Abstract
Animal tuberculosis (TB) is a contagious and chronic disease caused by mycobacteria belonging to theMycobacterium tuberculosis complex (MTBC) in domestic and wild animals. MTBC strains infection has been confirmed in many animal species in Nigeria, including captive wildlife, cattle, dromedary camels, goats, and pigs. Despite widespread infection and the potential impact of the disease on public health, active surveillance and control strategies are absent in Nigeria. This study aimed to conduct the first comprehensive meta-analysis to assess the distribution of tuberculosis and analyze the potential moderators of infection in animals in Nigeria. Eligible studies (sixty-one (Cadmus et al., 2014) [61] prevalence and seven (Menzies and Neill, 2000) [7] case reports) were retrieved and included in the analysis. The analyses showed an overall pooled TB prevalence of 7.0% (95% CI: 6.0–8.0) comprising of infection distributed in cattle (8.0%, 95% CI: 7.0–8.0), goats (0.47%, 95% CI: 0–1.2), sheep (0.27%, 95% CI: 0.14–0.46), camels (13.0%, 95% CI: 0–47), and wildlife (13.0%, 95% CI: 9–16) respectively. The occurrence of infection was significantly moderated by the publication periods, geographical location, sample size, and detection methods. TB prevalence was heterogeneous across several predictors, with the year of publication exhibiting a higher rate (46%) of the detected heterogeneity. These findings should provide policy-relevant information to guide the design and establishment of prevention and control measures amenable to the local situations in Nigeria.
Keywords: Animal tuberculosis, Livestock, Wildlife, meta-Analysis, Epidemiology, Nigeria
1. Introduction
Animal tuberculosis (TB), also referred to as TB in domestic and wild animals, is a zoonotic, contagious, and chronic infectious disease with similar pathology caused by mycobacteria belonging to the Mycobacterium tuberculosis complex (MTBC) [1,2]. Despite the causative agents, very often, Mycobacterium bovis is capable of infecting a wide variety of mammalian species, including humans, tuberculosis in animals is generally referred to as “bovine tuberculosis (bTB)" [3,4]. While cattle are the principal host for M. bovis, the intricacy of disease transmission is exacerbated by the possibility that it can be transmitted by other animals [5,6]. Transmission of tubercle bacilli among animals occurs directly through respiratory and oral contact and indirectly through contaminated fecal and oral secretions, particularly at sites where feed resources and water are shared [7,8]. The clinically inapparent diseased animals can shed tubercle bacilli in infectious droplets and milk, putting humans at risk of infection, which was the initial driver for bTB control [2]. The most usual means for transmission of M. bovis to humans is through contaminated food, most typically untreated dairy products or, less frequently, untreated meat products, while additional pathways (aerosol inhalation or direct contact with infected animals and offal) are also conceivable [[9], [10], [11]]. The highest incidence of zoonotic tuberculosis is found in impoverished, marginalized, remote populations that live in close contact with animals and have limited access to hygienic food and medical treatment.4 The risk of contracting M. bovis infection is enhanced in many sub-Saharan African communities, such as Nigeria, where humans have frequent contact with cattle and consume unpasteurized dairy products [12]. In Nigeria, more human infections were documented as a result of occupational exposure [13], foodborne transmission [14] or cohabitation with infected animals [15], and there are government neglect and lacking commitment to bTB control.
Animal TB is responsible for high economic losses due to decreased production, trade restrictions, and slaughter compensations for the test-positive animals, as well as the cost of preventive measures, and it continues to be a zoonotic threat in many countries across the world [2,16,17]. Azami and Zinsstag have attributed the economic costs of bTB to be due to a drop of 10–18% in milk yield, a loss of 10–25% of productive efficiency, higher condemnation of edible organs, a 15% reduction in meat production, and an increase in mortality [18]. Costs associated with bovine tuberculosis in underdeveloped nations are primarily due to livestock production losses, such as higher mortality and decreased milk and meat output [19]. The effective bTB elimination strategy relies on the widespread implementation of whole-herd test-and-slaughter and abattoir monitoring programs supported by animal identification, tracing, and movement restriction [20]. The use of the intradermal test and individual slaughter to eliminate bTB is challenging but necessary, as it can help to limit pathogen circulation and, as a result, minimize potential zoonotic risk and negative impact on animal health and welfare [21]. However, with the increasing size of the bTB-infected herd, the complexity of test-and-slaughter policy implementation grows, rendering it improbable, especially given the low finances available in many undeveloped countries [21], such as Nigeria, for compensating farmers for slaughtered animals. Routine slaughterhouse inspection of carcasses by veterinarians to identify animals with suspect lesions is the sole element of the bTB intervention program in Nigeria, where active national surveillance and control strategies are absent.
Mycobacterium bovis is a typical multi-host pathogen that thrives worldwide in various settings at the livestock-wildlife interface [22]. Notably, the infection caused by M. bovis has been confirmed in many animal species in Nigeria, including captive wildlife [23], cattle [[24], [25], [26]], camels [27], goats [25,28], and pigs [25]. The role played by these animals in the maintenance and transmission of tubercle bacilli infection is currently the subject of an investigation in Nigeria. Considering the infection is still largely undetected, the country lacks active surveillance and a mandated disease reporting mechanism, severely restricting TB control in animals and informed prevention. Data from surveillance are essential for formulating successful TB control programs, assessing disease burden, identifying risk factors and vulnerable populations, and tracking trends in morbidity and mortality [29]. However, in developing countries, there is a lack of comprehensive information concerning overall geographic TB prevalence, animal disease distribution, and diagnostic techniques under typical applications [30]. As a result, this study aimed to conduct a systematic review and meta-analysis to estimate the prevalence of TB in different animal species and analyze the potential moderators of infection to bridge data gaps for policy formulation on preventive and control measures in Nigeria.
2. Materials and methods
2.1. Study design
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines (S1 File) [31]. However, an a priori protocol was designed according to the previous description (S2 File) [32], and registered on the International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021273441). The registration guaranteed that no other meta-analysis on tuberculsosis in animals in Nigeria exists or was underway.
2.2. Search strategy
A search for the peer-reviewed literature on tuberculosis in animals in Nigeria was conducted through Web of Science, Scopus, PubMed, Academic Search Complete, and African Journal Online (AJOL). These databases were explored using a search string: ((“Prevalence” OR “Epidemiology”) AND (“Cattle” OR “Bovine” OR “Sheep” OR “Ovine” OR “Goats” OR “Caprine” OR “Camel” OR “Dromedary camel” OR “Pig” OR “Wildlife” OR “Animals”) AND (“Bovine TB” OR “Bovine tuberculosis” OR “Tuberculosis” OR “Mycobacterium tuberculosis complex” OR “Mycobacterium bovis” OR “Mycobacterium caprae” OR “Mycobacterium tuberculosis” OR “Mycobacterium africanum” OR “Tubercle bacilli” OR “Acid fast bacilli”) AND (“Nigeria”)). Furthermore, a grey search was conducted to avoid missing valid literature using the key terms “bovine tuberculosis”, “bTB”, “animal tuberculosis”, “Nigeria” in Google Scholar. However, there was no time restriction as regards the publication date during these searches.
2.3. Selection criteria
All retrieved articles from the database and grey search were exported to the Mendeley reference manager to remove the duplicates. The de-duplicated citations were then exported to the Rayyan Intelligent Systematic Review software for the title, abstract, and full-text screening based on the inclusion and exclusion criteria of the study. The literature was selected based on the following criteria: 1). It was an observational prevalence study or case report; 2). The data concerned animals in Nigeria; 3). Detection of tuberculosis was conducted using valid methods; 4). The full text was available. In contrast, studies that did not contain the relevant data were excluded from the review. Three independent reviewers conducted the screening process, and a fourth reviewer decided on areas of disagreement between the three reviewers.
2.4. Data extraction
Standardized form in Microsoft (MS) Excel was used to extract data from the included studies on characteristics such as first author, publication year, sampling time, study type, study location by state, sampling season, animal species, detection methods, mycobacterial isolation, MTBC identification, the number of samples positive for TB/total number of animals examined, prevalence, and study quality (S1 Table). Three independent reviewers carried out the entire data extraction process and, in case of discrepancies that may arise, were sorted out by the fourth reviewer. Data were extracted from text, tables, and graphs, and in case of missing data, the corresponding author of such an article was contacted by email not more than twice a week.
2.5. Quality assessment
Each article that meets the study inclusion criteria was subjected to a quality assessment using different appraisal tools for the two study types. First, the quality of the eligible papers was evaluated using a critical appraisal checklist for prevalence data described previously [33]. This appraisal checklist contains ten items that assess 1). Appropriate sampling frame; 2). Proper sampling technique; 3). Adequate sample size; 4). Adequate description of study subjects and setting; 5). Sufficient data analysis; 6). Use of valid detection techniques for the identified conditions; 7). Adequate training of those involved in the detection of the identified conditions; 8). Employment of appropriate statistical analysis (for prevalence); 9). Adequate identification and account of subgroups/confounding factors/differences; and 10). Use valid detection methods to identify subgroups (S2 Table). At the same time, the case report studies were evaluated using eight criteria (S3 Table) based on the described critical appraisal checklist [34], Yes (Y), no (N), unclear (U), or not applicable (NA) were assigned to answer each question on the checklists. Articles with ≤60% score were considered low-quality studies. Three reviewers conducted the quality assessment, and the fourth reviewer resolved discrepancies.
2.6. Data analysis
MetaXL software (add-in for Microsoft Excel) by Barendregt and Doi [35] was used for the meta-analysis. The meta-analysis and pooling of the prevalence estimate (with a 95% confidence interval) were done using the random effect (RE) model. In addition, the (transformed) double arcsine method was employed in the meta-analysis [36]. Statistical heterogeneity was estimated for the included studies using the chi-square (χ2) test, Cochrane Q test, tau, and I2 statistics [37]. An I2 value of 0 to <40% was considered not significant, >40%–60% was regarded as moderate heterogeneity, >60%–75% was considered substantial heterogeneity, and >75%–100% was considered significant heterogeneity [38].
