Abstract
Objectives
To evaluate the correlation between patient characteristics, operative variables and the risk of blood stream infection as well as the association of primary blood stream infection and adverse outcomes.
Methods
Clinical records of 6500 adult patients who underwent open heart surgery between February 2008 and October 2020 were analyzed. The microbiological pattern of the primary BSI and its association with adverse events, such as mortality and major cardiovascular events, were evaluated.
Results
Primary bloodstream infection was diagnosed in 1.7% (n = 108) of patients following cardiac surgery with the application of cardiopulmonary bypass. Most isolated bacteria were gram-negative bacillus groups, such as the Enterobacteriaceae family with Serrata marcescens in 26.26%, followed by the Enterococcaceae family with the Enterococcus faecalis in 7.39% and Enterococcus faecium in 9.14% as the most frequently identified bacteria. The postprocedural mortality, stroke rate p < 0.001, the incidence of postoperative new renal failure p < 0.001, and the renal replacement therapy p < 0.001 were significantly higher in the primary BSI group. Aortic cross-clamp time >120 min, OR 2.31 95%CI 1.34 to 3.98, perfusion time >120 min, OR 2.45 95%CI 1.63 to 3.67, and duration of the intervention >300min, OR 2.78 95%CI 1.47 to 5.28, were significantly related to the primary BSI.
Conclusion
The gram-negative bacillus was the most common microorganism identified in BSI after cardiovascular operations using cardiopulmonary bypass. Patients on dialysis prior to cardiac surgery are at higher risk for having BSI. Enteric bacterial translocation after prolonged cardiopulmonary bypass is a possible mechanism of early primary bloodstream infection in these patients. In patients at high risk, prophylactic use of an antibiotic regimen with broader gram-negative bacteria coverage should be considered, especially in those with prolonged cardiopulmonary bypass and intervention time.
Keywords: Cardiopulmonary bypass, Primary blood stream infection, Bacterial translocation, Gram negative
Nomenclature
Abbreviations
- AUROC
area under the receiver operating characteristics
- BSI
bloodstream infection
- CBP
cardiopulmonary bypass
- CI
confidence interval
- CT
computer tomography
- GNB
gram negative bacillus
- LVEF
left ventricular ejection fraction
- MACCE
major adverse cardiac and cerebrovascular events
- MRI
magnetic resonance imaging
- NYHA
new york heart association
- OR
odds ratio
- ROC
receiver operating characteristics
- ST
the society of thoracic surgery
- VARC
Valve Academic Research Consortium
1. Introduction
Infectious complications after cardiovascular surgery, especially in cardiopulmonary bypass (CPB) interventions, are associated with significant morbidity and increased perioperative mortality [[1], [2], [3], [4], [5]]. A perioperative mortality rate of up to 40% has been reported in cases of bloodstream infection (BSI) in patients following cardiovascular surgeries [3].
The risk factors for postoperative infection after cardiovascular surgery using CPB have been identified, such as older age, diabetes mellitus, obesity, emergency surgery, multiple blood transfusions, postoperative low cardiac output, and the use of mechanical circulatory support devices [[4], [5], [6]]. Due to the increased risk of blood product transfusion and immunomodulatory effect, CPB use can be theoretically considered a risk factor for infection after a cardiovascular operation. Practice guidelines for the choice of prophylactic antibiotics and other preventive measures in patients undergoing cardiovascular operations have been established and are widely accepted [7,8]. Nevertheless, the microbiological pattern in patients with nosocomial infection, which continues to change in the current modern antibiotic era, has been rarely investigated [9,10]. Understanding the prevalence and associated risk factors for different causative microorganisms in patients undergoing cardiac surgery conducted with CPB should be emphasized.
We hypothesized that prolonged CPB usage predicts early bloodstream infection following cardiac surgery. This single-center retrospective study aimed to analyze demographics and microbiological factors of patients undergoing cardio surgical interventions with BSI.
2. Materials and methods
The local ethics committee at the University of Basel, Basel, Switzerland approved the study protocol (EKNZ 2020-00210, clinicaltrials.gov NCT04548167). Written informed consent was waived due to the retrospective nature of the study.
2.1. Patient populations
This retrospective study included all adult patients who underwent open heart surgery using CPB from February 2008 to October 2020. Patients with positive blood culture and evidence of newly developed septicemia during the index hospital stay were included. Exclusion criteria were as follows; active endocarditis, patients with indications such as left ventricle aneurysma, cardiac trauma, isolated congenital defect and left ventricular assist device (LVAD) implantation.
Patient records were selected from our institutional database (Dendrite), which is harmonized with the surgical planning tool and checked for completeness and consistency monthly.
2.2. Study outcome: definition of bloodstream infection
Primary BSI was defined as any finding of a microorganism from a blood sample without any evidence of any other infection site or potential infection entry region diagnosed within 30 days after surgery. This definition does not comprise common skin colonized microorganisms, such as coagulase-negative Staphylococci, Viridans group Streptococci, Corynebacterium species, Bacillus species, Propionibacterium species, and Aerococcus species or Micrococcus species, which were isolated from only a single blood sample, where contamination was suspected. Primary outcome of this study was BSI within 30 days. As a sensitivity analysis, we repeated the analysis for any finding of the same microorganisms within 7 days after surgery to maintain phenotypical primary BSI and avoid confounding, further referred to as early BSI.
