Abstract
Objective: Schizophrenia is known as a severe psychiatric disorder with a broad range of clinical indications and symptoms such as positive and negative symptoms. This study was conducted with the aim of investigating the effect of melatonin on positive and negative symptoms of inpatients with schizophrenia.
Method : This study was conducted as a randomized placebo-controlled trial (double-blind) in the population of patients with schizophrenia. Study samples were selected from inpatients with schizophrenia, according to the DSM-5 criteria, who had not been diagnosed with a depressive episode of schizophrenia based on the Calgary questionnaire and who also met the inclusion criteria. 46 patients with schizophrenia were randomly assigned to the intervention (6 mg melatonin per day as two 3 mg pills for six weeks) and placebo groups. The positive and negative symptom scale (PANSS) was used to assess the effect of treatment at T1 (before intervention), T2 (three weeks after beginning the intervention) and T3 (six weeks after beginning the intervention). To check the research hypotheses, multiple comparison statistics were used by the SPSS 22 software.
Results: The placebo and melatonin groups had no significant difference in terms of PANSS scores (negative, positive, general and total symptom scores) at T1. Also, there was no difference in PANSS scores between the two groups at T2. However, at T3, there was a significant difference between the two groups only in the score of negative symptoms of PANSS (P = 0.036), so that negative symptoms of schizophrenia were significantly reduced in the intervention group compared to the placebo group. Furthermore, based on within-group analyzes, all PANSS scores were significantly reduced in the two groups at T2 and T3 (P < 0.05).
Conclusion: Long-term use (at least six weeks) of melatonin can improve the negative symptoms of schizophrenia. Since antipsychotics can better affect the positive symptoms, the use of melatonin in combination with these drugs may perhaps further improve the patients’ symptoms.
Key Words: Melatonin, Randomized Controlled Trial, Schizophrenia, Signs and Symptoms
Schizophrenia is a clinical syndrome with variable but highly destructive psychopathology that affects cognition, emotion, perception, and other aspects of behavior (1). Auditory or visual hallucinations and delusions (positive symptoms) and disturbed speech and thinking (negative symptoms) are among the symptoms of this disease (2). Schizophrenia is a neurodevelopmental disorder caused by genetic and environmental factors (3, 4). The positive and negative symptoms of the disease interfere with a person's ability to cope with the needs of life and lead to severe impairment in social skills and economic and social losses throughout the patient's life (5). Schizophrenia is a psychiatric disorder that affects approximately 1% of the population (6, 7). Patients with schizophrenia occupy about 50% of all psychiatric hospital beds and constitute 16% of all psychiatric patients receiving some form of treatment (8). Therefore, schizophrenia is associated with a high burden of chronic disability compared to other mental illnesses, and the economic and social costs of this disease are disproportionately high compared to its prevalence and incidence (9, 10).
With the discovery of antipsychotics in early 1950s, there was a revolution in the treatment of schizophrenia. However, over time, it became clear that the most important problem with typical antipsychotics is lack of proper effectiveness on the negative symptoms of schizophrenia (11). Also new generation antipsychotics, except clozapine, have no significant effect on the negative symptoms of schizophrenia (12). These drugs mainly improve the positive symptoms of schizophrenia, but do not significantly affect the negative symptoms, especially cognitive and functional symptoms (13). Therefore, negative symptoms of schizophrenia remain an important problem that plays a vital role in patients' disability, and the poor response of schizophrenic patients to antipsychotics has prompted researchers to find adjunctive therapies alongside antipsychotic treatments (14). In addition, antipsychotics have been demonstrated to cause serious side effects in patients, including considerable weight gains and, thus, increased risk for metabolic syndrome and different cardiovascular illnesses (15).
