Abstract
Background
Rhabdomyosarcoma (RMS) harboring EWSR1/FUS-TFCP2 fusions has been recently described as a distinct form of RMS with an aggressive course and predilection for the craniofacial bones, especially the jaws.
Methods
We report three new cases of this rare entity, two from Brazil and one from Guatemala, with detailed clinicopathologic, immunohistochemical, and molecular descriptions. Additionally, we explored the English-language literature searching RMS with TFCP2 rearrangement or typical immunophenotype with co-expression of AE1/AE3 and ALK in the head and neck region.
Results
Case 1 is a 58-year-old male with a 3-month history of painful swelling in the anterior maxilla. Case 2 is a 22-year-old male presenting with right facial swelling and proptosis. Case 3 is a 43-year-old female with a rapidly growing tumor located in the zygomatic region. Imaging examinations revealed highly destructive intraosseous masses in the first two cases, and a soft tissue tumor with bone invasion in case 3. Microscopically, all cases showed a hybrid spindle and epithelioid phenotype of tumor cells which expressed desmin, myogenin and/or Myo-D1, AE1/AE3, and ALK. FISH confirmed molecular alterations related to TFCP2 rearrangement in Cases 1–2. In case 3, there was no available material for molecular analysis. The patients were subsequently referred to oncologic treatment. Additionally, we summarized the clinicopathologic, immunohistochemical, and molecular features of 27 cases of this rare RMS variant in the head and neck region reported in the English-language literature.
Conclusion
RMS with TFCP2 rearrangement is a rare and aggressive tumor with a particular predilection for craniofacial bones, especially the jaws. Knowing its clinicopathologic and immunohistochemical profile can avoid misdiagnosis.
Keywords: Rhabdomyosarcoma, Head and neck, TFCP2, Jaws, AE1/AE3, ALK
Introduction
Rhabdomyosarcoma (RMS) is a high-grade malignant neoplasm characterized by tumor cells with myogenic differentiation showing different growth patterns and morphologic features [1]. This tumor may arise in any body part; however, it mainly occurs in the trunk, genitourinary tract, extremities, and the head and neck region [2]. RMS represents the most common soft tissue sarcoma in pediatric patients, and the head and neck region is affected in approximately 40% of cases. In contrast, RMS is uncommon in adults, and only 1% occur in the head and neck region [3].
In the most recent WHO Classification of Bone and Soft Tissue Tumors (2020), RMS is divided into alveolar, embryonal, pleomorphic, and spindle cell/sclerosing types [4]. Based on molecular features the spindle cell/sclerosing RMS is subdivided into: (a) congenital/infantile spindle cell RMS harboring gene fusions of VGLL2, NCOA1/2, and SRF, (b) spindle cell/sclerosing RMS with MYOD1 mutations; and (c) intraosseous RMS with EWSR1/FUS-TFCP2 fusions (collectively referred to as FET-TFCP2 fusion RMS) or MEIS–NCOA2 fusions [4, 5]. This latter variant was introduced in the current WHO Classification of Head and Neck Tumors (2022) as an independent entity in malignant maxillofacial bone tumors [6, 7].
