Abstract
The nitrogen use efficiency (NUE) of maize is usually below 60%. Considering future food supply and climate change, selective breeding of maize with high nitrogen (N)-efficient varieties, covering genetic diversities, is an effective strategy for identifying specific elements for controlling NUE and productivity per arable farming unit while reducing environmental damage. This study evaluated the yield and nitrous oxide (N2O) emission of 30 maize varieties under two different N doses of 57.5 kg N ha−1 (N1, N-sufficient) and 173 kg N ha−1 (N3, N-high) applied in two equal splits on 2 and 4 weeks after germination (WAG). Then, the tested maize varieties were categorized into four groups based on the grain yield and cumulative N2O, that is, efficient-efficient (EE) under both N1 and N3, high-N efficient (HNE) under N3 only, low-N efficient (LNE) under N1 only, and nonefficient-nonefficient (NN) under neither N1 nor N3. Maize yield was significantly positively correlated with shoot biomass, N-accumulation, and kernel-number under N1 and with N2O-flux at 5 WAG, NH4+, shoot biomass, and all of yield components under N3, whereas cumulative N2O showed a significant positive correlation with NO3− under N3 only and with N2O flux at 3 WAG under both N levels. The EE generally showed higher grain yield, yield components, N-accumulation, dry matter accumulation, root volume, and NH4+ in soil and lower cumulative N2O and NO3− in soil relative to NN maize varieties. The EE variety groups of maize can be a feasible strategy for increasing N fertilizer efficiency without reducing maize production as well as decrease the negative impact of N lost in agricultural system.
Keywords: Maize, Yield, Nitrous oxide, Nitrogen, Efficient
1. Introduction
Nitrogen (N) is an essential element for organisms, including plants, and plays a key role in certain ecosystem functions and biochemistry (a part of chlorophyll and enzyme) [1,2]. Nitrogen compounds in the biosphere are abundant in different physical and chemical properties giving nitrogen exceptionally complex and considerable variables with respect to their flow and transformation. The nitrogen cycle in the agricultural ecosystem is majorly controlled by the application of fertilizer and nitrogen uptake by plant trough harvesting [2,3]. The global demand for N-fertilizer increases annually, and rose from approximately 105 million tons in 2016 to approximately 163 million tons in 2022. Furthermore, the potential world balance of N gradually decreased by 27.44% in 2022 compared to that in 2019 [4].
The increasing use of N for fertilizer is used by some farmers to maintain and improve maize productivity considering the increasing demand for maize for food, livestock feed, and biofuel [5]. Maize (150–200 kg ha−1) has relatively higher N thresholds than wheat (140–210 kg ha−1) and rice (90–135 kg ha−1) [6]. Nitrogen can contribute to such environmental problems as air and groundwater pollution, and decreasing quality in water surface owing to the low level of nitrogen use efficiency (NUE) in agricultural land [7]. Maize NUE is the grain production per unit of available N in soil and is expressed by the N uptake of maize and conversion of the available N into grain yield [8,9]. The NUE in an agricultural system is usually below 50%; the rest of the nitrogen is lost from soil through leaching (NO3−, NH4+), run-off (NO3−), and volatilization in the form of N2O, N2, Nx, or NH3 [10,11].
Nitrous oxide (N2O), which is released through denitrification-nitrification processes in soil, plays an important role in the depletion of stratosphere ozone [12]. The emission of N2O from soil is the greatest contributor of global greenhouse gases majorly driven by soil properties [13]. Moreover, Ding et al. [14] stated that the largest source of N2O emission is dominated by the application of nitrogen fertilizer to improve plant yield. However, nitrogen availability is exceptionally important in improving plant yield. Therefore, the management of nitrogen is one of the essential factors for suppressing N2O emission without decreasing plant productivity [10,11].
Owing to their low level NUE and N-recovery during plant production, most agricultural systems exhibit inefficient use of nitrogen from both applied fertilizer and indigenous N thus inducing the loss of N and adversely affecting the environmental [15]. The NUE of maize is usually below 60% [16], which is mainly attributed to the interaction among genotypes, emerging environmental conditions, and agronomic management, particularly nitrogen application management [17]. Considering future food supply and climate change, selective breeding of high N efficient maize varieties covering genetic diversity is an effective strategy for identifying specific elements controlling NUE and productivity per arable land unit while reducing the negative environmental impact [5,18,19]. The high yielding rate of maize varieties with low potential loss-N particularly through emission-N₂O is a promising method for reducing N-loss and increasing NUE without decreasing plant productivity.
Maize is the second most important crop after rice in Indonesia. In Indonesia government decided on intensification methods such as cropping intensity and providing sufficient fertilizer as an agricultural development policy related to improving food crop productivity for more than 4 decades [20]. Moreover, farmers in Indonesia including maize farmers prefer to apply nitrogen fertilizer rather than K and P caused by the quick and high response of N application to the plant. This encourages the low efficient N fertilizer used in agricultural system and environmental damage. Most of maize farmers in lowland Java could apply N in form of urea up to 600 kg ha−1 [21]. This study primarily focused on the characterization of Indonesian maize varieties based on yield and cumulative N2O emission, particularly maize varieties that were common, superior, and new varieties used by farmers and considered to have adaptive responses to different N application rates. We hypothesize that certain Indonesian maize varieties are N-high tolerant and belong to the N-efficient (EE). The N-efficient varieties exhibit high yield and low cumulative N2O emission both under N-sufficient and N-high conditions.
2. Materials and methods
2.1. Experiment site description
The field experiment was conducted in Ngablak, Magelang Regency, Indonesia (7°22′44.7″S, 110°23′13.4″E) during the October 2020 and February 2021 growing season. The annual mean air temperature and precipitation from 2017 to 2021 were 25.1 °C (max 36.07 °C and min. 17.14 °C) and 2225 mm, respectively. The soil type was Andosols with a soil pH-H2O of 6.6 and soil pH-NaF of 11.1. Soil chemical properties of the experimental site were 6.3% organic C; 0.58 kg dm−3 bulk density; 14.05 (cmol(+) kg−1) cation change capacity; 0.24% total N; 1.1 mg kg−1 NH4+, and 0.2 mg kg−1 NO3−.
2.2. Experimental design
The experiment was performed according to the randomized complete block design (RCBD) with three replications. Thirty maize varieties including: (1) composite varieties from the Cereal Plant Research Institution in South Sulawesi, Indonesia (Bisma, Sukmaraga, Anoman, Pulut URI, Lamuru, Provit A1, Srikandi Putih, and Bima 10); (2) local varieties (Dale Lei (Gorontalo) and Lokal Poso (Poso, Sulawesi)) from Institute for Agricultural Technology in North Sulawesi, Indonesia, and Guluk-guluk, Manding and Talango (Madura, East Java) from Institute for Agricultural Technology in East Java, Indonesia; and (3) hybrid varieties obtained from certain private agricultural companies in Indonesia, that is, Arjuna (Cereal Plant Research Institution in Maros, South Sulawesi, Indonesia), Pioneer 35, Pioneer 36, Pioneer 27, and Pioneer 21 (PT. Dupont Indonesia), Bisi 2, Bisi 220, Bisi 228, Bisi 226, Bisi 18, Bisi 99, and Bisi 79 (PT. Bisi International, Tbk in Kediri, East Java, Indonesia), NK 33, NK 6172, NK 7202, and NK 007 (PT. Sygenta Indonesia), and Pertiwi 3 (PT. Agri Makmur Pertiwi in Kediri, East Java, Indonesia), were studied. First, each plot was set to a size of 150 × 100 cm with spacing between plants being 75 × 20 cm. Two maize seeds were then sown in each spot (one seed per hole) and after germination (approximately 7 days), the seedlings were reduced to one per spot. There were twelve individual plants in every plot. Potassium chloride (KCl) fertilizer and super phosphate (SP36) as sources of K and P, respectively, were added to the soil as basal fertilizer (1 week after germination, WAG) at 75 kg ha−1 (equal to 22.41 kg K+ ha−1) and 75 kg ha−1 (equal to 37.5 kg P2O5 ha−1) of the soil, respectively. Ammonium sulfate [(NH4)2SO4] as the source of N at 57.5 kg N ha−1 of soil (as sufficient N, N1) and 173 kg N ha−1 of soil (as high N application, N3) were applied in 2 equal splits 2 and 4 WAG. We determined the nitrogen application rate by considering the N uptake by maize, the indigenous N in the study site, and the N use efficiency in the agriculture system. The N uptake by maize is 115 kg N ha−1 (Setiyono et al. 2010); the indigenous N in the study site is 0.24% which is categorized as moderate concentration N in the soil; N use efficiency (NUE) in the agricultural system generally is <50%. Therefore, we considered 57.5 kg N ha−1 (half of total N uptake by maize plant) as the normal N application (N1) and 173 kg N ha−1 as high N application (1.5x than N-uptake by plant) representing the behaviour of high N application by the farmer which predicted as the main cause of low NUE in the agricultural ecosystem. Therefore, there were 180 plots in total. Fertilizer was uniformly applied in liquid form to each plot. The maize plant was grown to physiological maturity (15 WAG).
2.3. Nitrous oxide emission from soil
The nitrous oxide gas was initially collected using a static closed chamber made of fiber and that was 5 mm wide, and 60 × 40 × 120 cm3 in volume. The chamber was fitted with a thermometer to measure the temperature and a portable fan to homogenize the air inside the chamber (Fig. 1). The gas was collected at 3 and 5 WAG (1 week after N application). One sample set consisted of three sampling times at 10 min-intervals from 07.00 to 11.00 a.m. The collection process used polypropylene syringe (10 mL), which was then placed inside the vacuum tube (10 mL). The N2O concentration was measured using gas chromatography (Agilent Technology 7820A) equipped with an Electron Capture Detector (ECD). Afterward, daily N2O flux (F) was measured using linear regression and the ideal gas laws were assessed based on the concentration obtained from the closed chamber headspace for over 30 min, and calculated using Equation (1) reported by Ussiri et al. [22]. Lastly, cumulative N2O emission was measured based on the N2O flux at 3 WAG and 5 WAG.
| (1) |
where ΔC/Δt is the average of change in gas concentration inside the chamber (mg m−2 min−1, ρ is the gas density, V is the volume of the chamber (m3), A is the surface area circumscribed by the chamber (m2), T is the temperature in the chamber (°C), and k is the time of the conversion factor.
Fig. 1.
Static closed chamber (left) and chamber lid (right). Noted: (1) thermometer, (2) rubber stopper for collecting gas, (3) rubber stopper for thermometer, (4) portable fan, and (5) battery.
