Abstract
Background
Sclerosing odontogenic carcinoma is an exceedingly rare gnathic malignancy first described by Koutlas et al. in 2008, and was only recently designated as a distinct pathologic entity by World Health Organization in the 2017 Classification of Head and Neck Tumors. To date, fewer than fifteen cases of this neoplasm have been reported in the English language literature. This tumor is characterized by thin cords, strands, and small nests of epithelium in a densely sclerotic stroma. In some tumor foci, the density of the stroma may be sufficient to compress the epithelial component beyond detection in the absence of immunohistochemistry, thus rendering this entity a particularly challenging diagnosis in small sample sizes.
Methods
A 55-year-old male presented with an asymptomatic lesion of posterior left maxilla. Cone beam computed tomography (CBCT) demonstrated a large, well-defined bony lesion with scalloped border, spanning from canine to first molar. External root resorption of the adjacent teeth was also noted. Microscopic examination of the biopsy specimen revealed an odontogenic tumor with features consistent with sclerosing odontogenic carcinoma. Immunohistochemical staining was performed to confirm the diagnosis.
Results
The tumor was positive for CK5/6, CK19, E-cadherin, p63 and negative for CK20 and CK7.
Conclusion
Sclerosing odontogenic carcinoma is a rare, low-grade malignancy of odontogenic origin, which represents a diagnosis of exclusion in many cases. An immunohistochemical profile demonstrating positivity for markers including CK5/6, CK19, p63, and E-cadherin, in addition to a set of pertinent negative findings, can aid in the diagnosis of this tumor. This entity appears to lack metastatic potential despite its locally destructive behavior and a common histologic finding of perineural invasion.
Keywords: Sclerosing odontogenic carcinoma, Odontogenic carcinoma, Clear cell carcinoma
Introduction
Sclerosing odontogenic carcinoma (SOC) arises in the maxillary or mandibular jaws of patients in the 5–7th decades most commonly, with a slight male predilection. The pathophysiology of this tumor is not well understood, though it has been postulated to arise from either odontogenic epithelial rests (rests of Malassez or rests of Serres) or represent malignant transformation of odontogenic cyst epithelium [1]. Fewer than fifteen cases of SOC have been reported in the literature to date, with multiple of such cases reporting an initial diagnosis of benign or other malignant entities. Mischaracterization, particularly on biopsy, is likely attributable to the subtle and variable histologic features of SOC that microscopically mimic other lesions, including calcifying epithelial odontogenic tumor (CEOT), epithelial-rich variant of central odontogenic fibroma (COF), and clear cell odontogenic carcinoma (CCOC). The differential diagnoses may be expanded in some cases to include squamous cell carcinoma (SCC) and metastatic disease. In this paper we present a case of sclerosing odontogenic carcinoma with emphasis on clinical and histologic features that should raise suspicion for this lesion on biopsy. We will also discuss an immunohistochemical panel and molecular testing that can be applied to eliminate mimics on the histologic differential, as this entity frequently represents a diagnosis of exclusion.
Materials and Methods
Case Report
A 55-year-old male was referred to an oral surgeon for evaluation of an asymptomatic lesion of his left maxilla. The lesion was discovered by the patient’s periodontist who detected deep periodontal pocketing and slight mobility of the left posterior maxillary teeth. The patient reported a history of ‘inflamed’ gingiva in the area and intermittent bleeding. Significant past medical history included a 30-pack-year smoking history. Cone beam computed tomography (CBCT) of the area was performed to assess for pathology. On CBCT, a large, well-defined low-density bony lesion with scalloped borders was seen on the palatal aspect of the posterior left maxillary teeth (spanning from the canine to the first molar). The lesion was causing external root resorption of the adjacent teeth. The radiographic impression, as per radiology report, included entities such as unicystic ameloblastoma, odontogenic keratocyst, and localized advanced periodontal disease. Clinical evaluation of the site was negative for swelling, cortical expansion, or purulence. The remainder of the head and neck examination was negative for palpable cervical lymphadenopathy, extraoral swellings, or trismus; cranial nerves II–XII were intact. In an outpatient facility, the oral surgeon performed an incisional biopsy via the reflection of a palatal soft tissue flap and bony window. On surgical exploration the mass was uniformly composed of friable, tan-white, soft tissue without purulence, fluid, nor the presence of a cystic cavity. A representative biopsy was obtained and submitted for microscopic examination. The patient was dismissed the same day without complication.
