Highlights
-
•
Patients with T2D and CV disease are at a high risk of adverse CV events.
-
•
IPE therapy should be considered in patients with T2D with or at risk of CV disease.
-
•
26–45% of people from EMPA-REG OUTCOME would have been eligible for IPE.
-
•
Empagliflozin benefits were consistent, regardless of REDUCE-IT or FDA eligibility.
Keywords: Cardiovascular disease, Icosapent ethyl, Omega-3 fatty acids, Triglycerides, Type 2 diabetes
Abstract
Objectives
REDUCE-IT showed that icosapent ethyl (IPE) improved cardiovascular (CV) outcomes in participants with established CV disease (CVD) or type 2 diabetes (T2D) and at least one additional risk factor plus mild-moderate hypertriglyceridemia and reasonably controlled low-density lipoprotein cholesterol (LDL-C). As the generalizability of REDUCE-IT has not been investigated in a T2D population with established CVD, this post hoc analysis investigated how many participants from EMPA-REG OUTCOME, which tested the effects of empagliflozin versus placebo on CV outcomes in participants with T2D and CVD, would have been eligible for IPE treatment, and whether CV outcomes differed based on eligibility for IPE treatment.
Methods
Participants from EMPA-REG OUTCOME were screened for inclusion using both REDUCE-IT-like criteria (baseline statin therapy, triglycerides 135–499 mg/dL and LDL-C 41–100 mg/dL) and slightly amended FDA indication criteria (triglycerides ≥150 mg/dL). Analyses were conducted to characterize the study population and CV outcomes in participants eligible for IPE versus those not eligible for IPE.
Results
Of the 7020 participants from EMPA-REG OUTCOME, 1810 (25.8%) fulfilled REDUCE-IT criteria and 3182 (45.3%) fulfilled FDA criteria for IPE treatment. Treatment effects of empagliflozin versus placebo on CV and kidney outcomes and mortality were consistent in participants meeting REDUCE-IT and FDA criteria and those who did not.
Conclusions
These results indicate that a sizable proportion of patients with diabetes and established CVD, such as those in EMPA-REG OUTCOME, may be eligible for IPE treatment to lower residual CV risk. Treatment benefit with empagliflozin was consistent, regardless of REDUCE-IT or FDA eligibility criteria.
1. Introduction
Type 2 diabetes (T2D) is a highly prevalent disease associated with a plethora of downstream complications [1]. Elevated triglyceride levels are common in patients with T2D [2], and may serve as an independent marker for increased risk of cardiovascular (CV) events among patients with low-density lipoprotein cholesterol (LDL-C) levels that are controlled with statins [3].
The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) randomized 8179 participants with CV disease (CVD) or diabetes plus at least one additional CVD risk factor who were receiving statin therapy to either icosapent ethyl (IPE) or placebo [4]. In the REDUCE-IT trial, IPE demonstrated a significant relative risk reduction (25%, p<0.0001) in the primary composite endpoint of CV death, nonfatal myocardial infarction (MI), nonfatal stroke, coronary revascularization, or unstable angina requiring hospitalization. For the key secondary endpoint (CV death, nonfatal MI, and nonfatal stroke) IPE showed a similar benefit (26% risk reduction favoring treatment, p<0.0001) [4]. These results were consistent irrespective of diabetes status [5], in patients with a history of coronary artery bypass grafting [6], and in patients with prior percutaneous coronary intervention [7].
Following publication of the REDUCE-IT trial results, IPE received approval from the Food and Drug Administration (FDA) for reduction of CV event risk among patients with either established CVD or T2D plus at least one CVD risk factor with triglyceride levels ≥150 mg/dl [8]. However, it may be valuable to expand the external generalizability of the characteristics of the patient population studied in REDUCE-IT to other patient populations, potentially influencing future treatment initiatives.
In the EMPA-REG OUTCOME trial, the sodium-glucose cotransporter 2 (SGLT2) inhibitor empagliflozin, demonstrated a significant reduction in primary endpoint events (3-point major adverse cardiovascular events [3P-MACE], including death from CV causes, nonfatal MI, or nonfatal stroke) versus placebo (hazard ratio 0.86 [95% confidence intervals 0.74, 0.99], p = 0.04). This result was driven by a reduction in the risk of CV death. All-cause mortality, heart failure hospitalization and kidney outcomes were also significantly reduced [9,10]. The EMPA-REG OUTCOME trial included participants with T2D at high risk of CV events, using any background glucose-lowering agent, making this a representative cohort with a setting similar to real-life clinical practice [11].
Thus, in this analysis we aimed to investigate whether participants included in the EMPA-REG OUTCOME trial would be eligible for IPE according to the REDUCE-IT eligibility criteria or to the FDA label requirements. We also investigated whether the treatment benefits of empagliflozin observed in the EMPA-REG OUTCOME trial were consistent in participants fulfilling the eligibility criteria (i.e., those with elevated levels of triglycerides and at high risk of having a CV event), versus not fulfilling the criteria.
