Abstract
Protein posttranslation modifications (PTMs) are a critical regulatory mechanism of protein function. Protein α-N-terminal (Nα) methylation is a conserved PTM across prokaryotes and eukaryotes. Studies of the Nα methyltransferases responsible for Να methylation and their substrate proteins have shown that the PTM involves diverse biological processes, including protein synthesis and degradation, cell division, DNA damage response, and transcription regulation. This review provides an overview of the progress toward the regulatory function of Να methyltransferases and their substrate landscape. More than 200 proteins in humans and 45 in yeast are potential substrates for protein Nα methylation based on the canonical recognition motif, XP[KR]. Based on recent evidence for a less stringent motif requirement, the number of substrates might be increased, but further validation is needed to solidify this concept. A comparison of the motif in substrate orthologs in selected eukaryotic species indicates intriguing gain and loss of the motif across the evolutionary landscape. We discuss the state of knowledge in the field that has provided insights into the regulation of protein Να methyltransferases and their role in cellular physiology and disease. We also outline the current research tools that are key to understanding Να methylation. Finally, challenges are identified and discussed that would aid in unlocking a system-level view of the roles of Να methylation in diverse cellular pathways.
Keywords: protein N terminus, posttranslational modifications, alpha-N-terminal methylation, methyltransferases, substrates
Proteins are essential macromolecules responsible for various cellular functions, including signal transduction, transcription, and translation. A wide range of dynamic posttranslational modifications (PTMs) is employed to regulate protein function. For instance, protein acetylation on the N terminus is one of the most prevalent PTMs and regulates protein stability and degradation (1, 2, 3). Compared with N-terminal acetylation, protein α-N-terminal (Nα) methylation documented in prokaryotes and eukaryotes (4, 5, 6, 7, 8, 9, 10, 11, 12) has more specific protein substrates. Unlike lysine acetylation and methylation, N-terminal acetylation and methylation are viewed as irreversible with no identified erasers (13). Compared with lysine/arginine side chain methylation (14, 15), the physiological roles of Nα methylation are in relatively early stages (16, 17, 18).
Protein Να methylation and their related methyltransferases (MTases) are drawing attention because they are involved in various essential biological processes and their substrates span diverse protein families. Furthermore, an N6-methyladenosine (m6A)-mediated posttranscriptional mechanism (19) and transcriptional activation mediated by cAMP-response element-binding protein 1 (CREB1) have emerged as upstream regulators (20). The dysregulation of Nα methyltransferase is associated with a premature phenotype in mice (21) and multiple human cancers (22, 23, 24). Meanwhile, the advent of a suite of selective and potent inhibitors of human Να MTases through different mechanisms and chemical modalities expands the toolbox for probing the function of Να MTases (25, 26, 27).
Several reviews have summarized the catalytic mechanisms, substrate recognition, inhibitors, and physiological consequences of Να methylation (16, 18, 28). Since then, additional studies have been conducted with more identified substrates and inhibitors. This review will focus on the regulation of Να MTases and the function of substrates concerning Να methylation. We outline the diverse substrates and categorize the biological processes modulated by Να MTases. Proteins being Να methylated are involved in protein synthesis and degradation, cell cycle proteins, DNA repair, and cytoskeletal myosin proteins. As many substrates remain uncharted, an expanded and higher resolution map of the Να methylated substrate landscape is required to elucidate this modification in a systematic view. Herein, we explore the variability of the function of Να methylation based on the protein, cellular, and tissue settings. We also note that a cohesive concept for this variability is currently absent, so it remains unclear whether a single unifying theme is possible.
Protein Να MTases and the methylation process
Sequence analysis indicates that 1 to 2% of genes of eukaryote and prokaryote genomes encode protein MTases spanning three structural protein families, which include the seven-beta strand protein family (7βS), Su(Var) 3 to 9 Enhancer-of-Zeste Trithorax domain protein family (SET), and SpoU and TrmD (SPOUT) domain protein family (29, 30, 31, 32, 33). The human genome encodes approximately 200 MTases, while 23 MTases are found in the yeast genome (33). A 7βS domain is conserved in all identified eukaryotic protein Να MTases, including three Να MTases in humans (METTL13 (34) and NTMT1/2, alias NRMT1/2 or METTL11 A/B (12)); three homologs in mice, rabbit, African clawed frog, and zebrafish (EFNMT, NTM1A, and NTM1B); and two homologs in yeast (Efm7 (35) and Ntm1 (11)) and Drosophila (EFNMT and CG1675 (36)). Specifically, the Να MTases consist of seven alpha helices (α1–α7), nine beta strands (β1–β9), and one 310 helix (η1), which fold into a single domain with a highly conserved Rossmann fold structure (37). The NTMT family proteins also harbor two unique structural elements involved in substrate binding, one β hairpin and an N-terminal extension (37). There are two groups of eukaryotic Να MTases based on substrate specificity: N-terminal methyltransferase 1/2 (NTMT1/2) that methylate substrates with the XP[K/R] sequence motif and the eEF1α lysine and N-terminal dual methyltransferases (eEF1A-KNMT, METTL13), which exclusively methylate eukaryotic elongation factor 1α (eEF1α). Like METTL13, the prokaryotic Να MTase, PrmA (Protein L11 MTase), methylates both the Nα-amino and the ε-amino groups of lysine residues (Fig. 1A) (38). PrmA is also a member of the 7βS family but exhibits minimal sequence and structural similarity with other eukaryotic Να MTases through Basic Local Alignment Search Tool (BLAST) sequence alignment with amino acid sequences or structural alignment with PyMol software. Like other 7βS MTases, NTMT1/2 maintain deeply buried binding pockets for S-adenosyl-L-methionine (SAM), while the SAM-binding sites in eEF1A-KNMTs or PrmA are closer to the protein surface (Fig. 1A).
Figure 1.
The Να methyltransferases and the protein Να methylation reaction process.A, types of identified protein Να methyltransferases in eukaryotes and prokaryotes. The eukaryotic proteins have conserved 7βS domains. NTMTs include yeast Ntm1 (PDB: 7D8D), NTMT1 (PDB: 5E1M), and NTMT2 (PDB: 6DUB) and have similar structure and binding poses. METTL13 is a dual 7βS protein that modifies eEF1α with a C-terminal domain (MT13-C; PDB: 5WCJ) responsible for methylation of the N-terminal glycine and an N-terminal domain (MT13-N) responsible for methylating lysine-55. PrmA (PDB: 2NXE) is not a clear homolog to METTL13 in terms of amino acid sequence, but both are similar in their mode of recognition in requiring full-length substrates (magenta, SAH/SAM molecule; blue caged molecule, substrate peptides). hMT13C represents METTL13 C-terminal domain. B, scheme for the protein methylation process catalyzed by NTMTs demonstrating removal of methionine and sequential methylation. The methylation is currently thought to be irreversible. C, the substrate peptide-binding pockets of NTMT1 (PDB: 5E1M) and Ntm1 (PDB: 7D8D). D, the SAH-binding pockets of NTMT1 and Ntm1. The key amino acid residues in the peptide-binding pocket and SAH-binding pocket of NTMT1 are annotated in blue font. Residues of NTMT1 are colored blue, and residues of Ntm1 are colored magenta. Structures were realized using PyMOL 2.5.5. EEFKNMs, eEF1α lysine and N-terminal dual methyltransferases; NTMTs, N-terminal methyltransferases; SAH, S-adenosyl-L-homocysteine; SAM, S-adenosyl-L-methionine.
N-terminal methyltransferases
The NTMTs were first described in humans and yeast almost simultaneously. Substrates were found to be methylated at the α-amino group after the initiating methionine was cleaved, with methylation events including mono, di, and trimethylation (Fig. 1B). Yeast Ntm1 (YBR261C gene, also annotated as TAE1 for Translation Associated Element 1) methylates the ribosomal proteins Rps25 and Rpl12 (11), each encoded by a pair of paralog genes, RSP25A/RSP25B and RPL12A/RPL12B. Rpl12a/b are identical in sequence, and Rps25a/b only differ by a single amino acid at the C terminus. NTMT1 has multiple protein substrates in humans, including RCC1 (Regulator of chromosome condensation 1), SETα (SET Domain Containing 1A), and RB (Retinoblastoma 1) (11, 12). NTMTs are also modified histone proteins, such as the fly H2B (36) and human CENP-A (39). It is noteworthy that, in 1987, a hypothesis was put forward regarding the existence of a eukaryotic methyltransferase that methylates the α-NT of proteins harboring an XPK motif before the discovery of Nα MTases (17). NTMTs prefer an XPK motif (X = A, S, or P commonly observed), while methylation was diminished if P2 or K3 was substituted or acidic residues were present at the X1 position (11, 12). The recognition motif was further expanded based on biochemical NTMT1 methylation assays with synthetic peptides, where diverse amino acids can be tolerated at the X1 position (G, F, Y, C, M, K, R, N Q). Still, lysine or arginine is favored at the third residue, and the P2 position is preferred with limited variation (40). Bioinformatic analysis of the XP[KR] motif indicates 216 potential substrates in humans (40, 41) and 45 in yeast (38) (X1 can be any amino acid residue). Notably, the possibility of an even less stringent motif methylated by NTMT1 is discussed in the context of newly identified atypical substrates and when discussing useful tools in this review.
