Abstract
Background:
Western lifestyle has been associated with an increase in relapsing–remitting multiple sclerosis (RRMS). In mice, dietary wheat amylase–trypsin inhibitors (ATIs) activate intestinal myeloid cells and augment T cell-mediated systemic inflammation.
Objective:
The aim of this study was to assess whether a wheat- and thus ATI-reduced diet might exert beneficial effects in RRMS patients with modest disease activity.
Methods:
In this 6-month, crossover, open-label, bicentric proof-of-concept trial, 16 RRMS patients with stable disease course were randomized to either 3 months of a standard wheat-containing diet with consecutive switch to a > 90% wheat-reduced diet, or vice versa.
Results:
The primary endpoint was negative, as the frequency of circulating pro-inflammatory T cells did not decrease during the ATI-reduced diet. We did, however, observe decreased frequencies of CD14+ CD16++ monocytes and a concomitant increase in CD14++ CD16− monocytes during the wheat-reduced diet interval. This was accompanied by an improvement in pain-related quality of life in health-related quality of life assessed (SF-36).
Conclusion:
Our results suggest that the wheat- and thus ATI-reduced diet was associated with changes in monocyte subsets and improved pain-related quality of life in RRMS patients. Thus, a wheat (ATI)-reduced diet might be a complementary approach accompanying immunotherapy for some patients.
Registration:
German Clinical Trial Register (No. DRKS00027967).
Keywords: adaptive immune system, amylase–trypsin inhibitors, autoimmunity, gluten, inflammation, innate immune system, non-celiac wheat sensitivity, quality of life
Introduction
The contribution of dietary factors to the pathogenesis of multiple sclerosis (MS) and the disease-modifying potential of dietary interventions are topics of increasing interest. Recent studies demonstrated immunomodulatory effects of diets enriched in propionic acid 1 or conjugated linoleic acid, 2 and that a ketogenic diet improved signs of fatigue and depression in patients with relapsing–remitting multiple sclerosis (RRMS). 3 Furthermore, 5.5% of American MS patients follow a gluten-free diet (GFD) and believe it to be effective. 4
Wheat has become the most widely consumed food staple worldwide, which has been accompanied by an increase in hypersensitivities to wheat. 5 With gluten being the clearly identified trigger of celiac disease, it has long been suggested that gluten proteins also mediate the other hypersensitivities. However, recent findings have highlighted the important role of non-gluten wheat proteins, the amylase–trypsin inhibitors (ATIs), in the underlying pathogenesis of intestinal and extra-intestinal inflammatory diseases.6–12
ATIs constitute a small proportion of the protein fraction of various gluten-containing cereals (~2–4%). Thus, a GFD can also be considered ATI-free.8,13 ATIs act as triggers of the innate immune system. In myeloid cells, the binding of ATIs to the toll-like receptor 4 (TLR4) results in the activation of both the classical and the non-classical nuclear factor kappa B (NF-ĸB) pathway. 6 Moreover, ATIs promote a pro-inflammatory intestinal dysbiosis by a direct interaction with the microbiota. 14 We hypothesize that the mild pro-inflammatory intestinal signal induced by nutritional ATI is propagated toward extra-intestinal organs by migration of activated myeloid cells out of the gut where they can co-stimulate T cell-mediated preexisting inflammation. 8 This can induce stronger antigen-specific adaptive immune responses with a consecutive exacerbation of disease activity. 7 Indeed, in murine models of intestinal or allergic airway inflammation, and non-alcoholic fatty liver disease, ATI feeding in quantities comparable to the average consumption of humans, promoted airway, liver, and adipose tissue inflammation.8-11,14 In a recent first clinical pilot trial in patients with familial Mediterranean fever, we could show a marked pro-inflammatory effect of a wheat- and thus ATI-based diet compared to a wheat-free diet, both in clinical symptoms and immune-relevant parameters. 15 As the described effects of ATIs are dose-dependent, a reduction of daily ATI ingestion by 90–95% or even less may be sufficient to abolish their co-stimulatory effect on chronic T cell-mediated inflammation. 7
In experimental autoimmune encephalitis (EAE), the murine model of MS, a markedly more severe disease course paralleled by a shift toward a pro-inflammatory immune cell phenotype, was observed in mice that were fed purified ATI concentrations comparable to a standard western diet. 16 The aim of our current study was thus to evaluate if these promising preclinical findings could be translated into clinical strategies to modulate the immune response in MS by reducing dietary ATIs.
Materials and methods
Subjects
Subjects diagnosed with RRMS 17 were enrolled into this 6-month, crossover, open-label, bicentric pilot study. Among the 20 subjects initially included in the study, the 3- and 6-month visits were completed by 16 patients. Four patients decided to quit the study due to personal reasons, such as moving and extended traveling.
To be included, patients had to be aged between 18 and 60 years and to maintain an unchanged mild or moderate disease-modifying therapy (DMT). Furthermore, patients had to be relapse-free for at least 3 months prior to study enrollment. Exclusion criteria were the presence of other autoimmune diseases, previous limiting dietary habits, organ transplantation, or the intake of any other immunosuppressive drugs apart from the MS medication.
