Abstract
Background
Oral tongue squamous cell carcinoma (OTSCC) is a common malignancy of the oral cavity with poor survival rates. The aim of this project is to investigate the relationship between certain histopathological factors such as Worst Pattern of Invasion (WPOI) and Extranodal Extension (ENE) in patients with oral tongue squamous cell carcinoma (OTSCC) who underwent surgical resection at Loyola University Medical Center.
Methods
This was a retrospective cohort study at a tertiary care academic medical center. All patients that underwent primary surgical resection of OTSCC between 1/1/2015 and 1/1/2022 were reviewed. Patients were identified using the Cerner CoPath Laboratory Information System.
Results
A total of 82 patients met inclusion criteria and were included in the study. Higher grades of WPOI (WPOI 5) were not significantly associated with the presence of ENE in our study (P = 0.82), regardless of the presence of major or minor ENE. WPOI 5 was associated with a higher incidence of local recurrence (P = 0.011).
Conclusions
Higher grades of WPOI were not found to correlate with the presence of ENE, a common histopathological factor that is used as an important prognostic indicator in OTSCC. It is important for clinicians to consider these factors separately when determining whether a patient is high-risk and would benefit from aggressive multimodal treatment.
Keywords: Oral tongue squamous cell carcinoma, Worst pattern of invasion, Extranodal extension, Head and neck cancer
Introduction
Oral cavity squamous cell carcinoma comprises 95% of all head and neck malignancies [1]. Within the oral cavity, the oral tongue is the most common site of malignancy [2]. Despite advances in the treatment of oral tongue squamous cell carcinoma (OTSCC) over the decades, the 5-year survival of OTSCC has continued to decline, with a recent study estimating it to be about 65%. Early-stage OTSCC is typically managed with primary surgical resection. However, there is tremendous variability in treatment response, incidence of loco-regional recurrence, and disease-specific survival. Treatment failure is partially attributed to the difficulty of achieving adequate margins, particularly on the deep margin of the excision, due to the unique anatomy of the tongue [3]. In addition, there have been several studies exploring histopathologic prognostic indicators in OTSCC that could be more predictive of treatment response, regardless of resection margin status. The widely used Brandwein-Gensler model has been validated as a risk model for early-stage, high-risk head and neck squamous cell carcinoma (HNSCC). The model includes 3 histologic variables: worst tumor pattern of invasion (WPOI), perineural invasion (PNI) and lymphocytic host response [4]. WPOI, which categorizes a tumor by its highest pattern of invasion, has been shown to be associated with increased loco-regional recurrence and mortality in early-stage tumors [5, 6].
WPOI is stratified into five categories ranging from type 1 to type 5 and describes the nature of infiltrative growth of the tumor. WPOI 1–4 were previously described by Bryne et al. [7–9] who illustrated a spectrum of patterns at the host/tumor interface spanning from least invasive to most invasive. WPOI 1 demonstrates broad, pushing borders at the interface. WPOI 2 is represented by finger-like projections of invasiveness. WPOI 3 is defined by tumor islands of greater than 15 cells per island. WPOI 4 is characterized by tumor islands of less than 15 cells per island. WPOI 5 was introduced by Brandwein-Gensler and is the most invasive pattern of growth. It demonstrates a dispersed and infiltrative pattern with tumor satellite lesions at least 1 mm away from the main tumor bed [10]. According to the College of American Pathologists (CAP) Protocol, extratumoral PNI and LVI are also considered criteria for WPOI 5. Furthermore, it has been established that the distinction between WPOI 5 and all other patterns is considered the most important. Therefore, it is often only recommended to characterize the pattern as WPOI 5 or WPOI 1–4 [11].
Another histopathologic prognostic factor that has been investigated is extranodal extension (ENE). Some studies have shown that extent of ENE is an important factor for overall survival in OTSCC [6]. Therefore, ENE is considered one of two pathologic factors (along with positive surgical margins) that necessitates the addition of adjuvant chemotherapy to radiation treatment [12]. In our study, pathologic ENE was defined using the AJCC Cancer Staging Manual guidelines; it was defined as carcinoma within a lymph node that has extended outside of the lymph node, through the fibrous capsule, and into surrounding tissue. ENE can be further stratified into major and minor criteria, as recommended by the AJCC Cancer Staging Manual. Minor ENE (ENEmi) is defined as extension less than or equal to 2 mm beyond the lymph node capsule. Major ENE (ENEma) is characterized by either extension apparent to the pathologist’s naked eye, greater than 2 mm extension beyond the capsule, or deposits of soft tissue without lymph node architecture [13].