2. Materials and methods
The EMPA-REG OUTCOME trial analyzed 7020 adult (aged ≥ 18 years) participants with T2D and established CVD, randomized 1:1:1 and treated with empagliflozin (10 mg or 25 mg) or placebo once per day [9]. Key participant eligibility criteria included a body-mass index (BMI) of ≤45 kg/m2 and an estimated glomerular filtration rate (eGFR) of ≥30 ml/min/1.73 m2. The full list of inclusion/exclusion criteria of the EMPA-REG OUTCOME trial has been previously described [9]. There were no inclusion/exclusion criteria regarding triglyceride levels. Median follow-up time was 3.1 years.
Using baseline data, we categorized the study population into subgroups according to the REDUCE-IT inclusion criteria [12] and the FDA label criteria [13] in two separate analyses. For inclusion in the REDUCE-IT-like subgroup, all three of the following criteria had to be fulfilled: statin therapy at baseline, triglyceride levels of 135 to 499 mg/dl, and low-density lipoprotein cholesterol (LDL-C) levels of 41 to 100 mg/dl (Fig. 1A). REDUCE-IT exclusion criteria included severe heart failure, active severe liver disease, glycated hemoglobin >10%, planned coronary intervention or surgery, history of pancreatitis, or hypersensitivity to fish, shellfish, or ingredients of IPE or placebo. Since not all data from the REDUCE-IT exclusion criteria was recorded in EMPA-REG OUTCOME participants, they were not included in the current analysis. As all participants in EMPA-REG OUTCOME had established CVD and the maximal tolerated statin dose may have been zero in the case of statin intolerance, we used slightly amended FDA indication criteria and accepted any statin use as the maximally tolerated dose, with the assumption that those who did not use a statin did so due to intolerance or contraindication. Therefore, a triglyceride level at baseline of ≥150 mg/dl was considered sufficient for entering the subgroup fulfilling the FDA-label criteria (Fig. 1B). Outcomes included CV death, hospitalization for heart failure (HHF), the composite of CV death (excluding fatal stroke) and HHF, all-cause mortality, 3P-MACE, and incident or worsening nephropathy defined as the composite of macroalbuminuria (i.e., UACR >300 mg/g), doubling of s-creatinine with eGFR <45 ml/min/1.73 m2, need for dialysis or renal transplant, or renal death.
Fig. 1.
Proportion of participants eligible for IPE treatment. (A) A schematic outlining population from EMPA-REG OUTCOME eligible for IPE treatment fulfilling REDUCE-IT-like criteria. (B) A schematic outlining population from EMPA-REG OUTCOME eligible for IPE treatment fulfilling FDA label criteria. Abbreviations: FDA, Food and Drug Administration; LDL-C, low-density lipoprotein cholesterol.
2.1. Statistical analyses
Baseline characteristics are provided in subgroups of participants eligible and not eligible for IPE treatment based on REDUCE-IT and FDA label criteria with continuous variables presented as mean ± standard deviation (SD) or median (interquartile range), and categorical variables as number (n) and proportion (%). We assessed the effect of empagliflozin versus placebo in those fulfilling versus not fulfilling the REDUCE-IT criteria, and in those fulfilling versus not fulfilling the FDA label criteria, in two separate Cox regression models. The models included covariate terms for age, sex, BMI, glycated hemoglobin (HbA1c), eGFR, region, fulfillment of REDUCE-IT criteria/FDA label criteria (as subgroup), treatment, and treatment*subgroup interaction, enabling an evaluation of the treatment effect in participants fulfilling versus not fulfilling the REDUCE-IT/FDA criteria at baseline. For this analysis, empagliflozin dose groups (10 and 25 mg) were pooled for comparison versus placebo since, overall, no differences in efficacy or safety were seen between the 2 doses. All analyses were performed at the nominal alpha level of 0.05, without correction for multiple hypothesis testing.
3. Results
Of the 7020 participants included in the EMPA-REG OUTCOME trial, 25.8% of the population (n = 1810; 1202 receiving empagliflozin, 608 receiving placebo) fulfilled the REDUCE-IT criteria (Fig. 1). Overall, 45.3% of the study population fulfilled FDA label requirements (n = 3182; 2139 receiving empagliflozin,1043 receiving placebo).
There were minor differences in baseline characteristics between the REDUCE-IT-like cohort and participants not fulfilling REDUCE-IT-like eligibility criteria (Table 1). Compared with the non-fulfilling cohort, the REDUCE-IT-like cohort had lower estimated glomerular filtration rate (eGFR) levels, fewer participants with a history of stroke and/or peripheral arterial disease, and participants had lower lipid levels (total cholesterol, high-density lipoprotein [HDL-C] and low-density lipoprotein [LDL-C]; with the exception of triglycerides). The REDUCE-IT-like cohort also had a higher prevalence of coronary artery disease, greater waist measurements, and increased baseline use of CV medications including lipid lowering drugs (as per subgroup definition) compared with those not fulfilling REDUCE-IT-like eligibility criteria.
Table 1.
