig. 3g).
Fig. 3.
%BSA percentage of body surface area, EASI Eczema Area and Severity Index, EASI-90 ≥ 90% improvement from baseline in EASI, IGA Investigator’s Global Assessment, QD once daily, Q2W once every 2 weeks. EASI-90 rates at week 16 in patients with baseline a IGA 4, b EASI > 21, c EASI > 38, d Failure or intolerance to prior systemic agents,a e %BSA > 50, f %BSA > 65 and g in the combined subgroup.b *p < 0.05, **p < 0.01, and ***p < 0.001 for abrocitinib versus placebo. ****Significant difference between dupilumab versus placebo based on 95% CIs for the estimated difference. †p < 0.05 for abrocitinib versus dupilumab. aExcluding patients who took only corticosteroids. bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)
EASI-90 response was significantly greater (based on 95% CIs for estimated differences) with dupilumab versus placebo across all subgroups (Fig. 3a–f) except the combined group (Fig. 3g).
A significant difference was observed between abrocitinib 200 mg and dupilumab in the IGA 4 [estimated difference, 21.6% (95% CI 6.6–36.5), p < 0.0061; Fig. 3a], EASI > 21 [estimated difference, 11.1% (95% CI 0.4–21.7), p < 0.0430; Fig. 3b], EASI > 38 [estimated difference, 36.7% (95% CI 19.9–53.4), p < 0.0001; Fig. 3c], %BSA > 50 [estimated difference, 22.3% (95% CI 8.6–36.0), p < 0.0020; Fig. 3e], %BSA > 65 [estimated difference, 24.7% (95% CI 6.7–42.8), p < 0.0098; Fig. 3f] subgroups. Among those who failed or were intolerant to prior systemic therapy and in the combined subgroup, EASI-90 response with abrocitinib 200 mg was numerically greater than that with dupilumab but did not reach significance (Fig. 3d, g).
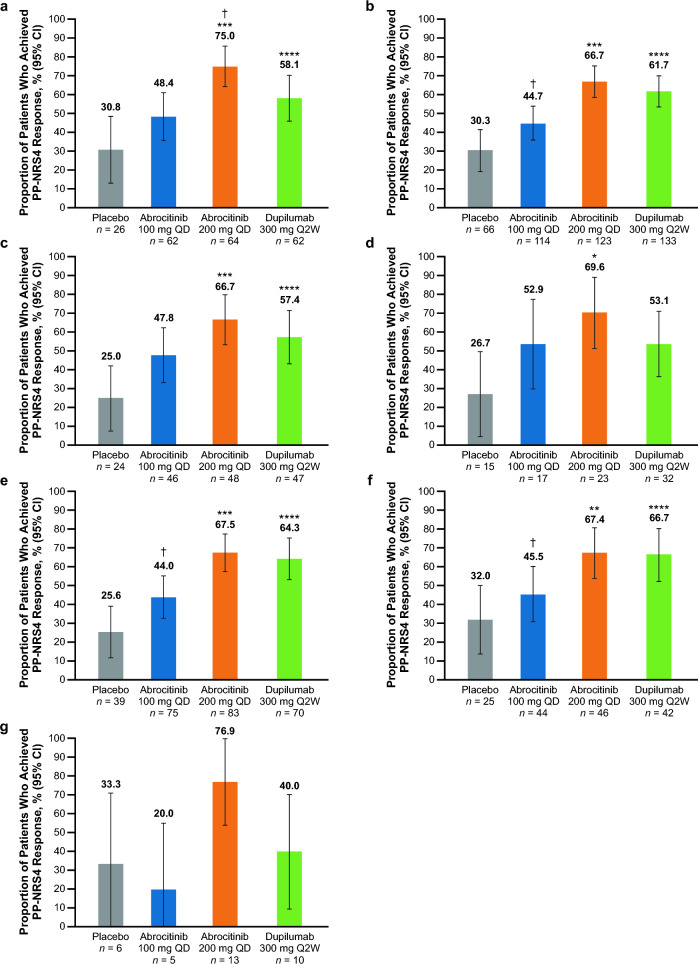
PP-NRS4 Response at Week 16
The proportion of patients who achieved a PP-NRS4 response was significantly greater with abrocitinib 200 mg than placebo (nominal p < 0.05) in all groups (Fig. 4a–f) except the combined subgroup, in which improvements were numerically greater than placebo but not statistically significant (Fig. 4g). PP-NRS4 response was numerically greater (not statistically significant) with abrocitinib 100 mg versus placebo across all subgroups except the combined subgroup (Fig. 4).