The primary study endpoint was defined as a decrease in pro-inflammatory T cell populations in the peripheral blood on a wheat/gluten- and therefore ATI-reduced diet (W/G/A−). Secondary study endpoints included the decrease in other pro-inflammatory immune cell populations in peripheral blood, an improvement in health-related quality of life assessed by the 36-item short form survey (SF-36), clinical disease stability assessed by the Expanded Disability Status Scale (EDSS) score, and a reduction of the annualized relapse rate (ARR). In addition, serum neurofilament light chain (sNfL) levels as a biomarker of neuroaxonal damage were assessed.
This study was registered in the German Clinical Trial Register (No. DRKS00027967).
Study procedures
At the baseline visit, patients were randomly assigned to one of the two study arms in a crossover study design (Figure 1). They were instructed to either continue on their normal wheat/gluten- and thus ATI-containing diet (W/G/A+) for 3 months and then switch to the largely wheat- and ATI-free diet (reduction of at least 90%) for the following 3 months (W/G/A−), or started with 3 months of the W/G/A− diet with a subsequent switch to their normal W/G/A+ diet. Wheat consumption was assessed and monitored by structured food questionnaires (see supplement) before study entry, at diet switch and at study end.
Figure 1.
Study design.
At baseline visit, patients were randomized to either continue on their normal W/G/A+ diet for 12 weeks and then switch to the W/G/A− diet for the following 12 weeks, or vice versa. Clinical outcome and adherence measures were assessed at every study visit, while immunophenotyping was only performed at baseline and at the end of each dietary intervention. If not stated otherwise, the consecutive analyses refer to the outcome measures at week 12 and week 24.
ATI, amylase–trypsin inhibitor; EDSS, Expanded Disability Status Scale; GIP, gluten immunogenic peptides; SF-36, 36-item short form survey; sNfL, serum neurofilament light chain levels; W/G/A, wheat/gluten/ATI.
Clinical and patient-reported outcomes
At baseline visit, demographic and MS-related data, including disease history, history of relapses, and past and current DMTs, were collected. The baseline visit and every consecutive study visit in 6-week intervals included general physical and neurological examination and the assessment of self-reported quality of life. Weight and height were measured, and the body mass index (BMI) was calculated. EDSS scores were assessed by an experienced neurologist blinded to the diet assignments. 18 The occurrence of new neurological symptoms indicative for a relapse was documented at each visit. Quality of life was determined using SF-36, which is a widely used patient-reported outcome covering eight domains of physical and mental quality of life (physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy and fatigue, emotional well-being, social functioning, pain, and general health). 19 Item reports were summed without weighting separately for each domain, and ranges were transformed to have a common range of 0 (worst health) to 100 (best health) as described previously. 20
Measurement of gluten immunogenic peptides
Diet adherence was monitored by the measurement of gluten immunogenic peptides (GIPs) in 4-h urine samples at every study visit (Glutenostics, Indianapolis, USA). GIPs are degradation products of gluten and can be detected in urine for 3–34 h after the consumption of at least 25–50 mg of gluten. 21
sNfL single-molecule array
sNfL levels of study participants were measured by sNfL single-molecule array (SiMoA) technology as previously described 22 (see supplemental material).
Immunophenotyping
High-quality peripheral blood mononuclear cells (PBMCs) were isolated from all patients at baseline, and at the end of the respective diet intervals at the 3- and 6-month time points. Multiparameter flow cytometry of immune cells in peripheral blood was performed as described previously. 23 Supplementary Figure 1 illustrates the immune cell parameters as determined by conventional gating.
Statistical analysis
The data from all subjects completing the 3- and 6-month time points of the crossover period were included into statistical analyses (per protocol analysis). Normality of distribution was assessed by Kolmogorov–Smirnov test. Changes in clinical and laboratory outcomes between the two dietary intervals were assessed using two-tailed, paired t-tests in case of normally distributed variables, and Wilcoxon test for not normally distributed variables. A two-sided p-value of less than 0.05 was defined as statistically significant. Of note, due to the explorative character of this pilot study, no correction for multiple testing was performed. Statistical analyses were conducted using SPSS 23.0 software (IBM Corp., USA); figures were generated using GraphPad Prism 8.0 for Windows (GraphPad Software, USA).
Results
Patient characteristics
At the baseline visit, patients were randomly assigned to one of the two study arms in a crossover study design (Figure 1). In total, 16 patients completed the 3- and 6-month visits [15 females, median 42.0 (interquartile range, IQR 35.5–51.5) years, details in Table 1].
Table 1.
Baseline characteristics of study cohort.
| Parameter | Patients completing the study (n = 16, per protocol analysis) |
|---|---|
| Age [median (IQR)] | 42.0 (35.5–51.5) |
| Sex (female/male) | 15/1 |
| Disease duration in years [median (IQR)] | 7.0 (4.0–14.5) |
| DMT (none/Copaxone/Interferon/other) | 1/2/11/2 |
| EDSS [median (IQR)] | 2.5 (1.0–3.0) |
| No. of relapses in 2 years before screening [median (range)] | 0 (0–3) |
| ARR in the 2 years before screening | 0.34 |
ARR, annualized relapse rate; DMT, disease-modifying treatment; EDSS, Expanded Disability Status Scale; IQR, interquartile range.