The biopsy specimen was submitted in a 10% formalin fixative and subsequently embedded in paraffin. The specimen was processed into four-micrometer-thick sections and stained with hematoxylin and eosin for routine microscopic examination. Additional immunohistochemical studies were performed and included antibodies against CK5/6, CK19, e-cadherin, CK7, CK20, p63, and Ki67 (Table 1).
Table 1.
Antibodies used for immunohistochemical evaluation of sclerosing odontogenic carcinoma
| Antibody | Clone/Source | Dilution |
|---|---|---|
| CK5/6 | D5 & 16B4, Cell Marque™• | Ready to use |
| CK19 | B170, BOND™* | Ready to use |
| CK7 | RN7, BOND™* | Ready to use |
| CK20 | Ks20.8, BOND™* | Ready to use |
| p63 | 4A4, Biocare Medical+ | 1:300 |
| Ki67 | MM1, BOND™* | 1:600 |
| e-cadherin | 36B5, BOND™* | Ready to use |
•Cell Marque, Sigma Aldrich Company, Rocklin, CA
*BOND™, Leica Biosystems, Deer Park, IL
+Biocare Medical, Biocare Medical, Pacheco, CA
Following biopsy diagnosis of sclerosing odontogenic carcinoma the patient was referred to a surgical oncology center for definitive treatment. The patient underwent left infrastructure maxillectomy with reconstruction with buccal fat pad flap. His hospital course was complicated by bilateral pulmonary emboli and the patient was later discharged with Eliquis. The patient is reportedly disease-free 19 months postoperatively.
Results
Microscopic Findings
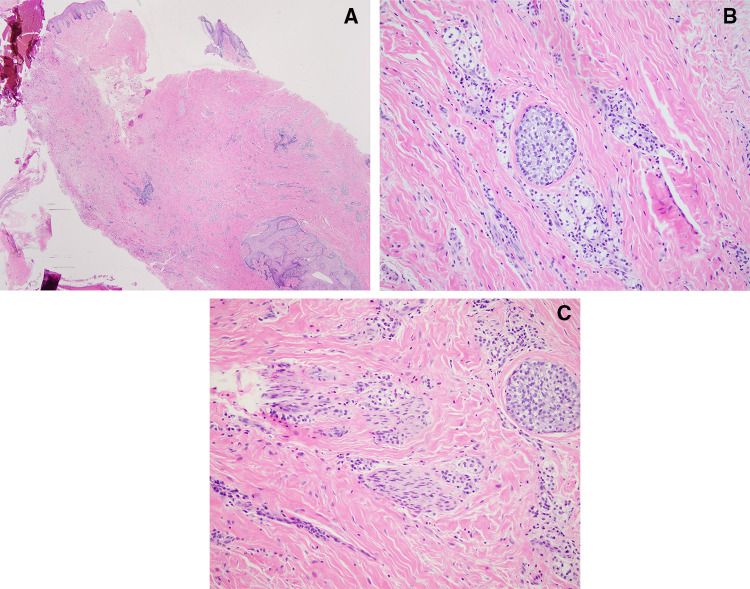
The biopsy specimen consisted of multiple pieces of soft tissue with benign-appearing overlying squamous epithelium. On low power the tumor consisted of predominantly dense fibrous connective tissue with scattered inflammation and nests of epithelium. Epithelial nests that were recognizable at low power were composed of approximately 10–20 cells and were similar to common benign odontogenic rests seen in both neoplastic and non-neoplastic odontogenic lesions (Fig. 1A). On high power small nests and single file cords composed of 2–4 tumor cells became distinguishable from the background inflammatory infiltrate. The neoplastic cells demonstrated bland cytology with minimal nuclear pleomorphism or hyperchromasia, and a moderate amount of cytoplasm which ranged from amphophilic to clear. (Fig. 1B) Mitotic activity was inconspicuous. Few scattered dystrophic calcifications were noted as well as fragments of residual reactive bone. Perineural invasion was readily apparent and frequent within the submitted specimen (Fig. 1C).
