Abstract
Multinodular and vacuolating neuronal tumor (MVNT) is a relatively new disease concept proposed in 2013 and was classified as a separate tumor type in 2021 by the World Health Organization (WHO) classification. MVNT can cause seizures but is a benign disease, with no cases of enlargement or postoperative recurrence reported. Recent reports described advanced MRI features in MVNT cases, but the diagnosis of MVNT is usually based on characteristic MRI findings of clusters of nodules. Here, we report advanced multiparametric MRI and FDG-PET/CT findings in a case of MVNT with epileptiform symptoms that was pathologically confirmed by surgery.
Keywords: Multinodular and vacuolating neuronal tumor (MVNT), Advanced multiparametric MRI, FDG-PET/CT
Introduction
Multinodular and vacuolating neuronal tumor (MVNT) is a new disease state described as a pathologically benign neurological lesion by Huse et al. in 2013 [1]. In 2016, MVNT was classified as a gangliocytoma by the World Health Organization (WHO) but was classified as a separate tumor type WHO grade 1 in 2021 [2]. It occurs primarily in the cerebral hemispheres of adults, most commonly in the temporal lobe, and can cause seizures [3]. It is a benign disease, with no cases of enlargement or postoperative recurrence reported.
We experienced a case of MVNT in the right temporal lobe with epileptiform symptoms that was pathologically confirmed by surgery. In this case, we describe advanced multiparametric MRI including apparent diffusion coefficient (ADC), MR spectroscopy (MRS), arterial spin labeling (ASL), and amide proton transfer (APT) imaging and FDG-PET/CT findings.
Presentation of the case
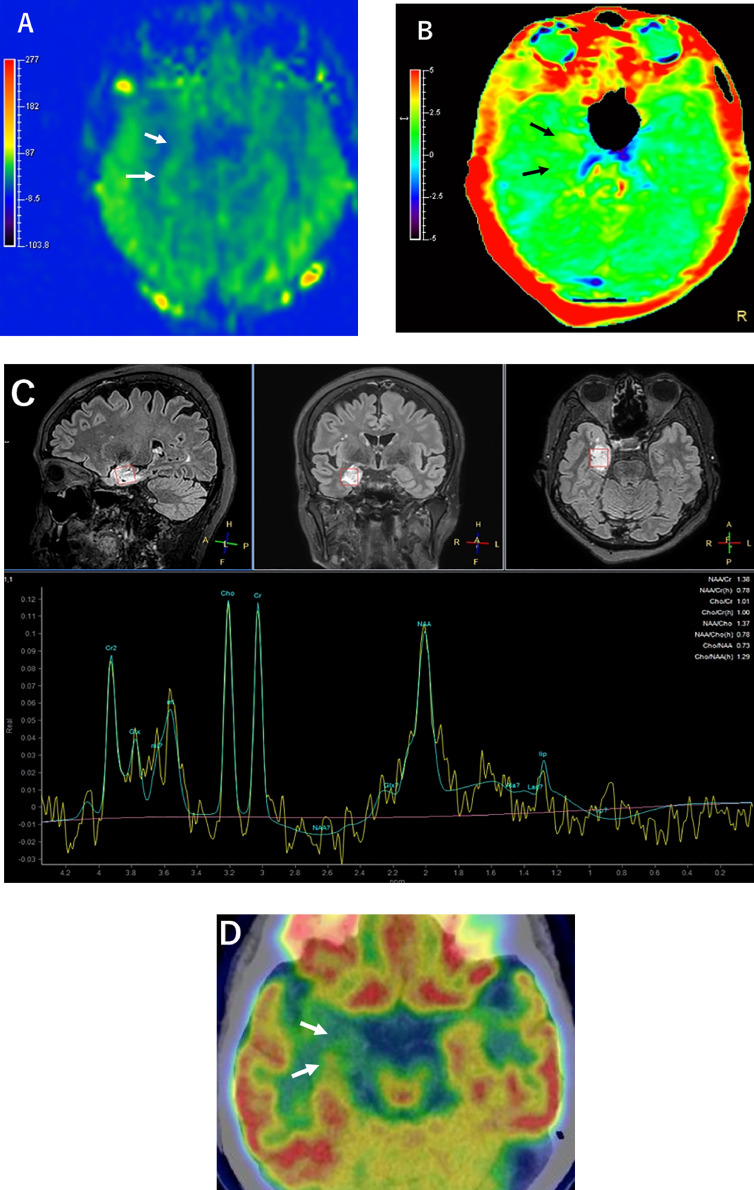
A 67-year-old woman presented with dizziness and decreased level of consciousness for 1 month. Her medical history, family history, and blood tests were unremarkable. MRI was performed on this patient. T2-weighted and fluid-attenuated inversion recovery (FLAIR) images revealed a mass up to 29 mm in diameter with clusters of multiple nodular hyperintense lesions of varying sizes in the amygdala, hippocampus, and