A funnel plot was constructed using the double arcsine prevalence against standard error to examine publication bias. A further assessment of the observed asymmetry on the funnel plot, indicating potential publication bias, was done using the Doi plot [36]. The Doi plot is to assess the degree of asymmetry seen in the funnel plot. More so, Egger's regression test was done to test the significance of the confirmed asymmetry. Sensitivity analysis was done based on the leave-one-out model to identify the study that greatly influenced the meta-analysis result. Subgroup and meta-regression analyses were conducted to identify the factors contributing to heterogeneity among the studies. The factors considered for the analyses include the year of publication, sampling season, study region (geographical zone), state of study, detection methods, sample size, age distribution, sex distribution, and study quality. For meta-regression, univariate analysis was performed for each of the covariates. Due to the low power of the test (meta-regression), 0.25 was considered significant. All factors with significant p values were included in the multivariate meta-regression analysis.
3. Results
3.1. Study selection process and characteristics of included studies
A total of 84 articles were subjected to full–text screening, and 68 remained after thorough screening steps based on inclusion criteria relating to the prevalence (n = 61) or case report (n = 7) of TB in a given host detected by any valid diagnostic test (Fig. 1). Data extracted from publications with multiple animals, states, or regions were considered separate-level data. Publications that provided prevalence data (61 articles) were included in the quantitative analyses (S4 Table). While seven case report studies were used for synthesis review to identify risks associated with exposure to infection (S5 Table). The reviewed literature revealed prevalence and case report of tuberculosis between 1979 and 2021 (42 years) across all the six geographical zones and from 23 states of the country involving five animal species, namely, cattle (number of articles, n = 64), goats (n = 5), sheep (n = 4), camels (n = 4), and wildlife (n = 3; zebras [n = 2], elands [n = 2], antelopes [n = 1], baboons [n = 1], African giant rats [n = 1], gorilla [n = 1], lioness [n = 1], wildebeest [n = 1], hares [n = 1], waterbucks [n = 1], grass cutters [n = 1]) (S1 Table). Prevalence data was provided on cattle from 58 articles comprising 70 studies, goats from five studies, sheep from four studies, camels from three, and wildlife from two studies (S6 Table). The included studies with prevalence data provided a sample size of 5,289,921, of which 3,829,482 were cattle, 611,118 were goats, 630,329 were sheep, 219,643 were camels, and 347 were wildlife. Varied detection methods were used in the included studies. These techniques include post-mortem examination (PM), Ziehl – Neelsen staining (ZN), histopathological examination (Hist), culture isolation (CI), and blood-based laboratory test (enzyme-linked immunosorbent assay [ELISA] or lateral flow test [LF]). Others are tuberculin tests (namely, single intradermal test [SIT] or single comparative intradermal tuberculin test [SCITT]), molecular methods (Mol), and Hain assay test (HAT) or GenoType MTBC analysis, or combined methods (e.g., PM, ZN, and culture isolation) as detailed in S1 Table.
Fig. 1.
PRISMA flowchart.
3.2. Study quality assessment
Ten (14.7%) of the included studies were shown to be of low quality after quality assessment. The detailed result of the quality assessment for each included study is provided in the S2 Table.
3.3. Outcomes
The prevalence of animal TB in Nigeria (comprising cattle, goats, sheep, camels, and wildlife) was obtained by pooling the prevalence data from individual publications. The meta-analysis was done using the RE model utilizing the transformed double arcsine method. The overall prevalence estimate was determined as 7.0% (95% CI: 6.0–8.0). To test for heterogeneity, the following statistics were computed: Cochrane Q value (Q; = 104079.288), χ2 p < 0.0001, and I2 = 99.9%. Fig. 2 shows the forest plot of the RE model meta-analysis. The pooled estimates for cattle, goat, sheep, camel, and wildlife are 8.0% (95% CI: 7.0–8.0), 0.47% (95% CI: 0–1.2), 0.27% (95% CI: 0.14–0.46), 13.0% (95% CI: 0–47), and 13.0% (95% CI: 9–16) respectively (Fig. 3). The meta-analysis summary is provided in Table 1. Sensitivity analysis was done for the cattle meta-analysis with no significant difference (Table 1).
Fig. 2.
Forest plot of meta-analysis of animal TB (overall) prevalence in Nigeria.
Fig. 3.
Forest plot of overall subgroup analysis (involving cattle, goat, sheep, camel, and wildlife).
Table 1.
Meta-analysis of prevalence of tuberculosis in Nigerian cattle, 1979–2021.
| Study or subgroup | Prevalence | LCI 95% | HCI 95% | Weight (%) |
|---|---|---|---|---|
| High | ||||
| Saidu et al. (2015) | 0.150000000 | 0.126063546 | 0.175615945 | 1.569182576 |
| Ibrahim et al. (2012) | 0.010845987 | 0.005026953 | 0.018705958 | 1.586436209 |
| Damina et al. (2011) | 0.044365572 | 0.037673579 | 0.051575171 | 1.674274611 |
| Alonge and Fasanmi (1979)p | 0.673913043 | 0.530639892 | 0.80281624 | 0.672467747 |
| Alonge and Fasanmi (1979)b | 0.473684211 | 0.251182543 | 0.701388015 | 0.365463637 |
| Alonge and Fasanmi (1979)bo | 0.360824742 | 0.267820089 | 0.45931105 | 0.985082036 |
| Alonge and Fasanmi (1979)ka | 0.428571429 | 0.292484214 | 0.570200586 | 0.698137881 |
| Alonge and Fasanmi (1979)s | 0.514492754 | 0.430843671 | 0.59773999 | 1.126418417 |
| Alonge and Fasanmi (1979)n | 0.206060606 | 0.147525025 | 0.271417948 | 1.192814265 |
| Alonge and Fasanmi (1979)kw | 1.000000000 | 0.949867117 | 1.000000000 | 0.555288279 |
| Alonge and Fasanmi (1979)kd | 0.327868852 | 0.215021602 | 0.451466272 | 0.789257812 |
| Jajere et al. (2018a) | 0.020901639 | 0.015576467 | 0.026980973 | 1.660971821 |
| Ibrahim et al. (2018g) | 0.137266528 | 0.125855423 | 0.149090678 | 1.674192143 |
| Ibrahim et al. (2018) | 0.109640832 | 0.096672116 | 0.123318035 | 1.653743611 |
| Cadmus et al. (2008a) | 0.042589438 | 0.0276074 | 0.060563003 | 1.523751768 |
| Agbalaya et al. (2020) | 0.256684492 | 0.196434567 | 0.32191843 | 1.236688432 |
| Jajere et al. (2018b) | 0.011322005 | 0.010701382 | 0.011959908 | 1.708661502 |
| Ejeh et al. (2013) | 0.089256064 | 0.087213518 | 0.09131991 | 1.708135715 |
| Ahmad et al. (2017a) | 0.061246612 | 0.053731102 | 0.069218818 | 1.677191538 |
| Yohanna et al. (2008) | 0.145728643 | 0.099850193 | 0.198428622 | 1.257619873 |
| Abubakar et al. (2013) | 0.146779303 | 0.12493006 | 0.170060539 | 1.589461865 |
| Cadmus et al. (2010)nw | 0.029312289 | 0.019133779 | 0.041528594 | 1.581935333 |
| Cadmus et al. (2010)nc | 0.165829146 | 0.117140059 | 0.22095235 | 1.257619873 |
| Cadmus et al. (2010)sw | 0.069343066 | 0.041961819 | 0.102753368 | 1.355568714 |
| Cadmus and Arinola (2007) | 0.414893617 | 0.31688701 | 0.516358564 | 0.972002957 |
| Bikom et al. (2021) | 0.041036717 | 0.032451416 | 0.050573755 | 1.646054217 |
| Ibrahim et al. (2010) | 0.010845987 | 0.005026953 | 0.018705958 | 1.586436209 |