To our knowledge, there has yet to be a study to investigate the potential association between higher grades of WPOI and ENE. Our hypothesis is that higher grades of WPOI (WPOI 5) are associated with ENE in OTSCC. OTSCC with WPOI 5 demonstrates a highly infiltrate front and aggressive nature that allow for invasion of local structures, including nerves, lymphatics, and blood vessels. Therefore, we believe that the invasive growth characteristics of cancers with WPOI 5 could display a similar infiltrative growth pattern within a lymph node, leading to ENE. A better understanding of the relationship between WPOI and ENE would allow surgeons to make better treatment planning decisions by identifying patients with high-risk OTSCC that would benefit from more aggressive multimodal treatment. The goal of this study is to evaluate the relationship between WPOI and ENE in patients who underwent primary surgical resection for oral tongue squamous cell carcinoma at a single academic center.
Materials and Methods
The study was given an IRB exemption by the Loyola University Medical Center institutional review board. A retrospective chart review of all patients who underwent primary surgical resection for OTSCC between 1/1/2015 and 1/1/2022 was performed. Patients were identified in the Cerner CoPath Laboratory Information System (Kansas City, MO) database using a text search of “squamous cell carcinoma” and part type of “tongue.” Patients who had p16 + disease or EBV-related squamous cell carcinoma, lack of lymph node involvement, or were previously treated with surgical resection, chemotherapy, or radiation therapy for OTSCC were not included in the study. The patients’ charts were reviewed, including patient history, operative reports, surgical pathology reports, and post-operative clinic notes. Information including race, tobacco and alcohol use history, immunosuppression status, tumor pathology details, loco-regional recurrence, and up to 5-year survival outcomes were recorded. Tumor staging was determined by the American Joint Committee of Cancer (AJCC) (8th edition) TNM staging system.
For patients who did not initially have WPOI reported on the pathology report of their surgical specimen, surgical specimen slides were obtained from the hospital archives to be reviewed retrospectively. These patients’ samples were analyzed microscopically by the pathology team. High-grade WPOI (WPOI 5) was determined by the presence of small tumor islands located at least 1 mm away from the main tumor bed or the nearest satellite, as described above by Brandwein-Gensler. Extratumoral PNI or LVI was also defined as WPOI 5. All other patterns of tumor growth were classified as low-grade WPOI (WPOI 1–4). Upon internal validation, concordance between scorers was high.
Patients with ENE-positive OTSCC were further categorized into ENEmi and ENEma as described above by the AJCC Cancer Staging Manual. For those patients who did not have an ENE subcategorization in their original pathology report, their surgical specimen slides were also obtained from the hospital archives and examined microscopically by the pathology team.
PNI was described dichotomously as present or not present. Any LVI that was present was considered extratumoral LVI.
Local recurrence was defined by recurrence that occurred at the primary site of the original tumor. Regional recurrence was defined as involvement of tissues near the primary site and/or involvement of lymph nodes in the neck. Distant metastases included all other recurrences that presented elsewhere in the body.
Statistical analysis was performed with chi-squared, unpaired t-test, univariate and multivariate logistic regression utilizing SPSS (28.0 Armonk, MY). A P-value of < 0.05 was defined as statistically significant.
Results
A total of 82 patients met inclusion criteria. 42 patients were classified as WPOI 1–4 whereas 40 patients were classified as WPOI 5. The majority of patients were male (64.6%) and the average age was 62 years (range: 28–87 years). The majority of patients (64.6%) were active smokers or had a history of smoking tobacco products. Mean follow up time was 25.6 months. There were no significant differences in TNM staging or overall staging between the low-grade and high-grade WPOI groups. Additionally, there were no significant differences in margin status or adjuvant treatment between the two groups. There were no significant demographic differences found between the groups (Table 1).
Table 1.