RMS with TFCP2 rearrangement has a predilection for the craniofacial bones, most commonly the mandible, and can affect patients of all age groups with an aggressive clinical course [5, 8]. Microscopically, the tumor shows a mixture of spindle and epithelioid cells with positivity for myogenic markers (desmin, myogenin, and Myo-D1), epithelial markers (pan-cytokeratins and EMA), and ALK overexpression [4, 8, 9].
Less than 30 cases of this rare variant have been reported in the head and neck region. We report three additional cases of head and neck rhabdomyosarcomas (HNRMS) with TFCP2 rearrangement or typical immunophenotype with co-expression of AE1/AE3 and ALK. In addition, we review the literature regarding the clinicopathologic, immunohistochemical and molecular features of this rare entity.
Case Reports
Case 1
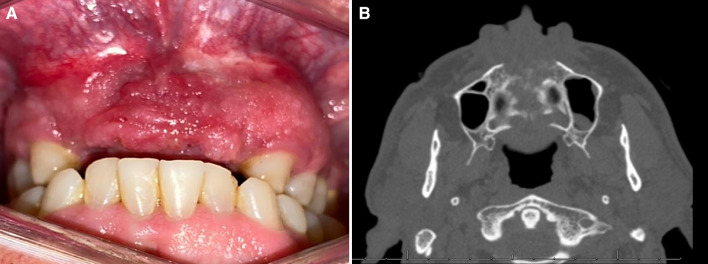
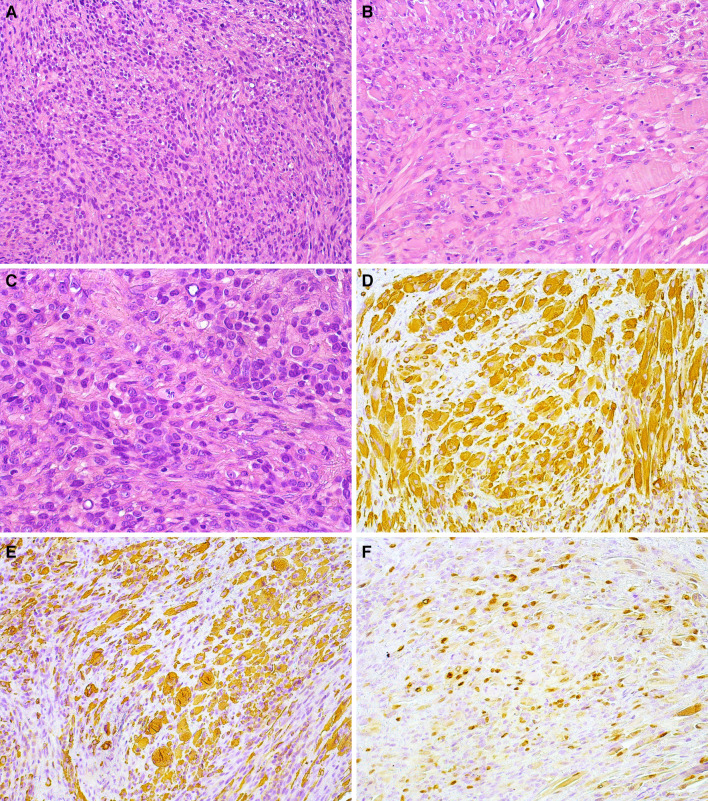
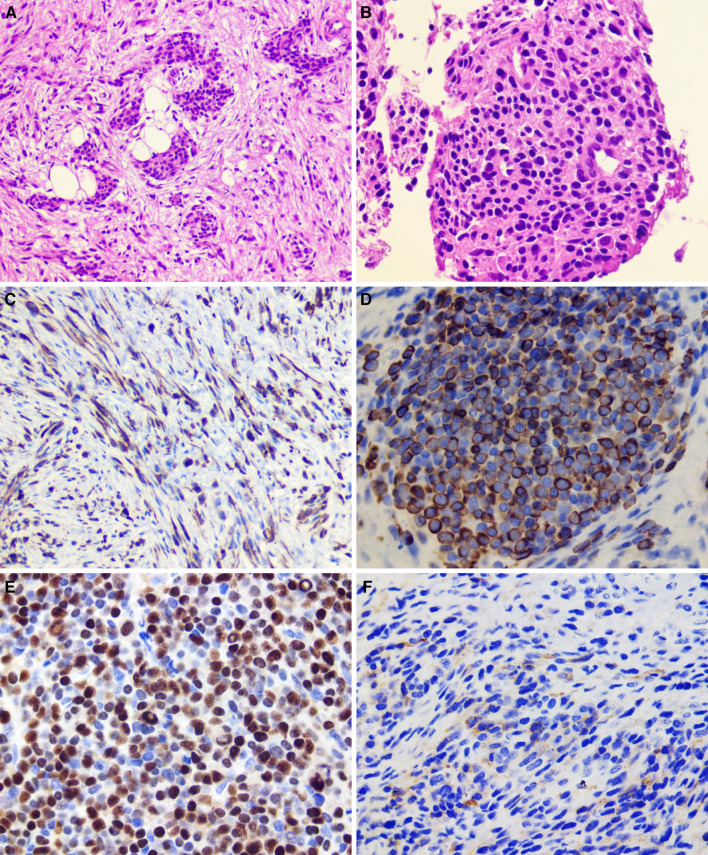
In February 2021, a 58-year-old Brazilian man presented with a 3-month history of a painful swelling in the anterior maxillary alveolar ridge. The patient’s medical history was unremarkable; however, he mentioned previous endodontic treatment of the upper right canine and extraction of the two upper central incisors. Despite treatment, the swelling and pain persisted. Intraoral examination revealed a swelling in the anterior portion of the maxilla, presenting a reddish irregular surface and ill-defined borders, extending into the palatal region (Fig. 1A). Computed tomography (CT) demonstrated a destructive lesion in the anterior maxilla with cortical bone destruction and ill-defined borders, measuring 5.0 × 4.5 × 4.0 cm (Fig. 1B). An incisional biopsy was performed and sent for histopathologic evaluation. Gross examination showed a yellowish soft-tissue fragment measuring 0.9 × 0.6 × 0.5 cm. Microscopically, the tumor revealed a solid proliferation of neoplastic cells arranged in fascicular and storiform patterns. Tumor cells were surrounded by scarce fibrous stroma and presented spindle to epithelioid morphology with abundant eosinophilic cytoplasm and variable sized nuclei with irregular contours and prominent nucleoli. Aggregates of small, round, blue cells were observed in focal areas. Mitotic figures and apoptotic cells were also identified within the tumor (Fig. 2A-C). Immunohistochemical evaluation showed diffuse positivity for desmin, myogenin was focally positive, and approximately 60% of cells stained for Myo-D1 (Fig. 2E–G). Strong and diffuse expression of AE1/AE3 (Fig. 2D) was observed in the spindle and epithelioid areas. Cytoplasmic expression of ALK (Fig. 2H) was focally positive in the neoplastic cells. The tumor showed a high proliferative cell index determined by the expression of Ki-67 in 90% of cells. Fluorescence in situ hybridization (FISH), using a dual color break-apart probe, showed the translocation of TFCP2 (Fig. 2I). The patient was treated with three cycles of neoadjuvant chemotherapy with partial response, but unfortunately, died 3 months after the diagnosis.
Fig. 1.
Case 1—Clinical and radiographical features: a A reddish irregular swelling with ill-defined borders in the anterior maxillary alveolar ridge b CT revealed a hypodense lytic lesion causing cortical destruction in the anterior maxilla
Fig. 2.