Baseline characteristics of participants in the REDUCE-IT-like cohort compared to participants not fulfilling REDUCE-IT-like criteria, and participants fulfilling the FDA label criteria compared to participants not fulfilling the FDA label criteria.
| Baseline Characteristics | EMPA-REG OUTCOME study population |
|||
|---|---|---|---|---|
| Not fulfilling REDUCE-IT-like criteria n = 5210 |
Fulfilling REDUCE-IT-like criteriaa n = 1810 |
Not fulfilling FDA label criteria n = 3753 |
Fulfilling FDA label criteria n = 3182 |
|
| Female Sex | 1552 (29.8) | 452 (25.0) | 1030 (27.4) | 945 (29.7) |
| Race | ||||
| White | 3675 (70.5) | 1406 (77.7) | 2546 (67.8) | 2467 (77.5) |
| Black/African American | 302 (5.8) | 55 (3.0) | 233 (6.2) | 119 (3.7) |
| Asian | 1193 (22.9) | 324 (17.9) | 946 (25.2) | 560 (17.6) |
| Native Hawaii/ Other Pacific |
5 (0.1) | 5 (0.3) | 6 (0.2) | 3 (0.1) |
| American Indian/ Alaska native |
35 (0.7) | 19 (1.0) | 22 (0.6) | 32 (1.0) |
| Age, years | 63.2 ± 8.7 | 62.9 ± 8.5 | 63.8 ± 8.7 | 62.3 ± 8.6 |
| BMI, kg/m2 | 30.26 ± 5.28 | 31.66 ± 5.05 | 29.85 ± 5.29 | 31.49 ± 5.07 |
| ≥ 30 | 2505 (48.1) | 1116 (61.7) | 1685 (44.9) | 1888 (59.3) |
| Time Since T2D Diagnosis, Years | ||||
| ≤ 1 | 121 (2.3) | 59 (3.3) | 75 (2.0) | 102 (3.2) |
| > 1 to 5 | 804 (15.4) | 279 (15.4) | 493 (13.1) | 574 (18.0) |
| > 5 to 10 | 1295 (24.9) | 451 (24.9) | 875 (23.3) | 852 (26.8) |
| > 10 | 2990 (57.4) | 1021 (56.4) | 2310 (61.6) | 1654 (52.0) |
| Insulin | 2478 (47.6) | 909 (50.2) | 1840 (49.0) | 1500 (47.1) |
| Metformin | 3798 (72.9) | 1395 (77.1) | 2711 (72.2) | 2422 (76.1) |
| UACR | 16.08 (6.19, 69.84) |
20.33 (7.07, 83.98) |
15.91 (6.19, 60.11) |
21.22 (7.07, 92.82) |
| Normal | 3120 (59.9) | 1051 (58.1) | 2332 (62.1) | 1789 (56.2) |
| Microalbuminuria | 1499 (28.8) | 514 (28.4) | 1042 (27.8) | 946 (29.7) |
| Macroalbuminuria | 540 (10.4) | 229 (12.7) | 350 (9.3) | 415 (13.0) |
| Missing | 51 (1.0) | 16 (0.9) | 29 (0.8) | 32 (1.0) |
| eGFR, ml/min/1.73 m2, MDRD |
74.91 ± 21.54 | 71.56 ± 20.83 | 75.09 ± 21.20 | 72.81 ± 21.65 |
| eGFR Category | ||||
| ≥ 90 | 1186 (22.8) | 352 (19.4) | 848 (22.6) | 675 (21.2) |
| 60 to < 90 | 2747 (52.7) | 915 (50.6) | 2004 (53.4) | 1607 (50.5) |
| < 60 | 1277 (24.5) | 542 (29.9) | 901 (24.0) | 899 (28.3) |
| Missing | 1 (< 0.1) | 1 (0.1) | 0 (0) | 1 (< 0.1) |
| Previous Stroke | 1296 (24.9) | 341 (18.8) | 864 (23.0) | 753 (23.7) |
| Previous CAD | 3795 (72.8) | 1513 (83.6) | 2851 (76.0) | 2394 (75.2) |
| Previous PAD | 1132 (21.7) | 329 (18.2) | 780 (20.8) | 657 (20.6) |
| Retinopathy | 1174 (22.5) | 372 (20.6) | 914 (24.4) | 616 (19.4) |
| Cardiac Failure | 525 (10.1) | 181 (10.0) | 361 (9.6) | 336 (10.6) |
| HbA1c,% | 8.06 ± 0.85 | 8.12 ± 0.84 | 7.99 ± 0.83 | 8.16 ± 0.86 |
| Waist Size, m | 1.038 ± 0.138 | 1.079 ± 0.133 | 1.028 ± 0.138 | 1.072 ± 0.133 |
| Systolic BP, beats/min | 135.4 ± 17.1 | 135.7 ± 16.7 | 134.9 ± 17.2 | 136.0 ± 16.9 |
| Diastolic BP, beats/min | 76.6 ± 9.8 | 76.9 ± 9.9 | 75.8 ± 9.9 | 77.7 ± 9.8 |
| Total Cholesterol, mg/dL | 158.2 (130.3, 197.2) |
152.4 (136.1, 166.3) |
145.4 (125.3, 170.1) |
169.4 (144.2, 202.2) |
| HDL-C, mg/dl | 44.1 (37.1, 52.2) |
39.1 (34.0, 45.2) |
46.0 (40.2, 54.1) |
39.1 (34.0, 45.2) |
| LDL-C, mg/dl | 85.1 (61.1, 114.1) |
71.2 (59.2, 83.1) |
76.2 (59.2, 98.2) |
83.1 (62.3, 112.1) |
| Triglycerides, mg/dl | 121.3 (93.9, 178.0) |
183.3 (155.9, 229.4) |
107.2 (85.0, 127.5) |
205.5 (174.5, 266.6) |
| Beta-blockers | 3207 (61.6) | 1347 (74.4) | 2366 (63.0) | 2134 (67.1) |
| Diuretics | 2165 (41.6) | 870 (48.1) | 1530 (40.8) | 1468 (46.1) |
| ACEi/ARBs | 4132 (79.3) | 1534 (84.8) | 3009 (80.2) | 2584 (81.2) |
| Statins | 3593 (69.0) | 1810 (100.0) | 2979 (79.4) | 2355 (74.0) |
| Niacin | 88 (1.7) | 38 (2.1) | 59 (1.6) | 65 (2.0) |
| Fibrates | 425 (8.2) | 205 (11.3) | 206 (5.5) | 415 (13.0) |
| Ezetimibe | 191 (3.7) | 79 (4.4) | 140 (3.7) | 129 (4.1) |
| Other lipid lowering drugs | 352 (6.8) | 188 (10.4) | 247 (6.6) | 283 (8.9) |
| ASA | 4223 (81.1) | 1580 (87.3) | 3126 (83.3) | 2606 (81.9) |
Data outputs are displayed as number (%) or mean ± standard deviation, median (Q1, Q3) for UACR, total cholesterol, HLD-C, LDL-C, and triglycerides. aThe REDUCE-IT-like Cohort. Abbreviations: ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ASA, acetylsalicylic acid; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol;
MDRD, Modification of Diet in Renal Disease formula; PAD, peripheral artery disease; Q1, quartile 1; Q3, quartile 3; T2D, type 2 diabetes; UACR, urine albumin-to-creatinine ratio.