| Makeri et al. (2018) | 0.117333333 | 0.095234521 | 0.141390738 | 1.560632216 |
| Oluwasile et al. (2013) | 0.017765186 | 0.01664983 | 0.018916004 | 1.707445104 |
| Adesokan et al. (2019a) | 0.020833333 | 0.002595255 | 0.052154989 | 1.142606009 |
| Akinseye et al. (2018) | 0.021702838 | 0.015436718 | 0.028991231 | 1.644178873 |
| Okeke et al. (2014) | 0.214285714 | 0.155282533 | 0.27980161 | 1.199270296 |
| Atuman et al. (2018) | 0.1 | 0.06840082 | 0.136712848 | 1.380310857 |
| Ogugua et al. (2021) | 0.007142857 | 0.00087563 | 0.018033608 | 1.460634146 |
| Akinbobola et al. (2017) | 0.007240125 | 0.006739968 | 0.007758033 | 1.70864025 |
| Okoro et al. (2014) | 0.118 | 0.091104182 | 0.147827601 | 1.495467122 |
| Lawan et al. (2020a) | 0.093373494 | 0.072350386 | 0.116747222 | 1.543208916 |
| Ejeh et al. (2014b) | 0.080321285 | 0.04946795 | 0.117638232 | 1.328001084 |
| Ahmad et al. (2018) | 0.163716814 | 0.11813251 | 0.215004014 | 1.298556812 |
| Musawa et al. (2013) | 0.041237113 | 0.017076856 | 0.07448595 | 1.249127753 |
| Bala et al. (2011) | 0.015779562 | 0.01539736 | 0.016166373 | 1.709485809 |
| Adang et al. (2015) | 0.265625 | 0.218580077 | 0.315478688 | 1.397111184 |
| Adamu et al. (2021) | 0.007493568 | 0.007143216 | 0.007852241 | 1.709250492 |
| Ameen et al. (2008) | 0.005487667 | 0.00444958 | 0.006633223 | 1.70287958 |
| Tinau et al. (2020) | 0.09623431 | 0.061783354 | 0.137162566 | 1.315733958 |
| Aliyu et al. (2009) | 0.040498844 | 0.039799506 | 0.041204013 | 1.709384591 |
| Bikom and Oboegbulem (2007) | 0.013348165 | 0.011075127 | 0.01582914 | 1.696256385 |
| Opara (2005) | 0.03408456 | 0.029391739 | 0.039110704 | 1.687250338 |
| Chukwu et al. (2013) | 0.3 | 0.179922873 | 0.435293963 | 0.70641457 |
| Nwanta et al. (2011) | 0.014016678 | 0.01303643 | 0.015031908 | 1.707494595 |
| Cadmus et al. (2004) | 0.105263158 | 0.063232951 | 0.156138247 | 1.205567069 |
| Adah et al. (1992) | 0.028846154 | 0.003627206 | 0.071856397 | 1.013868173 |
| Ejeh et al. (2014c) | 0.080645161 | 0.04967124 | 0.118103691 | 1.326808848 |
| Igbokwe et al. (2001) | 0.028 | 0.027752396 | 0.028248672 | 1.70971743 |
| Ibrahim et al. (2016a) | 0.138530067 | 0.12454161 | 0.153136889 | 1.656863691 |
| Opara et al. (2012) | 0.034477945 | 0.030373896 | 0.038831596 | 1.692841382 |
| Oragwa et al. (2017) | 0.085877892 | 0.083721061 | 0.088059545 | 1.707878254 |
| Kachalla et al. (2016) | 0.172972973 | 0.121612174 | 0.231105515 | 1.233009989 |
| Kwaghe et al. (2015) | 0.064128257 | 0.054838455 | 0.074089008 | 1.662017688 |
| Alaku and Moruppa (1993) | 0.042300133 | 0.041659547 | 0.042945388 | 1.709463975 |
| High subgroup | 0.082851891 | 0.074417218 | 0.091681256 | 84.28223648 |
| Low | ||||
| Okeke et al. (2016) | 0.090866529 | 0.088393665 | 0.093370052 | 1.707400574 |
| Oyekunle and Talabi (2013) | 0.002641753 | 0.001890921 | 0.003515698 | 1.701924096 |
| Cadmus et al. (2006) | 0.1 | 0.058932062 | 0.150084025 | 1.203485474 |
| Ejeh et al. (2014a) | 0.01900931 | 0.017946164 | 0.020102425 | 1.70780279 |
| Saidu et al. (2017) | 0.007776814 | 0.007344914 | 0.008220948 | 1.708996546 |
| Adesokan et al. (2019b)o | 0.149825784 | 0.121745372 | 0.180247506 | 1.520009299 |
| Adesokan et al. (2019b)e | 0.017676768 | 0.006637162 | 0.033408797 | 1.447863191 |
| Adesokan et al. (2019b)s | 0.014950166 | 0.006563035 | 0.026437473 | 1.527890671 |
| Hena et al. (2012) | 0.047297297 | 0.031533871 | 0.065996286 | 1.525152173 |
| Danbirni et al. (2015) | 0.083985765 | 0.074006171 | 0.094533968 | 1.667238706 |
| Low subgroup | 0.042227515 | 0.017382312 | 0.071333427 | 15.71776352 |
| Pooled | 0.076712692 | 0.068847637 | 0.084965314 | 100 |
| Statistics | ||||
| I2 | 99.83202574 | 99.82419777 | 99.83950514 | |
| Cochrane's Q | 41077.7216 | |||
| χ2, p | 0 | |||
| tau2 | 0.013941354 | |||
3.4. Publication bias
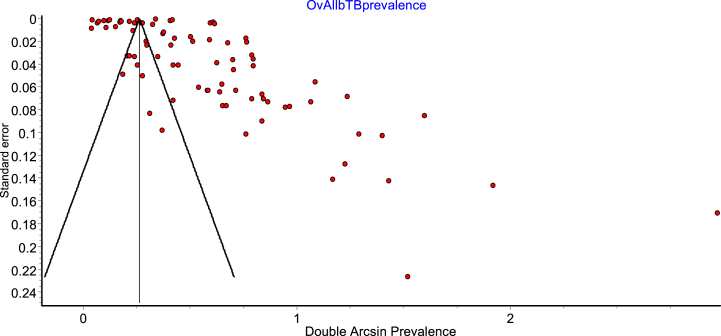
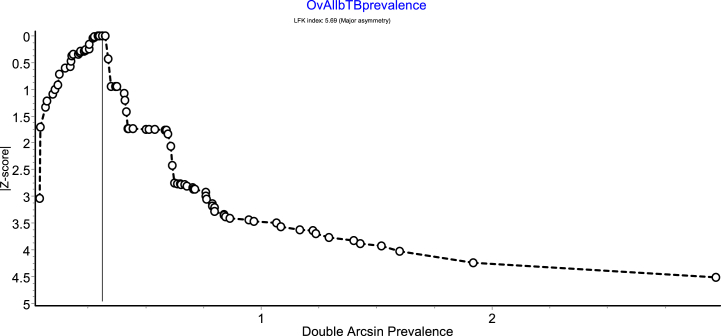
A lack of symmetry was seen in the funnel plot, which illustrates potential publication bias (Fig. 4). Thus, a Doi plot was done to ascertain the level of asymmetry. The LFK index of 5.69 (Fig. 5) detected from the Doi plot implies the existence of major asymmetry. Egger's regression test was also performed to measure the level of asymmetry and was not significant (p = 0.687).
Fig. 4.
Funnel plot of double arcsine prevalence against standard error showing observed asymmetry.
Fig. 5.
Doi plot of double arcsine prevalence against Z-score showing evidence of major asymmetry.
3.5. Subgroup and meta-regression analyses
Further subgroup analysis was done for the cattle population, the most reported animal population in the included studies. The subgroup analysis was used to investigate the detected high heterogeneity to determine the predictors (source(s) of high heterogeneity). The variables examined in the subgroup analysis were the study region (geographical zones), state of study, year of publication, detection methods, study quality, and sample size. The factors such as sampling season, age, and sex distribution were not used due to missing data. The subgroup analysis revealed the highest pooled prevalence of 15% (95% CI: 11.0–18.0) in the North-Central zone. The South-South zone recorded the lowest prevalence of 3% (95% CI: 3.0–4.0). Nigeria is divided into two regions comprising six geographical zones: northwest, northcentral and northeast, making up the northern part, while southwest, south-south, and southeast form the southern part. The state with the highest (significant) prevalence estimate is Kaduna (20%, 95% CI: 0–46.0), and Jigawa state had the lowest (1%, 95% CI: 1.0–2.0). The prevalence rates tend to be higher in northern States such as Niger (19.0%, 95% CI: 15.0–23.0), Kano (18.0%, 95% CI: 0–73.0), Nassarawa (15.0%, 95% CI: 10.0–20.0), and Sokoto (14.0%, 95% CI: 0–45.0) than areas in the south, which might be related with differences in the abundance of the animal population between the two regions. Based on sample size, the subgroup analysis showed that studies with a sample size below 100 had a significantly higher prevalence of 52% (95% CI: 34.0–70.0), followed by studies that sampled between 100 and 1000 animals (10.0%, 95% CI: 7.0–13.0), with the remaining sample size categories showing lower prevalence results. More so, the prevalence estimate in 2001 or before was 17% (95% CI: 15.0–20.0) and was higher than the other periods, with a particular increase in studies and publications starting in 2007, reaching a peak between 2017 and 2021. The high-quality studies had a higher pooled prevalence (8%, 95% CI: 7.0–9.0) than the low-quality studies. Regarding detection methods, the prevalence of animal TB estimated through ZN (15%, 95% CI: 0–35.0), lateral flow test (13.0%, 95% CI: 8.0–17.0), and SCITT (8.0%, 95% CI: 5.0–11.0) tallied significantly higher values than post-mortem examination (7.0%, 95% CI: 6.0–8.0) or culture isolation (7.0%, 95% CI: 2.0–13.0). The summary result of the subgroup analysis for each of the factors is detailed in Table 2. At the same time, the forest plots are presented in the S1 Figure.
Table 2.
Summary of subgroup analysis for cattle tuberculosis, Nigeria, 1979–2021.
