Summary
Background:
Broadly neutralizing anti-human immunodeficiency virus type I (HIV-1) antibodies (bNAbs) administered prior to latency reversal may facilitate elimination of HIV-1-infected CD4 T cells. We tested this concept by combining bNAb 3BNC117 with the latency reversing agent (LRA) romidepsin in HIV-1-infected individuals on suppressive antiretroviral therapy (ART).
Methods:
This phase 2a randomized open-label, international trial enrolled HIV-1-infected adults on long-term ART. Group A received 3BNC117 two days prior to two romidepsin cycles, with romidepsin administered at weeks 0/1/2, and weeks 8/9/10. Group B received romidepsin alone. An analytical treatment interruption (ATI) of ART was done at week 24. The primary endpoint was time to viral rebound during ATI. Secondary endpoints included changes in HIV-1 reservoir measures and safety assessments.
Findings:
19 of 22 enrolled participants completed both treatment cycles. The median time to viral rebound during ATI was 17.5 days for group A and 28 days for group B (p=0·02). From baseline to pre-ATI, median total HIV-1 DNA declined by −1 and −61 in groups A and B, respectively. Intact proviruses changed by −11 and 10 in groups A and B, respectively. The changes in HIV-1 reservoir were not statistically significant. The combination of 3BNC117 and romidepsin was overall safe, and two severe adverse events were observed during 48 weeks of follow-up.
Interpretation:
The combination of bNAb and LRA was safe, but did not reduce the HIV-1 reservoir or delay viral rebound during ATI in individuals on long-term ART.
INTRODUCTION
Durable viral control in the absence of antiretroviral therapy (ART) is the goal of strategies to cure human immunodeficiency virus type 1 (HIV-1)-infection.1 Despite effective suppression of viral replication by ART, proviral DNA integrated into immune cells allows HIV-1 to persist as latent infection. Upon interruption of ART, viral replication can rapidly resume from the reservoir of infected cells and result in rebound of viremia within weeks. Because ART does not cure HIV-1 infection, life-long ART is necessary to prevent disease progression.
The primary barrier to eradication of HIV-1 is a pool of long-lived latently infected memory CD4 T cells. In their resting state, these cells do not produce viral particles and elude recognition by the immune system. Reversing latency could expose these cells to immune-mediated elimination in an approach termed ‘shock and kill’.2 Latently infected cells can be induced to resume HIV-1 expression by different classes of latency-reversing agents (LRAs), of which histone deacetylase inhibitors (HDACis) are the most extensively studied to date.3–5
Clinical proof-of-concept trials demonstrated that LRAs can transiently increase HIV-1 RNA transcription in ART-treated individuals.3–6 However, this resulted in no or only modest reductions in the size of the HIV-1 reservoir, possibly due to insufficient stimulation of immune-mediated clearance of infected cells when LRAs are given alone.7 When the HDACi romidepsin was combined with an experimental HIV-1 peptide vaccine (Vacc-4x), a 40% reduction in the total HIV-1 DNA reservoir size was determined.8 However, HIV-1-specific T cell activity following Vacc-4x immunization was not significantly increased and no delay in the time to viral rebound during subsequent analytical treatment interruption (ATI) of ART was observed.8,9
An alternative approach that has been proposed to enhance elimination of latently infected cells is to combine LRAs with broadly neutralizing anti-HIV-1 antibodies (bNAbs) that target the HIV-1 envelope protein (Env).10–12 In clinical trials, bNAbs have been shown to suppress viremia and delay viral rebound during ATI.13 In addition to potently neutralizing HIV-1, bNAbs can engage different components of the host immune system. These interactions are mediated by the antibodies’ fragment crystallizable (Fc) regions and can result in accelerated viral clearance, induction of antibody-dependent cellular cytotoxicity (ADCC), and enhanced antigen presentation.10,11,14 In humanized mouse and non-human primate models of HIV-1 infection, treatment with a combination of bNAbs and LRAs resulted in a significant delay in time to viral rebound in the absence of ART compared to bNAbs given alone.12,15
To investigate this concept in humans, we conducted a clinical trial of the combination of the bNAb 3BNC117 and the LRA romidepsin. 3BNC117 targets the CD4 binding site (CD4bs) on the HIV-1 Env protein and has demonstrated high antiviral activity in clinical studies.16,17 Romidepsin is a pan-HDACi approved for treatment of peripheral and cutaneous T cell lymphoma, and is one of the most potent and extensively clinically tested LRAs in three trials,5,8,18 but in a recent dose-escalation trial among individuals on ART romidepsin failed to work as a LRA19. To evaluate the impact of the combination of 3BNC117 and romidepsin in individuals on ART compared to romidepsin alone, we performed quantitative assessments of the HIV-1 reservoir and determined the time to viral rebound during ATI.
METHODS
Study design and participants
This randomized, open-label, parallel-group, international, multi-center phase 2a trial was conducted among virologically suppressed HIV-1-infected adults (18–65 years) on ART. Prior to enrolment, participants were required to have plasma HIV-1 RNA levels <50 copies/ml for at least 12 months (one blip <500 copies/ml was allowed), be on ART for a minimum of 18 months, and have a CD4 T cell count >500 cells/μl. Individuals were switched to an integrase inhibitor-based regimen (raltegravir or dolutegravir) prior to enrolment if their ART regimen included non-nucleoside reverse transcriptase inhibitors (due to their long half-life), or cobicistat or protease inhibitors (due to the potential for interactions with romidepsin). Exclusion criteria included a CD4 T cell nadir of <200 cells/μl within the last 5 years, concomitant hepatitis B or C virus infection, receipt of any anti-HIV-1 monoclonal antibody or therapeutic HIV-1 vaccine in the past, receipt of any HDACi in the past 2 years, and QTc prolongation. The full list of inclusion and exclusion criteria is provided in the protocol. The study was conducted in accordance with Good Clinical Practice and is reported in accordance with the CONSORT 2010 statement20. The protocol was approved by the Paul-Ehrlich-Institute (#2944/01), the Food and Drug Administration (IND 118229), the Danish Medicine Authorities (#2016080161), the Institutional Review Boards at the University of Cologne (#16–452) and the Rockefeller University, and the National Committee on Health Research Ethics in Denmark (#1-10-72-355-15). Individuals were enrolled at the Departments of Infectious Diseases, Aarhus/Aalborg University Hospitals, Aarhus/Aalborg, Denmark, at the Rockefeller University Hospital, New York, USA, and at the University Hospital Cologne, Cologne, Germany. All participants provided written informed consent. The trial is registered at clinicaltrials.gov (NCT02850016).
Randomization
Participants were randomly assigned at a 1:1 ratio to receive 3BNC117 and romidepsin (group A) or romidepsin alone (group B) (figure 1A). Randomization was done using randomly permuted blocks of 2 or 4 per site, stratified by site, and implemented by the Institute of Medical Statistics, Informatics and Epidemiology (IMSIE) at the University of Cologne. The trial was conducted open-label with no blinding.
Figure 1.


(A) CONSORT flow diagram and (B) the ROADMAP study design. Circles indicate time points of assessments.
