Abstract
Head and neck squamous cell carcinoma (HNSCC) is the world’s 6th most common malignancy. Oral cavity SCC (OCSCC) represents approximately one third of the HNSCC cases diagnosed annually in the United States. Despite therapeutic advances, OCSCC is frequently lethal, with a modest 5-year survival. Because OCSCC is often preceded by premalignant lesions, it is an ideal disease for screening initiatives. The conventional visual and tactile exam (CVTE), coupled with a tissue biopsy, remains the gold standard. However, CVTE alone cannot reliably differentiate between reactive/inflammatory and dysplastic lesions. Further, the histologic diagnosis of dysplasia is subjective in nature and a highly imperfect predictor of malignant transformation. This prognostic uncertainty creates a significant clinical management dilemma—watchful waiting with increased patient psychological and economic burdens versus unnecessary aggressive treatment. As such, the development and validation of novel diagnostic platforms such as Artificial Intelligence (AI) and prognostic molecular biomarkers may help address these critical unmet clinical needs.
Keywords: Oral dysplasia, Grading, World Health Organization classification
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy in the world and is associated with significant morbidity and mortality. Oral cavity SCC (OCSCC) represents a subset of ~ 36% of the 54,000 cases of HNSCC diagnosed annually in the United States [1]. Tobacco use and excessive alcohol consumption are the major etiologic factors for OCSCC. While prominent in oropharyngeal SCC, high-risk human papilloma virus infection (HPV) is not a major etiologic factor for OCSCC [2–5]. Despite numerous therapeutic advances, the overall long-term survival for patients with HPV-negative OCSCC remains a modest ~ 68%, with early and late-stage survivorship of 86.3% and 40.4%, respectively [6]. Therefore, improved methods of screening, diagnosis, prognostication, and intervention are critical. OCSCC is often preceded by a clinically diverse group of lesions defined as ‘oral potentially malignant disorders’ (OPMDs, Table 1) that possess variable risks of malignant transformation [7]. The world-wide prevalence of OPMD is 4.47% and differs among populations with Asians and South Americans having the highest (10.54%) and North Americans with the lowest (0.1%) [8]. While OPMDs can involve any site in the oral cavity, lesions involving the lateral/ventral tongue and floor of mouth are associated with a higher risk of progression [9].
Table 1.
World Health Organization list of oral potentially malignant disorders found in the oral cavity [10]
| Oral potentially malignant disorders | |
|---|---|
| Risk/associate groups | Entities |
| Clinical diagnosis | Erythroplakia |
| Erythroleukoplakia | |
| Leukoplakia | |
| Clinical and histologic | Proliferative verrucous leukoplakia |
| Smoking/tobacco related | Submucous Fibrosis |
| Palatal lesions associated with reversed smoking | |
| Smokeless tobacco keratosis (dependent on tobacco type) | |
| Miscellaneous | Oral lichenoid lesions (resemble lichen planus but lack typical clinical or histopathologic features) |
| Oral graft versus host disease | |
| Autoimmune/inherited | Lupus erythematosus |
| Familial cancer syndromes | |
The conventional visual and tactile exam (CVTE) is the current gold standard for OPMD screening [11–14]. Despite its importance, the CVTE has significant limitations. First, the CVTE cannot reliably discriminate between reactive/inflammatory and premalignant/early malignant lesions. Second, some premalignant lesions cannot be readily identified during a CVTE [15–18]. Since premalignant oral lesions cannot be accurately identified solely on the basis of their clinical characteristics, biopsy and histologic evaluation are critical. Lesions are considered premalignant and at risk for progressing to OCSCC when the histologic diagnosis of dysplasia is rendered. However, from a biological, diagnostic and clinical management perspective, a definition of oral dysplasia is problematic for several reasons.