2.4. Ammonium and nitrate concentration in soil
To analyze ammonium-nitrate concentration, soil was collected using a small shovel at depths of 5–10 cm from the surface and around the maize root at 3 and 5 WAG. The fresh soil was immediately transferred to the laboratory, and the ammonium and nitrate in the soil were measured as inorganic nitrogen in accordance with the colorimetric determination method reported by Keeney and Nelson [23] and Kempers and Zweers [24]. First, 5 g of fresh soil sample was extracted with 50 mL 1 M potassium chloride, and then shaken for 30 min and filtered using Whatman filter paper Grade 42. Subsequently, the resulting extract was treated with a mixture solution by Keeney and Nelson [23] and Kempers and Zweers [24]. The concentration of ammonium-nitrate was measured through the colorimetric method using a UV-VIS spectrophotometer (Shimadzu A-06-22) at 655 nm and 540 nm of wavelength for ammonium and nitrate, respectively.
2.5. Maize physiology and yield analysis
The two plants in each plot were collected at 7 WAG (before silking) to measure the plant parameters according to fresh and dry weights of the shoot and root, root volume, and concentration and accumulation of nitrogen in the shoots and roots. Afterward, the plant samples were separated according to the roots and shoots, dried in oven at 60 °C for a week, and weighed as dry weight. The dried samples were later used to analyze the concentration and accumulation of nitrogen in the shoots and roots of the maize. Finally, maize plants were harvested at 15 WAG (physiological maturity), and the maize cob was collected for the measurement of yield parameters such as cob weight, kernel number per cob, 100-kernel weight, and maize grain yield.
2.6. Maize characterization method
Thirty maize varieties were divided into four categories based on the average grain yield and cumulative N2O emission of 30 maize varieties under N-sufficient (N1) and N-high (N3) conditions: (1) EE, more grain yield and less cumulative N2O than the average of all cultivars tested under N1 and N3; (2) HNE, more grain yield and lower cumulative N2O than the average of all cultivars tested only under N3; (3) LNE, more grain yield and lower cumulative N2O than the average of all cultivars tested only under N1; and (4) NN, less grain yield and more cumulative N2O than the average of all cultivars tested under both N1 and N3 [25]. Finally, Considering the variety numbers of LNE (only one for grain yield basis) and HNE (only one for cumulative N2O basis) group, the responses of the N dynamic, dry matter accumulation, and yield component under N application rates were only compared between the EE and NN groups.
2.7. Data analysis
Statistical analyses were performed using software R (x64.4.1.3.Ink [26]) (R Core Team 2022). Significant differences among maize varieties for all parameters and among maize groups (EE, HNE, LNE, and NN) under N1 and N3 treatment were assessed through analysis of variance (two-way ANOVA) and Tukey HSD test for multiple comparisons at 0.05 probability level. The relationship of cumulative N2O and grain yield and nitrogen dynamic, dry mater accumulation, N concentration in the plant, N accumulation in the plant, root system, and yield component were tested using correlation analysis. In addition, the differences between N treatments (N1 and N3) and between EE and NN groups were determined using Student t-test at 0.05 probability level using Microsoft Excel.
3. Results
3.1. Grain yield and cumulative nitrous oxide under different nitrogen-treatments
Maize yield from each plot was established at 15 WAG. For 13 of the 30 maize varieties, there was a significant difference between the grain yield under the following applications of sufficient and high N fertilizer: Anoman, Srikandi Putih, Arjuna, Manding, Lokal Poso, Bisi 226, NK 33, NK 6172, Pertiwi 3, Sukmaraga, Lamuru, Provit A1, and Dalei Lei, (Table 1). Grain yield of Arjuna, Anoman, Srikandi Putih, Manding, NK 6172, Pertiwi 3, and NK 33 decreased significantly by 5–100% at N3 relative to N1 conditions, respectively. The opposite trend was observed in the Sukmaraga, Lamuru, Provit A1, Dalei Lei, and Bisi 226varieties, which showed a significant increase (18–97%) in grain yield under N3 compared to N1 conditions. The other remaining 17 varieties did not show any response between N1 and N3.
Table 1.
Grain yield at 15 WAG and N2O Cumulative (3 WAG and 5 WAG) of maize cultivars under different N-supply conditions.
| Variety |
Grain yield (kg per m2) |
N₂O cumulative (μg N m−2h−1) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N3 | N1 | N3 | |||||||||||||
| Bisma | 2.120 | ± | 0.040 | 2.240 | ± | 0.073 | 3.488 | ± | 2.602 | 9.682 | ± | 4.028 | ||||
| Sukmaraga | 1.560 | ± | 0.000 | *** | 1.680 | ± | 0.000 | *** | 8.448 | ± | 1.411 | ** | 19.798 | ± | 3.496 | ** |
| Anoman | 0.880 | ± | 0.000 | ** | 0.720 | ± | 0.000 | ** | 21.430 | ± | 5.243 | 16.470 | ± | 0.406 | ||
| Pulut URI | 0.680 | ± | 0.000 | 0.640 | ± | 0.040 | 5.177 | ± | 0.260 | *** | 8.515 | ± | 0.056 | *** | ||
| Lamuru | 0.680 | ± | 0.000 | * | 0.940 | ± | 0.100 | * | 4.819 | ± | 1.710 | ** | 15.150 | ± | 1.792 | ** |
| Provit A1 | 0.440 | ± | 0.000 | *** | 1.440 | ± | 0.000 | *** | 3.986 | ± | 1.053 | *** | 13.004 | ± | 0.237 | *** |
| Srikandi Putih | 0.520 | ± | 0.000 | *** | 0.400 | ± | 0.000 | *** | 0.080 | ± | 0.001 | * | 3.491 | ± | 1.360 | * |
| Arjuna | 0.760 | ± | 0.016 | ** | 0.520 | ± | 0.040 | ** | 17.464 | ± | 0.688 | *** | 11.623 | ± | 0.409 | *** |
| Bima 10 | 3.067 | ± | 0.463 | 2.360 | ± | 0.069 | 20.674 | ± | 0.594 | ** | 14.336 | ± | 0.547 | ** | ||
| Dale Lei | 0.400 | ± | 0.000 | *** | 1.600 | ± | 0.000 | *** | 15.399 | ± | 3.298 | * | 6.925 | ± | 1.197 | * |
| Guluk-guluk | 1.020 | ± | 0.060 | 0.960 | ± | 0.000 | 14.953 | ± | 3.896 | 15.769 | ± | 1.975 | ||||
| Manding | 0.960 | ± | 0.000 | *** | 0.360 | ± | 0.000 | *** | 14.202 | ± | 4.769 | 14.195 | ± | 0.720 | ||
| Talango | 0.000 | ± | 0.000 | 0.000 | ± | 0.000 | 4.559 | ± | 0.000 | 1.653 | ± | 1.039 | ||||
| Lokal Poso | 1.040 | ± | 0.000 | ** | 0.000 | ± | 0.000 | ** | 9.095 | ± | 7.434 | 1.713 | ± | 1.230 | ||
| Pioneer 35 | 2.272 | ± | 0.153 | 2.453 | ± | 0.027 | 4.935 | ± | 2.526 | ** | 13.460 | ± | 1.269 | ** | ||
| Pioneer 36 | 2.060 | ± | 0.151 | 2.080 | ± | 0.000 | 13.849 | ± | 0.777 | * | 11.301 | ± | 0.000 | * | ||
| Pioneer 21 | 1.013 | ± | 0.035 | 1.180 | ± | 0.020 | 6.509 | ± | 0.409 | * | 12.584 | ± | 2.165 | * | ||
| Pioneer 27 | 1.080 | ± | 0.061 | 1.010 | ± | 0.122 | 7.798 | ± | 0.803 | *** | 0.209 | ± | 0.006 | *** | ||
| Bisi 2 | 2.240 | ± | 0.080 | 1.880 | ± | 0.052 | 5.723 | ± | 0.097 | *** | 0.519 | ± | 0.086 | *** | ||
| Bisi 220 | 2.027 | ± | 0.071 | 2.128 | ± | 0.219 | 6.548 | ± | 0.229 | 10.846 | ± | 2.744 | ||||
| Bisi 228 | 2.256 | ± | 0.117 | 2.613 | ± | 0.301 | 3.387 | ± | 0.529 | 2.182 | ± | 0.529 | ||||
| Bisi 226 | 1.160 | ± | 0.040 | * | 1.747 | ± | 0.093 | * | 0.000 | ± | 0.000 | * | 4.399 | ± | 2.309 | * |
| Bisi 18 | 1.888 | ± | 0.163 | 2.620 | ± | 0.038 | 4.447 | ± | 3.415 | 6.833 | ± | 1.566 | ||||
| Bisi 99 | 2.896 | ± | 0.230 | 2.260 | ± | 0.280 | 3.791 | ± | 3.584 | 3.245 | ± | 2.231 | ||||
| Bisi 79 | 2.187 | ± | 0.792 | 3.547 | ± | 0.334 | 16.913 | ± | 5.167 | 15.063 | ± | 2.804 | ||||
| NK 33 | 2.853 | ± | 0.116 | * | 1.360 | ± | 0.076 | * | 24.108 | ± | 1.968 | ** | 9.501 | ± | 2.347 | ** |
| NK 6172 | 1.020 | ± | 0.140 | ** | 0.947 | ± | 0.013 | ** | 8.492 | ± | 0.409 | * | 6.672 | ± | 0.854 | * |
| NK 7202 | 2.040 | ± | 0.106 | 1.787 | ± | 0.301 | 0.000 | ± | 0.000 | ** | 2.870 | ± | 0.859 | ** | ||
| NK 007 | 1.387 | ± | 0.035 | 0.960 | ± | 0.186 | 16.172 | ± | 4.397 | 15.865 | ± | 1.401 | ||||
| Pertiwi 3 | 1.390 | ± | 0.104 | * | 1.320 | ± | 0.037 | * | 21.017 | ± | 11.54 | 15.195 | ± | 1.523 | ||
N1 and N3 indicate 57.5 kg N/ha of soil (normal-N) and 173 kg N/ha of soil (high-N), respectively.
Data are means ± SE (n = 3). *, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, according to Student t-Test between N1 and N3.
N2O emission in maize was varied considerably among varieties and between sufficient and high N fertilizer application (Table 1). Of the 30 maize varieties, 17 (1 local variety, 7 composite varieties and 9 hybrid varieties) significantly affected cumulative N2O emission caused by N rate. N-high treatment had positive impact on the cumulative N2O of 8 varieties (Dalei Lei, Pioneer 36, Bisi 2, NK 6172, Pioneer 21, Pioneer 27, NK 33, Arjuna, and Bima 10) and negative impact on that of 9 varieties (Sukmaraga, Pulut Uri, Lamuru, Provit A1, Pioneer 35, Srikandi Putih, Pioneer 21, Bisi 226, and NK 7202). The cumulative N2O of composite varieties such as Sukmaraga, Pulut URI, Lamuru, Srikandi Putih and Provit A1 significantly increased under N3 treatment, while Arjuna and Bima 10 decreased drastically under N3 relative to N1 treatment. Hybrid varieties: Pioneer 35, Pioneer 21, Bisi 226 and NK 7202, exhibited relatively high cumulative N2O under N3 treatment, while Pioneer 36, Pioneer 27, Bisi 2, NK 6172, and NK 33 exhibited relatively low cumulative N2O under N3 treatment relative to N1 treatment.