Procedures
Screening visits occurred up to 8 weeks before the first administration of study medication. While participants were on ART, leukapheresis was performed at week −2 (baseline) and week 22 (pre-ATI). Participants assigned to group A received 3BNC117 as an intravenous infusion over 60 min at a dose of 30 mg/kg two days prior to each of two romidepsin treatment cycles. Romidepsin was administered intravenously at a dose of 5 mg/m2 over 120 min at weeks 0, 1, and 2 (treatment cycle 1), and weeks 8, 9, and 10 (treatment cycle 2). Dosing of 3BNC117 was based on previously observed antiviral efficacy16, and an interval of six weeks between the two LRA treatment cycles was based on clinical findings from a single romidepsin treatment cycle.5 Participants assigned to group B received 5 mg/m2 romidepsin alone in treatment cycles 1 and 2.
At week 24, participants initiated an ATI. This timepoint, 16 weeks after the last 3BNC117 infusion in group A, was chosen to ensure that 3BNC117 serum concentrations were sub-therapeutic (<10 μg/ml) at the start of the ATI (the serum half-life of 3BNC117 is approximately two weeks).16,21 During ATI, plasma HIV-1 RNA levels were monitored weekly and CD4 T cell counts were assessed every other week. Viral rebound was defined as the first of two consecutive plasma HIV-1 RNA measurements of ≥200 copies/ml. ART resumption criteria included viral rebound and a confirmed decreased of CD4 T cells to <350 cells/μl. After resumption of ART, plasma HIV-1 RNA levels were determined every other week until undetectable levels (<20 copies/ml) on two consecutive measurements. At that point, participants were followed every eight weeks until the final visit at week 48.
Blood samples were processed within 4 hours of collection. Serum and plasma samples were stored at −80 °C. Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation and cryopreserved in fetal bovine serum with 10% DMSO. Plasma HIV-1 RNA levels were measured at every visit. Cell-associated unspliced HIV-1 RNA (CA usHIV-1 RNA) levels were assessed at 14 times points (see figure 1B). Parameters of the HIV-1 reservoir size and T cell immunity were assessed at the leukapheresis time points before and after completion of the romidepsin treatment cycles (baseline and pre-ATI), with total HIV-1 DNA levels additionally determined between the two treatment cycles at week 3.
Safety data are reported through the end of study. Solicited adverse events (AEs) were recorded for two weeks following infusions. Unsolicited AEs were recorded at all visits. The Common Terminology Criteria for Adverse Events (CTCAE) scale (version 4·03) was used to grade infusion-related AEs, and the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events (version 2·0) was used to grade non-infusion-related AEs. Safety assessments included directed physical examinations, vital sign measurements, and clinical laboratory tests. Study participants were on continuous cardiac monitoring during romidepsin infusions and ECGs were repeatedly recorded throughout the treatment cycles. An independent safety monitoring committee regularly reviewed data to ensure trial participants’ safety.
Outcomes
The primary outcome was the time to viral rebound during ATI, defined as the first of two consecutive plasma HIV-1 RNA measurements of ≥200 copies/ml. Secondary outcomes included safety, the size of the CD4 T cell HIV-1 reservoir determined by total HIV-1 DNA levels and by intact proviral DNA levels per million unfractionated CD4 T cells using droplet digital PCR (ddPCR), and plasma HIV-1 RNA levels using standardized clinical assays. Additional exploratory outcomes included HIV-1 transcriptional activity determined by the levels of CA usHIV-1 RNA per million CD4 T cells using ddPCR HIV-1-specific T cell responses, and markers of T cell activation and exhaustion using flow cytometry. Additional analyses included assessments of viral 3BNC117 sensitivity by env sequencing of proviral DNA at baseline and by pseudovirus TZM-bl cell neutralization assays of plasma single genome amplification (SGA)-derived viruses at rebound (group A), and the determination of serum 3BNC117 concentrations by TZM-bl cell assay.
Statistical analysis
The sample size calculation was based on the primary endpoint. Assuming a standard deviation of 10 days, a sample size of 12 individuals in each of the two arms was calculated to have 80% power to detect ≥13 days difference in time to viral rebound between the two arms at a 5% significance level. A futility rule was implemented so that if 10 participants in a single group had shown viral rebound within two weeks of initiating ATI, no additional participants from the affected group would have undergone ATI. To accommodate for potential dropouts, we aimed to enroll 30 study subjects (15 in each arm). Due to slow enrollment, the study was terminated early (see below). The primary efficacy analysis population (time to viral rebound) included all participants completing both treatment cycles and ATI (n=17). The safety analysis population included all participants receiving any study drug (n=20). Reservoir and immunological assessments were performed in all participants completing both treatment cycles (n=19). Serum 3BNC117 levels were determined in all individuals receiving 3BNC117 (n=11) and antibody sensitivity assessments were attempted in all 3BNC117 recipients, although sequences could not be obtained from all individuals. Time to viral rebound during ATI was compared between the two treatment arms using the log-rank test. The Kaplan-Meier estimator was used as secondary analysis to assess the magnitude of the difference between the study groups. The Wilcoxon-Mann-Whitney or paired t-test were used as primary analyses for the secondary outcomes. A two-sided α value of less than 0.05 was considered significant. Statistical analyses were performed using Stata (version 16·0, StataCorp) and Prism (version 7·0, GraphPad).
Role of the funding source
Funding was provided by amfAR (The American Foundation for AIDS Research). Additional funding was provided through a grant by the German Center for Infection Research (DZIF). amfAR reviewed the study design and outcomes, but had no role in data collection, analysis, or interpretation. Celgene (now Bristol Myers Squibb) provided romidepsin free of charge.
RESULTS
Out of a total of 30 planned participants, 22 individuals were enrolled into the study between March 2017 and August 2018, with the final follow-up visit occurring in July 2019. Enrollment was terminated early in February 2019 due to significant difficulties in recruitment, with many potential participants citing the demanding visit schedule as main reason to decline entering the study. Of the 22 randomized participants, two individuals assigned to receive romidepsin alone withdrew consent prior to study drug administration due to scheduling problems or relocation (figure 1A and figures S1–S3, appendix pp 8–10). Of the 20 remaining randomized participants, 11 were allocated to receive 3BNC117 and romidepsin (group A) and 9 were allocated to receive to romidepsin alone (group B) (figure 1B). In group B, one participant (02–12-B) erroneously stopped ART at the start of the first treatment cycle and was prematurely discontinued from the trial and excluded from efficacy analyses. Nineteen (95%) participants, 11 in group A and 8 in group B, completed both treatment cycles. One participant in each group opted out of the subsequent ATI due to personal reasons, therefore 17 (89%) of the individuals receiving both treatment cycles entered the ATI (10 in group A and 7 in group B).
Demographic characteristics were similar in the two groups, although participants in group B were of a higher median age (table 1). HIV-1 parameters of reservoir size and transcription were also similar between the two groups, although the median time since diagnosis with HIV-1 infection and the median duration of ART were longer for participants receiving romidepsin only compared to those receiving romidepsin and 3BNC117 (table 1 + table S1, appendix p 11). Most participants had started ART in the calendar year of HIV-1 diagnosis (8 in group A and 5 in group B), but we did not select for individuals having initiated ART during primary HIV-1 infection and the time point of infection was generally not known.
Table 1.
