Abstract
Purpose
To evaluate and compare the clinical efficacy of transforaminal steroid and platelet-rich plasma (PRP) injections in patients with discogenic lumbar radiculopathy.
Methods
60 patients were randomized to be treated with single transforaminal injection of PRP (n = 29) or steroid (methylprednisolone acetate [n = 31]). Clinical assessment was done with Visual analogue scale (VAS), modified Oswestry low back pain disability index (MODI), and straight leg raise test (SLRT). Baseline assessment of outcomes was done followed by post-intervention evaluation at 1, 3, and 6 months. Both groups had similar baseline characteristics.
Results
There was a significant statistical improvement of VAS and MODI in both groups at follow-up (P < 0.05). In PRP group, minimal clinically important change (> 2 cm difference of mean for VAS and > 10-point change in MODI) for both outcome scores was achieved at all follow-up intervals (1, 3, 6 months), while as in steroid group, it was seen only at 1 and 3 months for both VAS and MODI. On intergroup comparison, better results were seen in steroid group at 1 month (P < 0.001 for both VAS and MODI), and in PRP group at 6 months (P < 0.001 for both VAS and MODI) with non-significant difference at 3 months (P = 0.605 for MODI and P = 0.612 for VAS). More than 90% tested SLRT negative in PRP group and 62% in steroid group at 6 months. No serious complications were seen.
Conclusion
Transforaminal injections of PRP and steroid improve short-term (up to 3 months) clinical outcome scores in discogenic lumbar radiculopathy, but clinically meaningful improvements sustaining for 6 months were provided by PRP only.
Keywords: Lumbar radiculopathy, Platelet-rich plasma (PRP), Methylprednisolone acetate, Visual analogue scale (VAS), Modified Oswestry low back pain disability index (MODI)
Introduction
The lifetime prevalence of low back ache is in the vicinity of around 60–80% in the general population [1]. It can present with or without radiculopathy. Radiculopathy refers to pain that radiates down the lower extremities along the distribution of a nerve root, often described by patients as sharp, electric, or burning type. Lumbar disc herniations are the most common cause of lumbar radiculopathy in young adults in their 3rd–5th decade with incidence reported to be about 5–20 cases per thousand annually, with a male preponderance, most common site (> 90%) being the region of L4–L5 or L5–S1 disc level [2]. Disc herniations posterolaterally due to their proximity are more likely to compress the nerve root. The pain characteristically increases on bending forward, sitting, coughing, sneezing, and straining, and can be relieved by lying down or occasionally by walking. In addition to back pain, patients may report paresthesias in afflicted dermatomes.
The treatment of lumbar radicular pain can be either operative or non-operative. Absolute indications for surgery are progressive and significant neurological deficit and cauda equina syndrome [3]. Non-operative treatment is the first-line treatment for patients without surgical indication, which is in the form of rest, analgesics (acetaminophen, NSAIDS, opioids, muscle relaxants, and sometimes GABA analogues like gabapentin and pregabalin), activity modification, physical therapy, and psychotherapy [1].
Epidural injections particularly steroids, with or without anaesthetic, as a surgery sparing treatment modality have been used for lumbar disc herniations for few decades with its effectiveness reported by multiple studies [4–7]. Epidural injections can be administered via three routes: interlaminar, caudal, or transforaminal. The transforaminal route is considered to be better than the other two, because it could reach the intended target site where a nerve root passes through its foramen while avoiding underlying roots and other important structures [7]. Most common approach for transforaminal injections is subpedicular approach, for which C-arm is positioned to obtain appropriate posteroanterior (PA), oblique (Scotty dog) views and lateral views. The needle is directed under the pedicle in the “safe triangle” to approach the superior neural foramen under fluoroscopy. The other approach is via Kambin’s triangle. To reach Kambin’s triangle, the fluoroscopic image is created in the oblique view, which aligns the superior articulate process (SAP) in the centre of the intervertebral disc, and then, the needle is advanced in a lateral, inferior direction to the SAP. When the needle contacts the SAP, the direction of the needle is changed to the lateral aspect of the bony landmark. Needle advancement and final placement are confirmed with a lateral view and contrast imaging. Both the subpedicular and Kambin’s triangle approaches are safe routes for administration of drugs into the intervertebral foramen with studies showing no significant differences in outcomes or complications between the two [8, 9].
Because of some concerns reported in the literature with epidural steroid use in the form of neurological injury, neurotoxicity and pharmacologic effect of steroids like hyperglycemia, hypercorticolism and adrenal suppression [6, 10] (rare with single injection), alternative injection therapies have been considered. One such therapy is platelet-rich plasma (PRP) which has anti-inflammatory, anti-nociceptive, and potential regenerative effects on extracellular matrix (ECM) which has led to its increased use in many orthopaedic conditions [11]. It contains high number of platelets which have been concentrated by centrifugation and growth factor release from platelet alpha-granules directly at the target site in addition have proliferative and anti-apoptotic effects on fibroblasts and neurons [12].