| Subgroups | Number of studies | Pooled prevalence |
Heterogeneity |
||
|---|---|---|---|---|---|
| % | 95% CI | I2 | P | ||
| Region (geographical zone) of study | 70.0 | ||||
|
21.0 | 8.0 | 6.0–9.0 | 100% | <0.00001 |
|
12.0 | 10.0 | 6.0–15.0 | 98.0% | <0.00001 |
|
17.0 | 15.0 | 11.0–18.0 | 100% | <0.00001 |
|
6.0 | 3.0 | 1.0–5.0 | 98.0% | <0.00001 |
|
11.0 | 7.0 | 5.0–9.0 | 99.0% | <0.00001 |
|
3.0 | 3.0 | 3.0–4.0 | 98.0% | <0.00001 |
| State of study | 70.0 | ||||
|
4.0 | 4.0 | 3.0–5.0 | 99.0% | <0.00001 |
|
2.0 | 9.0 | 8.0–9.0 | 0.0% | 0.34 |
|
1.0 | 3.0 | 3.0–4.0 | – | – |
|
7.0 | 10.0 | 4.0–17.0 | 99.0% | <0.00001 |
|
3.0 | 5.0 | 1.0–12.0 | 96.0% | <0.00001 |
|
8.0 | 5.0 | 4.0–6.0 | 100% | <0.00001 |
|
2.0 | 3.0 | 0–6.0 | 98.0% | <0.00001 |
|
2.0 | 2.0 | 1.0–3.0 | 0.0% | 0.71 |
|
3.0 | 3.0 | 0–9.0 | 98.0% | <0.00001 |
|
4.0 | 12.0 | 0–27.0 | 100% | <0.00001 |
|
1.0 | 3.0 | 3.0–4.0 | – | – |
|
2.0 | 1.0 | 1.0–2.0 | 0.0% | <0.00001 |
|
2.0 | 20.0 | 0–46.0 | 94.0% | <0.00001 |
|
2.0 | 18.0 | 0–73.0 | 98.0% | <0.00001 |
|
1.0 | 99.2 | 99–100 | – | – |
|
1.0 | 26.0 | 20.0–32.0 | – | – |
|
1.0 | 15.0 | 10.0–20.0 | – | – |
|
2.0 | 19.0 | 15.0–23.0 | 0.0% | 0.33 |
|
3.0 | 4.0 | 1.0–7.0 | 100% | <0.00001 |
|
7.0 | 8.0 | 3.0–13.0 | 98.0% | <0.00001 |
|
6.0 | 11.0 | 9.0–13.0 | 98.0% | <0.00001 |
|
3.0 | 14.0 | 0–45.0 | 99.0% | <0.00001 |
|
3.0 | 8.0 | 4.0–13.0 | 93.0% | <0.00001 |
| Sample size | 70.0 | ||||
|
8.0 | 52.0 | 34.0–70.0 | 93.0% | <0.00001 |
|
33.0 | 10.0 | 7.0–13.0 | 97.0% | <0.00001 |
|
13.0 | 6.0 | 3.0–8.0 | 99.0% | <0.00001 |
|
2.0 | 0.4 | 0.14–1.0 | 94.0% | <0.00001 |
|
8.0 | 3.0 | 1.0–6.0 | 100% | <0.00001 |
|
6.0 | 2.0 | 1.0–3.0 | 100% | <0.00001 |
| Year of publication | 70.0 | ||||
|
11.0 | 17.0 | 15.0–20.0 | 100% | <0.00001 |
|
3.0 | 7.0 | 2.0–14.0 | 93.0% | <0.00001 |
|
13.0 | 4.0 | 3.0–6.0 | 100% | <0.00001 |
|
21.0 | 9.0 | 6.0–11.0 | 100% | <0.00001 |
|
22.0 | 6.0 | 4.0–7.0 | 100% | <0.00001 |
| Detection methods | 70.0 | ||||
|
41.0 | 7.0 | 6.0–8.0 | 100% | <0.00001 |
|
14.0 | 8.0 | 5.0–11.0 | 98.0% | <0.00001 |
|
5.0 | 7.0 | 2.0–13.0 | 98.0% | <0.00001 |
|
6.0 | 13.0 | 8.0–17.0 | 89.0% | <0.00001 |
|
4.0 | 15.0 | 0–35.0 | 99.0% | <0.00001 |
| Study Quality | 70.0 | ||||
|
60.0 | 8.0 | 7.0–9.0 | 100% | <0.00001 |
|
10.0 | 4.0 | 2.0–7.0 | 100% | <0.00001 |
PM: post-mortem examination; SCITT: single comaparative intradermal tuberculin test; CI: culture isolation; LF: lateral flow test; ZN: Ziehl – Neelsen staining.
A univariable meta-regression was conducted to determine the effect of the covariates as moderators of the cumulative prevalence. The factors used in the subgroup analysis were used as the moderators in the meta-regression. Hence, geographical location (northcentral was compared with others), state of study (Plateau state was compared with others), detection methods (PM technique was compared with others), and study quality (high quality was compared with low). The univariable analysis showed that all the covariates except study quality are significant predictors, with the highest value of R2 being observed for publication year at 46% of the detected heterogeneity. The multivariable meta-regression analysis of all the five significant variables revealed that the factors (in combination) accounted for 81% (significant) of the detected heterogeneity. The proportion (R2) of each of the moderators’ effects on heterogeneity with their corresponding p values is shown in Table 3.
Table 3.
Univariate and multivariate meta-regression for cattle tuberculosis, Nigeria, 1979–2021.
| Univariate analysis |
Multivariate analysis (combined all 5 factors) |
|||
|---|---|---|---|---|
| Covariates | R2 (%) | P value | R2(%) | P value |
| State of study | 5.50 | <0.001 | 81.0 | <0.001 |
| Region of study | 9.10 | <0.001 | ||
| Detection methods | 1.60 | <0.001 | ||
| Sample size | 2.30 | <0.001 | ||
| Year of publication | 46.00 | <0.001 | ||
| Study quality | 3.30 | 0.33 | ||
R2: explains the proportion of between study variance (the effect of covariates on heterogeneity).
3.6. bTB presentation and associated risk of exposure to infection
The seven included case reports in this review have shown that bTB infection affects different animal species, from cattle and camels to wildlife. Furthermore, the affected animals were drawn from different settings, including university farms, zoological gardens, dairy farms, and local markets. Thus, putting different groups of the human population at risk. The bTB presentations and associated risk factors for exposure are given in Table 4.
Table 4.
Presentation of bovine tuberculosis (bTB) in selected studies and associated risk of exposure to infection, 2008–2020.
| Study | Cases | Source | Age (Years) | Presentation |
Affected organs/regions | Tuberculous mycobacteria species detected | Transmission link | At-risk human groups | |
|---|---|---|---|---|---|---|---|---|---|
| Postmortem pathology | Clinical observations | ||||||||
| Ahmad et al., 2019b | 1 cow | Community | 2–3 | Pronounced emaciation | – | Thoracic cavity, abdominal cavity and pre-mammary lymph nodes | M. bovis | – | Veterinary staff, butchers and meat consumers |
| 1 bull camel | Community | 4–6 | Lungs, liver, lymph nodes, diaphragm, and intestines | Spillover | |||||
| Tijani et al., 2020 | 1 bull cattle (Sokoto Gudali) | University farm | 4 | Anorexia, Lethargy, Recumbency | Congested and moist mucous membranes, weak pulse, tachypnoea, and fever (39 °C). | Lungs, spleen, and lymph nodes | M. bovis | – | Farmers, veterinary staff, students, and other workers |
| Cadmus et al., 2008b | 6 cows | – | – | – | – | – | - | – | – |
| Adeogun et al., 2016 | 1 female gorilla | Zoological Garden | 47 | Marked emaciation | Pale ocular and oral mucous membranes | Lungs, liver, spleen, and the serosa. | M. tuberculosis | Human transmission (anthropozoonotic) | Zookeepers and visitors |
| 1 lioness | 15 | sunken eyes and pale mucous membranes | Trachea, lungs, lymph nodes, liver, spleen, and kidneys |
M. tuberculosis M. bovis |
Human transmission (anthropozoonotic) and contaminated raw meat | ||||
| Kalu et al., 2019 | 1 bull cattle (White Fulani) | – | Adult | – | – | Thoracic and abdominal cavities, and liver | - | – | – |
| Ibrahim et al., 2016b | 1 cow (Heifer) | Dairy farm | 2 | – | – | Lymph nodes | M. tuberculosis | Anthropozoonotic | Heifers, farm workers and consumers of unpasteurized milk |
| Ahmad et al., 2017b | 2 cows (White Fulani) | Local market | 6 | Granulomatous lungs and mammary glands | – | Mammary glands and lungs | - | – | Veterinary staff, consumers of meat and unpasteurized milk |
4. Discussion
Animal tuberculosis is a paradigmatic shared disease at the wildlife-livestock interface, and the condition has complex global epidemiological scenarios impacting the livestock industry and human health. This impact transcends the economic welfare of farmers and valuable wildlife resources such as game and endangered species [22,39]. The detection of bovine tuberculosis in Nigeria dates back to 1932, and it has since been an endemic disease that has significantly contributed to economic losses in the animal industry [40,41]. Furthermore, the results of bacteriological confirmation through culture and molecular typing have demonstrated M. bovis as the leading causative agent. However, in rare cases, Mycobacterium tuberculosis and M. africanum were equally implicated as responsible for bTB observed during post-mortem examinations of animals in the country [14,25,27,28]. On the other hand, an emerging M. tuberculosis Uganda-I strain has also been implicated in reverse zoonotic tuberculosis transmission from human pastoralists to cattle in Nigeria [42]. Given the substandard animal healthcare system, shortage of veterinarians in most slaughterhouses, unbounded movement of animals, and lack of regulations for testing and slaughtering animals suspected of having tuberculosis in Nigeria, a deeper understanding of the epidemiology of bTB is essential for the development of prevention and control strategies.
