Abstract
The oncogene ERBB2 encoding the receptor tyrosine-protein kinase erbB-2 (HER2) is frequently overexpressed or amplified and occasionally mutated in a variety of human cancers. The early discovery of this oncogene, its established oncogenic relevance in diverse cancers, its substantial expression on surface of cancer cells, and its druggable catalytic activity have made it one of the most pursued targets in the history of cancer drug development. Initiatives targeting HER2 provided the early stimulus for several transformational pharmaceutical technologies including monoclonal antibodies, tyrosine kinase inhibitors, antibody-drug conjugates, and others. The seismic impact of these efforts has been felt in treatment of many cancers including breast, gastroesophageal, lung, colorectal and others. This impact continues to broaden with increasing indications on the horizon and a plethora of novel agents in development. However, implementation of these therapeutic strategies has been complex. The clinical translation of every one of these classes of agents has been notable for underperformance or overperformance characteristics that have informed new lines of research providing deeper insights into the mechanistic complexities and unrealized opportunities provided by this molecular target. Despite all the successes to date, the preponderance of scientific evidence indicates that the full potential of HER2 as a target for cancer therapeutics is far greater than currently realized and numerous lines of investigation are ongoing to deepen and broaden the scope of impact of HER2 as a signaling, homing, or immunologic target. In this review, we explore the existing data and evolving paradigms surrounding this remarkable target for cancer therapy.
Keywords: Amplification, Antibody drug conjugate, ERBB2, HER2, Targeted therapy
Introduction
ERBB2, commonly referred to as Human Epidermal Growth Factor Receptor-2 (HER2), is a proto-oncogene (chromosome 17q21) that encodes a transmembrane receptor tyrosine kinase involved in cell growth and differentiation (Figure 1). It was one of the first genes identified in the 1980’s efforts to discover oncogenes and was quickly shown to have relevance to diverse human cancers 1,2. What followed was an explosion of scientific inquiries that validated the role of HER2 in carcinogenesis and led to development of a variety of targeting approaches which have paved the way in transforming drug development. The oncogenic potential of HER2 was evident in the early days of mouse genetic engineering with several models of HER2-driven mammary tumorigenesis showing an aggressive and metastatic disease biology 3. As more elegant inducible mouse genetic models were developed, HER2 was shown to be continuously required throughout the entire disease process from initiation through advanced metastatic disease, nicely illustrating the concept of oncogene addiction and the potential of this oncogene as a target for cancer therapeutics 4. Advances in sequencing technologies brought about the era of widespread tumor genome sequencing with findings that extended the scope of HER2 amplification to include substantial subsets of gastric and esophageal cancers as well as smaller subsets of colon, bladder, endometrial, lung, salivary gland, and other cancers (Figure 2) 5-7. These surveys also discovered much smaller subsets of cancers harboring activating HER2 mutations without amplification 8. Whether these HER2 mutants are dominant tumor-drivers like HER2 amplifications, remains to be determined.
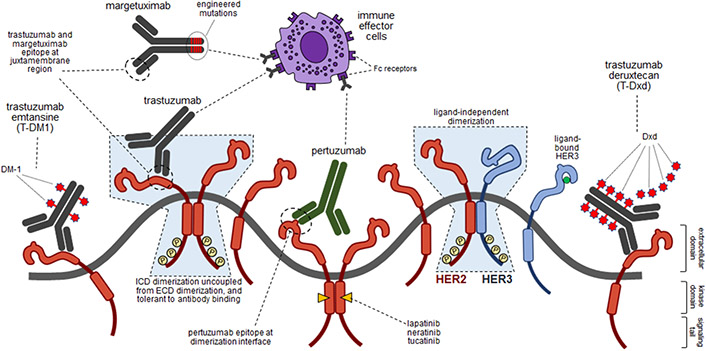
Figure 1. HER2 pathway and pharmacologic strategies.
Schematic showing the structures and events occurring in HER2-amplified cancers and the site of binding of various HER2-targeted therapies. HER2 is shown in red and HER3 is shown in blue. HER3 is shown in the activated ligand-bound state as well as the inactive unbound state. HER2 only has one conformation always poised for dimerization. The shaded light blue enclosures highlight aspects of dimerization that are non-physiologic but occur in cancers propelled by the massive expression of HER2, reflecting the uncoupling of kinase domain dimerization and signaling from the ECDs and tolerance to antibody binding.
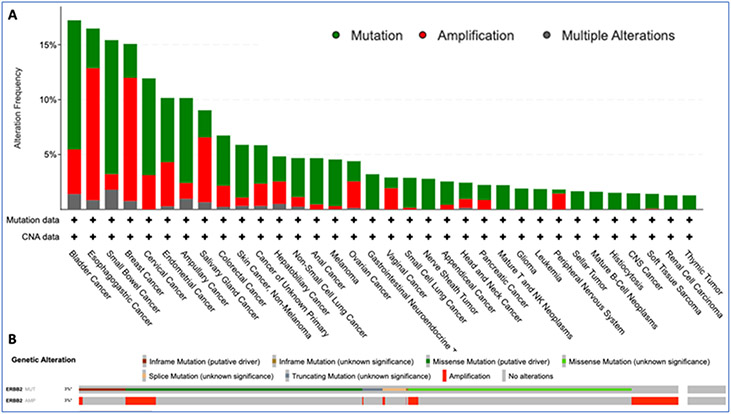
Figure 2. Pan-cancer prevalence of HER2 alterations (mutations and amplifications).
Panel A shows ERBB2 alterations seen in 6% of 85,575 patients with diverse cancers as per the cancer genomic data aggregated through AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE) effort (AACR Genie v11.0-public). Only tumor types with ≥ 10 cases and at least 1% prevalence were included. Panel B shows the oncoprint in the same cohort and highlights the type of mutations seen in these patients with limited overlap of mutations and amplifications.
The technology to generate monoclonal antibodies and to humanize them for repeated administration as therapeutic agents evolved in the late 1980s and HER2 was one of the first targets explored by this novel pharmaceutical technology 9. Trastuzumab, the anti-HER2 monoclonal antibody (mAb), entered clinical phase in 1992, eventually leading to a pivotal phase 3 study in HER2-amplified metastatic breast cancer, and landmark studies in HER2-amplified early-stage breast cancers, paving the way and establishing the role of mAbs in treatment of human cancers 10-13. As understanding of structure and function among the HER family receptors evolved in 1990s, these insights were applied to development of structure-guided mAbs such as pertuzumab to disrupt HER2 dimerization more effectively with additional, albeit incremental benefits demonstrated in clinical studies 14,15. Technologies to arm these mAbs with cytotoxic agents evolved in late 1990s with improvements in linker chemistries and HER2 was among the early targets pursued leading to development of the antibody drug conjugate (ADC) aldo-trastuzumab emtansine (T-DM1) 16. While the naked HER2 mAbs, trastuzumab or pertuzumab, showed only limited efficacies and their clinical benefits were mainly manifest in combination regimens with chemotherapeutics, T-DM1 was found to be highly efficacious in monotherapy with activities surpassing chemotherapies in treatment refractory tumors with a much more favorable toxicity profile 17. This remarkable breakthrough fueled efforts to further explore ADCs leading to development of trastuzumab deruxtecan (DS-8201 or T-Dxd) 18. Exploring T-Dxd revealed unforeseen findings of non-cross resistance with T-DM1 and much broader clinical activity in other HER2-amplified cancers and in cancers with lower expression of HER2 19-22. The high efficacy of HER2 ADCs has led to development of a plethora of ADCs, now in trials.
