Abstract
Introduction
It has been reported that some herbal products affect reproduction. To date, reproductive toxicity of Syzygium guineense has not been investigated although the plant is widely used in treating fertility related problems. Thus, the objective of the current study was to investigate the toxic effects of 70% ethanol extract of S. guineense leaves on the reproductive function and histopathology of reproductive organs in female rats.
Methods
Eighty female Wistar albino rats were randomly divided into four groups where each group consisted of 20 rats. Rats in the first three groups were treated with S. guineense extract at doses of 250, 500 and 1000 mg/kg body weight, respectively. The fourth group served as a control group. The rats were treated for ten consecutive weeks. The length of estrous cycle, reproductive indices, pregnancy outcomes, and number of postnatal deaths were recorded. At necropsy, organ weight was measured, gross and histopathological examinations of ovaries, uterus, and vagina were conducted.
Results
Treatment of rats, with high dose (1000 mg/kg) of S. guineense, significantly prolonged the duration of estrous cycle and reduced weight of uterus and ovaries as well as the number of total and live birth pups. However, there were no significant changes observed in reproductive indices and gross morphology as well as histopathology of ovaries, uterus, and vagina.
Conclusion
Administration of high doses of S. guineense could be toxic to some aspects of the reproductive system of female rats and might also affect reproduction. Therefore, consuming high dose of S. guineense leaves is not recommended.
Keywords: Female rats, Histopathology, Reproductive toxicity, Syzygium guineense leaf
1. Introduction
Recently, due to the global upsurge in the use of herbal products, WHO strongly recommends their safety evaluation. According to the recommendation of WHO, assessing the safety of herbal products is a fundamental principle prior to the incorporation into the health care system. Safety evaluation is also a vital component of quality control [1]. The deleterious effect of the test substances on sexual function and fecundity in females is investigated by reproductive toxicity studies. These effects can be observed on the onset of sexual maturity, sexual behavior and performance, germ cell synthesis and transportation, pattern of the sexual cycle, fertilization, implantation, labor, and pregnancy outcome. In addition, nesting and lactation behavior can be affected by reproductive toxicants [2]. Previous studies revealed the adverse effect of test substances on the reproductive system of laboratory animals and humans. As reported by Smith and Gilbeau [3], Zenick and Clegg [4], and Liu et al. [5], reproductive toxicants affected reproductive function, ovarian cycle, ovulation, and survival rate of litters during lactation.
Syzygium guineense Wall., also known as “water berry” or “water-pear”, is belongs to the family of Myrtaceae [6] It is distributed throughout tropical and subtropical regions of Africa [6,7]. S. guineense has been traditionally used for the treatment of menstrual cycle disorder, and infertility [8,9]. It also has scientifically proven potency against hypertension [10], diabetes mellites [11], cancer [12], malaria [13], and inflammation [14]. Phytochemical analysis done on S. guineense leaves indicated the presence of secondary metabolites such as: alkaloids, terpenoids, anthraquinones, flavonoids, tannins, saponins, and glycosides [13,15].
Studies indicated the toxic effects of some herbal products on reproduction. These effects were observed on the ovarian hormone synthesis, ovarian cycle, ovulation, and survival rate of litters during lactation [5,16]. S. guineense leaf, beyond its traditional and scientifically established pharmacologic importance, its toxic effect on the reproductive structures and functions has not been investigated yet. Thus, this study was aimed at investigating the toxic effects of ethanol extract of S. guineense leaves on the reproductive function and histopathology of reproductive organs in female rats.
2. Materials and methods
2.1. Collection, processing and extraction of plant material
Fresh leaves of the experimental plant were gathered from the field in Woliso, 113 km West of Addis Ababa, Ethiopia. The plant was identified and authenticated in the National Herbarium, Department of Plant Biology and Biodiversity Management, Addis Ababa University, Ethiopia. A reference number of the specimen (MS 001) was allocated for future utilization. Extraction was carried out as previously discussed by Abebe et al. [17] in line with Zhang et al. [18]. Briefly, the leaves were cleaned, shade dried, coarsely crushed by a grinder and blended with 70% ethanol and kept on an orbital shaker (Gallenkamp, UK) for 24 h at 130 g. The mixture was then filtered (Whatman No. 1, Whatman Ltd. England), and the filtrate concentrated using Rota Vapor (Büchi Rota Vapor R-205, Switzerland) at a temperature not exceeding 40 °C and stored in a refrigerator at −4 °C until use [19].