subcortical white matter of the right temporal lobe (Figs. 1A-C). ADC of the mass (1.17 × 10−3 mm2/s) was higher than that of the contralateral side (0.8 × 10−3 mm2/s) with a relative ratio of 1.46 (Fig. 1D). No enhancement was seen on T1-weighted images after gadolinium administration (Fig. 1E). ASL imaging showed that mean cerebral blood flow (CBF) of the mass (37.72 mL/100g/min) was nearly equivalent to that of the contralateral side (39.19 mL/100g/min) with a relative ratio of 0.96 (Fig. 2A). APT imaging showed mildly increased signal intensity value of the mass (mean 2.28%) compared to the contralateral side (mean 1.65%) (Fig. 2B). MRS showed a mild increase of the choline (Cho) to N-acetyl aspartate (NAA) ratio (1.29) in the lesion (Fig. 2C). FDG-PET/CT brain imaging showed focal hypometabolism in the right medial temporal lobe corresponding to the site of the lesion (Fig. 2D).
Fig. 1.
(A) T2WI and (B and C) FLAIR images demonstrate a hyperintense lesion (arrows) and clusters of nodules (arrowheads) in the right temporal lobe. (D) Apparent diffusion coefficient map shows increased diffusion of the mass (arrows). (E) Contrast-enhanced T1WI shows no enhancement of the mass.
Fig. 2.
(A) ASL imaging shows nearly equivalent CBF of the mass to the contralateral side (arrows). APT imaging shows focally increased signal intensity of the lesion (arrows). MRS shows mildly increased ratio of Cho to NAA. FDG-PET/CT shows focal hypometabolism in the site of the lesion (arrows).
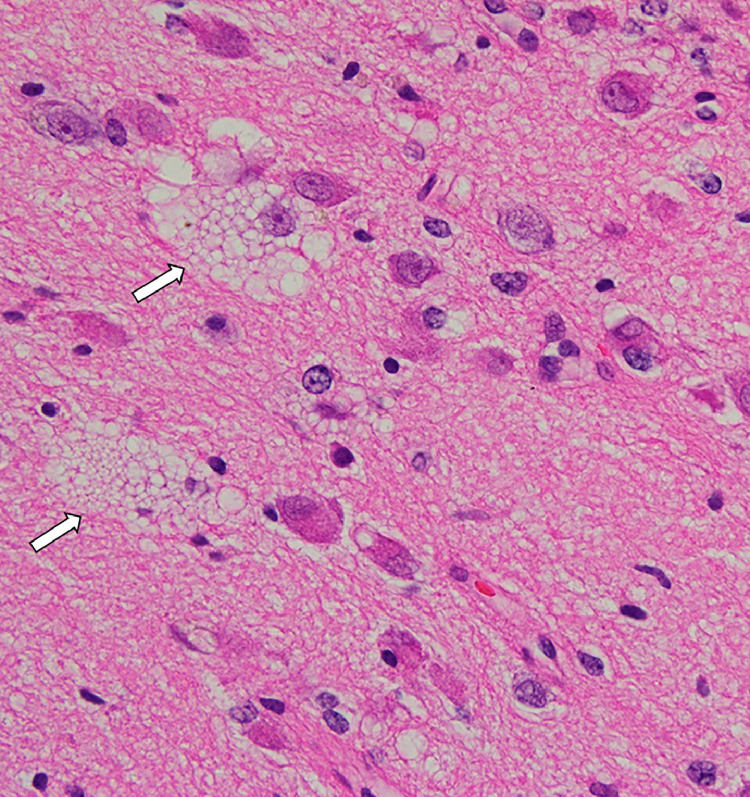
The mass was partially removed from the patient. Histologically, the lesion contained medium-to-large, mature-appearing neurons within an altered vacuolar matrix (Fig. 3). There was no evidence of mitotic figures, necrosis, or microvessel proliferation. The tumor cells were immunoreactive for GFAP, Olig2, synaptophysin, ATRX, and MGMT, but negative for neurofilament, IDH1, IDH1/2, and p53. The positive cell rate for Ki-67 was <1%. MVNT was confirmed based on these pathological and immunohistochemical findings. More than 3 years have passed since surgery, but no tumor progression has been observed.