There were also only minor differences in baseline characteristics between participants fulfilling and those not fulfilling the FDA label criteria (Table 1). When compared with the non-fulfilling criteria cohort, the FDA label fulfilling cohort had lower eGFR levels, greater waist measurements, higher lipid levels (total cholesterol, LDL-C and triglycerides; with the exception of HDL-C), increased use of CV medications including lipid-lowering drugs (with the exception of statins) but lower numbers of participants with retinopathy.
3.1. CV and kidney outcomes and mortality across subgroups
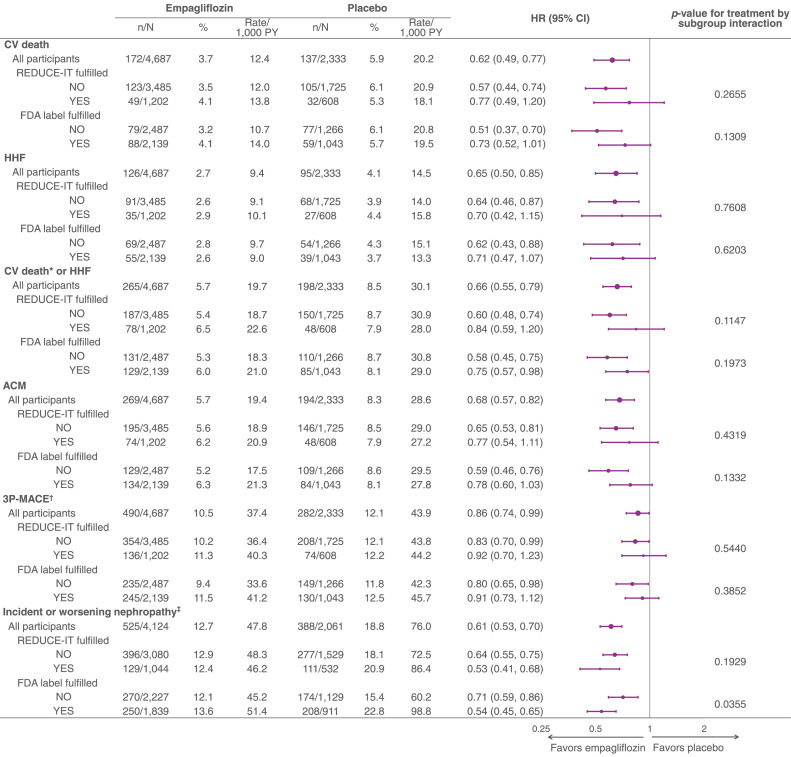
Considering the placebo group only, in general, the incidence rates of outcomes did not differ between those fulfilling versus not fulfilling the REDUCE-IT criteria (Fig. 2). Those fulfilling the FDA label requirements had a higher risk of incident or worsening nephropathy, but a similar risk of CV outcomes and mortality compared to those not fulfilling the FDA label criteria (Fig. 2). The treatment effect of empagliflozin versus placebo on CV and kidney outcomes and mortality was consistent across subgroups of participants fulfilling or not fulfilling the REDUCE-IT criteria as well as those fulfilling versus not fulfilling the FDA label criteria (Fig. 2). For incident or worsening nephropathy, there was an apparent greater magnitude of treatment effects in those fulfilling the FDA label criteria, compared with those not fulfilling the FDA criteria, but the effect of empagliflozin was shown to be significant within both subgroups.
Fig. 2.