All blood samples were taken from central and peripheral lines and/or phlebotomy. Blood cultures were drawn in case of suspected sepsis based on clinical and/or diagnostical evidence.
2.3. Perioperative prophylactic disinfection and antibiotic protocol
At our institution, all elective patients showered with octenidine-based skin cleanser (Octenisan®) wash lotion less than 24 h before the intervention. Preoperatively all patients are screened for MRSA. Afterward, the patient was transferred to the operating room, where a medical doctor was responsible for the disinfection. The first perioperative prophylactic antibiotic dosage was given before the surgical incision. According to the Society of Thoracic Surgery (STS) Practice Guidelines [7,8], all patients received six shots of 2nd generation cephalosporin within 48 h. In case of a previous allergic reaction to lactam or penicillin, vancomycin was used, usually with another antibiotic agent with additional gram-negative coverage. Prophylactic antibiotics were taken for 48 h postoperatively.
Postulating that the most common cause of surgical site infection is likely related to the colonized Staphylococcus, Vancomycin and third-generation cephalosporin were used as prolonged antibiotic therapy in patients with elevated risk for surgical site infection. Likely until the risk was eliminated, for example, in patients with open sternal wound until the wound has been closed.
2.4. Clinical parameters
In-hospital mortality was defined as death before discharge. A neurologic event was defined according to the Valve Academic Research Consortium-2 (VARC) [9] and a perioperative stroke was defined as a duration of a focal or global neurological deficit >24h or <24 h if available neuroimaging documents a new hemorrhage or infarct.
Aim of the study was analyze the demographics and microbiological factors of patients undergoing cardiac surgery using CPB complicated with primary BSI postoperatively. Secondary outcomes were the incidence of postoperative stroke, myocardial infarction during the hospitalization, and the incidence of composite adverse cardiac and cerebrovascular events (MACCE). MACCE was a composite endpoint of in-hospital mortality, stroke and myocardial infarction. The composite endpoints were defined according to the VARC [9].
2.5. Statistical methods
To investigate a crude association of our pre-specified prognostic variables, duration of operation, perfusion, or aortic clamping, with the risk of positive blood culture within 30 days after surgery, we first calculated odds ratios (OR) with 95% confidence intervals (CI) for categories of one or 2 h, respectively, according to the range and distribution of the predictive variable. We carried out nonparametric tests for trends among these ORs. We then calculated odds ratios per continuous increase of predictive variables using logistic regression, first crude then with adjustment. To avoid zero digits, ORs are expressed per 10-min increase. We back-transformed the crude odds to probabilities and plotted them against kernel density estimates of the distribution of the prognostic variable. For the adjustment, we included EuroSCORE, patient age, left ventricular ejection fraction (LVEF), as continuous covariates and type of cardiac surgery, diabetes, preoperative renal failure, New York Heart Association (NYHA) class III or IV, and emergency as categorical covariates. We plotted receiver operating characteristics (ROC) curves and calculated the area under the ROC curves (AUROC) to assess the predictive performance of duration, perfusion time, and clamping time. We repeated the main analysis for early BSI, e.g. BSI within 7 days after surgery.
Continuous variables were shown as mean ± sd or median with interquartile range, as appropriate, and were compared using Student's t-Test or Wilcoxon-Mann-Whitney test. Categorical variables were shown in percentages and tested using Fisher's exact test. For descriptive statistics, we grouped the cohort by the outcome. We refrained from applying weighting or matching methods by treatment (duration of cardio-pulmonary bypass time), as grouping would need a cut-off for which there is no evidence. Furthermore, the assumption that each patient would have a probability >0 to be in either group is not plausible. All analyses were carried out using Stata 16 (Stata Corp., College Station, Texas).
3. Results
Between February 2008 and October 2020, 7836 adult patients older than 18 underwent cardiac surgery. In the analysis, we included 6500 patients. Positive bloodstream infection was confirmed in 355 (5.46%) patients, whereas according to the exclusion criteria, 247 cases were not included in the final analysis. Primary BSI was found in 108 (1.7%) patients within the first 30 days. The patient enrolment flowchart is presented in Fig. 1. The demographics of patients included in the analysis are presented in Table 1.
Fig. 1.
Selection flow chart of patients undergoing cardiovascular operations using cardiopulmonary bypass
LVAD: left ventricular assist device.
Table 1.
Patient characteristics.
