Abstract
Background
Sinonasal mucosal melanoma (MM) is a rare, aggressive melanoma subtype. Complete surgical excision, with or without adjuvant radiotherapy, remains the cornerstone of treatment and yields adequate locoregional control. Metastatic MM is managed similarly to metastatic cutaneous melanoma but with poorer survival. PReferentially expressed Antigen in MElanoma (PRAME) has been identified as a potential diagnostic marker and therapeutic target in the treatment of cutaneous melanoma.
Methods
Retrospective analysis of the clinical characteristics and immunohistochemical features of all sinonasal MM patients referred to the department of Head and Neck Surgical Oncology, UMC Utrecht Cancer Center, between 2011 and 2021 was performed. Single nucleotide polymorphism (SNP) array and next-generation sequencing (NGS) were performed in selected cases.
Results
A total of 26 patients with an MM were included. The median follow-up duration was 15 months. At the end of follow-up, 13 patients had died due to progression of their disease, and one patient died of intercurrent disease. PRAME immunohistochemistry was performed in 23 out of 26 cases, all displaying PRAME expression. In two cases PRAME expression was present both within the melanoma cells and in melanocytes in adjacent mucosa. SNP array showed ≥ 5 copy number variants (CNV) in all tested cases, with a median of 29.5 CNVs (IQR 23.25–40). The three most common mutations identified by NGS were NRAS (7 cases) and NF1 (2 cases).
Conclusion
We show that expression of PRAME is common in sinonasal MM, making PRAME a useful ancillary diagnostic tool and a potential therapeutic target in sinonasal MM. The demonstrated occurrence of extensive presence of PRAME-positive melanocytes in the surrounding mucosa of sinonasal MM might explain the multifocal nature of melanoma in the (sinonasal) mucosa, and would be an extra argument for a PRAME targeting treatment in preventing local disease recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-022-01515-9.
Keywords: Sinonasal cancer, Mucosal melanoma, Field-melanomization, PRAME, Nasal mucosa, Immunotherapy
Introduction
Mucosal melanoma (MM) is a rare and aggressive type of melanoma. The incidence varies substantially by ethnicity, accounting for 1% of all melanomas in white patients and approximately 25% in Asians [1]. The most frequently affected sites in the upper aerodigestive tract are the nasal cavity, paranasal sinuses, and oral cavity.
Patients with an MM of the nasal cavity typically present with unilateral nasal obstruction and epistaxis, and are found to have a pigmented polyp or lesion. Mucosal melanomas in the head and neck area are a diagnostic challenge because 20% of the lesions are multifocal, and up to 40% are amelanotic [1]. Diagnostic workup includes clinical examination, imaging of the primary site (MR imaging and CT scan), tissue biopsy, and a (PET-)CT scan to evaluate lymphadenopathy and distant spread. Positive immunohistochemical staining for proteins SOX10, S-100, HMB-45, Melan-A, tyrosinase, Mart-I, and MITF supports the diagnosis [2]. TNM classification of MM starts at stage III, which is indicative of the poor prognosis of these patients [3]. Complete surgical excision remains the cornerstone in treatment, although this can be challenging in this complex anatomical region. Definitive or postoperative RT for MM of the head and neck yields fairly good locoregional control of disease but does not improve survival [4, 5]. In recent years, novel treatment options, such as targeted therapy (e.g. BRAF-inhibitors) and immunotherapy (e.g. anti-PD-1 antibodies), have revolutionized the treatment of metastatic cutaneous melanoma. In contrast to cutaneous melanoma, survival rates for advanced MM in the head and neck region remain poor, with 1-and 3-year survival rates of 42% and 15%, respectively [6].
In search of novel treatment options for melanoma, PRAME (PReferentially expressed Antigen in MElanoma) was identified as a potential target in the treatment of cutaneous melanoma [7]. The authors encountered a case of sinonasal MM that showed expression of PRAME in both the invasive melanoma and in multifocal pigmented lesions within the nasal cavity, leading to the hypothesis that PRAME expression predicts derailment of melanocytic lesions. Subsequently, the clinical characteristics, treatment strategies, and immunohistochemistry findings of 26 consecutive sinonasal MM patients were evaluated. In addition, Single Nucleotide Polymorphism (SNP) array and Next-Generation Sequencing (NGS) were performed in a selection of cases.