Central Illustration. Treatment effect of empagliflozin versus placebo, comparing the REDUCE-IT-like cohort to participants not fulfilling the REDUCE-IT-like criteria, and the participants fulfilling the FDA label criteria to those not fulfilling the FDA label criteria. p-values for treatment by subgroup interaction were obtained from tests of homogeneity of treatment group differences among subgroups with no adjustment for multiple testing. HR (95% CI) based on multivariable Cox regression including factors for age, sex, geographical region, Hemoglobin A1c, body mass index, eGFR, treatment, fulfillment of REDUCE-IT criteria/FDA label criteria (as subgroup), treatment-by-subgroup interaction term. *CV death without fatal stroke. †3P-MACE includes death from CV causes, nonfatal myocardial infarction, or nonfatal stroke. ‡Incident or worsening nephropathy defined as the composite of macroalbuminuria (UACR > 300 mg/g), doubling of s-creatinine with eGFR <45 ml/min/1.73 m2, need for dialysis or kidney transplant, or kidney death.
Abbreviations: 3P-MACE, 3-point major adverse cardiovascular events; ACM, all-cause mortality; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; FDA, Food and Drug Administration; HHF, hospitalization for heart failure; HR, hazard ratio; PY, patient-years; UACR, urine albumin-to-creatinine ratio.
4. Discussion
In this post hoc analysis, we demonstrate that among the participants included in the EMPA-REG OUTCOME trial, 25.8% were eligible for IPE according to the REDUCE-IT eligibility criteria and 45.3% were eligible according to the FDA label requirements. These participants had generally comparable risk of CV outcomes with those not fulfilling the criteria, with a consistent treatment effect of empagliflozin across all groups.
Despite well-controlled CV risk factors, a substantial residual CV risk remains in patients with T2D, warranting a search for additional treatments [14,15]. Following the REDUCE-IT trial, IPE was approved in the USA to reduce the risk of CV events for patients with either established CVD or T2D and ≥ 2 additional CV risk factors who were on a maximum tolerated statin dose and had high triglyceride levels. Identifying patient groups with a high prevalence of mild to moderate hypertriglyceridemia and CV risk for therapeutic targeting remains a clinical need. The current results, from a representative cohort of patients with T2D and high CV risk, suggest that a substantial proportion of a contemporary population of patients with T2D and CVD would be eligible for IPE treatment. It has previously been shown that, among the placebo group, risk of CV outcomes and mortality was higher in those participants in EMPA-REG OUTCOME with poor control of traditional CV risk factors versus those with good CV risk control when entering the trial [16]. Furthermore, despite a significant reduction in CV event occurrence, a rate of 37.4 participants with primary outcome event per 1000 patient-years was observed in the empagliflozin-treated population [9].
A persistent risk for CV events remained even in those with good CV risk factor control and when treated with empagliflozin, thus supporting the need for additional treatments [17]. The characteristics of the participants at baseline were largely comparable across the subgroups that fulfilled the REDUCE-IT criteria and the FDA requirements in EMPA-REG OUTCOME, and in those included in the REDUCE-IT trial (Table 2).
Table 2.
Comparisons of the REDUCE-IT-like, REDUCE-IT and FDA label cohorts.
| Baseline Characteristics/ Endpoint |
REDUCE-IT cohorta n = 8179 |
REDUCE-IT-like cohort in EMPA-REG OUTCOME n = 1810 |
FDA label criteria cohort in EMPA-REG OUTCOME n = 3182 |
|||
|---|---|---|---|---|---|---|
| Icosapent Ethyl | Placebo | Empagliflozin | Placebo | Empagliflozin | Placebo | |
| Number of participants analyzed | 4089 (100.0) | 4090 (100.0) | 1202 (100.0) | 608 (100.0) | 2139 (100.0) | 1043 (100.0) |
| Demographics | ||||||
| Age, years | 64.0 (57.0, 69.0) |
64.0 (57.0, 69.0) |
63.0 (57.0, 69.0) |
63.0 (57.0, 69.0) |
62.0 (56.0, 68.0) |
62.0 (57.0, 69.0) |
| Female Sex | 1162 (28.4) | 1195 (29.2) | 306 (25.5) | 146 (24.0) | 632 (29.5) | 313 (30.0) |
| BMI ≥ 30 kg/m2 | – | – | 724 (60.2) | 392 (64.5) | 1264 (59.1) | 624 (59.8) |
| Systolic BP, mmHg | – | – | 135.3 ± 16.6 | 136.3 ± 17.0 | 135.8 ± 16.9 | 136.4 ± 16.8 |
| Diastolic BP, mmHg | – | – | 76.8 ± 9.8 | 77.1 ± 10.2 | 77.9 ± 9.6 | 77.5 ± 10.2 |
| Smoking status | ||||||
| Never smoked | 1604 (39.2) | 1660 (40.6) | 430 (35.8) | 194 (31.9) | 833 (38.9) | 401 (38.4) |
| Ex-smoker | 1857 (45.4) | 1815 (44.4) | 604 (50.2) | 322 (53.0) | 972 (45.4) | 493 (47.3) |
| Currently smokes | 628 (15.4) | 613 (15.0) | 168 (14.0) | 92 (15.1) | 334 (15.6) | 149 (14.3) |
| T2D | 2367 (57.9) | 2363 (57.8) | 1202 (100.0) | 608 (100.0) | 2139 (100.0) | 1043 (100.0) |