Effects of wheat-reduced diet on cell populations of the adaptive immune system
The primary study endpoint defined as a decrease in pro-inflammatory T cell populations in the peripheral blood on W/G/A− diet was negative. We observed no significant changes in the frequency of pro-inflammatory Th1 [Figure 2(a)] and Th17 cells [Figure 2(b)] on the wheat-reduced diet. However, the CD8+ TEMRA (terminally differentiated effector memory T cells re-expressing CD45RA) subset (gated by CD3+ CD8+ CD45RO− CD27−) displayed significant alterations during the wheat-reduced diet, as the frequency of granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing [Figure 2(c)] and interleukin (IL)-17A-producing [Figure 2(d)] TEMRA cells was lower on the W/G/A− diet. However, the overall proportion of these cell populations was low and the data of six patients had to be excluded from the analysis since cell counts were too low for cytokine measurement (Supplementary Figure 2).
Figure 2.
Effects of dietary ATI reduction on peripheral blood cell subsets of the adaptive immune system in patients with RRMS. There was no significant change in (a) Th1 (CCR4−CCR6−CXCR3+) cells or (b) Th17 cells during the ATI-reduced diet interval. The proportion of (c) GM-CSF-producing and (d) IL-17-producing TEMRA cells was lower during the ATI interval. (e) Proportions of transitional B cells were decreased during the ATI-reduced diet interval, whereas (f) the proportion of class switch memory B cells (CD20+ CD19+ CD27+ IgD− IgM−) was increased.
Connected dots depict intra-individual changes. Bars comprise means, and whiskers correspond to the SD.
ATI, amylase–trypsin inhibitor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; NS, non-significant; RRMS, relapsing–remitting multiple sclerosis; TEMRA, terminally differentiated effector memory T cells re-expressing CD45RA.
*p < 0.05.
Within the B cell compartment, there was a decrease in transitional B cells (gated by CD19+ CD27+ IgD+ IgM+ CD38+ CD21+ CD24+) [Figure 2(e)] and an increase in class switch memory B cells (CD20+ CD19+ CD27+ IgD− IgM−) [Figure 2(f)] during the wheat-reduced diet.
Effects of wheat-reduced diet on cell populations of the innate immune system
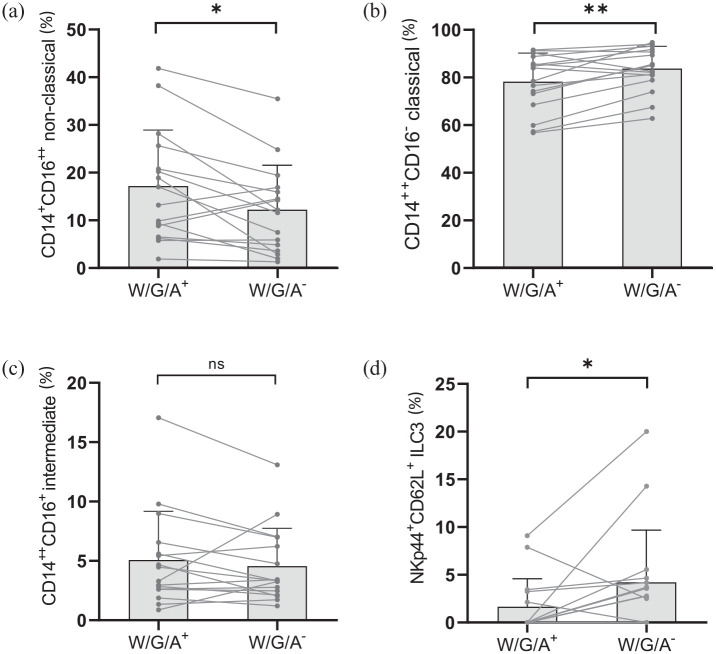
While there was no difference in the overall CD14+ monocyte frequency [percentage of lineage-negative (linX−) HLA-DR+ cells] between the two diet intervals, we observed relevant alterations of their composition. There was a reduction of circulating CD14+ CD16++ non-classical monocytes during the wheat-reduced compared to the wheat-containing diet [Figure 3(a)]. Correspondingly, the frequency of classical CD14++ CD16− monocytes increased [Figure 3(b)], whereas intermediate CD14++ CD16+ monocytes remained unchanged [Figure 3(c)].
Figure 3.
Dietary ATI reduction impacts peripheral blood cell subsets of the innate immune system in patients with RRMS. A reduction of dietary wheat/gluten and ATIs (W/G/A−) led to (a) a decreased proportion of CD14+ CD16++ non-classical monocytes (% of CD14+ monocytes), whereas (b) the proportion of CD14++ CD16− monocytes increased. (c) No change in the proportion of intermediate CD14++ CD16+ monocytes was observed. (d) The proportion of circulating type 3 innate lymphoid cells (ILCs) expressing NKp44 and CD62L (percentage of ILC3) was increased by ATI reduction.
