Abstract
Introduction
Despite rasburicase's proven efficiency in Caucasians, Japanese, and Koreans, studies evaluating the safety and effectiveness of rasburicase in Chinese pediatric patients with non-Hodgkin’s lymphoma (NHL) and acute leukemia (AL) in particular are lacking.
Objective
The aim was to evaluate the safety and effectiveness of rasburicase in Chinese pediatric patients with NHL and AL.
Methods
In this phase IV, open-label, non-randomized, single-arm, multi-center, interventional study (NCT04349306), children newly diagnosed with NHL or AL who received 0.20 mg/kg/day of rasburicase were included. The primary objective was to assess the safety of rasburicase by the incidence of adverse events (AEs). The secondary objective was to determine the effectiveness of rasburicase in the control of hyperuricemia.
Results
Out of 50 patients, 25 reported a total of 76 treatment-emergent adverse events (TEAEs), including eight TEAEs of grade ≥ 3 in 12 patients. A drug-related serious AE was reported in one patient, and there was no incidence of death. The response rate in the intent-to-treat population was 100.0% (95% confidence interval 82.4–100.0) in patients (n = 19) with baseline uric acid level of > 8.0 mg/dL. Similarly, the response rate was 86.2% (n = 25) among 29 patients (60.4%) with baseline uric acid levels of ≤ 8.0 mg/dL. The maximum mean percentage decrease of plasma uric acid level in the overall patients was 96.9%.
Conclusion
Rasburicase was well tolerated and effective in controlling hyperuricemia in Chinese pediatric patients with NHL and AL.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40268-023-00420-y.
Key Points
| Until this time, no studies had evaluated the safety and effectiveness of rasburicase in Chinese pediatric patients with non-Hodgkin’s lymphoma (NHL) and acute leukemia (AL). |
| The current study evaluated the safety and effectiveness of rasburicase in Chinese pediatric patients with NHL and AL and demonstrated tolerable safety and efficacy. |
| Being the first study to evaluate the safety and effectiveness of rasburicase in Chinese pediatric patients, the current study offers a new option in the treatment armamentarium for hematological malignancies having a risk of tumor lysis syndrome. |
Introduction
Tumor lysis syndrome (TLS) is a hemato-oncological emergency associated with life-threatening complications [1, 2]. It is prevalent in both adult and pediatric patients with leukemia, lymphoma, and solid tumor malignancies who are undergoing chemotherapy and results from the rapid destruction of malignant cells, thereby releasing intracellular contents into the extra-cellular space [1, 3]. Patients with a large tumor burden and/or tumors with rapidly dividing cells (lymphoma and leukemia) and/or high sensitivity to chemotherapy or cytolytic antibody therapy are at high risk for TLS [4]. If left undiagnosed or diagnosed at an advanced stage, mortality is observed in 20–50% of cases [5]. Hyperuricemia and TLS are known complications of non-Hodgkin’s lymphoma (NHL) and acute leukemia (AL) [6].
As per the guidelines of the American Society of Clinical Oncology, 2008, and the British Committee for Standards in Haematology, 2015 [7, 8], the most effective management of TLS is prevention of the syndrome, which includes hydration plus prophylactic rasburicase or hydration plus allopurinol in high- and intermediate-risk pediatric patients, respectively. Although few studies from east Asia including Japan and Korea are available [9, 10], with China’s larger population (1.3 billion), around 14.9% of new cases of NHL and 15.7% of deaths in the world are constituted by China [11, 12]. Similarly, among the population of 212 million people below 15 years of age in China, approximately 36.5 per million of the population suffer from leukemia [12]. Moreover, to date, there are no clinical guidelines for managing TLS in China, and the standard of care includes hydration, alkalization, allopurinol, furosemide, and low-molecular dextran. Allopurinol is a xanthine oxidase inhibitor widely used to prevent cancer-related hyperuricemia [13]. However, allopurinol cannot prevent the levels of pre-existing uric acid, xanthine, or hypoxanthine [14]. Hence, there is an unmet need of effective treatment for TLS in China. Rasburicase (Fasturtec®), a recombinant form of the enzyme urate oxidase produced in Saccharomyces cerevisiae from Aspergillus flavus, offers a potential advantage over allopurinol [15]. Approved by the US Food and Drug Administration (FDA) for treating hyperuricemia [16], this enzyme converts hypoxanthine and xanthine into more soluble allantoin, which gets easily excreted by the kidneys [17]. The efficacy of rasburicase lies in its rapid onset of action, promptness in reducing uric acid levels, improvement in electrolyte status, and reversal of renal insufficiency [15].
Previously conducted clinical trials have demonstrated the superiority of rasburicase compared to allopurinol in producing a steep and rapid decrease in plasma uric acid concentrations in pediatric patients with TLS [18–20]. A single low-dose of rasburicase has shown effectiveness in pediatric patients with NHL and AL in the management of TLS, with significant cost reductions [21, 22]. Rasburicase was also efficient in the prevention and management of TLS in resource-limited settings [22, 23].
Furthermore, rasburicase is generally well tolerated, with the most commonly reported adverse events (AEs) being headache, nausea, abdominal pain, mucositis, and mild allergic reactions [24, 25]. Previously conducted studies have demonstrated rasburicase has a tolerable safety profile, with no incidence of anaphylactic or severe acute hypersensitivity reactions [19, 26]. Few studies have evaluated the efficacy and safety of rasburicase in Chinese patients with hematological malignancies [27, 28], and observations and experiences of phase IV post-marketing clinical trials are limited. Therefore, the current phase IV study aimed to evaluate the safety and effectiveness of rasburicase in Chinese pediatric patients with NHL and AL.
Materials and Methods
Study Design
This was a multi-center, non-randomized, single-arm, open-label, interventional phase IV study conducted in 55 pediatric patients with NHL and AL spanning ten centers in China from May 2020 to March 2021. The study is registered at ClinicalTrials.gov (NCT04349306). The study protocol and its subsequent revisions were approved by the independent ethics committees (IECs) and/or institutional review boards (IRBs) (IEC-C-007-A01-V.05), while conforming to the standards of the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice (GCP), all applicable laws, rules, and regulations. All patients provided written informed consent before study entry.