Despite the promising role of PRP in pain relief, its effects in lumbar disc herniations with radiculopathy remains unclear as only a few studies have evaluated it. Henceforth, we carried out this randomized study to evaluate the safety and efficacy of transforaminal injection of PRP versus methyl prednisolone acetate in management of patients suffering from lumbar radiculopathy secondary to disc herniation.
Materials and Methods
This randomized study was carried out in a tertiary care centre from July 2021 to May 2022 after due approval from ethics committee of the institute. Patients aged > 18 years with lower back pain (VAS > 5) and well-established lumbar radiculopathy (> 3 month duration) secondary to posterolateral herniated disc (predominant unilateral leg pain more than back pain, with symptoms restricted to a single dermatome) were included.
Exclusion criteria: bleeding disorder, previous surgery of the spine, pregnancy, sepsis, raised intracranial pressure, any neurological deficit, known malignancy, systemic infections or skin lesion over injection site, any known drug allergies (for drugs used in the study), spinal stenosis, multi-level disc disease, spondylolisthesis, and previous epidural injections within 3 months.
Study Design
Patients who presented to us in the outpatient/inpatient department with lumbar radiculopathy of more than 3 month duration were screened. Since we have included Patients with symptom duration of > 3 months, majority of them had already taken conservative treatment in the form of oral NSAIDS and muscle relaxants which included physiotherapy, as well.
Imaging was done in the form of anteroposterior and lateral radiographs followed by magnetic resonance imaging (MRI). MR imaging was done with Siemens Avanto scanner, having 1.5 T grade, phase resolution of 75 and with sequences having 3 mm slice thickness. MRI images were assessed and reported by a senior radiologist in the institute and Pfirrmann grades II and III with normal facet joint and no ligamentum hypertrophy were included. An informed and written consent was taken as per the guidelines from institute Ethical Committee and the patients were randomized to be treated with single injection of either steroid or PRP. A computer-generated sequence was used for randomization. Each patient’s randomization number and the group allocation were concealed from the patients and outcome accessor throughout the study.
Routine blood investigations (total blood counts, bleeding profile, blood sugar, and C-reactive protein) were done pre-procedure for all patients.
Visual Analogue scale (VAS) was used for assessment of pain, measured on a 10 cm line (extremes of 10 and 0 denoting worst possible pain and no pain, respectively, while as 5 was considered moderate pain). Modified Oswestry Disability Index (MODI) was used for the assessment of function, with a lowest score of 0 and highest of 100% (higher scores denoting more disability). Straight leg raise test (SLRT) was also noted. An SLRT of < 75° was considered as positive.
These outcome scores were evaluated at baseline and then at 1, 3, and 6 months post-intervention by an independent accessor not involved in the study. Seventy patients in total received single injection of steroid (methylprednisolone acetate + 1% lignocaine) or PRP.
PRP Preparation
PRP was prepared in the well-equipped main laboratory of our pathology department with York centrifuge machine. Under all aseptic conditions, 34–45 ml of whole blood was obtained from the antecubital vein in 8.5 ml acid citrate dextrose tubes. First centrifugation of the whole blood was done using a soft spin (3000 rpm/minute for 3 min). Supernatant plasma obtained from this was collected and transferred to another sterile tube (10 ml, without anticoagulant). Then, this tube was centrifuged at hard spin that is @ 4000 rpm/minute for 15 min, to obtain a platelet-rich concentrate. In this concentrated plasma, the lower 1/3rd layer is platelet-rich plasma (PRP), and the upper 2/3rd layer is discarded as it was platelet poor.
The platelet-rich plasma was suspended in minimal plasma (2–5 ml) and the contents are mixed by gentle shaking. This collected (3–5 ml) PRP was injected within 2 h of preparation.
Procedure
All transforaminal injections were performed by a single senior orthopaedic surgeon in the main operation theatre under fluoroscopic guidance. After proper positioning of the patient (prone with lower abdomen supported by a pillow), the injection area was cleaned and painted with betadine and draped. C-arm was positioned to obtain appropriate posteroanterior (PA), oblique (Scotty dog) views and lateral views. At L5-S1, the C-arm was tilted, so that the sacral foramen appears oval shaped. Skin was injected with local anaesthetic using 25-gauge needle (1–2 ml of 1% lidocaine). A 22-gauge spinal needle (diamond tipped with a stylet) was directed under the pedicle in the “safe triangle “to approach the neural foramen under fluoroscopy. After checking for blood/cerebrospinal fluid in the needle on aspiration, correct position was confirmed by injecting 0.5–2 ml of iohexol dye which enhances the exiting nerve root coming out from the foramen. All patients received the same volume of drug, either 2 ml of methylprednisolone acetate (40 mg/ml) with 1 ml 1% lignocaine or 3 ml of autologous PRP.
Figures 1, 2, 3, 4, 5 show clinical (Fig. 1) and C-arm images of needle placement in PA, oblique, and lateral views (Fig. 2, 3, 4) and the dye spread along the nerve root (Fig. 5).
Fig. 1.