The well-characterized crystal structures of NTMTs reveal the enzyme–substrate interaction and explain the substrate preference. Peptide substrates bind to an extensively negatively charged, deep-buried channel. These general structural features and organization can be found in the crystallized protein structures of NTMT1, NTMT2, and Ntm1. The conserved DxGxGxG motif surrounds the ribosyl and methionyl moiety of the S-adenosyl-L-homocysteine (SAH) molecule as in other Rossmann-fold MTases. In the NTMT1 crystal structure, R74, W20, I92, V137, L119, and Q120 are the critical amino acids to position SAH in the binding pocket. N168, W136, D177, D180, and H140 are essential residues for substrate recognition and MTase activity (Fig. 1, C and D) (37). Specifically, the planar shape of W136 forms a ring–ring stacking interaction with the P2 residue on the substrate, while D177 and D180 form the electrostatic network with the K/R3 residue (24, 37). Several crystal structures of NTMT1 have been reported with both nonhistone peptides and histone peptides, such as the hexapeptide of fly histone H2B (Protein Data Bank [PDB]: 5CVE), monomethylated hexapeptide of CENP-A (PDB: 6KPQ), or with the dimethylated hexapeptide of CENP-A (PDB: 5CVD) (Table 1).
Table 1.
NTMTs crystal structures that are deposited in the PDB
| Methyltransferase | PDB | Cocrystallized molecules |
|---|---|---|
| NTMT1 | 2EX4 | SAH |
| 6KDQ | CENP-A monomethylated peptide | |
| 5CVD | CENP-A dimethylated peptide | |
| 5CVE | SAH, fly histone 2B peptide | |
| 7K3D | DC113 | |
| 6DTN | NAH-C3-PPKRIA | |
| 6WH8 | BM30 | |
| 6WJ7 | NAH-C2-GPARIA | |
| 6PVA | NAH-C3-GPKK | |
| 6PVB | NAH-C5-GPKRIA | |
| 5E2A, 5E1M, 5E1O, 5E1B, 5E1D, 5E2B | SAH, RCC1 peptide | |
| NTMT2 | 5UBB | SAM |
| 6DUB | SAH, RCC1 peptide | |
| Ntm1 | 7D8F | SAH |
| 7D8D | SAH, Rps25a peptide |
Abbreviations: NAH, N-adenosyl-L-homocysteine; NTMT, N-terminal methyltransferase; SAH, S-adenosyl-L-homocysteine; SAM, S-adenosyl-L-methionine.
NTMT1 and NTMT2 in humans share 50% amino acid sequence identity and 75% amino acid sequence similarity. NTMT1 can install different methylation states. NTMT2, a predominantly monomethylase, was initially proposed to prime substrates for further methylation by NTMT1 (42). However, increased methylation, including di/trimethylation by NTMT2, was observed with 2 of 20 different peptide substrates in vitro (43). The N89 residue of NTMT2 is a gatekeeper to influence the binding to methylated peptides because of steric hindrance from the N89 side chain compared with G33 in NTMT1 (43). Mutation of N89 to G89 increased the binding affinity of monomethylated peptides and generated trimethylated products (43). In addition, an NTMT2 monomer was found to form a heterotrimer with an NTMT1 dimer to enhance the protein stability and substrate binding affinity (44). Two crystal structures of Ntm1 in complex with SAH (PDB: 7D8F) or SAH/Rps25a hexapeptide (PDB: 7D8D) are also available in the PDB database (Table 1) (45). The two structures show a similar tertiary structure to the human homologs with RMSD around 0.5 to 0.7 Å (45). The core structure with the active site is highly conserved (Fig. 1, C and D).
Eukaryotic elongation factor lysine and N-terminal methyltransferases (eEF1A-KNMTs) exclusively target eEF1α
Contrary to the NTMTs, EF1A-KNMTs in eukaryotes are highly specific and exclusively methylate eEF1α. eEF1α delivers aminoacyl-tRNAs to the A-site of a ribosome powered by GTP during the translational process and is subject to extensive methylation by multiple MTases. EF1A-KNMTs exhibit dual N-terminal MTase and lysine MTase activities (46). The human enzyme, METTL13, modifies the G1 of eEF1α via the C-terminal orphan MTase (denoted as MT13-C) (Fig. 1). The N-terminal KMT-like MTase domain is responsible for mono-/dimethylating K55 (denoted as MT13-N in the original publication) (Fig. 1). MT13-C catalyzes the N-terminal methylation in a processive manner, producing mainly trimethylated products and a trace amount of mono-/dimethylated species. An in vitro peptide array assay revealed that G1 of human eEF1α is essential for METTL13 recognition since other amino acids at the first position either eliminated or decreased methylation (34). No evidence indicates that the K55 and G1 methylation events are coupled. Interestingly, nucleotides GTP or GDP suppressed K55 methylation by MT13-N but the protein Να methylation by MT13-C is insensitive to GTP/GDP. The closest homolog of MT13-C is SpdS, based on a similar topology from the crystal structures. G503, E524, and a short DG-motif (MT13-C D551-G552) are involved in the interaction between MT13-C and SAM/SAH, while D575 and D577 confer substrate recognition. E524 and D575 are critical for enzymatic activity (34). Interestingly, yeast Efm7 (systematic gene name, YLR285W) catalyzes the N-terminal G1 trimethylation and K2 methylation of yeast eEF1α, although it lacks the sequence homology to MT13-C. Thus, the methylated products span from monomethylation (+1 methyl group) to hexamethylation (+6 methyl group). The trimethylation of G1 with high stoichiometry in tri-, tetra-, and pentamethylated forms suggests the protein α-amino group is the more favored site than K2, possibly facilitated by the similar chemical environments of the Να amino G1 and K2 side chain (35). Unlike the MT13-C domain, Efm7 is sensitive to GDP/GTP and recognizes the conformational changes of eEF1α induced by GDP/GTP. Adding GDP or GTP increased the amount of di-/trimethylated products on full-length protein, while no methylation is detected when using truncated peptides of eEF1α (35).
Prokaryotic and plant Να MTases
Contrary to eukaryotic Να MTases with high substrate specificity, prokaryotic Να MTases are promiscuous in recognizing protein N termini. PrmA is a multifunctional enzyme that can methylate Να amino and lysine side chains on bacterial 50S ribosomal protein L11 (RPL11). Conserved among bacteria but absent in archaea (47, 48), PrmA is evolutionarily distinct from eukaryotic NTMTs. PrmA shows less than 11 to 13% sequence similarity to NTMT1/2 and does not share crucial residues in the active site of Ntm1/NTMT1/NTMT2. PrmA has a domain organization similar to that of the eEF1A-KNMTs and contains two domains linked by a flexible loop (Fig. 1). The N-terminal domain is known for substrate recognition, and the C-terminal domain has catalytic activity (49). The C-terminal domain PrmA has a recognition preference for the AK[A/G/K] or M[L/M/K][G/Q] sequence (16). PrmA orthologs appear to be restricted to bacteria and photosynthetic eukaryotes, while BLAST searches identified weak matches only for the C-terminal catalytic domain of PrmA with other eukaryotic methyltransferases (50). The structural flexibility of prokaryotic PrmA contributes to multiple-site methylations of RPL11, including the protein α-amino group, K3, and K39 side chain. A proposed mechanism is that RPL11 binds to PrmA with a random orientation to the active site, and then PrmA searches the substrate for potential methylation sites (47). Deletion of PrmA in E.coli is not essential for viability (51) but affects ribosome biogenesis (48). The PrmA gene is prevalent across prokaryotes and photosynthetic plants, where Arabidopsis PrmA was found to localize to plastids and mitochondria. Unlike its orthologue in E.coli, the plant PrmA catalyzes the trimethylation of K109 instead of the RPL11 N terminus, as the plant protein has a proline after the initial methionine. However, the plant PrmA can trimethylate bacterial Rpl11 in vitro, demonstrating its potential to methylate other potential substrates that have yet to be identified.