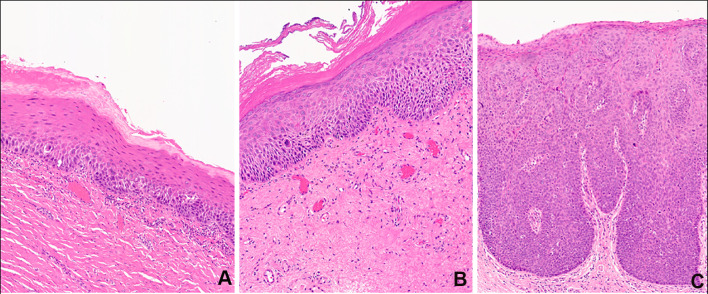
First, the criteria for diagnosing and grading oral dysplasia are not uniformly standardized, subjective in nature, and open to a wide range of interpretation. The concept of grading oral epithelial dysplasia was first introduced ~ 60 years ago. Since then, there have been many proposed grading systems including the Ljubljana system, the Brothwell system, and the binary and the three-tier grading systems [19]. Variations of the three-tier grading system have been in practice for several decades. This approach has traditionally classified dysplasia based on the number of thirds of the squamous epithelium affected by the cytologic and architectural atypia. Mild dysplasia is characterized by cytological atypia limited to the basal third, moderate dysplasia with extension into the middle third and severe dysplasia extending into the upper third (Fig. 1). However, using this arbitrary, one third approach oversimplifies the complexity of dysplasia grading as cytologic atypia confined to the basal layer can also be classified as severe dysplasia if the other features such as bulbous rete processes, budding, disorganization of the basal cells, and marked cytological atypia are present. To potentially address this, the most recent edition of the World Health Organization Blue Book maintained a three-tiered approach with an expanded criterion for oral epithelial dysplasia that includes sixteen architectural features and twelve cytological features (Table 2, 3) [10]. Alternatively, Kujan et al. recently proposed a binary grading system that segregated oral dysplasia into low-grade and high-grade groups depending on the number of observed architectural and cytological changes [20]. To be classified as high-grade dysplasia, a lesion required at least four architectural features (irregular epithelial stratification, loss of polarity of basal cells, drop-shaped rete ridges, increased number of mitotic figures, abnormally superficial mitoses, premature keratinization in single cells and keratin pearls within rete ridges) and five cytological features (abnormal variation in nuclear size, abnormal variation in nuclear shape, abnormal variation in cell size, abnormal variation in cell shape, increased nuclear-to-cytoplasmic ratio, increased nuclear size, atypical mitotic figures, increased number and size of nucleoli and hyperchromatism). Alternatively, low-grade dysplasia would have less than four architectural changes and less than five cytologic features [20]. Those that advocate for a two-tier system cite a greater inter-observer agreement than a three-tiered system. Conversely, others believe that the two-tiered system provides less information to the clinician than a three-grade system. However, despite these attempts to develop standardized criteria, numerous studies have found histologic grading to be notoriously unreliable with both poor inter- and intra-observer agreement [21–28]. For example, a study involving six board-certified oral & maxillofacial pathologists observed intra-examiner and inter-examiner agreement in the diagnosis of oral dysplasia of just 50.8% and 50.5%, respectively [29]. One potential solution for this challenge may be case adjudication involving multiple pathologists. In one recent study, an initial agreement of 69.9% by two pathologists was improved to 92.7% when a third pathologist was added to the case review [30]. While this study demonstrates the value of case adjudication for diagnostic consensus of oral dysplasia, in practice, it may be particularly difficult to implement such a system in a real-world setting.
Fig. 1.
Representative histological examples of A mild, B moderate, and C severe oral dysplasia using the three-tier grading system. If one were to utilize a two-tier grading system, the mild dysplasia would be characterized as low-grade dysplasia, while the moderate and severe lesion would be diagnosed as high-grade dysplasia
Table 2.