Materials and Methods
Study Design and Data Collection
All patients with a sinonasal MM who had been referred to the Department of Head and Neck Surgical Oncology, UMC Utrecht Cancer Center, between June 2011 and May 2021, were retrieved and included.
Patient characteristics were recorded, including sex, age at the time of diagnosis, symptoms, subsite of sinonasal MM, treatment strategy, metastatic disease, and outcome. The duration of follow-up was defined as the period between the date of histopathologic confirmation of MM diagnosis and the most recent outpatient clinic visit or date of death. Immunohistochemistry (IHC) was performed using the following antibodies, if indicated: PRAME, Melan-A, HMB-45, MART-A, SOX-10, and S-100. Positive PRAME expression was defined as ≥ 75% nuclear staining [8]. As stated previously, SNP array and NGS analysis were performed in selected cases [9].
DNA Isolation
For DNA isolation, formalin-fixed paraffin-embedded (FFPE) tissue blocks from all cases were cut at 4 μm. After hematoxylin-eosin staining, viable tumor tissue areas with the highest tumor cell percentage (TCP), estimated by a pathologist, were selected, marked, and macro-dissected. Non-lesional areas were not included in the sampling. DNA from the samples was subsequently extracted and purified using the Cobas® DNA Sample Preparation Kit (Roche, Basel, Switzerland), according to the manufacturer’s protocols.
SNP Array Analysis
SNP array was performed using the Infinium CytoSNP-850 K v1.2 BeadChip (Illumina, San Diego, California), according to the manufacturer’s protocol. This exon-centric oligo-array employs 850,000 single nucleotide polymorphism probes with enriched coverage for 3,262 cancer genes enabling copy number calling with a resolution of 10 kb in selected genomic regions. Briefly, 8–200 ng of isolated DNA was treated with the Infinium HD FFPE DNA Restore Kit (Illumina, San Diego, CA). After FFPE restoration, DNA was amplified, fragmented, and hybridized to the Infinium CytoSNP-850 K v1.2 BeadChip. After extension and staining, the Beadchip was scanned using the iScan (Illumina, San Diego, California) to generate probe intensity output files. Probe fluorescence was compared with a reference human genome. The results were visualized, analyzed, and interpreted using NxClinical Software (BioDiscovery, El Segundo, California). NxClinical provides information on B-allele frequency (BAF) and intensity, allowing accurate calling of copy number variants (CNVs) and copy-neutral loss of heterozygosity (CN-LOH) using a pre-defined ruleset, and chromothripsis (defined ≥ 10 CNVs on a chromosome or chromosome arm) [10]. A TCP of ≥ 30% was considered sufficient for reliable interpretation of the SNP array results.
NGS
As previously described, amplicon-based targeted Next-Generation Sequencing (NGS) was performed with Ion Ampliseq™ custom-designed panels (see supplementary Tables S1–S3 for a complete overview of the covered hotspot regions for each gene of the different panels) [11]. Library preparation was performed using the Ion Ampliseq™ Library kit 2.0 (Thermo Fisher Scientific, Waltham, USA), according to the manufacturer’s protocol, on the Janus Express (PerkinElmer, Waltham, USA). Template preparation and chip loading was performed by the Ion Chef System using the Ion 510™ & Ion 520™ & Ion 530™ Chef kit and protocol. Sequencing was performed on the Ion Torrent S5, followed by variant calling by the Torrent Variant Caller. Variant annotation was done by an in-house bioinformatics pipeline using Ensembl API12. Variants were visually inspected using the Integrative Genomics Viewer (IGV), and variant of uncertain significance (VUS, class 3), likely pathogenic (class 4), and pathogenic (class 5) are reported.
Statistical Analysis
Analyses were performed using IBM SPSS Statistics version 26.0.0.1.(32bit). Baseline characteristics are reported as mean with standard deviation (SD) or as numbers with corresponding percentages. If variables were not normally distributed, values are reported as median with interquartile range (IQR). Recurrence-free survival (RFS), Disease-specific survival (DSS), and overall survival (OS) were calculated via the Kaplan–Meier estimator. P-values ≤ 0.05 were considered statistically significant.