The current study revealed the overall prevalence of animal TB in Nigeria. The difference between the overall individual TB prevalence across species was pronounced, with camels and wildlife showing similar higher prevalence values of 13.0%. The small sample size, very few studies included in the model, and the confined geographic locations of those studies might have contributed to the higher TB prevalence rates in camels and wildlife in Nigeria. The bias in disease prevalence estimates can arise from different sample size studies, with small sample size studies overestimating TB prevalence while a higher sampling number shows more reliable prevalence rates [16]. The pooled individual TB prevalence (8.0%) in cattle in Nigeria is lower than the 20.4% reported in Morocco [43] and comparable to the 5.5% reported in Ethiopia [5]. The switch to more intensive cattle farming systems may explain the high prevalence of bTB in Morocco [43]. Intensive farming practices have been established to contribute to bTB in animals [44]. Mycobacteria may be transmitted more easily between animals in intensive farming conditions housed in confined spaces with limited sunlight, airflow, high humidity, and increased stocking densities [43]. On the other hand, compared to intensive farming, free-range production can lower the risk of tubercle bacilli infection due to the relatively high levels of sunlight outside and heat effect on mycobacteria environmental contaminants, lower farming density on communal pastures, and better air circulation [45]. The most significant percentage of the farm animals in Nigeria are raised mainly in the northern region. In this region, an extensive traditional livestock system (referring to pastoral and mixed livestock production) is practised. Where cattle, goats, sheep, and camels are herded together, often sharing a common pasture and watering points. This animal husbandry system can be an essential risk factor for animal-to-animal and inter-species M. bovis transmission. Given the transhumance and trade of animals, particularly from the northern part down south, in search of water and pasture during the dry season, this has consequences for the epidemiology and control of bTB in Nigeria. The movement dynamics could represent a common source of interregional transmission for MTBC strains [42]. M. bovis strains regularly isolated from cattle were also found in camels, goats, and pigs, implying possible transmission from cattle reservoirs to other animals in Nigeria [25,27,28]. Similar strains in several host species highlight the interaction of more susceptible hosts and the circulation of M. bovis strains. The interaction of multiple animal species in one environment, as exhibited in the extensive rearing system, might therefore be considered a factor favoring this interspecies transmission of M. bovis in Nigeria [25]. Previous studies suggest that small ruminants (e.g., goats and sheep) are mainly spillover hosts that cannot maintain the infection in a herd unless they are kept near cattle with high bTB prevalence [46,47], or raised under a scale farming system [[48], [49], [50]]. Camels frequently exposed to cattle were likewise observed to have increased TB lesions in the abattoir and a higher chance of acquiring M. bovis infection than camels not in contact with cattle [45,51,52]. M. bovis can infect wildlife hosts, which can subsequently serve as reservoirs for infection in livestock [53]. The precise transmission and dissemination mechanisms of M. bovis to wild animals remain unknown. Wild animals, however, may acquire infection directly through close contact with animal scavengers, indirectly through environmental contamination, or by consuming contaminated products [54]. MTBC strains can be spread between cattle and other reservoir species capable of sustaining infection transmission without prominent cattle involvement in the epidemiology, corroborating the concept that bTB should be addressed as a multi-host disease necessitating comprehensive control methods [55]. Furthermore, due to recurrent close contact at water points and resting areas where animals congregate and uncontrolled livestock movement, inter-species herd mixing has been proposed as a potential risk factor for the transmission of bTB within extensively mixed management systems [45]. A control strategy should be encouraged to prioritise routine animal screening and separate herding to prevent inter-species mixing in grazing areas or water sources. Other measures, such as restricting animal movements from crisscrossing the country during transhumance and housing different animal species (camels, goats, and sheep) separately from cattle, might help to lessen the burden of bTB in Nigeria.
The burden of animal TB was significantly concentrated in the North-Central than in other geographical zones of the country, followed by North-West, North-East, South-West, and the South-East, with the lowest prevalence recorded in the South-South. The correlation analysis shows that location explains 9.10% of the heterogeneity of the occurrence of animal tuberculosis in Nigeria. Nigeria is a tropical country with two main dry and wet seasons experienced in the northern and southern areas [56]. In the northern region, the dry season is characterized by low humidity and high temperatures (up to 45 °C) because of the warm winds from the Sahara Desert. In contrast, the rainy season is dominated by high humidity and low temperatures [56,57]. The temperature ranges from 17 °C to 24 °C during the dry cold season in the south and is as low as 12 °C in the north [57]. The climate of the northcentral geographical zone is generally monsoon [57], consisting of temperate-dry (in Nassarawa, Niger, and Federal capital territory), temperate-dry with cool climate (in Plateau), and temperate-humid (in Benue, Kwara, and Kogi) [58]. This zone is hot and wet in the rainy summer and cold and humid in the dry winter. Previous studies have speculated that warm and humid weather, characteristics of the monsoon climate in northcentral Nigeria, may exacerbate the prevalence of bTB [43,44,59,60]. It is thus recommended to strengthen preventive measures in moist and warm areas, aiming to reduce the incidence of bTB in Nigeria. Among states of Nigeria, Kaduna had the highest prevalence, while Jigawa had the lowest prevalence estimate. This may be associated with the fact that Kaduna is a major metropolis in northern Nigeria where cattle are pulled from parts of northern Nigeria, including but not limited to Jigawa state. Many of such animals are moved without prior ante-mortem inspection. Anecdotal evidence suggests that farmers tend to remove their sick animals for sale first. Animals are widely kept across Nigeria, with the highest densities registered in the northern region due to the predominant practice of livestock production compared to the southern part. Thus, this could explain the high prevalence of infection being more concentrated in areas in the northern region. However, the differences in the disease prevalence estimates between North and South might be influenced by the individuals' survey efforts, as most of the studies on bTB in the country were conducted mainly for academic research purposes, not to generate national data. Furthermore, although 23 States were featured in this study, the majority only had one or two reported studies, which could lead to skewed findings. Therefore, this analysis emphasizes the need for the Federal and State governments to fund surveillance efforts in all animals to demonstrate the diversity in the bTB prevalence throughout Nigeria.
The subgroup analysis showed a considerably higher annual prevalence of infection in 2001 or years prior to 2001 compared to other periods between 2002 and 2021. In addition, the correlation analysis shows that publication year accounted for 46.0% of the detected heterogeneity, implying that it has a significant influence on each subgroup. However, the substantial increase in the volume of studies and publications since 2001 could be attributed to the growing awareness and interest in animal TB research, enhanced by international capacitation programs for scientific and technological advancement in developing countries. Preliminary data on bTB based on postmortem examination in abattoirs gathered from the 1930s through 1958 in northern Nigeria and between 1937 and 1947 in the southern region revealed prevalence rates of between 0.02% and 15.9%.40 Even though there were a few investigations employing mycobacterial culture and molecular characterizations, studies conducted in the last 20 years have improved the diagnosis of TB infection in animals in the country. The prevalence has been low since 2002 (4%–9%). There could be a correlation between the variable decline of bTB prevalence and increased laboratory confirmation of infection through individual and targeted group projects. The Veterinary Laboratories Agency in the United Kingdom, for instance, assisted in funding research initiatives that provided baseline data on the strains of MTBC circulating among animals in Nigeria [61]. In underdeveloped countries where resources for diagnosis and molecular typing are limited, the development of control strategies is severely constrained by the lack of facilities and expertise to accurately determine disease prevalence, resulting in a poor understanding of the local epidemiology of infection [62]. This can culminate in disorderly activities and eventual campaign failures before the crucial data is available to guide the formulation of control plans [63]. Furthermore, due to logistical, political, and financial limitations, the test-and-slaughter policy which is the cornerstone of national bTB control programs in industrialized nations, is not yet viable in many developing countries, including Nigeria [61]. Therefore, the main priority in preventing the widespread occurrence of the bTB in Africa should be the evaluation and application of practicable, technically feasible, and economically viable alternative methods under these conditions [64].
The studies included in this analysis employed different diagnostic techniques. Thus, most analyzed publications used only PM or its combination as an initial step in the diagnostic workflow, followed by SCITT, LF, CI, and ZN. The heterogeneity explained by the detection method from covariate analysis was only 1.60%, which implied a minor influence on the prevalence. In order to detect the infection in routinely slaughtered animals or tuberculin test reactors, the postmortem examination is a critical component of bTB control programs in endemic areas [65]. In countries with high to low prevalences of infection, as well as in nations that are formally infection-free, where meat inspection is a prerequisite for establishing and maintaining the official TB-free (OTB) status, the detection of bTB during meat inspection is still significant for the surveillance and control of this disease in animals and herds [8]. Instead of histopathology, Ziehl-Neelsen staining is utilized to evaluate sampled animal products or lesions obtained postmortem in low-resource settings to diagnose tuberculosis. Even though there are risk factors for zoonotic transmission of Mycobacterium tuberculosis complex (MTC) species in Nigeria, TB diagnosis relies mainly on smear microscopy and relatively moderate cultural isolation [42]. Tuberculosis infection is identified and definitively confirmed by mycobacterial culture. Still, this procedure mainly depends on the growth rate of MTBC, which could take up to three months and delay rapid diagnosis [62]. It may not be practicable to monitor TB cases in animals in Nigeria utilizing mycobacterial culture because the method is time-consuming and occasionally risky in laboratories with inadequate facilities. Currently, molecular studies have provided evidence for the widespread distribution of MTBC strains among animal populations throughout Nigeria [14,[23], [24], [25], [26], [27], [28]]. The dataset generated from the numerous molecular studies of bTB in Nigeria is vital for developing the strategic control measures needed to lessen the burden of the disease in the country [26,61]. Furthermore, during M. bovis infection, the cell-mediated immune response is crucial, and in-vivo tuberculin skin tests, such as SIT and SCITT, as well as in-vitro interferon-gamma (IFN-γ) assay, are used to detect this response in animals [66]. Conversely, animals with advanced stages of infection or that tested negative for tuberculin can be identified using humoral marker detection assays like ELISA (lateral flow). Therefore, the results of cellular response tests can be supplemented with humoral marker detection assays [67]. Consequently, antemortem immunological diagnostic tests are essential for bTB epidemiological surveys and test and cull control programs [12]. The IFN-γ assay, in contrast to tuberculin skin tests, can be utilized in the field without needing a follow-up visit, making it potentially advantageous in epidemiological surveys in low-and middle-income countries (LMICs) [12], such as Nigeria. However, when using IFN-γ assay in remote field situations, laboratory equipment to incubate and harvest the plasma within less than 8 h should not be undervalued [12]. While numerous test options are readily available to determine M. bovis infection at the herd level, diagnosing bTB is frequently challenging due to the paucity of diagnostic methods that meet all the requirements for identifying diseased animals [68]. Identifying the origin and transmission of the disease and developing programs to eradicate tuberculosis from cattle and other domestic and wild animals depend on the accurate diagnosis of MTBC infection and adequate differentiation between isolates [62]. Given the prevalence of the disease in all locations, a sensitive and scalable detection method (e.g., IFN-γ assay) should be adopted for comprehensive testing across Nigeria to isolate and remove infected animals to maximize outbreak control, thereby preventing continuous transmission. Relative to tuberculin skin tests, IFN-γ has shown to be more sensitive in the field while maintaining comparable or lower specificity [69]. Accordingly, the IFN-γ assay was found to have a higher sensitivity (median: 67%; CI: 49–82%) and slightly lower specificity (median: 98%; CI: 96–99%) than the SCITT [70]. In an environment with no control measures to minimize false-negative results, a test with high sensitivity would be advantageous in determining the prevalence of a disease with zoonotic implications [12,71,72].