In the last decade, exogenous melatonin supplementation has been demonstrated to have therapeutic benefits in schizophrenia, especially for sleep dysfunctions, due to the substantial reduction in the endogenous secretion of this important hormone from the pineal gland in this disorder (16). The pineal gland of patients with schizophrenia shows noticeable gliosis and sclerosis and an important reduction in volume in comparison to healthy individuals, which could elucidate the reduced secretion of melatonin in these patients (17, 18). Meanwhile, melatonin affects the catabolic pathway of tryptophan through changes in cortisol levels, and thus influences cortical cognitive functions, amygdala-related affect, and striatal motivational processing (16). Therefore, the disorder in the secretion of melatonin can be involved in the pathophysiology, etiology and management of schizophrenia (19). However, it has been shown that treatment by antipsychotic drugs does not appear to alter the pattern of melatonin secretion (20). In a 24-week clinical trial, Baandrup et al. examined the effects of supplemental melatonin in patients with schizophrenia or bipolar disorder, compared to the placebo, and found no significant improvement in patients' psychological functioning, well-being, or cognition (21). Two recent systematic reviews showed that adjunctive melatonin treatment in patients with schizophrenia leads to the improvement of their sleep quality, metabolic status and symptoms of tardive dyskinesia (22, 23).
In general, research studies on melatonin and schizophrenia pursue diagnostic and therapeutic goals. Although melatonin was discovered more than 50 years ago, research findings linking melatonin to schizophrenia appear contradictory (17). Despite the fact that melatonin is n drug approved by the European Medicines Agency, investigation and evidence on its therapeutic use is very limited in comparison to the evidence on the use of melatonin as an indicator of schizophrenia prognosis (17, 19). In addition, the therapeutic researches conducted so far have mainly focused on the effect of melatonin on metabolic problems and cognitive and extrapyramidal side effects of antipsychotics; however, its effect on positive and negative symptoms of patients with schizophrenia has been much less considered. Meanwhile, improving the positive and negative symptoms of patients can be very beneficial in controlling the disease and its complications, as well as improving the patients’ personal and social life. Therefore, the purpose of this double-blind clinical trial with placebo control is to investigate the effect of melatonin on the positive and negative symptoms of hospitalized schizophrenia patients.
Materials and Methods
This study was conducted as a randomized placebo-controlled trial (double-blind) in the population of patients with schizophrenia. All steps of this study have been approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.HGOLESTAN.REC.1400.024). Also, this study has been registered in the Iranian Registry of Clinical Trials (IRCT20210528051416N1).
Participants
The samples were selected from patients admitted to the psychiatric ward of Golestan Hospital affiliated to Ahvaz University of Medical Sciences in 2021-2022, who were diagnosed with schizophrenia based on the Structured Clinical Interview for DSM-5 (SCID-5) (24), which is a semi-structured interview guide for making the major DSM-5 diagnoses. This interview was conducted by an experienced psychiatrist. Our inclusion criteria were as follows: (1) Adult patients in the age range of 18-65 years who have been treated with risperidone, (2) signing the informed consent form by the patient's guardian to participate in the study, (3) non-diagnosis of a depressive episode based on Calgary Depression Scale for Schizophrenia (CDSS) (25), (4) absence of any major comorbid psychiatric disorders such as schizoaffective disorder, bipolar disorder, personality disorders, anxiety disorders, and substance use disorders, (5) not taking typical and atypical antipsychotics (except for risperidone), antidepressants, sodium valproate, and lithium, (6) no history of cognitive and neurological disorders, and (7) not receiving ECT in the current hospitalization. Furthermore, our exclusion criteria were as follows: (1) dissatisfaction to continue the research project, (2) failure to adhere to research procedures and abandoning it, (3) incidence of allergy to melatonin or any of the placebo compounds during the study.
Based on the above criteria, 46 patients with schizophrenia were included in the study and were randomly assigned to the intervention (n = 23) and placebo (n = 23) groups. Random allocation was done using random permutation blocks with a block size of six. For this purpose, a random list was prepared by a statistician. The interventions used in this research were placed in sealed envelopes based on the randomized list by a third person according to the corresponding codes, then assigned to each patient who entered the study. It should be noted that in this research, based on a 20% decrease in the average overall positive and negative symptom scale (PANSS) score of the intervention group compared to the placebo group at the end of the study and referring to previous studies (26), and considering the error level of 5% and power of 80%, the sample size in each group was determined to be 23 people and 46 people in total.