World Health Organization architectural features for oral epithelial dysplasia
| Architectural features | |
|---|---|
| Group features | Specific features |
| Basal features | Irregular stratification |
| Drop-shaped rete processes | |
| *Basal cell clustering/nesting | |
| Loss of polarity or disorganization of basal cells | |
| *Expanded proliferative compartment | |
| Mitotic features | Mitoses high in epithelium |
| Mitoses in maturing cells | |
| Keratin features | Reduced keratinocyte cohesion |
| Keratin pearls in rete processes | |
| Generalized premature keratinization | |
| *Altered keratin pattern for oral subsite | |
| Structural features | *Verrucous or papillary architecture |
| *Multifocal or skip lesions | |
| *Sharply defined margin to changes | |
| *Multiple different patterns of dysplasia | |
| *Extension of changes along minor gland ducts | |
*Denotes new additions to the criteria [10]
Table 3.
World Health Organization cytological features for oral epithelial dysplasia
| Cytological features | |
|---|---|
| Group features | Specific features |
| Nuclear features | Abnormal variation in nuclear size (anisonucleosis) |
| Abnormal variation in nuclear shape (nuclear pleomorphism) | |
| *Increased nuclear size | |
| Hyperchromatic nuclei | |
| Nucleolar features | Increased number and size of nucleoli |
| Keratin features | *Single cell keratinization |
| Mitotic features | Increased mitotic activity |
| *Apoptotic mitoses | |
| Atypical mitotic figures | |
| Cell features | Abnormal variation in cell size |
| Abnormal variation in cell shape | |
| Increased nuclear:cytoplasm ratio | |
*Denotes new additions to the criteria [10]
In addition to formalizing an objective and reproducible grading scale for oral dysplasia, we face several additional conundrums in the context of oral premalignancies. For example, the interchangeable use of carcinoma in situ and severe dysplasia of the oral cavity is an additional contentious topic. The American Joint Committee on Cancer (AJCC) Cancer Staging Manual uses the term carcinoma in situ (Tis) instead of severe dysplasia [31]. However, most oral & maxillofacial/head & neck pathologists consider oral cavity carcinoma in situ and severe dysplasia to be synonymous. While both terms are technically an accurate description of oral mucosa demonstrating full thickness atypia, these terms often lead to confusion, particularly with the otolaryngology/head and neck surgeon community. Further, the current “Lip and Oral Cavity” AJCC chapter does include a discussion of premalignancy/dysplasia (other than Tis). Perhaps future editions will include a section describing the grading of oral dysplasia that also harmonizes these diagnostic terms. In addition, the 5th edition of the World Health Organization Blue Book introduced the concept of human papilloma virus (HPV)-associated oral epithelial dysplasia (HPVOED) [32]. These lesions are most commonly observed on the tongue and the floor of the mouth, and are characterized by histologically distinctive viral cytopathic changes such karyorrhectic and apoptotic cells [33]. At this time, there are no validated histologic grading criteria for HPVOED. Similarly, there are limited data regarding the risk of malignant transformation. Therefore, the current recommendations are that HPVOED should be histologically graded and clinically managed as conventional oral dysplasia. Finally, proliferative verrucous leukoplakia (PVL) is a distinctive clinico-pathologic entity that was recently described. It presents as a multifocal and progressive disease with high rates of progression to squamous cell carcinoma. At this time, the definitive diagnostic criteria for this entity, including the presence/grading of dysplasia, are still under development. For example, the 5th edition WHO Blue Book provides a list of essential criteria for diagnosis that include the clinical presentation consistent with PVL as well as the histologic presentation of either “early” or “late” lesions. The histologic features of early lesions consist of premature keratinization with minimal cytologic atypia. Conversely, the histologic features of late lesions are described as having verrucous, nodular, or bulky architecture with variable amounts of dysplasia [34]. Alternatively, an expert consensus panel created histologic categories for PVL lesions that included corrugated orthohyperkeratosis, bulky hyperkeratosis epithelial proliferation, and squamous cell carcinoma. Importantly, the working group found that architectural findings were more important than the presence of atypical cytologic features or degrees of dysplasia [35]. Overall, while it is possible that some degree of dysplasia may be observed in PVL, the histologic grading of this dysplasia does not appear to have significant diagnostic or prognostic utility.