2.2. Experimental animals
Female (nulliparous) Wistar albino rats, weighing 220–240 g, and 10–12 weeks old were included in this study. Experimental animals were acquired from the Ethiopian Public Health Institute (EPHI) animal breeding unit. The test animals were habituated for a week and maintained for the experiment in the laboratory of the Traditional and Modern Medicine Research Directorate (TMMRD) of EPHI. The rats were kept in a stainless-steel cage in a room where temperature (23 ± 3 °C) and relative humidity of 50 ± 10% were controlled as well as an alternating 12-h light-dark cycle was maintained. During the entire treatment period, test animals were provided with standard laboratory diet (75% carbohydrate, 16% protein, 55% fat, 3.6% calcium, and 0.4% phosphorus) and water with no limit.
Eighty female rats were randomly allocated to four groups, each consisting twenty rats per group. The first three groups (I-III) received S. guineense extract (group I: 250, group II: 500, and group III: 1000 mg/kg), based on a previous efficacy study [11]. The fourth group (control) administered distilled water. Administration of distilled water and extract was done via an intragastric tube [20]. The investigators blindly measured the respective outcomes.
Rats were treated for ten consecutive weeks: two premating, two mating, three gestational, and three lactational weeks. Following two weeks of the premating treatment period, female rats were mated with unrelated males of proven fertility in a 1:1 male-to-female ratio and placed in separate cage until pregnancy was confirmed. Female rats were kept in a cage containing a male partner for two weeks until the pregnancy was confirmed. Within two weeks mating period, every morning, female rats were inspected for the presence of a vaginal plug. Then, smear test was conducted detect sperm cells in the vaginal fluid. Once the sperm cell is confirmed in the smear, this day was considered as day zero of pregnancy or gestational day (GD) zero. After pregnancy is confirmed, female rats were separated from males and continue the treatment during pregnancy and lactation [20].
2.3. Measuring estrous cycle
The effect of S. guineense extract on the length of estrous cycle was evaluated by conducting vaginal smear test daily. The estrous cycle was measured in the first two weeks of the premating period. Ten rats per group were randomly selected for estrous cycle measurement. Rats were held in a restrainer and vaginal fluid was collected by a plastic pipette filled with 10 μl of normal saline (0.9% Na Cl). The tip of a plastic pipette was gently inserted into the rat's vagina. The aspirated vaginal fluid was fairly distributed over the labeled glass slides. The slides were kept at room temperature until they dry. For staining purposes, few droplets of crystal violet were added to it and kept for 1 min. The crystal violet was washed with distilled water and glycerol was added to increase the optical property. Finally, it was covered by cover-slip and examined using a binocular light microscope. Using microscope examination, each phase of the estrous cycle was identified. These were: proestrus, estrus, metestrus, and diestrus. The full duration of these phases was considered as the length of one estrous cycle [21,22]. Estrous cycle was always measured at 8:00–9:00 a.m. [23]. The difference in the estrous cycle length was compared among the experimental groups.
2.4. Reproductive indices and pregnancy outcomes
The date of pairing, insemination, and delivery was registered. The pre-coital interval (pairing to insemination) and the length of pregnancy (insemination to birth) were computed. In addition, the number of rats capable of fertilization and the number of pregnant rats was also recorded.
The mating index was computed by dividing the number of rats with evidence of mating by the number of paired and multiplied by hundred. Fertility index was computed: (number of females with evidence of pregnancy/number of paired) x 100. In addition, gestation index was also computed by dividing the number of females delivering a viable litter by the number of females with evidence of pregnancy and multiplied by hundred. The number of live births, stillbirth, and sex of the pups were recorded. Moreover, gestational survival index, number of postnatal deaths of litters on postnatal days (PNDs) 1, 4, 7, 14, 21 and sex ratio were calculated. The day on which rats gave birth was considered as PND zero for pups and lactational day (LD) zero for dams.
2.5. Weight and macroscopic examination of reproductive organs
Once the treatment schedule was completed, all rats were killed with an intraperitoneal administration of pentobarbital [24]. A median incision on the abdominal wall was performed to reveal visceral organs. Organs were macroscopically examined for the presence of structural malformations or treatment-related pathological changes. Paired ovaries and uterus were dissected free of fat and weighed separately.