| Total (N = 6500) | No BSI (N = 6392) | BSI (N = 108) | p | |
|---|---|---|---|---|
| Age | 66 ± 11 | 66 ± 11 | 70 ± 10 | <0.001 |
| Female | 1592 (24%) | 1568 (25%) | 24 (22%) | 0.65 |
| Diabetes | 1691 (26%) | 1645 (26%) | 46 (43%) | <0.001 |
| CAD | 3080 (47%) | 3029 (47%) | 51 (47%) | 1.00 |
| Main stem disease | 951 (15%) | 934 (15%) | 17 (16%) | 0.68 |
| Peripheral artery disease | 588 (9.0%) | 572 (8.9%) | 16 (15%) | 0.042 |
| Preoperative Stroke | 534 (8.2%) | 522 (8.2%) | 12 (11%) | 0.29 |
| Preoperative renal impairment | 386 (5.9%) | 366 (5.7%) | 20 (19%) | <0.001 |
| Terminal renal impairment with dialysis | 73 (1.1%) | 66 (1.0%) | 7 (6.5%) | <0.001 |
| COPD | 765 (12%) | 747 (12%) | 18 (17%) | 0.13 |
| History of MI | 2164 (33%) | 2107 (33%) | 57 (53%) | <0.001 |
| Hypertension | 5093 (78%) | 4997 (78%) | 96 (89%) | 0.006 |
| Hypercholesteremia | 3935 (61%) | 3864 (60%) | 71 (66%) | 0.28 |
| NYHA | <0.001 | |||
| n/a | 887 (14%) | 875 (14%) | 12 (11%) | |
| I | 1270 (20%) | 1255 (20%) | 15 (14%) | |
| II | 2283 (35%) | 2253 (35%) | 30 (28%) | |
| III | 1753 (27%) | 1718 (27%) | 35 (32%) | |
| IV | 307 (4.7%) | 291 (4.6%) | 16 (15%) | |
| NYHA ≥ III | 2060 (32%) | 2009 (31%) | 51 (47%) | <0.001 |
| AF preop. | 615 (9.5%) | 601 (9.4%) | 14 (13%) | 0.24 |
| Current Smoker | 1324 (20%) | 1300 (20%) | 24 (22%) | 0.63 |
| LVEF | 54 ± 12 | 54 ± 11 | 48 ± 15 | <0.001 |
BSI: blood stream infection, CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; MI: myocardial infarction; NYHA: New York heart association; AF: atrial fibrillation; LVEF: left ventricular ejection fraction.
Overall, patients with primary BSI were older (p < 0.001), had more frequently diabetes mellitus (p < 0.001), had a history of myocardial infarction (p = 0.002) and were on dialysis (p < 0.001). Patients with BSI were more often diagnosed with renal impairment p < 0.001 pre-intervention and were more frequently diagnosed with heart failure (NYHA > III) p < 0.001. Patients with primary BSI had significantly lower left ventricular ejection fraction p < 0.001 (Table 1).
The microbiological findings of cases with primary BSI are presented in Table 2. In 86.1% of cases, a single bacteria was identified, whereas two different bacteria were identified in 12.9% and three bacteria in 0.9% of the cases. The Serrata marcescens was the most frequently identified bacteria in 32.4% of the cases, followed by Enterococcus faecalis in 12% and Klebsiella pneumonia, Enterococcus faecium, Escherichia coli, and Enterobacter cloacae all in 8.3% of the cases. The Enterobacteriaceae family was the most frequently identified bacteria family, found in 66.5% of the cases, followed by Enterococcacea in 16.5% and Morganellaceae and Pseudomonadaceae family in 6.22%. The microbiological finding was positive on gram-negative bacillus (GNB) in 79.6% of cases.
Table 2.
Relevant bacteria identified in primary blood stream infection.
| Any finding |
Single finding |
Double finding |
Triple finding |
|
|---|---|---|---|---|
| N = 108 | N = 93 | N = 14 | N = 1 | |
| Serratia marcescens | 35 | 32 | 3 | 0 |
| Enterococcus faecalis | 12 | 7 | 4 | 1 |
| Enterobacter cloacae group | 9 | 5 | 3 | 1 |
| Enterococcus faecium | 9 | 6 | 3 | 0 |
| Escherichia coli | 9 | 6 | 3 | 0 |
| Klebsiella pneumoniae | 9 | 8 | 1 | 0 |
| Pseudomonas aeruginosa | 8 | 6 | 2 | 0 |
| Klebsiella oxytoca | 7 | 4 | 3 | 0 |
| Enterobacter aerogenes | 4 | 3 | 1 | 0 |
| Citrobacter koseri | 3 | 2 | 0 | 1 |
| Eggerthella lenta | 3 | 1 | 2 | 0 |
| Morganella morganii | 3 | 2 | 1 | 0 |
| Actinomyces species | 2 | 2 | 0 | 0 |
| Klebsiella aerogenes | 2 | 2 | 0 | 0 |
| Acinetobacter ursingii | 1 | 1 | 0 | 0 |
| Bacteroides thetaiotaomicron | 1 | 1 | 0 | 0 |
| Clostridium innocuum | 1 | 0 | 1 | 0 |
| Gemella species | 1 | 1 | 0 | 0 |
| Granulicatella adiacens | 1 | 1 | 0 | 0 |
| Prevotella melaninogenica/oralis | 1 | 1 | 0 | 0 |
| Proteus mirabilis | 1 | 1 | 0 | 0 |
| Proteus vulgaris | 1 | 0 | 1 | 0 |
| Ruminococcus gnavus | 1 | 1 | 0 | 0 |
Number of bacteria found per patient.
Surgical details are presented in Table 3. In the BSI group, the combined interventions were performed more often (p < 0.001) compared to the no BSI group, and there were more emergency procedures (p < 0.001). The intervention duration (p < 0.001), the aortic cross-clamp time (p < 0.001), and cardiopulmonary bypass time (p < 0.001) were significantly longer compared to the group with no evidence of infection (Table 3).
Table 3.