As technologies to develop selective tyrosine kinase inhibitors (TKIs) evolved, the HER family of kinases including EGFR and HER2 were among the first targets pursued leading to many compounds proceeding to clinical testing in HER2-amplified breast cancers. Of these the pan-HER reversible TKI lapatinib, pan-HER irreversible TKI neratinib, and HER2-selective reversible TKI tucatinib, have made it to clinical use with many others in development. TKIs have only modest activities in monotherapy of HER2-amplified cancers and their clinical use has been predominantly focused on combination therapies 23,24. In contrast, the HER2-mutated subtypes of breast and other cancers appear amenable to TKI monotherapy as shown in the basket neratinib study.25
Mechanics
We now have a good understanding of mechanisms by which HER family of receptors function. Signals are generated when two receptors from the family come together in a homo- or hetero-dimerization configuration. The receptor extracellular domains (ECD) are generally restrained by a closed conformation that is prohibitive to dimerization and it is the binding of extracellular ligands that exposes an interface that promotes dimerization 26. HER2 is unique in that it lacks this self-restraint or a physiologic ligand, and its ECD is always poised for dimerization 27. As such the only physiologic restraint built into HER2 is its low expression which is overwhelmed in cancer cells through amplification and overexpression. Dimerization leads to phosphorylation of C-terminal tails and consequent initiation of second messenger signaling pathways 28. A particularly strong relationship between HER2 and HER3 as dimerization partners is apparent from in vitro signaling assays 29. Many lines of investigation also confirm that HER3 is an important HER2 partner in HER2 overexpressing tumors 30-32. These structural insights provide ample mechanistic rationale to inactivate HER2 signaling through ECD targeting antibodies that disrupt dimerization or through TKIs that inactivate catalytic activity and eliminate phosphorylation. Yet, the story has turned out to be much more complicated.
The translational research in pursuit of mutationally activated oncogene-driven cancers such as EGFR- or ALK-mutant lung cancer among others has followed a somewhat direct path with the rational expectation that the catalytic inactivation of these disease drivers should be a highly effective treatment strategy. However, the pursuit of amplified HER2 as a target has followed a much more tortuous and unexpected path with many mechanistic complexities and ambiguities, new insights learned, and new opportunities discovered. It is evident now that in most cases HER2 is activated through massive overexpression rather than mutation and this makes for a mechanistically more challenging target, something that was not readily anticipated in 1990s. While in the early days following development of trastuzumab and pertuzumab it was thought that these mAbs could interfere with HER2 signaling by eliminating their expression or disrupting dimerization, this was clearly not the case as was evident in simple in vitro studies in HER2-amplified cancer cells 33-37. Pertuzumab was specifically developed based on a structure-based design to bind the HER2 dimerization interface and clearly does inhibit signaling in physiologic cell systems with low HER2 expression but fails to do the same in HER2-overexpressing cancer cells 35-38. Similarly, a variety of rationally designed HER2 or HER3-targeting mAbs, bispecific mAbs, DARPins, and other biotherapeutics have shown only limited ability to interfere with HER2-HER2 or HER2-HER3 signaling in HER2-amplified cancer cells. Experimental models now more clearly demonstrate the mechanistic futility of these approaches.
The kinase domain interactions in these cancers are driven entirely by massive HER2-overexpression with no promoting or restraining functions from the ECDs essentially uncoupling intracellular signaling from the ECDs (Figure 1) 37. Even strategies to add bulk to the ECDs and prevent receptor proximation leave kinase domain signaling intact due to conformational flexibilities across the span of these receptors and the curvatures in the plasma membrane (Figure 1) 37. Although mechanistic studies with trastuzumab failed to show effective inhibition of HER2 signaling, preclinical and clinical studies do show measurable anti-tumor effects 11,39. This has fueled efforts to understand the mechanistic basis for the observed in vivo effects and a large body of work now demonstrates this to have a substantial immunologic basis. This was initially shown through elegant mouse studies showing the critical role of host Fc receptor in mediating the in vivo anti-tumor activity of trastuzumab and subsequently shown to encompass a wider repertoire of immunologic activities including innate and adaptive immune functions, memory, and cytokines 40-43. Capitalizing on these insights, enhancements in the Fc portion of trastuzumab to optimize its immunologic activities has produced margetuximab with superior clinical activity compared with trastuzumab 44.
In contrast to mAbs, HER2 targeting TKIs do effectively inactivate HER2 signaling in cell-based studies in HER2-amplified cancer cells, leading to apoptotic cell death 45,46. Yet these agents fail to show substantial activities in patients as monotherapy 47,48. Mechanistic studies highlight limitations in target inactivation, in particular with regards to inactivation of HER2-HER3 heterodimer signaling. In HER2-amplified cancers HER3 is tightly linked with downstream PI3K-AKT signaling in a pathway that involves robust compensatory feedback regulation. As such, inhibition of HER2 with TKIs leads to a compensatory upregulation of HER3 unleashing a 100-fold reserve in signaling capacity that overpowers an incomplete inhibition of HER2 kinase and restores HER2-HER3 signaling 49-51. Only the complete inactivation of HER2 kinase can durably inactivate HER2-HER3 signaling in these cancers, and although this is feasible in cell culture models at higher concentrations of TKIs, it is beyond the therapeutic index of all current TKIs 50. High dosing of oral TKIs in patients has been studied but limited by a bioavailability ceiling 52. Irreversible TKIs, such as neratinib, that covalently bind their target have much higher molar potency, but this comes at a cost of substantial off-target activities, within and outside of the kinome, significantly limiting therapeutic index 53-56. A rational strategy for increasing potency would be co-targeting HER2 and HER3, but HER3 is a challenging target for pharmaceutical inactivation. Its function in HER2-amplified cancers is engaged in a ligand and ECD-independent manner limiting the efficacy of ECD-targeting mAbs in this disease, and its kinase domain functions in allostery, not catalysis, and conventional TKIs binding within its ATP pocket have no effects on its signaling function 37,57. Novel approaches to target HER3 through degradation are being pursued and may provide breakthroughs in this arena 58,59.
Although the massive overexpression of HER2 makes it a challenging target for inactivation, its surface expression provides significant opportunities for targeted delivery of cytotoxic molecules, radioisotopes, liposomes, nucleic acids, and other moieties designed to kill cancer cells. In this regard the pursuit of HER2-targeting ADCs has proven particularly fruitful. In contrast to HER2-targeting mAbs and TKIs which underperformed expectations as oncogene inhibitors when they entered the clinical arena, HER2 ADCs have overperformed expectations, and their success has spawned many lines of study to better understand the mechanistic basis for their activities and fueled substantial investments in the pharmaceutical sector with many new agents in development. The science underlying exact mechanisms of activity of ADCs continues to evolve. The key variables appear to be the linker chemistry, the activity, cell permeability and potency of the cytotoxic payload molecule, the payload-to-antibody ratio, and the characteristics of the mAb, all of which contribute to the observed clinical activities of these agents 60,61. Although the initial and purest vision for the development of ADCs was for the most precise and protected delivery of cytotoxic molecules intracellularly to cancer cells, it is now apparent that reducing the stringency of this vision can potentially increase, not decrease, their therapeutic index. Using cleavable linkers that more readily release their payloads or cell permeable cytotoxic agents that diffuse out of cells can lead to exposure beyond the target cells including non-targeted surrounding cells, a so-called bystander effect, and a measurable low exposure in the systemic circulation (Figure 1) 62. Clinical exploration of the cleavable HER2-targeting ADC, T-Dxd, has revealed an unexpected range of activity. The highest efficacy is still seen in patients with classic “HER2-high” (HER2-overexpressing/amplified) subtypes of cancers, consistent with the original and simplest concept of ADCs as agents for precise tumor-targeted cytotoxic delivery 19-21. However, pursuing an assumption that cancers with lower expression of HER2 may also afford a therapeutic index by mechanisms involving heterogenous expression and bystander effects, significant clinical efficacy was also observed in breast cancers with “HER2-low” expression (IHC 1+ or 2+) 22. Further exploration has shown no lower cutoff for this quantitative biomarker with efficacy spanning the entire spectrum from 0 - 2+ expression and across the hormone receptor status in breast cancer 19,63. This wide range of activity is also seen in HER2-mutant lung cancers wherein, there is no correlation of activity with tumor HER2 expression 64. The observed broad and marker-independent clinical activities of T-Dxd have upended the mechanistic hypotheses regarding the mode of action of ADCs. Tumor-specific targeted delivery of cytotoxic payloads related to high expressing and bystander effect in lower expressing cancers can only partially account for this observed broad range of activity and the mechanistic basis remains to be determined. Traditionally, mechanism of action forms the starting point for development of rationally designed therapeutics. But clinical development of T-Dxd and similar ADCs has clearly exited this mechanistic orbit and their clinical exploration continues in an open space until a ceiling is encountered, while mechanistic studies will have to follow to fill this ever-enlarging knowledge gap.