Conservation of Να MTase substrates and Να methylation functions
The presence of a canonical motif is a strong indicator that a protein is likely Να methylated. To examine the level of conservation of Να methylation across eukaryotes, we identified the orthologs of ten different eukaryotic species, from humans to yeast. We focused on model organisms as defined by the DIOPT database (Drosophila RNAi Screening Center Integrative Ortholog Prediction Tool) (52) and MARRVEL databases (Model Organism Aggregated Resources for Rare Variant ExpLoration) (53). We observed that the motif varies across the 10 eukaryotic species. For example, RCC1 has an SPK sequence motif in humans, whereas the Pro1 variant is present in most mammals, but the motif is not conserved in other model eukaryotes (Fig. 2).
Figure 2.
Conservation of the α-NT motif in ortholog genes across model organisms. Group 1 represents most of the known methylated substrates that have been identified and the corresponding ortholog sequence across model organism species—additional proteins have been demonstrated to be methylated but are included in other categories. Group 2 represents the eight yeast proteins that have one or more model organism orthologs with a conserved canonical motif. Group 3 represents the atypical substrates that were demonstrated to be methylated. Group 4 is the sole substrate for METTL13 and yeast Efm7. Yellow sequences conform to the canonical motif, XP[KR]; orange represents the XX[KR] motif based on MYL9 (41) and ZHX2 (61) methylated substrates; blue are nonconforming sequences within the same ortholog group; green represents the METTL13 motif. ∗ represents the species in which the substrate was demonstrated to be methylated. The sequence orthologs were retrieved from DIOPT/MARRVEL, and the closest ortholog to the human protein (gray) in each species is displayed. H.s., human; M.m., mouse; R.n., rat; X.t., frog; D.r., zebrafish; D.m., fly; C.e., worm; S.c., budding yeast; S.p., fission yeast; and A.t., thale cress. Note that orthologs are not present in some species or could not be detected. PSMC3IP and MND1 proteins have been reported as not present in fly or worms.
The gain or loss of the XP[K/R] motif across the evolutionary landscape may be explained by two reasons. First, many modifications are nonfunctional, and mutations do not affect cellular fitness (54). Indeed, several studies have posited that some phosphorylation and acetylation are functionless based on evolutionary and functional studies (55, 56). Second, the modification interchanges with other mechanisms, such as amino acid substitutions or different PTMs. Bioinformatic analysis indicates that PTMs coevolve, indicating a functional association between PTMs (57, 58). One possibility is that other PTMs could interchange with Να methylation, although these associations need validation. Evolutionarily, most low conservation sites are unlikely to impact cellular fitness and are easily mutated (59). Highly conserved sites are more likely to be critically functional sites. For example, 8 of 45 XP[K/R]-containing yeast proteins have 1 or more orthologs with a conserved canonical motif compared with other eukaryotes. Four proteins have a canonical motif across all orthologs (Rps25, Ola1, Rpl12, and Tma46 [human ortholog is ZC3H15]), suggesting these substrates may be vital for conserved eukaryotic biology. In addition, the recognition preference of the N-terminal sequences by Nα MTases from different species is similar in that they recognize the XP[K/R] motif suggesting selective pressure to maintain enzymatic sequence specificity. A similar trend has been observed for the Gid4 subunit, the substrate recognition subunit of the GID ubiquitin ligase, which targets N-terminal proline (Proline/N-degron) substrates (60).
The variance in the recognition motif could indicate if Να modifications are conserved across eukaryotic orthologs. A recent study suggests that peptides without a Pro2 can be methylated, potentially expanding the substrate pool to XX[K/R]-containing proteins. We observed the co-occurrence of the XP[K/R] and the XX[K/R] sequences within the same ortholog group (yellow and orange motifs in Fig. 2), suggesting plasticity and transition between these two motifs. Thus, examining motif conservation across species is a strategy to identify potential substrate candidates with the less stringent motif. The evidence for methylation of XX[KR]-containing proteins is emerging but needs further validation. Potential in vivo methylation of atypical motifs in myosins (MYL9 [Myosin Light Chain 9], MYL12A and MYL12B; also referred to as MLC9, MLCB-like, and MLC12B) was initially identified using a trimethylation antibody pulldown from mouse spleen lysates and confirmed by mass spectrometry (MS) (40). The methylation of the atypical substrates, MYL9 (41) and ZHX2 (Zinc Fingers and Homeoboxes 2) (61), was also demonstrated by mutational analysis and in vitro peptide studies. More evidence is required to verify the prevalence of permissiveness of the XX[KR] sequence. Future studies should endeavor to directly show the in vivo existence of Nα modifications on these atypical motifs. Interestingly, polybromo 1 protein (PBRM1) has a GSK sequence and was identified as a potential substrate via activity-based profiling of NTMT1 and subsequently demonstrated to be methylated in vitro (62). An early in vitro study of NTMT1 methylation of a range of synthetic peptides showed tolerance at the P2 position (40). However, another study found that peptides with mutations at the P2 position were not methylated by NTMT1 (37), agreeing with the reported in vitro preference of Ntm1 (11). These studies demonstrate a need to investigate atypical substrates to solidify or refute if the XX[KR] sequence is widely recognized by Να MTases. The N-terminal sequences and in vivo mutations used to probe functions of known Να methylated proteins are discussed below and summarized in Figure 3. The following subsections categorize substrates within overarching biological roles to aid in a system-level view of NTMT1 function. These categories will be expanded as additional roles for Να methylation are identified in substrate proteins.
Figure 3.
Known substrates and identified molecular functions of methylation.A, known substrates of N-terminal transferases (NTMTs) are clustered into different biological processes. The main biological themes for identified substrates are depicted, including DNA damage repair, myosin processes, protein synthesis, protein degradation, and cell division. B, molecular functions of methylation based on in vivo mutational analysis of substrates. Mutational analysis using in vitro methods are not listed. Most in vivo mutations are the K4Q substitution that eliminates methylation (ΔPK denotes deletion of the second and third amino acids [PK]; AGPK adds an alanine preceding the G1 residue). Identifying molecular functions of methylation is difficult, and this table lists the most known examples based on in vivo mutational analysis (h before a gene indicates human, sc indicates Saccharomyces cerevisiae).
Cell division
The link between cell division processes and Να methylation is supported by several Να-methylated proteins that function as chromatin regulators, histone proteins, or centromere components. However, there is considerable variation in the N-terminal sequence and methylation states of these proteins across different species, indicating a lack of consistency. NTMT1 methylates histone H2B, RCC1 (12), and CENP-A/B to maintain chromatin structure and function (Fig. 3). RCC1 is a Ran guanine nucleotide exchange factor (12) whose binding to chromatin is enhanced by nucleotide-free Ran and RCC1 methylation (63, 64). Methylation of RCC1 is important for its interaction with chromatin during mitosis (65). RCC1 methylation-defective mutant (66) leads to a multispindle phenotype and chromosome missegregation (63). Knockdown of NTMT causes a similar phenotype (12), indicating its role in cell cycle processes (Fig. 3B).
Centromere protein A (CENP-A) and CENP-B both have a canonical motif, and CENP-A marks the location of the centromere by assembling the constitutive centromere associated network. CENP-A is modified at its N-terminal tail by a combination of PTMs, including G1 phase trimethylation and two phosphorylation events (67). N-terminal methylation on CENP-A is associated with increased cell proliferation and tumorigenesis (39). The loss of trimethylation on G1 reduces the recruitment of CENP-T and CENP-I to centromeres. An increased trimethylation level of CENP-A is observed as the cell cycle progresses (39). Although CENP-A methylation appears to be involved in a fundamental biological process, the GPR is only conserved in mice and humans, but the rat GVR sequence is consistent with a less stringent XX[KR] motif (Fig. 2).
CENP-B, part of the constitutive centromere associated network complex, is also methylated by NTMT1. The GPK motif is conserved in humans, mice, and rats but not in other organisms. CENP-B is crucial for kinetochore assembly and connecting it to centromeric DNA. The N-terminal helix–loop–helix domain of CENP-B binds directly to the CENP-B motif, which is boosted by methylation. CENP-B methylation increases under cellular stress but is unaffected by colcemid treatment (68). Methylation-defective mutants (K4Q and AGPK) confirmed that methylation enhances CENP-B's DNA binding ability. H2B is methylated in various species, including Drosophila melanogaster, Tetrahymena, starfish, marine worms, and plants (7, 8, 69, 70, 71, 72, 73, 74). The N-terminal MTase, CG1675, methylates H2B in D. melanogaster. CG1675 physically interacts with dART8, an arginine methyltransferase, and modulation of dART8 protein levels is negatively correlated with Nα methylation levels (36). H2B methylation levels increase during fly development and can be induced by various stressors (36). Human isoforms, including the closest ortholog H2BC1, do not retain the PPK motif (Fig. 2), which is a striking example of the gain or loss of this modification across evolution.