| Previous CAD | – | – | 1000 (83.2) | 513 (84.4) | 1599 (74.8) | 795 (76.2) |
| Previous PAD | – | – | 231 (19.2) | 98 (16.1) | 453 (21.2) | 204 (19.6) |
| Previous MI | – | – | 619 (51.5) | 312 (51.3) | 1005 (47.0) | 481 (46.1) |
| Statin Use | 4089 (100.0)b | 4090 (100.0)b | 1202 (100.0) | 608 (100.0) | 1596 (74.6) | 759 (72.8) |
| HDL-C, mg/dl | 40.0 (34.5, 46.0) |
40.0 (35.0, 46.0) |
40.2 (34.0, 46.0) |
39.1 (34.0, 45.2) |
39.1 (34.0, 46.0) |
39.1 (34.0, 45.2) |
| LDL-C, mg/dl | 74.0 (61.5, 88.0) |
76.0 (63.0, 89.0) |
71.9 (58.0, 83.1) |
71.2 (59.2, 82.0) |
84.3 (62.3, 112.7) |
82.0 (62.3, 110.2) |
| Triglycerides, mg/dl | 216.5 (176.5, 272.0) |
216.0 (175.5, 274.0) |
183.3 (155.9, 228.5) |
184.2 (156.8, 231.6) |
204.6 (172.7, 263.1) |
209.9 (176.3, 271.0) |
| 3P-MACE | 459 (11.2)c | 606 (14.8)c | 136 (11.3)d | 74 (12.2)d | 245 (11.5)d | 130 (12.5)d |
| Incidence rate for 3P-MACE per 1000 PY | 32 | 44 | 40 | 44 | 41 | 46 |
Data outputs are displayed as number (%), mean ± standard deviation, or median (Q1, Q3), when available. For 3P-MACE: presented are n with event/N analyzed (%). aBaseline characteristics for the REDUCE-IT cohort are given in the Bhatt, D.L. et al. NEJM. 2019 publication[12], smoking status were given in the Miller, M. et al. Eur Heart J Cardiovasc Pharmacother. 2022 publication [30], and incidence rate for 3P-MACE per 1000 PY were given in the Bhatt D.L. et al. JACC. 2019 publication [31]. b0.42% of participants are missing statin use information although statin use was required for REDUCE-IT study inclusion. cThe key secondary outcome in REDUCE-IT was 3P-MACE. dThe primary endpoint in EMPA-REG OUTCOME was 3P-MACE.
Abbreviations: 3P-MACE, 3-point major adverse cardiovascular events; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MI, myocardial infarction; PAD, peripheral arterial disease; PY, patient years; Q1, quartile 1; Q3, quartile 3; T2D, type 2 diabetes; UACR, urine albumin-to-creatinine ratio.
Assessment of the generalizability of the REDUCE-IT results to diverse patient groups beyond those included in the trial has been limited [12]. However, some post hoc studies have examined the external validity of REDUCE-IT on unique datasets to ascertain the proportion of participants eligible for the potential benefits of IPE therapy. At present, researchers have demonstrated that within study populations of participants with atherosclerotic CVD, coronary artery disease, MI, and a history of coronary artery bypass graft surgery, there are subgroups that would be eligible for, and hence potentially also benefit from, IPE treatment according to the REDUCE-IT criteria [18], [19], [20], [21], [22]. The present post hoc analysis adds to the growing body of literature by exploring a contemporary diabetes population with known CVD. Table S1 includes a summary of REDUCE-IT external validity reports to date. Overall, in the absence of dedicated clinical trials, these analyses are invaluable to determine the generalizability of the patient population eligible for IPE across varying disease states.
While IPE has been associated with improvements in risk of CV events, this was not accompanied by a substantial effect on biomarkers associated with atherosclerotic disease [23]. This is in contrast to empagliflozin where data suggests that a multitude of mechanisms, associated with atherosclerotic disease, may be involved, including an amelioration of the inflammatory process in the cardiomyocytes [24], reduction in epi/pericardial fat [25,26] and improvement of endothelial dysfunction [24]. Although the exact biological mechanisms in which IPE or empagliflozin incur CV benefits are yet to be elucidated, it is likely that these two drugs work by different mechanisms and could act in a complementary manner to each other.
In this post hoc analysis, empagliflozin reduced the risk of CV events, mortality, and nephropathy versus placebo regardless of whether or not participants were eligible for IPE treatment based on the REDUCE-IT or FDA label criteria. This is in line with previously published data, demonstrating consistent effects of empagliflozin irrespective of CV risk [27], heart failure risk [28], and number of affected vascular beds [29].
4.1. Limitations of the study
There are several limitations to our analyses. Some participants had missing baseline lipid values such as triglyceride (n = 105) and LDL-C (n = 108) levels, however, we still report data from a large, contemporary clinical trial with independent adjudication of all CV endpoints. In addition, these are post hoc analyses, and the trial was not powered to assess treatment effect in subgroups. The potential benefits of IPE were not actually tested in our study. Lastly, inclusion of patients into EMPA-REG OUTCOME was based on the respective inclusion and exclusion criteria of that study [9].