The newly discovered broad clinical activity is not limited to T-Dxd and appears to be a class effect. Preliminary evidence from other HER2-targeting ADCs appears to show similar broad range of efficacies 65-67. This property of ADCs is also not unique to the target HER2 and appears to apply to other targets as well, as is evident with the experience with Trop-2 targeting ADCs. While, initially explored in triple-negative breast cancer which has the highest expression of Trop-2, its efficacy has poor correlation with Trop-2 expression levels and shows similar clinical activity in hormone receptor positive breast cancers with lower expression of Trop-2 68-71. A plethora of other ADCs in the investigational pipelines exploring other types of linkers, payloads, release properties, and antibodies and drug-antibody ratios will further expand the body of data available and allow us to formulate more informed hypotheses regarding potential mechanisms of action. Although initial pharmacokinetic studies of T-Dxd showed low levels of free deruxtecan in circulation, the role of systemic release products must be revisited 72,73. Much of the mechanistic foundation for the broad clinical activities of these ADCs remains unknown and eagerly awaits new hypotheses and experimental studies.
Distinct from cancers driven by amplification and overexpression of HER2, there are also rarer cancers that harbor somatic mutations in HER2 74,75. Most, but not all, are within the kinase domain and generally result in increased catalytic activity of the HER2 kinase. Individual mutations appear to have different characteristics with respect to catalytic activities and partner preferences and exhibit cell-context dependent characteristics 74. Most experimental studies have used overexpression systems and in these artificial systems many are more potent oncogenes compared with wildtype HER2. But in human cancers they are typically mutated in copy-neutral fashion without overexpression and whether they are primary disease drivers in human cancers remains speculative. The irreversible TKI neratinib has modest but short-lived activity in HER2-mutant cancers, confirming a limited biologic role for at least some of these mutants in the human disease. The diverse nature of these HER2 mutants makes for a complex arena for analysis and it remains difficult to know whether non-responsive mutants are not biologically relevant or whether they harbor intrinsic resistance to TKIs. There is much more to be learned about the biology of HER2 mutant cancer.
Distinct from HER2-amplified and HER2-mutant cancers are exceedingly rare cancers driven by ligand activation of HER2. These appear to be driven by genetic fusions of the NRG1 gene leading to neuregulin fusion proteins expressed at the plasma membrane 76,77. Anecdotal reports of these rare cases suggest that these tumors are responsive to HER2 TKIs or HER2 or HER3 targeting mAbs that block dimerization or ligand binding 78,79.
Clinical Implications
Breast Cancer
The field of HER2 amplified breast cancer has benefited greatly from decades of translational cancer research through the development of numerous targeted therapies that have made substantial impact in the clinical realm. Addition of trastuzumab and pertuzumab to chemotherapy significantly improves outcomes in patients with metastatic or early-stage breast cancer and is now the standard of care for the treatment of HER2 amplified breast cancer at all stages of disease 11-13,15,80. T-DM1 has shown significant clinical activity in trastuzumab-resistant disease and has become a standard second-line salvage option in patients with metastatic disease or in early stage patients who have significant residual disease following neo-adjuvant therapy 17,81. The second generation ADC, T-Dxd, has shown clinical activity superior to T-DM1 and activity in disease that is resistant to T-DM1 82. As discussed previously, T-Dxd has a much broader range of clinical activity that encompasses HER2-negative breast cancers, including cancers traditionally labeled HER2-low 22,63. This broad range of activity appears to somewhat uncouple T-Dxd from its target biomarker and it may be best considered a broadly active breast cancer agent without a biomarker association, exhibiting clinical activity in breast cancers spanning HER2 positive and negative and hormone receptor positive and negative subtypes, albeit with superior activity against HER2-amplified cancers. This has sparked substantial interest and numerous other investigational HER2 targeting ADCs are exploring this newly discovered terrain 83.
The HER2 TKIs lapatinib or tucatinib in combination with capecitabine are active regimens for the treatment of metastatic HER2 amplified breast cancers refractory to prior HER2 therapy, providing additional options for later lines of therapy 23,24. The combination of lapatinib and trastuzumab has clinical activity that provides a non-chemo option for patients with low disease burden, indolent disease, or not suitable candidates for chemotherapy 84.
The HER2-amplified subtype of breast cancer has a higher predilection for brain metastases and numerous studies have attempted to define the activities of HER2-targeted therapies in the treatment of brain metastases 85. Small molecule HER2 TKIs have better CNS penetration than mAbs and these and have been actively pursued for CNS activity. The CNS activity with lapatinib is minimal, but more significant activity is evident with neratinib in the treatment of brain metastases 86. The highest CNS activity is seen with the brain-penetrant TKI tucatinib and the tucatinib, capecitabine, trastuzumab combination has become a standard in the management of patients with brain metastases 87. Although the activity of ADCs in the CNS compartment were thought to be restricted by the large size of their mAb component, hints from subset analyses of their clinical studies suggested these agents may have activity in the CNS and this was followed by specific clinical studies showing modest activity with T-DM1 88-90 and substantial intracranial activity with T-Dxd 91,92.
Gastroesophageal Cancer
HER2-overexpression/amplification, to date, remains the only clinically usable biomarker for selection of targeted therapy in metastatic gastroesophageal adenocarcinoma (GEC) and is seen in about 12%-20% of cases (Figure 2) 6,7,93. HER2-amplification is seen in a greater proportion of patients with liver metastasis, GEJ adenocarcinomas and intestinal subtype tumors, although its prognostic significance is unclear 93,94. Compared to circumferential staining seen in breast cancer, HER2 expression in GEC is predominantly basolateral/lateral and has more notable intratumoral heterogeneity, making testing (ToGA criteria) and patient selection, more challenging 93,94.
Anti-HER2 therapy is mainstay of therapy for advanced HER2-overexpressed/amplified GEC. Addition of trastuzumab to chemotherapy for first-line treatment of HER2-positive advanced GEC demonstrated a clear survival benefit over chemotherapy alone 94. However further efforts, as in the case of breast cancer, such as addition of dual anti-HER2 therapy with pertuzumab and T-DM1, showed limited activity and failed to significantly improve overall survival in patients with HER2-positive metastatic GEC 95,96. More recently, addition of pembrolizumab to trastuzumab and chemotherapy significantly improved objective response rate (74% vs. 52%) highlighting the immune interactions of the HER2 pathway 97. T-Dxd has also shown significant activity in treatment of HER2-positive metastatic GEC after progression of first line anti-HER2 therapy with a significantly higher response rate (51%) compared to physician's choice of chemotherapy (14%) and longer overall survival (median: 12.5 vs. 8.4 months) 21.