Fig. 4.
%BSA percentage of body surface area, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, PP-NRS4 ≥ 4-point improvement in Peak Pruritus Numerical Rating Scale, QD once daily, Q2W once every 2 weeks. PP-NRS4 response rates at week 16 in patients with baseline a IGA 4, b EASI > 21, c EASI > 38, d Failure or intolerance to prior systemic agents,a e %BSA > 50, f %BSA > 65 and g in the combined subgroup.b *p < 0.05, **p < 0.01, and ***p < 0.001 for abrocitinib versus placebo. ****Significant difference between dupilumab versus placebo based on 95% CIs for the estimated difference. †p < 0.05 for abrocitinib versus dupilumab. aExcluding patients who took only corticosteroids. bThe combined subgroup was defined as patients with baseline IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)
PP-NRS4 response was significantly greater (based on 95% CIs for estimated differences) with dupilumab versus placebo across all subgroups (Fig. 4a–c, e–f) except the failure or intolerance to prior systemic subgroup (Fig. 4d) and the combined subgroup (Fig. 4g).
A significant difference was observed between abrocitinib 200 mg and dupilumab in the IGA 4 subgroup [estimated difference, 16.9% (95% CI 0.7–33.2), p < 0.0446; Fig. 4a]. In all other subgroups, PP-NRS4 response was numerically greater with abrocitinib 200 mg than dupilumab (Fig. 4b–g).
Time to PP-NRS4 Response
Across all subgroups, the estimated time to first PP-NRS4 response ranged from 4.5 days to 6.0 days with abrocitinib 200 mg, 5.0–17.0 days with abrocitinib 100 mg, 8.0–11.0 days with dupilumab, and 3.0–11.5 days with placebo (Supplementary Fig. S1).
LSM Change From Baseline in PP-NRS at Day 15
LSM change from baseline in PP-NRS response was significantly greater (nominal p < 0.05) with abrocitinib 200 mg than placebo at most timepoints in all but the combined subgroup (Supplementary Fig. S2). Similar trends were observed with abrocitinib at the 100 mg dose (Supplementary Fig. S2).
On day 15, LSM change from baseline in PP-NRS response was significantly greater (based on 95% CIs for LSM differences) with dupilumab versus placebo in the IGA 4, EASI > 21, and %BSA > 50 subgroups (Supplementary Fig. S2a, b, e).
On day 15, significant differences (based on 95% CIs for LSM differences) were observed between abrocitinib 200 mg and dupilumab in the IGA 4 [LSM difference −1.4 (95% CI −2.0 to −0.7); Supplementary Fig. S2a], EASI > 21 [LSM difference −1.4 (95% CI −1.9 to −0.9); Supplementary Fig. S2b], EASI > 38 [LSM difference −1.1 (95% CI −1.9 to −0.3); Supplementary Fig. S2c], %BSA > 50 [LSM difference −1.3 (95% CI −1.9 to −0.7); Supplementary Fig. S2e], and %BSA > 65 [LSM difference −1.3 (95% CI −2.2 to −0.5); Supplementary Fig. S2f] subgroups.
LSM Change From Baseline in POEM at Week 16
LSM change from baseline in POEM was significantly greater (nominal p < 0.001) with abrocitinib 200 mg than placebo across all subgroups of severe and/or difficult-to-treat AD (Supplementary Fig. S3). Similarly, LSM change from baseline in POEM with abrocitinib 100 mg was significantly greater than placebo in most subgroups (nominal p < 0.05; Supplementary Fig. S3a–f) and numerically, but not statistically significantly, greater in the combined subgroup (Supplementary Fig. S3g).
LSM change from baseline in POEM was significantly greater with dupilumab versus placebo across all subgroups based on 95% CIs for LSM differences (Supplementary Fig. S3a–f) except the combined group (Supplementary Fig. S3g).