Fig. 3.
Histopathology shows medium-to-large mature-appearing neurons occupied in this nodule with altered vacuolar matrix (arrows; original magnification, ×400).
Discussion
Approximately 50 pathologically proven MVNT cases have been reported to date. On the other hand, MR findings of MVNT were reported to be morphologically highly characteristic, showing clusters of nodules [4,5]. Clusters of nodules indicate subcortical nodular lesions located within the medial surface of the cerebral cortex, primarily deep cortical ribbons and superficial subcortical white matter, demonstrated on FLAIR [4]. Subsequently, many cases have been reported with no pathologic evidence of MVNT on imaging findings alone [5–8]. Diagnosis of MVNT may be achieved clinically based on characteristic findings without pathologic evidence, as many patients are asymptomatic and discovered incidentally. However, dysembryoplastic neuroepithelial tumor (DNT) may be diagnosed radiologically as a differential. DNT occurs mostly in children, but rarely in older people. Therefore, it was not considered in this case. Recently, advanced MR techniques including MRS, ADC, and perfusion-weighted imaging have been investigated for more definitive diagnosis [6–8]. It has been suggested that a comprehensive MR protocol should be performed to increase the diagnostic confidence of MVNT [7].
The current case demonstrated data of advanced multiparametric MRI in a case of MVNT pathologically proven. Lecler et al. [7] reported a large series of 64 patients with a lesion suggestive of MVNT using advanced MRI. They showed that the median relative ratios of ADC, CBF, and Cho/NAA were 1.13, 1.01, and 0.7, respectively, indicating no imaging patterns suggestive of malignancy in MVNT [7]. In our MVNT case, ADC and CBF support their results in that no restricted diffusion or hyperperfusion was seen. However, the choline peak was higher than that of NAA, unlike theirs. We speculate that this is due to differences in tumor size and anatomic localization. MVNT in our case was large and localized mainly to the amygdala and hippocampus, whereas their cases were small and scattered over subcortical areas. Therefore, in their cases, the MRS voxels may have included not only the MVNT lesion but also the surrounding normal brain. Considering the literature, several large MVNT cases with pathological evidence showed increased Cho peak in MRS [9], [10], [11]. This shows that in MVNT, one should be careful in interpreting MRS.
Only one case of hippocampal MVNT was studied using FDG-PET/CT [4]. The finding was consistent with that of our case, indicating localized hypometabolism at the lesion site. This case and ours suggest that FDG-PET/CT may also be characteristic of MVNT, but more cases need to be studied in the future.
APT imaging is an MR molecular imaging technique sensitive to mobile proteins and peptides in living tissue. Studies have shown that APT-associated signal intensity is consistent with glioma grade, enabling preoperative assessment of glioma grade [12]. Although the origin of APT signal intensity in tumors is unknown, it has been suggested that it is increased in brain tumors due to increased mobile protein concentrations in malignant cells associated with increased cellularity. Togao et al. [12] reported that the mean APT signal intensity values were 2.1 ± 0.4% in grade II gliomas, 3.2 ± 0.9% in grade III gliomas, and 4.1 ± 1.0% in grade IV gliomas. MVNT showed an APT signal intensity value of 2.28%. Therefore, the value is close to that for grade II gliomas. However, it remains unclear whether APT imaging also contributes to the grading of tumors other than glioma, as it has never been reported before. Further investigation is needed to clarify this issue.
The ultrasound fusion imaging system is a new promising imaging modality that combines live ultrasound investigations with preregistered CT, MRI or PET images [13]. Recently, it has been reported that this system can be applied to the brain with multiple reproducibility, enabling real-time monitoring [14]. Therefore, in the future, it may be possible to monitor MVNT using this method.