Baseline characteristics.
| Characteristics | Group A: | Group B: |
|---|---|---|
| 3BNC117+RMD | RMD | |
| (n=11) | (n=9) | |
| Age (years) | 40 (33–51) | 51 (38–62) |
| Sex | ||
| Female | 2 (18) | 1 (11) |
| Male | 9 (82) | 8 (89) |
| Ethnicity | ||
| African American | 2 (18) | 3 (33) |
| Caucasian | 8 (73) | 6 (67) |
| Hispanic | 1 (09) | 0 (00) |
| Time since HIV-1 diagnosis (years) | 6 (2–11) | 16 (6–31) |
| Time from HIV-1 diagnosis to ART initiation (years) | 0 (0–10) | 0 (0–2) |
| Time on ART (years) | 5 (2–11) | 10 (6–21) |
| HIV subtype | ||
| A | 1 | 1 |
| B | 9 | 5 |
| Not available | 1 | 3 |
| CD4+ T cell count (cells per mm3)* | 716 (450–1090) | 600 (450–1150) |
| Cell-associated unspliced HIV-1 RNA (copies per million CD4+ T cells)* | 9·2 (0·9–111) | 11·0 (4·9–84·4) |
| Total HIV-1 DNA (copies per million CD4+ T cells)* | 604 (57–3281) | 686 (75–2071) |
| Intact proviral HIV-1 DNA (intact proviruses per million CD4+ T cells)* | 58 (41–162) | 46 (7–124) |
CA usHIV-1 RNA=cell-associated unspliced HIV-1 RNA, IPDA=intact proviral DNA assay, RMD=romidepsin.
Data are n (%) or median (range). ART=antiretroviral therapy, RMD=romidepsin.
Analyses performed at first leukaphresis time point (day −14)
The effect of romidepsin on HIV-1 transcription and plasma viral load
Individual romidepsin infusions resulted in modest and diverse effects on the levels of CD4 T CA usHIV-1 RNA as a marker of HIV-1 transcription when assessed one day post-infusion and compared to pre-infusion levels (figure S4 and table S2, appendix pp. 12–13). The median fold-change in CA usHIV-1 RNA levels after romidepsin administration across all infusions for which pre- and post-infusion measurements were available was 1.14 (interquartile range [IQR] 0·71 – 1·95; p=0·003) and was similar in both groups (figures S4, appendix p 13). During the first treatment cycle, CA usHIV-1 RNA levels increased significantly after the third romidepsin infusion in group A (day 16/17; p=0 039; figure 2A). During the second treatment cycle, the increases in HIV-1 transcriptional activity remained modest for group A and appeared more pronounced for group B. Eight of 19 individuals had quantifiable plasma HIV-1 RNA (range 20 to 144 copies/ml) during one or both treatment cycles (4 individuals in each group) (table S3, appendix p 14). Collectively, 15 of the 19 individuals had detectable plasma HIV-1 RNA during the treatment cycles. Three individuals in group A and one individual in group B maintained undetectable HIV-1 RNA levels after romidepsin infusions during both treatment cycles.
Figure 2.

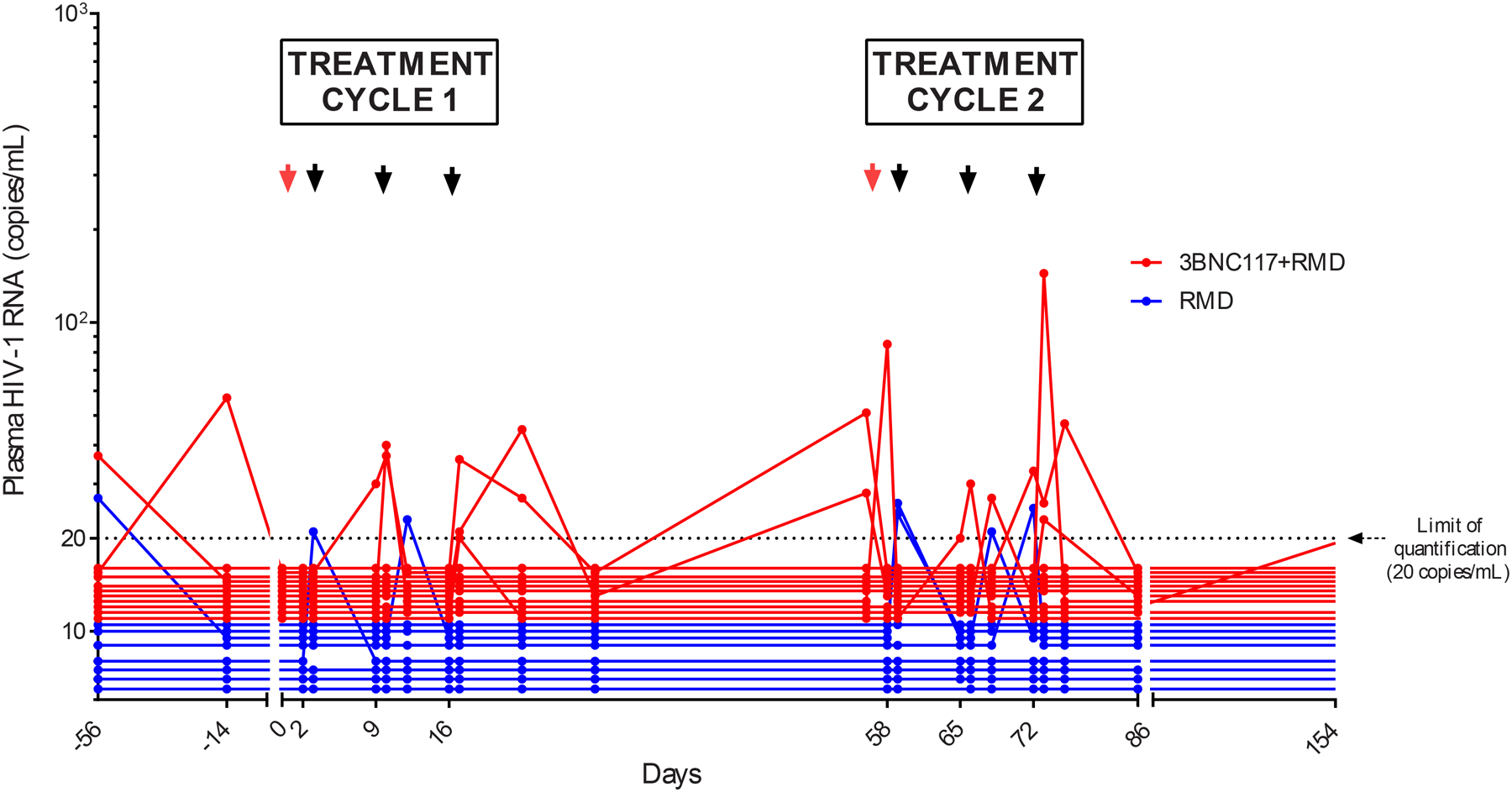
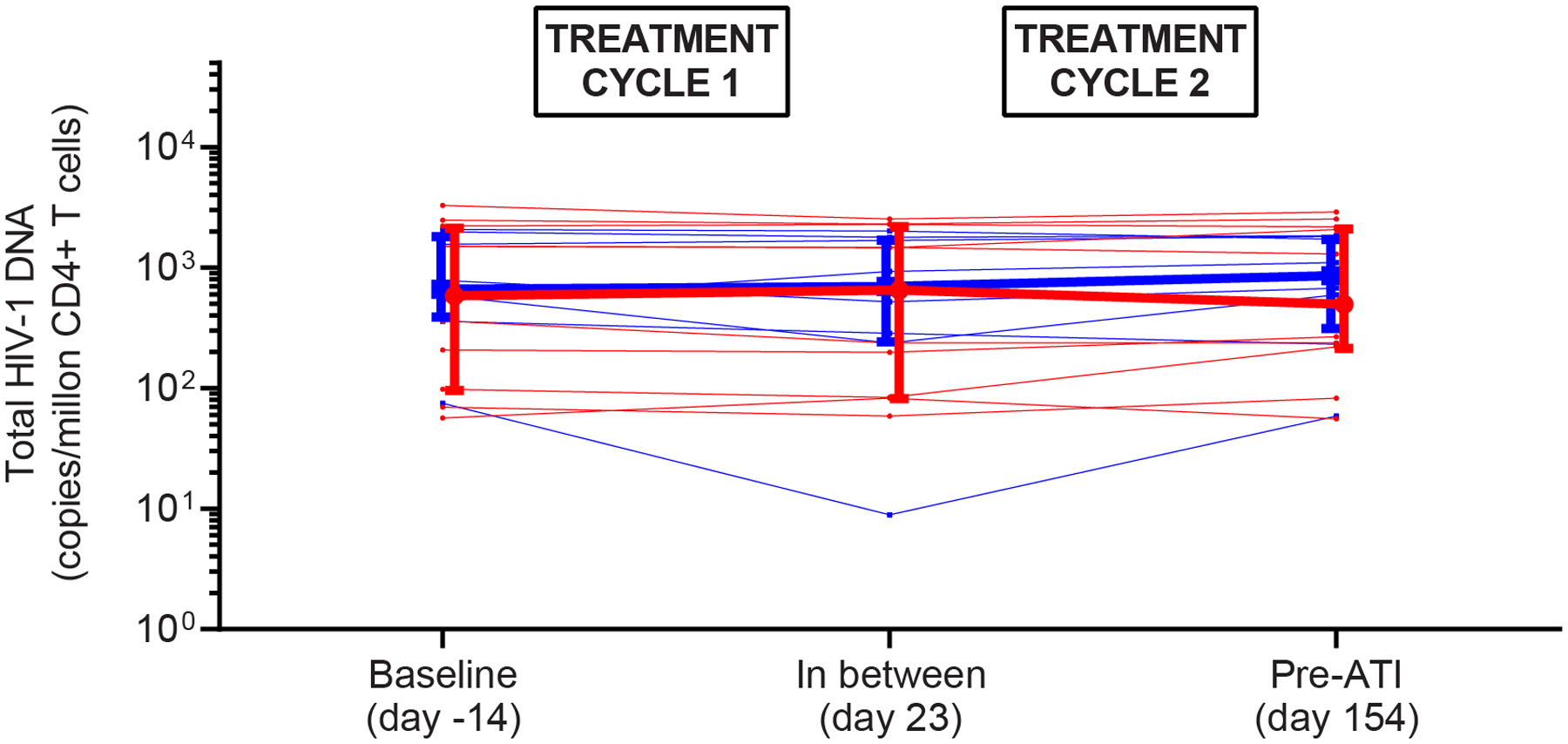

HIV-1 transcription, plasma HIV-1 RNA, total HIV-1 DNA, and intact proviruses. (A) Mean (SEM) fold increase in the level of cell-associated unspliced HIV-1 RNA from baseline (prior to first romidepsin infusion) to subsequent time points in each of the two treatment cycles. (B) Individual plasma HIV-1 RNA. The dotted line represents the limit of quantification (20 copies per mL). The arrows represent infusion time points. (C) Individual and median (IQR) level of total HIV-1 DNA per million CD4 T cells. (D) Individual and median (IQR) level of intact proviruses per million CD4 T cells. Two individuals in group B had 0 intact proviruses per million CD4 T cells at baseline. RMD=romidepsin. * P < 0·05.