Procedure
At the beginning of the study, the CDSS scale was completed by a psychiatrist for all patients to rule out those patients with a major depressive episode. Patients who scored less than 6 in this scale were included in the study. Demographic information including age, gender, duration of illness, marital status, place of residence and education level were collected for all eligible patients. Then, the patients were randomly divided into two groups. Both groups of patients were treated with risperidone as an antipsychotic at a dose of 6 to 8 mg. At first, anticholinergic drugs were not prescribed for patients. However, if a patient had a previous history of drug complications and if there was a need and the possibility for the incidence of drug complications during the study, anticholinergics were prescribed. The intervention group received two 3 mg melatonin tablets 30 minutes before bedtime (total of 6 mg per day). In case of excessive sleepiness of the patient, the dose of melatonin was reduced to 3 mg per day. In the placebo group, in addition to risperidone, the patients received a placebo tablet which was similar to melatonin tablets in terms of shape, smell, taste, size and color and was prepared by the Faculty of Pharmacy of Ahvaz Jundishapur University of Medical Sciences. The use of placebo tablets in the control group was similar to the intervention group. The duration of the study was six weeks and the patients received either melatonin or placebo for six weeks according to instructions, depending on which group they were in. The PANSS was used as the primary outcome measure in this study to assess the effects of melatonin treatment on schizophrenia symptoms at T1 (before intervention), T2 (three weeks after beginning the intervention) and T3 (six weeks after beginning the intervention).
Primary outcome measure
The severity of positive and negative symptoms of schizophrenia was evaluated as the primary outcome measure in this trial. As mentioned, the PANSS was used for this purpose. The PANSS is a psychiatric instrument for assessing the severity of symptoms in people with schizophrenia. It is commonly utilized in antipsychotic treatment trials. This instrument is a seven-point Likert-type scale that measures 30 different symptoms in patients based on a semi-structured clinical interview and a family/caregiver report. Seven items of this scale evaluate negative symptoms (e.g., emotional withdrawal, stereotyped thinking, etc.), seven items evaluate positive symptoms (delusions, hallucinations, hostility, etc.), and 16 items evaluate general psychopathology (e.g., anxiety, tension, depression, disorientation, etc.). According to the research conducted by the creators of the questionnaire, the Cronbach's alpha of the questionnaire was reported to be 0.83, and the correlation of this scale with the scale for the assessment of negative symptoms (SANS) was 0.58 (27). The psychometric properties of the Iranian version of this scale are also reported to be acceptable. Two studies in Iran obtained the Cronbach's alpha of the PANSS as 0.80 and 0.77, and its validity was reported as acceptable using factor analysis (26, 28).
Secondary outcome measure
The possible side effects of melatonin consumption were evaluated in this study as the secondary outcome measure at T2 and T3. This was done using a checklist of melatonin side effects, which included drowsiness, headache, abdominal cramp, irritability, daytime fatigue, circadian rhythm disruption, and decreased alertness.
Data analysis
In this research, descriptive statistics including mean and standard deviation for quantitative variables and frequencies and frequency percentages for qualitative variables along with graphs were used to describe the research variables. The changes in the subscales of the questionnaires were recorded separately in two intervention and control groups. After checking the normality of the data using the Kolmogorov-Smirnov test, the research hypotheses were checked. Parametric statistics (repeated measures ANOVA, t-test, chi-square) or non-parametric tests (Friedman test, Mann-Whitney) were used to check the research hypotheses. The significance level of all tests is considered less than 0.05. All analyzes were done using SPSS version 22 statistical software.
Results
Figure 1 presents the consort flow diagram of our trial. In this work, 46 patients were studied, of which 35 (76.1%) were men and 11 (23.9%) were women (mean age = 33.39 ± 8.03 years). All participants completed the study and none were excluded from the study. As shown in Table 1, there is no significant difference between the two groups in terms of baseline characteristics (P > 0.05).
Figure 1.
Consort Flow Diagram for a Randomized Controlled Trial to Evaluate the Effects of Melatonin on Positive and Negative Symptoms of Schizophrenia
Table 1.