Results
Index Patient
The index patient (patient no. 4), a 68-year-old female, presented with a history of right-sided nasal obstruction and recurrent epistaxis. Upon nasendoscopy, a highly vascular, pigmented polypoidal mass was visualized. Immunohistochemistry demonstrated positive stains for Melan-A, S-100, and SOX10, with the presence of an intraepithelial precursor lesion, leading to a diagnosis of primary mucosal sinonasal melanoma. No mutations were found in BRAF, NRAS, HRAS, or KIT. Under general anesthesia, a wide local excision of the lesion was performed. Fourteen months after initial treatment, the patient developed a local recurrence. Once again, wide local excision was performed. Besides the recurrence, multifocal pigmented mucosal lesions which had been visualized preoperatively were also resected (Fig. 1). The patient developed a second local recurrence in the left nasal cavity 4 months later. After a 5-month interval, there was a third local recurrence (23 months since initial treatment). Both tumors were also widely excised under general anesthesia, and the diagnosis of mucosal melanoma was confirmed by the pathologist with the additional unexpected finding of a widespread intra-epithelial presence of PRAME positive melanocytes in clinically pigmented mucosa, with separate invasive foci as well (Fig. 2).
Fig. 1.
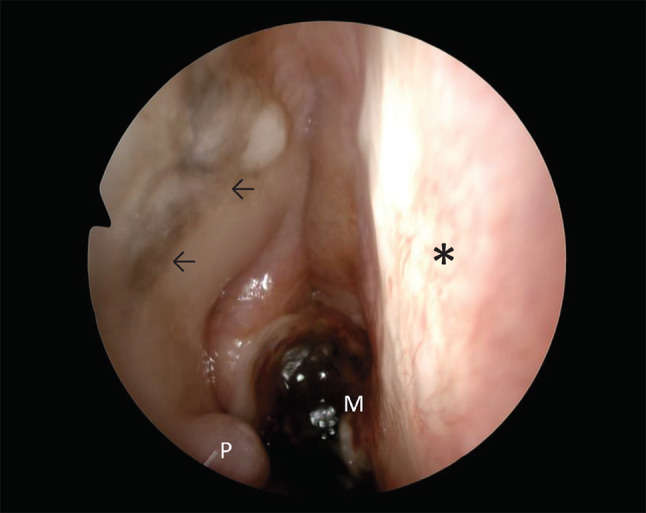
Endoscopic view of the right nasal cavity. Nasal septum (*), melanoma posterior nasal septum (M), polyp lateral wall with melanoma (P), adjacent pigmented lesion of the lateral wall (←) which proved to be non-invasive, but PRAME positive
Fig. 2.
Immunohistochemical staining of the index patient. A H&E stain of melanoma; B PRAME immunohistochemical stain of melanoma; C H&E stain of separate focus with invasive and in situ melanoma; D corresponding PRAME immunohistochemical stain; E H&E stain of surrounding mucosa with PRAME positive intra-epithelial melanocytes; F corresponding PRAME immunohistochemical stain. H&E hematoxylin and eosin stain; PRAME PReferentially expressed Antigen in Melanoma
Clinical Characteristics
A total of 26 sinonasal MM patients (including the index patient), 14 women (53.8%), and 12 men (46.2%), who were treated over a 10-year period were identified and included. The clinical characteristics and treatment strategies are outlined in Table 1. The median age at the time of diagnosis was 75 years (IQR 65–85). Tumors occurred most frequently laterally in the nasal cavity (53.8%), followed by the nasal septum (23.1%), maxillary sinus (11.5%), ethmoid sinus (7.7%), and nasal vestibule (3.8%). Out of 26 patients, the majority presented with stage III disease (50%), five (19.3%) had lymph node metastases at first clinical presentation, and five presented with distant metastases prior to treatment. Surgery was the primary treatment modality for 18 patients (69.2%). Depending on tumor size, localization, and patient fitness, surgery consisted of either endonasal endoscopic tumor resection (14; 53.8%), craniofacial resection (2; 7.7%) lateral rhinotomy (1; 3.8%), or nose amputation (1; 3.8%). One of the patients who presented with lymph node metastases also underwent neck dissection. Out of the remaining eight patients, all of whom were treated with palliative intent, two (7.7%) underwent radiotherapy, another two (7.7%) received immunotherapy, one (3.8%) had chemotherapy, and two (7.7%) received best supportive care until their death. Following treatment, 13 patients (50%) developed recurrent disease. Six patients (23.1%) developed a local recurrence, four (15.4%) of which had repeating recurrences up to four times. Four (15.4%) patients developed distant metastases, while a combination of (loco)regional and distant metastases occurred in three (11.%) patients. The estimated 3-year RFS was 16.4% (Supplementary Fig. 1A). The median follow-up duration was 18 months (IQR 5–30). Out of 26 patients, at the most recent moment of follow-up, 14 (53.8%) had died due to disease progression, seven (26.9%) displayed no evidence of disease, three (11.5%) were alive with active disease, and one (3.8%) had died to due to intercurrent disease. One patient who preferred to be treated in his country of birth was lost to follow-up. Estimated 3-year OS and DSS were 40.0% and 48.0%, respectively (Supplementary Fig. 1B, C).