The effect of romidepsin with or without 3BNC117 on the HIV-1 reservoir size
There are many different ways to quantify the HIV reservoir size. We first isolated CD4 T cells and quantified total HIV-1 DNA which includes both defective and replication-competent proviruses. Median total HIV-1 DNA levels at baseline were 603 (IQR 98 – 2223) and 686 (IQR 403 – 1880) copies/1m CD4 T cells in groups A and B, respectively. We found no significant changes in the levels of HIV-1 DNA from baseline to midway between the treatment cycles, or to the pre-ATI timepoint after completion of both treatment cycles in either group (figure 2C). After the first treatment cycle, the median decline in total HIV-1 DNA levels was −14 (interquartile range [IQR] −125;25; p=0·24) vs −71 (IQR −226;31; p=0·46) copies/1m CD4 T cells for groups A and B, respectively (p=0·41). From baseline to the pre-ATI timepoint, the median decline in total HIV-1 DNA was −1 (IQR −125;67; p=0·76) vs −61 (IQR −134;129; p=0·74) copies/1m CD4 T cells for groups A vs B, respectively (p=0·74). To more precisely quantify changes in the reservoir of replication-competent proviruses, we utilized the quantitative droplet digital PCR-based Intact Proviral DNA Assay (IPDA) that distinguishes defective from intact proviral sequences (figure 2D).22,23 Median levels of intact proviral HIV-1 DNA at baseline were 58 (IQR 38 – 201) and 46 (IQR 4 – 131) copies/1m CD4 T cells in groups A and B, respectively. From baseline to pre-ATI, the median level of intact proviruses declined by −11 (IQR −31;15, p=0·49) copies/1m CD4 T cells in group A and increased by 10 (IQR −19;10; p=0·74) copies/1m CD4 T cells in group B, respectively, but these changes as well as the difference between the two groups (p=0·89) were not statistically significant. We conclude that neither romidepsin alone nor its combined administration with the bNAb 3BNC117 had a significant impact on the size of the HIV-1 reservoir in chronically infected individuals on suppressive ART.
HIV-1-specific immunity and T cell activation
To determine the impact of the interventions on HIV-1-specific immunity and T cell function, we conducted a series of flow cytometry analyses. No significant changes in HIV-1-specific CD4 and CD8 T cell responses, measured by cytokine secretion following peptide stimulation, were observed from baseline o the pre-ATI time point in either group (figure 3A+B). Additionally, the expression of activation (figure 3C; subset analyses in figures S5+S6, appendix pp 15–16) and exhaustion (figure S7, appendix p 17) markers on CD4 and CD8 T cells remained unchanged after the two treatment cycles in both groups, but might have been temporarily increased during romidepsin infusions.5,9,24 These findings suggest that 3BNC117 in addition to romidepsin during maintained ART did not result in sustained alterations of HIV-1-specific T cell immunity or function.
Figure 3.