2.6. Histopathological investigation of reproductive organs
Histopathological examination of ovaries, uterus, and vagina was performed. Sample from each organ was fixed with 10% formalin and further processed following routine tissue processing steps. After tissue processing, tissues were stained with hematoxylin and eosin (H & E). Tissue processing and staining steps are as described elsewhere by Abebe et al. [17] in accordance with Bancroft's Theory and Practice of Histological Techniques [25]. A detailed microscopic examination, for any treatment-related changes, was performed using a binocular light microscope. The histological appearance of organs from treatment groups was compared with the control group. After investigation, representative photomicrographs were captured with an automated inbuilt digital microscope camera (Leica EC4, Germany) under total magnification of 40×, 100x, and 200x.
2.7. Data analysis
Data analysis was done by a statistical package for social science (SPSS) version 24. Variation between treatment and control groups was analyzed by one-way analysis of variance (ANOVA) followed by Turkey and Games-Howell post Hoc tests. Before conducting ANOVA, independence of observations, normality of data, and homogeneity of variance were checked. Shapiro-Wilk test of normality was conducted to check if the data were normally distributed or not. Data were subjected to Levene's test to meet the test of homogeneity of variance. Difference in the percentages of reproductive indicis was analyzed by chi square test. Results are expressed as mean ± standard deviation of mean (SDM) and percentages. P-value <0.05 was considered as a statistical level of significance.
3. Results
The experimental rats well tolerated the 10-week treatment of S. guineense. No significant signs and symptoms of sever toxicity on the skin, mucus membranes of the mouth and eyes, respiratory pattern, and motor activities were observed. In addition, no rats were dead during the entire treatment period.
3.1. Effects on the estrous cycle
The length of the estrous cycle and its four stages were recorded based on the examination of a vaginal smear. The result displayed in Table 1 indicated that the length of estrous cycle was significantly longer for the high dose treated group (5.8 ± 1.4) compared to those in the control group (4.3 ± 0.5), P-value <0.05.
Table 1.
Length of estrous cycle of rats treated with Syzygium guineense.
| Group |
||||
|---|---|---|---|---|
| Group I 250 mg/kg | Group II 500 mg/kg | Group III 1000 mg/kg | Group IV Control | |
| Length of estrous cycle (days/dam) | 5.1 ± 1.2 | 4.5 ± 0.9 | 5.8 ± 1.4a | 4.3 ± 0.5 |
Results are expressed as mean ± standard deviation of mean.
Significantly different from control group (P-value <0.05), One-Way ANOVA, Games-Howell post Hoc test.
3.2. Effects on the reproductive indices
Administration of S. guineense to rats did not bring significant change on the mating, fertility, and gestation indices. All female rats, except two (10%), in group three (1000 mg/kg) were pregnant. The pre-coital interval (the days required to be inseminated since pairing) was significantly longer (7 ± 3.6) in rats administered with 1000 mg/kg body weight of the test substance, compared to the control group (2.8 ± 1.2). However, the duration of pregnancy was not significantly altered by the treatment (Table 2).
Table 2.
Reproductive indices of rats treated with Syzygium guineense.
| Reproductive indices | Group |
|||
|---|---|---|---|---|
| Group I 250 mg/kg | Group II 500 mg/kg | Group III 1000 mg/kg | Group IV Control | |
| Mating Index (%) | 100 | 100 | 90 | 100 |
| Fertility index (%) | 100 | 100 | 90 | 100 |
| Gestation index (%) | 100 | 100 | 90 | 100 |
| Pre-coital interval (days/dam) | 5.6 ± 4.7 | 3.2 ± 2.8 | 7.0 ± 3.6a | 2.8 ± 1.2 |
| Pregnancy duration (days/dam) | 22.2 ± 0.9 | 21.5 ± 1.2 | 21.4 ± 0.7 | 21.5 ± 0.7 |
Results are expressed as mean ± standard deviation of mean and percentage.
Significantly different from control group (P-value <0.05), One-Way ANOVA and Chi-square.
3.3. Effects on pregnancy outcomes
Data related to the pregnancy outcomes are presented in Table 3. The mean number of litters (total), male, and live births were significantly reduced in the treatment groups, compared to those in the control group. The number of stillbirth litters did not reveal a significant change between the treatment and control groups. Concerning the sex ratio, a higher number of male litters (1.2:1, male: female) were reported from the high dose treated group. On the contrary, the number of male litters was lesser (0.58:1, male: female) in the low dose group, but not statistically significant.
Table 3.