Case 1—Histopathological, immunohistochemical and molecular features: a Solid neoplasm predominantly composed of epithelioid and spindle cells (H&E, 200 ×). b Epithelioid cells showing abundant eosinophilic cytoplasm (H&E, 200 ×). c Pleomorphic and hyperchromatic nuclei of spindle-to-epithelioid cells and some atypical mitotic figures (H&E, 400 ×). Diffuse cytoplasmic positivity for AE1/AE3 (d; 200 ×) and Desmin (e; 200 ×). Strong nuclear positivity for MyoD1 (f; 200 ×) and myogenin (g; 200 ×). h Cytoplasmic expression of ALK (200 ×). i A split signal is seen with the FISH assay using a break-apart probe for TFCP2
Case 2
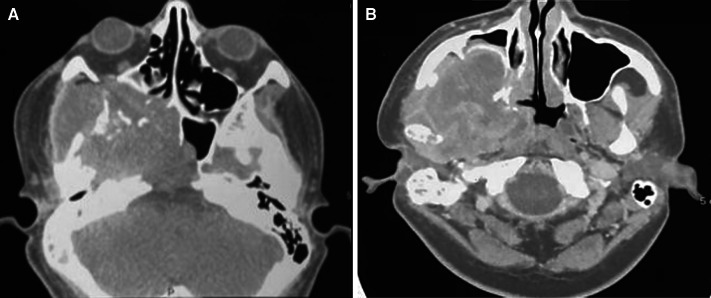
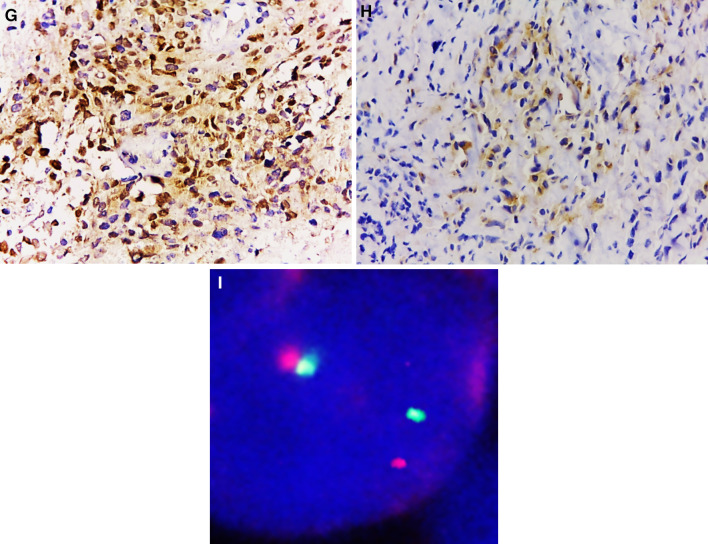
In November 2021, a 22-year-old Brazilian male was referred for evaluation due to an expansile lesion in the right maxilla. His past medical history was unremarkable. Extraoral examination showed a diffuse swelling causing facial asymmetry on the right side and exophthalmos. Intraoral examination showed extensive ulceration with a crater-like center and irregular borders located in the posterior portion of the right buccal mucosa. CT imaging revealed an infiltrative and destructive tumor located in the right posterior maxilla with extension into the maxillary sinus, nasal cavity, infratemporal fossa, and floor of the orbit (Fig. 3A, B). The patient underwent an incisional biopsy and gross examination revealed two irregular brownish soft tissues with homogenous white cut surfaces (Fig. 4A). Microscopically, a solid neoplasm was observed within the medullary bone tissue. The tumor presented a biphasic appearance with alternating hypocellular areas of spindle cells in a myxo-collagenized stroma and hypercellular areas composed of spindled-to-epithelioid cells with abundant eosinophilic cytoplasm. Nuclei were variable in size with evident pleomorphism and prominent nucleoli. Atypical mitotic figures, foci of necrosis, and residual bone fragments were noted (Fig. 4B–D). Immunohistochemical evaluation disclosed diffuse positivity for desmin, Myo-D1, and AE1/AE3, and focal cytoplasmic expression of ALK (Fig. 4E–H). The cell proliferation index measured by Ki-67 was 90%. TFCP2 translocation was confirmed by fluorescence in situ hybridization (FISH) using a dual color break-apart probe (Fig. 4I). The patient was subsequently referred to oncologic treatment, but was lost to follow-up.
Fig. 3.
Case 2—Radiographic features: a, b CT showing extensive mass in right maxilla affecting maxillary sinus and orbit, causing bone destruction and invading the surrounding soft tissue
Fig. 4.
Case 2—Macroscopic, microscopic, immunohistochemical and molecular features: a Macroscopic appearance of surgical specimens. b Tumor composed of hypocellular and hypercellular areas (H&E, 100 ×). c Spindle cells with pleomorphic and hyperchromatic nuclei (H&E, 200 ×). d Spindled-to-epithelioid cells with abundant eosinophilic cytoplasm (H&E, 200 ×). e Diffuse cytoplasmic positivity for AE1/AE3 (100 ×) and desmin (f, 200 ×). Strong nuclear positivity for MyoD1 (g, 200 ×) and focal expression of ALK (h; 200 ×). i FISH assay for TFCP2 dual color break-apart probe showing split signals
Case 3
In May 2021, a 43-year-old Guatemalan female patient with an unremarkable medical history presented with a rapidly growing tumor affecting the zygomatic region. Extraoral examination revealed an exophytic sessile mass in the right zygomatic region, causing diffuse swelling and facial asymmetry. Magnetic resonance imaging (MRI) revealed a hypo-and isointense soft tissue mass with regular borders (Fig. 5A, B). CT evidenced an extensive ill-defined lesion causing bone destruction of the zygomatic process. An incisional biopsy was performed, and the specimen was sent for histopathologic analysis. Microscopic examination showed a malignant neoplasm characterized by a diffuse proliferation of spindle cells arranged in a fascicular pattern and intermixed with aggregates and strands of epithelioid cells showing eosinophilic cytoplasm with hyperchromatic nuclei and surrounded by a fibromyxoid stroma (Fig. 6A, B). Multinucleated tumor cells and mitotic figures were also noted within the tumor. Immunohistochemical results revealed positivity for vimentin, AE1/AE3, desmin and Myo-D1 (Fig. 6C–E), and was negative for myogenin. In addition, focal positivity for ALK was observed (Fig. 6F). Considering the clinicopathologic and immunohistochemical features (AE1/AE3, ALK, desmin and Myo-D1) this tumor was diagnosed as epithelioid RMS ALK positive highly suspected of RMS with TFCP2 translocation, however it was not molecularly confirmed because of unavailability of the paraffin block sample. The patient was referred for oncologic treatment, but the follow-up information is unknown.