Lung Cancer
Unlike breast and gastric cancer, HER2 amplification are rare in lung cancer (0.88% cases) and HER2 mutations are the driver events in 3.5% cases, comprising 80% of all HER2 alterations (Figure 2) 6,7. These HER2 mutations, specifically exon 20 mutations are analogous to in-frame EGFR deletions and dysregulate the HER2 pathway due to constitutive tyrosine kinase activity, are enriched in non-smokers, adenocarcinomas, and are mutually exclusive of other oncogenic mutations in NSCLC 98. HER2 mutations, barring amplifications and overexpression, appear to be key determinant of response to HER2 targeted therapies. No clinical benefit was observed with addition of trastuzumab to chemotherapy in HER2-overexpressed/amplified non-small-cell lung cancer (NSCLC) 99. This aspect of aberrant HER2 pathway is unique to lung cancer. Although, HER2 kinase domain mutations are also seen in other tumor types, such as breast (4.3%), gastric (5.0%), and colorectal (2.9%) carcinomas, they are often missense mutations (not deletions/insertions) and involve exons other than exon 20, indicating tissue dependent oncogenic mechanisms 8. Additionally, HER2 mutation are not associated with HER2 overexpression/amplification 100.
HER2-mutant lung cancer responds to HER2 inhibitors, especially small-molecule TKIs and ADCs 101. Although, selective HER2 TKIs have yielded response rates of 20-35% in single-arm phase 2 studies, many of these have shown limited overall clinical benefit. Recent evidence has brought HER2 ADCs to the forefront in treatment of HER2-mutant NSCLC. T-DM1 demonstrated a response rate of 44% (N = 18) in treatment refractory HER2-mutant lung cancer, across a variety of HER2 exon 20 insertions and point mutations (in kinase, transmembrane, and extracellular domains) and regardless of HER2 expression or amplification status 102. Similarly, T-Dxd showed a confirmed objective response in 55% patients (N = 91) with refractory metastatic HER2-mutant NSCLC, again notwithstanding HER2 expression or amplification 103. Currently there is no mechanistic paradigm to account for this activity of ADCs and this is an active area of pursuit as discussed previously.
Colorectal Cancer
HER2 overexpression/amplification is seen in 2-3% of all colorectal cancers (CRC) and represents a very distinct subset of CRC (Figure 2) 6,7. Pre-clinical and retrospective clinical evidence shows that HER2 amplification is enriched in RAS/BRAF wild-type tumors (5-6%) compared to RAS-mutant CRC (1-2%) 104,105. In RAS//BRAF wild-type mCRC, HER2 amplification appears to be a negative predictive biomarker of response to the anti-EGFR monoclonal antibody based therapy, which is the current standard of care in these patients with metastatic CRC 105. Although a diverse array of HER2 somatic mutations are seen in a very small subset of CRC, only a small number appear to have oncogenic potential and show response to irreversible TKIs (such as neratinib and afatinib), and as such they have not been exploited clinically 106.
As opposed to gastric cancer, single agent anti-HER2 therapy shows limited efficacy in HER2-amplified metastatic CRC 104,107. However, dual-HER2 targeting with trastuzumab combined with lapatinib, pertuzumab and tucatinib has shown robust activity in treatment refractory metastatic CRC with response rates ranging from 28-40% in RAS wild-type tumors 107-110. Notably, dual anti-HER2 therapy benefits only patients with RAS wild-type tumors (response rate of 40% vs. 8% for RAS-mutant cases) 109. Along similar lines although the HER2 ADC, T-DM1 showed very limited activity (response rate 9.7% combined with pertuzumab) in HER2-amplified metastatic CRC, trastuzumab deruxtecan (DS-8201) showed very promising and durable activity in HER2-amplified metastatic CRC refractory to standard treatment with response rate of 45%, including those who had received prior anti-HER2 therapies 20,111.
Other Solid Tumors and HER2-mutant cancers
In addition to tumor types mentioned above, HER2 alterations can be seen in a variety of tumor types such as salivary gland tumors (particularly non-adenoid cystic carcinoma, non-secretory type), hepatobiliary tumors (principally gallbladder cancer) and others (Figure 2). Dual HER2 targeting with trastuzumab and pertuzumab showed a response rate of 23% (N = 39) in HER2-amplified metastatic biliary tract cancer 112. A phase 2 trial of trastuzumab and docetaxel in HER2-amplified salivary duct carcinoma (N = 57) showed an overall response rate of 70.2% and is the preferred treatment option for these rare cancers 113. Basket trials of HER2 targeted therapies to engage tumor types with lower prevalence of HER2 alterations are ongoing.
Numerous basket studies and disease-specific phase 2 studies of TKIs have been conducted in cancers harboring somatic mutation of HER2 (Figure 2) 6,7. As a class, TKIs do have activity, albeit modest, in these cancers although there is variation in activity according to disease type, specific mutations, and specific drug. The irreversible class of TKIs have clinically meaningful activity and neratinib has been studied the most with modest activity in breast and lung cancers but evidence of activity in other cancers 25,101,114-117. Pyrotinib and poziotinib also have similar efficacies while afatinib appears to lack efficacy 118-120.
Future Directions
The field of HER2 targeting has been an archetype for pan-cancer developmental therapeutics with promising clinical results, continuously evolving new mechanistic insights, profound translational efforts, and tremendous hope for patients suffering with cancer. While we have achieved unprecedented success, a substantial subset of patients derive limited benefits from current approaches and resistance develops eventually. Through a barrage of ongoing clinical trials (Table 1), three major themes are evolving: 1) therapeutics strategies involving combining anti-HER2 therapies with immunotherapy, inhibitors of DNA damage pathway, receptor cycling modulators among others which may work synergistically to increase the responses, 2) targeting a large subset of patients who have tumors that were traditionally considered HER2-low and not amenable to past generation anti-HER2 therapies, and 3) immune targeting of HER2 using novel immunotherapy approaches, such as CAR-Tcells, and vaccines. No matter the strategy, one thing is for sure, HER2 targeting remains the epitome of precision cancer medicine in oncology.
Table 1.