Second, in addition to the challenges related to reproducibly diagnosing and grading oral dysplasia, we currently lack validated histologic criteria for predicting the risk of malignant transformation. Currently, all dysplastic lesions are considered to be at risk for progressing to OCSCC. However, the vast majority of dysplasia will not progress to OCSCC, even over many years or decades of follow-up. As such, the histological finding of dysplasia, regardless of the grading system utilized, can only be used to indicate that a lesion has some undefined malignant potential. Several studies underscore this concept. Mincer et al. evaluated patients with oral dysplasia and followed them for up to 8 years. Only 11% of the lesions underwent malignant transformation during the observation period [36]. Likewise, Arduino et al. demonstrated that the 1-year outcomes of oral dysplasia were highly variable with ~ 40% disappearing, ~ 20% remaining stable, and ~ 7% progressing to OCSCC [37]. A more recent systematic review was performed with the meta-analysis of 992 patients with the purpose of investigating an evidence-based management policy for oral dysplastic lesions. The mean overall malignant transformation rate (MTR) was 12.1%, with a mean time to malignant transformation of 4.3 years. When stratified by grade, the MTR was 10.3% for mild/moderate dysplasia and 24.1% for severe dysplasia/carcinoma in situ [38]. Finally, a recent case–cohort study investigated the histologic predictors of progression to OCSCC within a large, integrated United States healthcare system. It found that the 5-year absolute risk of progression to OCSCC when stratified by histologic grade was 11.9% for mild dysplasia, 8.7% for moderate dysplasia, and 32.9% for severe dysplasia [39]. Overall, these findings emphasize the reality that we are currently unable to accurately prognosticate on the basis of histologic alterations and underscore the need to develop additional protocols to refine our diagnostic skills and address the diagnostic/prognostic dilemma outlined in Fig. 2.
Fig. 2.

Squamous cell carcinoma progression model. Mucosal lesion do not follow a linear progression pattern to oral cavity squamous cell carcinoma. While a small percentage of dysplastic lesion will progress to OSCC, the majority will either remain quiescent or regress
What are some potential alternative approaches to improving the diagnostic and prognostic accuracy of oral dysplasia? Two possibilities may include artificial intelligence (AI) and molecular diagnostics. AI can be defined as the field of computer science that seeks to reproduce human intelligence when analyzing large datasets. The term AI is an overarching term that includes the areas of machine learning and deep learning. There has been considerable interest in AI-based platforms in the fields of science and healthcare, particularly in the areas of imaging and diagnosis. In the context of diagnostic pathology, it is postulated that the combination of pattern recognition and the discovery of novel, and not readily identifiable, histopathologic characteristics may result in improved diagnostics and treatment [40–44]. In the context of OCSCC diagnosis, several studies have validated the potential diagnostic utility of AI, particularly deep learning [45–50]. Importantly, the inclusion of AI platforms may also reduce diagnosis delays and outcomes [51, 52]. AI has also been reported to facilitate and the identification of premalignant lesions in a number of anatomic sites including esophagus, cervix, and the oral cavity with high diagnostic accuracy [53–57]. To date, there are a limited number of studies that have specifically investigated the potential role of AI in the context of oral dysplasia and predicting the risk of malignant transformation. While some of the studies investigating AI in the context of oral dysplasia contained cohorts of lesions that did and did not progress to OCSCC, only one manuscript has reported the identification of potentially novel prognostic features. In sum, at this time, while there are data to support a potential role for AI in diagnosing oral dysplasia, a general consensus of the literature is that current AI platforms/algorithms may be best utilized to augment conventional diagnostic practices rather than replacing them all together. However, future studies may improve upon the ability of AI to aid in the diagnosis and prognostication of dysplastic oral lesions. Epithelial carcinogenesis is thought to be a multi-step process involving multiple genetic alterations in a population of cells that can be observed at the DNA, RNA, and protein levels. These genotypic changes result in concomitant phenotypic alterations which promote uncontrolled cell proliferation and survival. In recent years, the molecular landscape has been largely defined for a number of malignancies including HNSCC and some of these alterations can now be used to aid in the diagnosis, classification, and treatment [58–60]. A number of molecular changes have also been observed in oral dysplasia [61–64]. However, to date, the molecular landscape of oral premalignancy has not been fully characterized. While not currently available as standard of care, with further research and clinical validation, it is likely that both AI and molecular diagnostics will play an important role in more accurate diagnoses of oral dysplasia as well as improve our ability to prognosticate/quantify the risk of lesions to progress to OCSCC.