Fig. 5.
Case 3—Clinical and imaginological features: a MRI in T1-weighted image showed a hypo- and isointense MR signal in relation to soft tissues, and in T2-weighted image (b) evidenced a solid tumor component with hyperintense signal with apparently regular borders
Fig. 6.
Case 3—Histopathological and immunohistochemical features: a Tumor composed by short fascicles of spindle cells and aggregates of epithelioid cells (H&E, 200 ×). b Epithelioid cells with monotonous hyperchromatic nuclei and eosinophilic cytoplasm (H&E, 400 ×). Diffuse cytoplasmic positivity for AE1/AE3 (c; 200 ×) and desmin (d; 400 ×). e Strong nuclear positivity for MyoD1 (400 ×). f Focal expression of ALK (400 ×)
Discussion
In 2017, Watson et al. described for the first time a “new epithelioid RMS” characterized by TFCP2 rearrangement [8]. Since then, other cases of this rare entity have been reported in the literature using different terminology such as intraosseous RMS, epithelioid and spindle cell RMS with FUS/EWSR1-TFCP2 fusion, FET-TFCP2 RMS, RMS with FUS or TFCP2 rearrangements and RMS with TFCP2 fusions [9–15]. Despite the heterogeneous terminology, all authors agree that this is an aggressive tumor characterized by epithelioid and spindle cell phenotype with a striking predilection for the craniofacial skeleton [7–14, 16–20]. However, extraosseous tumors have also been described [9–11, 16].
So far, 27 cases of HNRMS with TFCP2 rearrangement have been reported in the English-language literature (Table 1) [8–20]. Most patients were young adults in the third and fourth decades of life. Nevertheless, 29.6% of cases (8/27) occurred in pediatric patients (19 years of age or younger). The median age at diagnosis was 26 years (range 11–74 years) with a slight male predilection. The majority of the cases were intraosseous (92.5%; 25/27), and the mandible was the most common site affected (40.7% of cases; 11/27), followed by the maxilla (14.8%; 4/27), skull, and occipital bone (11.1%; 3/27 cases each). Although, two cases affected the soft tissues of the neck [9] and oral cavity [11] without evidence of bone involvement. Similarly, our cases were in adults, two males with tumors located in the maxilla and one female presenting a zygomatic tumor. Cases 1 and 2 seem to be intraosseous lesions, but Case 3 arose in soft tissue causing bone destruction.
Table 1.
Clinicopathological features and outcome of head and neck rhabdomyosarcomas exhibiting TFCP2 rearrangement
| Author, year | Age/Gender | Location | Clinical Symptoms | Imaging features | Cells morphology | IHC | Molecular alteration | Metastasis | Treatment | Survival/Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Dashti et al. (2018) [16] | 72/M | Mandible | Gum swelling, tingling, numbness and loosening of the teeth in anterior left mandible | Destructive lytic lesion in left parasymphyseal mandible and cortex perforation | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; diffuse) Myogenin (+ ; focal) AE1/AE3 (+ ; diffuse) ALK (+ ; strong) |
FUS–TFCP2 fusion | No | Mandibulectomy |
ANED (2 mo.) |
| Wong et al. (2019) [17] and Lewin et al. (2019) [12] | 23/M | Nasal cavity | Nasal congestion related | Left nasal cavity tumor of 8 cm | Spindle, epithelioid and rhabdoid cells |
Desmin (+ ; patchy) MyoD1 (+ ; diffuse) Myogenin (+ ; rare cells) AE1/AE3 (−) ALK (+ ; strong) |
FUS rearrangement ALK gene deletion |
No | RT + CT and ALK inhibitor |
AWD (2 mo.) |
| Agaram et al. (2019) [18] | 33/F | Maxilla | NA | Large mass involving the maxilla and masticator space with extension into the maxillary and sphenoidal sinuses, orbit, and clivus | Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (+ ; diffuse) Myogenin (+ ; focal) AE1/AE3 (+) ALK (+) |
EWSR1–TFCP2 fusion | Yes (femur) | Surgical resection | ANED (108 mo.) |
| Le Loarer et al. (2019) [13] and Watson et al. (2018) [8] | 16/F | Sphenoid bone | Headache and left exophthalmos | NA | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 75%) Myogenin (+ ; 10%) AE1/AE3 (+) ALK (+ ; 50%) |
FUS–TFCP2 fusion | Yes (right femoral bone) | CT, surgical resection and cerebral RT |
DOD (15 mo.) |
| Le Loarer et al. (2019) [13] | 32/M | Hard palate and upper lip | Nodule of gingiva and hard palate of 3 cm | NA | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 75%) Myogenin (−) AE1/AE3 (+) ALK (−) |
EWSR–TFCP2 fusion | Yes (vertebra, ribs, pelvis) | CT |
DOD (8 mo.) |
| Le Loarer et al. (2019) [13] | 20/M | Orbito-temporo-sphenoid | Soft-tissue mass and left exophthalmos | NA | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 65%) Myogenin (+ ; 15%) AE1/AE3 (+) ALK (+ ; < 5%) |