Birth outcomes of parental rats treated with of Syzygium guineense.
| Birth outcomes | Group |
||||
|---|---|---|---|---|---|
| Group I 250 mg/kg (n = 20) | Group II 500 mg/kg (n = 20) | Group III 1000 mg/kg (n = 18) | Group IV Control (n = 20) | ||
| No of litter/dam | Male | 3.2 ± 1.3a | 3.8 ± 1.6a | 4.7 ± 1.7 | 6 ± 1.8 |
| Female | 5.4 ± 1.9 | 4.2 ± 2.1 | 4.0 ± 1.7 | 4.6 ± 1.6 | |
| Total | 8.6 ± 1.9a | 8.0 ± 2.3a | 8.7 ± 1.9 | 10.6 ± 0.8 | |
| Live birth | 8.2 ± 1.9a | 7.9 ± 2.2a | 8.3 ± 2.2 | 10.2 ± 0.8 | |
| Stillbirth | 0.4 ± 0.8 | 0.1 ± 0.3 | 0.4 ± 0.7 | 0.4 ± 0.7 | |
| Male: Female ratio | 0.58:1 | 0.9:1 | 1.2:1 | 0.77:1 | |
Results are expressed as %, mean ± standard deviation of mean, and ratio.
Significantly different from control group (P-value <0.05), One-Way ANOVA; n: means number of dams.
3.4. Effects on the postnatal survival of litters
Gestational survival index (viability at birth) was more than 95% in the treatment and control groups. Therefore, no significant variation was observed between treatment and control groups. The mean postnatal death of litters was calculated on postnatal days (PNDs) 1, 4, 7, 14, and 21. There was no pup death reported on PND 1 in any of the groups. From PNDs 4–21, the mean postnatal death of litters was not significantly varied between treatment and control groups (Table 4).
Table 4.
Gestation survival index and postnatal death of litters treated with Syzygium guineense.
| Reproductive indices | Group |
||||
|---|---|---|---|---|---|
| Group I 250 mg/kg | Group II 500 mg/kg | Group III 1000 mg/kg | Group IV Control | ||
| Gestation survival index % | 95.8 | 98.8 | 96.2 | 96.5 | |
| No of postnatal death | PND 1 | 0.0 | 0.0 | 0.0 | 0.0 |
| PND 4 | 0.33 ± 0.5 | 0.6 ± 1.6 | 1.4 ± 2.1 | 0.63 ± 1.4 | |
| PND 7 | 0.0 | 0.3 ± 0.7 | 0.1 ± 0.3 | 0.1 ± 0.4 | |
| PND 14 | 0.0 | 0.9 ± 1.4 | 0.0 | 0.1 ± 0.4 | |
| PND 21 | 0.0 | 0.3 ± 0.7 | 0.1 ± 0.3 | 0.0 | |
Results are expressed as mean ± standard deviation of mean and percentages, One-Way ANOVA and Chi square.
3.5. Gross examination and organ weight measurement results
Each reproductive organ was freshly examined for any grass abnormalities prior to microscopic examination. The result of macroscopic examination did not reveal any abnormalities in the treated or control groups.
In the current study, relative organ weight was calculated by dividing absolute organ weight by rat weight at necropsy and multiplied by a hundred. Administration of ethanol leaf extract of S. guineense produced a significant change in the relative weight of female reproductive organs: uterus and ovaries. There was a significant reduction in uterus and ovarian weight of rats treated with 500 and 1000 mg/kg of S. guineense extract compared to those in the control group (Table 5).
Table 5.
Relative organ weight of rats treated with Syzygium guineense.
| Organ weight (g) | Group |
|||
|---|---|---|---|---|
| Group I (250 mg/kg) | Group II (500 mg/kg) | Group III (1000 mg/kg) | Group IV (Control) | |
| Uterus | 0.173 ± 0.083 | 0.145 ± 0.035a | 0.123 ± 0.045a | 0.257 ± 0.103 |
| Ovary | 0.024 ± 0.005 | 0.018 ± 0.002a | 0.018 ± 0.003a | 0.025 ± 0.004 |
Results are expressed as mean ± standard deviation of mean.
Significantly different from control group (P-value was <0.05), One-Way ANOVA.
3.6. Histopathological examination results
Female reproductive organs, mainly ovaries, uterus, and vagina were microscopically examined following the routine tissue processing. The result of this examination showed that there were no significant microscopic changes in the above reproductive organs of the treated rats.