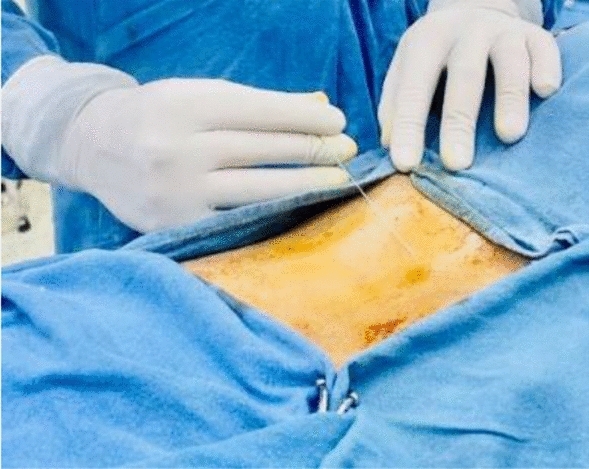
Clinical image of the injection procedure
Fig. 2.
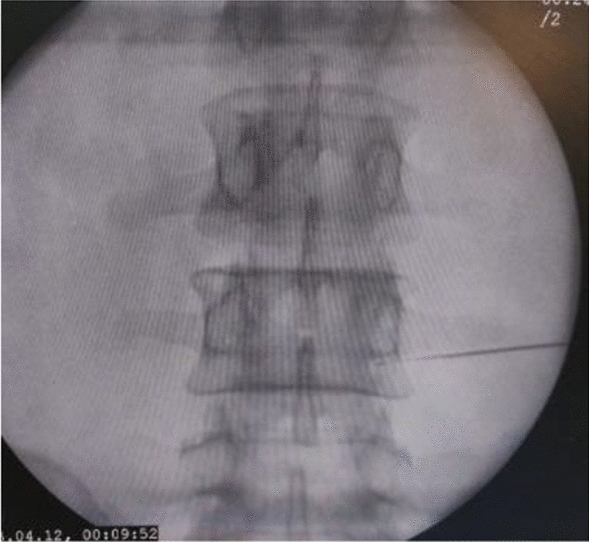
C-arm image of needle placement in PA view
Fig. 3.
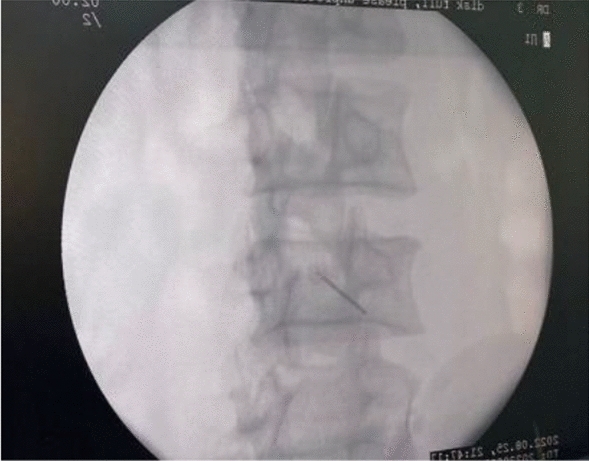
C-arm image of needle placement in oblique view
Fig. 4.
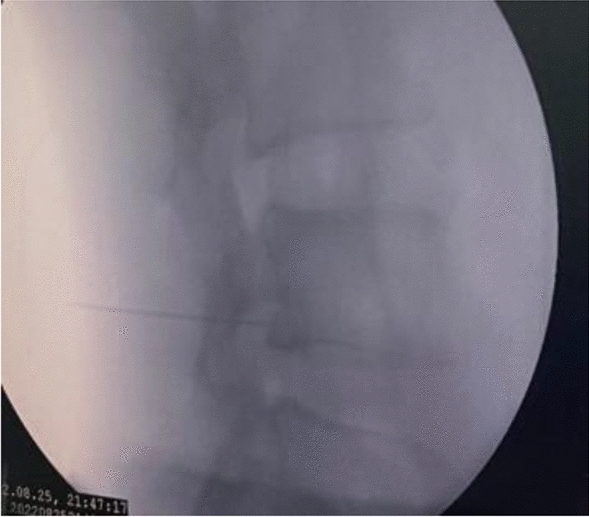
C-arm image of needle placement in Lateral view
Fig. 5.
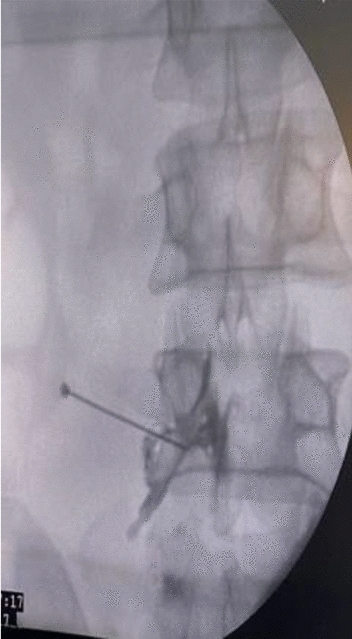
C-arm image showing dye spread post contrast injection
Post-procedure Protocol
Patients were observed for 2 h post-procedure and were sent home after instructing rest for 24 h. Oral tramadol (50 gm) twice daily was prescribed for pain management in first 72 h. They were prohibited from taking NSAIDS, corticosteroids, or other analgesics and were advised to carry out their normal daily activities except heavy weight lifting, bending forward, and prolonged standing.