Patient Eligibility
Patients aged between 2 and 18 years old and newly diagnosed with NHL or AL, having baseline blood uric acid levels > 8 mg/dL (473 µmol/L), with a performance status (PS) of ≤ 3 on the Eastern Cooperative Oncology Group (ECOG) scale or a PS of ≥ 30 on the Lansky score, and with a minimum life expectancy of 45 days were considered eligible. TLS risk was defined on the basis of Cairo and Bishop criteria [29, 30]. At the time of screening, if the newly diagnosed patient with NHL or AL had a blood uric acid level ≤ 8 mg/dL, the patient was classified as stage III or IV NHL with either Burkitt lymphoma/leukemia or lymphoblastic lymphoma and/or with at least one lymph node affected or tumor with a diameter of > 5 cm, and/or lactate dehydrogenase (LDH) ≥ 2 times the upper limit of normal (ULN); while patients with blood uric acid levels ≤ 8 mg/dL were considered at high risk for TLS with either a white blood cell (WBC) value of ≥ 100.0 × 109/L, or < 100.0 × 109/L with LDH ≥ 2 × ULN.
Patients were excluded from the study if they had acute promyelocytic leukemia, severe infection or active bleeding, a documented history of hereditary allergy or asthma, a known deficiency of glucose-6-phosphate dehydrogenase (G6PD), or a history of hemolytic disease or methemoglobinemia. Patients who had received previous therapy with urate oxidase, were treated or were planned to receive allopurinol within 72 h before rasburicase administration, had abnormal liver or renal function (alanine aminotransferase [ALT] > 5 × ULN, total bilirubin > 3 × ULN, and serum creatinine > 3 × ULN), or had a hypersensitive reaction against rasburicase or any of the other ingredients of the study drug were also excluded. Pregnant or lactating women and those not under birth control, male patients not protected by highly effective method(s) of birth control, and individuals judged unsuitable for participation by the investigator were considered ineligible for study participation.
Study Outcomes and Endpoints
The primary endpoint of this study was to evaluate the safety of rasburicase in pediatric patients with NHL and AL by the incidence of AEs and serious adverse events (SAEs). The safety parameters included clinical laboratory tests, physical examinations, vital signs, electrocardiograph (ECG) results, and ECOG scale score. The effectiveness of rasburicase for controlling hyperuricemia was assessed in terms of response rate (number of responders after completion of rasburicase treatment under chemotherapy, which was defined as the number of patients achieving normal uric acid levels [≤ 8.0 mg/dL] in patients whose uric acid levels were > 8.0 mg/dL), the proportion of patients maintaining normal uric acid levels from first administration to 120 h after the first dose in patients with baseline plasma uric acid levels ≤ 8 mg/dL but at a high risk of TLS, and the percentage of the maximum decreasing degree of plasma uric acid level from baseline after rasburicase treatment were considered as the secondary endpoints of the study.
The safety outcomes were assessed by the incidence of AEs observed from day 1 to 48 h after administration of the last dose, which were coded by the Medical Dictionary for Regulatory Activities (MedDRA), version 24.0. Data collected on AEs included treatment-emergent adverse events (TEAEs), drug-related TEAEs, TEAEs with Common Terminology Criteria for Adverse Events (CTCAE) of grade ≥ 3 and classified by System Organ Class (SOC) or Preferred Term (PT), serious TEAEs, drug-related serious TEAEs, TEAEs leading to treatment discontinuation, TEAEs leading to death, and adverse events of special interest (AESIs), and they were summarized by the number of events, the number of study participants, and the incidence rate.
Treatment Modalities and Patient Evaluation
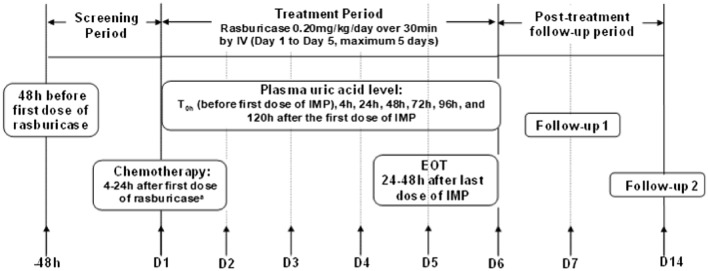
The eligible patients received rasburicase (Fasturtec®) at a dosage of 0.20 mg/kg/day by intravenous infusion over 30 min for 1–5 consecutive days as per the patients’ plasma uric acid level or the investigator’s judgement. All cytoreductive chemotherapy agents were initiated within 4–24 h after the initiation of the first dose of rasburicase. The entire study was divided into various sections, including a 48-h screening period, a 1- to 5-day treatment period, and post-treatment observation up to the 14th day after the first dose. AEs were assessed from screening until 14 days after the first dose of rasburicase, whereas the plasma uric acid levels were measured at baseline and 4, 24, 48, 72, 96, and 120 h. Biochemistry, hematology, urinalysis, vital signs, and physical examination investigations were also conducted. PS was assessed using the ECOG PS criteria/Lansky scale. Patients were followed up post-treatment on day 7 and day 14 (Fig. 1).
Fig. 1.
Study design. D day, EOT end of therapy, IMP investigational medicinal product, IV intravenous, T time. aAll participants received study medication before or during the first cycle of chemotherapy
Statistical Analysis
The safety and intent-to-treat (ITT) analysis sets were used to analyze safety and effectiveness data, respectively. The safety population was defined as any patients who received at least one dose of the drug, whereas the ITT population included patients who received at least one dose of study treatment and had one post-treatment assessment of plasma uric acid level. Descriptive statistics were used to analyze the safety parameters that included the incidences of AEs or SAEs with respect to the number and percentage of participants. The response rate based on the effectiveness endpoint definitions was presented as a percentage with 95% confidence interval (CI). Mean (standard deviation [SD]), median, minimum, and maximum were used to summarize the percentage decrease. In general, continuous data were described by the number of participants (n), mean (SD), interquartile range (IQR), minimum, and maximum unless otherwise specified. For categorical variables, the number and percentage of patients for each category were calculated. All statistical analyses were performed using SAS® software, version 9.4 (SAS Institute Inc. SAS/STAT, Cary, NC).