Operative variables.
| Total (N = 6500) | No BSI (N = 6392) | BSI (N = 108) | p | |
|---|---|---|---|---|
| Type of surgery | 0.002 | |||
| CABG | 2693 (41%) | 2658 (42%) | 35 (32%) | |
| CABG&Valve(s) | 1114 (17%) | 1083 (17%) | 31 (29%) | |
| Valve(s) | 1583 (24%) | 1565 (24%) | 18 (17%) | |
| Aortic surgery | 1110 (17%) | 1086 (17%) | 24 (22%) | |
| Emergency | 681 (10%) | 652 (10%) | 29 (27%) | <0.001 |
| Additive EUROScore | 6.4 ± 3.7 | 6.4 ± 3.7 | 10 ± 4.1 | <0.001 |
| Logistic EUROScore | 10 ± 14 | 10 ± 13 | 23 ± 21 | <0.001 |
| Euroscore II | 2.1 [1.1 to 4.8] | 2.1 [1.1 to 4.6] | 6.3 [3.2 to 19] | <0.001 |
| Intervention Time (min) | 218 ± 66 | 217 ± 65 | 260 ± 90 | <0.001 |
| Cardiopulmonary bypass time (min) | 118 ± 48 | 117 ± 47 | 151 ± 69 | <0.001 |
| Aortic cross clamping time (min) | 79 ± 35 | 79 ± 34 | 91 ± 41 | <0.001 |
BSI: blood stream infection, CABG: coronary artery bypass graft.
Hospital outcomes are summarized in Table 4. The in-hospital mortality and the incidence of perioperative myocardial infarction and stroke were significantly higher in the primary BSI group (p < 0.001). The intensive care unit stay and the incidence of prolonged intubation (p < 0.001) were longer in the BSI group. The incidence of MACCE was significantly higher in the primary BSI (p < 0.001) group.
Table 4.
In hospital outcomes.
| Total (N = 6500) | No BSI (N = 6392) | BSI (N = 108) | p | |
|---|---|---|---|---|
| Operative mortality | 235 (3.6%) | 203 (3.2%) | 32 (30%) | <0.001 |
| Length of ICU stay, days | 2.0 [1.0 to 3.0] | 2.0 [1.0 to 3.0] | 13 [5.0 to 23] | <0.001 |
| Intubation >72h | 281 (4.3%) | 235 (3.7%) | 46 (43%) | <0.001 |
| Reoperation for bleeding | 272 (4.2%) | 259 (4.1%) | 13 (12%) | <0.001 |
| Postoperative MI | 122 (1.9%) | 115 (1.8%) | 7 (6.5%) | 0.004 |
| Postoperative Stroke | 259 (4.0%) | 243 (3.8%) | 16 (15%) | <0.001 |
| AF at discharge | 1885 (29%) | 1833 (29%) | 52 (48%) | <0.001 |
| Permanent pacemaker | 274 (4.2%) | 264 (4.1%) | 10 (9.3%) | 0.024 |
| Sternal infection | 122 (1.9%) | 109 (1.7%) | 13 (12%) | <0.001 |
| Postoperative renal failure | 553 (8.5%) | 503 (7.9%) | 50 (46%) | <0.001 |
| Renal replacement therapy | 173 (2.7%) | 148 (2.3%) | 25 (23%) | <0.001 |
| Pulmonary infection | 465 (7.2%) | 415 (6.5%) | 50 (46%) | <0.001 |
| MACCE | 545 (8.4%) | 499 (7.8%) | 46 (43%) | <0.001 |
| Sepsis | 155 (2.4%) | 105 (1.6%) | 50 (46%) | <0.001 |
| Length of stay | 9.0 [7.0 to 12] | 8.0 [7.0 to 12] | 22 [14 to 36] | <0.001 |
BSI: blood stream infection, ICU: intensive care unit; MI: myocardial infarction; AF: atrial fibrillation; MACCE: major adverse cardiac and cerebrovascular events.
Prolonged cross-clamp time, prolonged perfusion time and prolonged intervention time were significantly associated with an elevated risk of primary BSI (Table 5). The perfusion time >120 min, OR 2.45 (1.63 to 3.67); aortic cross-clamp time >120min, OR 2.31 (1.34 to 3.98); and intervention time >300min, OR 2.78 (1.47 to 5.28), were related to primary BSI (Table 5).
Table 5.
Time categories and outcome.
| Category | N | events | OR | P | P* |
|---|---|---|---|---|---|
| Intervention time (min) | |||||
| ≤180 | 2232 | 24 | Reference | <0.001 | |
| >180 to 300 | 3657 | 61 | 1.56 (0.97 to 2.51) | 0.066 | |
| >300 to 420 | 545 | 16 | 2.78 (1.47 to 5.28) | 0.002 | |
| >420 | 66 | 7 | 10.9 (4.52 to 26.3) | <0.001 | |
| Perfusion time, (min) | |||||
| ≤120 | 3944 | 40 | Reference | <0.001 | |
| >120 to 240 | 2411 | 59 | 2.45 (1.63 to 3.67) | <0.001 | |
| >240 | 145 | 9 | 6.46 (3.07 to 13.6) | <0.001 | |
| Aortic clamping time, (min) | |||||
| ≤60 | 2170 | 30 | Reference | 0.008 | |
| >60 to 120 | 3566 | 54 | 1.10 (0.70 to 1.72) | 0.687 | |
| >120 | 764 | 24 | 2.31 (1.34 to 3.98) | 0.002 | |
OR: odds ratio. P* relates to non-parametric trends.