Statistical Analysis
Required minimum sample size was 48 patients (sample size calculation formula to compare 2 means, with power = 80%, significance level = 0.05).
Final assessment was done on 60 patients in total (PRP/steroid = 29/31) as 10 were lost to follow up. Patients and accessor were blinded to the group allocation.
VAS, MODI, and percentage of negative SLRT were noted at each follow-up.
Analysis was done using SPSS version 22. For quantitative data, mean/standard deviation was calculated, and for qualitative data, frequency/percentages were calculated. Comparison of mean was done with one-way ANOVA Test and independent t test. P < 0.05 was considered as significant. Post hoc test was done for intragroup comparison.
Results
Overall mean height and weight were 167.6 cm ± 7.36 and 64.44 kgs ± 6.42, and mean BMI was 22.9 kg/m2 ± 4.24.
Baseline characteristics with respect to mean age, gender, number of patients and body mass index (BMI), baseline VAS, and MODI were similar in both the groups (P > 0.05) (Table 1).
Table 1.
Baseline parameters
| Intervention groups | P value | ||
|---|---|---|---|
| PRP (N = 29) | Steroid (N = 31) | ||
| Age (years) | 42.03 + − 11.31 | 45.83 +− 12.35 | 0.65 |
| Sex, male/female, N/% | 15/14, 51.7/48.3 | 16/15, 51.6/48.4 | 0.99 |
| BMI (kg/m2) | 23.21 + − 4.68 | 22.05 + − 3.03 | 0.26 |
|
Level: L4–L5, N L5-S1, N L3–L4, N |
20 07 02 |
24 06 01 |
|
| MODI baseline | 57.3 + − 9.7 | 55.9 + − 10.6 | 0.60 |
| VAS baseline | 6.7 + − 1.4 | 6.5 + − 1.1 | 0.55 |
Majority were labourers by occupation (18), followed by housewives (21), drivers (6), teacher (4), shopkeeper (3), student (2), policeman (1), stenographer (1), paramedical staff (2), and rest had no job (2).
Outcome Scores in Steroid Group
VAS: Baseline VAS was 6.5 + − 1.1 which decreased to 3.2 + − 1.1 at 1 month, 4 + − 1 at 3 months, and 5.2 + − 1 at 6 months.
Mean difference in VAS at 1, 3 and 6 months compared to baseline was 3.29, 2.48, and 1.29, respectively. This improvement was statistically significant (P < 0.001). The mean difference in VAS among other time points 1 month vs 3 and 6 months (− 0.8 and – 2, respectively), 3 month vs 6 months (− 1.2) was negative. This change was statistically significant (P < 0.05) (Table 2).
Table 2.
Intra-group comparison of VAS and MODI among different time intervals in steroid group
| MODI | Mean difference | P value | 95% confidence interval | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Baseline | 1 month | 26.097 | 0.001 | 19.28 | 32.92 |
| 3 months | 18.419 | 0.001 | 11.60 | 25.24 | |
| 6 months | 9.355 | 0.003 | 2.54 | 16.17 | |
| 1 month | 3 months | − 7.677 | 0.021 | − 14.50 | − 0.86 |
| 6 months | − 16.742 | 0.001 | − 23.56 | − 9.92 | |
| 3 months | 6 months | − 9.065 | 0.004 | − 15.88 | − 2.25 |
| VAS | Mean difference | P value | 95% confidence interval | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Baseline | 1 month | 3.290 | 0.001 | 2.59 | 3.99 |
| 3 months | 2.484 | 0.001 | 1.79 | 3.18 | |
| 6 months | 1.290 | 0.001 | 0.59 | 1.99 | |
| 1 month | 3 months | − 0.806 | 0.017 | − 1.50 | − 0.11 |
| 6 months | − 2.000 | 0.001 | − 2.70 | − 1.30 | |
| 3 months | 6 months | − 1.194 | 0.001 | − 1.89 | − 0.50 |
MODI score: Mean baseline MODI score decreased from a baseline of 55.9 + − 10.6 to 29.8 + − 10.5 at 1 month, 37.5 + − 10.3 at 3 months, and 46.6 + − 9.7 at 6 months.
Mean difference in MODI at 1, 3, and 6 months compared to baseline was 26.09, 18.42, and 9.35, respectively. This improvement was statistically significant (P < 0.001). The mean difference in VAS among other time points 1 month vs 3 and 6 months (− 7.68 and − 16.74 respectively), 3 month vs 6 months (− 9.06) was negative. This change was statistically significant (P < 0.05) (Table 2). 72% had SLRT in the range of 35–75°, while as 28% had < 35° at baseline. Test negative SLRT were maximum at 1 month (84%), which decreased to 70% at 3 months and 62% at 6 months.