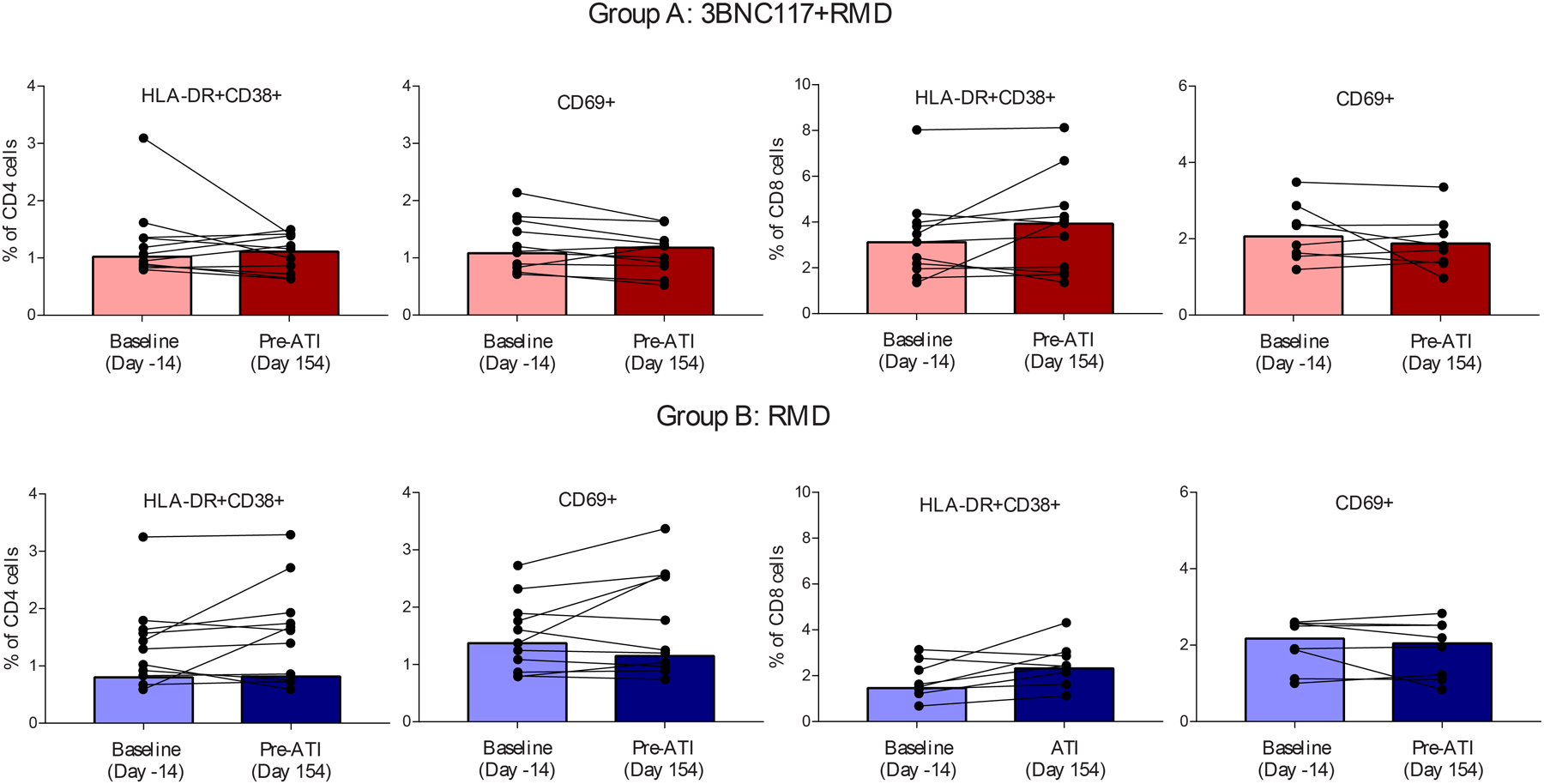
T cell immunity. (A) Proportion of polyfunctional HIV-1-specific memory CD8 T cells. Box-and-whisker plots show median values (line), 25th to 75th percentiles (box outline) and minimum and maximum values (whiskers) (B) Proportion of polyfunctional HIV-1-specific memory CD4 T cells. Box-and-whisker plots show median values (line), 25th to 75th percentiles (box outline) and minimum and maximum values (whiskers) (C) Individual proportion of the level of T-cell activation markers. Bars are median. RMD=romidepsin.
Time to viral rebound during ATI
The most important clinical outcome measure in HIV-1 cure trials is time to viral rebound after stopping ART.25 As expected, serum 3BNC117 concentrations had dropped to levels considered to be sub-therapeutic (mean concentration 1.3 μg/ml, range <0.2 to 5.2) by the time of ATI initiation in all individuals in group A (table S4, appendix 18). Viral rebound occurred by day 35 of the ATI in all individuals (figure 4A), except for one participant in group B (02–11-B) who maintained HIV-1 RNA levels <200 copies/ml until day 84 of the ATI (table S3, appendix p 14). The median time to viral rebound was 17.5 days (IQR 14–28) in group A and 28 days (IQR 21–35) in group B (figure 4B). Although this difference of 10.5 days in the median time to viral rebound after interruption of ART may not be clinically meaningful, it is statistically significant (p=0·02). All individuals achieved viral re-suppression upon ART re-initiation following viral rebound after a median of 28 days (IQR 14–42). We found weak correlations between the time to viral rebound and size of the HIV-1 reservoir at different time points (figure S8, appendix p 19). Of note, individual 02–11-B who maintained plasma HIV-1 RNA <200 copies/ml until day 84 had a small HIV-1 reservoir pre-ATI with a total HIV-1 DNA level of 59 copies and 9 intact proviruses per million CD4 T cells, respectively (figure S8, appendix p 19). To evaluate the sensitivity of the HIV-1 reservoir to 3BNC117 in individuals receiving the combination of bNAb and romidepsin, we obtained a total of 370 proviral env sequences from 7 out of 11 individuals (table S5A, no proviral env DNA could be amplified from the remaining four individuals despite the use of two different primer sets, appendix 20). Of all env sequences, 15 sequences (4%) obtained from two individuals (3/27 and 12/38 sequences respectively) were predicted to be associated with resistance to 3BNC117 (table S5A, appendix p 20). Based on a pre-defined threshold of 30% of resistant sequences, the reservoir of one of these individuals (02–15-A) was assessed as 3BNC117-resstant, although SGA-derived pseudoviruses obtained from plasma after viral rebound remained relatively sensitive to 3BN117 (table S5B, appendix p 20). Overall, all 28 SGA-derived rebound viruses obtained from six participants receiving 3BNC117 showed relative sensitivity to 3BNC117 (table S5B, no single genome sequences could be amplified from the remaining four individuals, appendix p 20).
Figure 4.


Time to viral rebound during analytical treatment interruption. (A) Individual plasma HIV-1 RNA levels after interruption of antiretroviral therapy; the dotted line represents the limit of quantification (20 copies per mL). (B) Proportion of individuals with plasma HIV-1 RNA of less than 200 copies per mL after interruption of antiretroviral therapy. ART=antiretroviral therapy, ATI=analytical treatment interruption, RMD=romidepsin.