Baseline Characteristics of Patients with Schizophrenia in Two Intervention and Placebo Groups
| Variables |
Intervention group
(n = 23) |
Placebo group
(n = 23) |
P-value | |
|---|---|---|---|---|
| Age (mean ± SD years) | 35.65 ± 10.26 | 33.39 ± 8.03 | 0.410a | |
| Duration of illness (mean ± SD years) | 7.04 ± 9.98 | 5.04 ± 4.66 | 0.389a | |
| Gender N (%) |
Men | 16 (69.6) | 19 (82.6) | 0.300b |
| Women | 7 (30.4) | 4 (17.4) | ||
| Marital status N (%) |
Single | 9 (39.1) | 12 (52.2) | 0.666b |
| Married | 10 (43.5) | 6 (26.1) | ||
| Widowed | 1 (4.3) | 1 (4.3) | ||
| Divorced | 3 (13) | 4 (17.4) | ||
| Place of residence N (%) |
Urban | 17 (73.9) | 13 (56.5) | 0.216b |
| Rural | 6 (26.1) | 10 (43.5) | ||
| Education level N (%) |
Illiterate | 1 (4.3) | 3 (13) | 0.402b |
| High school | 11 (47.8) | 12 (52.2) | ||
| High school diploma and postgraduate diploma |
11 (47.8) | 7 (30.4) | ||
| Bachelor's and Master's degrees |
0 (0) | 1 (4.3) | ||
Note: “a” indicates the use of independent samples t-test; “b” indicates the use of chi-square test.
The study groups (placebo and melatonin) were not significantly different from each other in terms of initial PANSS scores at T1 (negative, positive, and total symptom scores) (P > 0.05). Figure 2 indicates the PANSS scores for both groups at the beginning (T1), after the third week (T2) and after the sixth week (T3) of the intervention period. In addition, Table 2 shows the mean changes in PANSS scores for each group (6th Week – Baseline or T3 – T1). There was no difference in PANSS scores between the placebo and melatonin groups at T2. However, at T3, there was a significant difference between the two groups only in the score of negative symptoms of PANSS (P = 0.036), so that negative symptoms of schizophrenia were significantly reduced in the intervention group compared to the placebo group. As shown in Table 2, the mean change in negative symptoms in the melatonin group is significantly higher than the placebo group (P = 0.032, Cohen’s D = 0.65). However, there was no significant difference between the two groups in the mean change of other scores (P > 0.05). Furthermore, based on within-group analyzes, all PANSS scores showed a significant reduction in the two groups at T2 and T3 (P < 0.05).
Figure 2.
Changes in the Positive and Negative Symptom Scale (PANSS) Scores in the Intervention Group Receiving Melatonin Compared to the Placebo Group through Repeated Measures Analysis of Variance. * Indicates a Significant Difference between the Two Groups (P < 0.05)
Table 2.
Comparison of Mean Change (6th Week - Baseline) of Positive and Negative Symptom Scale (PANSS) Scores in Patients with Schizophrenia Receiving Melatonin or Placebo
| Variables |
Mean change (T3 – T1)
for intervention group (n = 23) |
Mean change
(T3 – T1) for placebo group (n = 23) |
Mean
difference |
P-value
(t-statistic) |
Cohen’s D |
|---|---|---|---|---|---|
| Negative symptoms | -7.09 ± 4.03 | -3.39 ± 6.97 | 3.70 | 0.032 (2.204)* | 0.65 |
| Positive symptoms | -11.56 ± 6.09 | -10.00 ± 6.24 | 1.56 | 0.395 (0.858) | 0.25 |
| General psychopathology | -15.00 ± 7.92 | -12.35 ± 8.83 | 2.65 | 0.289 (1.071) | 0.31 |
| PANSS total score | -33.65 ± 13.63 | -25.74 ± 21.60 | 7.91 | 0.144 (1.485) | 0.43 |
T1 = before intervention at baseline, T3 = six weeks after beginning the intervention.
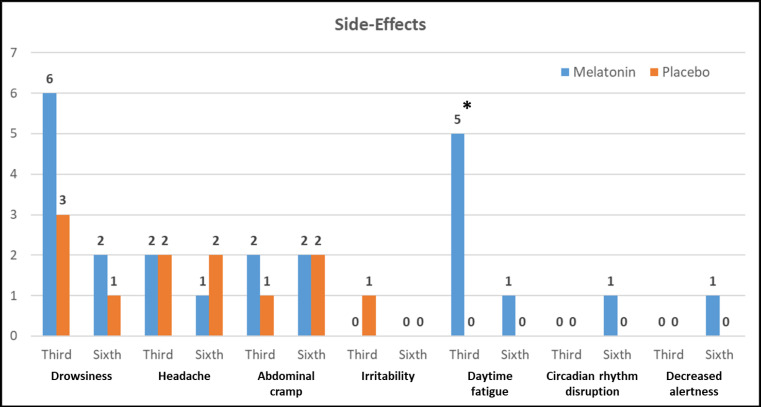
At T2, a total of 22 patients in the two groups exhibited drug side effects. The most common side effect was drowsiness with 9 cases (19.6%). Also, at T3, 13 patients were observed in the two groups with drug side effects. The most common side effect was abdominal cramp with 4 cases (8.7%). Figure 3 shows the frequency of each medication side effect for each of the intervention and placebo groups at T2 and T3 measurement times. As shown, there was a significant difference between the two groups in terms of daytime fatigue at T2, so that five patients in the melatonin group showed this side effect, but none of the patients in the placebo group had such a side effect at this time. However, in general, none of the reported side effects were of high severity and did not lead to drug discontinuation for any of the patients.