Connected dots depict intra-individual changes. Bars comprise means, and whiskers correspond to the SD.
ATI, amylase–trypsin inhibitor; ILC, innate lymphoid cell; NS, non-significant; RRMS, relapsing–remitting multiple sclerosis; SD, standard deviation; W/G/A, wheat/gluten/ATI.
*p < 0.05; **p < 0.01.
Furthermore, there was an increase in type 3 innate lymphoid cells (ILCs) bearing the natural cytotoxicity receptor (NCR+ ILC3, gated by CD45+ linX− NCR+ CD117+) expressing both NKp44 and CD62L [Figure 3(d)]. There were no significant changes within the dendritic cell compartment.
Patient adherence
In the dietary questionnaires, all patients declared a reduced consumption of wheat and other gluten-containing foods (as proxy for ATI consumption since both are highly correlated) by about 88% (from an estimated 6.6 ± 6.3 to an estimated 0.8 ± 2.4 g of gluten daily). However, 3 patients went on a strict GFD and 10 consumed gluten-containing foods less than once a week. Another 3 patients reported to be consuming gluten-containing foods more than once a week. Urinary GIP levels of 10 patients were reduced during the W/G/A− interval compared with the W/G/A+ interval, while there was no difference in GIP levels between the W/G/A− diet and W/G/A+ diet in 4 patients. Two patients had GIP levels below the detection limit at both time points. Overall, the median GIP levels were lower during the W/G/A− diet compared with the W/G/A+ diet (Supplementary Figure 3).
Wheat-reduced diet is tolerated well and leads to an improvement in pain-related quality of life in RRMS patients
The EDSS scores during the wheat-reduced diet interval did not differ from those during the wheat-containing diet interval. One patient suffered from an MS relapse with new gait ataxia during the W/G/A+ diet interval, while no MS relapses were reported during the W/G/A− diet interval. Levels of sNfL remained stable throughout the study independent of diet assignments. In total, the occurrence of 11 adverse events was reported (Table 2).
Table 2.
Comparison of outcome measures between wheat-containing (W/G/A+) and wheat-reduced (W/G/A−) diet.
| W/G/A+ | W/G/A− | p value | |
|---|---|---|---|
| EDSS | 2.0 ± 1.5 | 2.3 ± 1.3 | 0.096 |
| No. of relapses (total number) | 1 | 0 | N/A |
| sNfL (pg/ml) | 11.1 ± 7.0 | 11.4 ± 7.9 | 0.187 |
| Weight (kg) | 69.4 ± 16.1 | 68.7 ± 15.5 | 0.074 |
| BMI (kg/m2) | 24.2 (22.0–26.0) | 24.2 (20.6–25.7) | 0.313 |
| SF-36 | |||
| Physical functioning | 85.0 ± 20.9 | 85.8 ± 19.8 | 0.684 |
| Role limitations due to physical health | 87.5 ± 31.1 | 81.3 ± 35.6 | 0.276 |
| Role limitations due to emotional problems | 83.3 ± 38.9 | 94.4 ± 13.0 | 0.285 |
| Energy/fatigue | 55.0 ± 20.6 | 56.7 ± 24.1 | 0.403 |
| Emotional well-being | 77.0 ± 18.7 | 77.7 ± 15.5 | 0.720 |
| Social functioning | 88.5 ± 18.0 | 90.6 ± 17.8 | 0.655 |
| Pain | 72.3 ± 30.4 | 79.5 ± 25.6 | 0.008 |
| General health | 62.5 ± 18.8 | 62.7 ± 16.4 | 0.968 |
| Any adverse events | 6 (37.5%) | 5 (31.3%) | 0.709 |
| Upper respiratory infection | 1 (6.3%) | 2 (12.5%) | N/A |
| Urinary tract infection | 0 (0%) | 1 (6.3%) | N/A |
| Diarrhea | 1 (6.3%) | 0 (0%) | N/A |
| Edema of the legs | 1 (6.3%) | 0 (0%) | N/A |
| Not specified | 3 (18.8%) | 2 (12.5%) | N/A |
BMI, body mass index; EDSS, Expanded Disability Status Scale; SD, standard deviation; SF-36, 36-item short form survey; sNfL, serum neurofilament light chain; W/G/A, wheat/gluten/ATI.
Comparison of mean clinical outcome measures during the W/G/A+ and the W/G/A− diet interval. During the W/G/A− diet interval, patients scored higher in the category ‘Pain’ of the SF-36 questionnaire, indicating a higher quality of life with regard to pain. If not stated otherwise, results within this table represent mean ± SD. Statistically significant differences are marked in bold.
In the SF-36, we observed a significant improvement in the category ‘pain’ during the wheat-reduced diet (72.3/100 ± 30.4 versus 79.5/100 ± 25.6, p = 0.008). The self-reported perception of the the other categories did not differ significantly between the wheat-reduced and the wheat-containing diets.
A comparison of clinical outcome measures between wheat-reduced and wheat-containing diet is reported in Table 2.