A significant difference (based on 95% CIs for LSM differences) was observed between abrocitinib 200 mg and dupilumab in the IGA 4 [LSM difference −2.9 (95% CI −5.2 to −0.6); Supplementary Fig. S3a], EASI > 21 [LSM difference −1.6 (95% CI −3.1 to −0.1); Supplementary Fig. S3b], EASI > 38 [LSM difference − 3.9 (95% CI − 6.7 to −1.2); Supplementary Fig. S3c], failure or intolerance to prior systemic agents [LSM difference −4.0 (95% CI −7.3 to −0.6); Supplementary Fig. S3d], %BSA > 50 [LSM difference −2.6 (95% CI −4.6 to −0.6); Supplementary Fig. S3e], %BSA > 65 [LSM difference −3.5 (95% CI −6.2 to −0.7); Supplementary Fig. S3f], and combined [LSM difference −5.7 (95% CI −11.3 to −0.1); Supplementary Fig. S3g] subgroups.
LSM Change From Baseline in DLQI at Week 16
LSM change from baseline in DLQI was significantly greater (nominal p < 0.05) with abrocitinib 200 mg and 100 mg than placebo across all subgroups of severe and/or difficult-to-treat AD (Supplementary Fig. S4).
LSM change from baseline in DLQI was significantly greater (based on 95% CIs for LSM differences) with dupilumab versus placebo across all subgroups (Supplementary Fig. S4a–g).
A significant difference (based on 95% CIs for LSM differences) was observed between abrocitinib 200 mg and dupilumab in the IGA 4 subgroup [LSM difference −2.1 (95% CI −3.8 to −0.5); Supplementary Fig. S4a]. In all other subgroups, LSM change in DLQI was numerically greater with abrocitinib 200 mg than dupilumab (Supplementary Fig. S4b–g).
Safety
A total of 634 patients were included in the subgroup with baseline IGA 4, or EASI > 21, or %BSA > 50, or failure or intolerance to prior systemic agents. Of those, 354 patients (55.8%) experienced all-cause TEAEs (Table 2). Rates of TEAEs were 65.9% with abrocitinib 200 mg, 49.7% with abrocitinib 100 mg, 55.6% with placebo, and 51.9% with dupilumab. Nausea and nasopharyngitis were the most common TEAEs with abrocitinib. The proportion of patients with conjunctivitis was greater with dupilumab (7.1%) than abrocitinib 200 mg (1.7%), abrocitinib 100 mg (0.0%), or placebo (2.0%).
Table 2.
All-cause and treatment-emergent adverse eventsa
| Placebo n = 99 |
Abrocitinib 100 mg QD n = 173 |
Abrocitinib 200 mg QD n = 179 |
Dupilumab 300 mg Q2W n = 183 |
|
|---|---|---|---|---|
| Patients with AEs, n (%) | 55 (55.6) | 86 (49.7) | 118 (65.9) | 95 (51.9) |
| Patients with serious AEs, n (%) | 5 (5.1) | 5 (2.9) | 1 (0.6) | 2 (1.1) |
| Patients with severe AEs, n (%) | 2 (2.0) | 3 (1.7) | 3 (1.7) | 2 (1.1) |
| Discontinuations due to AEs, n (%) | 2 (2.0) | 2 (1.2) | 6 (3.4) | 5 (2.7) |
| Most common TEAEs, n (%) | ||||
| Nasopharyngitis | 7 (7.1) | 15 (8.7) | 13 (7.3) | 17 (9.3) |
| Headache | 6 (6.1) | 7 (4.0) | 11 (6.1) | 11 (6.0) |
| Nausea | 1 (1.0) | 7 (4.0) | 20 (11.2) | 4 (2.2) |
| Upper respiratory tract infection | 3 (3.0) | 7 (4.0) | 5 (2.8) | 7 (3.8) |
| Conjunctivitis | 2 (2.0) | 0 (0.0) | 3 (1.7) | 13 (7.1) |
| Blood creatine phosphokinase increased | 2 (2.0) | 5 (2.9) | 4 (2.2) | 2 (1.1) |
| Diarrhea | 1 (1.0) | 3 (1.7) | 4 (2.2) | 3 (1.6) |
AE adverse event, QD once daily, Q2W once every 2 weeks, TEAE treatment-emergent adverse event
aSubgroup with baseline IGA 4, or EASI > 21, or %BSA > 50 or failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids).