As presented in Fig. 1(a–d), the ovaries appeared normal, containing ovarian follicles of different stages, corpus luteum, vascular stroma, and covering germinal epithelium. No follicular cyst, interstitial stromal cell hyperplasia/hypertrophy, or increased/decreased number of follicular cells were observed in the ovaries of the treated groups compared to the control group.
Fig. 1.
Photomicrograph of rat ovary showing normal ovarian cortex, (a &b) Sections taken from the rat treated with 1000 mg/kg of 70% ethanol extract of Syzygium guineense, (c & d) Sections taken from control group. F: Different stage follicular cell, GF: Graafian follicle, GC: Granulosa cells; H and E stain, a & c 40x, b & d 100x total magnification.
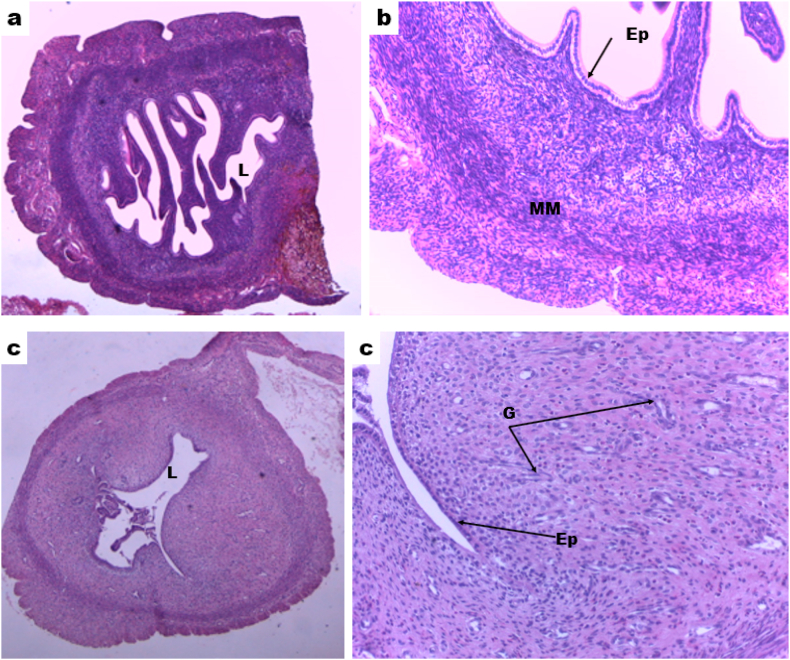
Moreover, in the uteri of the experimental and control group rats, the epithelial lining, endometrial glands, and the myometrium were not significantly affected by the treatment. Thus, no metaplastic change, hypertrophy, or hyperplasia of the epithelial lining, neutrophil infiltration in the endometrial stroma, cystic changes of endometrial glands were seen in any of the uteri from all groups (Fig. 2(a–d)).
Fig. 2.
Photomicrograph of rat uterus showing normal uterine epithelium and musculature, (a & b) Sections taken from rat treated with 1000 mg/kg body weight of 70% ethanol extract of Syzygium guineense, (c & d) Sections taken from control group. L: Uterine lumen, Ep: Epithelium, G: Uterine glands, and MM: Muscle; H and E stain, a & c 40x, b & d 100x total magnification.
In the microscopic examination of the vagina, only one, from twenty rats treated with 1000 mg/kg of the plant extract showed fibroma in the vagina (Fig. 3 a & b). The rest of the rats in all groups revealed normal histological appearance of the vagina in both the epithelium and the other supporting tissues (Fig. 3 c & d).
Fig. 3.
Photomicrograph of rat uterus, (a & b) Sections taken from rat treated with 1000 mg/kg body weight of 70% ethanol extract of Syzygium guineense, showing fibroma (F) in the vaginal musculature, (c & d) Sections taken from control group showing normal vaginal epithelium, musculature, and adventitia. L: Vaginal lumen, Ep: Epithelium, BV: Blood vessels, and MM: Muscle; H and E stain, a & c 100x, b & d 200x total magnification.
4. Discussion
The effect of a test plant on the reproductive system of rats can be manifested by alteration in the phases or duration of the estrous cycle and changes in: normal structure of the reproductive organs, mating performance, pregnancy outcomes, and postnatal survival of litters [26]. In the current study, the reproductive toxicity of S. guineense was investigated by measuring the length of the estrous cycle, time taken for mating/insemination, and duration of pregnancy. Moreover, pregnancy outcomes and histopathologic investigation of reproductive organs also were evaluated. The results of the current study indicate that treatment with high dose of S. guineense prolonged the duration of estrous cycle and reduced relative organ weight of uterus and ovaries as well as the number of total and live births.