Conclusion
We report a case of MVNT in the right temporal lobe with epileptiform symptoms that was pathologically confirmed by surgery. In this case, we described advanced multiparametric MRI including ADC, MRS, ASL, APT, and FDG-PET/CT findings. MVNT exhibited no restricted diffusion or hyperperfusion that may be characteristic of this tumor. However, in MVNT, one must be careful in interpreting MRS. More cases are needed for APT imaging and FDG-PET/CT.
Patient consent
Written informed consent for publication of the case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no conflict of interest.
References
- 1.Huse JT, Edgar M, Halliday J, Mikolaenko I, Lavi E, Rosenblum MK. Multinodular and vacuolating neuronal tumors of the cerebrum: 10 cases of a distinctive seizure-associated lesion. Brain Pathol. 2013;23(5):515–524. doi: 10.1111/bpa.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenbulm MK, Giangaspero F, Giannini C, Huse JT, Komori T, Pekmezci M. Multinodular and vacuolating neuronal tumour. In: WHO classification of tumours of the central nervous system. 5th Edition. IARC; Lyon: 2021. [Google Scholar]
- 3.Yamaguchi M, Komori T, Nakata Y, Yagishita A, Morino M, Isozaki E. Multinodular and vacuolating neuronal tumor affecting amygdala and hippocampus: a quasi-tumor? Pathol Int. 2016;66(1):34–41. doi: 10.1111/pin.12366. [DOI] [PubMed] [Google Scholar]
- 4.Nunes RH, Hsu CC, da Rocha AJ, do Amaral LLF, Godoy LFS, Watkins TW, et al. Multinodular and vacuolating neuronal tumor of the cerebrum: a new “leave me alone” lesion with a characteristic imaging pattern. AJNR Am J Neuroradiol. 2017;38(10):1899–1904. doi: 10.3174/ajnr.A5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buffa GB, Chaves H, Serra MM, Stefanoff NI, Gangliardo AS, Yanez P. Multinodular and vacuolating neuronal tumor of the cerebrum (MVNT): a case series and review of the literature. J Neuroradiol. 2020;47(3):216–220. doi: 10.1016/j.neurad.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Makrakis D, Veneris S, Papadaki E. Multinodular and vacuolating neuronal tumor incidentally discovered in a young man: conventional and advanced MRI features. Radiol Case Rep. 2018;13(5):960–964. doi: 10.1016/j.radcr.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecler A, Broquet V, Bailleux J, Carsin B, Adele-Biassette H, Baloglu S, et al. Advanced multiparametric magnetic resonance imaging of multinodular and vacuolating neuronal tumor. Eur J Neurol. 2020;27(8):1561–1569. doi: 10.1111/ene.14264. [DOI] [PubMed] [Google Scholar]
- 8.Turan A, Tatar IG, Hekimoglu A, Coskun H, Yildirim F. Advanced magnetic resonance imaging findings of multinodular and vacuolating neuronal tumor. Turk Neurosurg. 2021;31(5):725–730. doi: 10.5137/1019-5149.JTN.32215-20.3. [DOI] [PubMed] [Google Scholar]
- 9.Nagaishi M, Yokoo H, Nobusawa S, Fujii Y, Sugiura Y, Suzuki R, et al. Localized overexpression of alpha-internexin within nodules in multinodular and vacuolating neuronal tumors. Neuropathology. 2015;35(6):561–568. doi: 10.1111/neup.12217. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Nakahara Y, Wakamiya T, Koguchi M, Yoshioka F, Inoue K, et al. Multinodular and vacuolating neuronal tumors with suggested slow progression. Jpn J Neurosurg (Tokyo) 2020;29:580–585. [Google Scholar]
- 11.Sirbu CA, Stefani C, Tuta S, Manole AM, Sirbu OM, Ivan R, et al. New imaging features of multinodular and vacuolating neuronal tumor revealed by alcohol and illicit drugs consumption. Diagnostics (Basel) 2022;12(11):2779. doi: 10.3390/diagnostics12112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neurol Oncol. 2014;16(3):441–448. doi: 10.1093/neuonc/not158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoll J. Ultrasound fusion imaging. Perspect Med. 2012;1(112):80–81. [Google Scholar]
- 14.Peycheva MV, Chervenkov L, Haizanova Z, Ahmed-Popova F, Zahariev ZI. Ultrasound fusion imaging system in neurology practice. Folia Med (Plovdiv) 2022;64(4):667–671. doi: 10.3897/folmed.64.e64271. [DOI] [PubMed] [Google Scholar]