Outcome Scores in PRP Group
VAS: Baseline VAS was 6.7 +− 1.2 which decreased to 4.7 + − 1.5 at 1 month, 3.9 + − 1 at 3 months and 3.5 + − 1.4 at 6 months.
Mean difference in VAS at 1, 3, and 6 months compared to baseline was 2.03, 2.79, and 3.21, respectively. This progressive improvement was statistically significant (P < 0.001). The mean difference in VAS among other time points (1 vs 3 months, 3 vs 6 months) except 1 month vs 6 months (P = 0.004) was not significant (P > 0.05). (Table 3).
Table 3.
Intra-group comparison of VAS and MODI among different time intervals in PRP group
| MODI | Mean difference | P value | 95% confidence interval | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Baseline | 1 month | 14.828 | 0.001 | 7.06 | 22.59 |
| 3 months | 21.138 | 0.001 | 13.37 | 28.90 | |
| 6 months | 24.034 | 0.001 | 16.27 | 31.80 | |
| 1 month | 3 months | 6.310 | 0.153 | − 1.45 | 14.07 |
| 6 months | 9.207 | 0.013 | 1.44 | 16.97 | |
| 3 months | 6 months | 2.897 | 0.765 | − 4.87 | 10.66 |
| VAS | Mean difference | P value | 95% confidence interval | ||
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Baseline | 1 month | 2.034 | 0.001 | 1.16 | 2.91 |
| 3 months | 2.793 | 0.001 | 1.92 | 3.67 | |
| 6 months | 3.207 | 0.001 | 2.33 | 4.08 | |
| 1 month | 3 months | 0.759 | 0.113 | − 0.12 | 1.63 |
| 6 months | 1.172 | 0.004 | 0.30 | 2.05 | |
| 3 months | 6 months | 0.414 | 0.607 | − 0.46 | 1.29 |
MODI: MODI score decreased from baseline 57.3 + − 9.7 to 42.5 + − 14.8 at 1 month, 36.2 + − 9.7 at 3 months, and 33.3 + − 10.3 at 6 months.
Mean difference in MODI at 1, 3, and 6 months compared to baseline was 14.83, 21.14, and 24.03, respectively. This progressive improvement was statistically significant (P < 0.001). The mean difference in MODI among other time points (1 vs 3 months, 3 vs 6 months) except 1 month vs 6 months (P = 0.0013) was not significant (P > 0.05) (Table 3).
68% had SLRT in the range of 35–75°, while as 32% had < 35° at baseline. At 6 months, there was maximum percentage of test negative SLRT (81%), 12% had an SLRT of 35–75, and 7% had below 35°
Inter-group Comparison (Steroid vs PRP) (Table 4)
Table 4.
Inter-group comparison of VAS and MODI at different time intervals
| Test variable | Group | P value | |
|---|---|---|---|
| PRP | Steroid | ||
| MODI score (baseline) | 57.3 ± 9.7 | 55.9 ± 10.6 | 0.603 |
| VAS score (baseline) | 6.7 ± 1.2 | 6.5 ± 1.1 | 0.559 |
| MODI score (1 months) | 42.5 ± 14.8 | 29.8 ± 10.5 | 0.001 |
| VAS score (1 months) | 4.7 ± 1.5 | 3.2 ± 1.1 | 0.001 |
| MODI score (3 months) | 36.2 ± 9.7 | 37.5 ± 10.3 | 0.605 |
| VAS score (3 months) | 3.9 ± 1 | 4 ± 1 | 0.612 |
| MODI score (6 months) | 33.3 ± 10.3 | 46.6 ± 9.7 | 0.001 |
| VAS score (6 months) | 3.5 ± 1.4 | 5.2 ± 1 | 0.001 |
There was no difference in Baseline VAS and MODI scores among the two groups (P = 0.603).
At 1 month, both VAS (diff = 1.5, 95% CI 0.82–2.18, P < 0.001) and MODI (diff = 12.7, 95% CI 6.10–19.3, P < 0.001) were significantly better in the steroid group.
At 3 months, the difference was statistically not significant among the two groups for both the scores (P = 0.605 for MODI and P = 0.612 for VAS).
At 6 months, VAS (diff = − 1.70, 95% CI − 2.3 to − 1.07, P < 0.001) and MODI (diff = − 13.30, 95% CI − 18.47 to − 8.13, P < 0.001) were significantly better in the PRP group.