Safety
Both treatment cycles were completed without interruption by all participants initiating study medication (with the exception of individual 02–12-B who was discontinued from the study because of erroneous early ART interruption). Adverse events were reported for 11 [100%] of 11 in group A and 9 [100%] of 9 in group B (table 2). Most adverse events were mild to moderate and similar between the groups. In total, 223 AEs (174 grade 1, 48 grade 2, and 1 grade 3) were reported of which 106 were solicited and 117 unsolicited AEs. Of the reported AEs, 159 were considered at least possibly related to study medications (table S6, appendix pp. 21–22). Two severe adverse events were reported in group B (concussion following a traffic incident requiring hospitalization was unrelated to study medication and increased direct bilirubin related to romidepsin: table S6+S7, appendix pp. 21–23) and both resolved without intervention. The most common solicited AEs were nausea (37; 75% of participants), headache (23; 35%), malaise (11, 25%), vomiting (10; 25%) and chills (9; 25%). Romidepsin infusions were associated with more drug-related AEs than 3BNC117 (table S6 + S7, appendix pp 20–22). Three participants (two in group A and one in group B) experienced transient QTc prolongations (>450 ms) at single post-romidepsin infusions (one day after infusion), and seven participants (9 infusions) had an increased QTc of >10 ms post-infusion compared to the ECG at pre-infusion, but none of the QTc changes were associated with clinical symptoms. No other clinically significant ECG changes were observed among the other 107 romidepsin infusions. Additionally, CD4 T cell counts were unaffected between and after the treatment cycles in both groups (figure S9, appendix p 24).
Table 2.
Adverse events.
| Adverse events | Group A | Group B |
|---|---|---|
| 3BNC117+RMD | RMD | |
| (n=11) | (n=9) | |
| Any clinical adverse event | 11 (100) | 9 (100) |
| Nausea | 11 (100) | 5 (56) |
| Fatigue | 5 (45) | 4 (44) |
| Vomiting | 5 (45) | 2 (22) |
| Headache | 4 (36) | 3 (33) |
| Constipation | 4 (36) | 1 (11) |
| Chills | 3 (27) | 2 (22) |
| Malaise | 3 (27) | 2 (22) |
| Arthralgia | 3 (27) | 1 (11) |
| Xerostomia | 3 (27) | 1 (11) |
| Rhinitis | 2 (18) | 3 (33) |
| Heartburn | 2 (18) | 2 (22) |
| Myalgia | 2 (18) | 1 (11) |
| Cough | 2 (18) | 0 (00) |
| Diarrhea | 2 (18) | 0 (00) |
| Pharyngitis | 2 (18) | 0 (00) |
| QTc>450 ms post-infusion | 1 (09) | 2 (22) |
| Blurred vision | 1 (09) | 1 (11) |
| Borborygmi | 1 (09) | 1 (11) |
| Dizziness | 1 (09) | 1 (11) |
| Hyperhidrosis | 1 (09) | 1 (11) |
| Upper respiratory tract infection | 1 (09) | 1 (11) |
| Abdominal discomfort | 1 (09) | 0 (00) |
| Acute stress reaction | 1 (09) | 0 (00) |
| Change in body odor | 1 (09) | 0 (00) |
| Cystitis | 1 (09) | 0 (00) |
| Epistaxis | 1 (09) | 0 (00) |
| Eye accomadation | 1 (09) | 0 (00) |
| Feverishness | 1 (09) | 0 (00) |
| Gonorrhea | 1 (09) | 0 (00) |
| Herpes labialis | 1 (09) | 0 (00) |
| Hordeolum | 1 (09) | 0 (00) |
| Hot flush | 1 (09) | 0 (00) |
| Hypotension | 1 (09) | 0 (00) |
| Increased lacrimal fluid | 1 (09) | 0 (00) |
| Increase of diastolic blood pressure | 1 (09) | 0 (00) |
| Lumbago with sciatica | 1 (09) | 0 (00) |
| Mastodynia | 1 (09) | 0 (00) |
| Musceloskeletal pain | 1 (09) | 0 (00) |
| Nose fracture | 1 (09) | 0 (00) |
| Pain lumbar spine | 1 (09) | 0 (00) |
| Rash | 1 (09) | 0 (00) |
| Secondary amenorrhea | 1 (09) | 0 (00) |
| Superficial injury of finger | 1 (09) | 0 (00) |
| Urethritis | 1 (09) | 0 (00) |
| Ageusia | 0 (00) | 1 (11) |
| Circulatory insufficiency | 0 (00) | 1 (11) |
| Cold intolerance | 0 (00) | 1 (11) |
| Concussion | 0 (00) | 1 (11) |
| Conjunctival erythema | 0 (00) | 1 (11) |
| Decreased libido | 0 (00) | 1 (11) |
| Dry skin | 0 (00) | 1 (11) |
| Dysesthesia | 0 (00) | 1 (11) |
| Epileptic seizure | 0 (00) | 1 (11) |
| Hematoma | 0 (00) | 1 (11) |
| Hoarse | 0 (00) | 1 (11) |
| Intermittent hematuria | 0 (00) | 1 (11) |
| Leg cramping | 0 (00) | 1 (11) |
| Loss of appetite | 0 (00) | 1 (11) |
| Palpitations | 0 (00) | 1 (11) |
| Toothache | 0 (00) | 1 (11) |
| Stomach ache | 0 (00) | 1 (11) |
| Maximum grade | ||
| Mild | 3 (27) | 4 (44) |
| Moderate | 8 (73) | 4 (44) |
| Severe | 0 (00) | 1 (11) |
Data are n (%). RMD=romidepsin.
DISCUSSION
In this phase 2a randomized trial, we evaluated the effect of combining a potent bNAb with the LRA romidepsin as a novel approach to reduce the HIV-1 reservoir in individuals on long-term suppressive ART. The two groups were overall comparable in their baseline characteristics (except group B had been diagnosed with HIV-1 infection significantly longer than group A) and both interventions were safe and relatively well-tolerated and the majority of the included participants completed all trial-related procedures. We observed no effect of the combined intervention with 3BNC117 and romidepsin administered during suppressive ART on the size of the HIV-1 reservoir and time to viral rebound during the ATI.
The goal of administering 3BNC117 prior to latency reversal was to enhance killing of reactivated latently infected cells (e.g. through antibody-dependent cellular cytotoxicity). Although a recent study did not detect increases in HIV-1 transcription following romidepsin infusions in ART-treated individuals, increased levels of CD4 CA usHIV-1 RNA peaking within hours of romidepsin infusion have previously been reported in several trials of participants on suppressive ART.5,8,18,19 Despite multiple romidepsin infusions, we observed only modest latency reversal and inconsistent blips in plasma HIV-1 RNA levels in a subset of participants in both groups. However, we collected blood samples one day after romidepsin infusions and may therefore have missed early post-infusion changes in HIV-1 transcriptional activity.