Demographic and clinical information
| Total | WPOI 1–4 | WPOI 5 | P Value | |
|---|---|---|---|---|
| Number | 82 | 42 | 40 | |
| Gender (male) | 53 (64.6%) | 27 (64.3%) | 26 (65.0%) | 0.95 |
| Average age (years) | 62.1 (28–87) | 61.1 (28–87) | 63.1 (36–87) | 0.51 |
| Smokers | 53 (64.6%) | 26 (61.9%) | 27 (67.5%) | 0.59 |
| T stage | 0.67 | |||
| T1/T2 | 39 (47.6%) | 19 (45.2%) | 20 (50.0%) | |
| T3/T4 | 43 (52.4%) | 23 (54.8%) | 20 (50.0%) | |
| N stage | 0.91 | |||
| N1 | 26 (31.7%) | 13 (31.0%) | 13 (32.5%) | |
| N2a | 6 (7.3%) | 3 (7.1%) | 3 (7.5%) | |
| N2b | 25 (30.5%) | 13 (31.0%) | 12 (30.0%) | |
| N2c | 10 (12.2%) | 4 (9.5%) | 6 (15.0%) | |
| N3a | 2 (2.4%) | 1 (2.4%) | 1 (2.5%) | |
| N3b | 13 (15.9%) | 8 (19.0%) | 5 (12.5%) | |
| Overall Stage | 0.52 | |||
| III | 23 (28.0%) | 13 (31.0%) | 10 (25.0%) | |
| IVa | 44 (53.7%) | 20 (47.6%) | 24 (60.0%) | |
| IVb | 15 (18.3%) | 9 (21.4%) | 6 (15.0%) | |
| + ENE | 48 (58.5%) | 22 (52.4%) | 26 (65.0%) | 0.25 |
| miENE | 29 (35.4%) | 12 (28.5%) | 17 (42.5%) | 0.19 |
| maENE | 19 (23.2%) | 10 (23.8%) | 9 (22.5%) | 0.89 |
| + PNI | 52 (63.4%) | 19 (45.2%) | 33 (82.5%) | 0.00046 |
| + LVI | 39 (47.6%) | 14 (33.3%) | 25 (62.5%) | 0.0082 |
| Margins | 0.98 | |||
| Negative margins (> 3 mm) | 30 (31.7%) | 16 (38.1%) | 14 (35.0%) | |
| Close margins (1–3 mm) | 44 (52.4%) | 22 (52.4%) | 22 (55.0%) | |
| Positive margins | 8 (9.8%) | 4 (9.5%) | 4 (10.0%) | |
| Adjuvant RT alone | 24 (29.3%) | 12 (28.6%) | 12 (30.0%) | 0.89 |
| Adjuvant CRT | 39 (47.6%) | 19 (45.2%) | 20 (50.0%) | 0.67 |
Statistical analysis performed with chi-squared and unpaired t-test
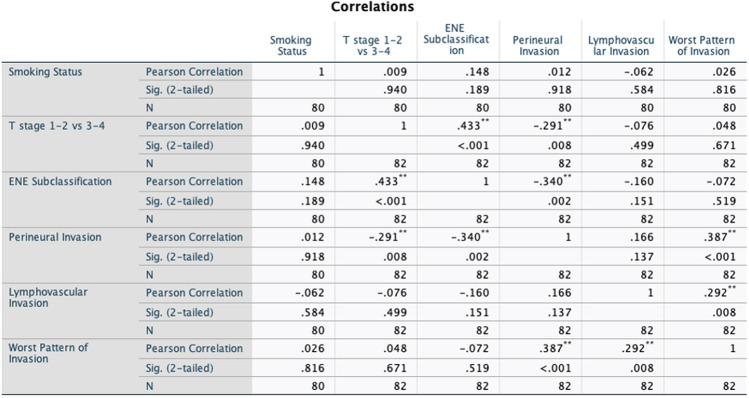
Using multivariate analysis, we found that higher grades of WPOI (WPOI 5) were not significantly associated with the presence of ENE in our study (OR = 1.150; 95% CI 0.352–3.762; P-value = 0.82). Upon stratification of ENE into major and minor categories, we did not find a significant association with WPOI 5 and major ENE using univariate analysis (OR 0.778, 95% CI 0.251–2.408, 0.66). Given the lack of significant difference found when ENE was subdivided on univariate analysis, only the presence vs. absence of ENE was used in the multivariate model (Tables 2). The clinicopathologic factors that were included in the multivariate analysis can be found in the correlation matrix in Fig. 1.
Table 2.