Discussion
The current study shows that both steroid and PRP were able to improve pain and function scores significantly when these injections were given via transforaminal route for patients suffering from radicular symptoms secondary to posterolateral disc herniation. Maximum improvement of outcome scores in the steroid group was seen at 1 month (mean difference of VAS and MODI 3.29 and 26.09, respectively), beyond which they worsened. The increase in mean VAS and mean MODI (worsening) for the time periods between 1 and 3 months and 3 and 6 months was significant (P < 0.05), but they remained below baseline values. In the PRP group, the scores continued to improve beyond 1st month with maximum improvement seen at 6 months for both the scores (mean difference of VAS and MODI, 3.20 and 24.03, respectively). The improvement in outcome scores at 6 months compared to 1 month was significant (P = 0.004 for VAS, P = 0.013 for MODI), but it was not significant for periods between 1 and 3 months (P = 0.11 for VAS, P = 0.15 for MODI) and 3 and 6 months (P = 0.60 for VAS, P = 0.76 for MODI). Minimal clinically important change (MCIC) (considering MCIC to be more than 10-point change for MODI and > 2 cm difference in mean for VAS [13]) was achieved at 1 and 3 months for both the outcome scores in steroid group (at 6 months, mean VAS difference = 1.29 and mean MODI difference = 9.35, both below MCIC), but in the PRP group, it was achieved at all follow-up intervals (1, 3, 6 months) for VAS and MODI. Above findings suggest that the effects of steroids peak at 1 month and clinically meaningful improvements may last for 3 months, beyond which the scores slowly decline, but in case of PRP, there is a continuous clinical meaningful improvement extending for at least 6 months. On intergroup comparison, steroid group showed better results at 1 month while as PRP was better at 6 months.
The exact cause for disc herniations and the symptom production is not known, but several changes related to the disc have been implicated, in the form of reduced water retention, increase in collagen type 1 in nucleus and inner fibrosus, ECM, and collagen destruction as well as an upregulation in matrix metalloproteinases (MMP), inflammatory and apoptotic pathways, ultimately leading to increase in the inflammatory cytokines locally and mechanical compression of the nerve by protruding disc [14]. The rationale for use of transforaminal injections as a surgery sparing treatment method is direct delivery of drugs at the local site with minimal complications. Steroids have been extensively used for this purpose for a long time. PRP and related products have come into focus in recent years. Steroids are potent anti-inflammatory agents and they act via activation of anti-inflammatory signalling and inhibition of pro-inflammatory pathways as well as curtailment of ectopic discharges (from unmyelinated C-fibres) and direct decrease in central sensitization of pain [15, 16]. The various growth factors released from PRP directly at the target site not only have anti-inflammatory, anti-nociceptive properties [12] but also have matrix regenerative potential [11, 17] and may result in resorption of herniated disc [18], and neural regeneration [19–22].
Wilby et al. [23] compared the results of transforaminal steroid injections with microdiscectomy and reported that these injections should be considered as the first treatment option for patients with sciatica of up to 1 year duration, secondary to lumbar disc herniation. In a systemic review, Helm et al. [5] concluded that there was high evidence (level-1) for the use of transforaminal injections in discogenic radicular pain. On the other hand, Cohen et al. [7] found no differences between saline, TNF alfa, and steroids. Concerns have been raised with steroid injections in the form of reports of serious complications like cord infarction, hematoma formation, and paralysis [24], due to which alternate therapies like PRP have been explored. In a recent trial, Xu et al. [25] compared the results of transforaminal PRP and steroid injections in 136 patients suffering from lumbar disc disease, which reported that both showed significant improvements in VAS, ODI, which was sustained up to 1 year, with no inter group differences. In another prospective study by Le et al. [26], patients with lumbar disc herniation were given single transforaminal PRP injection and observed a significant sustained improvement in VAS, ODI, and SLRT over a period of 12 months with no associated complications. However, this study had no comparison group and the sample size was small. Bise et al. [27], in a study of 60 patients who were given CT-guided epidural PRP injections reported significant pain reduction and functional improvement with effects sustaining for a period of 6 weeks without any complications, but this study was non-randomized. Centeno et al. [28] used platelet lysate epidural injections and reported its effectiveness in improving lumbar radicular pain through a 2 year follow-up, suggesting it to be good alternative to steroids, but they did not have any comparison group. Symptomatic improvements with effects sustaining up to 1 year after intradiscal injections of PRP have been reported by Akeda et al. [29] and Tukali-wosornu et al. [30] in patients of low back pain of discogenic origin. The first study did not have a control group while as contrast agent was used as control in second study. Kubrova et al. [31], in a systemic review of 12 studies, found that the epidural PRP, platelet-derived growth factors, and steroids improve short-term pain and function scores but long-term effects lasting 1–2 years maybe be provided by PRP and related products only. They reported them to be safe, and apart from minor complications in the form of injection related pain, soreness, and signs of dural puncture (3 patients, which resolved with conservative treatment), no serious adverse effects were seen.
Our results are in accordance with above studies which report the clinical efficacy of PRP and steroids in lumbar disc herniation with both the groups showing short-term improvements in pain and function scores for up to 3 months but only PRP showed sustained MCIC in MODI and VAS for 6 months.
Established studies have reported no major complications with PRP, while as cord ischemia, hematoma formation, and infection have been reported by a few studies with steroid use [24]. We did not observe any major complications or side effects in both the groups.
Our study has several limitations in the form of small sample size, the lack of long-term follow-up beyond 6 months, lack of platelet quantification in PRP, and the use of subjective outcome scores. Large trails using objective outcome scores and highly sensitive modern tools of assessment are further needed.