Neither romidepsin alone nor the combination of 3BNC117 and romidepsin led to a significant reduction in the size of the HIV-1 reservoir measured by total HIV-1 DNA and IPDA. Moreover, we observed no clinically meaningful delay in viral rebound during ATI in either of the groups and in contrast to our hypothesis, the median time to viral rebound was longer in individuals receiving romidepsin only compared to those receiving the combination of 3BNC117 and romidepsin. Notably, group B had been diagnosed with HIV-1 infection for a longer time than group A, but size of the HIV-1 reservoir and HIV-1 transcriptional activity between the two cohorts were comparable. An insufficient levels of antigen expression on reactivated infected cells following latency reversal may have limited 3BNC117 binding and immune-mediated elimination of these cells during suppressive ART. Similar aspect may have contributed to the lack of effects on reducing the latent reservoir and/or time to viral rebound observed in clinical trials investigating combinations of LRAs and therapeutic vaccines aimed at enhancing autologous antiviral immunity.8,18,26 Moreover, reactivated latently infected cells may be relatively resistant to elimination.27
Since accurate quantification of the replication-competent viral reservoir in humans is notoriously difficult to do and virological control in the absence of ART hard to predict, the impact HIV-1 cure interventions should ideally be assessed by evaluating HIV-1 control in the absence of ART during an ATI.25 Criteria for ART resumption was two consecutive plasma HIV-1 RNA measurements ≥200 copies/ml, which might have been a too strict threshold to observe post-treatment control of HIV-1 as recent studies show that some post-treatment controllers have peak viral loads >100,000 c/mL prior to regaining immunological control of viral replication1,25. CD8 T cell immunity against HIV-1 seems critical for achieving HIV-1 remission, but we observed no enhancement of HIV-1-specific cellular immunity following the treatment cycles.11,28,29 Importantly, we also did not observe detrimental effects of romidepsin on T cell HIV-1-specific immunity over the course of the study.5,9,24 Romidepsin infusions have previously been shown to temporarily impact activation and exhaustion markers on T cells over the interventional period, but reassuringly as seen in our study the expression of these markers pre-ATI were comparable to baseline levels.5,9,24 However, studies have indicated that HDACis do not inhibit natural killer cell function.30
Sensitivity of archived proviruses to bNAbs appear to be critical for the success of these monoclonal antibodies. In a phase Ib study, three infusions of a combination of two potent bNAbs (3BNC117 + 10–1074; both at 30 mg/kg) into an ATI led to durable HIV-1 control in the absence of ART among individuals with sensitive archived proviruses, whereas individuals with bNAbs-resistant reservoir showed no prolonged time to viral rebound.31 Pre-screening for bNAbs-sensitivity remains a challenge and was not feasible at the start of this study. However, post-hoc reservoir sequencing analyses at baseline and/or neutralization assays at rebound suggested relative antibody sensitivity in the 3BNC117 group.
The lack of effect of the combinations of LRA and passive or active immunization on the HIV-1 reservoir may also be partly due to immune exhaustion among individuals on long-term suppressive ART. As a result of persistent exposure to viral antigen during HIV-1 infection, expression of coinhibitory molecules such as programmed death 1 (PD-1) may be high on cells such as CD8 T cells which reduces their ability to eliminate infected cells. In the present study, all participants were on ART for a minimum of 5 years. PD-1 expression on T cells is only partially restored by ART, thus CD8 T cells functions are expected to remain impaired despite ART.
This was the first reported study evaluating the combination of potent bNAb and a LRA designed to target the HIV-1 reservoir. However, our study has some limitations. The study size was substantially smaller than planned due to difficulties in recruitment, reducing statistical power to find differences between the two groups in terms of immunologic and virologic parameters. Additionally, no placebo control arm was included, which would have enabled us to distinguish the impact of the interventions. Also, the study may not be generalizable to all HIV-1-infected individuals due to our stringent inclusion/exclusion criterions.
Based on the findings in this study and other recent trials testing the “shock and kill” approach, we conclude that latency reversal by a single HDAC inhibitor and modulation of autologous HIV-1-specific immunity by a single bNAb during suppressive ART is not effective in achieving HIV-1 remission among long-term ART suppressed individuals. There are several potential explanations for the lack of effect on the HIV-1 reservoir including insufficient potency of the LRA used, but escalating the dose of romidepsin is not an option due to higher toxicity,32 and anatomical reservoir ‘sanctuaries’ e.g. CNS where LRAs like HDACis have poor penetration. Alternative HIV-1 cure strategies based on overall similar concepts are under investigation. For example, some HIV-1 curative strategies are moving towards intervening immediately prior to and/or into an ATI rather than during suppressive ART, which may allow for controlled release of antigen from the HIV-1 reservoir and enhanced immunologic responses. Other studies select study participants who initiated ART during primary infection, or try to intervene at the time of ART initiation instead of after years of ART.33 These approaches can be seen as a departure from the classical ‘shock and kill’ hypothesis. Instead of using LRAs that appear to have limited capacity to induce of HIV-1 transcription, newer studies suggest that immunomodulators such as toll-like receptor agonists or interleukin-15 super-agonist in combination with bNAbs can lead to sustained viral control in simian–human immunodeficiency virus-infected non-human primates.15 Outcomes from such combinations in HIV-1-infected individuals are greatly anticipated. The results of our trial may serve as a benchmark for further optimization of HIV-1 curative strategies among HIV-1 infected individuals on suppressive ART.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for clinical trials with the terms “HIV remission”, “HIV cure”, “HIV eradication”, “HIV reservoir”, “HIV latency”, “Broadly anti-HIV neutralizing antibodies (bNAbs)”, “Latency-reversing agent (LRA)”, and “Histone deacetylase inhibitor (HDACi)”. We did not restrict our search by date or language. In clinical phase I-II trials, bNAb infusions have been shown to transiently decrease plasma HIV-1 RNA levels in viremic HIV-1-infected individuals. Multiple infusions of bNAbs alone or in combination prolonged HIV-1 control after interruption of antiretroviral therapy (ART). Latency reversal with HDACis (vorinostat, panobinostat, or romidepsin) as a single intervention has shown enhanced HIV-1 transcriptional activity as measured by increased levels of cellular and/or plasma HIV-1 RNA. However, none of these interventions alone have led to significant decreases in the HIV-1 reservoir or HIV-1 remission. In pre-clinical animal models, combinations of latency-reversing agents and HIV-1-neutralizing antibodies have resulted in reductions of HIV-1 reservoir measures.
Added value of this study
We report results from the first randomized clinical trial evaluating the concept of combining a potent bNAb (3BNC117) and a LRA (romidepsin) as a novel approach to reduce the viral reservoir in HIV-1-infected individuals on suppressive ART. In this trial, group A received 3BNC117 (30 mg/kg) two days prior to each romidepsin cycle, with romidepsin (5 mg/m2) administered weekly for three weeks in two treatment cycles. Group B received the two romidepsin treatment cycles without 3BNC117. Assessments of the cell-associated viral reservoir and the time to viral rebound after analytical treatment interruption of ART indicated that the combination of a single bNAb and romidepsin did not substantially affect the HIV-1 reservoir. The results demonstrate the clinical feasibility of combining a bNAb with a LRA, but highlight the challenges in achieving HIV-1 clearance in chronically infected individuals on ART.
Implications of all the available evidence
Combining a single LRA with a single bNAb did not significantly impact the latent HIV-1 reservoir. Taken together with all the available evidence, eradication of the HIV-1 reservoir among individuals on long-term ART using a ‘shock and kill’ strategy may be very difficult to achieve.
Acknowledgments
We thank all study participants who devoted time to our research. We thank members of the Nussenzweig, Klein, and Søgaard groups for support and discussion; the clinical study teams for trial conduct; the Clinical Trials Center Cologne (ZKS Köln) for help with data management and monitoring; and the Institute of Medical Statistics, Informatics, and Epidemiology (IMSIE) Cologne for support with randomization. We acknowledge Bristol-Myers Squibb Company (Celgene Corporation) for providing romidepsin.
Footnotes
Declaration of interests
HG and FK are listed as inventors on a patent application on HIV-1 neutralizing antibodies filed by the University of Cologne. MCN is listed as an inventor on patents for antibody 3BNC117.