FUS–TFCP2 fusion | No | CT + RT |
DOD (6 mo.) |
| Le Loarer et al. (2019) [13] and Brunac et al. (2019) [15] | 17/F | Cervico-occipital junction |
Insomnia and headaches related to neck pain for 3 months not relieved by standard painkillers. Loss- weight 3 kg |
NA | Round cells |
Desmin (+ ; 20%) MyoD1 (+ ; 75%) Myogenin (−) EMA (+ ; focal) ALK (+ ; 100%) |
FUS–TFCP2 fusion | No |
CT and adjuvant RT Anti-ALK therapy |
AWD (15 mo.) |
| Le Loarer et al. (2019) [13] | 31/M | Left occipital bone | Headache | NA | Spindle, epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; diffuse) Myogenin (+ ; 15%) AE1/AE3 (+ ; 25%) ALK (+ ; 100%) |
FUS–TFCP2 fusion | Yes (lung, mediastinum) | Fragmented resection and adjuvant CT |
DOD (6 mo.) |
| Le Loarer et al. (2019) [13] | 32/M | Mandible | Toothache | NA | Spindle cells |
Desmin (+ ; 20%) MyoD1 (+ ; diffuse) Myogenin (+ ; 15%) AE1/AE3 (+ ; 20%) ALK (+ ; 65%) |
FUS–TFCP2 fusion | Yes (lung) | Partial mandibulectomy and adjuvant CT |
AWD (14 mo.) |
| Le Loarer et al. (2019) [13] | 58/F | Mandible | NA | NA | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 70%) Myogenin (+ ; 15%) AE1/AE3 (+) ALK (+ ; 70%) |
FUS–TFCP2 fusion | No | Surgery |
ANED (21 mo.) |
| Le Loarer et al. (2019) [13] | 12/F | Mandible | Local painful swelling for 4 months | Osteolysis of the body, angle and ramus of the mandible and extension into surrounding soft-tissue | Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 40%) Myogenin (+ ; 50%) AE1/AE3 (+) ALK (+ ; 50%) |
FUS–TFCP2 fusion | No | Neoadjuvant CT |
ANED (21 mo.) |
| Le Loarer et al. (2019) [13] | 11/F | Maxilla | NA | NA | Epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; 80%) Myogenin (+ ; 30%) AE1/AE3 (+ ; weak) ALK (+ ; < 5%) |
EWSR–TFCP2 fusion | No | CT | DOD (Unknown) |
| Le Loarer et al. (2019) [13] | 25/M | Mandible | Local painful swelling for 1 month | NA | Epithelioid cells |
Desmin (−) MyoD1 (+ ; 80%) Myogenin (−) AE1/AE3 (+) ALK (+ ; 80%) |
EWSR–TFCP2 fusion | No | Surgery |
ANED (20 mo.) |
| Chrisinger et al. (2020) [10] | Mid-20 s–30 s / F | Frontal bone | Rapidly growing right scalp swelling associated with headache for 6 weeks |
Destructive lesion arising from the right frontal bone with cortical breach, which measured 5 × 4.6 × 4 cm |
Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (NA) Myogenin (−) AE1/AE3 (+ ; diffuse) ALK (+) |
EWSR–TFCP2 fusion | No | CT, RT and surgical resection |
DOD (17 mo.) |
| Flaitz et al. (20,200 [20] | 15/F | Mandible | Left mandibular enlargement | NA | Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (+ ; diffuse) Myogenin (+ ; focal) AE1/AE3 (NA) ALK (+ ; focal) |
FUS–TFCP2 fusion | NA | Surgical resection, CT and RT | Unknown |
| Koutlas et al. (2021) [14] | 15/M | Mandible | Pain and swelling in the left posterior mandible | Destructive lesion exhibiting moth eaten-like irregular and ill-defined borders and loss of both buccal and lingual plates | Spindle, epithelioid and round cells |
Desmin (+ ; patchy) MyoD1 (+ ; diffuse) Myogenin (+ ; focal) AE1/AE3 (+ ; diffuse) ALK (−) β-catenin (+) |
EWSR–TFCP2 fusion | Yes (ipsilateral cervical lymph nodes) | Surgical resection, homolateral lymph node dissection, CT and proton beam therapy |
AWD (7 mo.) |
| Xu et al. (2021) [9] | 22/M | Mandible | NA | NA | Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (+) Myogenin (+ ; focal) AE1/AE3 (+) ALK (+) |
FUS–TFCP2 fusion ALK wild type |
Yes (lymph node) | NA | NA |
| Xu et al. (2021) [9] | 34/M | Mandible | NA | NA | Spindle, epithelioid and rhabdoid cells |
Desmin (+) MyoD1 (+ ; patchy) Myogenin (NA) AE1/AE3 (−) ALK (−) |
FUS–TFCP2 fusion ALK deletion |
No | NA |
AWD (10 mo.) |
| Xu et al. (2021) [9] | 16/M | Mandible | NA | NA | Spindle, epithelioid and rhabdoid cells |
Desmin (+ ; focal) MyoD1 (+ ; focal) Myogenin (+ ; focal) AE1/AE3 (+) ALK (+) |
FUS–TFCP2 fusion ALK deletion |
Yes (bone, lung, lymph node) | NA |
DOD (20 mo.) |
| Xu et al. (2021) [9] | 43/F | Mandible | NA | NA | Spindle and epithelioid cells |
Desmin (+) MyoD1 (+) Myogenin (+ ; rare cells) AE1/AE3 (+) ALK (+) |
FUS–TFCP2 fusion ALK not performed |
NA | NA | NA |
| Xu et al. (2021) [9] | 20/F | Maxilla | NA | NA | Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (NA) Myogenin (+) AE1/AE3 (+) ALK (+) |
EWSR1–TFCP2 fusion ALK not performed |
Yes (bone) | NA | NA |
| Xu et al. (2021) [9] and Zhu et al. (2019) [19] | 74/F | Maxilla/ gingiva |
Growing lesion on right maxillary gingiva |
Expansile lytic lesion within the right maxillary alveolar ridge extending beyond midline, involving the hard palate |