Human Obg-like ATPase1 (OLA1) was identified as a substrate of NTMT1 and almost fully methylated in HEK293 cells, but the function of Nα methylation of OLA1 is unknown. OLA1 regulates protein–protein interactions and acts as a tumor suppressor in breast, colon, and ovarian cancers but an oncogene in lung cancer by regulating interactions with glycogen synthase kinase 3 and protein phosphatase 1 (75). OLA1 interacts with BRCA1/BARD1 (Breast Cancer gene 1/BRCA1-associated RING domain protein 1) and regulates centromere formation, but the effect of Nα methylation on this interaction is unclear (76). The defective methylation mutant was only used as a control substrate (Fig. 3B). Interestingly, the PPK motif is conserved across model organisms, including yeast Ola1 (Fig. 2).
Retinoblastoma protein 1, RB1, a tumor suppressor containing the PPK N-terminal motif, was one of the earliest substrates identified (17). RB is involved in neural stem cell development, and its defective methylation causes premature depletion of quiescent neural stem cells and expansion of progenitor cells (77). RB inhibits the cell cycle by repressing E2F-mediated gene transcription, and its hyperphosphorylation may promote cell cycle entry (77). Another substrate, SETα, is a multitasking protein implicated in nucleosome assembly in which Nα methylation may play a role in its stability (Fig. 3B). Knockout of NTMT1 leads to a reduced half-life of SETα (44).
DNA damage repair and transcription
NTMT1 loss reduces DNA repair and increases sensitivity to DNA damage. NTMT1 methylates substrates involved in DNA repair, including PARP3 (Poly(ADP-ribose) polymerase family member 3) and DDB2 (Damage-specific DNA-binding protein 2). DDB2 is part of the nucleotide excision repair pathway that repairs UV-induced DNA damage and bulky DNA adducts. DDB2 can be methylated by NTMT1 at its N-terminal APK motif, and loss of methylation compromises its nuclear localization and recruitment to DNA-damage foci, hindering ATM activation and CPD repair (Fig. 3B). NTMT1 knockdown or DDB2 N-terminal mutation leads to reduced ATM activation, impaired DNA repair, and increased UV light damage sensitivity (76).
PARP3 transfers ADP-ribose to itself and other proteins in the presence of DNA breaks, and it regulates the nonhomologous end joining pathway during DNA double-strand break repair (77). Overexpression of a methylation-defective PARP3 mutant in knockdown cells can still clear γH2AX foci, suggesting that methylation may not be involved in this PARP3-mediated DNA double-strand repair pathway (78).
Ntmt1−/− mice display premature liver degeneration, highlighting the potential significance of NTMT1 in maintaining liver tissue integrity. ZHX2 is a newly discovered substrate of NTMT1, with its ASK N terminus methylated in vitro and a methylation-deficient K4Q mutant showing impaired transcriptional activity (61). Notably, other members of the ZHX family (ZHX1 and ZHX3) have ASK/R N-terminal sequences. Other eukaryotes possess similar conserved N-terminal ASK sequences, whereas evolutionarily distant species lack them. Human Kelch-like family member 3 (KLHL31), a transcription factor that regulates transcriptional activity in muscle and heart, is also identified as methylated, but the impact of its Να methylation on transcriptional regulation remains unknown (12).
Protein synthesis, degradation, and folding
Protein synthesis and degradation are also regulated by Να methylation. Protein components of the ribosomal machinery are Να methylated across multiple species, including Rpl12ab and Rps25a/Rps25b in humans (11) and yeast (79, 80, 81), ribosomal protein L23a in humans (12), and ribosomal protein L2 in plants (Spinacia oleracea chloroplasts) (82). Rpl12ab and Rps25a/Rps25b have the XP[KR] motif conserved across eukaryotes (Fig. 2) and have been demonstrated to be methylated in Schizosaccharomyces pombe (81) and Saccharomyces cerevisiae (79, 80). Some evidence indicates that Να methylation of the ribosome is implicated in translation or ribosome assembly. In yeast, deletion of the NTM1 gene reduces translation efficiency and fidelity, leading to fewer polysomes, more 80S monosomes, and increased sensitivity to protein synthesis inhibitors (83). NTM1 deletion also causes resistance to translational inhibitors, suggesting a termination deficiency. However, these effects may not be solely due to loss of Rpl12ab, Rps25a/b, and Rpl23a methylation, as other ribosomal biogenesis factors, Lsg1 and Loc1, may also be involved (11).
eEF1α is the exclusive substrate of Efm7 (encoded by YLR285W) in yeast and METTL13 in humans. Both enzymes can methylate the Να amino group and an additional lysine side chain. The knockout of METTL13 results in altered translational dynamics and changes in the translation rate of specific codons. An increase in the levels of METTL13 and its dimethylation species was observed in Ras-driven cancer and is negatively associated with pancreatic and lung cancer survival (84). Further investigation showed that Ras-driven cancers depend on the METTL13-eEF1α methylation axis to meet increased protein synthesis requirements and promote neoplastic growth (84).
The 26S proteasome degrades proteins in cells and consists of a 20S proteolytic core and a 19S regulatory particle composed of ATPase (Rpt) and non-ATPase subunits (Rpn) (85, 86). Yeast Rpt1 contains a PPK N-terminal motif. Removal of potential methylation by deleting the PK sequence in Rpt1 caused delayed growth and stress sensitivity (Fig. 3B) (87). Another recently identified substrate is the yeast Hsp31 (89), a multifunctional chaperone and deglycase/methylglyoxalase enzyme in the DJ-1 superfamily (89). Hsp31 is involved in protein repair (88) and oxidative stress response (90). Hsp31 was identified through reanalysis of proteomic data and confirmed to be N-terminally methylated by MS and immunodetection, but the biological function of the PTM was not investigated (91). Hsp31 has an ASK motif, absent in other DJ-1 orthologs such as human PARK7, linked to Parkinson's disease (Fig. 2).
Cytoskeletal myosin protein functions
Myosin proteins of skeletal muscle fiber, MYL2, MYL3, and MYL9, are another set of conserved substrate proteins (Fig. 2). Human MYL2 and MYL3 can be fully methylated by NTMT1 (12), but the function of MYL3 and MYL2 methylation is unknown. MYL9, MYL12A, and MYL12B are atypical substrates that contain an SSK sequence derivative of the canonical motif that was demonstrated to be methylated (40). Methylation of MYL9 supports a more relaxed recognition than the XP[KR] motif, and this sequence is conserved in seven eukaryotic species but not in yeast or Arabidopsis thaliana (Fig. 2).
MYL9 is a regulatory subunit of nonmuscle myosin II (NMII) with roles in cell shape, polarity, adhesion, migration, and signaling (92). Phosphorylation of MYL9 at T18 and S19 increases its association with actin filaments and ATPase activity. Myosins, including MYL9, are translocated into the nucleus and act as transcriptional activators (93). MYL9 can be methylated by NTMT1 and acetylated by NatA (Να-terminal acetyltransferase A), and different modifications affect its localization and function. Να methylation promotes MYL9 nuclear function and facilitates the interaction with DNA, which was supported using an S3P mutant that is methylated but not acetylated. The MYL9 K4Q mutation prevents methylation but permits acetylation, thereby facilitating localization to the cytoplasm. This leads to heightened cytoskeletal NMII activity, ultimately enhancing cell migration. Neither modification affects MYL9's half-life, and only Cofilin-1 was confirmed to interact preferentially with the methylated peptide (41).
Emerging system-level view of Να methylation
In general, protein Να methylation and MTases are responsible for regulating a wide range of biological pathways. Although some specific examples have been studied in detail, most research suggests that the resulting phenotypic changes are relatively minor. Protein Να methylation may operate as a modulator, adjusting activity levels rather than acting as an on/off switch which has been suggested as role of protein methylation at a global level based on the yeast methylation network (94). At this stage, there is not enough to clearly establish a unifying theme, but common elements of the diverse pathways involved include stress response, development, and cellular and protein homeostasis. Together, studies show the varied substrate-specific effects that are context dependent. As discussed below, additional tools and studies are needed to gain further molecular details of Να methylation and a global view of the cellular roles of MTases.