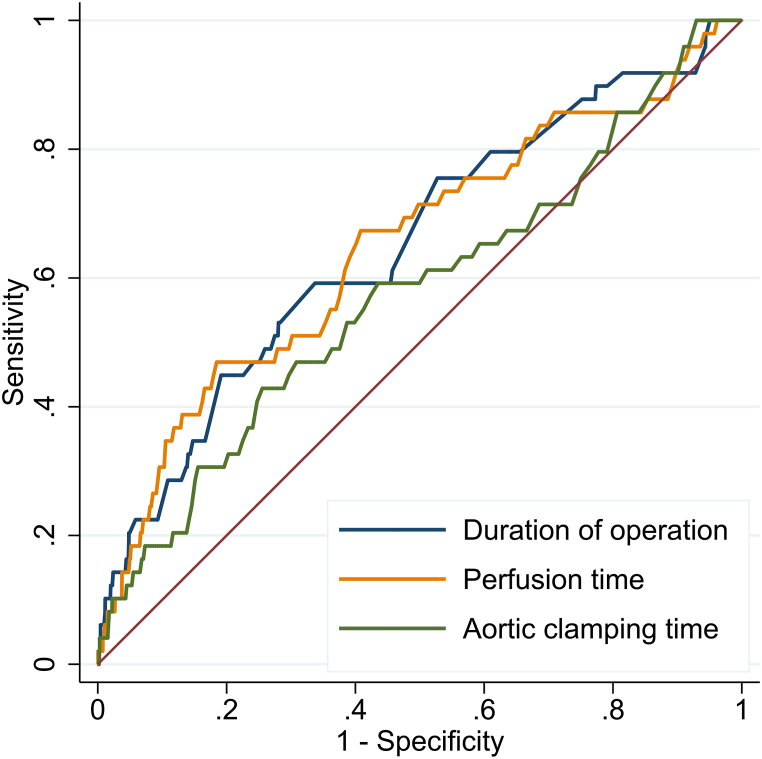
The receiver operating characteristic curve for cross-clamp time, perfusion time, and consequently the intervention time showed areas under curves of 0.58 (95%CI 0.53 to 0.64), 0.66 (95%CI 0.60 to 0.71), and 0.64 (95%CI 0.59 to 0.70) for primary BSI (Fig. 2).
Fig. 2.
Receiver operation characteristics curve of main prognostic variables such as duration of operation, cardiopulmonary bypass time and aortic clamping time. Area under the curve were as follows for duration of operation; 0.64 (0.59 to 0.70), for perfusion time; 0.66 (0.60 to 0.71) and for aortic clamping time; 0.58 (0.53 to 0.64).
Concerning all main prognostic variables, ORs for primary BSI increased monotonically compared to the lowest category, which we used as a reference, exhibiting a nonparametric trend, see also Table 5. For the duration of operation (Fig. 3A), perfusion time (Fig. 3B) and aortic clamping time (Fig. 3C), the increase became significant only for higher categories, indicating that risk increased in a relatively small subgroup at the upper tail of the distribution (Fig. 3).
Fig. 3.
Distribution of main prognostic variables and of outcome; 3A) Distribution of operation duration and outcome probability, 3B) Distribution of perfusion time and outcome probability, 3C) Distribution of Aortic clamping time and outcome probability.
Table 6 shows crude and multivariable odds ratios (ORs) for our main prognostic variables and outcome. Duration of operation was associated with a small increase in the risk of the outcome. More specifically, risk of BSI increased by 0.12% per 10 min increase in the duration of the operation corresponding to an OR of 1.07, given the overall risk of BSI being 1.7% (e.g. 108/6500). Adjusting for EuroSCORE and variables in the full model reduced this increase to 0.084%, yielding robust results. We found the same pattern for perfusion and clamping time.
Table 6.
Crude and multivariable odds ratios (ORs) for our main prognostic variables and primary blood stream infection.
| Outcome | Adjustment | OR (95% CI) | p |
|---|---|---|---|
| Operation duration, 10 min | Crude | 1.07 (1.05 to 1.09) | <0.001 |
| adj. logisticeuroscore | 1.05 (1.02 to 1.07) | <0.001 | |
| adj. age | 1.07 (1.05 to 1.09) | <0.001 | |
| adj. diabetes | 1.07 (1.05 to 1.09) | <0.001 | |
| adj. preoprenalfailure | 1.07 (1.05 to 1.09) | <0.001 | |
| adj. NYHA III-IV | 1.07 (1.05 to 1.09) | <0.001 | |
| adj. LVEF | 1.07 (1.05 to 1.09) | <0.001 | |
| adj. emergency | 1.06 (1.04 to 1.09) | <0.001 | |
| adj. CABG | 1.07 (1.04 to 1.09) | <0.001 | |
| adj. full modela | 1.05 (1.03 to 1.08) | <0.001 | |
| Perfusion time, 10 min | Crude | 1.10 (1.07 to 1.12) | <0.001 |
| adj. logisticeuroscore | 1.07 (1.04 to 1.10) | <0.001 | |
| adj. age | 1.10 (1.07 to 1.13) | <0.001 | |
| adj. diabetes | 1.10 (1.07 to 1.13) | <0.001 | |
| adj. preoprenalfailure | 1.09 (1.07 to 1.12) | <0.001 | |
| adj. NYHA III-IV | 1.09 (1.06 to 1.12) | <0.001 | |
| adj. LVEEF | 1.10 (1.07 to 1.13) | <0.001 | |
| adj. emergency | 1.09 (1.06 to 1.12) | <0.001 | |
| adj. CABG | 1.09 (1.06 to 1.12) | <0.001 | |
| adj. full model | 1.08 (1.05 to 1.12) | <0.001 | |
| Aortic clamping time, 10 min | Crude | 1.08 (1.04 to 1.13) | <0.001 |
| adj. logisticeuroscore | 1.05 (1.01 to 1.10) | 0.029 | |
| adj. age | 1.09 (1.04 to 1.14) | <0.001 | |
| adj. diabetes | 1.09 (1.04 to 1.14) | <0.001 | |
| adj. preoprenalfailure | 1.08 (1.04 to 1.13) | <0.001 | |
| adj. NYHA III-IV | 1.08 (1.03 to 1.13) | 0.001 | |
| adj. LVEF | 1.09 (1.04 to 1.14) | <0.001 | |
| adj. emergency | 1.08 (1.04 to 1.13) | <0.001 | |
| adj. CABG | 1.06 (1.01 to 1.12) | 0.030 | |
| adj. full model | 1.07 (1.01 to 1.13) | 0.014 |
NYHA: New York heart association; LVEF: left ventricular ejection fraction, CABG: coronary artery bypass graft.