The length of the estrous cycle was affected by administration of a high dose of S. guineense. Rats that received 1000 mg/kg body weight of the extract of S. guineense had longer duration of estrous cycle. This was also supported by delayed pre-coital interval (time to insemination since they were mated) in the high dose treated group. The pre-coital interval was 7 ± 3.6 days for 1000 mg/kg dose treated rats while that of the control was 2.8 ± 1.2 days. This observation indicated that the plant extract affected the estrous cycle of rats. These findings can be a benchmark for investigating the effect of S. guineense on fertility. The exact mechanism of how the plant extract delayed the estrous cycle still needs further investigation. However, other researchers have reported that the presence of active components like alkaloids, tannins, and flavonoids in the crude extract exert an antigonadotrophic effect and suppress ovulation [27,28]. Alkaloids, tannins, and flavonoids are predominant components of S. guineense leaves [13,29]. Therefore, this may have been the reason for the delay in estrous cycle observed in the current study. The other reproductive indices: mating index, fertility index, and pregnancy duration were not significantly affected by treatment with S. guineense extract.
In the pregnancy outcomes, the number of total and live births was reduced by treatment with S. guineense leaf extract in low and middle doses but not in high dose group. In another study, it was reported that administration of S. guineense reduced the crown-rump length of rat fetuses [30]. S. guineense's secondary metabolites such as alkaloids might have been responsible for the reduced number of total and live births. As reported by other investigators [31], plant's alkaloids cross placental membrane and affect development of the embryo. However, it needs further investigation to explore why not the high dose group did not significantly affected.
In the current study, we did not find significant variation in the number of postnatal deaths across all groups. The pup's weights measured at PND 0, 4, 7, 14, and 21 were not different in the treated and control groups. Also, insignificant difference in the AGD of male and female pups recorded at PND 4 was observed. Moreover, there was no nipples/areola developed in male pups. Therefore, it may be possible to conclude that S. guineense did not interfere with the normal growth of survived pups.
The relative weight of uterus and ovary was significantly reduced by treatment with 500 and 1000 mg/kg doses of the plant extract. As reported by Wolfsegger et al. [32], alteration in organ weight can be due to the direct toxic effect of the test substance even in the absence of structural change in the organ. Therefore, the observed weight changes of the uterus and ovaries may be attributed to the toxic effect of S. guineense. S. guineense's alkaloids may be responsible for this effect because another research reports indicated that alkaloids in the plant were toxic [33,34].
Histopathologic data are essential part of toxicological studies that investigate hazards and elucidate toxic mechanisms [35,36]. In female rats, contrary to the observed reduction in weights of the ovary and uterus in the high dose treated group, pathological microscopic changes in the uterus or ovaries were absent in any of the treatment groups. A vaginal fibroma is a very rare benign, noninfiltrating, and smooth muscle-derived tumor [37]. The presence of fibroma in one of the rats treated with 1000 mg/kg body weight of the extract was not statistically significant and may not have been treatment-related. It has been reported by another investigator that tannins present in the S. guineense repress Wnt signaling and Wnt-dependent tumor cell proliferation [38].
5. Conclusion
Even though the test plant did not produce significant microscopic structural changes on female reproductive organs, consuming S. guineense leaf could be toxic to some aspects of the reproductive system of female rats and might affect reproduction. Because we found prolonged duration of estrous cycle and reduced weight of uterus and ovaries as well as number of live births have been observed in the current study following consumption of this plant. Therefore, utilization of high dose of S. guineense leaf is not recommended.
Ethics approval and consent to participate
All the experimental procedures were done in accordance with the relevant guidelines and regulations. Ethical approval by institutional review board (IRB) of the College of Health Sciences, Addis Ababa University (AAU), with protocol number AAUMF03-008, in accordance with Organization for the Economic Co-operation and Development's (OECD) guideline, test number 443 [20] were received prior to the study. In addition, experimental plant collection was conducted after getting a permission letter from EPHI and Oromia region where the plant was collected. Plant collection process was following the standard of EPHI herbarium in line with Convention on the International Trade in Endangered Species of Wild Fauna and Flora [39]. Our study is reported in accordance with arrival guidelines on animal research.
Author contribution statement
Melese Shenkut Abebe: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kaleab Asres; Yonas Bekuretsion; Samuel Woldekidan; Eyob Debebe; Abiy Abebe; Bihonegn Sisay; Girma Seyoum: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
Warmest gratitude is extended to Addis Ababa University and EPHI for their support.