The role of NTMTs under physiological and disease contexts
The physiological role of NTMTs across different tissues and cell types in mammalian systems
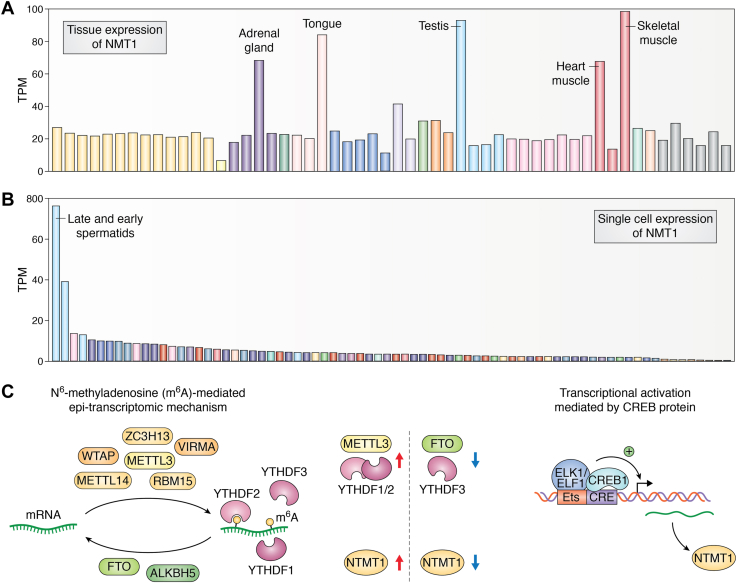
NTMT1 is an intracellular protein and exists in both the cytosol and nucleoplasm NTMT1 is expressed in various tissues and cells, as shown in the Human Protein Atlas (HPA) (95) (Fig. 4A). It is highly transcribed in the adrenal gland, tongue tissue, liver, testis, heart muscle, and skeletal muscle. Single-cell sequencing analysis shows increased transcription in early and late spermatids (Fig. 4B). NTMT2 RNA expression in the HPA suggests heart and skeletal muscle expression. In addition, NTMT2 was reported to be expressed in cardiomyocytes (22). Protein expression data for NTMT2 are not available in the HPA. NTMT1 levels increase during myogenic differentiation (96), while NTMT2 levels increase during osteogenic and myogenic differentiation (20).
Figure 4.
Tissue and single-cell expression of NTMT1 and regulation of NTMT1.A, tissue expression level of NTMT1 from a consensus dataset that includes RNA-seq data from two sources, the Human Protein Atlas dataset and the Genotype-Tissue Expression project. The normalized expression level (nTPM) of NTMT1 in 55 tissue types is plotted into a column graph. Tissue groups are coded by different colors. The top five tissues are labeled. B, single-cell sequencing of many cell types ordered by expression level reveals enrichment of NTMT1 in late spermatids followed by early spermatids. C, the two regulation mechanisms identified for NTMT1 are N6-methyladenosine (m6A)-mediated regulation and CREB1-mediated transcriptional activation. Tissue and single-cell expression images were adapted from the Human Protein Atlas, www.proteinatlas.org. Original images are available at the following URL: v21.1.proteinatlas.org/humancell. NTMT, N-terminal methyltransferase.
The association of NTMTs with disease development
NTMT1/2 genes are nonessential in many species, but their misregulation is linked to diseases. This section explores their roles in cancer and aging. A multiomics study suggests that NTMT1/2 play a context-dependent role in cancer, with NTMT2 and eEF1A-KNMTs being amplified, deleted, and mutated in several cancer types. NTMT1 is less altered but upregulated in lung adenocarcinoma, and its upregulation is associated with poor prognosis in multiple cancer types (22).
NTMT1 has context-dependent regulatory roles in cancer, seen in its contrasting effects on oncogenic or tumor suppressor pathways. Decreased NTMT1 expression is found in testicular seminomas, stroma cells surrounding breast tumors, glioblastoma, and leukemia, suggesting a tumor suppressor role (97, 98, 99, 100, 101). NTMT1 acts as a tumor suppressor in estrogen receptor–positive breast cell lines, inducing cell growth and increasing susceptibility to DNA damage reagents (21, 23). Poor prognosis was observed in patients with estrogen receptor–positive tumors if they carried an RB mutation (102). Inactivation of the Rb protein can activate E2F-dependent transcription and upregulate cell cycle–associated genes (103). A proposed mechanism is that the loss of NTMT1 in breast cancer mimics RB mutation, thus contributing to tumorigenesis (12, 23). Finally, Ntmt1−/− mice have decreased protein levels of RB (104) and overexpression of NTMT1 slows down cell proliferation (23). In contrast, NTMT1 can contribute to oncogenesis in several cancers, such as colorectal cancers and lymphomas (103, 104, 111). Overexpression of NTMT1 is most prevalent in colon cancer and is associated with a low survival rate (100). Recent studies also implied the oncogenic roles of NTMT1 in cervical cancer and retinoblastoma. NTMT1 appears to mediate resistance of retinoblastoma cells to cisplatin treatment, potentially by a CENPA-mediated process (105). NTMT1 was recently reported to be overexpressed in cervical cancer based on analysis of the TCGA database, and subsequent experiments demonstrated the ability to promote cervical cancer cell line proliferation and migration (106).
Να methylation is implicated in modulating the aging process by affecting genome maintenance and DNA damage repair pathway. The homozygous Ntmt1−/− mouse has developmental defects and premature aging phenotypes, such as early graying and hair loss, neurodegeneration, and transcripts levels in the liver similar to those of an aged mouse liver (21). The neurodegenerative phenotypes and cognitive defects are potentially mediated by the derepression of RB gene expression, suggesting NTMT1 has a regulatory role in RB-mediated transcription (104).
Regulation of NTMTs function
N6-methyladenosine-mediated RNA modification posttranscriptional regulation
Despite the progress in the function of protein Ναmethylation in pathophysiology, mechanisms for the regulation of NTMT1 protein expression have only recently emerged. Interestingly, m6A-mediated RNA modification regulates the expression of NTMT1 protein level upon genetic depletion of reader, writer, or eraser proteins of m6A methylation in HEK293T cells. The N6 position on adenosine in RNA can be dynamically methylated and is crucial for normal development. The proper functioning of m6A-mediated regulation, which governs various processes in mRNA metabolism, necessitates a group of reader, writer, and eraser proteins. The m6A modification has been implicated in various biological processes and disease states (107). The major MTase complex of m6A comprises a catalytic subunit, METTL3, along with other regulatory subunits (108). Several reader proteins, including the YTH domain–containing family proteins 1/2/3 (YTHDF 1/2/3), can recognize the m6A methylation of RNA (109). FTO (Fat mass and obesity-associated protein) and ALKBH5 (alkB homolog 5 RNA demethylase) are known to perform demethylation of m6A (108). An elevated level of NTMT1 is associated with an increased level of METTL3 writer or YTHDF1/2 readers, while genetic depletion of FTO eraser or YTHDF3 reader results in diminished expression (19). These m6A modulators' regulatory impact results in a concomitant change in the N-terminal methylation level of MRG15, a known substrate protein of NTMT1 (19). The close link between the m6A modification and concomitant alteration of N-terminal methylation is intriguing and suggests that these pathways are involved in similar biological processes. It is worth noting that METTL3 is essential for mouse gametogenesis (110) and NTMT1 is also expressed at a high level in the testis (Fig. 4A), indicating a possible shared role in development, which needs to be investigated further.
Transcriptional activation mediated by CREB1 protein
CREB1 was recently identified as the first known transcriptional-level regulator of α-protein MTases (20). The CREB1 transcription factor is activated by the phosphorylation of S133 through multiple cellular signaling cascades (111). Phosphorylated CREB1 allows binding to cAMP response elements (CRE) in the promoter region of the target genes and the subsequent recruitment of CREB binding protein to initiate transcription (111). CREB1-mediated transcription is activated under oxidative stress and ionizing radiation, which is important in the differentiation of many stem cell populations. CREB1 was shown to regulate myoblast differentiation through its downstream target, NTMT1. The main regulatory unit promoting NTMT1 transcription appears to be an Ets motif and a CRE/ATF motif (20). The CRE/ATF motif in the promoter region binds to CREB1, and the Ets motif selectively binds to the ETS transcription factors, ELK1 (ETS domain-containing protein Elk-1) or ELF1 (E74-like ETS transcription factor 1). CREB1 was predicted as the main transcriptional driver of the NTMT1 gene with synergistic effects from ELK1/ELF1 (20). It was later determined that the cell cycle entry and exit processes affected NTMT1 expression through a CREB1-dependent mechanism. However, the upregulation of NTMT1 transcription requires sustained signaling by CREB1 and is not induced by serum stimulation associated with CREB1-driven early transcription of myogenesis (Fig. 4C) (20). NTMT1 could modulate downstream signaling and transcription of myogenic factors, possibly by Να methylation of transcription factor targets, but further investigation is required to validate this model (20). CREB1 is associated with inducing gene expression in response to oxidative or DNA damage but did not induce transcription of NTMT1 under these stresses (20). However, NTMT1 activity was increased in HEK293T cells under stress resulting in elevated CENP-B methylation (68), suggesting that expression or NTMT1 activity may vary by cell type and conditions.