Sample Size Determination
Since there was no clear epidemiology data about hyperuricemia or TLS during chemotherapy in Chinese pediatric hematological malignancies, the sample size was determined based on the requirement of the Center for Drug Evaluation [31]. A post-marketing study with a sample size similar to the phase IV studies conducted in Taiwan [L8637, n = 45] and South Korea [L8720, n = 45] was considered for sample size calculation. According to a previous study, EFC2975, in the USA [19], a total of 52 pediatric patients were finally included. Thus, a sample size of 55 patients was determined for this study.
Results
Baseline Demographics
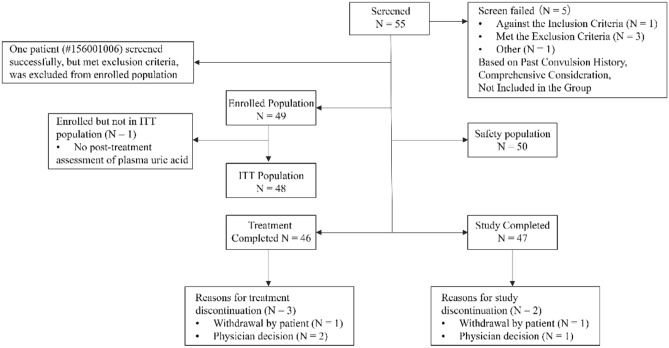
A total of 55 patients were screened, of which five patients failed the screening, resulting in 50 patients in the final safety analysis set. Out of the 49 enrolled patients (NHL, n = 8; AL, n = 41), 48 were included in the ITT analysis set, of which, 47 patients (95.9%) completed the study (one patient withdrew from the study and one patient discontinued treatment based on physician’s decision) and 46 patients (93.9%) completed the planned treatment (one patient withdrew from the study and two patients discontinued treatment based on physician’s decision) (Fig. 2). The mean age of patients was 6.9 ± 3.4 years, mean body mass index was 16.7 ± 3.4 kg/m2, and the majority of patients were male 34 (69.4%) (Table 1). The mean baseline blood uric acid level was 7.4 ± 3.0 mg/dL at baseline. There were 30 (61.2%) and 19 patients (38.8%) with blood uric acid levels ≤ 8 and > 8 mg/dL, respectively. Thirty-five patients (71.4%) had at least one medical history, including infections and infestations in 21 patients (42.9%), followed by gastrointestinal disorders (n = 8, 16.3%), metabolism and nutrition disorders (n = 7, 14.3%), and general disorders and administration site conditions (n = 7, 14.3%). Twenty-three patients (46.9%) had past medication history, and 32 patients (65.3%) had received concomitant medication related to the study disease (NHL or AL). Most of the enrolled patients (n = 48, 98.0%) received cytoreductive chemotherapy agents for NHL or AL.
Fig. 2.
Patient disposition flow chart. ITT intent-to-treat
Table 1.
Baseline demographics of study population
| Parameters | Rasburicase (N = 49) |
|---|---|
| Age (years) | |
| N | 49 |
| Mean (SD) | 6.9 (3.4) |
| Median | 7.0 |
| Q1, Q3 | 4.0, 10.0 |
| Min, Max | 2, 13 |
| Age group (years), n (%) | |
| N | 49 |
| < 6 | 21 (42.9) |
| ≥ 6 to < 12 | 22 (44.9) |
| ≥ 12 to < 18 | 6 (12.2) |
| Sex, n (%) | |
| N | 49 |
| Female | 15 (30.6) |
| Male | 34 (69.4) |
| Race, n (%) | |
| N | 49 |
| Han | 47 (95.9) |
| Other | 2 (4.1) |
| Weight (kg) | |
| N | 49 |
| Mean (SD) | 27.6 (14.1) |
| Median | 25.5 |
| Q1, Q3 | 16.9, 32.5 |
| Min, Max | 10.0, 77.0 |
| Height (cm) | |
| N | 48 |
| Mean (SD) | 125.8 (24.8) |
| Median | 130.0 |
| Q1, Q3 | 107.5, 139.0 |
| Min, Max | 81.0, 187.0 |
| BMI (kg/m2) | |
| N | 48 |
| Mean (SD) | 16.7 (3.4) |
| Median | 15.9 |
| Q1, Q3 | 14.6, 17.5 |
| Min, Max | 12.4, 29.0 |
| BMI (kg/m2), n (%) | |
| N | 48 |
| < 24 | 46 (95.8) |
| ≥ 24 to < 28 | 0 |
| ≥ 28 | 2 (4.2) |
| Missing | 1 |
| < 25 | 46 (95.8) |
| ≥ 25 to < 30 | 2 (4.2) |
| ≥ 30 | 0 |
| Missing | 1 |
| NHL, n (%) | 8 (16.3) |
| NHL stage | |
| I | 0 |
| II | 0 |
| III | 4 (50.0) |
| IV | 4 (50.0) |
| Diagnosis of classification | |
| Mature B cell neoplasms | 2 (25) |
| Burkitt lymphoma | 4 (50.0) |
| B cell lymphoblastic leukemias/lymphoma | 1 (12.5) |
| T lymphoblastic leukemia/lymphoma | 1 (12.5) |
| AL, n (%) | 41 (83.7) |
| AL type | |
| ALL | 35 (85.4) |
| AML | 6 (14.6) |
| Unknown | 0 |
| Baseline blood uric acid (mg/dL) | |
| N | 49 |
| Mean (SD) | 7.4 (3.0) |
| Median | 6.4 |
| Q1, Q3 | 5.3, 10.0 |
| Min, Max | 2.3, 17.7 |
| Baseline Blood Uric Acid group (mg/dL), n (%) | |
| ≤ 8 | 30 (61.2) |
| > 8 | 19 (38.8) |
| ECOG scale, n (%) | |
| N | 31 |
| Mean (SD) | 1.0 (0.9) |
| Median | 1.0 |
| Q1, Q3 | 0.0, 2.0 |
| Min, Max | 0, 3 |
| Lansky scale, n (%) | |
| N | 25 |
| Mean (SD) | 80.8 (16.6) |
| Median | 80.0 |
| Q1, Q3 | 70.0, 100.0 |
| Min, Max | 40, 100 |
AL acute leukemia, ALL acute lymphoblastic leukemia, AML acute myeloid leukemia, BMI body mass index, ECOG Eastern Cooperative Oncology Group, Max maximum, Min minimum, NHL non-Hodgkin’s lymphoma, Q1 25% quantile, Q3 75% quantile, SD standard deviation
Safety Outcomes