Abbreviations
- AAU
Addis Ababa University
- ANOVA
Analysis of variance
- EPHI
Ethiopian Public Health Institute
- GD
Gestational day
- H & E
hematoxylin and eosin
- IRB
Institutional review board
- LD
Lactational day
- OECD
Organization for Economic Co-operation and Development
- PND
Postnatal day
- SDM
Standard deviation of mean
- SPSS
Statistical package for social science
- TMMRD
Traditional and Modern Medicine Research Directorate
References
- 1.WHO . World Health Organization; Geneva, Switzerland: 2004. WHO Guidelines on Safety Monitoring of Herbal Medicines in Pharmacovigilance Systems. [Google Scholar]
- 2.Osha U. United Nations Economic Commission for Europe (UNECE); 2013. Globally Harmonized System of Classification and Labelling of Chemicals (GHS) p. 224. [Google Scholar]
- 3.Smith C.G., Gilbeau P.M. In: Endocrine Toxicology. Thomas J.A., Korach K.S., McLachlan J.A., editors. Raven Press; New York: 1985. Drug abuse effects on reproductive hormones; pp. 249–267. [Google Scholar]
- 4.Zenick H., Clegg E. Issues in risk assessment in male reproductive toxicology. J. Am. Coll. Toxicol. 1986;5(4):249–259. [Google Scholar]
- 5.Liu Q., et al. The reproductive toxicity of mequindox in a two-generation study in Wistar rats. Front. Pharmacol. 2018;9:870. doi: 10.3389/fphar.2018.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dharani N. A review of traditional uses and phytochemical constituents of indigenous Syzygium species in east Africa. Pharm J Kenya. 2016;22(4):123–127. [Google Scholar]
- 7.Ahmad B., et al. Syzygium (Myrtaceae): monographing a taxonomic giant via 22 coordinated regional revisions. Peer J Preprints. 2016;4 [Google Scholar]
- 8.Nguyen T.L., et al. Flavonoids, gallotannins and ellagitannins in Syzygium guineense and the traditional use among Malian healers. J. Ethnopharmacol. 2016;192:450–458. doi: 10.1016/j.jep.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Nigatu B. MSc Thesis submitted to the school of graduate studies, department of pharmacology, Addis Ababa University; 2004. Antispasmodic, Antidiarrheal and LD50 Determination of Syzygium Guineense in Animal Models. [Google Scholar]
- 10.Ayele Y., Urga K., Engidawork E. Evaluation of in vivo antihypertensive and in vitro vasodepressor activities of the leaf extract of Syzygium guineense (Willd) DC. Phytother Res. 2010;24(10):1457–1462. doi: 10.1002/ptr.3141. [DOI] [PubMed] [Google Scholar]
- 11.Ezenyi I.C., et al. Antidiabetic potentials of Syzygium guineense methanol leaf extract. J. Phytopharmacol. 2016;5(4):150–156. [Google Scholar]
- 12.Esubalew S.T., et al. Review of ethnobotanical and ethnopharmacological evidences of some Ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiop. J. Health Dev. 2017;31(3):161–187. [Google Scholar]
- 13.Tadesse S.A., Wubneh Z.B. Antimalarial activity of Syzygium guineense during early and established Plasmodium infection in rodent models. BMC Compl. Alternative Med. 2017;17(1):21. doi: 10.1186/s12906-016-1538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ior I., Otimenyin I., Umar M. Anti-inflammatory and analgesic activities of the ethanolic extract of the leaf of Syzygium guineense in rats and mice. IDSR Journal of pharmacy. 2012;2(4):33. [Google Scholar]
- 15.Djoukeng J., et al. Antibacterial triterpenes from Syzygium guineense (myrtaceae) J. Ethnopharmacol. 2005;101(1–3):283–286. doi: 10.1016/j.jep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Smith C., Asch R. Drug abuse and reproduction. Fertil. Steril. 1987;48(3):355–373. doi: 10.1016/s0015-0282(16)59400-x. [DOI] [PubMed] [Google Scholar]
- 17.Abebe M.S., et al. Sub-chronic toxicity of ethanol leaf extract of Syzygium guineense on the biochemical parameters and histopathology of liver and kidney in the rats. Toxicol Rep. 2021;8:822–828. doi: 10.1016/j.toxrep.2021.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q.-W., Lin L.-G., Ye W.-C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13(1):1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abebe M.S. Acute and subacute toxicity of rhamnus prinoides leaves on histopathology of liver, kidney, and brain tissues, and biochemical profile of rats. J. Toxicol. 2023:2023. doi: 10.1155/2023/3105615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.OECD . 2018. Guideline for Testing of Chemicals. Extended One-Generation Reproductive Toxicity Study. Test No. 443; pp. 1–25. [Google Scholar]