Odds ratio for WPOI 5 with univariable and multivariable logistic regression
| Univariate | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| T stage ( 3–4 v 1–2) | 1.211 | 0.508–2.884 | 0.67 |
| Smoking status (any vs never smoker) n = 79 | 1.118 | 0.442–2.827 | 0.81 |
| + ENE | 1.688 | 0.695–4.104 | 0.24 |
| miENE | 0.494 | 0.181–1.352 | 0.17 |
| maENE | 0.778 | 0.251–2.408 | 0.66 |
| + PNI | 5.707 | 2.064–15.779 | < 0.001 |
| + LVI | 3.333 | 1.347–8.250 | 0.009 |
| Multivariate | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| T stage ( 3–4 v 1–2) | 2.417 | 0.758–7.713 | 0.14 |
| Smoking status (any vs never smoker) n = 79 | 1.191 | 0.400–3.548 | 0.75 |
| + ENE | 1.150 | 0.352–3.762 | 0.82 |
| + PNI | 8.312 | 2.314–29.850 | 0.001 |
| + LVI | 3.667 | 1.298–10.360 | 0.014 |
Fig. 1.
Correlation matrix of variables included in the logistic regression
Not surprisingly, patients with PNI were over 8 times more likely to have WPOI 5 than patients without PNI (OR 8.312; 95% CI 2.314–29.850; P-value = 0.001) using multivariate analyses. Similarly, patients with lymphovascular invasion (LVI) were over 3 times more likely to have WPOI 5 than patients without LVI (OR 3.667; 95% CI 1.298–10.360; P-value = 0.014) (Tables 2).
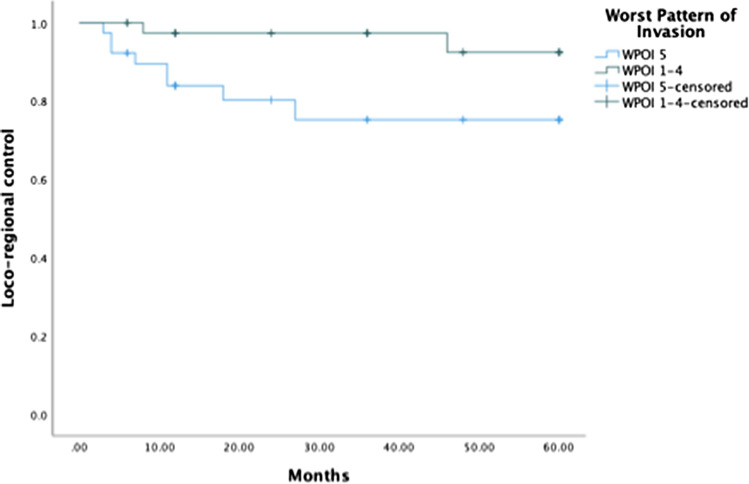
WPOI 5 was associated with a higher rate of local recurrence (P = 0.011) but was not significantly associated with a higher rate of regional recurrence or distant metastases (P = 0.60 and P = 0.345, respectively) (Table 3). Hazards ratio (HR) for loco-regional recurrence using Cox logistic regression demonstrated a HR for loco-regional recurrence with WPOI 5 of 5.161 (95% CI 1.023–26.052, P = 0.047) compared to WPOI 1–4 (Fig. 2).
Table 3.
Loco-regional recurrence and distant metastases
| WPOI 1–4 | WPOI 5 | P-Value | |
|---|---|---|---|
| Local Recurrence | 0 | 6 | 0.011 |
| Regional Recurrence | 2 | 3 | 0.60 |
| Distant Metastases | 1 | 4 | 0.15 |
Fig. 2.
Hazards ratio for loco-regional recurrence using Cox logistic regression
Discussion
The National Comprehensive Cancer Network (NCCN) guidelines recommend treatment of early-stage (T1-2, N0) OSTCC with surgical resection of the primary tumor with or without neck dissection. Adjuvant treatment (eg, radiation therapy, systemic therapy, or a combination) is recommended in patients who have advanced disease or adverse features on pathology. It has been well established that certain histopathological factors, such as PNI, LVI, and loss of differentiation are important prognostic factors in OTSCC. Depth of invasion (DOI) is also an important histologic factor to consider and has been correlated with increased rates of nodal positivity. A substantial increase in nodal positivity was noted when depth of invasion increased from 3 to 4 mm [14]. Therefore, the NCCN recommends an elective neck dissection for DOI 4 mm [15]. ENE is also considered one of the most important prognostic indicators of oral squamous cell carcinoma. It is associated with increased mortality, regional recurrence, and distant metastases [16]. DOI and ENE were both included in a 2018 update to the TNM classification system by the AJCC and upstages the cancer as DOI increases and if ENE is present [17].