Table 1.
Clinical characteristics of 26 patients with a sinonasal mucosal melanoma
| Patient No. | Sex | Age at diagnosis* | Tumor localization | Staging at diagnosis** | Treatment strategy | Recurrent disease | Recurrent disease treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 78 | Maxillary sinus | cT4aN0M0 (IVa) | BSC | – | – | DOD |
| 2 | F | 76 | Nasal cavity (lateral) | cT3N0M0 (III) | S | – | – | NED |
| 3 | M | 79 | Nasal cavity (septum) | cT3N1M0 (IVa) | S + RT | Local (×2) | S (×2) (c) | DID |
| 4a | F | 68 | Nasal cavity (septum) | cT3N0M0 (III) | S |
Local (×2); Local; Regional + distant |
S (×2) (c); S + RT (c); I (p) |
AWD |
| 5 | F | 63 | Nasal cavity (lateral) | cT4bN1M0 (IVb) | S + RT + I | – | – | AWD |
| 6 | M | 71 | Ethmoid sinus | cT4bN0M0 (IVb) | S + RT | Distant | BSC | DOD |
| 7 | F | 82 | Nasal cavity (lateral) | cT3N0M0 (III) | S | Local + distant | RT + I (p) | DOD |
| 8 | M | 73 | Nasal cavity (lateral) | cT3N0M0 (III) | S | Local | S (c) | NED |
| 9 | F | 59 | Nasal cavity (septum) | cT3N0M0 (III) | S | Local (4x) | S (×2) (c) | NED |
| 10 | F | 73 | Maxillary sinus | cT41N0M0 (IVa) | RT (p) | – | – | DOD |
| 11 | F | 77 | Nasal cavity (lateral) | cT4aN0M1 (IVc) | I + RT (p) | – | – | AWD |
| 12 | F | 74 | Nasal cavity (lateral) | cT3N0M0 (III) | S | Distant | BSC | DOD |
| 13 | F | 59 | Nasal cavity (septum) | cT4aN0M0 (IVa) | S + RT | Regional + distant | BSC | DOD |
| 14 | M | 85 | Nasal cavity (lateral) | cT4aN1M0 (IVb) | S + RT | . | DOD | |
| 15 | F | 60 | Nasal cavity (lateral) | cT3N0M0 (III) | S | Local | S + RT (c) | NED |
| 16 | M | 80 | Maxillary sinus | cT1N0M1 (IVc) | I (p) | – | – | DOD |
| 17 | M | 92 | Nasal cavity (lateral) | cT3N0M0 (III) | S | Local | RT (c) | NED |
| 18 | M | 75 | Nasal cavity (lateral) | cT3N0M0 (III) | S + RT | Distant | RT (p) | DOD |
| 19 | F | 90 | Nasal cavity (lateral) | cT3N0M0 (III) | RT (p) | – | – | DOD |
| 20 | M | 58 | Ethmoid sinus | cT4aN0M1 (IVc) | . | – | – | LTF |
| 21 | M | 77 | Nasal cavity (lateral) | cT4bN1M1 (IVc) | BSC | – | – | DOD |
| 22 | M | 46 | Nasal vestibule | cT4aN1M1 (IVc) | CT | – | – | DOD |
| 23 | F | 51 | Nasal cavity (lateral) | cT3N0M0 (III) | S + RT | Distant | I (p) | DOD |
| 24 | F | 84 | Nasal cavity (lateral) | cT4aN0M0 (IVa) | S + RT | – | – | NED |
| 25 | M | 51 | Nasal cavity (septum) | cT4aN0M0 (IVa) | S + RT |
Local; Local; Local |
S + RT (c); S (c) S + I (c) |