Author Contributions
Each author confirms he/she has contributed to reading and approving the final manuscript.
Funding
This study was not supported by any funding.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
For this type of study, formal consent is not required.
Informed Consent
For this type of study, informed consent is not required.
Consent for Publication
For this type of study, consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics. CA Cancer J Clin. 2023 doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Poling JS, Ma XJ, Bui S, Luo Y, Li R, Koch WM, Westra WH. Human papillomavirus (HPV) status of non-tobacco related squamous cell carcinomas of the lateral tongue. Oral Oncol. 2014 doi: 10.1016/j.oraloncology.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsague X, et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 patients. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 4.Lingen MW, et al. Low etiologic fraction for high-risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013 doi: 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Zafereo ME, et al. Squamous cell carcinoma of the oral cavity often overexpresses p16 but is rarely driven by human papillomavirus. Oral Oncol. 2016 doi: 10.1016/j.oraloncology.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SEER Cancer Statistics Factsheets . Oral cavity and pharynx cancer—cancer stat facts. NIH; 2022. [Google Scholar]
- 7.Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007 doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 8.Mello FW, et al. Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med. 2018 doi: 10.1111/jop.12726. [DOI] [PubMed] [Google Scholar]
- 9.Woo SB. Oral epithelial dysplasia and premalignancy. Head Neck Pathol. 2019 doi: 10.1007/s12105-019-01020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization Classification of Tumors online (2023) Head and neck tumors, oral epithelial dysplasia (5th edition), https://tumourclassification.iarc.who.int/chapters/52. Accessed 1 January 2023
- 11.Lingen MW, Abt E, Agrawal N, et al. Evidence-based clinical practice guideline for the evaluation of potentially malignant disorders in the oral cavity. J Am Dent Assoc. 2017 doi: 10.1016/j.adaj.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Lingen MW, Tampi M, Urquhart O, et al. Adjuncts for the evaluation of potentially malignant disorders in the oral cavity; Diagnostic test accuracy systematic review and meta-analysis. J Am Dent Assoc. 2017 doi: 10.1016/j.adaj.2017.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh T, Warnakulasuriya S, Lingen MW, et al. Clinical assessment for the detection of oral cavity cancer and potentially malignant disorders in apparently healthy adults. Cochrane Database Syst Rev. 2021 doi: 10.1002/14651858.CD010173.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh T, Macey R, Kerr AR, et al. Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. 2021 doi: 10.1002/14651858.CD010276.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang WW, Fujii H, Shirai T, Mega H, Takagi M. Accumulative increase of loss of heterozygosity from leukoplakia to foci of early cancerization in leukoplakia of the oral cavity. Cancer. 2001 doi: 10.1002/1097-0142(20011101)92:9<2349::aid-cncr1582>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Mao L, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996 doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 17.Partridge M, et al. Detection of minimal residual cancer to investigate why oral tumors recur despite seemingly adequate treatment. Clin Cancer Res. 2000;6(7):2718–2725. [PubMed] [Google Scholar]