Discussion
In this proof-of-concept study, we were able to translate some of our preclinical findings on the immunomodulatory effects of reducing dietary wheat consumption, and thus nutritional ATIs, to a potential clinical application in RRMS patients. While the study’s primary endpoint (decrease in circulating pro-inflammatory T cell populations) was negative, we observed a reduction in non-classical monocytes, an ILC subset (ILC3), and two small TEMRA subpopulations in the peripheral blood of RRMS patients during the > 90% wheat- and thus ATI-reduced diet interval. Furthermore, the wheat-reduced diet was tolerated well and led to an improvement in pain-related quality of life.
Rodent studies have shown that oral ingestion of wheat or purified ATIs as active ingredient leads to the activation of pro-inflammatory monocytes, macrophages, and dendritic cells (DCs) in the intestine via activated TLR4-signaling, resulting in elevated numbers of (activated) myeloid cells in the intestinal lamina propria and mesenteric lymph nodes as compared to animals on a wheat- and ATI-free control diet6,8–12,14 Moreover, this activation exacerbated inflammatory diseases of the intestine, the liver, and the lungs in mice, including mice with a humanized immune system10–12,14 This supported the hypothesis that after local activation of intestinal myeloid cells by nutritional ATIs, these myeloid cells may migrate to the mesenteric lymph nodes, where they may encounter already primed autoreactive T cells to aggravate tissue-specific inflammation at distant sites. 8 In our preliminary data on the role of ATI in EAE, this translated into a more severe disease course. 16
Depending on their expression of CD14 and CD16, myeloid cells can be subdivided into classical CD14++ CD16−, non-classical CD14+ CD16++, and intermediate CD14++ CD16+ monocytes. 24 Functionally, the roles of these monocyte subsets are still a matter of controversial debate. Some have described the classical monocytes as mainly phagocytic with few inflammatory attributes, 25 while others have suggested that they are critical for initial inflammatory response.26,27 Non-classical monocytes display antigen-presenting properties and are usually described to express an inflammatory phenotype, 25 although they can also be viewed as anti-inflammatory as they were found to maintain vascular homeostasis.28,29 Intermediate monocytes show both moderate phagocytic and inflammatory features. 25 In the current study, the reduction of dietary ATIs was associated with decreasing frequencies of circulating non-classical monocytes and a concomitant increase in classical monocytes. This is of interest as in comparison to healthy controls, MS patients show an elevation of non-classical and a reduction of classical monocytes in peripheral blood. 24 Furthermore, a recent study identified higher numbers of classical monocytes as a predictor of therapeutic efficacy in dimethyl fumarate-treated MS patients, 30 which might argue for a favorable effect of avoiding wheat/nutritional ATIs in MS. Notably, classical monocytes display a lower expression of toll-like receptors than intermediate and non-classical monocytes. 25 Since ATIs stimulate myeloid cells via TLR-4 signaling, this provides a mechanistic link to our observation of a shift from non-classical to classical monocytes during the diet low in TLR4-activating ATI.
Apart from monocytes and DCs, the innate immune system also comprises ILCs, which function as potent immune effector cells during inflammation. ILC3 can promote an immunologically tolerogenic state in the intestine in response to nutrients, commensal bacteria, or bacterial metabolites that limits the magnitude of potentially damaging T cell responses. 31 In the current study, the reduction of dietary ATIs led to an increase in activated (NKp44+) ILC3, which are prominent in the intestine and which are able to invade lymphoid tissues via CD62L+. While the exact consequence of this finding is unclear, it might indicate that the reduction of the modest intestinal immune cell activation during the wheat (ATI)-reduced diet, which has been observed in murine models, might be partly mediated by a recruitment of ILC3 to the mesenteric lymphoid tissue. Studies on the contribution of ILCs in the pathogenesis of MS remain inconclusive so far.23,32,33
Based on the aforementioned rodent studies on the effects of wheat or ATI consumption,6,8–12,14 we hypothesized that reducing dietary ATIs might diminish pro-inflammatory T cell responses in the periphery. Although we did not observe a general reduction of peripheral inflammatory T cell populations, there was a decrease in two small T cell subpopulations, namely GM-CSF- and IL-17A-producing CD8+ TEMRA cells. These cells, which represent the most differentiated type of memory cells, express high levels of cytotoxic effector molecules, such as perforin and Fas ligand. 34 An increased occurrence of clonally expanded CD8+ TEMRA cells in the blood of MS patients has been reported, 35 and the decreased proportion of subsets producing the pro-inflammatory cytokines GM-CSF and IL-17A on the ATI-reduced diet may reflect a downregulation of detrimental adaptive immune responses. However, the absolute number of TEMRA cells was low and therefore our findings should be interpreted with caution.