Macromolecular interactions regulate NTMT1/2 and METTL13 activities
Another mode of regulation is through complex formation with close MTase family members. NTMT1 is activated through the binding of its close homolog NTMT2, while METTL13 inhibits NTMT2 activity (112). This is an interesting example of MTases being regulated oppositely by different family members. Moreover, catalytic activity is not a requirement for these regulatory effects, demonstrating new, noncatalytic functions for NTMT1 and METTL13 (112). When all three are present, the regulatory effects of METTL13 override those of NTMT2. These findings result in a model where these three MTases use the formation of a macromolecular complex to regulate translation by methylating eEF1α, with separate roles for each enzyme (113). eEF1α is the only substrate for METTL13, but macromolecular complex regulation involving these enzymes may extend to additional substrates.
Tools for studying protein Να MTases
Protein methylation is a widespread PTM catalyzed by a cohort of MTases and is involved in various cellular processes, including transcriptional regulation, DNA replication, damage repair, and ribosomal protein synthesis. The role of dysregulated MTases in disease varies from cancer to neurodegenerative disease. Hence, precision medicine selectively targeting individual disease-related MTases would be beneficial and reduce potential side effects due to off-target inhibition. Moreover, a toolbox of potent, selective, and cell-permeable inhibitors of individual MTases would facilitate a better understanding of their biological functions.
Selective bisubstrate inhibitors
Kinetics studies on NTMT1 show that the catalysis proceeds in a random sequential Bi-Bi mechanism in a distributive manner (114). This Bi-Bi mechanism involves SAM and substrate N-terminal binding to the enzyme, followed by the binding of the other to form a transient ternary structure. Based on the Bi-Bi mechanism, a novel class of bisubstrate inhibitors of NTMT1 was developed to mimic the ternary complex formed during the transition state and designed to occupy the two adjacent binding pockets in NTMT1. The first two NTMT1 bisubstrate inhibitors, 1 and 2, are covalently linked to a stable SAM analog (N-adenosyl-L-homocysteine [NAH]) via a linker to SPKRIA or GPRRRS peptide. The IC50 for both inhibitors is in the sub-micromolar range, with a 30- to 60-fold selectivity for NTMT1 over other protein lysine MTases (G9a) and the arginine MTase 1 (PRMT1) (115). In the light of the cocrystal structure of NTMT1 in complex with both substrates and in vitro binding affinity data suggesting PPKRIA peptide has the tightest binding affinity, the second generation of bisubstrate inhibitors were synthesized to optimize the potency and selectivity of the early generation by conjugating Pro-containing peptide of varied length with a SAM analog. Among the new series of inhibitors, NAH-C3-PPKRIA showed the most improved IC50 and Ki by SAH hydrolase-coupled fluorescence assay with both values in the nanomolar range (27). It also showed about 100- to 600-fold selectivity over other SAM-dependent enzymes, such as PKMT (G9a and SETD7), PRMT (PRMT1, TbPRMT7), and NNMT. The cocrystal structure of NTMT1 and NAH-C3-PPKRIA (PDB: 6DTN) superimposed well with the substrate-bound crystal structure (PDB: 5E1M), displaying similar orientations at the binding pockets and retaining the same interactions with NTMT1 as the corresponding substrate and cofactor in 5E1M (27).
Later, a series of NTMT1 inhibitors with a GPKRIA or GPKR peptide segment and a linker of varying lengths were synthesized to explore the plasticity in the active site of NTMT1. Compound NAH-C4-GPKRIA with a four-carbon linker demonstrated the most potent inhibition (IC50 = 82 ± 17 nM and Ki,app = 130 pM) and more than 100,000-fold selectivity over other MTases (116). However, on substituting the four-carbon linker with a slightly longer five-carbon linker, it behaves as a single substrate without an inhibitory effect, as reflected by the cocrystal structure (116). A chemoproteomic profiling approach was applied to understand the selectivity and off-target engagement within the cellular context with an immobilized probe (NAH-C3-GPKK-biotin) (117). This probe was generated as a biotinylated derivative of NTMT1 bisubstrate inhibitor 1 (NAH-C3-GPKK) and subsequently immobilized to streptavidin beads (Fig. 5). While NTMT1 was efficiently enriched from HeLa cell lysate, another protein MTase complex, KMT9-Trm112, was pulled down and confirmed as a new target for NAH-C3-GPKK (117).
Figure 5.
Inhibitors targeting N-terminal methyltransferases. Two types of selective inhibitors targeting N-terminal methyltransferases are bisubstrate inhibitors and peptidomimetic inhibitors. The bisubstrate inhibitors (left panel) target both the substrate-binding pocket and the S-adenosyl-L-methionine (SAM)-binding pocket by linking a SAM analog, N-adenosyl-L-homocysteine (NAH) (orange), with a peptide moiety (blue). Linker is colored purple. Peptidomimetics inhibitors and small molecule inhibitors are colored green (right side) and only target the substrate-binding pocket.
Selective and cell-permeable peptidomimetic inhibitors
Although the bisubstrate inhibitors (NAH-C4-GPKRIA and its derivatives) display high potency, the poor cell permeability and protease stability limit their application in cell-based studies and in vivo applications. Enlighted by the crystal structures of NTMT1 with its peptide substrates, a new series of selective tetrapeptidomimetic inhibitors bearing a general R1-PKR structure were designed to target the unique peptide-binding pocket of NTMT1/2. The lead compound, BM30, has a 4-hydroxyphenyl group at the R1 position to mimic the tyrosine in YPKRIA peptide binding (25). BM30 shows the highest selectivity for NTMT1/2 against a panel of 41 MTases and modest inhibitory activity at the submicromolar range in vitro, yet low in vivo inhibitory activity due to minimal cell permeability. Modifications at the BM30 N and C termini increased the inhibitor potency while maintaining cell permeability. DC431 and DC432 were then generated to increase cell permeability and improve the inhibitory effect in vitro by conjugating a cell-permeable peptide TAT and five arginines to the C terminus of BM30, respectively (25). Another improved inhibitor, DC113, was generated by modifying the N terminus of BM30 to a more hydrophobic naphthyl group (26). This compound binds to NTMT1 in a similar posture as BM30 as indicated in the X-ray cocrystal structure (PDB ID: 7K3D) and shows 5-fold increased inhibitory activity in vitro, more than 1000-fold selectivity for NTMT1 over other tested MTs, modest cell penetration, and increased cellular inhibition activity. DC541 with a relatively hydrophobic naphthyl group substitution and meta-aminobenzoic amide group addition on the N and C terminus of BM30, respectively, shows the best cellular methylation inhibition with no apparent cytotoxicity in two colorectal cancer cell lines (26). High selectivity for NTMT1 is maintained in DC541 (Fig. 5). A recent study reported the development of a novel inhibitor in this class, GD562, which improves the inhibition of cellular N-terminal methylation by 6-fold compared with DC541 (118). Overall, NTMT1 inhibitors have steadily improved in cell potency and are staged to be further employed for mechanistic and cellular studies.
Small molecule inhibitors
In recent studies, cell-potent and selective inhibitors for NTMT1/2 were identified. Through high-throughput screening, venglustat was identified and validated as a substrate-competitive inhibitor of NTMT1/2 (119). Notably, venglustat is in clinical trials for multiple diseases through its allosteric inhibition of glucosylceramide synthase. Through structural optimization, GD433 was developed as an enhanced cell-potent and selective small molecule inhibitor for NTMT1/2 (120). Biochemical and cocrystallization studies demonstrate that GD433 is competitive to peptide substrate and uncompetitive to cofactor SAM. Furthermore, GD433 inhibits the cellular methylation level of both trimethylated RCC1 and trimethylated SETα, with a cellular IC50 of ∼30 nM in HEK293 cells. GD433 also displays a maximum tolerated dose of 350 mg/kg with oral bioavailability. In addition, a close analog (YD2160) of GD433 was inactive against NTMT1. The active inhibitor and negative control will serve as valuable tools to examine the physiological and pharmacological functions of NTMT1 catalytic activity in cellular and animal studies.
An additional approach to modulating cellular levels of NTMT1 includes the proteolysis-targeting chimera (PROTAC) strategy. A recent degradation-inducing small molecule was developed that used the NTMT1 peptidomimetic inhibitor, DC541, to direct the degradation of the enzyme (121). This was the first example of using a PROTAC strategy for an NTMT1 cellular probe, and further improvements could result in even greater potencies.