Key contemporary clinical trials exemplifying the landscape of HER2 targeting strategies in solid tumors
| NCT Number | Title of Study | Cancer Type | Drug | Mechanism of Action |
Ph. | Sample Size |
|---|---|---|---|---|---|---|
| NCT04941339 | A Study of MRG002 in the Treatment of Patients with HER2-positive Advanced Solid Tumors | HER2+ Advanced Solid Tumor | MRG002 | ADC | 1 | 74 |
| NCT03944499 | Phase 1 Study of FS-1502 in Patients with HER2 Expressed Advanced Solid Tumors and Breast Cancer. | HER2+ Advanced Solid Tumor | FS-1502 | ADC | 1 | 92 |
| NCT04513223 | A Phase 1 Study of SHR-A1811 in Patients With Selected HER2 Expressing Tumors | HER2+ Advanced Solid Tumor | SHR-A1811 | ADC | 1 | 114 |
| NCT05311397 | A Study of A166 in Patients With Advanced Solid Malignant Tumors | HER2+ Advanced Solid Tumor | A166 | ADC | 1 | 120 |
| NCT03821233 | A Dose Finding Study of ZW49 in Patients With HER2-Positive Cancers | HER2+ Advanced Solid Tumor | ZW49 | ADC | 1 | 174 |
| NCT03255070 | A Dose-escalation, Expansion Study of ARX788, in Advanced Solid Tumors Subjects With HER2 Expression (ACE-Pan Tumor 01) | HER2+ Advanced Solid Tumor | ARX788 | ADC | 1 | 190 |
| NCT04257110 | A First-in-human Study of Multiple Doses of BB-1701 in Subjects With Locally Advanced/Metastatic HER2 Expressing Solid Tumors | HER2+ Advanced Solid Tumor | BB-1701 | ADC | 1 | 208 |
| NCT05018676 | ARX788 in Breast Cancer With Low Expression of HER2 | HER2-Low Breast Cancer | ARX788 | ADC | 2 | 54 |
| NCT02675829 | Trial of Ado-Trastuzumab Emtansine for Patients With HER2 Amplified or Mutant Cancers | HER2+/MUT Advanced Solid Tumors | Trastuzumab emtansine | ADC | 2 | 135 |
| NCT04829604 | ARX788 in HER2-positive, Metastatic Breast Cancer Subjects (ACE-Breast-03) | HER2+ Breast Cancer | ARX788 | ADC | 2 | 210 |
| NCT04714190 | A Study of RC48-ADC in Local Advanced or Metastatic Gastric Cancer With the HER2-Overexpression | HER2+ GEC | RC-48 ADC | ADC | 3 | 351 |
| NCT04400695 | A Study of RC48-ADC for the Treatment of Locally Advanced or Metastatic Breast Cancer With Low Expression of HER2 | HER2-Low Breast Cancer | RC-48 ADC | ADC | 3 | 366 |
| NCT04704934 | Trastuzumab Deruxtecan for Subjects With HER2-Positive Gastric Cancer or Gastro-Esophageal Junction Adenocarcinoma After Progression on or After a Trastuzumab-Containing Regimen (DESTINY-Gastric04) | HER2+ GEC | Trastuzumab deruxtecan | ADC | 3 | 490 |
| NCT04494425 | Study of Trastuzumab Deruxtecan (T-DXd) vs Investigator's Choice Chemotherapy in HER2-low, Hormone Receptor Positive, Metastatic Breast Cancer | HER2-Low Breast Cancer | Trastuzumab deruxtecan | ADC | 3 | 850 |
| NCT04622319 | A Study of Trastuzumab Deruxtecan (T-DXd) Versus Trastuzumab Emtansine (T-DM1) in High-risk HER2-positive Participants With Residual Invasive Breast Cancer Following Neoadjuvant Therapy (DESTINY-Breast05) | HER2+ Breast Cancer | Trastuzumab deruxtecan | ADC | 3 | 1600 |
| NCT04704661 | Testing the Combination of Two Anti-cancer Drugs, DS-8201a and AZD6738, for The Treatment of Patients With Advanced Solid Tumors Expressing the HER2 Protein or Gene, The DASH Trial | HER2+ Advanced Solid Tumor | DS8201 + AZD6738 | ADC + ATRi | 1 | 39 |
| NCT04042701 | DS8201a and Pembrolizumab in Participants With Locally Advanced/Metastatic Breast or Non-Small Cell Lung Cancer | HER2+ Breast Cancer/NSCLC | DS8201 + Pembrolizumab | ADC + IO | 1 | 115 |
| NCT04585958 | Testing the Combination of DS-8201a and Olaparib in HER2-Expressing Cancers With Expansion in Patients With Endometrial Cancer | HER2+ Advanced Solid Tumor | DS8201 + Olaparib | ADC + PARPi | 1 | 36 |
| NCT03272334 | Her2-BATS and Pembrolizumab in Metastatic Breast Cancer | HER2+ Breast Cancer | Her2-BATS | BATS (IO) | 1 | 33 |
| NCT03842085 | Phase I Clinical Study of MBS301 in Treatment of HER2 Positive Recurrent or Metastatic Malignant Solid Tumor | HER2+ Advanced Solid Tumor | MBS301 | Bispecific Ab | 1 | 34 |
| NCT04162327 | A Phase Ia/Ib Study of IBI315 in Patients With HER2-expressing Advanced Solid Tumor | HER2+ Advanced Solid Tumor | IBI315 | Bispecific Ab | 1 | 191 |
| NCT03448042 | A Study of Runimotamab in Participants With Locally Advanced or Metastatic HER2-Expressing Cancers | HER2+ Advanced Solid Tumor | Runimotamab | Bispecific Ab | 1 | 521 |
| NCT05152147 | A Study of Zanidatamab in Combination With Chemotherapy Plus or Minus Tislelizumab in Patients With HER2-positive Advanced or Metastatic Gastric and Esophageal Cancers | HER2+ GEC | Zanidatamab (ZW25) | Bispecific Ab | 3 | 714 |
| NCT04224272 | A Study of ZW25 (Zanidatamab) With Palbociclib Plus Fulvestrant in Patients With HER2+/HR+ Advanced Breast Cancer | HER2+/HR+ Breast Cancer | Zanidatamab (ZW25) + Palbociclib | Bispecific Ab + CDKi | 2 | 86 |
| NCT03929666 | A Safety and Efficacy Study of ZW25 (Zanidatamab) Plus Combination Chemotherapy in HER2-expressing Gastrointestinal Cancers, Including Gastroesophageal Adenocarcinoma, Biliary Tract Cancer, and Colorectal Cancer | HER2+ GI Cancers | Zanidatamab (ZW25) + Chemo | Bispecific Ab + Chemo | 2 | 362 |
| NCT04040699 | KN026 Combined With KN046 in Subjects With HER2 Positive Solid Tumor | HER2+ Advanced Solid Tumor | KN026 + KN046 | Bispecific Ab + IO | 1 | 24 |
| NCT04660929 | CAR-macrophages for the Treatment of HER2 Overexpressing Solid Tumors | HER2+ Advanced Solid Tumor | CAR-Macrophage | CAR-Macrophage | 1 | 18 |
| NCT04511871 | A Phase I Trial of CCT303-406 in Patients With Relapsed or Refractory HER2 Positive Solid Tumors | HER2+ Advanced Solid Tumor | CCT303-406 | CAR-Tcells | 1 | 15 |
| NCT05325632 | Study of HER2 Directed Dendritic Cell (DC1) Vaccine + Weekly Paclitaxel, Trastuzumab & Pertuzumab | HER2+ Breast Cancer | DC1 | DCV | 2 | 34 |
| NCT04029922 | Study of MT-5111 in HER2-positive Solid Tumors | HER2+ Advanced Solid Tumor | MT-5111 | ETB | 1 | 178 |
| NCT04278144 | A First-in-human Study Using BDC-1001 as a Single Agent and in Combination With Nivolumab in Advanced HER2-Expressing Solid Tumors | HER2+ Advanced Solid Tumor | BDC-1001 | ISAC | 1 | 390 |
| NCT04908813 | Study of HLX22 in Combination With Trastuzumab and Chemotherapy Versus Placebo in Combination With Trastuzumab and Chemotherapy for Treatment of Locally Advanced or Metastatic Gastric Cancer | HER2+ GEC | HLX22 | ISAC | 2 | 150 |
| NCT04319757 | ACE1702 in Subjects With Advanced or Metastatic HER2-expressing Solid Tumors | HER2+ Advanced Solid Tumor | ACE1702 | NK-cell | 1 | 36 |
| NCT04147819 | A First in Human Study of BAY2701439 to Look at Safety, How the Body Absorbs, Distributes and Excretes the Drug, and How Well the Drug Works in Participants With Advanced Cancer Expressing the HER2 Protein | HER2+ Advanced Solid Tumor | BAY2701439 | Radionuclide | 1 | 213 |
| NCT04982926 | A Study of TAS2940 in Participants With Locally Advanced or Metastatic Solid Tumor Cancer | HER2+ Advanced Solid Tumor | TAS2940 | RTKI | 1 | 42 |
| NCT05315700 | Study of ORIC-114 in Patients With Advanced Solid Tumors Harboring an EGFR or HER2 Alteration | HER2+ Advanced Solid Tumor | ORIC-114 | RTKI | 1 | 42 |
| NCT04487236 | Trial of ZN-A-1041 Enteric Capsules in Patients With HER2-Positive Advanced Solid Tumors | HER2+ Advanced Solid Tumor | ZN-A-1041 | RTKI | 1 | 84 |
| NCT04886804 | A Study to Test Different Doses of BI 1810631 in People With Different Types of Advanced Cancer (Solid Tumours With Changes in the HER2 Gene) | HER2+ Advanced Solid Tumor | BI 1810631 | RTKI | 1 | 96 |
| NCT05245058 | SPH5030 Tablets in Subjects With Advanced Her2-positive Solid Tumors | HER2+ Advanced Solid Tumor | SPH5030 | RTKI | 1 | 105 |
| NCT04447118 | Phase 3 Study of Pyrotinib Versus Docetaxel in Patients With Advanced Non-squamous NSCLC Harboring a HER2 Exon 20 Mutation Who Failed Platinum Based Chemotherapy | HER2-MUT NSCLC | Pyrotinib | RTKI | 3 | 150 |
| NCT04539938 | A Study of Tucatinib Plus Trastuzumab Deruxtecan in HER2+ Breast Cancer | HER2+ Breast Cancer | Tucatinib + Trastuzumab deruxtecan | RTKI + ADC | 2 | 70 |