- 21.Mandl A.M. The phases of the oestrous cycle in the adult white rat. J. Exp. Biol. 1951;28(4):576–584. [Google Scholar]
- 22.Marcondes F., Bianchi F., Tanno A. Determination of the estrous cycle phases of rats: some helpful considerations. Braz. J. Biol. 2002;62(4A):609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- 23.Goldman J.M., Murr A.S., Cooper R.L. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. Part B Dev. Reproductive Toxicol. 2007;80(2):84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 24.Underwood W., Anthony R. American Veterinary Medical Association; 2020. AVMA Guidelines for the Euthanasia of Animals: 2020; pp. 60–63. [Google Scholar]
- 25.Bancroft J.D., Floyd A.D., Suvarna S.K. seventh ed. Cherchil Livingstone; 2013. Bancroft's Theory and Practice of Histological Techniques; pp. 70–216. [Google Scholar]
- 26.Westwood F.R. The female rat reproductive cycle: a practical histological guide to staging. Toxicol. Pathol. 2008;36(3):375–384. doi: 10.1177/0192623308315665. [DOI] [PubMed] [Google Scholar]
- 27.Benie T., Thieulant M.-L. Mechanisms underlying antigonadotropic effects of some traditional plant extracts in pituitary cell culture. Phytomedicine. 2004;11(2–3):157–164. doi: 10.1078/0944-7113-00326. [DOI] [PubMed] [Google Scholar]
- 28.Benie T., Thieulant M.L. Interaction of some traditional plant extracts with uterine oestrogen or progestin receptors. Phytother Res. 2003;17(7):756–760. doi: 10.1002/ptr.1208. [DOI] [PubMed] [Google Scholar]
- 29.Pieme C.A., et al. Syzyguim guineense extracts show antioxidant activities and beneficial activities on oxidative stress induced by ferric chloride in the liver homogenate. Antioxidants. 2014;3(3):618–635. doi: 10.3390/antiox3030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abebe M., et al. Teratogenic effect of high dose of Syzygium guineense (myrtaceae) leaves on wistar albino rat embryos and fetuses. Evid. base Compl. Alternative Med. 2021:2021. doi: 10.1155/2021/6677395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schröder-Van Der Elst J., et al. Synthetic flavonoids cross the placenta in the rat and are found in fetal brain. Am. J. Physiol. Endocrinol. Metabol. 1998;274(2):E253–E256. doi: 10.1152/ajpendo.1998.274.2.E253. [DOI] [PubMed] [Google Scholar]
- 32.Wolfsegger M.J., et al. A note on statistical analysis of organ weights in non-clinical toxicological studies. Toxicol. Appl. Pharmacol. 2009;240(1):117–122. doi: 10.1016/j.taap.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Chen T., Mei N., Fu P.P. Genotoxicity of pyrrolizidine alkaloids. J. Appl. Toxicol. 2010;30(3):183–196. doi: 10.1002/jat.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaz G.J. Toxicosis by plant alkaloids in humans and animals in Colombia. Toxins. 2015;7(12):5408–5416. doi: 10.3390/toxins7124892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dua P.N., Jackson B.A. Review of pathology data for regulatory purposes. Toxicol. Pathol. 1988;16(4):443–450. doi: 10.1177/019262338801600404. [DOI] [PubMed] [Google Scholar]
- 36.James R. The relevance of clinical pathology to toxicology studies. Comparat. Haematol. Int. 1993;3(4):190–195. [Google Scholar]
- 37.Lőrincz J., Jakab A., Török P. Vaginal Fibroma: an unusual vaginal tumor. J. Gynecol. Surg. 2017;33(3):114–116. [Google Scholar]
- 38.Koval A., et al. Tannins from Syzygium guineense suppress Wnt signaling and proliferation of Wnt-dependent tumors through a direct effect on secreted Wnts. Cancer Lett. 2018;435:110–120. doi: 10.1016/j.canlet.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 39.CITES Convention on international trade in endangered species of Wild Fauna and Flora. 2009. http://www.cites.org [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.