Conclusion
In conclusion, this study suggests that transforaminal injections of steroid and PRP improve short-term (3 months) clinical outcome scores in discogenic lumbar radiculopathy, but clinically meaningful improvements sustaining for at least 6 months were provided by PRP only.
Acknowledgements
We appreciate the help from the technical manager in our Hospital Laboratory as well as laboratory haematologist for their immense cooperation and for providing us with laboratory equipment needed for the study. We thank our laboratory technical manager and haematologist for help.
Funding
None to declare.
Data availability
Data supoorting the study is avaialable upon reasonable request from authors.
Declarations
Conflict of Interest
There is no conflict of interest to declare and this study was carried out in accordance with ethical guidelines of Helsinki declaration (revisited in Tokyo 2004).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amit Saraf, Email: amitsaraf_75@yahoo.com.
Altaf Hussain, Email: khan.altaf7@gmail.com.
Angad Singh Sandhu, Email: angadss1384@gmail.com.
Sandeep Bishnoi, Email: sandeepbishnoi.bishnoi@gmail.com.
Vaneet Arora, Email: dr.vaneet06@gmail.com.
References
- 1.Fujii K, Yamazaki M, Kang JD, Risbud MV, Cho SK, Qureshi SA, Hecht AC, Iatridis JC. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR Plus. 2019 doi: 10.1002/jbm4.10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan J, Konstantinou K, O'Dowd J. Herniated lumbar disc. BMJ Clinical Evidence. 2011;28(2011):1118. [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon WW, Koch J. Herniated discs: When is surgery necessary? EFORT Open Reviews. 2021;6(6):526–530. doi: 10.1302/2058-5241.6.210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CC, McCormick ZL, Mattie R, MacVicar J, Duszynski B, Stojanovic MP. The effectiveness of lumbar transforaminal injection of steroid for the treatment of radicular pain: A comprehensive review of the published data. Pain Medicine. 2020;21(3):472–487. doi: 10.1093/pm/pnz160. [DOI] [PubMed] [Google Scholar]
- 5.Helm Ii S, Harmon PC, Noe C, Calodney AK, Abd-Elsayed A, Knezevic NN, Racz GB. Transforaminal epidural steroid injections: A systematic review and meta-analysis of efficacy and safety. Pain Physician. 2021;24(S1):S209–S232. [PubMed] [Google Scholar]
- 6.Oliveira CB, Maher CG, Ferreira ML, Hancock MJ, Oliveira VC, McLachlan AJ, Koes BW, Ferreira PH, Cohen SP, Pinto RZ. Epidural corticosteroid injections for lumbosacral radicular pain. Cochrane Database of Systematic Reviews. 2020;4(4):CD013577. doi: 10.1002/14651858.CD013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, Bicket M, Jamison D, Wilkinson I, Rathmell J. Epidural steroids: A comprehensive, evidence-based review. Regional Anesthesia and Pain Medicine. 2013;38(3):175–200. doi: 10.1097/AAP.0b013e31828ea086. [DOI] [PubMed] [Google Scholar]
- 8.Botwin KP, Gruber RD, Bouchlas CG, Torres-Ramos FM, Sanelli JT, Freeman ED, Slaten WK, Rao S. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. American Journal of Physical Medicine and Rehabilitation. 2002;81(12):898–905. doi: 10.1097/00002060-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Park JW, Nam HS, Cho SK, Jung HJ, Lee BJ, Park Y. Kambin's triangle approach of lumbar transforaminal epidural injection with spinal stenosis. Annals of Rehabilitation Medicine. 2011;35(6):833–843. doi: 10.5535/arm.2011.35.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatia A, Flamer D, Shah P, Cohen S. Transforaminal epidural steroid injections for treating lumbosacral radicular pain from herniated intervertebral discs: A systematic review and meta-analysis. Anesthesia and Analgesia. 2016;122(3):857–870. doi: 10.1213/ANE.0000000000001155. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed S, Yu J. Platelet-rich plasma injections: An emerging therapy for chronic discogenic low back pain. Journal of Spine Surgery. 2018;4(1):115–122. doi: 10.21037/jss.2018.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang J, Wang X, Jiang W, Zhu Y, Hu Y, Zhao Y, Song X, Zhao J, Zhang W, Peng J, Wang Y. Platelet-rich plasma therapy in the treatment of diseases associated with orthopedic injuries. Tissue Engineering Part B: Reviews. 2020;26(6):571–585. doi: 10.1089/ten.teb.2019.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Practice & Research Clinical Rheumatology. 2005;19(4):593–607. doi: 10.1016/j.berh.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Al Qaraghli MI, De Jesus O. Lumbar disc herniation. StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 15.Viswanathan VK, Kanna RM, Farhadi HF. Role of transforaminal epidural injections or selective nerve root blocks in the management of lumbar radicular syndrome—a narrative, evidence-based review. Journal of Clinical Orthopaedics and Trauma. 2020;11(5):802–809. doi: 10.1016/j.jcot.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makkar JK, Gourav KKP, Jain K, Singh PM, Dhatt SS, Sachdeva N, Bhadada S. Transforaminal versus lateral parasagittal versus midline interlaminar lumbar epidural steroid injection for management of unilateral radicular lumbar pain: A randomized double-blind trial. Pain Physician. 2019;22(6):561–573. doi: 10.36076/ppj/2019.22.561. [DOI] [PubMed] [Google Scholar]