Clinically, we did not detect any evidence of increased MS disease activity, with stable EDSS scores and unchanged sNfL levels as a marker of neuroaxonal damage. There were no reported relapses during the wheat-reduced diet interval, whereas one patient suffered from a relapse during the wheat-containing diet (difference not statistically significant). However, in the patient-reported quality of life assessment, the wheat-reduced diet was associated with a reduction of pain. A plausible pathomechanistic explanation might be a reduction of ATI-mediated activation of intestinal myeloid cell TLR4 and downstream adaptive effector functions. This is in line with growing evidence suggesting that TLR4-signaling plays an important role in the induction, conversion, and maintenance of chronic systemic and neuropathic pain in MS. 36 Therefore, our current finding argues for a complementary role of reducing dietary ATIs in the symptomatic treatment of chronic pain and possibly also long-term sequelae in MS patients.
In an effort to verify dietary compliance, we used a validated test to quantify urinary gluten peptides (GIP). These peptides were indeed decreased in the majority of patients on the wheat-reduced versus the wheat-containing diet, but still detectable in the majority of patients on the wheat-reduced diet. Recent studies showed that urinary GIP have a high sensitivity – but a low specificity – to detect minor gluten ingestion in patients with celiac disease who need to comply with a strict gluten-free diet.37,38 In contrast, participants of our study were allowed to consume some gluten as long as the total amount was reduced by at least 90%. This explains why we were able to detect GIP in the majority of patients even during the wheat-reduced diet and underlines that urinary GIP cannot be considered a reliable biomarker to monitor dietary compliance in studies where minor amounts of wheat and gluten are allowed.
It should be noted that due to the explorative character of this pilot study, we did not employ corrections for multiple testing. Furthermore, the observed changes in immune cell populations are partly small or driven by outliers. Thus, statistically significant differences in these immune cell populations do not necessarily imply physiological relevance. Therefore, a larger-scale clinical trial is necessary to validate the currently observed shifts within the PBMC compartment and to evaluate the impact of a reduction of wheat consumption, and thus dietary ATIs on the clinical disease course of MS. This trial should also enroll patients with more severe MS and follow-up MRT to further classify CNS disease activity.
To conclude, this pilot study suggests that reducing dietary wheat/ATIs may be helpful for some RRMS patients as a complementary treatment. Along with good tolerability, following a wheat/ATI-reduced diet improved pain-related quality of life and exerted a potentially immunomodulating effect, which was most pronounced in the myeloid cell compartment.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231170928 for Attenuation of immune activation in patients with multiple sclerosis on a wheat-reduced diet: a pilot crossover trial by Sinah Engel, Luisa Klotz, Timo Wirth, Ann-Katrin Fleck, Geethanjali Pickert, Melanie Eschborn, Samia Kreuzburg, Valentina Curella, Stefan Bittner, Frauke Zipp, Detlef Schuppan and Felix Luessi in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pptx-2-tan-10.1177_17562864231170928 for Attenuation of immune activation in patients with multiple sclerosis on a wheat-reduced diet: a pilot crossover trial by Sinah Engel, Luisa Klotz, Timo Wirth, Ann-Katrin Fleck, Geethanjali Pickert, Melanie Eschborn, Samia Kreuzburg, Valentina Curella, Stefan Bittner, Frauke Zipp, Detlef Schuppan and Felix Luessi in Therapeutic Advances in Neurological Disorders
Acknowledgments
SK performed her MD thesis on the topic of the present study. The authors thank Cheryl Ernest for proofreading the article.
Footnotes
ORCID iDs: Sinah Engel  https://orcid.org/0000-0002-9051-9062
https://orcid.org/0000-0002-9051-9062
Stefan Bittner  https://orcid.org/0000-0003-2179-3655
https://orcid.org/0000-0003-2179-3655
Felix Luessi  https://orcid.org/0000-0003-4334-4199
https://orcid.org/0000-0003-4334-4199
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Sinah Engel, Department of Neurology and Focus Program Translational Neuroscience (FTN), Rhine Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Luisa Klotz, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, University of Münster, Münster, Germany.
Timo Wirth, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, University of Münster, Münster, Germany.
Ann-Katrin Fleck, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, University of Münster, Münster, Germany.
Geethanjali Pickert, Institute of Translational Immunology and Research Center for Immunotherapy, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Melanie Eschborn, Department of Neurology with Institute of Translational Neurology, University Hospital Münster, University of Münster, Münster, Germany.
Samia Kreuzburg, Department of Neurology and Focus Program Translational Neuroscience (FTN), Rhine Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Valentina Curella, Institute of Translational Immunology and Research Center for Immunotherapy, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Stefan Bittner, Department of Neurology and Focus Program Translational Neuroscience (FTN), Rhine Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Frauke Zipp, Department of Neurology and Focus Program Translational Neuroscience (FTN), Rhine Main Neuroscience Network (rmn2), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Detlef Schuppan, Institute of Translational Immunology and Research Center for Immunotherapy, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany; Division of Gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Felix Luessi, Department of Neurology and Focus Program Translational Neuroscience (FTN), Rhine Main Neuroscience Network (rmn²), University Medical Center of the Johannes Gutenberg University Mainz, Langenbeckstrasse 1, 55131 Mainz, Germany.
Declarations
Ethics approval and consent to participate: The study was approved by the local ethics committees [No. 837.163.16 (10482)]. All patients provided written informed consent.
Consent for publication: Not applicable.