The mean duration of treatment and mean dose of hyperuricemic treatment were 3.7 (1–5) days and 5.39 (1.50–15.30) mg, respectively. There was a decrease in the number of patients on rasburicase therapy over time, with 50 (100.0%), 44 (88.0%), 41 (82.0%), 26 (52.0%), and 22 (44.0%) on days 1, 2, 3, 4, and 5, respectively. In total, 25 out of 50 patients (50.0%) in the safety analysis set reported 76 TEAEs, including eight drug-related TEAEs in six patients (12.0%). The observed AEs were due to rasburicase and not due to chemotherapy or underlying disease. The most common TEAEs classified by SOC were gastrointestinal disorders (n = 8, 16.0%), abnormal blood investigations (n = 8, 16.0%), and metabolism and nutrition disorders (n = 8, 16.0%). Based on the PT, there were 20 patients (40.0%) with the most common TEAEs (≥ 5%). The most common among them was pyrexia (n = 6, 12.0%), followed by hypokalemia (n = 5, 10.0%), increase in ALT (n = 4, 8.0%), increase in aspartate aminotransferase (AST) (n = 4, 8.0%), and decrease in WBC (n = 4, 8.0%).
There were 18 CTCAE grade ≥ 3 TEAEs reported in 12 patients (grade ≥ 3: n = 8, 16.0%; grade ≥ 4: n = 4, 8.0%). Of all the reported TEAEs, only TEAEs reported by four patients (8.0%) were serious, of which only one event was considered to be related to the study drug. Due to TEAEs, three patients (6.0%) discontinued the treatment (Table 2). No death was reported to be accountable to the study drug, and no event was considered as an AESI. There were six SAEs, including four serious TEAEs in four patients and two post-treatment SAEs in two patients; however, only one serious TEAE and none of the post-treatment SAEs were related to the study treatment/study procedure. All SAEs were recovered/resolved with concomitant medication or treatment.
Table 2.
Treatment-emergent adverse events (safety analysis)
| TEAEs type | Total (N = 50) n (%) |
|---|---|
| Total participants with an event | 25 (50.0) |
| Gastrointestinal disorders | 8 (16.0) |
| Abdominal pain | 3 (6.0) |
| Nausea | 3 (6.0) |
| Vomiting | 3 (6.0) |
| Abdominal pain upper | 1 (2.0) |
| Ascites | 1 (2.0) |
| Gingival bleeding | 1 (2.0) |
| Hematochezia | 1 (2.0) |
| Blood investigations abnormality | 8 (16.0) |
| Alanine aminotransferase increased | 4 (8.0) |
| Aspartate aminotransferase increased | 4 (8.0) |
| WBC count decreased | 4 (8.0) |
| Neutrophil count decreased | 3 (6.0) |
| Blood bilirubin increased | 2 (4.0) |
| Bilirubin conjugated increased | 1 (2.0) |
| Blood bilirubin unconjugated increased | 1 (2.0) |
| Blood fibrinogen decreased | 1 (2.0) |
| Coagulation test abnormal | 1 (2.0) |
| Gamma-glutamyltransferase increased | 1 (2.0) |
| Metabolism and nutrition disorders | 8 (16.0) |
| Hypokalemia | 5 (10.0) |
| Hyponatremia | 2 (4.0) |
| TLS | 2 (4.0) |
| Hypocalcemia | 1 (2.0) |
| Hypophosphatemia | 1 (2.0) |
| General disorders and administration site conditions | 7 (14.0) |
| Pyrexia | 6 (12.0) |
| Asthenia | 1 (2.0) |
| Chest discomfort | 1 (2.0) |
| Blood and lymphatic system disorders | 5 (10.0) |
| Anemia | 3 (6.0) |
| Febrile neutropenia | 1 (2.0) |
| Hypercoagulation | 1 (2.0) |
| Hepatobiliary disorders | 4 (8.0) |
| Hepatic function abnormal | 3 (6.0) |
| Cholelithiasis | 1 (2.0) |
| Infections and infestations | 2 (4.0) |
| Pneumonia | 2 (4.0) |
| Nervous system disorders | 2 (4.0) |
| Headache | 2 (4.0) |
| Dizziness | 1 (2.0) |
| Respiratory, thoracic and mediastinal disorders | 2 (4.0) |
| Cough | 1 (2.0) |
| Pleural effusion | 1 (2.0) |
| Cardiac disorders | 1 (2.0) |
| Sinus bradycardia | 1 (2.0) |
| Eye disorders | 1 (2.0) |
| Eyelid oedema | 1 (2.0) |
| Immune system disorders | 1 (2.0) |
| Anaphylactic shock | 1 (2.0) |
| Injury, poisoning and procedural complications | 1 (2.0) |
| Refractoriness to platelet transfusion | 1 (2.0) |
| Musculoskeletal and connective tissue disorders | 1 (2.0) |
| Back pain | 1 (2.0) |
| TEAEs leading to treatment discontinuation | 3 (6.0) |
| Hepatobiliary disorders (abnormal hepatic function) | 1 (2.0) |
| Immune system disorders (anaphylactic shock) | 1 (2.0) |
| Metabolism and nutritional disorders (TLS) | 1 (2.0) |
| Most common (≥ 5%) TEAEs | |
| Total participants with an event | 20 (40.0) |
| Pyrexia | 6 (12.0) |
| Hypokalemia | 5 (10.0) |
| Alanine aminotransferase increased | 4 (8.0) |
| Aspartate aminotransferase increased | 4 (8.0) |
| WBC count decreased | 4 (8.0) |
| Abdominal pain | 3 (6.0) |
| Anemia | 3 (6.0) |
| Hepatic function abnormal | 3 (6.0) |
| Nausea | 3 (6.0) |
| Neutrophil count decreased | 3 (6.0) |
| Vomiting | 3 (6.0) |
TEAE treatment emergent adverse event, TLS tumor lysis syndrome, WBC white blood cell count
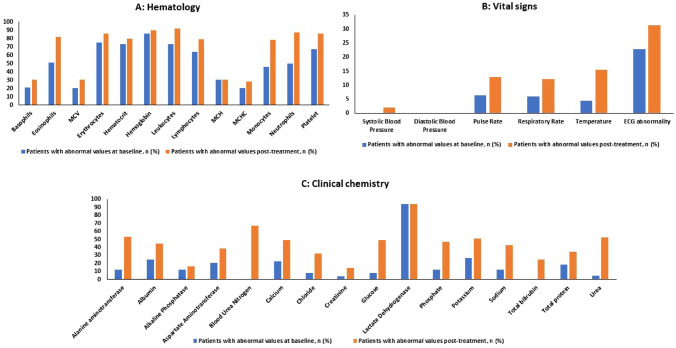
A greater number of patients experienced abnormalities after rasburicase therapy in the clinical laboratory evaluations, vital signs, and ECG when compared to baseline (Fig. 3).