DOD |
| 26 | F | 77 | Nasal cavity (septum) | cT3N0M0 (III) | S | – | – | NED |
M male; F female; BSC best supportive care; S surgery; RT radiotherapy; I immune therapy; CT chemotherapy; (p) palliative; (c) curative; DOD died of disease; AWD alive with disease; DID died of intercurrent disease; NED no evidence of disease; LTF lost to follow-up
*Age at diagnosis determined on the date of histopathologic confirmation
**Staged in accordance with the TNM 8th edition; Head and Neck; Tumors of the nose and paranasal sinuses; Staging prior to treatment
aIndex patient
Immunohistochemistry and Molecular Characteristics
Expression of PRAME was assessed in 23 patients. Tissue samples for PRAME immunohistochemistry were not available for three patients. Additionally, NGS and SNP array analyses were performed in 18 and eight cases, respectively. Immunohistochemistry and molecular characteristics are displayed in Table 2. PRAME expression was found in all assessed patients (100%), in two cases (no. 2 and 4) both in the melanoma and in melanocytes in the adjacent epithelium. The most commonly occurring mutations were found in NRAS (7; 38.9%), followed by NF1 (2; 11.1%). Mutations within the PIK3CA, PTEN, TERT and TP53 genes were all found once. All tumors in which SNP array was performed showed ≥ 5 CNVs with a median of 29.5 CNVs (IQR 23.25–40).
Table 2.
Immunohistochemical and molecular findings in 26 patients with sinonasal mucosal melanoma
IHC: ‘+’ = positive; ‘+/-‘ = moderate; ‘-‘ = negative. NGS: WNT pathway mutations (purple), MAPK pathway mutations (pink), PI3K pathway mutations (green), and other mutations (blue). Variant allele frequency provided for each identified mutation. IHC immunohistochemistry; NGS next generation sequencing; SNP single nucleotide polymorphism; NP not performed; CN-LOH copy-neutral loss of hetrozigosity
*Tumor cells displayed varying degrees of PRAME expression, but PRAME staining was suboptimal due to tissue damage
Discussion
Mucosal melanomas arise from melanocytes of the mucosal lining. In the head and neck area, these tumors are predominantly found in the nasal cavity and paranasal sinuses. There has been an unexplained increase in the incidence of sinonasal MM [12]. In addition, different mutational signatures have been observed in MM occurring in facial subsites compared to MM in lower body sites, further stressing the unique biology of sinonasal MM.