Fig. 1.
Microscopic examination of the biopsy specimen demonstrating a nests of epithelium with scattered inflammation in a background of dense fibrous connective tissue, 20 × (hematoxylin–eosin), b neoplastic cells with bland cytology, amphophilic to clear cytoplasm and minimal nuclear pleomorphism or hyperchromasia, 200× (hematoxylin–eosin), c perineural invasion, 200 × (hematoxylin–eosin)
Immunohistochemical Results
The immunohistochemical findings in this case and all previously reported cases are summarized in Table 2.
Table 2.
Immunohistochemical staining and molecular testing of reported cases of sclerosing odontogenic carcinoma
| Immunohistochemistry | Histologic features of malignancy | Molecular | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publication | CK5/6 | E-Cadherin | CK19 | CK14 | CK7 | CK20 | p40 | p63 | Other (+) | Other (−) | PNI | Mitotic activity | Necrosis | EWSR1 fish study |
| Current case | (+) | (+) | (+) | N/P | (−) | (−) | N/P | (+) | N/P | N/P | (+) | Inconspicuous Ki67 5% | (−) | N/P |
| Seyiti et al. [2] | (+) | N/P | N/P | N/P | N/P | N/P | N/P | (+) | N/P | SMA, S100, desmin | (+) | Ki67 10% | (−) | (−) |
| Todorovic et al. [3] | (+) | N/P | (−) | (+) | (−) | (−) | (+) | (+) | N/P | EBER ISH, ER, PAX8, CDX2 | (−) | 1/10 HPFs Ki67 10% | (−) | (−) |
| Hanisch et al. [4] | (+) | N/P | N/P | N/P | N/P | N/P | (+) | (+) | MNF116 | N/P | (+) | N/P | (−) | N/P |
| Wood et al. [1] | (+) | (+) | (+) | (+) | (−) | (−) | (−) | (+) | N/P | CEA, ER, PR, S100 | (+) | (−) | (−) | (−) |
| Tan et al. [5] | (+) | (+) | (+) | N/P | (+) Diffuse | (−) | N/P | (+) | p16 (30%), p53, PR,CAM5.2 | CEA, EMA, SMA, S100, Desmin, Calretinin, CD34, Vimentin, ER, CD1a | (−) | 1/50 HPFs Ki67 2% | (−) | (−) |
| Hussain et al. [6] | (+) | N/P | ( +) | N/P | N/P | N/P | N/P | N/P | N/P | AE1/AE3 | (+) | Inconspicuous | (−) | N/P |
| Saxena et al. [7] | (+) | N/P | N/P | N/P | N/P | N/P | N/P | (+) | N/P | S100, SMA, desmin | (+) | Occasional | (+) | N/P |
| Irie et al. [8] | (+) | N/P | (+) | N/P | (+) Focal | (−) | N/P | (+) | AE1/AE3 | S100, CEA, carletinin, CD34, vimentin, CK8 | (+) | (−) | (−) | N/P |
| Koutlas et al. [9] | (+) | (+) | (+) | N/P | ( +) Focal and weak | (−) | N/P | (+) | N/P | CK8/18, CEA, S100, SMA, desmin | (+) | (−) | (−) | N/P |
| Koutlas et al. [9] | (+) | (+) | (+) | N/P | N/P | (−) | N/P | (+) | N/P | CK8/18, CEA, S100, SMA, desmin | (+) | (−) | (−) | N/P |
| Koutlas et al. [9] | (+) | (+) | (+) | N/P | N/P | (−) | N/P | (+) | N/P | CK8/18, CEA, S100, SMA, desmin | (+) | (−) | (−) | N/P |
N/P not performed
Positive Markers
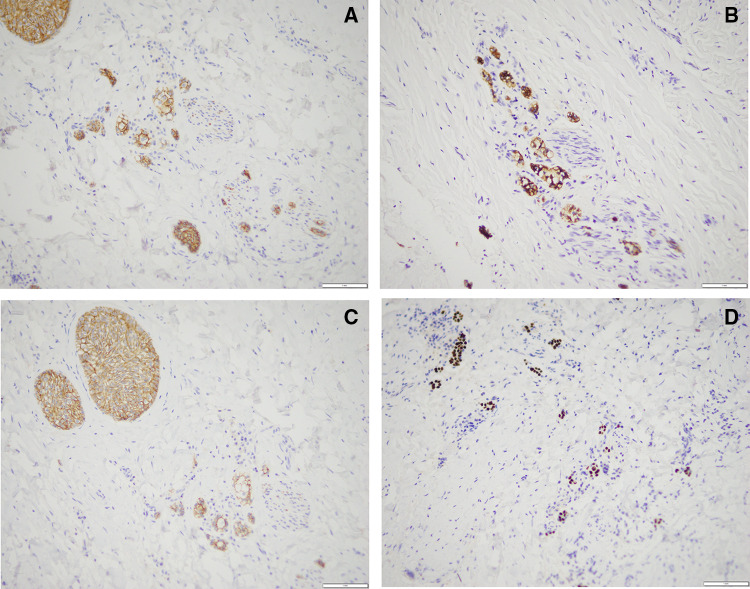
The epithelial component of the tumor showed strong and diffuse cytoplasmic immunoreactivity with markers CK5/6 and CK19 typical of non-keratinizing epithelium and basal cell phenotypes [9] (Fig. 2A, B). Though non-specific, CK19 expression can be suggestive of odontogenic origin as expression has been previously reported in secretory ameloblasts and pre-ameloblasts [10]. Other positive markers seen within the neoplastic epithelial component of the tumor included diffuse nuclear staining with p63 and membranous staining with e-cadherin. (Fig. 2C, D).