Spindle and epithelioid cells |
Desmin (+ ; diffuse) MyoD1 (+ ; patchy) Myogenin (+ ; focal) AE1/AE3 (−) ALK (+) |
FUS–TFCP2 fusion ALK wild type |
Yes (lymph node) | NA | DOD (21mo.) |
| Xu et al. (2021) [9] and Agaram et al. (2019) [18] | 27/F | Skull | NA | NA | Spindle and epithelioid cells |
Desmin (+ ; focal) MyoD1 (+ ; diffuse) Myogenin (+ ; focal) AE1/AE3 (+) ALK (+) |
EWSR1–TFCP2 fusion ALK not performed |
Yes (bone) | NA |
AWD (1 mo.) |
| Xu et al. (2021) [9] | 18/M | Skull | NA | NA | Spindle and epithelioid cells |
Desmin (−) MyoD1 (NA) Myogenin (−) AE1/AE3 (+) ALK (+) |
FUS–TFCP2 fusion ALK wild type |
NA | NA | NA |
| Xu et al. (2021) [9] | 29/M | Skull (base) | NA | NA | Spindle and epithelioid cells |
Desmin (+) MyoD1 (+) Myogenin (+) AE1/AE3 (+) ALK (NA) |
EWSR1–TFCP2 fusion ALK wild type |
Yes (Lung) | NA |
AWD (2 mo.) |
| Xu et al. (2021) [9] | 40/F | Neck superficial soft tissue | NA | NA | Spindle, epithelioid and round cells |
Desmin (+) MyoD1 (NA) Myogenin (+ ; rare cells) AE1/AE3 (+) ALK (+) |
FUS–TFCP2 fusion ALK deletion |
NA | NA | NA |
| Ochsner and Foss (2022) [11] | 48/M | Maxillary gingiva |
Rapidly growing exophytic lesion with rolled borders, erythematous surface and central ulceration and necrosis located on the anterior maxillary gingiva with extension into the labial vestibule |
Periapical radiograph revealed no evidence of a lytic lesion or intra-osseous involvement |
Spindle, epithelioid and round cells |
Desmin (+ ; focal) MyoD1 (+ ; strong and diffuse) Myogenin (−) AE1/AE3 (+) ALK (+) |
FUS rearrangement | NA | NA | NA |
Clinical manifestations were described in 55.5% of cases (15/27). Most patients referred painful swelling with rapid progression (10%; 10/15). Other signs and symptoms such as headache, nasal congestion, exophthalmos, and toothache were also reported. Imaging characterization of HNRMS with TFCP2 rearrangement was identified in 29.6% (8/27) of the cases, and most tumors were described as large osteolytic masses causing bone destruction and invasion of adjacent tissues, as also observed in our three reported cases.
Microscopically, 85.2% of tumors (23/27), showed a mixed spindle and epithelioid phenotype. Some cases also contained areas of round or rhabdoid cells (3/23; 13% for each) admixed with spindle and epithelioid cells. However, four HNRMS with TFCP2 rearrangement (14.8%) exhibited monotonous cell morphology; two with epithelioid cells, one with spindle cells, and another with round cells. All present cases exhibited a mixture of spindle and epithelioid cytomorphology. Case 1 also showed focal areas with small round cells.
The immunohistochemical profile of this rare entity was characterized by myogenic differentiation, cytokeratins, and ALK expression. Positivity for desmin, myogenin, and MyoD1 were 92.6% (25/27), 76.9% (20/26), and 100% (23/23), respectively. Desmin and MyoD1 were more sensitive and diffusely positive in most cases when compared with myogenin, as already described by Xu et al. [9] and Le Loarer et al. [13]. In addition, to the hybrid cell morphology and positivity for myogenic markers, diffuse and strong expression of AE1/AE3 is considered a hallmark of RMS with TFCP2 rearrangement. Of the reported cases, 84.6% (22/26) expressed diffuse positivity for AE1/AE3. Positive immunostaining for ALK was observed in 88% of cases (22/25). Similar findings of myogenic immunophenotype, pan-cytokeratin and ALK expression were observed in our cases.
It is important to highlight the common positivity of cytokeratins in about 50% of alveolar RMS [21], although only focally, contrasting with the diffuse expression displayed by most RMS with TFCP2 rearrangement [13]. Moreover, epithelial membrane antigen (EMA) and other keratins, including CK7, CAM5.2, and CK5/6, can also be positive in TFCP2 translocated RMS [6]. Therefore, it could be a potential diagnostic pitfall considering that keratin positivity is traditionally used to distinguish epithelial neoplasms from mesenchymal tumors [8, 13, 21].
The clinicopathological and immunohistochemical features of RMS with TFCP2 rearrangements could make diagnosing this neoplasm challenging for pathologists. Therefore, the differential diagnosis, in intraosseous tumors, includes metastatic sarcomatoid carcinoma, mesenchymal chondrosarcoma, hemangioendothelioma, osteosarcoma, dedifferentiated chondrosarcoma and leiomyosarcoma [10, 11]. For soft tissue tumors, malignant peripheral nerve sheath tumors, inflammatory myofibroblastic tumors, spindle cell and round cell sarcomas with EWSR1-PATZ1 fusion must be considered in the differential diagnosis [11].