Recently, WPOI has also been shown to be a potential prognostic indicator in OTSCC and is an optional reportable component to the oral cavity protocol according to CAP. Some studies have shown higher grades of WPOI to be associated with higher risk for recurrence, lymph node involvement and distant metastasis, and increased mortality [6, 18]. Our study confirmed that higher grades of WPOI are associated with higher rates of local recurrence but not regional or distant metastases.
Given that both WPOI and ENE have been independently found to be associated with increased loco-regional recurrence and mortality, the present study assessed the potential correlation between the presence of ENE and higher grades of WPOI. Interestingly, we found that ENE was not associated with the presence of WPOI 5. Historically, there are head and neck cancer sites, such as the tonsillar fossa, base of tongue, supraglottic larynx, and hypopharynx that are observed to have a lack of correlation between incidence of multiple nodal metastases and the staging of the primary, suggesting an aggressiveness of the primary lesions [19]. Aggressive primary lesions without nodal disease (eg, T4N0) could lead to mortality due to locally advanced invasion, regardless of ENE. Studies evaluating outcomes in patients with tumors of different WPOI grades would be needed to further explore the feasibility of this explanation.
Additionally, our study found that WPOI is associated with higher rates of PNI and LVI. This is somewhat expected given that extratumoral PNI and angiolymphatic invasion are considered diagnostic criteria for WPOI 5. A potential limitation in our study related to PNI includes our lack of stratification between extratumoral, intratumoral, and peripheral PNI. Unfortunately, our institution does not report subcategorization of PNI. A future study should include further stratification of PNI to assess its correlation with WPOI.
Since adequate surgical resection is imperative for tumor control in OSCC, it is important to understand the relationship between WPOI and the adequacy of surgical margins. One study suggested that the extent of surgical margins depended on WPOI. Furthermore, the definition of adequate surgical margins should be adjusted with more invasive grades of WPOI [20]. This is an important consideration to make as the understanding between WPOI and other histopathological factors improves, allowing for more educated treatment plans to be made. Further studies are needed to evaluate whether specific surgical margin cut-offs are warranted with differing grades of WPOI.
Limitations to our study include that this study was conducted as a retrospective cohort study at a single center. Given this study took place at a single institution, the power was limited. Additionally, there was limited stratification between grades of WPOI (WPOI 5 vs. WPOI 1–4), limiting our ability to identify differentiating factors between individual WPOI grades. Lastly, limited median follow-up (25.6 months) may have underestimated the five-year recurrence rate for OTSCC. In a recent study, the five-year recurrence rate for node-positive OTSCC was 29.4% [21].
We recommend a well-powered, multi-institutional prospective study with a large sample to further investigate the relationship between WPOI and ENE. Studies evaluating outcomes in patients with tumors of different WPOI grades and ENE status are needed to further investigate the correlation of WPOI with patient outcomes. Further studies may also include the evaluation of other histopathological grading systems, including the one described by Lewis Jr et al. that describes a tiered ENE grading system in oropharyngeal SCC that could potentially be applied to oral cavity SCC in the future [22, 23].
To our knowledge, this study is the first to explore the correlation between WPOI and ENE in OTSCC. We have demonstrated that WPOI and ENE are not correlated and should be considered independently when formulating treatment plans for OTSCC; however, further prospective studies with larger sample sizes are needed to confirm this relationship.
Author Contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis. The first draft of the manuscript was written by MY and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was not supported by any funding.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
The software application code used in SPSS for statistical analysis is available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
For this type of study formal consent is not required. The study was given an IRB exemption by the Loyola University Medical Center Institutional Review Board.
Consent to Participate
For this type of study informed consent is not required.
Consent for Publication
For this type of study consent for publication is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mayuri Yasuda, Email: myasuda@luc.edu.
Celina Chiodo, Email: cchiodo1@luc.edu.
Cullen Lilley, Email: clilley@luc.edu.
Swati Mehrotra, Email: smehrotra@lumc.edu.
Vijayalakshmi Ananthanarayanan, Email: vananth@lumc.edu.
Andrea Ziegler, Email: andrea.ziegler@luhs.org.
Eric Thorpe, Email: eric.thorpe@lumc.edu.