Fig. 2.
Neoplastic epithelial component of the tumor demonstrating positivity for a CK5/6 (200×), b CK19 (200×), c e-cadherin (200×), d p63 (200×)
Negative Markers
CK7 and CK20 were performed and found to be non-reactive in our case. In conjunction with clinical history these were used to eliminate the possibility of metastatic carcinoma. Though infrequent, metastasis to the jaws has been reported as the first sign of disease in numerous cases in the literature and should be ruled out when clinical history is unclear [11].
Proliferative Index
Mitotic activity was inconspicuous on routine H&E. Ki67 stain demonstrated rare nuclear positivity accounting for less than 5% of the overall neoplastic epithelial cell population.
Discussion
We describe a case of sclerosing odontogenic carcinoma (SOC) presenting in a 55-year-old male. On microscopic examination, SOC is characterized by small to medium sized nests, cords, and strands of epithelial cells in a densely sclerotic background. The epithelial cells demonstrate mild to moderate atypia, with minimal nuclear pleomorphism and inconspicuous nucleoli. Vacuolar change with resultant cytoplasmic clearing is a variable, but commonly identified feature. Features of malignancy include an infiltrative growth pattern and perineural invasion, as well as one reported case with the finding of tumor necrosis [7]. Despite these features this tumor appears to be low-grade as evidenced by rare mitoses (and/or low proliferative index) and no reported cases of regional or distant metastatic disease. SOC represents a diagnosis of exclusion in most cases, and therefore requires a combination of clinical context, morphologic features, appropriate immunohistochemical panel, and occasional molecular testing.
A comprehensive histologic differential may be difficult to devise in cases of small biopsy, in which the epithelial component of the tumor is not readily appreciated. Evidence of this is found in the literature in the form of initial diagnosis as a benign fibro-osseous lesion [3], Garre’s osteomyelitis [2], or a non-neoplastic process [4]. In such cases clinical and radiographic correlation is essential and should dictate a recommendation of repeat sampling for diagnosis before definitive management. Interestingly, nearly every case of SOC reported in the literature, including those reported after the inclusion of SOC in the 2017 WHO, underwent multiple biopsy procedures, and in some cases definitive resection before a diagnosis of SOC was rendered (Table 3). This highlights the fact that though SOC has been recognized by the WHO it remains a rare and little-known entity.