Discussion
In this post hoc analysis of JADE COMPARE, abrocitinib 200 mg was associated with greater improvements in both objective and subjective measures of disease severity compared with placebo and dupilumab in patients with severe and/or difficult-to-treat AD. Treatment with abrocitinib resulted in improvements in disease severity at week 16, with a greater proportion of patients achieving IGA 0/1, EASI-75, and EASI-90 responses with abrocitinib 200 mg compared with placebo and dupilumab. In the combined subgroup with severe and/or difficult-to-treat AD [i.e., those with IGA 4, and EASI > 21, and %BSA > 50, and failure or intolerance to prior systemic agents (excluding patients who took only corticosteroids)], the proportion of patients who achieved EASI-75 was numerically greater with dupilumab than abrocitinib 200 mg; however, the difference was not likely to be clinically relevant due to the small sample sizes and overlapping CIs. Patients in all subgroups of severe and/or difficult-to-treat AD experienced rapid itch relief with abrocitinib 200 mg within 6 days of initiating treatment, which was sustained through week 16. Notably, these improvements also occurred in patients who had failed or were intolerant to previous systemic therapy. These findings are particularly relevant given that abrocitinib is approved in several countries for the treatment of patients whose AD is not adequately controlled with other systemic drug products, including biologics, or when use of those therapies is inadvisable [7].
The differences in efficacy between abrocitinib and dupilumab in this study may be attributable to their respective mechanisms of action. Through selective inhibition of JAK1, abrocitinib directly inhibits the signaling of multiple inflammatory cytokines involved in AD pathogenesis, including IL-31, IL-4, IL-13, T-helper 1 cytokines (interferon-γ), and thymic stromal lymphopoietin [8, 9]. Dupilumab binds to the shared alpha chain subunit of the IL-4 and IL-13 receptors, thereby inhibiting the signalling of IL-4 and IL-13 [10]. Previous reports in the literature showed a faster onset of action with JAK inhibitors than dupilumab. In the JADE COMPARE overall population, abrocitinib 200 mg yielded rapid and significantly greater improvements in itch at week 2 compared with dupilumab [6]. In the JADE DARE head-to-head trial of abrocitinib versus dupilumab, 48% of patients achieved PP-NRS4 at week 2 and 29% achieved EASI-90 at week 4 with abrocitinib compared with 26% and 15% of dupilumab-treated patients [11]. In another head-to-head trial of upadacitinib, a JAK1-selective inhibitor, versus dupilumab, 44% of patients achieved EASI-75 at week 2 with upadacitinib compared with 17% of dupilumab-treated patients [12].
The improvements observed in disease severity and itch with abrocitinib corresponded with improvements in overall severity of eczema and patients’ quality of life, as measured by POEM and DLQI, across all subgroups of severe and/or difficult-to-treat AD. Given the substantial impairment in the quality of life of patients with AD, treatments that provide clinically meaningful improvements in disease symptoms fulfill an unmet need, particularly in the population with severe and/or difficult-to-treat AD. Both doses of abrocitinib demonstrated a favorable safety profile; most TEAEs were mild or moderate in severity.
Data on patients with severe and/or difficult-to-treat AD are limited. There is also a lack of consensus in the literature on the definition of difficult-to-treat AD [13]. In a registry study of patients with AD receiving dupilumab, the definition of difficult to treat was limited to those who were refractory to AD treatment (i.e., treatment for ≥ 4 months with ≥ 1 conventional systemic therapy at an adequate dose) [14]. In another study, patients who were unresponsive to simple moisturizers and topical corticosteroids were defined as difficult to treat [15]. Treatment with baricitinib 2 mg, another oral JAK-selective inhibitor, improved the signs and symptoms of AD in a subgroup of patients with baseline %BSA > 50, although the improvements were not significantly greater than placebo [16]. A key strength of our analysis is the use of a broad and comprehensive definition of patients with severe and/or difficult-to-treat AD that encompasses baseline measures of IGA, EASI, and BSA. The utility of these definitions in clinical practice remains to be determined.