| NCT03975647 | A Study of Tucatinib vs. Placebo in Combination With Ado-trastuzumab Emtansine (T-DM1) for Patients With Advanced or Metastatic HER2+ Breast Cancer | HER2+ Breast Cancer | Tucatinib + T-DM1 | RTKI + ADC | 3 | 460 |
| NCT05132582 | A Study of Tucatinib or Placebo With Trastuzumab and Pertuzumab for Metastatic HER2+ Breast Cancer | HER2+ Breast Cancer | Tucatinib + Trastuzumab | RTKI + mAb | 3 | 650 |
| NCT05253651 | A Study of Tucatinib With Trastuzumab and mFOLFOX6 Versus Standard of Care Treatment in First-line HER2+ Metastatic Colorectal Cancer | HER2+ CRC | Tucatinib + Trastuzumab + Chemo | RTKI + mAb + Chemo | 3 | 400 |
| NCT04430738 | Tucatinib Plus Trastuzumab and Oxaliplatin-based Chemotherapy or Pembrolizumab-containing Combinations for HER2+ Gastrointestinal Cancers | HER2+ GI Cancers | Tucatinib + Chemo | RTKI+ Chemo | 1 | 120 |
| NCT05356741 | To Access the Safety and Effects of Intravenous Administration of AMX 818 Alone and in Combination With Pembrolizumab in Adult Participants With Locally Advanced or Metastatic HER2-Expressing Cancers | HER2+ Advanced Solid Tumor | AMX 818 + Pembrolizumab | T cell engager | 1 | 560 |
| NCT04727151 | TAC T-cells for the Treatment of HER2-positive Solid Tumors | HER2+ Advanced Solid Tumor | TAC-Tcells | TAC-Tcells | 1 | 70 |
| NCT05013554 | Dose Escalation and Expansion Study of SAR443216 in Participants With Relapsed/Refractory HER2 Expressing Solid Tumors | HER2+ Advanced Solid Tumor | SAR443216 | Trispecific Ab | 1 | 184 |
| NCT04246671 | TAEK-VAC-HerBy Vaccine for Brachyury and HER2 Expressing Cancer | HER2+ Advanced Solid Tumor | TAEK-VAC-HerBy | Vaccine | 1 | 55 |
| NCT03632941 | A Study to Evaluate Concurrent VRP-HER2 Vaccination and Pembrolizumab for Patients With Breast Cancer | HER2+ Breast Cancer | VRP-HER2 | Vaccine | 2 | 39 |
Abbreviations: Ab, antibody; ADC, antibody drug conjugate; Chemo, chemotherapy; ETB, engineered toxin body; GEC, gastroesophageal cancer; GI, gastrointestinal; i, inhibitor; IO, immunotherapy; ISAC, immune stimulating antibody conjugate; mAb, monoclonal antibody; MUT, mutated; Ph., study phase; RTKI, receptor tyrosine kinase inhibitor.
Funding acknowledgment:
This work was supported by the National Cancer Institute (P30 CA016672 to K.P.S. Raghav).
Footnotes
Conflict of interest disclosure statement: No relevant disclosures
References
- 1.Schechter AL, Stern DF, Vaidyanathan L, et al. : The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312:513–6, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. : Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182, 1987 [DOI] [PubMed] [Google Scholar]
- 3.Guy CT, Webster MA, Schaller M, et al. : Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A 89:10578–82, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moody SE, Sarkisian CJ, Hahn KT, et al. : Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell 2:451–461, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Chen S, Feng W, et al. : A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine 62:103074, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerami E, Gao J, Dogrusoz U, et al. : The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2:401–4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Aksoy BA, Dogrusoz U, et al. : Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6:pl1, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JW, Soung YH, Seo SH, et al. : Somatic mutations of ERBB2 kinase domain in gastric, colorectal, and breast carcinomas. Clin Cancer Res 12:57–61, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Shepard HM, Lewis GD, Sarup JC, et al. : Monoclonal antibody therapy of human cancer: taking the HER2 protooncogene to the clinic. J Clin Immunol 11:117–27, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Shak S: Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Herceptin Multinational Investigator Study Group. Semin Oncol 26:71–7, 1999 [PubMed] [Google Scholar]
- 11.Slamon DJ, Leyland-Jones B, Shak S, et al. : Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–92, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. : Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353:1673–84, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. : Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353:1659–72, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Franklin MC, Carey KD, Vajdos FF, et al. : Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5:317–328, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Cortes J, Kim SB, et al. : Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366:109–19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis Phillips GD, Li G, Dugger DL, et al. : Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Research 68:9280–9290, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Verma S, Miles D, Gianni L, et al. : Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367:1783–91, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogitani Y, Aida T, Hagihara K, et al. : DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin Cancer Res 22:5097–5108, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Modi S, Saura C, Yamashita T, et al. : Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med 382:610–621, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siena S, Di Bartolomeo M, Raghav K, et al. : Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): a multicentre, open-label, phase 2 trial. Lancet Oncol 22:779–789, 2021 [DOI] [PubMed] [Google Scholar]
- 21.Shitara K, Bang YJ, Iwasa S, et al. : Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N Engl J Med 382:2419–2430, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Modi S, Park H, Murthy RK, et al. : Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol 38:1887–1896, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geyer CE, Forster J, Lindquist D, et al. : Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–43, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Murthy RK, Loi S, Okines A, et al. : Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine 382:597–609, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Hyman DM, Piha-Paul SA, Won H, et al. : HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554:189–194, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson KM: Structure-based view of epidermal growth factor receptor regulation. Annu Rev Biophys 37:353–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho HS, Mason K, Ramyar KX, et al. : Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 421:756–60, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Schulze WX, Deng L, Mann M: Phosphotyrosine interactome of the ErbB-receptor kinase family. Molecular Systems Biology:doi: 10.1038/msb4100012, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzahar E, Waterman H, Chen X, et al. : A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Molecular and Cellular Biology 16:5276–5287, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holbro T, Beerli RR, Maurer F, et al. : The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A 100:8933–8, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaught DB, Stanford JC, Young C, et al. : HER3 is required for HER2-induced preneoplastic changes to the breast epithelium and tumor formation. Cancer Res 72:2672–82, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee-Hoeflich ST, Crocker L, Yao E, et al. : A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res 68:5878–87, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Weigelt B, Lo AT, Park CC, et al. : HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat 122:35–43, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scaltriti M, Rojo F, Ocana A, et al. : Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99:628–38, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Cai Z, Zhang G, Zhou Z, et al. : Differential binding patterns of monoclonal antibody 2C4 to the ErbB3-p185her2/neu and the EGFR-p185her2/neu complexes. Oncogene 27:3870–4, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao E, Zhou W, Lee-Hoeflich ST, et al. : Suppression of HER2/HER3-mediated growth of breast