- 17.Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO, Fortier LA. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. American Journal of Sports Medicine. 2014;42(1):35–41. doi: 10.1177/0363546513507766. [DOI] [PubMed] [Google Scholar]
- 18.Rawson B. Platelet-rich plasma and epidural platelet lysate: Novel treatment for lumbar disk herniation. Journal of the American Osteopathic Association. 2020;120:201–207. doi: 10.7556/jaoa.2020.032. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz-Lopez R, Tsai YC. A randomized double-blind controlled pilot study comparing leucocyte-rich platelet-rich plasma and corticosteroid in caudal epidural injection for complex chronic degenerative spinal pain. Pain Practice. 2020;20:639–646. doi: 10.1111/papr.12893. [DOI] [PubMed] [Google Scholar]
- 20.Anjayani S, Wirohadidjojo YW, Adam AM, Suwandi D, Seweng A, Amiruddin MD. Sensory improvement of leprosy peripheral neuropathy in patients treated with perineural injection of platelet-rich plasma. International Journal of Dermatology. 2014;53:109–113. doi: 10.1111/ijd.12162. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi M, Kamei N, Shinomiya R, Sunagawa T, Suzuki O, Kamoda H, Ohtori S, Ochi M. Human platelet-rich plasma promotes axon growth in brain-spinal cord coculture. NeuroReport. 2012;23:712–716. doi: 10.1097/WNR.0b013e3283567196. [DOI] [PubMed] [Google Scholar]
- 22.Bies M, Ashmore Z, Qu W, Hunt C. Injectable biologics for neuropathic pain: A systematic review. Pain Medicine. 2022;23:1733–1749. doi: 10.1093/pm/pnac066. [DOI] [PubMed] [Google Scholar]
- 23.Wilby MJ, et al. Surgical microdiscectomy versus transforaminal epidural steroid injection in patients with sciatica secondary to herniated lumbar disc (NERVES): A phase 3, multicentre, open-label, randomised controlled trial and economic evaluation. Lancet Rheumatology. 2021;3(5):e347–e356. doi: 10.1016/S2665-9913(21)00036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voelker A, Pirlich M, Heyde CE. Complications of injections in conservative treatment of degenerative spine disease: A prospective unicentric study. BMC Musculoskeletal Disorders. 2022;23(1):1002. doi: 10.1186/s12891-022-05970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Z, Wu S, Li X, Liu C, Fan S, Ma C. Ultrasound-guided transforaminal injections of platelet-rich plasma compared with steroid in lumbar disc herniation: A prospective, randomized, controlled study. Neural Plasticity. 2021;27(2021):5558138. doi: 10.1155/2021/5558138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le V-T. Transforaminal injection with autologous platelet-rich plasma in lumbar disc herniation: A single-center prospective study in Vietnam. Asian Journal of Surgery. 2022 doi: 10.1016/j.asjsur.2022.05.047. [DOI] [PubMed] [Google Scholar]
- 27.Bise S, Dallaudiere B, Pesquer L, Pedram M, Meyer P, Antoun MB, Hocquelet A, Silvestre A. Comparison of interlaminar CT-guided epidural platelet-rich plasma versus steroid injection in patients with lumbar radicular pain. European Radiology. 2020;30(6):3152–3160. doi: 10.1007/s00330-020-06733-9. [DOI] [PubMed] [Google Scholar]
- 28.Centeno C, Markle J, Dodson E, Stemper I, Hyzy M, Williams C, Freeman M. The use of lumbar epidural injection of platelet lysate for treatment of radicular pain. Journal of Experimental Orthopaedics. 2017;4(1):38. doi: 10.1186/s40634-017-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akeda K, Ohishi K, Masuda K, Bae WC, Takegami N, Yamada J, Nakamura T, Sakakibara T, Kasai Y. Sudo A intradiscal injection of autologous platelet-rich plasma releasate to treat discogenic low back pain: A preliminary clinical trial. Asian Spine Journal. 2017;11(3):380–389. doi: 10.4184/asj.2017.11.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tuakli-Wosornu YA, Terry A, Boachie-Adjei K, Harrison JR, Gribbin CK, LaSalle EE, Nguyen JT, Solomon JL, Lutz GE. Lumbar intradiskal platelet-rich plasma (PRP) injections: a prospective, double-blind, randomized controlled study. PM & R : The Journal of Injury, Function, and Rehabilitation. 2016;8(1):1–10. doi: 10.1016/j.pmrj.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Kubrova E, Martinez Alvarez GA, Her YF, Pagan-Rosado R, Qu W, D'Souza RS. Platelet rich plasma and platelet-related products in the treatment of radiculopathy—a systematic review of the literature. Biomedicines. 2022;10(11):2813. doi: 10.3390/biomedicines10112813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supoorting the study is avaialable upon reasonable request from authors.