Proteomics approaches and antibodies
MS has been frequently applied to assess the presence and stoichiometry of protein Να methylation on almost all the known substrate proteins. Proteomics also allows protein Να methylation substrate profiling by using artificial cofactor in combination with click chemistry (62). Although proteomics approaches fully optimized for global Να methylation have not been reported, reanalysis of public MS datasets suggests about 6 to 7% of the total proteome in humans and yeast is subjected to Να methylation (91). The presence of Να methylation on noncanonical motif sequences was prevalent, and an [S][S/A/Q] or [A/N/G][A/S/V][A/G] pattern was observed in yeast and humans, respectively. The process or enzymes responsible for these noncanonical methylations are unclear, but NTMT1 has the potential to methylate noncanonical substrates with an XX[KR] sequence (61). Many of these methylation events were identified in low abundance, possibly due to incomplete proteome coverage or an indication of spurious or chemically induced methylation previously reported for other PTMs (55, 122). Methylated peptides are difficult to confirm, and the site of methylation is difficult to locate because of peptide misassignment and limited fragmentation detection (91). For example, formylation and acetylation have similar mass shifts to dimethylation and trimethylation, respectively. The presence of numerous noncanonical sequences could suggest that methyltransferases have a more lenient specificity. However, it is also plausible that the detection of these methylated sequences is an artifact. The definition of the Nα motif has primarily relied on peptide arrays, resulting in a useful and mostly precise motif. However, there is potential for further refinement of the complete range of leniency and specificity of the Nα motif. Additional in vitro quantitative proteomic approaches, such as MT-MAMS (methyltransferase motif analysis by MS), may provide a further definition (123). The MT-MAMS method was used to confirm an expanded and more lenient motif-recognition mode for several lysine and human arginine methyltransferases, which may also be the case with Να MTases. The combination of such quantitative proteomics with other in vivo approaches that confirm methylation would greatly enhance the understanding of specificity.
Another convenient tool for analyzing the level of Να methylation on purified substrate proteins is specific antibodies for distinct methylation species (mono-, di-, and trimethylation species). The anti-me2-SPK, anti-me3-SPK, and anti-me2-PPK antibodies were purified from the serum of rabbits immunized with corresponding methylated synthetic peptides and pan-selected against unmethylated peptides (12). However, cross-reactivity of antibodies to other methylated synthetic peptide sequences and different methylation species has been observed. Cross-reactivity was evident in that immunoprecipitation with an anti-me3-SPK antibody immunoprecipitated proteins with PPK and APK motifs (12). An additional antibody was raised using an in vitro methylated GPR-containing peptide antigen and was used effectively in immunofluorescence and immunoblots to investigate the role of CENP-A methylation (39). Further development of Να methylation-specific antibodies or antibody variants is clearly warranted.
Future directions
The function and physiological and pathological roles of the Nα MTases remain largely underexplored, especially NTMTs. NTMTs appear dispensable, and their deletion in human cell lines or yeast does not result in significant growth defects under standard culture conditions. However, evidence suggests that NTMTs are involved in the development process, as in mice and fruit flies (23, 36). Additional studies from the knockout mouse suggest an early role for NTMT1 in various aspects of mammalian development (21, 61, 104). NTMTs appear to have increased activity when HEK293 cells (68) or Drosophila (36) are exposed to various stress conditions suggesting that Να methylation is an integral part of the cellular stress response. NTMTs are clearly involved in developmental phenotypes and stress responses (heat stress, arsenite, dense cell culture), but exploring their detailed mechanism remains challenging.
One obstacle is the large cohort of potential methylation substrates. About two dozen are confirmed as substrates of NTMTs, and few have been described with detailed and precise regulatory or molecular mechanisms. The most characterized NTMTs in humans and yeast are known to selectively target a canonical recognition motif, XP[K/R]. Recent evidence has emerged indicating an expanded substrate pool because ZHX2, MYL9, MYL12A, and MYL12B, which lack the proline in the canonical motif, were identified as Nα methylated. Identifying substrates for NTMTs relies on developing MS techniques and selective antibodies recognizing the Να methylation species on canonical motif sequences. The lack of methodology to systematically determine substrates of N-terminal methylation impedes progress in this field. Searching for N-terminal methylation events with traditional proteomic methods or repurposing proteomic datasets has been successful and could yield methylated hit proteins (91).
A challenge for confirmation of substrate methylation is that the modification may have low stoichiometry or protein abundance. Employing sensitive and targeted purification strategies could improve identification, including affinity purification, cross-linking, BioID, or optimized chromatography methods followed by MS (124, 125). Recently, activity-based substrate profiling with clickable SAM analog successfully identified 72 potential substrate hits and confirmed OLA1 as a substrate of human NTMTs (62). Other possible methods might be N-terminal enrichment MS analysis such as iNrich (126), which should increase the capability to detect low-abundance N-terminal peptides and the presence of Να methylation in proteome samples. Most efforts are characterizing N-terminal modifications that traditionally do not include Να methylation. Thus, developing a workflow to confidently monitor the methylation of purified proteins or on a proteome scale would delineate the levels of Να methylation under various conditions and could reveal the regulatory roles of methylation. Global profiling and identifying Να methylation under multiple conditions and associating it with phenotypes will provide insight into the role of Να MTases in normal and disease physiology. Advances in tracking Να methylation levels across conditions may reveal if the modification is truly irreversible. A question remains, namely, what is the fate of methylated substrates? Is the methylation reversible? Although Να methylation is currently viewed as irreversible, possible mechanisms for methylation removal include demethylation by an unknown demethylase or clipping of the N terminus by aminopeptidases.
Beyond substrate identification, the investigation of the mechanism is more challenging and intriguing. On a molecular interaction level, Να methylation has been demonstrated to affect protein–DNA interactions in a few substrates, such as CENP-B and DDB2, and it may regulate interactions in large protein complexes or at the centromere. Evidence for modulation of molecular interactions involving other Να methylated substrates, such as ribosomal proteins, has not emerged. We note that acetylation and methylation of the N terminus reduced MYL9 interactions with many cytoskeletal proteins and might promote interaction with Cofilin-1 (41). The methylation site at the N termini of the known substrates is usually at a flexible or an unstructured region (around 80% of yeast proteins were predicted to have disordered N-terminal regions (127)). Thereby, it has been perplexing how the addition of small methyl groups affects the interaction between macromolecules in currently identified substrates with published structures (128, 129, 130, 131, 132, 133, 134, 135). Structural characterization of substrates in the fully methylated state would reveal critical molecular details of how the modification could mediate protein–protein or protein–nucleic acid interactions. The role of Να methylation has been confirmed in four significant processes: cell mitotic process, DNA damage repair, protein degradation and synthesis, and proper myosin functions.
Although it has been proposed that Να methylation might regulate protein stability through the N-end rule, this mechanism has not been confirmed in most substrates but may play a role in a subset of substrates such as SETα (44). The N-end rule involves the recognition of Να-terminally acetylated proteins that are targeted for degradation by the proteasome (136). The proline at the second position of the canonical motif naturally excludes Να acetylation, so the competition between Να methylation and Να acetylation rarely exists on substrates with this subset of the canonical motif. Interestingly, Να methylation might coordinate with Να acetylation to regulate the translocation of differentially localized proteins, as shown with MYL9. Although proteins with proline at position 2 exclude Να acetylation, such proteins may be subject to the recently discovered N-terminal proline degron involving the GID4 (GID Complex Subunit 4 Homolog) ubiquitin ligase complex targeting of gluconeogenic enzymes (137). The substrate specificity of the GID ubiquitin ligase recognition subunit, GID4, was recently expanded to include hydrophobic nonproline sequences (138), and X-P proteins (X is any amino acid) are also subject to degradation (139). The X-P proteins are trimmed by aminopeptidases that remove N-terminal serine or alanine, which exposes the proline to GID4-GID ubiquitin ligase degradation (139). Potential MTase substrates with the XP[KR] motif have similarities to these GID4-GID substrates, but the impact of Να methylation is unknown and needs clarification.
Cellular studies usually depend on the limited use of exogenous methylation mutants. The most popular loss-of-methylation mutant used is K4Q, and only one case of a gain-of-methylation mutant has been employed where S3P increases methylation and prevents acetylation of MYL9 (Fig. 3). The use of methylation-deficient mutants has successfully demonstrated the role of methylation in regulating protein–DNA or protein–chromatin interactions. The reliance on the K4Q mutation may have a caveat because lysine to glutamic acid mutation is a commonly used acetylated lysine mimic (140). Hence, the K4Q substitution introduces an acetylation mimic with unknown effects in proximity to the methylated N terminus. Expanding the range of mutants could mitigate the issue of introducing proximal PTM mimics and aid in further mechanistic studies. Further proteomic analysis of N-terminal mutants is warranted to assess if there are alterations in PTMs.