cancer cells with combinations of GDC-0941 PI3K inhibitor, trastuzumab, and pertuzumab. Clin Cancer Res 15:4147–56, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Campbell MR, Ruiz-Saenz A, Zhang Y, et al. : Extensive conformational and physical plasticity protects HER2-HER3 tumorigenic signaling. Cell Rep 38:110285, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agus DB, Akita RW, Fox WD, et al. : Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2:127–37, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Hudziak RM, Lewis GD, Winget M, et al. : p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol 9:1165–72, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clynes RA, Towers TL, Presta LG, et al. : Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 6:443–6, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Park S, Jiang Z, Mortenson ED, et al. : The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 18:160–170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gianni L: The "other" signaling of trastuzumab: antibodies are immunocompetent drugs. J Clin Oncol 26:1778–80, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Bianchini G, Gianni L: The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 15:e58–68, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Rugo HS, Im SA, Cardoso F, et al. : Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 7:573–584, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia W, Gerard CM, Liu L, et al. : Combining lapatinib (GW572016), a small molecule inhibitor of ErbB1 and ErbB2 tyrosine kinases, with therapeutic anti-ErbB2 antibodies enhances apoptosis of ErbB2-overexpressing breast cancer cells. Oncogene 24:6213–21, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Xia W, Mullin RJ, Keith BR, et al. : Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–63, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Burstein H, Storniolo A, Franco S, et al. : A phase II, open-label, multicenter study of lapatinib in two cohorts of patients with advanced or metastatic breast cancer who have progressed while receiving trastuzumab-containing regimens. Annals of Oncology 15(suppl 3):27 (abstract 1040), 2004 [Google Scholar]
- 48.Burstein HJ, Storniolo AM, Franco S, et al. : A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19:1068–74, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Sergina NV, Rausch M, Wang D, et al. : Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature 445:437–41, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amin DN, Sergina N, Ahuja D, et al. : Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med 2:16ra7, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrett JT, Olivares MG, Rinehart C, et al. : Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A 108:5021–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chien AJ, Koch KM, Auerback G, et al. : A phase I dose-escalation study of 5-day intermittent oral lapatinib therapy with biomarker analysis in patients with HER-2-overexpressing breast cancer. J Clin Oncol 27:(suppl; abstr e11077), 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dittus L, Werner T, Muelbaier M, et al. : Differential Kinobeads Profiling for Target Identification of Irreversible Kinase Inhibitors. ACS Chem Biol 12:2515–2521, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Davis MI, Hunt JP, Herrgard S, et al. : Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol 29:1046–1051, 2011 [DOI] [PubMed] [Google Scholar]
- 55.Niessen S, Dix MM, Barbas S, et al. : Proteome-wide Map of Targets of T790M-EGFR-Directed Covalent Inhibitors. Cell Chem Biol 24:1388–1400 e7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanning BR, Whitby LR, Dix MM, et al. : A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. Nat Chem Biol 10:760–767, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell MR, Ruiz-Saenz A, Peterson E, et al. : Targetable HER3 functions driving tumorigenic signaling in HER2-amplified cancers. Cell Rep 38:110291, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie T, Lim SM, Westover KD, et al. : Pharmacological targeting of the pseudokinase Her3. Nat Chem Biol 10:1006–12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ruiz-Saenz A, Sandhu M, Carrasco Y, et al. : Targeting HER3 by interfering with its Sec61-mediated cotranslational insertion into the endoplasmic reticulum. Oncogene 34:5288–94, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khongorzul P, Ling CJ, Khan FU, et al. : Antibody-Drug Conjugates: A Comprehensive Review. Mol Cancer Res 18:3–19, 2020 [DOI] [PubMed] [Google Scholar]
- 61.Pegram MD, Miles D, Tsui CK, et al. : HER2-Overexpressing/Amplified Breast Cancer as a Testing Ground for Antibody-Drug Conjugate Drug Development in Solid Tumors. Clin Cancer Res 26:775–786, 2020 [DOI] [PubMed] [Google Scholar]
- 62.Staudacher AH, Brown MP: Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 117:1736–1742, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diéras V, Deluche E, Lusque A, et al. : Trastuzumab deruxtecan (T-DXd) for advanced breast cancer patients (ABC), regardless HER2 status: A phase II study with biomarkers analysis (DAISY) [abstract]. . Cancer Research 82(4 Suppl):Abstract nr PD8-02., 2022 [Google Scholar]
- 64.Li BT, Smit EF, Goto Y, et al. : Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386:241–251, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saura C, Thistlethwaite F, Banerji U, et al. : A phase I expansion cohorts study of SYD985 in heavily pretreated patients with HER2-positive or HER2-low metastatic breast cancer. Journal of Clinical Oncology 36:1014–1014, 2018 [Google Scholar]
- 66.Wang J, Liu Y, Zhang Q, et al. : RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. Journal of Clinical Oncology 39:1022–1022, 2021 [Google Scholar]
- 67.Lu J, Budd GT, Frentzas S, et al. : HER2-positive and HER2-low Breast Cancer Patients in the ACE-Pan tumor-01 study: Phase 1 Study evaluating ARX788 as Monotherapy in Advanced Solid Tumors with HER2-expression or mutation. Annals of Oncology 33 (suppl_3):S194–S223., 2022 [Google Scholar]
- 68.Jabbarzadeh Kaboli P, Shabani S, Sharma S, et al. : Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates. Am J Cancer Res 12:1671–1685, 2022 [PMC free article] [PubMed] [Google Scholar]
- 69.Bardia A, Hurvitz SA, Tolaney SM, et al. : Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N Engl J Med 384:1529–1541, 2021 [DOI] [PubMed] [Google Scholar]
- 70.Bardia A, Tolaney SM, Punie K, et al. : Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol 32:1148–1156, 2021 [DOI] [PubMed] [Google Scholar]
- 71.Rugo HS, Bardia A, Marmé F, et al. : Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2−) advanced breast cancer. Journal of Clinical Oncology 40:LBA1001–LBA1001, 2022 [Google Scholar]
- 72.Doi T, Shitara K, Naito Y, et al. : Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 18:1512–1522, 2017 [DOI] [PubMed] [Google Scholar]
- 73.Nagai Y, Oitate M, Shiozawa H, et al. : Comprehensive preclinical pharmacokinetic evaluations of trastuzumab deruxtecan (DS-8201a), a HER2-targeting antibody-drug conjugate, in cynomolgus monkeys. Xenobiotica 49:1086–1096, 2019 [DOI] [PubMed] [Google Scholar]
- 74.Bose R, Kavuri SM, Searleman AC, et al. : Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov 3:224–37, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wen W, Chen WS, Xiao N, et al. : Mutations in the Kinase Domain of the HER2/ERBB2 Gene Identified in a Wide Variety of Human Cancers. J Mol Diagn 17:487–95, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fernandez-Cuesta L, Plenker D, Osada H, et al. : CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 4:415–22, 2014 [DOI] [PubMed] [Google Scholar]
- 77.Dhanasekaran SM, Balbin OA, Chen G, et al. : Transcriptome meta-analysis of lung cancer reveals recurrent aberrations in NRG1 and Hippo pathway genes. Nat Commun 5:5893, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heining C, Horak P, Uhrig S, et al. : NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discov 8:1087–1095, 2018 [DOI] [PubMed] [Google Scholar]