Note that ORs are expressed per 10 min increase to avoid zero digits.
Full model comprises all variables adjusted for one by one, hence all variables shown in the table.
Sensitivity analysis of risk elements for early BSI, e.g. primary BSI during the first 7 days following intervention is presented in supplementary materials. We found early BSI in 49 (0.75%) patients. The relative frequency of bacteria was similar to our main analysis, and all associations showed a similar pattern, however with smaller statistical power due to small number of patients in the early BSI group.
4. Discussion
In this retrospective data analysis, the gram-negative bacillus (GNB) was the predominant cause of primary BSI in patients undergoing open heart surgery using CPB. More than 60% of the identified bacteria belonged to the Enterobacteriaceae family, followed by the Enterococcaceae family in 16.5% of the cases, the two most representative gut flora bacteria. The primary BSI was strongly related to the prolonged cross-clamp and cardiopulmonary bypass time.
The incidence of primary BSI following cardio surgical procedure associated with the GNB infection is considerable, ranging from 18% to 70% [[10], [11], [12], [13], [14], [15]]. Unfortunately, in most reports, primary and secondary BSI are not differentiated, and hence does not reflect the different pathological pathways. According to the recent healthcare-associated infections (HAI), septicemia associated with a well-defined site of infection is classified as secondary BSI, whereas primary BSI is defined as an event with positive microbiological findings without evidence of another site of infection [15]. Consequently, the incidence of primary BSI following cardiac surgery may be wrongly assessed.
Ryna et al. set the primary BSI as any infection <96 h post cardiac surgery. Of 16 cases, 13 (81.3%) were GNB-related infections [10]. The time frame for the primary BIS was set to no more than 96 h post-intervention, an element that definitively restricted the results. Authors postulated that disrupting the mucosal-blood barrier in the gastrointestinal tract resulted in bacterial translocation, which would explain the high prevalence of GNB in primary BSI. Loss of intestinal barrier function is considered to play a key role in the onset of systemic inflammatory response syndrome, and it may be associated with intestinal ischemia-reperfusion injury [16]. Ischemia-reperfusion injury is caused by various pathological conditions that involve a critical reduction of blood flow to the intestine, such as acute obstruction of mesenteric arterial blood flow, hypoperfusion associated with major vascular or abdominal surgical procedures, and hemorrhagic shock or reduced intestinal perfusion during the CPB [17,18]. Indeed, splanchnic perfusion may be reduced during the CPB, and the prolonged CPB time may induce intestinal injury [19]. Moreover, the ischemic reperfusion injury following CPB may damage the intestinal mucosal barrier [20].
In our study, mainly intestinal flora, predominantly the Enterobacteriaceae family, caused the primary BSI following cardiac surgery with CPB, supporting the assumption of perioperative endogenous bacterial translocation as the predominant etiology after prolonged CPB.
The duration of intestinal hypoperfusion on CPB as catalysat or for ischemic injury as a key mechanism of bacterial translocation is an important factor to discuss. It was shown in an experimental setting that the extent of the ischemic mucosal injury is time-dependent. Specifically, a mesenteric hypoperfusion of 60 min results in loss of epithelium from the upper third of the intestinal villus, whereas ischemia of 120 min or more results in near-complete loss of epithelium and considerable damage to the lamina propria [21]. In our statistical model, where the perfusion time and cross-clamp time of more than 120 min were strongly associated with the increased risk for primary BSI, any prolongation of cross-clamp time and/or of the perfusion time on CPB considerably augmented the risk of primary BSI.
Restoring the intestinal barrier is a complex mechanism and is one of the key elements in regulating the uncontrolled translocation of noxa. Repairing the intestinal surface integrity is a complex process that depends on several elements. We postulated that vasoconstrictive drugs might affect the restoration process besides the extant intestinal damage of individual vascular status resulting in unequally distributed flow in splanchnic vessels.
In our study, the time frame to investigate the primary BSI incidence was set to 30 days, and this arbitrarily defined period is debatable.