References
- 1.Taghavi N, Yazdi I. Prognostic Factors of survival rate in oral squamous cell carcinoma: Clinical, Histologic, Genetic and Molecular Concepts. Arch Iran Med. 2015;18(5):314–319. [PubMed] [Google Scholar]
- 2.Almangush A, Heikkinen I, Mäkitie AA, et al. Prognostic biomarkers for oral tongue squamous cell carcinoma: a systematic review and meta-analysis. Br J Cancer. 2017;117(6):856–866. doi: 10.1038/bjc.2017.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli A, Bondi S, Canevari C, et al. High-risk early-stage oral tongue squamous cell carcinoma, when free margins are not enough: critical review. Head Neck. 2021;43(8):2510–2522. doi: 10.1002/hed.26718. [DOI] [PubMed] [Google Scholar]
- 4.Brandwein-Gensler M, Smith R, Wang B, et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34(5):676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi A, Husain N, Misra S, et al. Validation of the brandwein gensler risk model in patients of oral cavity squamous cell carcinoma in North India. Head Neck Pathol. 2020;14(3):616–622. doi: 10.1007/s12105-019-01082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatterjee D, Bansal V, Malik V, et al. Tumor budding and worse pattern of invasion can predict nodal metastasis in oral cancers and associated with poor survival in early-stage tumors. Ear Nose Throat J. 2019;98(7):E112–E119. doi: 10.1177/0145561319848669. [DOI] [PubMed] [Google Scholar]
- 7.Bryne M, Koppang HS, Lilleng R, Kjærheim Å. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J Pathol. 1992;166(4):375–381. doi: 10.1002/path.1711660409. [DOI] [PubMed] [Google Scholar]
- 8.Bryne M, Jenssen N, Boysen M. Histological grading in the deep invasive front of T1 and T2 glottic squamous cell carcinomas has high prognostic value. Virchows Archiv. 1995 doi: 10.1007/BF00203395. [DOI] [PubMed] [Google Scholar]
- 9.Bryne M, Koppang HS, Lilleng R, Stene T, Bang G, Dabelsteen E. New malignancy grading is a better prognostic indicator than Broders’ grading in oral squamous cell carcinomas. J Oral Pathol Med. 1989;18(8):432–437. doi: 10.1111/j.1600-0714.1989.tb01339.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 11.RR Seethala I Weinreb MJ Bullock et al (2017) Protocol for the examination of specimens from patients with cancers of the lip and oral cavity. Retrieved from https://cap.objects.frb.io/protocols/cp-headandneck-lip-oralcavity-17protocol-4001.pdf
- 12.Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 13.Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 14.D’Cruz AK, Vaish R, Kapre N, et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N Engl J Med. 2015;373(6):521–529. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network (2021) Head and Neck Cancers (Version 1.2021). Retrieved from https://www.nccn.org/guidelines/guidelines-process/transparency-process-and-recommendations/GetFileFromFileManager?fileManagerId=11180.
- 16.Pilborough AE, Lambert DW, Khurram SA. Extranodal extension in oral cancer: a role for the nodal microenvironment? J Oral Pathol Med. 2019;48(10):863–870. doi: 10.1111/jop.12870. [DOI] [PubMed] [Google Scholar]
- 17.Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the american joint committee on cancer staging of head and neck cancer: rationale and implications. Curr Oncol Rep. 2019 doi: 10.1007/s11912-019-0799-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almangush A, Bello IO, Keski-Säntti H, et al. Depth of invasion, tumor budding, and worst pattern of invasion: prognostic indicators in early-stage oral tongue cancer. Head Neck. 2014;36(6):811–818. doi: 10.1002/hed.23380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::AID-CNCR2820290604>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Köhler HF, Vartanian JG, Pinto CAL, Silva Rodrigues IFP, Kowalski LP. The impact of worst pattern of invasion on the extension of surgical margins in oral squamous cell carcinoma. Head Neck. 2022;44(3):691–697. doi: 10.1002/hed.26956. [DOI] [PubMed] [Google Scholar]
- 21.Sharma P, Shah SV, Taneja C, Patel AM, Patel MD. A prospective study of prognostic factors for recurrence in early oral tongue cancer. J Clin Diagn Res. 2013;7(11):2559–2562. doi: 10.7860/JCDR/2013/6890.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JS, Carpenter DH, Thorstad WL, Zhang Q, Haughey BH. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24(11):1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhu RS, Hanasoge S, Magliocca KR, et al. Extent of pathologic extracapsular extension and outcomes in patients with nonoropharyngeal head and neck cancer treated with initial surgical resection. Cancer. 2014;120(10):1499–1506. doi: 10.1002/cncr.28596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The software application code used in SPSS for statistical analysis is available from the corresponding author on reasonable request.