- 18.Thomson PJ, Soames JV, Booth C, O'Shea JA. Epithelial cell proliferative activity and oral cancer progression. Cell Prolif. 2002 doi: 10.1046/j.1365-2184.35.s1.12.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odell E, Kujan O, Warnakulasuriya S, Sloan P. Oral epithelial dysplasia: recognition, grading and clinical significance. Oral Dis. 2021 doi: 10.1111/odi.13993. [DOI] [PubMed] [Google Scholar]
- 20.Kujan O, et al. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006 doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Karabulut A, et al. Observer variability in the histologic assessment of oral premalignant lesions. J Oral Pathol Med. 1995 doi: 10.1111/j.1600-0714.1995.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 22.Nankivell P, et al. The binary oral dysplasia grading system: validity testing and suggested improvement. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013 doi: 10.1016/j.oooo.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Dost F, Lê Cao K, Ford PJ, Ades C, Farah CS. Malignant transformation of oral epithelial dysplasia: a real-world evaluation of histopathologic grading. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014 doi: 10.1016/j.oooo.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan L, et al. Inter- and intra-observer variability in three grading systems for oral epithelial dysplasia. J Oral Maxillofac Pathol. 2016 doi: 10.4103/0973-029X.185928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008 doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 26.Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral Oncol. 2007 doi: 10.1016/j.oraloncology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Ranganathan K, Kavitha L, Sharada P, Bavle RM, Rao RS, Pattanshetty SM, Hazarey VK, Madhura MG, Nagaraj T, Lingappa A, Warnakulasuriya S. Intra-observer and inter-observer variability in two grading systems for oral epithelial dysplasia: a multi-centre study in India. J Oral Pathol Med. 2020 doi: 10.1111/jop.13056. [DOI] [PubMed] [Google Scholar]
- 28.Fischer DJ, Epstein JB, Morton TH, Schwartz SM. Interobserver reliability in the histopathologic diagnosis of oral pre-malignant and malignant oral lesions. J Oral Pathol Med. 2004 doi: 10.1111/j.1600-0714.2004.0037n.x. [DOI] [PubMed] [Google Scholar]
- 29.Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, Eisenberg E, Krutchkoff DJ, Cushing M. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995 doi: 10.1016/s1079-2104(05)80201-x. [DOI] [PubMed] [Google Scholar]
- 30.Speight PM, et al. Interobserver agreement in dysplasia grading: toward an enhanced gold standard for clinical pathology trials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015 doi: 10.1016/j.oooo.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, et al. AJCC cancer staging manual (8th edition) Springer International Publishing; 2017. [Google Scholar]
- 32.World Health Organization Classification of Tumors online (2023) Head and neck tumors, HPV-associated oral epithelial dysplasia (5th edition), https://tumourclassification.iarc.who.int/chapters/52/101. Accessed 1 January 2023.
- 33.Lerman MA, Almazrooa S, Lindeman N, Hall D, Villa A, Woo SB. HPV-16 in a distinct subset of oral epithelial dysplasia. Mod Pathol. 2017 doi: 10.1038/modpathol.2017.71. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization Classification of Tumors online. Head and Neck Tumors, Proliferative verrucous leukoplakia (5th edition). https://tumourclassification.iarc.who.int/chapters/52/104. Accessed 1 January 2023.