Author contributions: Sinah Engel: Formal analysis; Investigation; Methodology; Writing – original draft.
Luisa Klotz: Conceptualization; Investigation; Methodology; Writing – review & editing.
Timo Wirth: Data curation; Investigation; Methodology; Writing – review & editing.
Ann-Katrin Fleck: Data curation; Investigation; Methodology; Writing – review & editing.
Geethanjali Pickert: Data curation; Methodology; Writing – review & editing.
Melanie Eschborn: Methodology; Writing – review & editing.
Samia Kreuzburg: Data curation; Investigation; Project administration; Writing – review & editing.
Valentina Curella: Methodology; Writing – review & editing.
Stefan Bittner: Conceptualization; Supervision; Writing – review & editing.
Frauke Zipp: Conceptualization; Supervision; Writing – review & editing.
Detlef Schuppan: Conceptualization; Investigation; Supervision; Writing – review & editing.
Felix Luessi: Conceptualization; Investigation; Methodology; Supervision; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the German Research Council (DFG, CRC-TR-128).
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LK received honoraria for lecturing and travel expenses for attending meetings from Alexion, Biogen, Janssen, Merck, Sanofi Genzyme, Novartis, Teva, Viatris, and Roche; her research is funded by the Deutsche Forschungsgemeinschaft (DFG), Interdisciplinary Center for Clinical Studies (IZKF) Muenster, Biogen, Merck, and Novartis. ME received speaker honoraria and travel support from Sanofi Genzyme; she received research support from the Deutsche Multiple Sklerose Gesellschaft (DMSG) Landesverband, Nordrhein-Westfalen (NRW), and the Innovative Medical Research (IMF) program of the University Münster. SB has received honoraria and compensation for travel from Biogen Idec, Merck Serono, Novartis, Sanofi Genzyme, and Roche. FZ has recently received research grants and consultation funds from DFG, BMBF, PMSA, MPG, Genzyme, Merck Serono, Roche, Novartis, Sanofi-Aventis, Celgene, ONO, and Octapharma. DS consults for, advises for, received grants from, and holds intellectual property rights with NorthSea; he consults for, advises for, and received grants from Boehringer Ingelheim; and consults for and advises for Pliant, UCB, Inversago, and Prometik. FL received consultancy fees from Roche and support with travel cost from Teva Pharma. The remaining authors have nothing to disclose.
Availability of data and materials: The raw data used in preparation of the figures and tables will be shared in an anonymized format on request of a qualified investigator to the corresponding author.
References
- 1.Duscha A, Gisevius B, Hirschberg S, et al. Propionic acid shapes the multiple sclerosis disease course by an immunomodulatory mechanism. Cell 2020; 180: 1067–1080.e16. [DOI] [PubMed] [Google Scholar]
- 2.Fleck AK, Hucke S, Teipel F, et al. Dietary conjugated linoleic acid links reduced intestinal inflammation to amelioration of CNS autoimmunity. Brain 2021; 144: 1152–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenton JN, Banwell B, Bergqvist AGC, et al. Pilot study of a ketogenic diet in relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2019; 6: e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald KC, Tyry T, Salter A, et al. A survey of dietary characteristics in a large population of people with multiple sclerosis. Mult Scler Relat Disord 2018; 22: 12–18. [DOI] [PubMed] [Google Scholar]
- 5.Fasano A, Sapone A, Zevallos V, et al. Nonceliac gluten sensitivity. Gastroenterology 2015; 148: 1195–1204. [DOI] [PubMed] [Google Scholar]
- 6.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med 2012; 209: 2395–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuppan D, Zevallos V. Wheat amylase trypsin inhibitors as nutritional activators of innate immunity. Dig Dis 2015; 33: 260–263. [DOI] [PubMed] [Google Scholar]
- 8.Zevallos VF, Raker V, Tenzer S, et al. Nutritional wheat amylase-trypsin inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology 2017; 152: 1100–1113. [DOI] [PubMed] [Google Scholar]
- 9.Zevallos VF, Raker VK, Maxeiner J, et al. Dietary wheat amylase trypsin inhibitors exacerbate murine allergic airway inflammation. Eur J Nutr 2019; 58: 1507–1514. [DOI] [PubMed] [Google Scholar]
- 10.Ashfaq-Khan M, Aslam M, Qureshi MA, et al. Dietary wheat amylase trypsin inhibitors promote features of murine non-alcoholic fatty liver disease. Sci Rep 2019; 9: 17463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellinghausen I, Weigmann B, Zevallos V, et al. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J Allergy Clin Immunol 2019; 143: 201–212. [DOI] [PubMed] [Google Scholar]
- 12.Caminero A, McCarville JL, Zevallos VF, et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology 2019; 156: 2266–2280. [DOI] [PubMed] [Google Scholar]
- 13.Dupont FM, Vensel WH, Tanaka CK, et al. Deciphering the complexities of the wheat flour proteome using quantitative two-dimensional electrophoresis, three proteases and tandem mass spectrometry. Proteome Sci 2011; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickert G, Wirtz S, Matzner J, et al. Wheat consumption aggravates colitis in mice via amylase trypsin inhibitor-mediated dysbiosis. Gastroenterology 2020; 159: 257–272. [DOI] [PubMed] [Google Scholar]