Funding:
amfAR, The Foundation for AIDS Research; DZIF, German Center for Infection Research.
Data sharing
The protocol (MCA-896, 10 October 2017) is available online at www.ClinicalTrials.gov. Data are not available for download due to privacy/ethical restrictions. Specific requests for access to the trial data may be sent to the corresponding author and access may be provided to a named individual in agreement with the rules and regulations of the national laws.
References
- 1.Namazi G, Fajnzylber JM, Aga E, et al. The Control of HIV after Antiretroviral Medication Pause (CHAMP) study: post-treatment controllers identified from 14 clinical studies. J Infect Dis. 2018;(August):1–10. doi: 10.1093/infdis/jiy479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deeks SG. HIV: Shock and kill. Nature. 2012;487(7408):439–440. doi: 10.1038/487439a [DOI] [PubMed] [Google Scholar]
- 3.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–485. doi: 10.1038/nature11286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen TA, Tolstrup M, Brinkmann CR, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. lancet HIV. 2014;1(1):e13–21. doi: 10.1016/S2352-3018(14)70014-1 [DOI] [PubMed] [Google Scholar]
- 5.Søgaard OS, Graversen ME, Leth S, et al. The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. Siliciano RF, ed. PLoS Pathog. 2015;11(9):e1005142. doi: 10.1371/journal.ppat.1005142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott JH, McMahon JH, Chang CC, et al. Short-term administration of disulfiram for reversal of latent HIV infection: A phase 2 dose-escalation study. Lancet HIV. 2015;2(12):e520–e529. doi: 10.1016/S2352-3018(15)00226-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shan L, Deng K, Shroff NS, et al. Stimulation of HIV-1-Specific Cytolytic T Lymphocytes Facilitates Elimination of Latent Viral Reservoir after Virus Reactivation. Immunity. 2012;36(3):491–501. doi: 10.1016/j.immuni.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leth S, Schleimann MH, Nissen SK, et al. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3(10):e463–e472. doi: 10.1016/S2352-3018(16)30055-8 [DOI] [PubMed] [Google Scholar]
- 9.Tapia G, Højen JF, Ökvist M, et al. Sequential Vacc-4x and romidepsin during combination antiretroviral therapy (cART): Immune responses to Vacc-4x regions on p24 and changes in HIV reservoirs. J Infect. 2017;75(6):555–571. doi: 10.1016/j.jinf.2017.09.004 [DOI] [PubMed] [Google Scholar]
- 10.Lu C-L, Murakowski DK, Bournazos S, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science (80- ). 2016;352(6288):1001–1004. doi: 10.1126/science.aaf1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niessl J, Baxter AE, Mendoza P, et al. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat Med. 2020;26(2):222–227. doi: 10.1038/s41591-019-0747-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halper-Stromberg A, Lu C-LL, Klein F, et al. Broadly Neutralizing Antibodies and Viral Inducers Decrease Rebound from HIV-1 Latent Reservoirs in Humanized Mice. Cell. 2014;158(5):989–999. doi: 10.1016/j.cell.2014.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caskey M, Klein F, Nussenzweig MC. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat Med. 2019;25(4):547–553. doi: 10.1038/s41591-019-0412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly Neutralizing Anti-HIV-1 Antibodies Require Fc Effector Functions for In Vivo Activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borducchi EN, Liu J, Nkolola JP, et al. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. October 2018. doi: 10.1038/s41586-018-0600-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caskey M, Klein F, Lorenzi JCC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–560. doi: 10.1038/nature18929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mothe B, Rosás-Umbert M, Coll P, et al. HIVconsv Vaccines and Romidepsin in Early-Treated HIV-1-Infected Individuals: Safety, Immunogenicity and Effect on the Viral Reservoir (Study BCN02). Front Immunol. 2020;11(May):1–15. doi: 10.3389/fimmu.2020.00823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon DK, Zheng L, Cyktor JC, et al. A phase I/II randomized, placebo-controlled trial of romidepsin in persons with HIV-1 on suppressive antiretroviral therapy to assess safety and activation of HIV-1 expression (A5315). J Infect Dis. 2020;6(2):106192. doi: 10.1093/infdis/jiaa777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials. 2010. doi: 10.1186/1745-6215-11-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen YZ, Butler AL, Millard K, et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10–1074 in healthy adults: A randomized, phase 1 study. Landay A, ed. PLoS One. 2019;14(8):e0219142. doi: 10.1371/journal.pone.0219142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruner KM, Wang Z, Simonetti FR, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566(7742):120–125. doi: 10.1038/s41586-019-0898-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinloch NN, Ren Y, Conce Alberto WD, et al. HIV-1 diversity considerations in the application of the Intact Proviral DNA Assay (IPDA). Nat Commun. 2021;12(1):165. doi: 10.1038/s41467-020-20442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosás-Umbert M, Ruiz-Riol M, Fernández MA, et al. In vivo Effects of Romidepsin on T-Cell Activation, Apoptosis and Function in the BCN02 HIV-1 Kick&Kill Clinical Trial. Front Immunol. 2020;11(March):1–11. doi: 10.3389/fimmu.2020.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julg B, Dee L, Ananworanich J, et al. Recommendations for analytical antiretroviral treatment interruptions in HIV research trials—report of a consensus meeting. Lancet HIV. 2019;6(4):e259–e268. doi: 10.1016/S2352-3018(19)30052-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidler S, Stöhr W, Pace M, et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet. 2020;6736(19):1–11. doi: 10.1016/S0140-6736(19)32990-3 [DOI] [PubMed] [Google Scholar]
- 27.Ren Y, Huang SH, Patel S, et al. BCL-2 antagonism sensitizes cytotoxic T cell–resistant HIV reservoirs to elimination ex vivo. J Clin Invest. 2020;130(5):2542–2559. doi: 10.1172/JCI132374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura Y, Gautam R, Chun T, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543(7646):559–563. doi: 10.1038/nature21435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim S-Y, Osuna CE, Hraber PT, et al. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. 2018;10(439):eaao4521. doi: 10.1126/scitranslmed.aao4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrido C, Tolstrup M, Søgaard OS, et al. In-vivo administration of histone deacetylase inhibitors does not impair natural killer cell function in HIV+ individuals. Aids. 2019;33(4):605–613. doi: 10.1097/QAD.0000000000002112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–484. doi: 10.1038/s41586-018-0531-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunst JD, Tolstrup M, Rasmussen TA, Søgaard OS. The potential role for romidepsin as a component in early HIV-1 curative efforts. Expert Rev Anti Infect Ther. 2016;14(5):447–450. doi: 10.1586/14787210.2016.1164031 [DOI] [PubMed] [Google Scholar]
- 33.Gunst JD, Tolstrup M, Søgaard OS. Beyond antiretroviral therapy: early interventions to control HIV-1 infection. AIDS. 2017;31(12):1665–1667. doi: 10.1097/QAD.0000000000001524 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol (MCA-896, 10 October 2017) is available online at www.ClinicalTrials.gov. Data are not available for download due to privacy/ethical restrictions. Specific requests for access to the trial data may be sent to the corresponding author and access may be provided to a named individual in agreement with the rules and regulations of the national laws.