Table 3.
Demographics, radiographic findings, histologic diagnoses and clinical outcomes of reported cases of sclerosing odontogenic carcinoma
| Demographics | Radiographic findings | Histologic diagnosis | Clinical | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pub | Age | Sex | Tumor site | Radiology | Initial diagnosis | Second sample | Third sample | Final excision | Definitive management | Recurrence/Management | F/u (mos) |
| Current case | 55 | M | Left maxilla | Large, well-defined low-density bony lesion with scalloped borders; external root resorption of adjacent teeth | SOC | N/A | N/A | SOC | Left infrastructure maxillectomy | (−) | 19 |
| Seyiti et al. [2] | 54 | F | Right mandibular body to left mandibular ramus | Irregular, extensive lytic changes with ill-defined borders and patchy calcifications. Buccal and lingual cortices with irregular resorption | Garrè’s osteomyelitis | SOC | N/A | SOC | Mandibulectomy | Not reported | Not reported |
| Todorovic et al. [3] | 62 | M | Left maxilla | Infiltrating lesion concerning for malignancy | Benign fibrosseous lesion | SOC | N/A | SOC | Resection | Recurrence at 5 months; 66 Gy in 33 fractions | 19 |
| Hanisch et al. [4] | 60 | M | Left mandible | Ill-defined lytic osseous changes with expansion, erosion, and perforation, highly suspicious of a malignant tumor | No signs of a neoplastic process | Low-grade squamous cell carcinoma | N/A | SOC |
1. Left hemi-mandibulectomy 2. Radical ipsilateral neck dissection |
(−) | 9 |
| Wood et al. [1] | 43 | F | Right anterior hard palate | 12 mm × 4.3 mm × 5.1 mm enhancing soft tissue mass with no evidence of bone destruction | Adenocarcinoma NOS | N/A | N/A | SOC | Right maxillectomy with wide margins | (−) | 17 |
| Tan et al. [5] | 31 | F | Right mandible | Well-circumscribed round radiolucent lesion measuring 1 cm, with scattered specks of radiopacities and a distinct sclerotic peripheral margin | SOC | N/A | N/A | N/A | Excisional biopsy by enucleation | (−) | 12 |
| Hussain et al. [6] | 54 | M | Right maxilla | Well demarcated radiolucency related to the upper right lateral incisor and canine teeth with loss of the lamina dura around the roots and irregular resorption of the canine | Poorly differentiated squamous cell carcinoma (metastatic). Revised to odontogenic carcinoma | N/A | N/A | SOC | Resection with 5 mm margins | (−) | 19 |
| Saxena et al. [7] | 42 | M | Left mandible | Well-defined unilocular lytic lesion in the left anterior region of the mandible with a smooth outline and no sclerotic margins. Perforation of buccal and lingual cortices | Epithelium-rich variant of central odontogenic fibroma | Primary intraosseous carcinoma of odontogenic origin | N/A | SOC |
1. Hemi-mandibulectomy 2. Radical neck dissection 3. Postoperative radiotherapy |
(−) | 10 |
| Irie et al. [8] | 67 | M | Left mandible | Mixed well and ill-defined radiolucency without definite root resorption, focally expansile with thinning and perforation of buccal cortex | Compatible with benign fibrosseous lesion | BFOL with hyperplastic and metaplastic odontogenic epithelia | Atypical odontogenic epithelium w/ fibrous component | SOC with BFOL |
1. Left segmental mandibulectomy 2. Postoperative chemotherapy |
(−) | 15 |
| Koutlas et al. [9] | 72 | M | Left mandible | Unavailable | Unavailable | N/A | N/A | SOC | 1. Extensive surgery 2. Ipsilateral neck dissection | (−) | 5 |
| Koutlas et al. [9] | 46 | F | Right mandible | Poorly defined osteolytic process with perforation of the buccal cortical plate and thinning of the lingual cortical bone | Unavailable | N/A | N/A | SOC | Extensive surgery | (−) | 12 |
| Koutlas et al. [9] | 73 | F | Right maxilla | Diffuse radiolucency that filled the alveolar ridge and extended into sinus | Poorly differentiated squamous cell carcinoma | N/A | N/A | SOC | 1. Extensive surgery 2. Postoperative radiation | (−) | 42 |
N/A not applicable