Recent literature has postulated that the intestinal barrier after CPB is restored a few days after intervention [22]. However, our sensitivity analysis revealed that the GNB with the predominant Enterobacteriaceae family was the main microbiological finding in patients with primary BSI up to 7 days post-intervention. Besides, primary BSI occurred in more than 50% of the included population after 7 days post-intervention, with the peak incidence between days 10 and 14. Intestinal barrier damage repair in patients with prolonged CPB is much more time-consuming, resulting in prolonged noxa translocation.
Consequently, efforts to prevent and adequately treat GNB-related BSI after cardiac surgery should be emphasized and might require adjustments. The first generation of cephalosporin is widely used as the first choice of prophylactic antibiotic therapy in patients undergoing cardiac surgery, and it was proven that the second generation of cephalosporine could reduce gram-positive and gram-negative infection after cardiac surgery [23].
In our study, more than half of the isolated Enterobacteriaceae were Enterobacter species. Based on the antibiotic sensitivity results from patients undergoing cardiac surgery and our institutional database, the Enterobacter species is generally resistant to first-generation and second-generation cephalosporins. Consequently, applying a third-generation or even fourth-generation cephalosporin as a prophylactic antibiotic regimen against both gram-negative and gram-positive bacteria in patients with prolonged CPB should be reasonable. That's why there is a need for further prospective randomized trials focusing on the strategy of antibiotic therapy in the field of cardiac surgery. In high-risk patients with infectious complications, even with the use of the third-generation or fourth-generation cephalosporin prophylaxis, a prompt escalation to a carbapenems-based antibiotic regimen could provide even better GNB coverage (i.e., covering GNBs with extended-spectrum b-lactamases) as an adaptive stepwise choice. However, the routine use of carbapenems to treat infectious complications after cardiac surgery before a definite culture result should be appraised with careful microbiological monitoring to prevent the emergence of multidrug-resistant GNB.
Some limitations should be considered when interpreting these findings. First, this is a single center retrospective study and there is a lack of random assignment. Second, we defined the timepoint of blood stream infection within 30 days after cardiac surgery. One should consider that potential confounders could mediate the connection between long bypass times and bacteremia, considering that the infection period was defined as 30 days post-op, The patients with long bypass times have longer lengths of stay and require more support than patients without long bypass times. However the subgroup analysis of patients with BSI till 7th postoperative day, provided similar results.
Further to tackle the mentioned potential limitation and to validate our results a larger randomized controlled study should be conducted in future. Additionally one should as well keep in mind that the practice of routinely collecting specimens via indwelling catheters must be acknowledged to involve a risk of false positives and is problematic because of colonization of lines [[24], [25]]).
5. Conclusion
Operative variables such as surgery over 5 h duration, aortic cross clamp of 120 min or cardiopulmonary time greater than 240 min as well as patient characteristics, especially preoperative need for hemodialysis were associated with increased risk of primary blood stream infection. Primary blood stream infection at out institution after cardiac surgery was most commonly caused by gram negative bacteria. Current guidelines for perioperative prophylaxis recommend first and second generation cephalosporins, which are not routinely effective against gram negative bacteria. Further prospective study is needed to determine the mechanism for BSI after cardiac surgery to improve the outcome for at risk patients.
Production notes
Author contribution statement
Constantin Mork MD.; Brigita Gahl PhD,; Friedrich Eckstein MD,; Denis A. Berdajs MD: Conceived and designed the experiments, performed the experiments, Analyzed and interpreted the data, Contributed reagents, materials analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Rebollo M.H., Bernal J.M., Llorca J., Rabasa J.M., Revuelta J.M. Nosocomial infections in patients having cardiovascular operations: a multivariate analysis of risk factors. J. Thorac. Cardiovasc. Surg. 1996;112:908–913. doi: 10.1016/S0022-5223(96)70090-9. [DOI] [PubMed] [Google Scholar]
- 2.Michalopoulos A., Geroulanos S., Rosmarakis E.S., Falagas M.E. Frequency, characteristics, and predictors of microbiologically documented nosocomial infections after cardiac surgery. Eur. J. Cardio. Thorac. Surg. 2006;29:456–460. doi: 10.1016/j.ejcts.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 3.De Santo L.S., Bancone C., Santarpino G., et al. Microbiologically documented nosocomial infections after cardiac surgery: an 18-month prospective tertiary care centre report. Eur. J. Cardio. Thorac. Surg. 2008;33:666–672. doi: 10.1016/j.ejcts.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 4.Fowler V.G., Jr., O'Brien S.M., Muhlbaier L.H., Corey G.R., Ferguson T.B., Peterson E.D. Clinical predictors of major infections after cardiac surgery. Circulation. 2005;112(9 Suppl):I358–I365. doi: 10.1161/CIRCULATIONAHA.104.525790. [DOI] [PubMed] [Google Scholar]