- 35.Thompson LDR, Fitzpatrick SG, Müller S, et al. Proliferative verrucous leukoplakia: an expert consensus guideline for standardized assessment and reporting. Head Neck Pathol. 2021 doi: 10.1007/s12105-020-01262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mincer HH, Coleman SA, Hopkins KP. Observations on the clinical characteristics of oral lesions showing histologic epithelial dysplasia. Oral Surg Oral Med Oral Pathol. 1972 doi: 10.1016/0030-4220(72)90468-9. [DOI] [PubMed] [Google Scholar]
- 37.Arduino PG, Surace A, Carbone M, Elia A, Massolini G, Gandolfo S, Broccoletti R. Outcome of oral dysplasia: a retrospective hospital-based study of 207 patients with a long follow-up. J Oral Pathol Med. 2009 doi: 10.1111/j.1600-0714.2009.00782.x. [DOI] [PubMed] [Google Scholar]
- 38.Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia—a systematic review and meta-analysis. Head Neck. 2009 doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 39.Chaturvedi AK, Udaltsova N, Engels EA, Katzel JA, Yanik EL, Katki HA, Lingen MW, Silverberg MJ. Oral Leukoplakia and risk of progression to oral cancer: a population-based cohort study. J Natl Cancer Inst. 2020 doi: 10.1093/jnci/djz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology-new tools for diagnosis and precision oncology. Nature Rev Clin Oncol. 2019 doi: 10.1038/s41571-019-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolezal JM, Srisuwananukorn A, Karpeyev D, Ramesh S, Kochanny S, Cody B, Mansfield AS, Rakshit S, Bansal R, Bois MC, Bungum AO, Schulte JJ, Vokes EE, Garassino MC, Husain AN, Pearson AT. Uncertainty-informed deep learning models enable high-confidence predictions for digital histopathology. Nat Commun. 2022 doi: 10.1038/s41467-022-34025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, Moreira AL, Razavian N, Tsirigos A. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018 doi: 10.1038/s41591-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kather JN, Heij LR, Grabsch HI, Loeffler C, Echle A, Muti HS, Krause J, Niehues JM, Sommer KAJ, Bankhead P, Kooreman LFS, Schulte JJ, Cipriani NA, Buelow RD, Boor P, Ortiz-Brüchle NN, Hanby AM, Speirs V, Kochanny S, Patnaik A, Srisuwananukorn A, Brenner H, Hoffmeister M, van den Brandt PA, Jäger D, Trautwein C, Pearson AT, Luedde T. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat Cancer. 2020 doi: 10.1038/s43018-020-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Echle A, Rindtorff NT, Brinker TJ, Luedde T, Pearson AT, Kather JN. Deep learning in cancer pathology: a new generation of clinical biomarkers. Br J Cancer. 2021 doi: 10.1038/s41416-020-01122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Araújo ALD, da Silva VM, Kudo MS, de Souza ESC, Saldivia-Siracusa C, Giraldo-Roldán D, Lopes MA, Vargas PA, Khurram SA, Pearson AT, Kowalski LP, de Carvalho ACPLF, Santos-Silva AR, Moraes MC. Machine learning concepts applied to oral pathology and oral medicine: a convolutional neural networks' approach. J Oral Pathol Med. 2023 doi: 10.1111/jop.13397. [DOI] [PubMed] [Google Scholar]
- 46.Majumder SK, Gupta A, Gupta S, Ghosh N, Gupta PK. Multi-class classification algorithm for optical diagnosis of oral cancer. J Photochem Photobiol B. 2006 doi: 10.1016/j.jphotobiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Das DK, Bose S, Maiti AK, Mitra B, Mukherjee G, Dutta PK. Automatic identification of clinically relevant regions from oral tissue histological images for oral squamous cell carcinoma diagnosis. Tissue Cell. 2018 doi: 10.1016/j.tice.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Rahman TY, Mahanta LB, Das AK, Sarma JD. Automated oral squamous cell carcinoma identification using shape, texture and color features of whole strip images. Tissue Cell. 2020 doi: 10.1016/j.tice.2019.101322. [DOI] [PubMed] [Google Scholar]