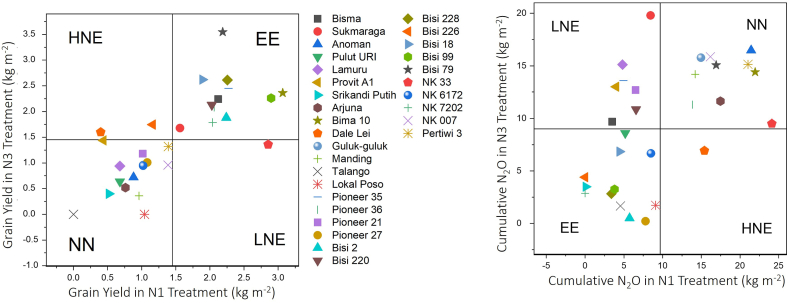
3.2. Categorization of the maize varieties based on the grain yield
Based on the grain yield, 12 of the 30 varieties (Bisi 79, Bisi 228, Bisi 18, Pioneer 35, Bisma, Pioneer 36, Bisi 2, Bisi 99, Bima 10, NK 7202, Bisi 220 and Sukmaraga) were categorized as EE group (Fig. 2) with means of 2.22 and 2.30 kg m−2 under N1 and N3 (Table 3), respectively. Meanwhile, two varieties (Bisi 226 and Dalei Lei) were categorized under HNE with mean yields of 0.78 kg m−2 and 1.67 kg m−2 under N1 and N3 treatment, respectively. Only NK 33 was classified as LNE with mean yields of 2.852 kg m−2 and 1.360 kg m−2 under N1 and N3 treatment, respectively. Fifteen varieties (Pioneer 21, Pioneer 27, Pertiwi 3, NK 007, Anoman, NK 6172, Provit A1, Lamuru, Arjuna, Pulut Uri, Manding, Guluk-guluk, Lokal Poso, Talango, and Srikandi Putih) were categorized under the NN group with mean yields of 0.86 kg m−2 and 0.76 kg m−2 under N1 and N3 conditions, respectively (Fig. 2; Table 3). Under N1 treatment, the mean yields of EE and LNE conditions of variety groups increased by 51.57% and 95.01%, compared to those of all tested varieties, respectively, while the mean yields of HNE and NN decreased by 46.69% and 41.36% compared to those of all test varieties, respectively. The mean yields of EE and HNE increased by 3.89% and 114.53% compared to those of N1 and decreased by 57.98% and 14.74% compared to those of all tested varieties, respectively. Moreover, the mean yields of LNE and NN declined by 52.34% and 11.45% compared to N1 and decreased by 6.75% and 47.90% compared to those of all tested varieties.
Fig. 2.
Relationship between maize grain yield and N2O cumulative under N-normal (N1) and N-high (N3) condition. N1 and N3 indicate 57.5 kg N ha−1 of soil (normal-N) and 173 kg N ha−1 of soil (high-N), respectively. EE, efficient-efficient (EE) varieties; HNE, high-nitrogen efficient (HNE) varieties; LNE, low-nitrogen efficient (LNE) varieties; NN, nonefficient-nonefficient (NN) varieties, respectively.
Table 3.
Grain yield, cumulative N2O, and N responsiveness of four varieties group.
| EE | HNE | LNE | NN | ||
|---|---|---|---|---|---|
| Grain Yield (kg m−2) | |||||
| N1 | Average of variety group (kg m−2) | 2.22a | 0.78b | 2.85a | 0.86b |
| Average of all tested cultivar (kg m−2) | 1.46 | 1.46 | 1.46 | 1.46 | |
| Yield of variety group increase compared to yield of all tested varieties (%) | 51.57 | −46.69 | 95.01 | −41.36 | |
| N3 | Average of variety group (kg m−2) | 2.30a | 1.67b | 1.36c | 0.76d |
| Average of all tested cultivar (kg m−2) | 1.46 | 1.46 | 1.46 | 1.46 | |
| Yield reduction compared to N1 (%) | 3.89 | 114.53 | −52.34 | −11.45 | |
| Yield of variety group increase compared to yield of all tested varieties (%) | 57.98 | 14.74 | −6.75 | −47.90 | |
| Cumulative N₂O (μg N m−2h−1) | |||||
| N1 | Average of variety group (μg N m−2h−1) | 4.38b | 15.40a | 5.53b | 18.08a |
| Average of all tested cultivar (μg N m−2h−1) | 9.58 | 9.58 | 9.58 | 9.58 | |
| Yield of variety group increase compared to yield of all tested varieties (%) | −54.30 | 60.71 | −42.25 | 88.67 | |
| N3 | Average of variety group (μg N m−2h−1) | 3.52b | 6.93b | 13.50a | 13.93a |
| Average of all tested cultivar (μg N m−2h−1) | 9.44 | 9.44 | 9.44 | 9.44 | |
| Yield reduction compared to N1 (%) | −19.51 | −55.03 | 144.04 | −22.94 | |
| Yield of variety group increase compared to yield of all tested varieties (%) | −62.64 | −26.60 | 43.11 | 47.65 | |
* and ** indicate significant correlation at P < 0.05 and P < 0.01, respectively.
1) The data for one season serve as repetitions (n = 30).
2) N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Different lowercase letters indicate significant differences at P < 0.5 according to Tukey HSD among group.
3.3. Categorization of the maize varieties based on cumulative nitrous oxide
Based on the cumulative N2O emission, 12 varieties (Bisi 228, Talango, Bisi 2, Pioneer 27, Lokal Poso, Srikandi Putih, NK 7202, Bisi 99, Bisi 226, Bisi 18, Pulut URI, and NK 6172) were grouped under the EE group; a variety (Dalei Lei) under HNE; 7 varieties (Bisma, Bisi 220, Pioneer 21, Provit A1, Pioneer 35, Lamuru, and Sukmaraga) under LNE; and 10 varieties (Pioneer 36, NK 33, Arjuna, Manding, Bisi 79, Guluk-guluk, NK 007, Anoman, Bima 10, and Pertiwi 3) under the NN group with mean cumulative N2O emissions of 18.08 and 13.93 μg N m−2 h−1 under N1 and N3 conditions, respectively (Fig. 2). The EE variety group can potentially achieve a N2O reduction of approximately 54% under N1 and 63% under N3 compared to all tested varieties. The NN group potentially achieved an N2O increase of 88.67% and 47.65% under N1 and N3, respectively (Table 3). The HNE trend exhibited a trend opposite to that of LNE; the HNE group showed a 60.71% increase in N2O under N1 treatment and a 26.60% reduction of N2O under N3 treatment. The LNE group showed a 42.25% reduction in N2O under N1 treatment and a 43.11% increase in N2O under N3 treatment (Table 3). Increasing the N-rate reduced the cumulative N2O of EE, HNE, and NN groups by 19.51%, 55.03% and 22.94%, respectively, and increased the cumulative N2O of the LNE group by 144.704% relative to N1 treatment.
3.4. Nitrogen response-associated traits of efficient-efficient and nonefficient-nonefficient varieties groups
In this study, the N-accumulation and N-concentration in plant tissue were measured at the end of the vegetative stage (before silking, 7 WAG). Different varieties and N rates individually as well as their interaction had a significant impact on the accumulation and concentration of N in maize regardless of the variety group (Table 4). Considering the variety group, the two variety groups performed differently with respect to N-accumulation, but did not differ in N-concentration across N-treatments (Fig. 3; Table 4). The N-accumulation average of the EE variety group was 146.03 mg plant−1, which was 33.98% higher than that of the NN variety group.
Table 4.
N-concentration and N-content in plant tissue of the two varieties groups under different N condition.
| Group | Variety | N-concentration in plant tissue (g 100gˉ1) |
N-content in plant tissue (mg plant−1) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N3 | N1 | N3 | ||||||||||||||
| EE | Bisi 2 | 1.59 | ab | 1.83 | a | 118.56 | d | 159.17 | bc | ||||||||
| Bisi 228 | 1.80 | ab | 1.88 | a | 117.27 | d | 118.31 | d | |||||||||
| Bisi 18 | 1.40 | b | 1.73 | ab | 188.60 | b | 237.99 | a | |||||||||
| Bisi 99 | 1.51 | ab | 1.92 | a | 130.74 | cd | 110.69 | d | |||||||||
| NK 7202 | 1.74 | ab | 1.62 | ab | 167.59 | b | 111.36 | d | |||||||||
| Mean | 1.61 | ± | 0.07 | aA | 1.80 | ± | 0.05 | aA | 144.55 | ± | 14.29 | aA | 147.50 | ± | 24.33 | aA | |
| Group mean | 1.70 | 146.03 | |||||||||||||||
| ANOVA | |||||||||||||||||
| Variety | ns | *** | |||||||||||||||
| N rate | ** | ns | |||||||||||||||
| Variety*N Rate | * | *** | |||||||||||||||
| NN | Anoman | 2.07 | ab | 1.69 | cd | 103.18 | cd | 83.92 | ef | ||||||||
| Arjuna | 1.88 | abc | 1.74 | cd | 56.71 | g | 109.88 | bc | |||||||||
| Manding | 1.59 | d | 2.11 | a | 94.96 | de | 71.64 | f | |||||||||
| NK 007 | 1.70 | cd | 1.84 | bc | 117.98 | ab | 108.70 | bc | |||||||||
| Pertiwi 3 | 1.55 | d | 1.71 | cd | 128.27 | a | 119.69 | ab | |||||||||
| Guluk-Guluk | 1.93 | abc | 1.89 | abc | 118.00 | ab | 43.99 | h | |||||||||
| Mean | 1.79 | ± | 0.08 | aA | 1.83 | ± | 0.06 | aA | 103.19 | ± | 10.48 | aB | 89.64 | ± | 11.71 | aA | |
| Group mean | 1.81 | 96.41 | |||||||||||||||
| ANOVA | |||||||||||||||||
| Variety | *** | *** | |||||||||||||||
| N rate | ns | *** | |||||||||||||||
| Variety*N Rate | *** | *** | |||||||||||||||
| Reduction of NN compared to EE (%) | 6.30 | −33.98 | |||||||||||||||
1) EE, efficient-efficient varieties; NN, nonefficient-nonefficient varieties.
2) N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Different lowercase letters in Mean indicate significant differences at P < 0.5 according to Student t-Test between N0 and N1.
Different uppercase letters indicate significant differences at P < 0.5 according to Student t-Test between EE and NN.
Different lowercase letters in each variety indicate significant differences at P < 0.5 according to Tukey HSD test among variety under N1 and N3 in each group.
*, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, and ns means not significant according to Two-ways ANOVA.
Fig. 3.