Anecdotal case reports of tuberculosis have been recorded in livestock and captive wildlife across Nigeria. Outbreaks of infection caused by M. tuberculosis in a heifer [73] and M. bovis in a heifer and a bull-camel [74] have been documented in different parts of the country. Camel TB is mainly the result of spill-over exposure, facilitated by close and repeated contact between camels and infected cattle in extensively mixed transhumance commonly practised in Nigeria [74]. Although extensive surveillance can frequently identify many risk factors related to bTB exposure, the maintenance and transmission of infection between cattle and other domestic animal species are poorly understood [62]. In addition, tuberculosis caused by M. tuberculosis and M. bovis has been identified in a gorilla and a lioness kept in a private zoo [23]. Animal attendants with active tuberculosis constitute the primary source of M. tuberculosis infection in animals following contamination of the environment with cough aerosol, sputum, urine, or feces [75,76]. Thus, an account of M. tuberculosis infection transmission between a farm worker and a cow has been confirmed in Nigeria [73]. More so, the most likely source of M. bovis infection to captive wildlife could be contaminated raw meat since TB has been confirmed in slaughtered livestock in Nigeria [24,25,28]. The zoo animals are at risk of exposure to M. bovis infection since the meat fed to them is not subjected to prior postmortem inspection [23]. Consequently, the risk of exposure and zoonotic transmission increases due to the endemicity of bTB in animals, and as a result, cases of human infection have been confirmed in Nigeria [13,15,25]. Therefore, there is a need for a national policy mandating multidisciplinary research efforts to improve understanding of the ecology of MTBC infection across the geographical zones. Implementing this policy will assist in determining the dynamics of the disease, understanding the role of different animal species in environmental contamination, and developing sustainable bTB prevention and control strategies in Nigeria. Sharing resources and strengthening interactions between public health and veterinary medical scientists can increase awareness of the ‘shared risk' of bTB between humans and animals and, in resource-constrained settings, such as Nigeria, can enhance the use of existing facilities and lessen duplication of effort in disease control programs [77]. In addition, urgent initiatives are required to implement and enforce strict regulations, including a coordinated approach to regulate milk pasteurization enforcement, detailed routine meat inspection, and routine TB screening of animal workers to prevent MTBC transmission at the human-animal interface [42]. Protecting livestock, wildlife, and humans demands immediate political acceptance of vaccination as the key to affordable and effective tuberculosis control in Africa, particularly where the prevalence of M tuberculosis infection in animals is evident, and the test-and-slaughter schemes used in Europe are not economically feasible [78,79].
5. Conclusions
This study has shown that tuberculosis is widespread among different animal species and across the geographical locations of Nigeria. The overall multivariable heterogeneity (81.0%) demonstrated that the infection moderators (publication year, geographical location, sample size, and detection methods) included in the meta-regression model account for most of the observed variance in this study. However, a few other variables not taken into account in the models, such as age, sex, sampling season, animal movements, animal-human interactions, and livestock-wildlife interactions, which are infrequently documented in the research conducted in Nigeria and hence unavailable for rigorous meta-analyses, may explain the remaining variability. Therefore, based on the analyzed moderators contributing to the prevalence of the disease, prevention and control policies that suit the local situations need to be adopted in the country. Importantly, regulate random animal movement along with a strict restriction on inter-species contacts, establish regular surveillance by the use of INF-γ test together with the phasing-out of infected animals, create a sustainable compensation plan for farmers, mandate postmortem examination with tracing back in all slaughterhouses, and pasteurization of milk and dairy products nationwide. Giving adequate consideration to vaccination of livestock as a strategic prevention and control programme against tuberculosis, contextualised to the Africa setting will be beneficial [78,79]. For example, though the use of BCG vaccination against TB in cattle is not permitted generally by European Union legislation, due to potential to induce cellular immune response thereby leading to diagnostic interference and complicating the eradication programmes, UK and Spain have now considered vaccination against M tuberculosis infection in animals and the wildlife [[80], [81], [82]]. Furthermore, standardized record-taking is recommended when reporting occurrences of animal tuberculosis to generate sufficient data that can guide a robust investigation.
Author contributions
Conceived and designed the experiments (Ibrahim Ahmad); Performed the experiments (Ibrahim Ahmad, Yakubu Egigogo Raji); Analyzed and interpreted the data (Ibrahim Ahmad, Yakubu Egigogo Raji, Abdullahi Samaila, Folorunso O. Fasina and Basiru Aliyu); Contributed reagents, materials, analysis tools or data (Ibrahim Ahmad, Yakubu Egigogo Raji and Folorunso O. Fasina); Wrote the paper (all authors).
Data availability statement
Data used to support the findings of this study are included within the supplementary information file(s).
Funding statement
None.
Declaration of competing interest
None of the authors has any interest that should prevent the publication of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17215.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gortázar C., Fernández-Calle L.M., Collazos-Martínez J.A., Mínguez-González O., Acevedo P. Animal tuberculosis maintenance at low abundance of suitable wildlife reservoir hosts: a case study in northern Spain. Prev. Vet. Med. 2017;146:150–157. doi: 10.1016/j.prevetmed.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Stański K., Lycett S., Porphyre T., Bronsvoort B.M., de C. Using machine learning improves predictions of herd-level bovine tuberculosis breakdowns in Great Britain. Sci. Rep. 2021;11(2208) doi: 10.1038/s41598-021-81716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gormley E., Corner L.A.L. Wild animal tuberculosis: stakeholder value systems and management of disease. Front Vet Sci.. 5. 2018 doi: 10.3389/fvets.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inlamea O.F., Soares P., Ikuta C.Y., Heinemann M.B., Achá S.J., Machado A., Ferreira Neto J.S., Correia-Neves M., Rito T. Evolutionary analysis of Mycobacterium bovis genotypes across Africa suggests co-evolution with livestock and humans. PLoS Negl Trop Dis. 14, e0008081. 2020. [DOI] [PMC free article] [PubMed]
- 5.Dejene S.W., Heitkönig I.M.A., Prins H.H.T., Lemma F.A., Mekonnen D.A., Alemu Z.E., Kelkay T.Z., de Boer W.F. Risk Factors for Bovine Tuberculosis (bTB) in Cattle in Ethiopia. PLoS One. 11, e0159083. 2016. [DOI] [PMC free article] [PubMed]
- 6.Macedo Couto R., Ranzani O.T., Waldman E.A. Zoonotic tuberculosis in humans: control, surveillance, and the one health approach. Epidemiol. Rev. 2019;41:130–144. doi: 10.1093/epirev/mxz002. [DOI] [PubMed] [Google Scholar]
- 7.Menzies F.D., Neill S.D. Cattle-to-Cattle transmission of bovine tuberculosis. Vet. J. 2000;160:92–106. doi: 10.1053/tvjl.2000.0482. [DOI] [PubMed] [Google Scholar]
- 8.Domingo M., Vidal E., Marco A. Pathology of bovine tuberculosis. Res. Vet. Sci. 2014;97:S20–S29. doi: 10.1016/j.rvsc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Buddle B.M., Vordermeier H.M., Chambers M.A., de Klerk-Lorist L.-M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Front. Vet. Sci. 2018;5(259) doi: 10.3389/fvets.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good M., Bakker D., Duignan A., Collins D.M. The history of in vivo tuberculin testing in bovines: tuberculosis, a “one health” issue. Front. Vet. Sci. 2018;5(59) doi: 10.3389/fvets.2018.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sichewo P.R., Vander Kelen C., Thys S., Michel A.L. Risk practices for bovine tuberculosis transmission to cattle and livestock farming communities living at wildlife-livestock-human interface in northern KwaZulu Natal, South Africa. PLoS Negl Trop Dis. 14, e0007618. 2020. [DOI] [PMC free article] [PubMed]
- 12.Kelly R.F., Gonzaléz Gordon L., Egbe N.F., Freeman E.J., Mazeri S., Ngwa V.N., Tanya V., Sander M., Ndip L., Muwonge A., Morgan K.L., Handel I.G., Bronsvoort B.M.D.C. Bovine tuberculosis epidemiology in Cameroon, central Africa, based on the interferon-gamma assay. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.877541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adesokan H.K., Jenkins A.O., van Soolingen D., Cadmus S.I.B. Mycobacterium bovis infection in livestock workers in Ibadan, Nigeria: evidence of occupational exposure. The International Journal of Tuberculosis and Lung Disease. 2012;16:1388–1392. doi: 10.5588/ijtld.12.0109. [DOI] [PubMed] [Google Scholar]