- 79.Wu X, Zhang D, Shi M, et al. : Successful targeting of the NRG1 fusion reveals durable response to afatinib in lung adenocarcinoma: a case report. Ann Transl Med 9:1507, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Piccart M, Procter M, Fumagalli D, et al. : Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years' Follow-Up. J Clin Oncol 39:1448–1457, 2021 [DOI] [PubMed] [Google Scholar]
- 81.von Minckwitz G, Huang CS, Mano MS, et al. : Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med 380:617–628, 2019 [DOI] [PubMed] [Google Scholar]
- 82.Cortés J, Kim S, Chung W, et al. : Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (Pts) with HER2+ metastatic breast cancer (mBC): Results of the randomized phase III DESTINY-Breast03 study. Annals of Oncology 32 (suppl 5):S1287, 2021 [Google Scholar]
- 83.Tarantino P, Hamilton E, Tolaney SM, et al. : HER2-Low Breast Cancer: Pathological and Clinical Landscape. Journal of Clinical Oncology 38:1951–1962, 2020 [DOI] [PubMed] [Google Scholar]
- 84.Xu ZQ, Zhang Y, Li N, et al. : Efficacy and safety of lapatinib and trastuzumab for HER2-positive breast cancer: a systematic review and meta-analysis of randomised controlled trials. BMJ Open 7:e013053, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pestalozzi BC, Zahrieh D, Price KN, et al. : Identifying breast cancer patients at risk for Central Nervous System (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann Oncol 17:935–44, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Saura C, Oliveira M, Feng YH, et al. : Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 38:3138–3149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin NU, Borges V, Anders C, et al. : Intracranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J Clin Oncol 38:2610–2619, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Montemurro F, Delaloge S, Barrios CH, et al. : Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial(☆). Ann Oncol 31:1350–1358, 2020 [DOI] [PubMed] [Google Scholar]
- 89.Jacot W, Pons E, Frenel JS, et al. : Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat 157:307–318, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Fabi A, Alesini D, Valle E, et al. : T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast 41:137–143, 2018 [DOI] [PubMed] [Google Scholar]
- 91.Bartsch R, Berghoff AS, Furtner J, et al. : Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jerusalem GHM, Park YH, Yamashita T, et al. : Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: A subgroup analysis of the DESTINY-Breast01 trial. Journal of Clinical Oncology 39:526–526, 2021 [Google Scholar]
- 93.Abrahao-Machado LF, Scapulatempo-Neto C: HER2 testing in gastric cancer: An update. World J Gastroenterol 22:4619–25, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bang YJ, Van Cutsem E, Feyereislova A, et al. : Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–97, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Tabernero J, Hoff PM, Shen L, et al. : Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol 19:1372–1384, 2018 [DOI] [PubMed] [Google Scholar]
- 96.Thuss-Patience PC, Shah MA, Ohtsu A, et al. : Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): an international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 18:640–653, 2017 [DOI] [PubMed] [Google Scholar]
- 97.Janjigian YY, Kawazoe A, Yanez P, et al. : The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 600:727–730, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mazieres J, Peters S, Lepage B, et al. : Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 31:1997–2003, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Gatzemeier U, Groth G, Butts C, et al. : Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 15:19–27, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Arcila ME, Chaft JE, Nafa K, et al. : Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 18:4910–8, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Passaro A, Peters S: Targeting HER2-Mutant NSCLC - The Light Is On. N Engl J Med 386:286–289, 2022 [DOI] [PubMed] [Google Scholar]
- 102.Li BT, Shen R, Buonocore D, et al. : Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 36:2532–2537, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li BT, Smit EF, Goto Y, et al. : Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 386:241–251, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertotti A, Migliardi G, Galimi F, et al. : A molecularly annotated platform of patient-derived xenografts ("xenopatients") identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov 1:508–23, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Raghav K, Loree JM, Morris JS, et al. : Validation of HER2 Amplification as a Predictive Biomarker for Anti–Epidermal Growth Factor Receptor Antibody Therapy in Metastatic Colorectal Cancer. JCO Precision Oncology:1–13, 2019 [DOI] [PubMed] [Google Scholar]
- 106.Kavuri SM, Jain N, Galimi F, et al. : HER2 activating mutations are targets for colorectal cancer treatment. Cancer Discov 5:832–41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strickler J, Cercek A, Siena S, et al. : LBA-2 Primary analysis of MOUNTAINEER: A phase 2 study of tucatinib and trastuzumab for HER2-positive mCRC. Annals of Oncology 33:S375–S376, 2022 [Google Scholar]
- 108.Sartore-Bianchi A, Trusolino L, Martino C, et al. : Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol 17:738–746, 2016 [DOI] [PubMed] [Google Scholar]
- 109.Meric-Bernstam F, Hurwitz H, Raghav KPS, et al. : Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 20:518–530, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamura Y, Okamoto W, Kato T, et al. : Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: a phase 2 trial. Nat Med 27:1899–1903, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sartore-Bianchi A, Lonardi S, Martino C, et al. : Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: the phase II HERACLES-B trial. ESMO Open 5:e000911, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Javle M, Borad MJ, Azad NS, et al. : Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol 22:1290–1300, 2021 [DOI] [PubMed] [Google Scholar]
- 113.Takahashi H, Tada Y, Saotome T, et al. : Phase II Trial of Trastuzumab and Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2-Positive Salivary Duct Carcinoma. J Clin Oncol 37:125–134, 2019 [DOI] [PubMed] [Google Scholar]
- 114.Li B, Gandhi L, Besse B, et al. : FP14.15 Neratinib-Based Combination Therapy in HER2-Mutant Lung Adenocarcinomas: Findings from two International Phase 2 Studies. Journal of Thoracic Oncology 16:S234, 2021 [Google Scholar]
- 115.Ma CX, Luo J, Freedman RA, et al. : The Phase II MutHER Study of Neratinib Alone and in Combination with Fulvestrant in HER2-Mutated, Non-amplified Metastatic Breast Cancer. Clin Cancer Res 28:1258–1267, 2022 [DOI] [PubMed] [Google Scholar]
- 116.Harding JJ, Piha-Paul SA, Shah RH, et al. : Targeting HER2 mutation–positive advanced biliary tract cancers with neratinib: Final results from the phase 2 SUMMIT basket trial. Journal of Clinical Oncology 40:4079–4079, 2022 [Google Scholar]
- 117.Oaknin A, Friedman CF, Roman LD, et al. : Neratinib in patients with HER2-mutant, metastatic cervical cancer: Findings from the phase 2 SUMMIT basket trial. Gynecol Oncol 159:150–156, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Song Z, Li Y, Chen S, et al. : Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: a multicenter, single-arm, phase II trial. BMC Med 20:42, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Elamin YY, Robichaux JP, Carter BW, et al. : Poziotinib for Patients With HER2 Exon 20 Mutant Non-Small-Cell Lung Cancer: Results From a Phase II Trial. J Clin Oncol 40:702–709, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fan Y, Chen J, Zhou C, et al. : Afatinib in patients with advanced non-small cell lung cancer harboring HER2mutations, previously treated with chemotherapy: A phase II trial. Lung Cancer 147:209–213, 2020 [DOI] [PubMed] [Google Scholar]