Mucosal melanomas are different from cutaneous melanomas in many respects. Compared to cutaneous melanoma, MM displays a relatively low mutational burden with a reduced UV radiation-related mutation load, but a greater load of structural chromosomal variants [13]. The types of common driver mutations differ considerably between cutaneous melanoma and MM [14]. Targetable BRAF and KIT mutations do not play a significant role, being detected in only as much as 4% of sinonasal MM, while NRAS mutations are found in up to 42% of cases [15–17]. The present study found a high frequency of CNVs as all analysed cases displayed a minimum of five CNVs with a median of 29.5 CNVs, which is in accordance with previous reports [14, 16, 17]. Furthermore, a relatively high frequency of activating mutations in NRAS in 38.9% of the analysed cases was observed. Currently, no NRAS-targeted drug is available. However, up to 20% of NRAS-mutated mucosal melanoma patients have shown a partial response to MEK-inhibitors (i.e. binimetinib), the safety and efficacy of which have been confirmed in an ongoing phase II trial [18]. Other mutations occurred in APC, BRAF, NF1, NRAS, PIK3CA, PTEN, SMADA4, TERT, and TP53 genes. Our findings are mostly in line with a recent analysis of 90 sinonasal MM cases by Chłopek et al., who found mutations in the aforementioned genes, as well as CNNB1, mTOR, and AMER1 mutants [17]. Other earlier studies did not report specifically on sinonasal MM. For instance, a systematic review and meta-analysis on MM by Broit et al. identified significant driver mutations in KIT, NF1, BRAF, NRAS, SF3B1, and SPRED1, as well as a wide variety of other genomic alterations, but a subanalysis for sinonasal MM was not performed [14]. Newell et al. have reported mutations in NRAS, BRAF, NF1, KIT, SF3B1, TP53, SPRED1, ATRX, HLA-A, CHD8, and CTNNB1 in a cohort of 67 MM patients with tumors of varying anatomic origin [13]. Notably, the majority of their cohort (70%) harbored mutations that would render the tumor potentially responsive to CDK4/6 inhibitors (e.g. abemaciclib). A similar finding was reported by Jurmeister et al., who found chromosomal or epigenetic alterations of genes associated with the CDK4/6 pathway (CCND1, CDKN2A, CDK4, RB1) in the majority of a cohort of 63 patients. The same study also reported a loss of PTEN positivity in immunohistochemistry of all sinonasal MM within said cohort, suggesting that PTEN promotor methylation is potentially an important step in the development of sinonasal MM [16]. These studies underline the genetic differences between cutaneous melanoma and MM, which can in part be responsible for their different biological behaviour and response to treatment.
Accordingly, response rates to checkpoint inhibition in metastatic mucosal melanoma have been poor compared to metastatic cutaneous melanoma. Following the introduction of immunotherapy in the treatment of melanoma in The Netherlands, the median overall survival for metastatic mucosal melanoma barely increased from 8.7 months (IQR 6.9–17.7) in 2013–2014 to 8.9 months (IQR 6.8–13.5) in 2015–2017. Meanwhile, the median survival for patients with metastatic cuteanous melanoma improved from 11.3 months (IQR 10.2–12.4) to 16.9 months (IQR 15.4–18.2) over the same period [6]. Due to the low frequency of BRAF mutations in (sinonasal) MM in comparison to cutaneous melanoma, systemic treatment options for patients with recurrent, unresectable, or metastatic sinonasal melanoma are very limited. Therefore, there is a need for the identification of new therapeutic targets. Previous studies identified the PReferential expressed Antigen in MElanoma (PRAME) as a potential therapeutic target in sinonasal melanoma. PRAME is a melanoma-associated antigen coded by the PRAME-gene, located on 22q11.22. It was discovered through autologous T-cell epitope cloning in a patient with metastatic cutaneous melanoma and was first reported in 1997 [7]. It was subsequently discovered that PRAME is expressed not only in cutaneous melanoma but also in MM, ocular melanoma, and various non-melanocytic malignant neoplasms (e.g. non-small cell lung cancer, breast carcinoma, ovarian carcinoma, renal cell carcinoma, haematological malignancies, various sarcoma subtypes) [19, 20]. Within our cohort, PRAME expression occurred in all assessed patients. This exceeds the findings of previous studies which reported evident staining for PRAME in MM in 83.3–86% of cases, with no significant difference between locations [21, 22]. Both NRAS mutation and PRAME expression are associated with worse prognosis in mucosal melanoma, but there seems to be no correlation between both [21].
In our index patient, we evaluated all resected material for PRAME expression. Besides PRAME expression in the mucosal melanoma, immunohistochemistry surprisingly revealed PRAME expression in all resected clinically non-suspect pigmented mucosal lesions as well. This finding suggests that similar to squamous cell carcinoma of the head and neck, field-cancerization (“field-melanomization”) could be responsible for developing new recurrent lesions in surrounding mucosal subsites. This would call for a multimodal approach, including resection of the MM in combination with systemic treatment rather than a local approach consisting of (multiple) resections.