- 14.Cadmus S., Palmer S., Okker M., Dale J., Gover K., Smith N., Jahans K., Hewinson R.G., Gordon S. v. Molecular analysis of human and bovine tubercle bacilli from a local setting in Nigeria. J. Clin. Microbiol. 2006;44:29–34. doi: 10.1128/JCM.44.1.29-34.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danbirni S., Okaiyeto S.O., Joshua I.A., Sackey K.B., Anthony K.C., Abdulkadir I.A. Prevalence of tuberculosis in a herd of cattle of a tuberculosis herdman following trace back Information from a Hospital in Taraba State, Nigeria. J. Anim. Prod. Adv. 2012;2:325–328. [Google Scholar]
- 16.Ramos B., Pereira A.C., Reis A.C., Cunha M.V. Estimates of the global and continental burden of animal tuberculosis in key livestock species worldwide: a meta-analysis study. One Health. 2020;10 doi: 10.1016/j.onehlt.2020.100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden J.M., McGrath G., Sweeney J., Murray G., Tratalos J.A., More S.J. Spatio-temporal models of bovine tuberculosis in the Irish cattle population, 2012-2019. Spat Spatiotemporal Epidemiol. 2021;39 doi: 10.1016/j.sste.2021.100441. [DOI] [PubMed] [Google Scholar]
- 18.Azami H.Y., Zinsstag J. In: Bovine Tuberculosis. Chambers M., Gordon S., Olea-Popelka F., Barrow P., editors. vol. 3. 2018. Economics of bovine tuberculosis: a One Health issue; pp. 31–42. [Google Scholar]
- 19.Caminiti A. Panorama 2019-1: the socio-economic costs of bovine tuberculosis. Bulletin de l’OIE. 2019;2019:1–2. doi: 10.20506/bull.2019.1.2916. [DOI] [Google Scholar]
- 20.Romero M.P., Chang Y.-M., Brunton L.A., Prosser A., Upton P., Rees E., Tearne O., Arnold M., Stevens K., Drewe J.A. A comparison of the value of two machine learning predictive models to support bovine tuberculosis disease control in England. Prev. Vet. Med. 2021;188(105264) doi: 10.1016/j.prevetmed.2021.105264. [DOI] [PubMed] [Google Scholar]
- 21.Picasso-Risso C., Alvarez J., VanderWaal K., Kinsley A., Gil A., Wells S.J., Perez A. Modelling the effect of test-and-slaughter strategies to control bovine tuberculosis in endemic high prevalence herds. Transbound Emerg Dis. 2021;68:1205–1215. doi: 10.1111/tbed.13774. [DOI] [PubMed] [Google Scholar]
- 22.Gortazar C., Diez-Delgado I., Barasona J.A., Vicente J., de La Fuente J., Boadella M. The wild side of disease control at the wildlife-livestock-human interface: a review. Front Vet Sci.. 1. 2015 doi: 10.3389/fvets.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adeogun A., Omobowale O., Owuamanam C., Alaka O., Taiwo V., van Soolingen D., Cadmus S. Mycobacterium tuberculosis and dual M. tuberculosis/M. bovis infection as the cause of tuberculosis in a Gorilla and a lioness, respectively, in Ibadan zoo, Nigeria. Case Rep Vet Med. 2016. 2016:1–4. doi: 10.1155/2016/8568237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cadmus S.I.B., Gordon S.V., Hewinson R.G., Smith N.H. Exploring the use of molecular epidemiology to track bovine tuberculosis in Nigeria: an overview from 2002 to 2004. Vet. Microbiol. 2011;151:133–138. doi: 10.1016/j.vetmic.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins A.O., Cadmus S.I.B., Venter E.H., Pourcel C., Hauk Y., Vergnaud G., Godfroid J. Molecular epidemiology of human and animal tuberculosis in Ibadan, Southwestern Nigeria. Vet. Microbiol. 2011;151:139–147. doi: 10.1016/j.vetmic.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Adesokan H.K., Streicher E.M., van Helden P.D., Warren R.M., Cadmus S.I.B. Genetic diversity of Mycobacterium tuberculosis complex strains isolated from livestock workers and cattle in Nigeria. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawan F.A., Ejeh F.E., Kwanashie C., Kadima K. Molecular characterization of Mycobacterium bovis isolated from camels slaughtered for human consumption in Northeastern Nigeria and the public health implication. PAMJ - One Health. 2. 2020 doi: 10.11604/pamj-oh.2020.2.4.21916. [DOI] [Google Scholar]
- 28.Cadmus S.I., Adesokan H.K., Jenkins A.O., van Soolingen D. Mycobacterium bovis and M. tuberculosis in goats, Nigeria. Emerg. Infect. Dis. 2009;15(12):2066–2067. doi: 10.3201/eid1512.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Macedo Couto R., Santana G.O., Ranzani O.T., Waldman E.A. One Health and surveillance of zoonotic tuberculosis in selected low-income, middle-income and high-income countries: a systematic review. PLoS Negl Trop Dis. 16, e0010428. 2022. [DOI] [PMC free article] [PubMed]
- 30.Reis A.C., Ramos B., Pereira A.C., Cunha M. v. Global trends of epidemiological research in livestock tuberculosis for the last four decades. Transbound Emerg Dis. 2021;68:333–346. doi: 10.1111/tbed.13763. [DOI] [PubMed] [Google Scholar]
- 31.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 2021;10(89) doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. g7647. [DOI] [PubMed] [Google Scholar]
- 33.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int. J. Health Pol. Manag. 2014;3:123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barendregt J.J., Doi S.A. 2016. MetaXL User Guide. Version 4. 2011–2016. [Google Scholar]
- 36.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J. Epidemiol. Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 37.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 38.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. 2019 doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martínez-Guijosa J., Lima-Barbero J.F., Acevedo P., Cano-Terriza D., Jiménez-Ruiz S., Barasona J.Á., Boadella M., García-Bocanegra I., Gortázar C., Vicente J. Description and implementation of an on-farm wildlife risk mitigation protocol at the wildlife-livestock interface: tuberculosis in mediterranean environments. Prev. Vet. Med. 2021;191 doi: 10.1016/j.prevetmed.2021.105346. [DOI] [PubMed] [Google Scholar]
- 40.Alhaji I. Bovine tuberculosis: a general review with special reference to Nigeria. Veterinary Bulletin. Weybridge. 1976;46(11):829–841. [Google Scholar]
- 41.Alonge D.O., Ayanwale F.O. Economic importance of bovine tuberculosis in Nigeria. J. Anim. Prod. Res. 1984;4(2):165–170. [Google Scholar]
- 42.Adesokan H.K., Akinseye V.O., Streicher E.M., van Helden P., Warren R.M., Cadmus S.I. Reverse zoonotic tuberculosis transmission from an emerging Uganda I strain between pastoralists and cattle in South-Eastern Nigeria. BMC Vet Res.. 15(1) 2019;437 doi: 10.1186/s12917-019-2185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yahyaoui Azami H., Ducrotoy M.J., Bouslikhane M., Hattendorf J., Thrusfield M., Conde- Álvarez R., Moriyón I., Zúñiga-Ripa A., Muñoz Álvaro P.M., Mick V., Bryssinckx W., Welburn S.C., Zinsstag J. The prevalence of brucellosis and bovine tuberculosis in ruminants in Sidi Kacem Province, Morocco. PLoS One. 13(9) 2018 doi: 10.1371/journal.pone.0203360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghebremariam M.K., Michel A.L., Nielen M., Vernooij J.C.M., Rutten V.P.M.G. Farm-level risk factors associated with bovine tuberculosis in the dairy sector in Eritrea. Transbound Emerg Dis. 2017;65(1):105–113. doi: 10.1111/tbed.12622. [DOI] [PubMed] [Google Scholar]
- 45.Ghebremariam M.K., Michel A.L., Vernooij J.C.M., Nielen M., Rutten V.P.M.G. Prevalence of bovine tuberculosis in cattle, goats, and camels of traditional livestock raising communities in Eritrea. BMC Vet Res.. 14(1) 2018;73 doi: 10.1186/s12917-018-1397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malone F.E., Wilson E.C., Pollock J.M., Skuce R.A. Investigations into an outbreak of tuberculosis in a flock of sheep in contact with tuberculous cattle. J. Vet. Med. Ser. B. 2003;50(10):500–504. doi: 10.1046/j.1439-0450.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 47.Tschopp R., Bobosha K., Aseffa A., Schelling E., Habtamu M., Iwnetu R., Hailu E., Firdessa R., Hussein J., Young D., Zinsstag J. Bovine tuberculosis at a cattle-small ruminant-human interface in Meskan, Gurage region, Central Ethiopia. BMC Infect Dis. 11(1) 2011;318 doi: 10.1186/1471-2334-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharpe A.E., Brady C.P., Johnson A., Byrne W., Kenny K., Costello E. Concurrent outbreak of tuberculosis and caseous lymphadenitis in a goat herd. Vet. Rec. 2010;166(19):591–592. doi: 10.1136/vr.b4825. [DOI] [PubMed] [Google Scholar]
- 49.Bezos J., Álvarez J., Romero B., Aranaz A., de Juan L. Tuberculosis in goats: assessment of current in vivo cell-mediated and antibody-based diagnostic assays. Vet. J. 2012;191(2):161–165. doi: 10.1016/j.tvjl.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Napp S., Allepuz A., Mercader I., Nofrarías M., López-Soria S., Domingo M., Romero B., Bezos J., Pérez de Val B. Evidence of goats acting as domestic reservoirs of bovine tuberculosis. Vet. Rec. 2013;172(25):663. doi: 10.1136/vr.101347. 663vr.101347, 10.1136/ [DOI] [PubMed] [Google Scholar]
- 51.Kinne J., Johnson B., Jahans K.L., Smith N.H., Ul-Haq A., Wernery U. Camel tuberculosis–a case report. Trop. Anim. Health Prod. 2006;38(3):207–213. doi: 10.1007/s11250-006-4366-8. [DOI] [PubMed] [Google Scholar]