Molecular alterations of this RMS variant are characterized by FET-TFCP2 and MEIS1-NCOA2 fusions [18]. HNRMS with TFCP2 translocations displayed genetic fusions with FUS and EWSR1 in 59.2% (16/27) and 3.7% (7/27) of cases, respectively. These genes are a member of the FET (FUS, EWS, TAF15) RNA binding protein family involved in deleterious genomic rearrangements with other transcription factor genes in some carcinomas, sarcomas, and acute leukemia [14, 22]. The gene TFCP2 regulates the expression of epidermal growth factor receptor (EGFR) and accelerates tumor cell motility, invasion, and metastasis in breast cancer. So, Koutlas et al. postulated that mutated stem cells with FET-TFCP2 fusion develop a myogenic phenotype through EWSR1 or FUS while the TFCP2 translocation induces invasion and metastasis of the tumor cells, which could explain the aggressive clinical behavior of this rare variant of RMS [14]. Hence, we confirmed the TFCP2 rearrangement in two of our cases. Unfortunately, we could not perform molecular analysis in Case 3. However, we favor clinical, microscopic, and immunohistochemical features as enough evidence to diagnose this tumor. Furthermore, the scenario of developing countries must be considered in this case due to the high cost and difficult access to molecular testing.
On the other hand, only two cases with MEIS1-NCOA2 fusion have been reported. Both are located in the iliac bones, and are characterized by pure spindle cells without cytokeratin and ALK expression [18]. HNRMS with MEIS1-NCOA2 fusion has not been described in the literature.
Moreover, 4/8 cases (50%) showed ALK deletion. Interestingly, ALK inhibitors have been used as potential target therapies in two patients affected by RMSs with TFCP2 rearrangements, despite inconclusive outcome reported [9, 12, 15]. ALK expression by immunohistochemical assay does not correlate with ALK rearrangement and it seems that not all patients may benefit with use of ALK inhibitors [9, 12]. We observed focal immunopositivity for ALK in our cases.
RMS with TFCP2 rearrangement has the potential to spread to regional lymph nodes and distant sites [9]. Among the 27 patients with TFCP2-translocated HNRMS, 54.5% (12/22) developed regional and/or distant metastasis. Despite the fact that no treatment protocol has been established for this aggressive neoplasm, most patients have been treated by surgical resection which may be with chemotherapy and/or radiotherapy. Two patients were treated with ALK inhibitors, one of them treated with combined chemotherapy had a good response [15]. The median follow-up time was 20 months, ranging between 1 to 108 months, but follow-up data was not provided in all cases. The vital status of 20/27 patients (74.1%) were available, and 25% were alive without evidence of disease, 35% were alive with the tumor, and 40% were dead. We reported only one case with follow-up information of three months who died of disease.
Finally, as previously discussed by Le Loarer et al. [13], the so-called “epithelioid rhabdomyosarcomas” present a purely epithelioid pattern, typically affecting deep soft tissues, and scarcely express epithelial markers. This morphologic subset contrasts with features of RMS with TFCP2 rearrangements; and only three cases have been reported presenting pure epithelioid morphology [13].
In summary, HNRMS with TFCP2 rearrangement was recently categorized as an independent entity due to its unique predilection for craniofacial bones, immunohistochemical profile, and genetic alteration. We add 2 cases with molecular confirmation of TFCP2 translocation affecting the maxilla and one additional suspected case in soft tissue. Microscopic evaluation of a high-grade malignant neoplasm with spindle and epithelioid cells and co-expression of myogenic markers, pan-cytokeratin, and ALK are essential diagnostic criteria. Molecular testing for TFCP2 translocation is desirable to confirm the diagnosis [6]. Furthermore, the limited follow-up information indicates the aggressive behavior and poor prognosis for this rare variant of RMS.
Acknowledgements
We would like to thank “Getulio Sales Diagnósticos—Natal, Brazil” for helping with the molecular testing of our cases.
Author Contributions
All authors contributed substantially to the conception, draft, and design of these cases reported, as well as participation in the acquisition, analysis, and interpretation of data. All authors have contributed equally.
Funding
This study was not supported by any funding.
Data Availability
The authors declare that all data supporting the findings of this study are available in the article.
Code Availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The tumors tissues included in the manuscript were obtained as part of the standard of care for the patient and were retrospectively collected for publication.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Footnotes
Roman Carlos—Deceased on 5th November 2021.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kohashi K, Kinoshita I, Oda Y. Soft tissue special issue: skeletal muscle tumors: a clinicopathological review. Head Neck Pathol. 2020;14(1):12–20. doi: 10.1007/s12105-019-01113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasgupta R, Fuchs J, Rodeberg D. Rhabdomyosarcoma. Semin Pediatr Surg. 2016;25(5):276–283. doi: 10.1053/j.sempedsurg.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Häußler SM, Stromberger C, Olze H, Seifert G, Knopke S, Böttcher A. Head and neck rhabdomyosarcoma in children: a 20-year retrospective study at a tertiary referral center. J Cancer Res Clin Oncol. 2018;144(2):371–379. doi: 10.1007/s00432-017-2544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The WHO Classification of Tumours Editorial Board . WHO classification of tumours soft tissue and bone tumours. 5. Lyon: IARC Press; 2020. [Google Scholar]
- 5.Agaram NP. Evolving classification of rhabdomyosarcoma. Histopathology. 2022;80(1):98–108. doi: 10.1111/his.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Classification of Tumours Editorial Board. Head and neck tumours. Lyon (France): International Agency for Research on Cancer. (WHO classification of tumours series, 5th ed. https://publications.iarc.fr/ (2022).