Morphologic features of SOC may mimic benign neoplasms of odontogenic epithelial origin. Calcifying epithelial odontogenic tumor (CEOT) has a similar low power appearance to SOC, with scattered nests of epithelial cells in a predominantly fibrotic stroma. However, CEOT characteristically produces odontogenic ameloblast-associated protein (ODAM), an amyloid-like material. ODAM, like amyloid, demonstrates apple-green birefringence when stained with Congo red and viewed with polarized light. Central odontogenic fibroma (COF), particularly the epithelial-rich variant, also has a similar morphologic appearance to SOC, demonstrating nests and strands of odontogenic epithelium in a fairly cellular stroma. Perineural invasion, a hallmark feature that distinguishes SOC from COF, has been called into question [14]. Earlier research has suggested that the close proximity of the residual intraosseous epithelial rests and neurovascular bundles are anatomically normal and should not be misinterpreted as perineural invasion [2]. A lack of sclerosis in the stroma of COF has been suggested as a possible distinguishing feature between the two entities [9]. In addition, SOC has an infiltrative growth with “areas of tissue invasion which extend beyond what is expected clinically and intraoperatively,” as described by Todorovic et al. [3]. In contrast, COF can be readily separated from the bone and lacks evidence of bone infiltration [15]. Further classification of the defining diagnostic criteria for SOC is likely warranted. At this time, we believe diagnosis should be based on the microscopic features coupled with locally aggressive clinical behavior, as seen in this case.
SOC also characteristically lacks certain high-grade features frequently seen in other malignant entities that could be considered on the differential diagnoses. In contrast to SOC, primary intraosseous SCC exhibits overt cytological features of malignancy. Such features include nuclear pleomorphism as well as frequent and atypical mitotic activity. When present, keratinization typical of low-to-moderate grade SCC can also help easily distinguish it from SOC. Another common malignant mimic of SOC is clear cell odontogenic carcinoma (CCOC). While cases of CCOC frequently display a biphasic pattern and/or high-grade malignant features, easily distinguishing it from SOC, there are cases in which a monophasic pattern predominates and high-grade cytologic features are absent. In such cases the features of CCOC are nearly identical to SOC, with infiltrative nests and cords of clear cells with bland cytology in a densely fibrous stoma and perineural invasion. It is important to delineate these two entities as SOC demonstrates only a locally aggressive behavior profile, whilst CCOC presents with regional metastasis in 20–25% of cases and has shown the capacity for distant metastases [12]. Molecular testing for translocation between EWSR1 and ATF1, which presents in > 80% of cases of CCOC and is absent in SOC [13], can be used in challenging cases.
Immunohistochemical staining can be helpful in highlighting the key features of SOC as well as in eliminating metastatic disease from the differential diagnoses. Although infrequent, metastasis to the jaws can represent the first sign of disease in rare cases and thus should be considered. In conjunction with clinical findings and morphology, immunohistochemical stains CK7, CK20, and p63 aid in the exclusion of metastasis from the differential diagnoses. SOC is characteristically positive for p63, negative for CK20, and infrequently CK7 positive (one case reporting strong/diffuse staining [5]). Neoplastic cells also stain positive for epithelial markers common to, though not specific for, odontogenic epithelium including CK5/6 and CK19. In addition, the epithelial nests of SOC demonstrate diffuse membranous staining with e-cadherin, a feature more typical of a low-grade malignancy.
Appropriate classification of this entity allows for optimal clinical management of these patients. SOC is typically treated with conservative surgical excision with negative margins. There are no reported cases of metastases of SOC, despite the apparent capacity for local destruction, infiltrative histologic growth pattern, and frequent perineural invasion. A definitive diagnosis of SOC on biopsy has the capacity to shift clinical management away from more radical treatment modalities previously reported in the literature (both before and after the designation and recognition of SOC as a histologic entity) and towards conservatism. From all collected cases in the literature, it appears that local excision with negative margins is sufficient for disease control. Given the subtle features of SOC, which often mimic other benign and malignant processes, sufficient clinical context and suspicion for this entity are necessary to make this important diagnosis that will ultimately impact clinical care.