- 5.Cove M.E., Spelman D.W., MacLaren G. Infectious complications of cardiac surgery: a clinical review. J. Cardiothorac. Vasc. Anesth. 2012;26:1094–1100. doi: 10.1053/j.jvca.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorski A., Hamouda K., Ozkur M., et al. Cardiac surgery antibiotic prophylaxis and calculated empiric antibiotic therapy. Asian Cardiovasc. Thorac. Ann. 2015;23:282–288. doi: 10.1177/0218492314546028. [DOI] [PubMed] [Google Scholar]
- 7.Edwards F.H., Engelman R.M., Houck P., Shahian D.M., Bridges C.R., Society of Thoracic Surgeons The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part I: duration. Ann. Thorac. Surg. 2006;81:397–404. doi: 10.1016/j.athoracsur.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 8.Engelman R., Shahian D., Shemin R., et al. Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: antibiotic prophylaxis in cardiac surgery, part II: antibiotic choice. Ann. Thorac. Surg. 2007;83:1569–1576. doi: 10.1016/j.athoracsur.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Kappetein A.P., Head S.J., Généreux P., Piazza N., van Mieghem N.M., Blackstone E.H., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur. Heart J. 2012 Oct;33(19):2403–2418. doi: 10.1093/eurheartj/ehs255. [DOI] [PubMed] [Google Scholar]
- 10.Ryan T., Mc Carthy J.F., Rady M.Y., et al. Early bloodstream infection after cardiopulmonary bypass: frequency rate, risk factors, and implications. Crit. Care Med. 1997;25:2009–2014. doi: 10.1097/00003246-199712000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Sahu M.K., Siddharth B., Choudhury A., et al. Incidence, microbiological profile of nosocomial infections, and their antibiotic resistance patterns in a high volume cardiac surgical intensive care unit. Ann. Card Anaesth. 2016;19:281–287. doi: 10.4103/0971-9784.179625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn D.M., Weinstein R.A., Kabins S.A. Infections with gram negative bacilli in a cardiac surgery intensive care unit: the relative role of Enterobacter. J. Hosp. Infect. 1988;11(Suppl A):367–373. doi: 10.1016/0195-6701(88)90212-5. [DOI] [PubMed] [Google Scholar]
- 13.Tago S., Hirai Y., Ainoda Y., Fujita T., Kikuchi K. Gram-negative rod bacteremia after cardiovascular surgery: clinical features and prognostic factors. J. Microbiol. Immunol. Infect. 2017;50:333–338. doi: 10.1016/j.jmii.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y.C., Wu H.Y., Luo C.Y., Lin T.W. Cardiopulmonary bypass time predicts early postoperative Enterobacteriaceae bloodstream infection. Ann. Thorac. Sur. 2019;107:1333–1341. doi: 10.1016/j.athoracsur.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Ahlborg G., Weitzberg E., Lundberg J. Metabolic and vascular effects of circulating endothelin-1 during moderately heavy prolonged exercise. J. Appl. Physiol. 1995;78:2294–2300. doi: 10.1152/jappl.1995.78.6.2294. [DOI] [PubMed] [Google Scholar]
- 16.Wright M.O., Allen-Bridson K., Hebden J.N. Assessment of the accuracy and consistency in the application of standardized surveillance definitions: a summary of the American Journal of Infection Control and National Healthcare Safety Network case studies, 2010–2016. Am. J. Infect. Control. 2017;45:607–611. doi: 10.1016/j.ajic.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMeo M.T., Mutlu E.A., Keshavarzian A., Tobin M.C. Intestinal permeation and gastrointestinal disease. J. Clin. Gastroenterol. 2002;34:385–396. doi: 10.1097/00004836-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Derikx J.P., Poeze M., van Bijnen A.A., Buurman W.A., Heineman E. Evidence for intestinal and liver epithelial cell injury in the early phase of sepsis. Shock. 2007;28:544–554. doi: 10.1097/shk.0b013e3180644e32. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen S.J., Derikx J.P., Vermeulen Windsant I.C., Heijmans J.H., Koeppel T.A., Schurink G.W., Buurman W.A., Jacobs M.J. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann. Surg. 2008;248:117–125$. doi: 10.1097/SLA.0b013e3181784cc5. [DOI] [PubMed] [Google Scholar]
- 20.Ohri S.K., Somasundaram S., Koak Y., et al. The effect of intestinal hypoperfusion on intestinal absorption and permeability during cardiopulmonary bypass. Gastroenterology. 1994;106:318–332. doi: 10.1016/0016-5085(94)90588-6. [DOI] [PubMed] [Google Scholar]
- 21.Ohri S.K., Bjarnason I., Pathi V., et al. Cardiopulmonary bypass impairs small intestinal transport and increases gut permeability. Ann. Thorac. Surg. 1993;55:1080–1086. doi: 10.1016/0003-4975(93)90011-6. [DOI] [PubMed] [Google Scholar]
- 22.Blikslager T., Roberts M.C., Rhoads J.M. Argenzio RA Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery. 1997;121:526–534. doi: 10.1016/s0039-6060(97)90107-0. [DOI] [PubMed] [Google Scholar]
- 23.Salomon J., Ericsson A., Price A., Manithody C., Murry D.J., Chhonker Y.S., Buchanan P., Lindsey M.L., Singh A.B., Jain A.K. Dysbiosis and intestinal barrier dysfunction in pediatric congenital heart disease is exacerbated following cardiopulmonary bypass. JACC Basic tTrans. Sci. 2021 Mar 3;6(4):311–327. doi: 10.1016/j.jacbts.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelijns A.C., Moskowitz A.J., Acker M.A., et al. Cardiothoracic Surgical Trials Network (CTSN). Management practices and major infections after cardiac surgery. J. Am. Coll. Cardiol. 2014;64:372–381. doi: 10.1016/j.jacc.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller J.M., Binnicker M.J., Campbell S., Carroll K.C., Chapin K.C., Gilligan P.H., et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the infectious diseases society of America and the American society for microbiology. Clin. Infect. Dis. 2018 Aug 31;67(6) doi: 10.1093/cid/ciy584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.