In recent years, PRAME has increasingly gained interest as a potential target for immunotherapy (e.g. cancer vaccine or adoptive T cell therapies) [20, 23]. In 2006 the FDA approved MKC1106-PP (Mannkind Corporation, USA), a vaccine targeting PRAME as well as PSMA (prostate-specific membrane antigen). In a phase 1 study, MKC1106-PP was well tolerated and showed some signs of efficacy with immune responses in 15/24 patients, which unfortunately did not translate into clinical responses [24]. In a phase I study, Gutzmer evaluated recPRAME, a recombinant PRAME immunotherapeutic, and found an acceptable safety profile with PRAME-specific-CD4 + T-cell responses between 46 and 75%. Since no (probably necessary) CD8 + T cell responses against PRAME could be detected, this vaccine has not been taken into further development [25]. Similarly, ISA pharmaceuticals has developed a PRAME-based vaccine which is currently in preclinical development [26].
In conclusion, sinonasal mucosal melanoma harbors a unique mutational signature and seems to be rising in incidence. The peritumoral PRAME positive melanocytes in non-invasive pigmented lesions in the presented case indicate that besides therapeutic possibilities, PRAME might also be of value in understanding the oncogenesis of multifocal melanoma of the sinonasal mucosa.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1 (TIF 353.4 kb)
Kaplan–Meier estimates of 26 patients with asinonasal mucosal melanoma. A = 3-year recurrence-free survival; B = 3-yeardisease-specific survival; C = 3-year overall survival
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by WFJS, WWB, RWJM, and GEB. The first draft of the manuscript was written by WFJS and WWB and all authors commented on previous versions of the manuscript. RdB, JAR, and GEB supervised the study. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Data Availability
Upon reasonable request.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study formal consent is not required.
Consent to Participate
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent of publication is not required.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
W. F. Julius Scheurleer and W. Weibel Braunius have contributed equally to this manuscript.
References
- 1.Ascierto P, Accorona R, Botti G, et al. Mucosal melanoma of the head and neck. Crit Rev Oncol Hematol. 2017;112:136–152. doi: 10.1016/j.critrevonc.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Prera JC. Update from the 5th Edition of the World Health Organization classification of head and neck tumors: the neck and lymph nodes, metastasis, and melanocytic tumors. Head Neck Pathol. 2022;16(1):110–122. doi: 10.1007/s12105-022-01433-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brierley JD, Gospodarowicz MK, Wittekind C. The TNM classification of malignant tumours. 8. Hoboken: Wiley; 2016. [Google Scholar]
- 4.Christopherson K, Malyapa RS, Werning JW, et al. Radiation therapy for mucosal melanoma of the head and neck. Am J Clin Oncol. 2015;38(1):87–89. doi: 10.1097/COC.0b013e31828d73bf. [DOI] [PubMed] [Google Scholar]
- 5.Owens JM, Roberts DB, Myers JN. The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch Otolaryngol Head Neck Surg. 2003;129(8):864–868. doi: 10.1001/archotol.129.8.864. [DOI] [PubMed] [Google Scholar]
- 6.van Zeijl MCT, Boer FL, van Poelgeest MIE, et al. Survival outcomes of patients with advanced mucosal melanoma diagnosed from 2013 to 2017 in the Netherlands—a nationwide population-based study. Eur J Cancer. 2020;137:127–135. doi: 10.1016/j.ejca.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda H, Lethé B, Lehmann F, et al. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6(2):199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 8.Lezcano C, Jungbluth AA, Busam KJ. Comparison of immunohistochemistry for PRAME with cytogenetic test results in the evaluation of challenging melanocytic tumors. Am J Surg Pathol. 2020;44(7):893–900. doi: 10.1097/PAS.0000000000001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebbelaar CF, Schrader AMR, van Dijk M, et al. Towards diagnostic criteria for malignant deep penetrating melanocytic tumors using single nucleotide polymorphism array and next-generation sequencing. Mod Pathol. 2022;35(8):1110–1120. doi: 10.1038/s41379-022-01026-6. [DOI] [PubMed] [Google Scholar]