N-content (A) and N-concentration (B) in plant tissue for two variety groups under N-sufficient and N-high treatment. EE, efficient-efficient cultivars; NN, nonefficient-nonefficient cultivars. N1 and N3 indicate 57.5 kg N ha−1 of soil (normal-N) and 173 kg N ha−1 of soil (high-N), respectively. Within cultivars, the means followed by different lowercase letters indicate significant differences at P < 0.05 according to Student t-Test between N1 and N3 and different uppercase letters indicate significant differences at P < 0.05 according to Student t-Test between EE and NN.
The variety and N rate interaction showed a significant effect on soil NH4+ and NO3− concentrations at 3 WAG (Fig. 4; Table 5). Soil NH4+ at 3 WAG decreased from 12.15 mg kg−1 soil under N1 to 6.64 mg kg−1 soil under N3 and from 11.13 mg kg−1 soil under N1 to 8.71 mg kg−1 soil under N3 of the EE and NN groups, respectively. Conversely, soil NO3− increased from 3.28 mg kg−1 under N1 to 8.2 mg kg−1 under N3 and from 3.91 mg kg−1 under N1 to 36.11 mg kg−1 under N3 of the EE and NN group, respectively. At 5 WAG, the variety and N rate interaction showed a significant effect only on soil NH4+ concentration in soil. Different variety and N rate individually had a significant effect on NO3− concentrations in NN group but not in EE group. The mean soil NH4+ and NO3− concentrations of the EE variety group were 58.55% and 41.30% higher than that of the NN group across the N treatments, respectively (Fig. 4). The increase in soil NH4+ concentration at 5 WAG of the EE group was attributed to higher grain yield (2.25 kg m−2 vs. 0.94 kg m−2 for the EE and NN variety group) under N3 treatment with a coefficient correlation of 0.42 (P < 0.05).
Fig. 4.
NH4+ and NO3− concentration in soil of the two varieties groups under different N condition at 3 WAG (A) and 5 WAG (B). EE, efficient-efficient cultivars; NN, nonefficient-nonefficient cultivars. N1 and N3 indicate 57.5 kg N ha−1 of soil (sufficient-N) and 173 kg N ha−1 of soil (high-N), respectively. Within cultivars, the means followed by different lowercase letters indicate significant differences at P < 0.05 according to Student t-Test between N1 and N3 and different uppercase letters indicate significant differences at P < 0.05 according to Student t-Test between EE and NN.
Table 5.
Ammonium and nitrate concentration in soil of the two varieties groups under different N condition at 3 and 5 WAG.
| 3 weeks after germination |
5 weeks after germination |
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammonium (mg kg−1) |
Nitrate (mg kg−1) |
Ammonium (mg kg−1) |
Nitrate (mg kg−1) |
||||||||||||||||||||||||||||||
| N1 | N3 | N1 | N3 | N1 | N3 | N1 | N3 | ||||||||||||||||||||||||||
| EE | Bisi 2 | 6.01 | ± | 1.00 | d | 16.81 | ± | 3.78 | a | 4.20 | ± | 0.49 | bc | 8.76 | ± | 0.00 | b | 9.96 | ± | 0.70 | bc | 6.61 | ± | 0.85 | c | 3.70 | ± | 0.78 | a | 5.66 | ± | 1.44 | a |
| Bisi 228 | 5.89 | ± | 0.85 | d | 14.27 | ± | 5.02 | b | 1.67 | ± | 0.38 | c | 21.41 | ± | 1.40 | a | 10.76 | ± | 0.76 | bc | 11.27 | ± | 4.47 | bc | 3.89 | ± | 0.47 | a | 4.24 | ± | 0.39 | a | |
| Bisi 99 | 5.84 | ± | 0.55 | d | 0.51 | ± | 0.44 | d | 4.78 | ± | 1.65 | bc | 4.93 | ± | 1.08 | bc | 9.64 | ± | 0.55 | bc | 18.25 | ± | 1.58 | a | 4.16 | ± | 0.58 | a | 1.82 | ± | 0.85 | a | |
| Bisi 18 | 36.38 | ± | 2.04 | c | 0.96 | ± | 1.80 | d | 2.58 | ± | 0.81 | c | 3.29 | ± | 0.86 | c | 7.42 | ± | 0.41 | c | 17.91 | ± | 4.29 | ab | 2.15 | ± | 0.30 | a | 3.54 | ± | 0.44 | a | |
| NK 7202 | 6.64 | ± | 0.45 | d | 0.63 | ± | 0.69 | d | 3.15 | ± | 0.85 | c | 2.62 | ± | 0.44 | c | 7.41 | ± | 0.99 | c | 8.33 | ± | 0.88 | c | 3.09 | ± | 0.21 | a | 2.73 | ± | 0.30 | a | |
| Mean | 12.15 | ± | 6.06 | aA | 6.64 | ± | 3.66 | aA | 3.28 | ± | 0.56 | aA | 8.20 | ± | 3.47 | aA | 9.04 | ± | 0.69 | aA | 12.47 | ± | 2.41 | aA | 3.40 | ± | 0.36 | aA | 3.60 | ± | 0.66 | aA | |
| Group mean | 9.39 | 5.74 | 10.75 | 3.50 | |||||||||||||||||||||||||||||
| ANOVA | *** | *** | *** | ns | |||||||||||||||||||||||||||||
| Variety | *** | *** | *** | ns | |||||||||||||||||||||||||||||
| N rate | *** | *** | *** | ns | |||||||||||||||||||||||||||||
| Variety*N Rate | |||||||||||||||||||||||||||||||||
| NN | Anoman | 7.78 | ± | 0.15 | e | 11.37 | ± | 6.44 | b | 4.65 | ± | 1.78 | b | 78.69 | ± | 14.10 | a | 6.69 | ± | 0.98 | abc | 6.29 | ± | 0.00 | bc | 5.86 | ± | 0.76 | a | 6.76 | ± | 1.75 | a |
| Arjuna | 31.95 | ± | 1.11 | d | 17.07 | ± | 1.16 | a | 3.80 | ± | 1.34 | b | 39.02 | ± | 19.86 | ab | 6.08 | ± | 0.27 | c | 7.68 | ± | 1.28 | abc | 1.29 | ± | 0.13 | bc | 4.10 | ± | 0.95 | bc | |
| Manding | 5.20 | ± | 0.68 | e | 16.37 | ± | 4.14 | a | 8.56 | ± | 1.37 | b | 80.95 | ± | 7.96 | a | 8.78 | ± | 0.22 | abc | 7.27 | ± | 1.16 | abc | 5.10 | ± | 0.44 | ab | 6.15 | ± | 0.99 | ab | |
| NK 007 | 6.19 | ± | 0.39 | e | 0.57 | ± | 0.48 | e | 3.10 | ± | 0.59 | b | 2.54 | ± | 0.66 | b | 7.23 | ± | 0.42 | abc | 8.89 | ± | 0.53 | abc | 8.45 | ± | 3.82 | c | 1.77 | ± | 0.75 | c | |
| Pertiwi 3 | 5.98 | ± | 0.57 | e | 0.68 | ± | 0.35 | e | 2.04 | ± | 0.72 | b | 2.15 | ± | 0.34 | b | 6.83 | ± | 0.45 | abc | 11.43 | ± | 2.41 | a | 1.09 | ± | 0.13 | ab | 4.74 | ± | 0.56 | ab | |
| Guluk-guluk | 9.70 | ± | 3.85 | e | 6.18 | ± | 6.81 | c | 1.27 | ± | 0.52 | b | 13.29 | ± | 5.06 | b | 11.17 | ± | 2.81 | ab | 6.38 | ± | 0.59 | bc | 2.59 | ± | 0.10 | abc | 4.93 | ± | 0.45 | abc | |
| Mean | 11.13 | ± | 4.21 | aA | 8.71 | ± | 3.02 | aA | 3.91 | ± | 1.05 | aA | 36.11 | ± | 14.87 | aA | 7.80 | ± | 0.77 | aA | 7.99 | ± | 0.79 | aA | 4.06 | ± | 1.19 | aA | 4.74 | ± | 0.72 | aA | |
| Group mean | 9.92 | 20.01 | 7.89 | 4.40 | |||||||||||||||||||||||||||||
| ANOVA | |||||||||||||||||||||||||||||||||
| Variety | *** | *** | ns | *** | |||||||||||||||||||||||||||||
| N rate | *** | *** | ns | * | |||||||||||||||||||||||||||||
| Variety*N Rate | *** | *** | *** | ns | |||||||||||||||||||||||||||||
| Reduction of NN compared to EE (%) | 5.61 | 248.51 | −26.60 | 25.83 | |||||||||||||||||||||||||||||
1) EE, efficient-efficient varieties; NN, nonefficient-nonefficient varieties.
2) N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Different lowercase letters in Mean indicate significant differences at P < 0.5 according to Student t-Test between N1 and N3.
Different uppercase letters indicate significant differences at P < 0.5 according to Student t-Test between EE and NN.
Different lowercase letters in each variety indicate significant differences at P < 0.5 according to Tukey HSD test among variety under N1 and N3 in each group.
*, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, and ns means not significant according to Two-ways ANOVA.
The interaction between variety × N rate was significant in the DWR, DWS, and RV (Table 6). Dry matter accumulation performed a great variation between two variety groups N1, of which, the EE variety group resulted in significantly higher of dry weight shoot than that of NN variety group (Table 6). Across the N treatments, the average DWS and DWR of the EE groups were 112.46 g plant−1 and 19.91 g plant−1, respectively, which were 38.152% and 41.53% higher than those of the NN group, respectively. The root volume of the EE group was 33.44% more than that of the NN group across all N treatments (Table 4).
Table 6.