Fig. 3.
Summary of patients with abnormal parameters. ECG electrocardiogram, MCH mean corpuscular hemoglobin, MCHC mean corpuscular hemoglobin concentration, MCV mean corpuscular volume
A few patients’ qualitative urinalysis results changed during the study period (Supplementary Table 1; see the electronic supplementary material). Compared with the baseline of the physical examination, no significant changes were observed in the percentage of patients after therapy.
Effectiveness Outcomes
The response rate in the ITT population was 100.0% (95% CI 82.4–100.0) in patients (n = 19) with baseline uric acid levels > 8.0 mg/dL. Similarly, the response rate was 86.2% (95% CI 68.3–96.1) among 29 patients (60.4%) with baseline uric acid levels of ≤ 8.0 mg/dL. Of the enrolled population, post-treatment assessment of plasma uric acid level was not performed in one patient and, hence, they were excluded from the analysis.
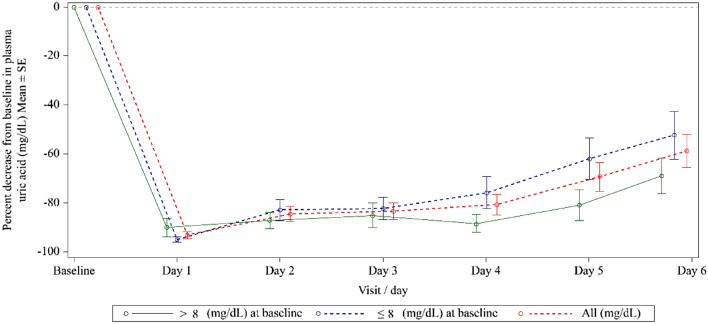
The mean maximum percentage decrease in plasma uric acid level for patients with baseline levels ≤ 8 mg/dL and > 8 mg/dL, and in the ITT population were 96.7%, 97.3%, and 96.9%, respectively (Table 3). Plasma uric acid levels for patients with baseline plasma uric acid levels ≤ 8 mg/dL and > 8 mg/dL, and the ITT population decreased dramatically from their baseline, reaching the lowest level on day 1, and then showed increasing trends in the following 5 days (Fig. 4).
Table 3.
Summary of the change from baseline in plasma uric acid level
| Subgroup statistics | Total (N = 48) |
|---|---|
| Baseline blood uric acid ≤ 8 (mg/dL) | |
| Maximum decrease visit—scheduled visits | |
| N | 29 |
| Mean (SD) | 0.2 (0.2) |
| Median | 0.1 |
| Q1, Q3 | 0.0, 0.3 |
| Min, Max | 0.0, 0.5 |
| Percentage change from baseline to maximum decrease visit—scheduled visits | |
| N | 29 |
| Mean (SD) | − 96.7 (3.5) |
| Median | − 98.5 |
| Q1, Q3 | − 99.5, − 93.5 |
| Min, Max | − 100.0, − 89.8 |
| Baseline blood uric acid > 8 (mg/dL) | |
| Maximum decrease visit—scheduled visits | |
| N | 19 |
| Mean (SD) | 0.3 (0.4) |
| Median | 0.1 |
| Q1, Q3 | 0.0, 0.5 |
| Min, Max | 0.0, 1.7 |
| Percentage change from baseline to maximum decrease visit—scheduled visits | |
| N | 19 |
| Mean (SD) | − 97.3 (3.7) |
| Median | − 98.5 |
| Q1, Q3 | − 99.8, − 94.5 |
| Min, Max | − 99.9, − 87.4 |
| Overall | |
| Maximum decrease visit—scheduled visits | |
| N | 48 |
| Mean (SD) | 0.2 (0.3) |
| Median | 0.1 |
| Q1, Q3 | 0.0, 0.4 |
| Min, Max | 0.0, 1.7 |
| Percentage change from baseline to maximum decrease visit—scheduled visits | |
| N | 48 |
| Mean (SD) | − 96.9 (3.5) |
| Median | − 98.5 |
| Q1, Q3 | − 99.7, − 94.0 |
| Min, Max | − 100.0, − 87.4 |
Max maximum, Min minimum, Q1 25% quantile, Q3 75% quantile, SD standard deviation
Fig. 4.