- 7.Vered M, Wright JM. Update from the 5th edition of the World Health Organization classification of head and neck tumors: odontogenic and maxillofacial bone tumours. Head Neck Pathol. 2022;16(1):63–75. doi: 10.1007/s12105-021-01404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson S, Perrin V, Guillemot D, Reynaud S, Coindre JM, Karanian M, Guinebretière JM, Freneaux P, Le Loarer F, Bouvet M, Galmiche-Rolland L, Larousserie F, Longchampt E, Ranchere-Vince D, Pierron G, Delattre O, Tirode F. Transcriptomic definition of molecular subgroups of small round cell sarcomas. J Pathol. 2018;245(1):29–40. doi: 10.1002/path.5053. [DOI] [PubMed] [Google Scholar]
- 9.Xu B, Suurmeijer AJH, Agaram NP, Zhang L, Antonescu CR. Head and neck rhabdomyosarcoma with TFCP2 fusions and ALK overexpression: a clinicopathological and molecular analysis of 11 cases. Histopathology. 2021;79(3):347–357. doi: 10.1111/his.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrisinger JS, Wehrli B, Dickson BC, et al. Epithelioid and spindle cell rhabdomyosarcoma with FUS-TFCP2 or EWSR1-TFCP2 fusion: report of two cases. Virchows Arch. 2020;477(5):725–732. doi: 10.1007/s00428-020-02870-0. [DOI] [PubMed] [Google Scholar]
- 11.Ochsner AR, Foss RD. Epithelioid and spindle cell rhabdomyosarcoma of the oral mucosa with FUS rearrangement. Head Neck Pathol. 2022;16:823–827. doi: 10.1007/s12105-022-01424-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewin J, Desai J, Smith K, Luen S, Wong D. Lack of clinical activity with crizotinib in a patient with FUS rearranged rhabdomyosarcoma with ALK protein overexpression. Pathology. 2019;51(6):655–657. doi: 10.1016/j.pathol.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Le Loarer F, Cleven AHG, Bouvier C, Castex MP, Romagosa C, Moreau A, Salas S, Bonhomme B, Gomez-Brouchet A, Laurent C, Le Guellec S, Audard V, Giraud A, Ramos-Oliver I, Cleton-Jansen AM, Savci-Heijink DC, Kroon HM, Baud J, Pissaloux D, Pierron G, Sherwood A, Coindre JM, Bovée JVMG, Larousserie F, Tirode F. A subset of epithelioid and spindle cell rhabdomyosarcomas is associated with TFCP2 fusions and common ALK upregulation. Mod Pathol. 2020;33(3):404–419. doi: 10.1038/s41379-019-0323-8. [DOI] [PubMed] [Google Scholar]
- 14.Koutlas IG, Olson DR, Rawwas J. FET(EWSR1)-TFCP2 rhabdomyosarcoma: an additional example of this aggressive variant with predilection for the gnathic bones. Head Neck Pathol. 2021;15(1):374–380. doi: 10.1007/s12105-020-01189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunac AC, Laprie A, Castex MP, Laurent C, Le Loarer F, Karanian M, Le Guellec S, Guillemot D, Pierron G, Gomez-Brouchet A. The combination of radiotherapy and ALK inhibitors is effective in the treatment of intraosseous rhabdomyosarcoma with FUS-TFCP2 fusion transcript. Pediatr Blood Cancer. 2020;67(5):e28185. doi: 10.1002/pbc.28185. [DOI] [PubMed] [Google Scholar]
- 16.Dashti NK, Wehrs RN, Thomas BC, Nair A, Davila J, Buckner JC, Martinez AP, Sukov WR, Halling KC, Howe BM, Folpe AL. Spindle cell rhabdomyosarcoma of bone with FUS-TFCP2 fusion: confirmation of a very recently described rhabdomyosarcoma subtype. Histopathology. 2018;73(3):514–520. doi: 10.1111/his.13649. [DOI] [PubMed] [Google Scholar]
- 17.Wong DD, van Vliet C, Gaman A, Giardina T, Amanuel B. Rhabdomyosarcoma with FUS re-arrangement: additional case in support of a novel subtype. Pathology. 2019;51(1):116–120. doi: 10.1016/j.pathol.2018.09.056. [DOI] [PubMed] [Google Scholar]
- 18.Agaram NP, Zhang L, Sung YS, Cavalcanti MS, Torrence D, Wexler L, Francis G, Sommerville S, Swanson D, Dickson BC, Suurmeijer AJH, Williamson R, Antonescu CR. Expanding the spectrum of intraosseous rhabdomyosarcoma: correlation between 2 distinct gene fusions and phenotype. Am J Surg Pathol. 2019;43(5):695–702. doi: 10.1097/PAS.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu G, Benayed R, Ho C, Mullaney K, Sukhadia P, Rios K, Berry R, Rubin BP, Nafa K, Wang L, Klimstra DS, Ladanyi M, Hameed MR. Diagnosis of known sarcoma fusions and novel fusion partners by targeted RNA sequencing with identification of a recurrent ACTB-FOSB fusion in pseudomyogenic hemangioendothelioma. Mod Pathol. 2019;32(5):609–620. doi: 10.1038/s41379-018-0175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flaitz CM, Hicks MJ. Primary intraosseous rhabdomyosarcoma: rare subtype involving mandible with unique translocation. 74th Annual Meeting, American Academy of Oral and Maxillofacial Pathology, Nashville; Abstract ID:43; poster No:55; 2020.
- 21.Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 22.Kovar H. Dr. Jeckyll and. Mr Hyde: the two faces of the FUS/EWS/TAF15 protein family. Sarcoma. 2011;2011:837474. doi: 10.1155/2011/837474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the findings of this study are available in the article.
Not applicable.