Figure 3.
Frequency Distribution of Side Effects Observed in the Third and Sixth Weeks after Starting the Treatment with Melatonin or Placebo in Patients with Schizophrenia
Discussion
In the current research, we assessed the effect of melatonin on positive and negative symptoms of patients with schizophrenia. Our results showed that melatonin can alleviate the negative symptoms of schizophrenia by 33.5% after six weeks of treatment without serious side effects, and this improvement was statistically significant compared to the placebo. Although few studies have been conducted on the effects of melatonin on the main symptoms of schizophrenia and most of them are animal studies, their results are almost in line with the present study and demonstrate the antipsychotic effects of melatonin in patients with schizophrenia (26, 27, 29). Previous studies showed that the level of melatonin and the circadian rhythm are significantly reduced in patients with schizophrenia, and the adjunctive use of melatonin in schizophrenia can lead to antipsychotic effects through its anti-inflammatory and antioxidant effects (17). Considering that one of the etiological hypotheses of schizophrenia is the role of oxidative stress (30), melatonin can affect the treatment of schizophrenia symptoms from different aspects due to its anti-oxidative properties (31).
One of the early mechanisms of action of melatonin was associated with its hypnogenic properties (32). In addition, melatonin has also resynchronizing mechanisms (33). Most of melatonin's actions are mediated via its interaction with certain membrane-bound MT1 and MT2 receptors (34). Although these receptors have been the subject of research for many years, they are still poorly understood. Furthermore, it has been hypothesized that abnormality of the suprachiasmatic nucleus, the main circadian pacemaker, may be involved in the pathogenesis of schizophrenia (35). Therefore, it has been suggested that bypassing the suprachiasmatic nucleus by oral melatonin may correct abnormal gene expression caused by suprachiasmatic nucleus damage and thereby improve psychotic symptoms (36).
In a case report, Hardman and O'Mahony found that 12 weeks of melatonin treatment could improve both positive and negative symptoms of schizophrenia in an adult with treatment-resistant schizophrenia. They attributed this symptom improvement to the restoration of the physiological pattern of melatonin secretion (37). Modabbernia et al. investigated the effectiveness of eight weeks of melatonin treatment in improving negative and positive symptoms of schizophrenia as well as in preventing olanzapine-related side effects. They reported a significant reduction in PANSS scores as well as metabolic side effects (such as weight gain) induced by olanzapine in the melatonin group compared to the placebo group (26). However, while the above-mentioned two studies have reported improvement of positive and general symptoms in patients in addition to negative symptoms, this study found only a significant improvement in negative symptoms of patients as a result of melatonin consumption. This difference could be due to the shorter duration of our study (six weeks) compared to the previous two studies (8 to 12 weeks).
Limitation
The limited sample size is one of the limitations of the present study, which limits the generalizability of the findings. However, we tried to minimize this limitation by the proper design of the study and careful implementation of different stages of the clinical trial. Also, not measuring the level of melatonin serum in patients in different stages of the study was another important limitation of this study, which was caused by the limited research budget.
Conclusion
Based on the findings of this study, it can be concluded that melatonin medication can be effective in improving the negative symptoms of schizophrenic patients, without serious side effects, within six weeks. Also, considering the decreasing slope of symptoms in the melatonin group, it can be concluded that long-term use of melatonin may be effective in improving positive and general symptoms of schizophrenia. In addition, since antipsychotics available in the market have a greater effect on positive symptoms, the adjunctive use of melatonin in combination with these drugs may result in greater clinical improvement in patients with schizophrenia.
Conflict of Interest
None.