Mean percentage decrease from baseline in plasma uric acid level (ITT population). ITT intent-to-treat, SE standard error
Discussion
Multiple studies have demonstrated the efficacy and safety of rasburicase in patients with hematological malignancies at high risk of TLS. In a multi-center study in Korea, rasburicase 0.2 mg/kg/day once daily was effective in treatment of hyperuricemia associated with hematological malignancies in pediatric patients, and only mild and reversible drug-related toxicities were observed [9]. Similarly, rasburicase was effective and well tolerated in Japanese pediatric patients with hematological malignancies at high risk of developing TLS [10]. In a study by Chiang et al. [32], rasburicase 6 mg effectively lowered uric acid levels in Asian lymphoma patients consisting of mostly Chinese individuals (91%) who had a risk of developing TLS. However, the study had a limited sample size, and the safety of rasburicase was not evaluated in detail to draw a conclusion on the safety profile of rasburicase in Chinese patients. In the present study with a sufficient sample size, rasburicase demonstrated a tolerable safety profile in Chinese pediatric patients and was effective in controlling hyperuricemia in pediatric patients with NHL and AL. The most commonly observed TEAEs were gastrointestinal disorders (16.0%), abnormal laboratory parameters (16.0%), metabolism and nutrition disorders (16.0%), pyrexia (12.0%), hypokalemia (10.0%), increase in ALT (8.0%) and AST (8.0%), and decrease in WBC (8.0%). Similar observations were noted in a multi-center, open-label, randomized, parallel-group study where the most common grade 3/4 AEs were decrease in WBC (86.7% of patients), neutropenia (83.3%), lymphocytopenia (80.0%), and increased ALT levels (70.0%) in pediatric patients with newly diagnosed hematological malignancies [10].
A study reported that using Aspergillus-derived non-recombinant urate oxidase in patients at risk of TLS showed approximately 4.5% of patients developed allergic reactions, manifested primarily by urticaria, bronchospasm, and hypoxia [33]. The recombinant form of the enzyme rasburicase also has a potential to cause anaphylaxis; hence, it has been suggested to be used with caution [34, 35]. One patient in our study experienced a drug-related anaphylactic shock, and the treatment with rasburicase was discontinued immediately, followed by subsequent recovery without concomitant medication or treatment. The result of our study is in line with the previous studies conducted by Goldman et al. [19] and Pui et al. [26], who reported no anaphylactic or severe acute hypersensitivity reactions among the enrolled 27 and 173 children, respectively. Jeha et al. [36] reported 1.2% of patients with likely hypersensitivity reactions, demonstrating a low prevalence of hypersensitivity reactions, and one patient who experienced anaphylactic shock, among those treated with rasburicase. After treatment with rasburicase, a greater number of patients experienced clinically significant abnormalities, including with regard to clinical laboratory evaluations, vital signs, and ECG, when compared with baseline. However, most of the abnormalities were unrelated to rasburicase therapy and were suspected to be a consequence of concomitant chemotherapy, which is in accordance with previously published literature. Jeha et al. [36] reported a grade 3 increase in liver transaminases in one child, whereas Shin et al. [9] reported serum glutamic-oxalacetic transaminase (SGOT)/serum glutamic-pyruvic transaminase (SGPT) elevation (n = 4), hyperbilirubinemia (n = 1), and hypokalemia (n = 1), which were either attributed as probably related to the study or of unknown etiology.
Overall, the response rate was 100% in patients with uric acid levels > 8.0 mg/dL at baseline, and 86.2% were able to maintain the normal uric acid levels throughout the study. A trial reporting data from 76 pediatric patients with hyperuricemia who were treated with 0.2 mg/kg intravenous rasburicase demonstrated uric acid level reduction below 8 mg/dL in 80% and 100% of patients within 24 h and 72 h, respectively [37]. Similarly, in a study by Jeha et al.[36], 98.5% of patients with malignancy-associated hyperuricemia achieved/maintained normal uric acid levels at 24–48 h after the last dose of rasburicase treatment. The results of the current study further support the effectiveness of rasburicase in line with the US trial that reported a significant (P < 0.001) decrease in mean plasma uric acid level (decreased from 11.3 to 0.2 mg/dL) among 122 evaluable hyperuricemic pediatric patients, along with a 100% response rate in patients [38]. Evidence from comparative analyses suggested that rasburicase was a more potent and rapid-acting drug than oral allopurinol with regard to the hypouricemic effect [19, 33].
This study demonstrated a tolerable safety profile and effectiveness in the control of hyperuricemia, thus providing supporting results for the use of rasburicase 0.20 mg/kg in pediatric patients with NHL and AL in China. The uric acid level decreased remarkably after 4 h of the first dose of rasburicase administration. A similar trend was observed in multiple studies where 4 h after the first dose, patients treated with rasburicase achieved a significant reduction (P < 0.001) of initial plasma uric acid levels, attributing it to the rapid onset of action of the drug [39, 40]. A retrospective study evaluating data from 18 hyperuricemic children reported a fall of 31.18%, 64.8%, and 74.5% in mean serum uric acid levels after a single dose of rasburicase at 4, 24, and 48 h respectively. However, six children (33.3%) required one more additional dose of rasburicase, which is similar to the decrease in the number of patients on rasburicase treatment over time as observed in this study [41]. Overall, owing to the availability of a limited number of studies using rasburicase 0.20 mg/kg/day in pediatric patients with NHL and AL in China, this evidence on rasburicase might play an essential role in improving the management of hyperuricemia, providing a new therapeutic option to patients and physicians.
There are some limitations of our study. First, this is a single-arm study without any comparator/control arm, making it difficult to conclude about whether rasburicase is more clinically beneficial than the standard treatment. Second, being an observational study, it has its own limitations of susceptibility to confounders and biases. Third, owing to the shorter follow-up period, the long-term effectiveness and safety of rasburicase in improving the risk of chronic kidney complications remains to be elucidated. In addition, the evaluation of efficacy with respect to clinical TLS and/or acute kidney injury (AKI) was not done, even though TLS at advanced stage may lead to severe clinical conditions such as cardiac arrhythmia, neurological complications, hypotension, and AKI, collectively known as clinical TLS [42]. Comparison of single and multiple doses of rasburicase is warranted, and may provide further insightful information on the efficacy of rasburicase. Moreover, studies comparing the efficacy, safety, and cost-effectiveness of rasburicase with renal replacement therapy in the management of TLS are warranted.