Dry weight of shoot and root, root length and root volume of the two varieties groups under different N condition.
| Group | Varieties | Dry weight of shoot (gr plant−1) |
Dry weight of root (gr plant−1) |
Root volume (cc plant−1) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N3 | N1 | N3 | N1 | N3 | ||||||||||||||||||||
| EE | Bisi 2 | 94.67 | ± | 10.32 | bcd | 117.87 | ± | 7.28 | b | 10.39 | ± | 0.69 | d | 11.14 | ± | 0.20 | d | 105 | ± | 5.0 | e | 135 | ± | 5.0 | cde |
| Bisi 228 | 87.83 | ± | 3.51 | de | 83.29 | ± | 2.47 | de | 20.96 | ± | 1.69 | bc | 10.95 | ± | 0.21 | d | 215 | ± | 5.0 | ab | 120 | ± | 0.0 | de | |
| Bisi 18 | 166.29 | ± | 67.62 | a | 180.24 | ± | 18.70 | a | 25.36 | ± | 11.80 | ab | 19.18 | ± | 4.81 | c | 200 | ± | 90.0 | ab | 178 | ± | 32.5 | bc | |
| Bisi 99 | 96.33 | ± | 6.17 | bcd | 66.51 | ± | 2.20 | e | 30.97 | ± | 2.38 | a | 28.59 | ± | 2.60 | a | 170 | ± | 20.0 | bcd | 245 | ± | 35.0 | a | |
| NK 7202 | 117.19 | ± | 9.87 | bc | 93.76 | ± | 42.69 | cd | 11.86 | ± | 2.47 | d | 9.75 | ± | 0.85 | d | 125 | ± | 25.0 | cde | 110 | ± | 7.5 | e | |
| Mean | 112.46 | ± | 14.32 | aA | 108.33 | ± | 19.81 | aA | 19.91 | ± | 3.93 | aA | 15.92 | ± | 3.58 | aA | 163.00 | ± | 21.13 | aA | 157.50 | ± | 24.72 | aA | |
| Group mean | 110.40 | 17.92 | 160.25 | ||||||||||||||||||||||
| ANOVA | |||||||||||||||||||||||||
| Variety | *** | *** | *** | ||||||||||||||||||||||
| N rate | ns | *** | ns | ||||||||||||||||||||||
| Variety*N Rate | *** | ** | *** | ||||||||||||||||||||||
| NN | Anoman | 63.82 | ± | 18.60 | de | 58.68 | ± | 20.35 | e | 6.10 | ± | 2.34 | e | 8.75 | ± | 5.53 | de | 60 | ± | 25.0 | f | 100 | ± | 55.0 | def |
| Arjuna | 35.55 | ± | 2.04 | fg | 80.34 | ± | 10.66 | bc | 8.87 | ± | 0.84 | de | 6.67 | ± | 2.10 | e | 105 | ± | 15.0 | cde | 93 | ± | 22.5 | ef | |
| Manding | 73.57 | ± | 24.95 | bcd | 42.36 | ± | 18.79 | f | 6.67 | ± | 6.41 | e | 14.90 | ± | 4.54 | ab | 70 | ± | 50.0 | ef | 110 | ± | 35.0 | bcde | |
| NK 007 | 85.94 | ± | 4.30 | ab | 83.97 | ± | 0.48 | bc | 12.60 | ± | 1.45 | bcd | 11.89 | ± | 0.19 | bcd | 150 | ± | 20.0 | ab | 140 | ± | 0.0 | abcd | |
| Pertiwi 3 | 98.35 | ± | 4.01 | a | 97.55 | ± | 8.82 | a | 14.64 | ± | 3.00 | abc | 10.99 | ± | 2.11 | cd | 180 | ± | 35.0 | a | 55 | ± | 5.0 | def | |
| Guluk-guluk | 71.54 | ± | 17.06 | cde | 27.68 | ± | 3.74 | g | 16.59 | ± | 5.03 | a | 7.04 | ± | 0.24 | e | 145 | ± | 42.5 | abc | 73 | ± | 2.5 | ef | |
| Mean | 71.46 | ± | 8.73 | aB | 65.10 | ± | 10.95 | aA | 10.91 | ± | 1.77 | aA | 10.04 | ± | 1.29 | aA | 118.33 | ± | 19.52 | aA | 95.00 | ± | 12.09 | aB | |
| Group mean | 68.28 | 10.48 | 106.67 | ||||||||||||||||||||||
| ANOVA | |||||||||||||||||||||||||
| Variety | *** | *** | *** | ||||||||||||||||||||||
| N rate | *** | ns | ** | ||||||||||||||||||||||
| Variety*N Rate | *** | *** | *** | ||||||||||||||||||||||
| Reduction of NN compared to EE (%) | −38.15 | −41.53 | −33.44 | ||||||||||||||||||||||
1) EE, efficient-efficient varieties; NN, nonefficient-nonefficient varieties.
2) N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Different lowercase letters in Mean indicate significant differences at P < 0.5 according to Student t-Test between N1 and N3.
Different uppercase letters indicate significant differences at P < 0.5 according to Student t-Test between EE and NN.
Different lowercase letters in each variety indicate significant differences at P < 0.5 according to Tukey HSD test among variety under N1 and N3 in each group.
*, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, and ns means not significant according to Two-ways ANOVA.
The variety and N rate interaction effect on yield components such as cob weight, kernel number per cob, 100-kernel weight per cob and grain yield tended to increase in the EE group than in the NN group and under N1 than under N3. The average kernel number per cob for the EE group was 164.42 g, which was 28.81% higher than that for the NN group across the N treatments; the average cob weight and 100-kernel number between two variety groups was comparable (Table 7). The cob weight of the NN group decreased from 156.35 g under N1 to 120.45 g under N3, whereas that of the EE group across the N treatment was comparable. Regardless of the N-treatment, the cob weight of EE (171.31 g) was 19.21% higher than that for the NN group (138.40 g).
Table 7.
Yield component of the two varieties groups under different N condition.
| Group | Variety | Cob weight (g) |
Kernel number per cob (g) |
100-kernel weight per cob(g) |
Grain yield (kg m−2) |
||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N3 | N1 | N3 | N1 | N3 | N1 | N3 | ||||||||||||||||||||||||||
| EE | Bisi 2 | 89.55 | ± | 7.96 | b | 108.07 | ± | 6.68 | b | 140 | ± | 5.00 | b | 118 | ± | 3.23 | b | 25 | ± | 0.00 | c | 25 | ± | 0.00 | c | 2.240 | ± | 0.08 | ab | 1.880 | ± | 0.05 | ab |
| Bisi 228 | 229.06 | ± | 40.18 | a | 201.58 | ± | 19.42 | a | 141 | ± | 7.31 | ab | 163 | ± | 18.78 | ab | 30 | ± | 0.00 | b | 28 | ± | 1.44 | b | 2.256 | ± | 0.12 | a | 2.613 | ± | 0.30 | a | |
| Bisi 18 | 168.65 | ± | 14.24 | a | 189.72 | ± | 8.65 | a | 118 | ± | 10.20 | ab | 164 | ± | 2.39 | ab | 35 | ± | 1.58 | a | 33 | ± | 1.44 | a | 1.888 | ± | 0.16 | a | 2.620 | ± | 0.04 | a | |
| Bisi 99 | 196.10 | ± | 17.45 | a | 169.80 | ± | 22.73 | a | 181 | ± | 14.35 | ab | 141 | ± | 17.49 | ab | 31 | ± | 1.00 | b | 30 | ± | 0.00 | b | 2.896 | ± | 0.23 | ab | 2.260 | ± | 0.28 | ab | |
| NK 7202 | 177.12 | ± | 28.80 | a | 183.41 | ± | 21.46 | a | 255 | ± | 13.23 | a | 223 | ± | 37.68 | a | 35 | ± | 0.00 | a | 35 | ± | 0.00 | a | 2.040 | ± | 0.11 | b | 1.787 | ± | 0.30 | b | |
| Mean | 172.09 | ± | 23.10 | aA | 170.52 | ± | 16.43 | aA | 167.00 | ± | 24.23 | aA | 161.83 | ± | 17.57 | aA | 31.20 | ± | 1.85 | aA | 30.00 | ± | 1.77 | aA | 2.26 | ± | 0.17 | aA | 2.23 | ± | 0.18 | aA | |
| Group mean | 171.31 | 164.42 | 30.60 | 2.25 | |||||||||||||||||||||||||||||
| ANOVA | |||||||||||||||||||||||||||||||||
| Variety | *** | * | *** | ** | |||||||||||||||||||||||||||||
| N rate | ns | ns | * | ns | |||||||||||||||||||||||||||||
| Variety*N Rate | ns | ns | ns | ns | |||||||||||||||||||||||||||||
| NN | Anoman | 127.68 | ± | 11.15 | cdef | 98.94 | ± | 14.01 | def | 110 | ± | 0.00 | bc | 90 | ± | 0.00 | bcd | 30 | ± | 0.00 | c | 20 | ± | 0.00 | d | 0.880 | ± | 0.00 | bc | 0.720 | ± | 0.00 | bcd |
| Arjuna | 253.04 | ± | 0.00 | a | 120.00 | ± | 17.23 | cdef | 95 | ± | 2.04 | bc | 65 | ± | 5.00 | cd | 34 | ± | 2.39 | abc | 30 | ± | 0.00 | c | 0.760 | ± | 0.02 | bc | 0.520 | ± | 0.04 | cd | |
| Manding | 66.98 | ± | 5.12 | f | 59.66 | ± | 0.00 | f | 120 | ± | 0.00 | ab | 45 | ± | 0.00 | d | 20 | ± | 0.00 | d | 10 | ± | 0.00 | e | 0.960 | ± | 0.00 | d | 0.360 | ± | 0.00 | ab | |
| NK 007 | 188.20 | ± | 11.10 | abcd | 143.22 | ± | 31.50 | bcde | 173 | ± | 4.41 | a | 120 | ± | 23.29 | ab | 37 | ± | 1.67 | ab | 30 | ± | 0.00 | c | 1.387 | ± | 0.04 | a | 0.960 | ± | 0.19 | ab | |
| Pertiwi 3 | 205.95 | ± | 24.86 | ab | 210.52 | ± | 28.18 | abc | 174 | ± | 12.97 | ab | 165 | ± | 4.56 | a | 33 | ± | 1.44 | bc | 40 | ± | 0.00 | a | 1.390 | ± | 0.10 | ab | 1.320 | ± | 0.04 | a | |
| Guluk-guluk | 96.25 | ± | 5.45 | ef | 90.34 | ± | 6.60 | ef | 128 | ± | 7.50 | ab | 120 | ± | 0.00 | ab | 30 | ± | 0.00 | c | 35 | ± | 0.00 | abc | 1.020 | ± | 0.06 | ab | 0.960 | ± | 0.00 | ab | |
| Mean | 156.35 | ± | 29.52 | aA | 120.45 | ± | 22.92 | aA | 133.26 | ± | 15.03 | aA | 100.83 | ± | 19.29 | aB | 30.49 | ± | 2.61 | aA | 27.50 | ± | 4.65 | aA | 1.07 | ± | 0.12 | aB | 0.81 | ± | 0.15 | aB | |
| Group mean | 138.40 | 117.05 | 28.99 | 0.94 | |||||||||||||||||||||||||||||
| ANOVA | |||||||||||||||||||||||||||||||||
| Variety | *** | *** | *** | *** | |||||||||||||||||||||||||||||
| N rate | *** | *** | *** | *** | |||||||||||||||||||||||||||||
| Variety*N Rate | ** | *** | *** | *** | |||||||||||||||||||||||||||||
| Reduction of NN compared to EE (%) | −19.21 | −28.81 | −5.25 | −58.35 | |||||||||||||||||||||||||||||
1) EE, efficient-efficient varieties; NN, nonefficient-nonefficient varieties.
2) N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Different lowercase letters in Mean indicate significant differences at P < 0.5 according to Student t-Test between N1 and N3.
Different uppercase letters indicate significant differences at P < 0.5 according to Student t-Test between EE and NN.
Different lowercase letters in each variety indicate significant differences at P < 0.5 according to Tukey HSD test among variety under N1 and N3 in each group.
*, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, and ns means not significant according to Two-ways ANOVA.
4. Discussion
4.1. Contrasting effect of nitrogen-treatment on the grain yield and cumulative nitrous oxide among maize varieties
The global demand for N-fertilizer increases annually from approximately 105 million tons in 2016 to approximately 163 million tons in 2022, which is 2–3 times higher than that of phosphorus and potassium demand for fertilizer. Meanwhile, the potential world balance of N, the difference between N-supply and total demand of N, decreased gradually after reaching the peak (approximately 15 million tons) in 2019 to date. In 2022, the potential global balance of N was approximately 11 million tons, which is 27.44% lower than that in 2019 [4]. The increasing demand for N-fertilizer related to the increased use of N-fertilizer by farmers is a strategy for increasing the maize yield to meet the high demand for its consumption as food and in the commercial industry. Furthermore, nitrogen is an essential nutrient for maize grain yield, and NUE in maize exceptionally exceeds 60% [15]. The excessive doses of N fertilizer application and/or the lack of precise timing of N fertilizer application with respect to the time of crop demand mainly caused the low efficiency of N use in the agricultural field [27]. Additionally, Gheith et al. [28] stated that NUE significantly increased as nitrogen application time varied, while it sharply decreased as nitrogen levels increased, and Davies et al. [29] demonstrated that split supplication of N or applied N near planting could increase maize grain yield and NUE.
The results showed that maize yield varied among varieties, and exhibited inconsistent responses to N-rate application. Of the 30 maize varieties, only 13 showed a significant response to the N-rate treatments, while the others did not show any response. This indicated different genotypes among maize varieties with varying optimal demand for and adaptability to nitrogen; thus showing varying responses to N-application related to varying abilities and capacities to uptake, transport, and utilize N for plant metabolism. The results are consistent with those obtained by Qiu et al. [30] who found that the maize grain yield increased inconsistently under nitrogen application exceeding the optimum amount, and Preza-Fontes [31] stated that the N application treatment had no effect on the grain yield of maize. Certain varieties such as Bisi 226, Sukmaraga, Lamuru, Dalei Lei, and Provit A1 resulted in increasing grain yield ranging between 8% and 300% under N-high treatment, indicating that these varieties had either significantly higher demand for nitrogen or higher tolerance for N-high treatment. This indicated a considerably positive response to N-high application. Tofa et al. [32] stated that increased N application resulted in increased grain yield of maize, which doubled under 60 kg N kg−1 and tripled under 120 kg N ha−1 compared to the control. Additionally, Gautam et al. [33] reported maize grain yield treated with 180 kg N ha−1 doubled compared to untreated maize (3 tons ha−1 to –6 tons ha−1). The opposite trend was observed for Arjuna, Anoman, Srikandi Putih, Manding, Lokal Poso, NK 6172, Pertiwi 3, and NK 33, which showed a significant decrease in grain yield under N-high than that of N-sufficient by 5%–100%. These indicate that they had either considerably lower nitrogen-demand or lower N-high tolerance. Qiu et al. [30] declared that the increase in nitrogen application beyond the optimal nitrogen demand the N recovery efficiency (up to 88%), N agronomic efficiency (up to 99%), N internal efficiency (up to 42%), and N partial factor productivity (up to 16%). Additionally, Stevens et al. [16] reported that needless N application negatively affected plant response to N, including the decreasing fertilizer nitrogen uptake efficiency (FNUE), thus affecting NO3−-N balance and the availability of easy mineralization of organic-N in soil. Su et al. [34] reported that maize grain yield could be greater under 225 kg N ha−1 than under 300 kg N ha−1 application related to the high net photosynthetic rate, stomatal conductance, root weight, and deep root distribution.
In this study, maize grain yield was found to be significantly correlated with N2O flux and NH4+-soil at 5 WAG (before silking) under N-high, but not with NO3− (Table 2). Maize yield was significantly correlated with shoot biomass across N-rate treatments. This result was consistent with that of a previous study where maize grain yield showed a significant correlation with dry matter accumulation [9]. Moreover, Mdlambuzi et al. [35] reported that higher dry matter accumulation was followed by higher grain yield. Our study revealed that maize grain yield is strongly correlated with N-accumulation in the plants under N-sufficient treatment at 6 WAG (before silking) in accordance with previous studies on the maize yield highly correlated with plant dry matter accumulation before silking under N-sufficient rate [9]. In addition, the yield component such as kernel number, cob weight, and 100-kernel weight was strongly correlated with the grain yield. The kernel number was positively correlated with grain yield across N-treatments, which was consistent with the study results obtained by Xiang-Ling et al. [9]. Moreover, cob weight and 100-kernel weight were only positively correlated under N-high treatment. Xiang-Ling et al. [9] reported that maize grain weight per ear was positively correlated with grain yield under N-sufficient treatment, while the 1000-kernel number had no correlation with the N-rate application. These finding indicated that the yield component, dry weight of the shoot, and N2O flux and soil NH4+ concentration at 5 WAG were significant factors for the evaluation of nitrogen responses on the grain yield of all varieties under N-high status in soil.
Table 2.
Correlation coefficients between grain yield and N2O cumulative and nitrogen dynamic, dry matter accumulation, and yield component under normal-N (N1) and high-N (N3) conditions.
| Item | Correlation coefficient (r) |
||||
|---|---|---|---|---|---|
| Grain yield |
N₂O cumulative |
||||
| N1 | N3 | N1 | N3 | ||
| Nitrogen dynamic | N₂O flux 3 WAG (μg N m−2h−1) | −0.05 | 0.05 | 1.00** | 1.00** |
| N₂O flux 5 WAG (μg N m−2h−1) | 0.24 | 0.38* | 0.10 | −0.10 | |
| N₂O cumulative (μg N m−2h−1) | −0.05 | 0.06 | 1.00 | 1.00 | |
| NH4+ 3 WAG (mg kg−1) | 0.21 | −0.09 | 0.06 | 0.08 | |
| NO3− 3 WAG (mg kg−1) | −0.28 | −0.08 | −0.11 | 0.49** | |
| NH4+ 5 WAG (mg kg−1) | −0.05 | 0.42* | −0.16 | −0.08 | |
| NO3− 5 WAG (mg kg−1) | 0.17 | 0.07 | 0.06 | 0.03 | |
| Dry matter accumulation | Shoot 7 WAG (g plant−1) | 0.57** | 0.38* | −0.24 | 0.02 |
| Root 7 WAG (g plant−1) | 0.4 | 0.25 | −0.17 | −0.09 | |
| N concentration | Shoot at 7 WAG (g 100g−1) | −0.13 | 0.00 | 0.04 | 0.03 |
| Root at 7 WAG (g 100g−1) | −0.30 | −0.20 | −0.03 | −0.24 | |
| Plant at 7 WAG (g 100g−1) | −0.30 | −0.11 | 0.01 | −0.11 | |
| N accumulation | Shoot at 7 WAG (g plant−1) | 0.50** | 0.10 | −0.01 | −0.14 |
| Root at 7 WAG (g plant−1) | 0.22 | 0.20 | 0.03 | −0.13 | |
| Plant at 7 WAG (g plant−1) | 0.50** | 0.11 | −0.01 | −0.14 | |
| Root system | Root length (cm plant−1) | 0.25 | −0.05 | 0.09 | −0.13 |
| Root volume (cc plant−1) | 0.36 | 0.34 | −0.04 | 0.18 | |
| Yield component | Grain yield (kg per m2) | 1.00 | 1.00 | −0.05 | 0.06 |
| Kernel number per cob (g) | 0.66** | 0.76** | −0.08 | 0.23 | |
| 100-kernel weight (g) | 0.03 | 0.41* | −0.3 | −0.24 | |
| Cob weight (g) | 0.18 | 0.66** | 0.07 | 0.09 | |
N1 and N3 indicate 57.5 kg N ha-1 of soil (normal-N) and 173 kg N ha-1 of soil (high-N), respectively.
Data are means ± SE (n = 3). * means significant differences at P < 0.5 according to Student t-Test between N1 and N3.
*, **, and *** means significant differences at P < 0.5, P < 0.01, and P < 0.001, respectively, according to Student t-Test between N1 and N3.
Furthermore, maize requires high nitrogen application, which potentially contributes to the high risk of nitrogen loss trough N2O emission. The N2O emission factors depend on the management practices particularly relating to N-treatment such as dose, time, and application method [36,37]. In this study, we used (NH4)2SO4 as the source of N applied in two equal splits at 2 and 4 WAG. Seventeen of tested maize cultivar showed a significant response to cumulative N2O across N-rate application. Varieties with significantly lower cumulative N2O under high-N showed higher dry matter and nitrogen accumulation under high-N ranging from 24% to 125% and 33%–139% relative to N-sufficient, respectively, and vice versa. However, these were not expressed in the correlation analysis. This was in line with the study by Stevens et al. [16] who reported that the high N level supported increased N-mineralization, particularly by improving plant biomass production and/or supporting N-organic accumulation which is easier to mineralize than that of indigenous-N. Therefore, it may contribute to the relatively low N-loss in form N2O emission under N-high treatment. Mdlambuzi et al. [35] found that increased N rate application increased the nitrogen availability in soil, and was strongly positively correlated with higher dry matter accumulation owing to the increase in nitrogen uptake increasing the growth and development of plants.
Cumulative emission of N2O was significantly positively correlated with N2O flux at 3 WAG for N treatments and NO3− concentration in soil under N-high treatment at 3 WAG (Table 2). This finding is consistent with that obtained by Yuttitham et al. [38] but inconsistent with that obtained by Liu et al. [36]. This difference may be attributed to the different genotypes, soil characteristics, and environmental conditions used in the experiment. Generally, nitrate is essential to increasing N2O emission related to its role as the substrate of the denitrification process in soil, but this was strongly affected by the soil moisture, revealing the availability of O₂ for transforming NO3− to N2O [38]. However, there was no correlation between cumulative N₂O emission and dry matter accumulation, root system, and yield components. These findings indicated that yield components, dry weight of shoot, and N2O flux and soil NH4+ concentrations at 5 WAG as well as N2O flux and soil NO3− at 3 WAG are significant indices for evaluating nitrogen responses to N2O emission of maize varieties under N-high status in soil.
4.2. Nitrogen responses-associated traits of efficient-efficient and nonefficient-nonefficient groups
The election of maize cultivar is an important way of improving maize production considering the potential yield and nutrient-efficiency. In addition, breeding high-yielding and high N-efficient maize varieties can be a feasible strategy for improving and maintaining maize yield and decreasing potential N-loss from soil as well as adverse impact on the environment [25]. Bisi 228, Bisi 2, Bisi 99, Bisi 18, and NK 7202 revealed consistent high grain yield and low emission both under N1 and N3, and were therefore were categorized under the EE group. Afterward, Arjuna, Guluk-guluk, Manding, NK 007, Anoman, and Pertiwi 3 were grouped under NN owing to their consistent result in low grain yield and high N2O emission both under N1 and N3 treatment. Therefore, the N efficient variety groups of maize can be a promising strategy for increasing N fertilizer efficiency without reducing maize production, and for mitigating the adverse impact of N-loss in the agricultural system, which is consistent with the findings of previous studies [5,9,17,19,[25], [39], [40]].