References
- 1.Mohammadi MR, Ahmadi N, Khaleghi A, Mostafavi SA, Kamali K, Rahgozar M, et al. Prevalence and Correlates of Psychiatric Disorders in a National Survey of Iranian Children and Adolescents. Iran J Psychiatry. 2019;14(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- 2.Alavi SS, Mohammadi MR, Hooshyari Z, Mohammadi Kalhori S, Salehi M, Salmanian M, et al. Epidemiology of Psychotic Disorders Based on Demographic Variables in Iranian Children and Adolescents. Iran J Psychiatry. 2021;16(1):1–12. doi: 10.18502/ijps.v16i1.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mongan D, Ramesar M, Föcking M, Cannon M, Cotter D. Role of inflammation in the pathogenesis of schizophrenia: A review of the evidence, proposed mechanisms and implications for treatment. Early Interv Psychiatry. 2020;14(4):385–97. doi: 10.1111/eip.12859. [DOI] [PubMed] [Google Scholar]
- 4.Khaleghi A, Mohammadi MR, Shahi K, Nasrabadi AM. Computational Neuroscience Approach to Psychiatry: A Review on Theory-driven Approaches. Clin Psychopharmacol Neurosci. 2022;20(1):26–36. doi: 10.9758/cpn.2022.20.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Häfner H. From Onset and Prodromal Stage to a Life-Long Course of Schizophrenia and Its Symptom Dimensions: How Sex, Age, and Other Risk Factors Influence Incidence and Course of Illness. Psychiatry J. 2019;2019:9804836. doi: 10.1155/2019/9804836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khaleghi A, Mohammadi MR, Zandifar A, Ahmadi N, Alavi SS, Ahmadi A, et al. Epidemiology of psychiatric disorders in children and adolescents; in Tehran, 2017. Asian J Psychiatr. 2018;37:146–53. doi: 10.1016/j.ajp.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Talepasand S, Mohammadi MR, Alavi SS, Khaleghi A, Sajedi Z, Akbari P, et al. Psychiatric disorders in children and adolescents: Prevalence and sociodemographic correlates in Semnan Province in Iran. Asian J Psychiatr. 2019;40:9–14. doi: 10.1016/j.ajp.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Nefzi R, Larnaout A, Ammar HB, Khelifa E, Aissa A, El Hechmi Z. S200. HYPOVITAMINOSIS D IN SCHIZOPHRENIA: PREVALENCE AND ASSOCIATED CLINICAL CHARACTERISTICS. Schizophr Bull. 2018;44(Suppl 1):S403–S. [Google Scholar]
- 9.Barlati S, Deste G, Gregorelli M, Vita A. Autistic traits in a sample of adult patients with schizophrenia: prevalence and correlates. Psychol Med. 2019;49(1):140–8. doi: 10.1017/S0033291718000600. [DOI] [PubMed] [Google Scholar]
- 10.Cohen CI, Freeman K, Ghoneim D, Vengassery A, Ghezelaiagh B, Reinhardt MM. Advances in the Conceptualization and Study of Schizophrenia in Later Life: 2020 Update. Clin Geriatr Med. 2020;36(2):221–36. doi: 10.1016/j.cger.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. P t. 2014;39(9):638–45. [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 13.Yang YS, Marder SR, Green MF. Repurposing Drugs for Cognition in Schizophrenia. Clin Pharmacol Ther. 2017;101(2):191–3. doi: 10.1002/cpt.529. [DOI] [PubMed] [Google Scholar]
- 14.Goff DC. The Pharmacologic Treatment of Schizophrenia-2021. Jama. 2021;325(2):175–6. doi: 10.1001/jama.2020.19048. [DOI] [PubMed] [Google Scholar]
- 15.Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi: 10.1016/S2215-0366(19)30416-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson G, Maes M. Melatonin: an overlooked factor in schizophrenia and in the inhibition of anti-psychotic side effects. Metab Brain Dis. 2012;27(2):113–9. doi: 10.1007/s11011-012-9307-9. [DOI] [PubMed] [Google Scholar]
- 17.Morera-Fumero AL, Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int J Mol Sci. 2013;14(5):9037–50. doi: 10.3390/ijms14059037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bastos MAV Jr, Oliveira Bastos PRH, Portella RB, Soares LFG, Conde RB, Rodrigues PMF Jr, et al. Pineal gland and schizophrenia: A systematic review and meta-analysis. Psychoneuroendocrinology. 2019;104:100–14. doi: 10.1016/j.psyneuen.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Simek J. Schizophrenia, Mental Disintegration, and Melatonin. Act Nerv Super. 2018;60(3):86–9. [Google Scholar]