Conclusions
This is the first study to evaluate the safety and effectiveness of rasburicase in Chinese pediatric patients with NHL and AL. The standard 5-day rasburicase therapy demonstrated a tolerable safety profile with no additional safety concerns in pediatric patients. In addition, this study provided further evidence on the effectiveness of rasburicase in the control of hyperuricemia, a component of TLS in Chinese patients with NHL and AL. The results of this study support the use of rasburicase for Chinese pediatric patients, thereby, offering a new option in the treatment armamentarium of pediatric patients with NHL and AL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical writing assistance was provided by Dr. Sunita Rana and Anwesha Mandal of Indegene Pvt Ltd, Bengaluru, India, which was funded by Sanofi. Jinchuan Sun is an employee of Sanofi.
Declarations
Funding
This phase IV trial was sponsored by Sanofi.
Conflict of interest
Jinchuan Sun and Minlu Zhang are Sanofi employees and may hold shares and/or stock options in the company. The other authors declare no conflicts of interest.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval
The study protocol and its subsequent revisions were approved by the independent ethics committees (IECs) and/or institutional review boards (IRBs) (IEC-C-007-A01-V.05), while conforming to the standards of the Declaration of Helsinki and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for Good Clinical Practice (GCP), all applicable laws, rules, and regulations.
Consent to participate
All patients provided written informed consent before study entry.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
All authors provided substantial contributions to the interpretation of data for the work and read and approved the final version of the manuscript to be published.
References
- 1.Adeyinka A, Bashir K. Tumor Lysis Syndrome. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. 2021. http://www.ncbi.nlm.nih.gov/books/NBK518985/. Accessed 2 Dec 2021.
- 2.Myint PT, Butt HW, Alrifai T, Marin C. Spontaneous tumor lysis syndrome secondary to small-cell neuroendocrine carcinoma of unknown origin: a rare case report and literature review. Case Rep Oncol Med. 2019;2019:e6375693. doi: 10.1155/2019/6375693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 4.Coiffier B, Mounier N, Bologna S, Fermé C, Tilly H, Sonet A, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-hodgkin’s lymphoma: results of the GRAAL1 (Groupe d’Etude des Lymphomes de l’Adulte Trial on Rasburicase Activity in Adult Lymphoma) Study. J Clin Oncol. 2003;21:4402–4406. doi: 10.1200/JCO.2003.04.115. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B. Acute tumor lysis syndrome—a rare complication in the treatment of solid tumors. Onkologie. 2010;33:498–499. doi: 10.1159/000320581. [DOI] [PubMed] [Google Scholar]
- 6.Annemans L, Moeremans K, Lamotte M, Garcia Conde J, van den Berg H, Myint H, et al. Incidence, medical resource utilisation and costs of hyperuricemia and tumour lysis syndrome in patients with acute leukaemia and non-Hodgkin’s lymphoma in four European countries. Leuk Lymphoma. 2003;44:77–83. doi: 10.1080/1042819021000054661. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Altman A, Pui C-H, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 8.Jones GL, Will A, Jackson GH, Webb NJA, Rule S, British Committee for Standards in Haematology Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2015;169:661–671. doi: 10.1111/bjh.13403. [DOI] [PubMed] [Google Scholar]
- 9.Shin HY, Kang HJ, Park ES, Choi HS, Ahn HS, Kim SY, et al. Recombinant urate oxidase (Rasburicase) for the treatment of hyperuricemia in pediatric patients with hematologic malignancies: results of a compassionate prospective multicenter study in Korea. Pediatr Blood Cancer. 2006;46:439–445. doi: 10.1002/pbc.20555. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi A, Kigasawa H, Tsurusawa M, Kawa K, Kikuta A, Tsuchida M, et al. A study of rasburicase for the management of hyperuricemia in pediatric patients with newly diagnosed hematologic malignancies at high risk for tumor lysis syndrome. Int J Hematol. 2009;90:492–500. doi: 10.1007/s12185-009-0402-6. [DOI] [PubMed] [Google Scholar]
- 11.Liu W, Liu J, Song Y, Zeng X, Wang X, Mi L, et al. Burden of lymphoma in China, 2006–2016: an analysis of the Global Burden of Disease Study 2016. J Hematol OncolJ Hematol Oncol. 2019;12:115. doi: 10.1186/s13045-019-0785-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Tang J, Zheng H, Fang J, Sun X. Treatment of childhood cancer in China: current status and future direction. Pediatr Investig. 2020;4:153–156. doi: 10.1002/ped4.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krakoff IH. Prevention of hyperuricemia in leukemia and lymphoma: use of allopurinol, a xanthine oxidase inhibitor. JAMA. 1965;193:1. doi: 10.1001/jama.1965.03090010007001. [DOI] [PubMed] [Google Scholar]
- 14.Alakel N, Middeke JM, Schetelig J, Bornhäuser M. Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. OncoTargets Ther. 2017;10:597–605. doi: 10.2147/OTT.S103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pession A, Melchionda F, Castellini C. Pitfalls, prevention, and treatment of hyperuricemia during tumor lysis syndrome in the era of rasburicase (recombinant urate oxidase) Biol Targets Ther. 2008;2:129–141. doi: 10.2147/BTT.S1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazel R, Zarei N, Ghaemi N, Namvaran MM, Enayati S, Mirabzadeh Ardakani E, et al. Cloning and expression of Aspergillus flavus urate oxidase in Pichia pastoris. Springerplus. 2014;3:395. doi: 10.1186/2193-1801-3-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammalleri L, Malaguarnera M. Rasburicase represents a new tool for hyperuricemia in tumor lysis syndrome and in gout. Int J Med Sci. 2007;4:83–93. doi: 10.7150/ijms.4.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinnel J, Moore BL, Skiver BM, Bose P. Rasburicase in the management of tumor lysis: an evidence-based review of its place in therapy. Core Evid. 2015;10:23–38. doi: 10.2147/CE.S54995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood. 2001;97:2998–3003. doi: 10.1182/blood.V97.10.2998. [DOI] [PubMed] [Google Scholar]