The EE groups showed increased yield and >50% reduction in cumulative N2O both under N1 and N3 treatment compared to those of all tested varieties, which was consistent with the findings of previous studies reporting that the EE group exhibited high maize yield under various N-treatments [9,[25], [39], [40]]. The EE group could potentially enhance their yield through the high N uptake at the post-silking stage [9,41], and yielded a large amount of N-grain, indicating their ability to immobilize N, particularly under N-low [9,39,42]. Moreover, the EE group showed high N uptake at the post-silking stage, related to the plant longevity, resulting in high dry matter at the post-silking stage, thus increasing plant yield [9], which is in agreement with our finding that the effect of maize variety and N application rate interaction for grain yield and dry matter accumulation was linear. This was related to the high capability of the EE group to remobilize N from vegetative organs to grain, thus controlling the group’s relatively high capacity for N-accumulation as reported by Xiang-Ling et al. [9] and Tolessa et al. [40]. They stated that the EE group represented higher N uptake efficiency (NUpE), N use efficiency (NUE), and N remobilization efficiency (NRE) than NN, indicating their ability to improve NUE and N-related traits relative to other groups [9,40]. Our results are consistent with those findings, whereas N-accumulation in maize demonstrated a significant effect by the interaction between variety and N application rate, which was linear with grain yield and dry matter accumulation. Additionally, cumulative N2O was significantly correlated with NO3−-N in soil at 3 WAG (a week after 1st N-application), and the EE showed lower NO3−-N concentration than NN groups, as a significant effect of the interaction between variety and N rate. Nitrogen fertilizer was applied in the form of NH4+ which is the main substrate of the nitrification process; oxidizing NH4+ to NO3−; therefore, the higher NO3− concentration in soil the higher nitrification process. This indicated the higher potential N-loss both through N2O or NO3−, confirming that every oxidation and reduction process in aerobic and anaerobic conditions of the nitrogen cycle produce N2O. In aerobic environments, nitrification is the primary source of N2O production and the source of NO3−, which is the primary substrate for denitrification and a very mobile source of N-loss through runoff and leaching [13]. The higher remaining N in form of NH4+ intepreted to reduced possibility of nitrification, and indicating the lower potential production of N₂O emission and N-loss from soil. In this study, the effect of variety and N rate was also significant, and ammonium concentration in the soil of the EE group was higher than that of the NN group which was related to the varying capabilities of the different groups with respect to adaptation to a rhizosphere condition. Subbarao et al. [15] reported that certain plants had certain mechanisms for suppressing the nitrifying activity of the soil to reduce N loss and nitrification-denitrification through the production and release of nitrification inhibitor from roots known as biological nitrification inhibition (BNI). Additionally, BNI could only be released by several plant species whose root systems are exposed to NH4+ in the rhizosphere, whereas nitrification is probably at the maximum rate. The composition of root exudate is affected by root development and the maturation zone as the major site of exudation for allelochemicals, including BNI [43]. Biological nitrification in maize was first identified in 2021 by Otaka et al. [44] and is referred to as zeanone.
N efficiency among maize varieties was affected by the N rate in soil and mainly controlled by the N uptake at high-N level and the N utilization at low N levels. Both N uptake and NUE were highly affected by the root system [40]. In this study, the dry weight and root volume significantly were affected by the interaction between variety and N rate, and the EE maize group had 38.15% and 33.44% higher dry weight and root volume, respectively, than the NN group across N treatment levels. Tolessa et al. [40] stated that the nitrogen use efficient maize cultivars allow for the uptake and utilization of N under various soil conditions proposed to have the ability to develop more optimized root systems allowing the maize root to extract N from relatively deep soil levels.
The amount of N cycled in the phloem and transported in the xylem of maize was relatively high under high-N. Generally, the N taken up by maize was significantly less than that simultaneously transported in the xylem. Therefore, it should be balanced with exports through the phloem; the amount of N cycled in phloem that simultaneously taken up [39]. In this study, the variety and N rate interaction significantly effected N-content of maize, whereas EE group was considerably higher N-content than that of NN group under both N levels, but did not differ between N1 and N3 treatments. Different cultivars of maize performed exhibited varying levels of NUE mainly owing to the difference in remobilizing N and utilizing accumulated N. N-efficient maize cultivars showed 10% higher contribution of N cycled in phloem on the xylem transported N than that of N-inefficient maize varieties [39,42]. N-efficient maize cultivars revealed higher N uptake and N cycling particularly when grown under N-limited conditions. N-cycling in maize changed depending on the N conditions, whereas the N reutilization by re-translocation through phloem increased under limited-N by shifting NO3− reduction towards root [39].
Some previous studies reported that yield and N2O emission in agricultural land increased as N levels increased [9,38,40,45,46], which was not consistent with the results of this study. In this study, the yield and cumulative N2O of all maize varieties were nonlinear with the increasing N application rate, and consistent with the findings of previous studies [36,25,47], indicating that the N2O emission and yield of maize were not always consistently linear with the N availability in soil whereas different N application rates exhibited varying of N-loss potential. The mean yield and cumulative N2O emission of all tested maize under N1 (57.5 kg N ha−1) and N3 (173 kg N ha−1) were not significantly different, indicating that the tested maize varieties did not respond significantly to increased N application levels. This finding was inconsistent with those of previous studies [40,45,47]. Roy et al. [45] reported that the maize yield and N2O emission responded significantly to the N application until a rate of 218 kg N ha−1, at which point they peaked, thereby reaching a maximum N-rate, while the N application effect at a rate of 145–218 kg N ha−1 was relatively decreased and comparable to that of the lower N-level (i.e., below 142 kg N ha−1) treatment, which was with the same outcome of the study by Tolessa et al. [40]. McSwiney and Robertson [47] stated the nitrous oxide fluxes remained low (∼20 g N ha−1 day−1) following N-application at a rate ranging from 0 to 101 kg N ha−1, and then relatively showed an increasing trend with the increasing N levels and reached peak at 134 kg N ha−1. The maize grain yield showed an increasing trend and reached the peak at 101 kg N ha−1 then leveled off at a higher N fertilizer level [47]. The results of this study confirm that maize yield and N-dynamics varied among varieties, while exhibiting inconsistent responses to the N-rate application, which indicates the possible difference in nitrogen demand and/or nitrogen use efficiency among various genotypes of maize varieties.
5. Conclusion
The EE maize varieties, including Bisi 228, Bisi 2, Bisi 99, Bisi 18, and NK 7202 which revealed consistent high grain yield and low emission under N1 and N3, showed increased levels of grain yield, yield components, N-accumulation, dry matter accumulation, root volume, and NH4+ in soil, and reduced cumulative N2O and NO3− in soil compared to N-inefficient maize varieties (NN), including Arjuna, Manding, Guluk-guluk, NK 007, Anoman, and Pertiwi 3 which were consistent result in low grain yield and high N2O emission both under N1 and N3. The EE variety groups of maize can be a feasible strategy for increasing N fertilizer efficiency without reducing maize production and can reduce the negative impact of N-loss in the agricultural system. Results in this study contribute to contemporary topics such as NUE increases and greenhouse gas mitigation, and further our understanding of how maize and soil react to N under different N statuses.
Funding acknowledgement statement
Supported by the Kementerian Pendidikan, Kebudayaan, Riset, dan Teknologi, Republik Indonesia through Pendidikan Magister menuju Doktor untuk Sarjana Unggul (PMDSU) Scholarship to J.K.
Author contribution statement
Firdausi Nur Azizah: Conceived and designed the experiments; performed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Benito Heru Purwanto: Conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper.