- 52.Wernery U., Kinne J., Jahans K., Vordermeier H., Esfandiari J., Greenwald U., Johnson B., Ulhaq A., Lyashchenko K. Tuberculosis outbreak in a dromedary racing herd and rapid serological detection of infected camels. Vet. Microbiol. 2007;122(1–2):108–115. doi: 10.1016/j.vetmic.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Blanco Vázquez C., Barral T.D., Romero B., Queipo M., Merediz I., Quirós P., Armenteros J.Á., Juste R., Domínguez L., Domínguez M., Casais R., Balseiro A. Spatial and temporal distribution of Mycobacterium tuberculosis complex infection in eurasian badger (Meles meles) and cattle in Asturias, Spain. Animals. 11(5) 2021;1294 doi: 10.3390/ani11051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hussain R., Jamal A., Ahmed Z., Mohamed B.B., Siddique A.B., Khan I., Mansoor M.K., Du X., Khan A. Pathological, histological, and molecular based investigations confirm novel Mycobacterium bovis infection in Boselaphus tragocamelus. BioMed Res. Int. 2022;2022:1–9. doi: 10.1155/2022/7601463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Ciaravino G., Vidal E., Cortey M., Martín M., Sanz A., Mercader I., Perea C., Robbe-Austerman S., Allepuz A., Pérez de Val B. Phylogenetic relationships investigation of Mycobacterium caprae strains from sympatric wild boar and goats based on whole genome sequencing. Transbound Emerg Dis. 2021;68(3):1476–1486. doi: 10.1111/tbed.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogunrinde A.T., Oguntunde P.G., Akinwumiju A.S., Fasinmirin J.T. Analysis of recent changes in rainfall and drought indices in Nigeria, 1981–2015. Hydrol. Sci. J. 2021;64(14):1755–1768. doi: 10.1080/02626667.2019.1673396. [DOI] [Google Scholar]
- 57.Shiru M.S., Shahid S., Chung E.-S., Alias N. Changing characteristics of meteorological droughts in Nigeria during 1901–2010. Atmos. Res. 2019;223:60–73. doi: 10.1016/j.atmosres.2019.03.010. [DOI] [Google Scholar]
- 58.Dorcas Mobolade T., Pourvahidi P. Bioclimatic approach for climate classification of Nigeria. Sustainability. 2020;12(10):4192. doi: 10.3390/su12104192. [DOI] [Google Scholar]
- 59.Kemal J., Sibhat B., Abraham A., Terefe Y., Tulu K.T., Welay K., Getahun N. Bovine tuberculosis in eastern Ethiopia: prevalence, risk factors and its public health importance. BMC Infect. Dis. 2019;19(1):39. doi: 10.1186/s12879-018-3628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gong Q.-L., Chen Y., Tian T., Wen X., Li D., Song Y.-H., Wang Q., Du R., Zhang X.-X. Prevalence of bovine tuberculosis in dairy cattle in China during 2010–2019: a systematic review and meta-analysis. PLoS Negl Trop Dis. 15(6), e0009502. 2021. [DOI] [PMC free article] [PubMed]
- 61.Cadmus S.I., Ayanwale F.O. In: Zoonotic Tuberculosis: Mycobacterium Bovis and Other Pathogenic Mycobacteria. Thoen C.O., Steele J.H., Kaneene J.B., editors. John Wiley & Sons, Inc.; 2014. Bovine tuberculosis: epidemiology, zoonotic transmission, activities, and challenges toward its control in Nigeria; pp. 149–158. [Google Scholar]
- 62.Gormley E., Corner L.A.L., Costello E., Rodriguez-Campos S. Bacteriological diagnosis and molecular strain typing of Mycobacterium bovis and Mycobacterium caprae. Res. Vet. Sci. 2014;97:S30–S43. doi: 10.1016/j.rvsc.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Marcotty T., Matthys F., Godfroid J., Rigouts L., Ameni G., Gey van Pittius N., Kazwala R., Muma J., van Helden P., Walravens K., de Klerk, M L., Geoghegan C., Mbotha D., Otte M., Amenu K., Abu Samra N., Botha C., Ekron M., Jenkins A., van den Bossche P. Zoonotic tuberculosis and brucellosis in Africa: neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Ann. Trop. Med. Parasitol. 2009;103(5):401–411. doi: 10.1179/136485909X451771. [DOI] [PubMed] [Google Scholar]
- 64.Awah-Ndukum J., Egbe N.F., Ngu-Ngwa V. In: Tuberculosis in Animals: an African Perspective. Dibaba A., Kriek N., Thoen C., editors. Springer International Publishing; 2019. The status of bovine tuberculosis in Cameroon; pp. 283–303. [DOI] [Google Scholar]
- 65.Pascual-Linaza A.v., Gordon A.W., Stringer L.A., Menzies F.D. Efficiency of slaughterhouse surveillance for the detection of bovine tuberculosis in cattle in Northern Ireland. Epidemiol. Infect. 2017;145(5):995–1005. doi: 10.1017/S0950268816003095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyashchenko K.P., Vordermeier H.M., Waters W.R. Memory B cells and tuberculosis. Vet. Immunol. Immunopathol. 2020;221 doi: 10.1016/j.vetimm.2020.110016. [DOI] [PubMed] [Google Scholar]
- 67.Garbaccio S.G., Garro C.J., Delgado F., Tejada G.A., Eirin M.E., Huertas P.S., Leon E.A., Zumárraga M.J. Enzyme-linked immunosorbent assay as complement of intradermal skin test for the detection of mycobacterium bovis infection in cattle. Tuberculosis. 2019;117:56–61. doi: 10.1016/j.tube.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 68.Borham M., Oreiby A., El-Gedawy A., Hegazy Y., Khalifa H.O., Al-Gaabary M., Matsumoto T. Review on bovine tuberculosis: an emerging disease associated with multidrug-resistant Mycobacterium species. Pathogens. 2022;11(7):715. doi: 10.3390/pathogens11070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Keshavarz R., Mosavari N., Geravand M.M., Tadayon K., Pajoohi R.A., Solemani K., Ameri M., Gilani G.V. Interferon-γ assay, a high-sensitivity, specific and appropriate method for detection of bovine tuberculosis in cattle. Int J Mycobacteriol. 2016;5:S219. doi: 10.1016/j.ijmyco.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Nuñez-Garcia J., Downs S.H., Parry J.E., Abernethy D.A., Broughan J.M., Cameron A.R., Cook A.J., de la Rua-Domenech R., Goodchild A.v., Gunn J., More S.J., Rhodes S., Rolfe S., Sharp M., Upton P.A., Vordermeier H.M., Watson E., Welsh M., Whelan A.O., Greiner M. Meta-analyses of the sensitivity and specificity of ante-mortem and post-mortem diagnostic tests for bovine tuberculosis in the UK and Ireland. Prev. Vet. Med. 2018;153:94–107. doi: 10.1016/j.prevetmed.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 71.Gormley E., Doyle M.B., Fitzsimons T., McGill K., Collins J.D. Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam®) assay. Vet. Microbiol. 2006;112(2–4):171–179. doi: 10.1016/j.vetmic.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 72.Okafor C.C., Grooms D.L., Bolin S.R., Averill J.J., Kaneene J.B. Evaluation of the interferon-γ assay on blood collected at exsanguination of cattle under field conditions for surveillance of bovine tuberculosis. Transbound Emerg Dis. 2014;61(6):e68–e75. doi: 10.1111/tbed.12080. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim S., Abubakar U., Danbirni S., Usman A., Ballah F., Kudi A., Lawson L., Abdulrazak H., Abdulkadir I. Molecular identification of Mycobacterium tuberculosis transmission between cattle and man: a case report. J Microbiol Exp. 2016;3(3) doi: 10.15406/jmen.2016.03.00091. [DOI] [Google Scholar]
- 74.Ahmad I., Kudi C.A., Magaji A.A., Yakubu Y., Salisu M.D., Shuaibu S., Daninna Z.M. Disseminated tuberculosis in a cow and a dromedary bull-camel in Zamfara State in Nigeria. Vet. Med. Sci. 2019;5(1):93–98. doi: 10.1002/vms3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fritsche A., Engel R., Buhl D., Zellweger J.-P. Mycobacterium bovis tuberculosis: from animal to man and back. International Union Against Tuberculosis and Lung Disease. 2004;8(7):903–904. [PubMed] [Google Scholar]
- 76.Krajewska M., Kozińska M., Zwolska Z., Lipiec M., Augustynowicz-Kopeć E., Szulowski K. Human as a source of tuberculosis for cattle. First evidence of transmission in Poland. Vet. Microbiol. 2012;159(1–2):269–271. doi: 10.1016/j.vetmic.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Thoen C.O., Kaplan B., Thoen T.C., Gilsdorf M.J., Shere J.A. Zoonotic tuberculosis: a comprehensive one health approach. Medicina. 2016;76:159–165. [PubMed] [Google Scholar]
- 78.Marais B.J., Buddle B.M., Klerk-Lorist L., Nguipdop-Djomo P., Quinn F., Greenblatt C. BCG vaccination for bovine tuberculosis; conclusions from the Jerusalem One Health workshop. Transbound Emerg Dis. 2019;66(2):1037–1043. doi: 10.1111/tbed.13089. [DOI] [PubMed] [Google Scholar]
- 79.Zumla A., Yeboah-Manu D., Michel A.L., Azhar E.I., Torrelles J.B., Cadmus S.I., Kendall S.L., Chakaya J.M., Marais B., Kock R. Zoonotic tuberculosis—a call for an open One Health debate. Lancet Infect. Dis. 2020;20(6):642–644. doi: 10.1016/S1473-3099(20)30166-3. [DOI] [PubMed] [Google Scholar]
- 80.Buddle B.M., Vordermeier H.M., Chambers M.A., de Klerk-Lorist L.M. Efficacy and safety of BCG vaccine for control of tuberculosis in domestic livestock and wildlife. Front. Vet. Sci. 2018;5(259) doi: 10.3389/fvets.2018.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Animal and Plant Health Agency (APHA) Field trials for bovine TB cattle vaccine and skin test move to next phase. Available at. 2023. https://www.gov.uk/government/news/field-trials-for-bovine-tb-cattle-vaccine-and-skin-test-move-to-next-phase2
- 82.Balseiro A., Thomas J., Gortázar C., Risalde M.A. Development and challenges in animal tuberculosis vaccination. Pathogens. 2020;9(6) doi: 10.3390/pathogens9060472. 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used to support the findings of this study are included within the supplementary information file(s).