The main limitation of this study is the post hoc nature of the efficacy assessments and the small sample sizes of the subgroups. Treatment duration was relatively short, lasting 16 weeks; assessment over a longer period will further inform abrocitinib efficacy in this patient population. This trial was not designed as a direct comparison of efficacy endpoints between abrocitinib and dupilumab (except for PP-NRS4 itch response from day 2 to day 15); further study in a head-to-head trial is needed to confirm these findings. Notably, results from a recent study of patients who received dupilumab in the JADE COMPARE trial and subsequently received abrocitinib (200 mg or 100 mg) in the long-term JADE EXTEND (NCT03422822) trial showed improvements in itch (PP-NRS4) and disease severity (EASI-75) after treatment with abrocitinib regardless of prior dupilumab response status [17]. Finally, results reported in patients in a clinical trial may not be applicable to a real-world setting. Nevertheless, abrocitinib efficacy was consistent across all subgroups of severe and/or difficult-to-treat AD.
Conclusions
Abrocitinib 200 mg provided rapid and substantially greater improvements in itch, skin clearance, and quality of life compared with placebo and dupilumab in patients with severe and/or difficult-to-treat AD, including those who had failed or were intolerant to previous systemic therapy. These findings support the use of abrocitinib for the treatment of severe and/or difficult-to-treat AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Editorial/medical writing support under the guidance of authors was provided by Renata Cunha, PharmD, at ApotheCom, San Francisco, CA, USA, and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022; 10.7326/M22-1460).
Declarations
Funding
This study and the Open Access fee were sponsored by Pfizer Inc.
Conflicts of interest
ELS received grants from Pfizer Inc., Eli Lilly and Company, Kyowa Kirin, LEO Pharma, Merck, and Regeneron and personal fees from Pfizer Inc., Bausch Health (Valeant), Dermira, Eli Lilly and Company, Galderma, LEO Pharma, Menlo Therapeutics, Novartis, Regeneron, and Sanofi Genzyme. JIS served as an investigator for Celgene, Eli Lilly and Company, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, Regeneron, and Sanofi; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly and Company, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron, and Sanofi Genzyme; and as a speaker for Regeneron and Sanofi-Genzyme. JPT is an advisor for Pfizer Inc., AbbVie, Almirall, Arena Pharmaceuticals, Aslan Pharmaceuticals, Coloplast, Eli Lilly and Company, LEO Pharma, OM Pharma, Regeneron, Sanofi-Genzyme, and Union Therapeutics; a speaker for Pfizer Inc., AbbVie, Almirall, Eli Lilly and Company, LEO Pharma, Regeneron, and Sanofi-Genzyme; and has received research grants from Pfizer Inc., Regeneron, and Sanofi-Genzyme. MV is an investigator, board member, and consultant for AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly and Company, Janssen, Medac, Novartis, and Sanofi. DT is a consultant, advisory board member, and investigator for Pfizer Inc., AbbVie, Almirall, Amgen, Beiersdorf, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly and Company, Janssen-Cilag, LEO Pharma, Novartis, Regeneron, Samsung, Sandoz, Sanofi, Sun Pharma, and UCB Pharma. MdBW is a consultant, advisory board member, and/or speaker for Pfizer, AbbVie, Almirall, Arena, Aslan, Eli Lilly and Company, Galderma, Janssen, LEO Pharma, Regeneron, and Sanofi Genzyme. SW has received institutional research grants from La Roche-Posay, LEO Pharma, and Sanofi Deutschland GmbH; has performed consultancies for Pfizer Inc., AbbVie, Eli Lilly and Company, Kymab, LEO Pharma, Novartis, Regeneron, and Sanofi Genzyme; has lectured at educational events sponsored by AbbVie, Galderma, LEO Pharma, Novartis, Regeneron, and Sanofi Genzyme; and is involved in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis and atopic dermatitis. GC, MD, and PB are employees and shareholders of Pfizer Inc. CF is an employee and shareholder of Pfizer Ltd. CK is an employee of Pfizer Hellas S.A. MJC has been a clinical trial investigator for Pfizer Inc., Atopix, Galapagos, Hyphens, Johnson & Johnson, Kymab, LEO Pharma, L’Oreal/La Roche-Posay, Novartis, Regeneron, and Sanofi Genzyme and an advisory board member, consultant, and/or invited lecturer for Pfizer Inc., AbbVie, Amlar, Astellas, Atopix, Boots, Dermavant, Galapagos, Galderma, Hyphens, Johnson & Johnson, Kymab, LEO Pharma, L’Oreal/La Roche-Posay, Menlo Therapeutics, Novartis, Oxagen, Procter & Gamble, Reckitt Benckiser, Regeneron, and Sanofi-Genzyme.