Keitaro Tawaraya; Diah Rachmawati: Conceived and designed the experiments; analyzed and interpreted the data; wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Vitousek P.M., Aber J.D., Howarth R.W., Likens G.E., Matson P.A., Schindler D.W., Schlesinger W.H., Tilman D.G. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 1997;7:737–750. [Google Scholar]
- 2.Ussiri D., Lal R. Springer Dordrecth Hidelberg; New York, London: 2013. Soil Emission of Nitrous Oxide and its Mitigation. [Google Scholar]
- 3.De Vries W., Van der Salm C., Reinds G.J., Erisman J.W. Element fluxes through European forest ecosystems and their relationships with stand and site characteristics. Environ. Pollut. 2007;148:501–513. doi: 10.1016/j.envpol.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 4.FAO . 2019. World Fertilizer Trends and Outlook to 2022.https://www.fao.org/3/ca6746en/ca6746en.pdf Rome. [Google Scholar]
- 5.Grassini P., Cassman K.G. High-yield maize with large net energy yield and small global warming intensity. Proc. Natl. Acad. Sci. U. S. A. 2012;109:1074–1079. doi: 10.1073/pnas.1116364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z., Hu Y., Zhang S., Raza S., Wei X., Zhao X. The thresholds and management of irrigation and fertilization earning yields and water use efficiency in maize, wheat, and rice in China: a meta-analysis (1990–2020) Agronomy. 2022;12:709. [Google Scholar]
- 7.Delgado J.A., Shaffer M., Hu C., Lavado R., Cueto-Wong J., Joosse P., Sotomayor D., Colon W., Follett R., DelGrosso S., Li X., Rimski-Korsakov H. An index approach to assess nitrogen losses to the environment. Ecol. Eng. 2008;32:108–120. doi: 10.1016/j.ecoleng.2007.10.006. [DOI] [Google Scholar]
- 8.Mi G.H., Chen F.J., Wu Q.P., Lai N.W., Yuan L., Zhang F.S. Ideotype root architecture for efficient nitrogen acquisition by maize in intensive cropping systems. Sci. China Life Sci. 2010;53:1369–1373. doi: 10.1007/s11427-010-4097-y. [DOI] [PubMed] [Google Scholar]
- 9.Xiang-ling L., Guo L.G., Zhou B.Y., Tang X.M., Chen C.C., Zhang L., Zhang S.Y., Li C.F., Xiao K., Dong W.X., Yin B.Z. Characterization of low-N responses in maize (Zea mays L.) cultivars with contrasting nitrogen use efficiency in the North China Plain. J. Integr. Agric. 2019;18:2141–2152. doi: 10.1016/S2095-3119(19)62597-9. [DOI] [Google Scholar]
- 10.Britto D.T., Kronzucker H.J. Bioengineering nitrogen acquisition in rice: can novel initiatives in rice genomics and physiology contribute to global food security? Bioessays. 2004;26:683–692. doi: 10.1002/bies.20040. [DOI] [PubMed] [Google Scholar]
- 11.Sun L., Lu Y., Yu F., Kronzucker H.J., Shi W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016;212:646–656. doi: 10.1111/nph.14057. [DOI] [PubMed] [Google Scholar]
- 12.Ravishankara A.R., Daniel J.S., Portmann R.W. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 13.Sorai M., Yoshida N., Ishikawa M. Biogeochemical simulation of nitrous oxide cycle based on the major nitrogen processes. J. Geophys. Res. Biogeo. 2007;112 doi: 10.1029/2005JG000109. [DOI] [Google Scholar]
- 14.Ding W., Cai Y., Cai Z., Yagi K., Zheng X. Nitrous oxide emissions from an intensively cultivated maize–wheat rotation soil in the North China Plain. Sci. Total Environ. 2007;373:501–511. doi: 10.1016/j.scitotenv.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 15.Subbarao G.V., Ito O., Sahrawat K.L., Berry W.L., Nakahara K., Ishikawa T., Watanabe T., Suenaga K., Rondon M., Rao I.M. Scope and strategies for regulation of nitrification in agricultural systems—challenges and opportunities. CRC Crit. Rev. Plant Sci. 2006;25:303–335. doi: 10.1080/07352680600794232. [DOI] [Google Scholar]
- 16.Stevens W.B., Hoeft R.G., Mulvaney R.L. Fate of nitrogen-15 in a long-term nitrogen rate study: II. Nitrogen uptake efficiency. Agron. J. 2005;97:1046–1053. doi: 10.2134/agronj2003.0313. [DOI] [Google Scholar]
- 17.Cañas R.A., Amiour N., Quilleré I., Hirel B. An integrated statistical analysis of the genetic variability of nitrogen metabolism in the ear of three maize inbred lines (Zea mays L.) J. Exp. Bot. 2011;62:2309–2318. doi: 10.1093/jxb/erq373. [DOI] [PubMed] [Google Scholar]
- 18.Liang C., Farjun C., Fu-so Z., Guo-hua M. Root growth, nitrogen uptake and yield formation of hybrid maize with different N efficiency. Plant Nutr. Fert. Sci. 2005;11:615–619. [Google Scholar]
- 19.Garnett T., Plett D., Conn V., Conn S., Rabie H., Rafalski J.A., Dhugga K., Tester M.A., Kaiser B.N. Variation for N uptake system in maize: genotypic response to N supply. Front. Plant Sci. 2015;6:936. doi: 10.3389/fpls.2015.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bantacut T., Pradifta J. Nitrogen cycling in Indonesian agriculture around 1968 to 2008 and its environmental impacts. Jurnal Pengelolaan Sumberdaya Alam Dan Lingkungan (J. Nat. Resour. Environ. Manag.) 2018;8(3):308–318. doi: 10.29244/jpsl.8.3.308-318. [DOI] [Google Scholar]
- 21.Adiy, L., Idowati, R. W., Nursy Amsi, E., Ochay Ati, S. R., and al Sarwani, M. (n.d.). Seminar on Increased Agricultural Nitrogen Circulation in Asia: Technological Challenge to Mitigate Agricultural N Emissions.
- 22.Ussiri D.A.N., Lal R., Jarecki M.K. Nitrous oxide and methane emissions from long-term tillage under a continuous corn cropping system in Ohio. Soil Tillage Res. 2009;104:247–255. doi: 10.1016/j.still.2009.03.001. [DOI] [Google Scholar]
- 23.Keeney D.R., Nelson D.W. In: Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties. second ed. Page A.L., editor. ASA; Madison, WI, SSSA: 1983. Nitrogen-inorganic forms; pp. 643–698. [DOI] [Google Scholar]
- 24.Kempers A.J., Zweers A. Ammonium determination in soil extracts by the salicylate method. Commun. Soil Sci. Plant Anal. 1986;17:715–723. doi: 10.1080/00103628609367745. [DOI] [Google Scholar]
- 25.Chen F.J., Fang Z.G., Gao Q., Ye Y.L., Jia L.L., Yuan L.X., Mi G.H., Zhang F.S. Evaluation of the yield and nitrogen use efficiency of the dominant maize hybrids grown in North and Northeast China. Sci. China Life Sci. 2013;56:552–560. doi: 10.1007/s11427-013-4462-8. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. R: A Language and Environment for Statistical Computing.https://www.R-project.org/ [Google Scholar]
- 27.IPCC . In: Climate Change and Land: an IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. Shukla P.R., Skea J., Calvo Buendia E., Masson-Delmotte V., Pörtner H.-O., Roberts D.C., Zhai P., Slade R., Connors S., van Diemen R., Ferrat M., Haughey E., Luz S., Neogi S., Pathak M., Petzold J., Portugal Pereira J., Vyas P., Huntley E., Kissick K., Belkacemi M., Malley J., editors. 2019. [Google Scholar]
- 28.Gheith E.M.S., El-Badry O.Z., Lamlom S.F., Ali H.M., Siddiqui M.H., Ghareeb R.Y., El-Sheikh M.H., Jebril J., Abdelsalam N.R., Kandil E.E. Maize (Zea mays L.) productivity and nitrogen use efficiency in response to nitrogen application levels and time. Front. Plant Sci. 2022:13. doi: 10.3389/fpls.2022.941343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies B., Coulter J.A., Pagliari P.H. Timing and rate of nitrogen fertilization influence maize yield and nitrogen use efficiency. PLoS One. 2020;15(5) doi: 10.1371/journal.pone.0233674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu S.J., He P., Zhao S.C., Li W.J., Xie J.G., Hou Y.P., Grant C.A., Zhou W., Jin J.Y. Impact of nitrogen rate on maize yield and nitrogen use efficiencies in northeast China. Agron. J. 2015;107:305–313. doi: 10.2134/agronj13.0567. [DOI] [Google Scholar]
- 31.Preza-Fontes G., Christianson L.E., Greer K., Bhattarai R., Pittelkow C.M. In-season split nitrogen application and cover cropping effects on nitrous oxide emissions in rainfed maize. Agric. Ecosyst. Environ. 2022:326. doi: 10.1016/j.agee.2021.107813. [DOI] [Google Scholar]
- 32.Tofa A.I., Kamara A.Y., Babaji B.A., Aliyu K.T., Ademulegun T.D., Bebeley J.F. Maize yield as affected by the interaction of fertilizer nitrogen and phosphorus in the Guinea savanna of Nigeria. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam S., Tiwari U., Sapkota B., Sharma B., Parajuli S., Pandit N.R., Gaihre Y.K., Dhakal K. Field evaluation of slow-release nitrogen fertilizers and real-time nitrogen management tools to improve grain yield and nitrogen use efficiency of spring maize in Nepal. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su W., Ahmad S., Ahmad I., Han Q. Nitrogen fertilization affects maize grain yield through regulating nitrogen uptake, radiation and water use efficiency, photosynthesis and root distribution. PeerJ. 2020;8 doi: 10.7717/peerj.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mdlambuzi T., Muchaonyerwa P., Tsubo M., Moshia M.E. Nitrogen fertiliser value of biogas slurry and cattle manure for maize (Zea mays L.) production. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y.T., Li Y.E., Wan Y.F., Chen D.L., Gao Q.Z., Li Y., Qin X.B. Nitrous oxide emissions from irrigated and fertilized spring maize in semi-arid northern China. Agric. Ecosyst. Environ. 2011;141:287–295. doi: 10.1016/j.agee.2011.03.002. [DOI] [Google Scholar]
- 37.Franco-Luesma S., Lafuente V., Alonso-Ayuso M., Bielsa A., Kouchami-Sardoo I., Arrúe J.L., Álvaro-Fuentes J. Maize diversification and nitrogen fertilization effects on soil nitrous oxide emissions in irrigated Mediterranean conditions. Front. Environ. Sci. 2022:10. doi: 10.3389/fenvs.2022.914851. [DOI] [Google Scholar]
- 38.Yuttitham M., Chidthaisong A., Ruangchu U. N2O fluxes and direct N2O emission factors from maize cultivation on oxisols in Thailand. Geoderma Reg. 2020;20 doi: 10.1016/j.geodrs.2019.e00244. [DOI] [Google Scholar]
- 39.Niu J., Chen F., Mi G., Li C., Zhang F. Transpiration, and nitrogen uptake and flow in two maize (Zea mays L.) inbred lines as affected by nitrogen supply. Ann. Bot. 2007;99:153–160. doi: 10.1093/aob/mcl237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tolessa D., Du Preez C.C., Ceronio G.M. Comparison of maize genotypes for grain yield, nitrogen uptake and use efficiency in Western Ethiopia. S. Afr. J. Plant Soil. 2007;24:70–76. doi: 10.1080/02571862.2007.10634784. [DOI] [Google Scholar]
- 41.Worku M., Bänziger M., Erley G.S.A.E., Friesen D., Diallo A.O., Horst W.J. Nitrogen uptake and utilization in contrasting nitrogen efficient tropical maize hybrids. Crop Sci. 2007;47:519–528. doi: 10.2135/cropsci2005.05.0070. [DOI] [Google Scholar]
- 42.Hirel B., Bertin P., Quilleré I., Bourdoncle W., Attagnant C., Dellay C., Gouy A., Cadiou S., Retailliau C., Falque M., Gallais A. Towards a better understanding of the genetic and physiological basis for nitrogen use efficiency in maize. Plant Physiol. 2001;125:1258–1270. doi: 10.1104/pp.125.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Badri D.V., Vivanco J.M. Regulation and function of root exudates. Plant Cell Environ. 2009;32:666–681. doi: 10.1111/j.1365-3040.2009.01926.x. [DOI] [PubMed] [Google Scholar]
- 44.Otaka J., Subbarao G.V., Ono H., Yoshihashi T. Biological nitrification inhibition in maize—isolation and identification of hydrophobic inhibitors from root exudates. Biol. Fertil. Soils. 2022;58:251–264. doi: 10.1007/s00374-021-01577-x. [DOI] [Google Scholar]
- 45.Roy A.K., Wagner-Riddle C., Deen B., Lauzon J., Bruulsema T. Nitrogen application rate, timing and history effects on nitrous oxide emissions from corn (Zea mays L.) Can. J. Soil Sci. 2014;94:563–573. doi: 10.4141/CJSS2013-118. [DOI] [Google Scholar]
- 46.Atakora W.K., Kwakye P.K., Weymann D., Brüggemann N. Stimulus of nitrogen fertilizers and soil characteristics on maize yield and nitrous oxide emission from Ferric Luvisol in the Guinea Savanna agro-ecological zone of Ghana. Sci. Afr. 2019;6 doi: 10.1016/j.sciaf.2019.e00141. [DOI] [Google Scholar]
- 47.McSwiney C.P., Robertson G.P. Nonlinear response of N2O flux to incremental fertilizer addition in a continuous maize (Zea mays L.) cropping system. Global Change Biol. 2005;11:1712–1719. doi: 10.1111/j.1365-2486.2005.01040.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.