- 20.Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone–results of a multicenter phase III study. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28:4207–4213. doi: 10.1200/JCO.2009.26.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu S, Han Y, Zhang W, Zhang T, Yao X, Liu L. Cost-effectiveness analysis of rasburicase over standard of care for the prevention and treatment of tumor lysis syndrome in children with hematologic malignancies in China. J Med Econ. 2019;22:742–750. doi: 10.1080/13696998.2019.1603155. [DOI] [PubMed] [Google Scholar]
- 22.Gupta G, Seth T, Garg V, Juneja R, Mahapatra M, Datta SK, et al. Efficacy of single low-dose rasburicase in management of tumor lysis syndrome in leukemia and lymphoma patients. Clin Lymphoma Myeloma Leuk. 2021;21:e99–104. doi: 10.1016/j.clml.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Philips A, Radhakrishnan V, Ganesan P, Ganesan TS, Ramamurthy J, Dhanushkodi M, et al. Efficacy of single dose rasburicase (1.5 mg) for prophylaxis and management of laboratory tumor lysis syndrome. Indian J Hematol Blood Transfus Off J Indian Soc Hematol Blood Transfus. 2018;34:618–622. doi: 10.1007/s12288-018-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheuk DKL, Chiang AKS, Chan GCF, Ha SY. Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2014;2014:CD006945. doi: 10.1002/14651858.CD006945.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Hochberg J, Cairo MS. Rasburicase: future directions in tumor lysis management. Expert Opin Biol Ther. 2008;8:1595–1604. doi: 10.1517/14712598.8.10.1595. [DOI] [PubMed] [Google Scholar]
- 26.Pui CH, Jeha S, Irwin D, Camitta B. Recombinant urate oxidase (rasburicase) in the prevention and treatment of malignancy-associated hyperuricemia in pediatric and adult patients: results of a compassionate-use trial. Leukemia. 2001;15:1505–1509. doi: 10.1038/sj.leu.2402235. [DOI] [PubMed] [Google Scholar]
- 27.Lee AC, Li CH, So KT, Chan R. Treatment of Impending tumor lysis with single-dose rasburicase. Ann Pharmacother. 2003;37:1614–1617. doi: 10.1345/aph.1D111. [DOI] [PubMed] [Google Scholar]
- 28.Pei Y, Li Y, Liang Y, Xu L, Huang X, Li Y, et al. Evaluation of the safety and efficacy of low-dose rasburicase in critically ill children with haematological malignancies. Int J Clin Pharm. 2020;42:1440–1446. doi: 10.1007/s11096-020-01144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification: new therapeutic strategies and classification of TLS. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 30.Cairo MS, Coiffier B, Reiter A, Younes A, Panel on behalf of the TE Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- 31.Technical guidelines for clinical pharmacokinetic research of chemical drugs. 2005.https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=4247ffceca57f2b47aa0f67a3fdc7c43.
- 32.Chiang J, Chan A, Lian T, Tay K, Quek R, Tao M, et al. Management of tumor lysis syndrome with a single fixed dose of rasburicase in Asian lymphoma patients: a case series and literature review. Asia Pac J Clin Oncol. 2011;7:351–356. doi: 10.1111/j.1743-7563.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 33.Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, et al. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia. 1997;11:1813–1816. doi: 10.1038/sj.leu.2400850. [DOI] [PubMed] [Google Scholar]
- 34.Allen KC, Champlain AH, Cotliar JA, Belknap SM, West DP, Mehta J, et al. Risk of anaphylaxis with repeated courses of rasburicase: a Research on Adverse Drug Events and Reports (RADAR) project. Drug Saf. 2015;38:183–187. doi: 10.1007/s40264-014-0255-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung WL, Hon KL, Fung CM, Leung AK. Tumor lysis syndrome in childhood malignancies. Drugs Context. 2020;9:2019. doi: 10.7573/dic.2019-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeha S, Kantarjian H, Irwin D, Shen V, Shenoy S, Blaney S, et al. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy-associated hyperuricemia in pediatric and adult patients: final results of a multicenter compassionate use trial. Leukemia. 2005;19:34–38. doi: 10.1038/sj.leu.2403566. [DOI] [PubMed] [Google Scholar]
- 37.Galardy PJ, Hochberg J, Perkins SL, Harrison L, Goldman S, Cairo MS. Rasburicase in the prevention of laboratory/clinical tumour lysis syndrome in children with advanced mature B-NHL: a Children’s Oncology Group Report. Br J Haematol. 2013;163:365–372. doi: 10.1111/bjh.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosly A, Sonet A, Pinkerton CR, McCowage G, Bron D, Sanz MA, et al. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients with cancer: report of an international compassionate use study. Cancer. 2003;98:1048–1054. doi: 10.1002/cncr.11612. [DOI] [PubMed] [Google Scholar]
- 39.Rényi I, Bárdi E, Udvardi E, Kovács G, Bartyik K, Kajtár P, et al. Prevention and treatment of hyperuricemia with rasburicase in children with leukemia and non-Hodgkin’s lymphoma. Pathol Oncol Res POR. 2007;13:57–62. doi: 10.1007/BF02893442. [DOI] [PubMed] [Google Scholar]
- 40.Tatay VS, Castilla JDL, Ponce JMC, Hurtado JMP, Cantero EQ, Abril ML. Rasburicase versus allopurinol in the treatment of hyperuricaemia in tumour lysis syndrome. An Pediatr Barc Spain. 2003;2010(72):103–110. doi: 10.1016/j.anpedi.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Gopakumar KG, Thankamony P, Seetharam S, Kusumakumary P. Treatment of tumor lysis syndrome in children with leukemia/lymphoma in resource-limited settings-efficacy of a fixed low-dose rasburicase. Pediatr Hematol Oncol. 2017;34:206–211. doi: 10.1080/08880018.2017.1348415. [DOI] [PubMed] [Google Scholar]
- 42.Matuszkiewicz-Rowinska J, Malyszko J. Prevention and treatment of tumor lysis syndrome in the era of onco-nephrology progress. Kidney Blood Press Res. 2020;45:645–660. doi: 10.1159/000509934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.