Funding
This study was not supported by any funding.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
For this type of study, informed consent is not required.
Consent to Participate
For this type of study, consent for participation is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wood A, Young F, Morrison J, Conn BI. Sclerosing odontogenic carcinoma presenting on the hard palate of a 43-year-old female: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(6):e204–e208. doi: 10.1016/j.oooo.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Seyiti P, Feng Y, Gao A, Lin Z, Huang X, Sun G, Zhang L, Wang T. An extensive sclerosing odontogenic carcinoma in mandible: a case report and literature review. Dentomaxillofac Radiol. 2020;49(6):20190426. doi: 10.1259/dmfr.20190426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todorovic E, Berthelet E, O’Connor R, Durham JS, Tran E, Martin M, Hayes MM, Ng TL. Sclerosing odontogenic carcinoma with local recurrence: case report and review of literature. Head Neck Pathol. 2019;13(3):371–377. doi: 10.1007/s12105-018-0975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanisch M, Baumhoer D, Elges S, Fröhlich LF, Kleinheinz J, Jung S. Sclerosing odontogenic carcinoma: current diagnostic and management considerations concerning a most unusual neoplasm. Int J Oral Maxillofac Surg. 2017;46(12):1641–1649. doi: 10.1016/j.ijom.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Tan SH, Yeo JF, Kheem Pang BN, Petersson F. An intraosseous sclerosing odontogenic tumor predominantly composed of epithelial cells: relation to (so-called) sclerosing odontogenic carcinoma and epithelial-rich central odontogenic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(4):e119–e125. doi: 10.1016/j.oooo.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Hussain O, Rendon AT, Orr RL, Speight PM. Sclerosing odontogenic carcinoma in the maxilla: a rare primary intraosseous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(4):e283–e286. doi: 10.1016/j.oooo.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Saxena S, Kumar S. Sclerosing odontogenic carcinoma–an enigma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(6):840. doi: 10.1016/j.oooo.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Irie T, Ogawa I, Takata T, et al. Sclerosing odontogenic carci-noma with benign fibro-osseous lesion of the mandible: anextremely rare case report. Pathol Int. 2010;60:694–700. doi: 10.1111/j.1440-1827.2010.02583.x. [DOI] [PubMed] [Google Scholar]
- 9.Koutlas IG, Allen CM, Warnock GR, Manivel JC. Sclerosing odontogenic carcinoma: a previously unreported variant of a locally aggressive odontogenic neoplasm without apparent metastatic potential. Am J Surg Pathol. 2008;32(11):1613–1619. doi: 10.1097/PAS.0b013e31817a8a58. [DOI] [PubMed] [Google Scholar]
- 10.Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC. Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med. 2012;41(3):272–280. doi: 10.1111/j.1600-0714.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 11.Irani S. Metastasis to the jawbones: a review of 453 cases. J Int Soc Prev Community Dent. 2017;7(2):71–81. doi: 10.4103/jispcd.JISPCD_512_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Liu L, Pan J, Tian X, Tan J, Zhou J, Duan Y. Clear cell odontogenic carcinoma: report of 6 cases and review of the literature. Med Oncol. 2011;28(Suppl 1):S626–S633. doi: 10.1007/s12032-010-9668-z. [DOI] [PubMed] [Google Scholar]
- 13.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, Barker B, Seethala RR. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37(7):1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 14.Ide F, Kikuchi K, Sakashita H, Muramatsu T, Kusama K. Neurovascular involvement in central odontogenic fibroma: a potential source of confusion with invasive carcinoma. Histopathology. 2015;66(7):1044–1046. doi: 10.1111/his.12567. [DOI] [PubMed] [Google Scholar]
- 15.Chhabra V, Chhabra A. Central odontogenic fibroma of the mandible. Contemp Clin Dent. 2012;3(2):230–233. doi: 10.4103/0976-237X.96845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.