- 20.Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8(2):133–48. doi: 10.1016/S1087-0792(02)00158-2. [DOI] [PubMed] [Google Scholar]
- 21.Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well-being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add-on melatonin versus placebo. Eur Arch Psychiatry Clin Neurosci. 2017;267(2):163–71. doi: 10.1007/s00406-016-0711-8. [DOI] [PubMed] [Google Scholar]
- 22.Duan C, Jenkins ZM, Castle D. Therapeutic use of melatonin in schizophrenia: A systematic review. World J Psychiatry. 2021;11(8):463–76. doi: 10.5498/wjp.v11.i8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miola A, Fornaro M, Sambataro F, Solmi M. Melatonin and melatonin-agonists for metabolic syndrome components in patients treated with antipsychotics: A systematic review and meta-analysis. Hum Psychopharmacol. 2022;37(2):e2821. doi: 10.1002/hup.2821. [DOI] [PubMed] [Google Scholar]
- 24.Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatric Assoc. 2013;21(21):591–643. [Google Scholar]
- 25.Rostami R, Kazemi R, Khodaie-Ardakani MR, Sohrabi L, Ghiasi S, Sadat Kamali Z, et al. The Persian version of the Calgary Depression Scale for Schizophrenia (CDSS-P) Asian J Psychiatr. 2019;45:44–9. doi: 10.1016/j.ajp.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133–40. doi: 10.1016/j.jpsychires.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Shirayama Y, Takahashi M, Suzuki M, Tsuruoka Y, Sato K. Effects of Add-on Ramelteon on Cognitive Impairment in Patients with Schizophrenia: An Open-label Pilot Trial. Clin Psychopharmacol Neurosci. 2014;12(3):215–7. doi: 10.9758/cpn.2014.12.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghamari GH, Molavi P, Heshmati R. Exploration of the factor structure of positive and negative syndrome scale in schizophrenia spectrum disorders. 2010;J Clin Psychol:10–1. [Google Scholar]
- 29.Andrabi SS, Vishnoi S, Kaushik M, Parveen K, Tabassum H, Akram M, et al. Reversal of Schizophrenia-like Symptoms and Cholinergic Alterations by Melatonin. Arch Med Res. 2019;50(5):295–303. doi: 10.1016/j.arcmed.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Okusaga OO. Accelerated aging in schizophrenia patients: the potential role of oxidative stress. Aging Dis. 2014;5(4):256–62. doi: 10.14336/AD.2014.0500256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naguy A. Therapeutic use of melatonin in schizophrenia-more than meets the eye! World J Psychiatry. 2022;12(3):533–5. doi: 10.5498/wjp.v12.i3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lushington K, Pollard K, Lack L, Kennaway DJ, Dawson D. Daytime melatonin administration in elderly good and poor sleepers: effects on core body temperature and sleep latency. Sleep. 1997;20(12):1135–44. doi: 10.1093/sleep/20.12.1135. [DOI] [PubMed] [Google Scholar]
- 33.Kayumov L, Brown G, Jindal R, Buttoo K, Shapiro CM. A randomized, double-blind, placebo-controlled crossover study of the effect of exogenous melatonin on delayed sleep phase syndrome. Psychosom Med. 2001;63(1):40–8. doi: 10.1097/00006842-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Gobbi G, Comai S. Differential Function of Melatonin MT(1) and MT(2) Receptors in REM and NREM Sleep. Front Endocrinol (Lausanne). 2019;10:87. doi: 10.3389/fendo.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trbovic SM. Schizophrenia as a possible dysfunction of the suprachiasmatic nucleus. Med Hypotheses. 2010;74(1):127–31. doi: 10.1016/j.mehy.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 36.Ashton A, Jagannath A. Disrupted Sleep and Circadian Rhythms in Schizophrenia and Their Interaction With Dopamine Signaling. Front Neurosci. 2020;14:636. doi: 10.3389/fnins.2020.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardman S, O'Mahony E. Is there a role for melatonin in the treatment of schizophrenia? Prog Neurol Psychiatry. 2022;26(3):16–9. [Google Scholar]