5. Conclusion
This analysis is the first to evaluate the proportion of participants with T2D and known CVD who would be eligible for treatment with IPE, based on REDUCE-IT-like criteria or FDA label requirements. Overall, 25.8% of the EMPA-REG OUTCOME population fulfilled key eligibility criteria of the REDUCE-IT trial, whereas 45.3% fulfilled the FDA prescription requirements, suggesting that up to half of a contemporary population of participants with T2D and CVD may be eligible for IPE treatment to reduce residual CV risk. Those fulfilling the REDUCE-IT-like or FDA criteria had a similar risk of CV, heart failure, and mortality outcomes compared with those not fulfilling the criteria, whereas the rates of nephropathy were somewhat higher in those fulfilling the FDA criteria. There was an increased benefit of using empagliflozin over placebo on all analyzed outcomes, irrespective of whether the above-mentioned criteria were fulfilled or not. The different mechanisms of action of the two medications may suggest that they may complement each other, and that addition of IPE to existing treatment with an SGLT2 inhibitor may assist to further reduce the risk of CV events in patients with diabetes and established CVD or with additional risk factors for CVD.
Financial support
The EMPA-REG OUTCOME trial was funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance. Boehringer Ingelheim was involved in the design and conduct of the study and the concept, execution, and interpretation of this analysis.
Data transparency statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript and secondary analyses in peer-reviewed journals and regulatory and reimbursement activities are completed, normally within 1 year after the marketing application has been granted by major Regulatory Authorities. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information
Author contributions
A.K., S.V. and A.P.O. conceived the study idea, wrote the first draft of the manuscript, and contributed to all subsequent versions. All authors reviewed, contributed to, and approved all drafts of the manuscript.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
S.V. declares that he has received research and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, CMS, HLS, Janssen, Merck Novartis, Novo Nordisk, PhaseBio, and Sanofi. He is also the President of the Canadian Medical and Surgical Knowledge Translation Research Group and holds the Tier 1 Canada Research Chair in Cardiovascular Surgery.
A.K. declares no conflict of interest.
D.L.B. discloses the following relationships - Advisory Board: AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Eli Lilly and Company, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda.
D.F. has received honoraria from Sanofi, Merck & Co., Amgen, AstraZeneca, Eli Lilly and Company, and Boehringer Ingelheim and has served on the data and safety monitoring board for Novo Nordisk.
A.P.O. and M.M. are employees of Boehringer Ingelheim.
C.W. reports personal fees from Boehringer Ingelheim during the conduct of the study; personal fees from Akebia, AstraZeneca, Bayer, Eli Lilly and Company, GSK, GILEAD, MSD, Mundipharma, Sanofi-Genzyme and Vifor Fresenius outside the submitted work.
B.Z. has received research grants awarded to his institution from Boehringer Ingelheim, AstraZeneca, and Novo Nordisk and honoraria from Janssen, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Novo Nordisk, and Merck.
P.R.L. is supported by a Heart and Stroke Foundation of Canada National New Investigator Award.
L.A.L. has received research funding from, has provided CME on behalf of, and/or has acted as an advisor to Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Esperion, HLS, Kowa, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Servier.
Acknowledgements
The authors thank the investigators, coordinators, and patients who participated in this trial. Editorial support was provided by Jonathon Gibbs of Elevate Scientific Solutions, which was contracted, and compensated by Boehringer Ingelheim. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE) and were fully responsible for all content and editorial decisions and were involved at all stages of manuscript development.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100510.
Appendix. Supplementary materials
References
- 1.Papatheodorou K., Banach M., Bekiari E., Rizzo M., Edmonds M. Complications of diabetes 2017. J Diabetes Res. 2018 doi: 10.1155/2018/3086167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginsberg H.N., Zhang Y.L., Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Nichols G.A., Philip S., Reynolds K., Granowitz C.B., Fazio S. Increased residual cardiovascular risk in patients with diabetes and high versus normal triglycerides despite statin-controlled LDL cholesterol. Diabetes Obes Metab. 2019;21:366–371. doi: 10.1111/dom.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaba P., Bhatt D.L., Mason R.P., Miller M., Verma S., Steg P.G., et al. Benefits of icosapent ethyl for enhancing residual cardiovascular risk reduction: a review of key findings from REDUCE-IT. J Clin Lipidol. 2022;16:389–402. doi: 10.1016/j.jacl.2022.05.067. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt D., Brinton E., Miller M., Steg P., Jacobson T., Ketchum S., et al. Substantial cardiovascular benefit from icosapent ethyl in patients with diabetes: REDUCE-IT DIABETES. Diabetologia. 2020;63:S302–S3S3. [Google Scholar]
- 6.Verma S., Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., et al. Icosapent ethyl reduces ischemic events in patients with a history of previous coronary artery bypass grafting: REDUCE-IT CABG. Circulation. 2021;144:1845–1855. doi: 10.1161/CIRCULATIONAHA.121.056290. [DOI] [PubMed] [Google Scholar]
- 7.Peterson B.E., Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., et al. Treatment with icosapent ethyl to reduce ischemic events in patients with prior percutaneous coronary intervention: insights from REDUCE-IT PCI. J Am Heart Assoc. 2022;11 doi: 10.1161/JAHA.121.022937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boden W.E., Baum S., Toth P.P., Fazio S., Bhatt D.L. Impact of expanded FDA indication for icosapent ethyl on enhanced cardiovascular residual risk reduction. Future Cardiol. 2021;17:155–174. doi: 10.2217/fca-2020-0106. [DOI] [PubMed] [Google Scholar]
- 9.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 10.Wanner C., Inzucchi S.E., Lachin J.M., Fitchett D., von Eynatten M., Mattheus M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 11.Zinman B., Inzucchi S.E., Lachin J.M., Wanner C., Ferrari R., Fitchett D., et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME) Cardiovasc Diabetol. 2014;13:102. doi: 10.1186/1475-2840-13-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 13.Amarin Pharma, Inc. Vascepa (isosapent ethyl) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202057s035lbl.pdf. Revised December 2019. Accessed December 12, 2022.