- 49.Rahman TY, Mahanta LB, Choudhury H, Das AK, Sarma JD. Study of morphological and textural features for classification of oral squamous cell carcinoma by traditional machine learning techniques. Cancer Rep. 2020 doi: 10.1002/cnr2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Rawi N, Sultan A, Rajai B, Shuaeeb H, Alnajjar M, Alketbi M, Mohammad Y, Shetty SR, Mashrah MA. The effectiveness of artificial intelligence in detection of oral cancer. Int Dent J. 2022 doi: 10.1016/j.identj.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ilhan B, Guneri P, Wilder-Smith P. The contribution of artificial intelligence to reducing diagnostic delay in oral cancer. Oral Oncol. 2021 doi: 10.1016/j.oraloncology.2021.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ilhan B, Lin K, Guneri P, Wilder-Smith P. Improving oral cancer outcomes with imaging and artificial intelligence. J Dent Res. 2020 doi: 10.1177/0022034520902128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan C, Yao Y, Cheng B, Cheng Y, Li Y, Li Y, Liu X, Cheng X, Xie X, Wu J, Wang X, Lu W. The application of deep learning based diagnostic system to cervical squamous intraepithelial lesions recognition in colposcopy images. Sci Rep. 2020 doi: 10.1038/s41598-020-68252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guleria S, Shah TU, Pulido JV, Fasullo M, Ehsan L, Lippman R, Sali R, Mutha P, Cheng L, Brown DE, Syed S. Deep learning systems detect dysplasia with human-like accuracy using histopathology and probe-based confocal laser endomicroscopy. Sci Rep. 2021 doi: 10.1038/s41598-021-84510-411:5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahmood H, Shaban M, Indave BI, Santos-Silva AR, Rajpoot N, Khurram SA. Use of artificial intelligence in diagnosis of head and neck precancerous and cancerous lesions: a systematic review. Oral Oncol. 2020 doi: 10.1016/j.oraloncology.2020.104885. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen PT, Sakamoto K, Ikeda T. Deep-learning application for identifying histological features of epithelial dysplasia of tongue. J Oral and Maxillofac Surg Med Pathol. 2022;34:514–522. doi: 10.1016/j.ajoms.2021.12.008. [DOI] [Google Scholar]
- 57.Liu Y, Bilodeau E, Pollack B, Batmanghelich K. Automated detection of premalignant oral lesions on whole slide images using convolutional neural networks. Oral Oncol. 2022 doi: 10.1016/j.oraloncology.2022.106109. [DOI] [PubMed] [Google Scholar]
- 58.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, Fakhry C, Xie TX, Zhang J, Wang J, Zhang N, El-Naggar AK, Jasser SA, Weinstein JN, Treviño L, Drummond JA, Muzny DM, Wu Y, Wood LD, Hruban RH, Westra WH, Koch WM, Califano JA, Gibbs RA, Sidransky D, Vogelstein B, Velculescu VE, Papadopoulos N, Wheeler DA, Kinzler KW, Myers JN. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011 doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, Kryukov GV, Lawrence MS, Sougnez C, McKenna A, Shefler E, Ramos AH, Stojanov P, Carter SL, Voet D, Cortés ML, Auclair D, Berger MF, Saksena G, Guiducci C, Onofrio RC, Parkin M, Romkes M, Weissfeld JL, Seethala RR, Wang L, Rangel-Escareño C, Fernandez-Lopez JC, Hidalgo-Miranda A, Melendez-Zajgla J, Winckler W, Ardlie K, Gabriel SB, Meyerson M, Lander ES, Getz G, Golub TR, Garraway LA, Grandis JR. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015 doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sathasivam HP, Kist R, Sloan P, Thomson P, Nugent M, Alexander J, Haider S, Robinson M. Predicting the clinical outcome of oral potentially malignant disorders using transcriptomic-based molecular pathology. Br J Cancer. 2021 doi: 10.1038/s41416-021-01411-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monteiro L, Mello FW, Warnakulasuriya S. Tissue biomarkers for predicting the risk of oral cancer in patients diagnosed with oral leukoplakia: a systematic review. Oral Dis. 2021 doi: 10.1111/odi.13747. [DOI] [PubMed] [Google Scholar]
- 63.Yap T, Celentano A, Seers C, McCullough MJ, Farah CS. Molecular diagnostics in oral cancer and oral potentially malignant disorders—A clinician's guide. J Oral Pathol Med. 2020 doi: 10.1111/jop.12920. [DOI] [PubMed] [Google Scholar]
- 64.Nikitakis NG, Pentenero M, Georgaki M, Poh CF, Peterson DE, Edwards P, Lingen M, Sauk JJ. Molecular markers associated with development and progression of potentially premalignant oral epithelial lesions: Current knowledge and future implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2018 doi: 10.1016/j.oooo.2018.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.