- 15.Carroccio A, Mansueto P, Soresi M, et al. Wheat consumption leads to immune activation and symptom worsening in patients with familial Mediterranean fever: a pilot randomized trial. Nutrients 2020; 12: 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zevallos V, Yogev N, Nikolaev A, et al. Nutritional wheat alpha-amylase/trypsin inhibitors (ATIs) favor the development of murine autoimmune encephalopathy. Z Gastroenterol 2015: 53–KG248. [Google Scholar]
- 17.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 20.Hobart J, Freeman J, Lamping D, et al. The SF-36 in multiple sclerosis: why basic assumptions must be tested. J Neurol Neurosurg Psychiatry 2001; 71: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreno ML, Cebolla Á, Muñoz-Suano A, et al. Detection of gluten immunogenic peptides in the urine of patients with coeliac disease reveals transgressions in the gluten-free diet and incomplete mucosal healing. Gut 2017; 66: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engel S, Steffen F, Uphaus T, et al. Association of intrathecal pleocytosis and IgG synthesis with axonal damage in early MS. Neurol Neuroimmunol Neuroinflamm 2020; 7: e679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross CC, Schulte-Mecklenbeck A, Hanning U, et al. Distinct pattern of lesion distribution in multiple sclerosis is associated with different circulating T-helper and helper-like innate lymphoid cell subsets. Mult Scler 2017; 23: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 24.Gjelstrup MC, Stilund M, Petersen T, et al. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunol Cell Biol 2018; 96: 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee R, Kanti Barman P, Kumar Thatoi P, et al. Non-classical monocytes display inflammatory features: validation in sepsis and systemic lupus erythematous. Sci Rep 2015; 5: 13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fingerle G, Pforte A, Passlick B, et al. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood 1993; 82: 3170–3176. [PubMed] [Google Scholar]
- 27.Narasimhan PB, Marcovecchio P, Hamers AAJ, et al. Nonclassical monocytes in health and disease. Annu Rev Immunol 2019; 37: 439–456. [DOI] [PubMed] [Google Scholar]
- 28.Carlin LM, Stamatiades EG, Auffray C, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 2013; 153: 362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapellos TS, Bonaguro L, Gemünd I, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol 2019; 10: 2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlstrom KE, Ewing E, Granqvist M, et al. Therapeutic efficacy of dimethyl fumarate in relapsing-remitting multiple sclerosis associates with ROS pathway in monocytes. Nat Commun 2019; 10: 3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artis D, Spits H. The biology of innate lymphoid cells. Nature 2015; 517: 293–301. [DOI] [PubMed] [Google Scholar]
- 32.Gillard GO, Saenz SA, Huss DJ, et al. Circulating innate lymphoid cells are unchanged in response to DAC HYP therapy. J Neuroimmunol 2016; 294: 41–45. [DOI] [PubMed] [Google Scholar]
- 33.Gross CC, Ahmetspahic D, Ruck T, et al. Alemtuzumab treatment alters circulating innate immune cells in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 2003; 101: 4260–4266. [DOI] [PubMed] [Google Scholar]
- 35.Salou M, Garcia A, Michel L, et al. Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol 2015; 2: 609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruno K, Woller SA, Miller YI, et al. Targeting toll-like receptor-4 (TLR4)-an emerging therapeutic target for persistent pain states. Pain 2018; 159: 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Carnicer A, Garzon-Benavides M, Fombuena B, et al. Negative predictive value of the repeated absence of gluten immunogenic peptides in the urine of treated celiac patients in predicting mucosal healing: new proposals for follow-up in celiac disease. Am J Clin Nutr 2020; 112: 1240–1251. [DOI] [PubMed] [Google Scholar]
- 38.Silvester JA, Comino I, Rigaux LN, et al. Exposure sources, amounts and time course of gluten ingestion and excretion in patients with coeliac disease on a gluten-free diet. Aliment Pharmacol Ther 2020; 52: 1469–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231170928 for Attenuation of immune activation in patients with multiple sclerosis on a wheat-reduced diet: a pilot crossover trial by Sinah Engel, Luisa Klotz, Timo Wirth, Ann-Katrin Fleck, Geethanjali Pickert, Melanie Eschborn, Samia Kreuzburg, Valentina Curella, Stefan Bittner, Frauke Zipp, Detlef Schuppan and Felix Luessi in Therapeutic Advances in Neurological Disorders
Supplemental material, sj-pptx-2-tan-10.1177_17562864231170928 for Attenuation of immune activation in patients with multiple sclerosis on a wheat-reduced diet: a pilot crossover trial by Sinah Engel, Luisa Klotz, Timo Wirth, Ann-Katrin Fleck, Geethanjali Pickert, Melanie Eschborn, Samia Kreuzburg, Valentina Curella, Stefan Bittner, Frauke Zipp, Detlef Schuppan and Felix Luessi in Therapeutic Advances in Neurological Disorders