- 14.Rawshani A., Rawshani A., Franzen S., Sattar N., Eliasson B., Svensson A.M., et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 15.Lawler P.R., Rosenson R.S., Ko D.T. Triglyceride reduction in secondary atherosclerotic cardiovascular disease prevention: core concepts in contemporary therapeutic targeting. Eur Heart J. 2020;41:1521–1522. doi: 10.1093/eurheartj/ehaa078. [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi S.E., Khunti K., Fitchett D.H., Wanner C., Mattheus M., George J.T., et al. Cardiovascular benefit of empagliflozin across the spectrum of cardiovascular risk factor control in the EMPA-REG OUTCOME trial. J Clin Endocrinol Metab. 2020;105:3025–3035. doi: 10.1210/clinem/dgaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawler P.R., Bhatt D.L., Godoy L.C., Luscher T.F., Bonow R.O., Verma S., et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42:113–131. doi: 10.1093/eurheartj/ehaa099. [DOI] [PubMed] [Google Scholar]
- 18.Kosmopoulos A., Verma S., Meglis G., Bhatt D.L., Verma R., Mazer C.D., et al. Generalizability of reduction of cardiovascular events with icosapent ethyl-intervention trial in patients with a history of coronary artery bypass graft surgery. Curr Opin Cardiol. 2021;36:172–178. doi: 10.1097/HCO.0000000000000800. [DOI] [PubMed] [Google Scholar]
- 19.Picard F., Bhatt D.L., Ducrocq G., Elbez Y., Ferrari R., Ford I., et al. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019;73:1362–1364. doi: 10.1016/j.jacc.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Ferrieres J., Bataille V., Puymirat E., Schiele F., Simon T., Danchin N., et al. Applicability of the REDUCE-IT trial to the FAST-MI registry. Are the results of randomized trials relevant in routine clinical practice? Clin Cardiol. 2020;43:1260–1265. doi: 10.1002/clc.23437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawler P.R., Kotrri G., Koh M., Goodman S.G., Farkouh M.E., Lee D.S., et al. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86–94. doi: 10.1093/eurheartj/ehz767. [DOI] [PubMed] [Google Scholar]
- 22.Picard F., Bhatt D.L., Ducrocq G., Ohman E.M., Goto S., Eagle K.A., et al. Generalizability of the REDUCE-IT trial and cardiovascular outcomes associated with hypertriglyceridemia among patients potentially eligible for icosapent ethyl therapy: an analysis of the REduction of Atherothrombosis for Continued Health (REACH) registry. Int J Cardiol. 2021;340:96–104. doi: 10.1016/j.ijcard.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 23.Ridker P.M., Rifai N., MacFadyen J., Glynn R.J., Jiao L., Steg P.G., et al. Effects of randomized treatment with icosapent ethyl and a mineral oil comparator on interleukin-1beta, interleukin-6, c-reactive protein, oxidized low-density lipoprotein cholesterol, homocysteine, lipoprotein(a), and lipoprotein-associated phospholipase A2: a REDUCE-IT biomarker substudy. Circulation. 2022;146:372–379. doi: 10.1161/CIRCULATIONAHA.122.059410. [DOI] [PubMed] [Google Scholar]
- 24.Kolijn D., Pabel S., Tian Y., Lodi M., Herwig M., Carrizzo A., et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Galpha oxidation. Cardiovasc Res. 2021;117:495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda T., Bouchi R., Terashima M., Sasahara Y., Asakawa M., Takeuchi T., et al. Ipragliflozin reduces epicardial fat accumulation in non-obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Ther. 2017;8:851–861. doi: 10.1007/s13300-017-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi S., Hirata Y., Ise T., Kusunose K., Yamada H., Fukuda D., et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2017;9:78. doi: 10.1186/s13098-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitchett D., Inzucchi S.E., Cannon C.P., McGuire D.K., Scirica B.M., Johansen O.E., et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME Trial. Circulation. 2019;139:1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verma S., Sharma A., Zinman B., Ofstad A.P., Fitchett D., Brueckmann M., et al. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across thrombolysis in myocardial infarction risk score for heart failure in diabetes categories: post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes Metab. 2020;22:1141–1150. doi: 10.1111/dom.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma S., Mazer C.D., Inzucchi S.E., Wanner C., Ofstad A.P., Johansen O.E., et al. Impact of polyvascular disease with and without co-existent kidney dysfunction on cardiovascular outcomes in diabetes: a post hoc analysis of EMPA-REG OUTCOME. Diabetes Obes Metab. 2021;23:1173–1181. doi: 10.1111/dom.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller M., Bhatt D.L., Steg P.G., Brinton E.A., Jacobson T.A., Jiao L., et al. Potential effects of icosapent ethyl on cardiovascular outcomes in cigarette smokers: REDUCE-IT smoking. Eur Heart J Cardiovasc Pharmacother. 2022;11 doi: 10.1093/ehjcvp/pvac045. pvac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.