Ethics Approval
All study documents and procedures were approved by the appropriate institutional review boards/ethics committees at each study site. The studies were conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Council for Harmonization Good Clinical Practice Guidelines. All local regulatory requirements were followed. This research was approved by institutional review boards or ethics committees at each study site. An internal review committee monitored the safety of patients throughout the studies.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Not applicable.
Data and/or Code Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Author Contributions
GC, PB, and CK contributed to the study concept and design. PB conducted the statistical analysis of the data. ELS, JIS, JPT, MV, DT MBW, SW, GC, MD, PB, CF, CK, and MJC interpreted the data, provided critical feedback on the manuscript, approved the final manuscript for submission, and are accountable for the accuracy and integrity of the manuscript.
References
- 1.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, et al. Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy. 2018;73(6):1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 2.Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. Euroguiderm guideline on atopic eczema. 2022. https://guidelines.edf.one/uploads/attachments/cl9nz0zci00m9l7jn5ayr281q-0-atopic-eczema-gl-full-version-oct-2022.pdf. Accessed 28 Oct 2022.
- 3.Gooderham MJ, Forman SB, Bissonnette R, Beebe JS, Zhang W, Banfield C, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155(12):1371–1379. doi: 10.1001/jamadermatol.2019.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/S0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bieber T, Simpson EL, Silverberg JI, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384(12):1101–1112. doi: 10.1056/NEJMoa2019380. [DOI] [PubMed] [Google Scholar]
- 7.Pfizer Inc. CIBINQO (abrocitinib) tablets, for oral use [prescribing information]. 2022. Pfizer Inc.,: New York; 2022.
- 8.Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi: 10.3389/fimmu.2019.02342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira S, Guttman-Yassky E, Torres T. Selective JAK1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol. 2020;21(6):783–798. doi: 10.1007/s40257-020-00548-6. [DOI] [PubMed] [Google Scholar]
- 10.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 11.Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. 2022;400(10348):273–282. doi: 10.1016/S0140-6736(22)01199-0. [DOI] [PubMed] [Google Scholar]
- 12.Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157(9):1047–1055. doi: 10.1001/jamadermatol.2021.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section II: tools for assessing the severity of atopic dermatitis. J Cutan Med Surg. 2018;22(1):10S–16S. doi: 10.1177/1203475418803628. [DOI] [PubMed] [Google Scholar]
- 14.Ariëns LFM, van der Schaft J, Spekhorst LS, Bakker DS, Romeijn GLE, Kouwenhoven TA, et al. Dupilumab shows long-term effectiveness in a large cohort of treatment-refractory atopic dermatitis patients in daily practice: 52-week results from the Dutch BioDay registry. J Am Acad Dermatol. 2021;84(4):1000–1009. doi: 10.1016/j.jaad.2020.08.127. [DOI] [PubMed] [Google Scholar]
- 15.Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1(2):142–151. doi: 10.1016/j.jaip.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg JI, Boguniewicz M, Waibel J, Weisman J, Strowd L, Sun L, et al. Clinical tailoring of baricitinib 2 mg in atopic dermatitis: baseline body surface area and rapid onset of action identifies response at week 16. Dermatol Ther (Heidelb). 2022;12(1):137–148. doi: 10.1007/s13555-021-00640-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi VY, Bhutani T, Fonacier L, Deleuran M, Shumack S, Valdez H, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND) J Am Acad Dermatol. 2022;87(2):351–358. doi: 10.1016/j.jaad.2022.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.