- 10.Ebbelaar CF, Jansen AML, Bloem LT, Blokx WAM. Genome-wide copy number variations as molecular diagnostic tool for cutaneous intermediate melanocytic lesions: a systematic review and individual patient data meta-analysis. Virchows Arch. 2021;479(4):773–783. doi: 10.1007/s00428-021-03095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strengman E, Barendrecht-Smouter FAS, de Voijs C, et al. Amplicon-based targeted next-generation sequencing of Formalin-Fixed, paraffin-embedded tissue. Methods Mol Biol. 2019;1908:1–17. doi: 10.1007/978-1-4939-9004-7_1. [DOI] [PubMed] [Google Scholar]
- 12.Marcus DM, Marcus RP, Prabhu RS, et al. Rising incidence of mucosal melanoma of the head and neck in the United States. J Skin Cancer. 2012 doi: 10.1155/2012/231693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newell F, Kong Y, Wilmott JS, et al. Whole-genome landscape of mucosal melanoma reveals diverse drivers and therapeutic targets. Nat Commun. 2019;10(1):3163. doi: 10.1038/s41467-019-11107-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broit N, Johansson PA, Rodgers CB, et al. Meta-analysis and systematic review of the genomics of mucosal melanoma. Mol Cancer Res. 2021;19(6):991–1004. doi: 10.1158/1541-7786.MCR-20-0839. [DOI] [PubMed] [Google Scholar]
- 15.Zebary A, Jangard M, Omholt K, Ragnarsson-Olding B, Hansson J. KIT, NRAS and BRAF mutations in sinonasal mucosal melanoma: a study of 56 cases. Br J Cancer. 2013;109(3):559–564. doi: 10.1038/bjc.2013.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurmeister P, Wrede N, Hoffmann I, et al. Mucosal melanomas of different anatomic sites share a common global DNA methylation profile with cutaneous melanoma but show location-dependent patterns of genetic and epigenetic alterations. J Pathol. 2022;256(1):61–70. doi: 10.1002/path.5808. [DOI] [PubMed] [Google Scholar]
- 17.Chłopek M, Lasota J, Thompson LDR, et al. Alterations in key signaling pathways in sinonasal tract melanoma. A molecular genetics and immunohistochemical study of 90 cases and comprehensive review of the literature. Mod Pathol. 2022 doi: 10.1038/s41379-022-01122-7. [DOI] [PubMed] [Google Scholar]
- 18.Ma Y, Xia R, Ma X, Judson-Torres RL, Zeng H. Mucosal melanoma: pathological evolution, pathway dependency and targeted therapy. Front Oncol. 2021;11:702287. doi: 10.3389/fonc.2021.702287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roszik J, Wang WL, Livingston JA, et al. Overexpressed PRAME is a potential immunotherapy target in sarcoma subtypes. Clin Sarcoma Res. 2017;7:11. doi: 10.1186/s13569-017-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Y, Zou R, Wang J, Wang ZW, Zhu X. The role of the cancer testis antigen PRAME in tumorigenesis and immunotherapy in human cancer. Cell Prolif. 2020;53(3):e12770. doi: 10.1111/cpr.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toyama A, Siegel L, Nelson AC, et al. Analyses of molecular and histopathologic features and expression of PRAME by immunohistochemistry in mucosal melanomas. Mod Pathol. 2011;32(12):1727–1733. doi: 10.1038/s41379-019-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hovander D, Allen J, Oda D, Moshiri AS. PRAME immunohistochemistry is useful in the diagnosis of oral malignant melanoma. Oral Oncol. 2022;124:105500. doi: 10.1016/j.oraloncology.2021.105500. [DOI] [PubMed] [Google Scholar]
- 23.Al-Khadairi G, Decock J. Cancer testis antigens and immunotherapy: where do we stand in the targeting of PRAME? Cancers. 2019;11(7):984. doi: 10.3390/cancers11070984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber JS, Vogelzang NJ, Ernstoff MS, et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother. 2011;34(7):556–567. doi: 10.1097/CJI.0b013e3182280db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutzmer R, Rivoltini L, Levchenko E, et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: results of a phase I dose escalation study. ESMO Open. 2016;1(4):e000068. doi: 10.1136/esmoopen-2016-000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ISA103 (PRAME). ISA pharmaceuticals. https://doi.org/https://www.isa-pharma.com/isa103-prame/. Accessed Nov 16 2022
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (TIF 353.4 kb)
Kaplan–Meier estimates of 26 patients with asinonasal mucosal melanoma. A = 3-year recurrence-free survival; B = 3-yeardisease-specific survival; C = 3-year overall survival
Data Availability Statement
Upon reasonable request.
Not applicable.