Accumulating evidence suggests a proper control of Να methylation levels in different contexts might be essential. Knockout of a potentially responsible enzyme is a typical way to confirm linkage to a modification, but other approaches including overexpression or a tunable expression system could be useful. CRISPR-based genome editing that introduces seamless N-terminal mutations closely matching native expression levels of the substrate would be an important tool. We also note that investigation of Να methylation in model organisms has been mostly restricted to mice, with a few studies in yeast or Drosophila. Thus, further studies in several species could reveal important insights into the role of this modification in conserved cellular pathways and potentially point to orthologous proteins that have retained the less stringent XX[KR] motif. The recent development of bisubstrate inhibitors and peptidomimetic inhibitors targeting NTMTs is promising and will help probe NTMT functions. However, reports on using these new tools in examining Να methylation on specific substrates are rare. The path toward potential therapeutic applications would involve additional validation of the target in disease contexts and improvements in cellular inhibition of inhibitors.
The information on the tissue-specific role of NTMTs in humans and their relevance to disease development is still missing. The high enrichment level in spermatids compared with the general low tissue specificity of NTMTs is notable (Fig. 3). However, the role of NTMTs in the development of sperm and meiosis has not been studied. NTMTs are associated with cancer development, but their role as oncogenes or suppressor genes appears to be context dependent. Therefore, the fundamental studies on identifying N-terminal methylation substrates and exploring the regulation mechanism coupled with a system-level view would be the key to unraveling the various roles of NTMTs in cellular physiology, development, and disease.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful for the constructive critiques from the reviewers. BioRender was used to conceptualize the figures, and we thank Luciana Giono for the artistic rendering of figures. We apologize to researchers who we could not cite due to space restrictions.
Author contributions
T. R. H. conceptualization; P. C. writing – original draft; T. R. H. and R. H. writing – review & editing; P. C. and T. R. H visualization; R. H. and T. R. H. funding acquisition.
Funding and additional information
Research in our laboratories (R. H. and T. R. H) was supported by the Purdue University Center for Cancer Research, NIH grant P30 CA023168, and the Richard and Anne Borch MCMP Research Award. R.H. acknowledges support from NIH grant R01GM117275. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Reviewed by members of the JBC Editorial Board. Edited by Brian Strahl
References
- 1.Hershko A., Heller H., Eytan E., Kaklut G., Roset I.A. Role of the a-amino group of protein in ubiquitin-mediated protein breakdown. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7021–7025. doi: 10.1073/pnas.81.22.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang C.-S., Shemorry A., Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 2010;327:973–977. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varshavsky A. Discovery of cellular regulation by protein degradation. J. Biol. Chem. 2008;283:34469–34489. doi: 10.1074/jbc.X800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosius J., Chen R. The primary structure of protein L16 located at the peptidyltransferase center of Escherichia coli ribosomes. FEBS Lett. 1976;68:105–109. doi: 10.1016/0014-5793(76)80415-2. [DOI] [PubMed] [Google Scholar]
- 5.Wittmann-Liebold B., Pannenbecker R. Primary structure of protein L33 from the large subunit of the Escherichia coli ribosome. FEBS Lett. 1976;68:115–118. doi: 10.1016/0014-5793(76)80417-6. [DOI] [PubMed] [Google Scholar]
- 6.Chen R., Brosius J., Wittmann-Liebold B. Occurrence of methylated amino acids as N-Termini of proteins from Escherichia coil ribosomes. J. Mol. Biol. 1977;111:173–181. doi: 10.1016/s0022-2836(77)80121-6. [DOI] [PubMed] [Google Scholar]
- 7.Martinage A., Briand G., Dorsselaer A., Turner C.H., Sautiere P. Primary structure of histone H2B from gonads of the starfish Asterias rubens. Identification of an N-dimethylproline residue at the amino-terminal. Eur. J. Biochem. 1985;147:351–359. doi: 10.1111/j.1432-1033.1985.tb08757.x. [DOI] [PubMed] [Google Scholar]
- 8.Nomoto M., Kyogoku Y., Iwai K. N-trimethylalanine, a novel blocked N-terminal residue of Tetrahymena histone H2B. J. Biol. Chem. 1982;92:1675–1678. doi: 10.1093/oxfordjournals.jbchem.a134096. [DOI] [PubMed] [Google Scholar]
- 9.Henry G.D., Trayer I.P., Brewer S., Levine B.A. The widespread distribution of alpha-N-trimethylalanine as the N-terminal amino acid of light chains from vertebrate striated muscle myosins. Eur. J. Biochem. 1985;148:75–82. doi: 10.1111/j.1432-1033.1985.tb08809.x. [DOI] [PubMed] [Google Scholar]
- 10.Trayer I.P., Trayer H.R., Levine B.A. Evidence that the N-terminal region of A1-light chain of myosin interacts directly with the C-terminal region of actin. A proton magnetic resonance study. Eur. J. Biochem. 1987;164:259–266. doi: 10.1111/j.1432-1033.1987.tb11019.x. [DOI] [PubMed] [Google Scholar]
- 11.Webb K.J., Lipson R.S., Al-Hadid Q., Whitelegge J.P., Clarke S.G. Identification of protein N-terminal methyltransferases in yeast and humans. Biochemistry. 2010;49:5225–5235. doi: 10.1021/bi100428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tooley C.E.S., Petkowski J.J., Muratore-Schroeder T.L., Balsbaugh J.L., Shabanowitz J., Sabat M., et al. NRMT is an α-N-methyltransferase that methylates RCC1 and retinoblastoma protein. Nature. 2010;466:1125–1128. doi: 10.1038/nature09343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnesen T. Towards a functional understanding of protein N-terminal acetylation. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhat K.P., Ümit Kaniskan H., Jin J., Gozani O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat. Rev. Drug Discov. 2021;20:265–286. doi: 10.1038/s41573-020-00108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q., Schapira M., Arrowsmith C.H., Barsyte-Lovejoy D. Protein arginine methylation: from enigmatic functions to therapeutic targeting. Nat. Rev. Drug Discov. 2021;20:509–530. doi: 10.1038/s41573-021-00159-8. [DOI] [PubMed] [Google Scholar]
- 16.Huang R. Chemical biology of protein N-terminal methyltransferases. ChemBioChem. 2019;20:976–984. doi: 10.1002/cbic.201800615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stock A., Clarke S., Clarke C., Stock J. N-Terminal methylation of proteins: structure, function and specificity. FEBS Lett. 1987;220:8–14. doi: 10.1016/0014-5793(87)80866-9. [DOI] [PubMed] [Google Scholar]
- 18.Diaz K., Meng Y., Huang R. Past, present, and perspectives of protein N-terminal methylation. Curr. Opin. Chem. Biol. 2021;63:115–122. doi: 10.1016/j.cbpa.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bade D., Cai Q., Li L., Yu K., Dai X., Miao W., et al. Modulation of N-terminal methyltransferase 1 by an N6-methyladenosine-based epitranscriptomic mechanism. Biochem. Biophysical Res. Commun. 2021;546:54–58. doi: 10.1016/j.bbrc.2021.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tooley J.G., Catlin J.P., Tooley C.E.S. CREB-mediated transcriptional activation of NRMT1 drives muscle differentiation. Transcription. 2021;12:72–88. doi: 10.1080/21541264.2021.1963627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonsignore L.A., Tooley J.G., Van Hoose P.M., Wang E., Cheng A., Cole M.P., et al. NRMT1 knockout mice exhibit phenotypes associated with impaired DNA repair and premature aging. Mech. Ageing Dev. 2015;146–148:42–52. doi: 10.1016/j.mad.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campeanu I.J., Jiang Y., Liu L., Pilecki M., Najor A., Cobani E., et al. Multi-omics integration of methyltransferase-like protein family reveals clinical outcomes and functional signatures in human cancer. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-94019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonsignore L.A., Butler J.S., Klinge C.M., Tooley C.E.S. Loss of the N-terminal methyltransferase NRMT1 increases sensitivity to DNA damage and promotes mammary oncogenesis. Oncotarget. 2015;6:12248–12263. doi: 10.18632/oncotarget.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shields K.M., Tooley J.G., Petkowski J.J., Wilkey D.W., Garbett N.C., Merchant M.L., et al. Select human cancer mutants of NRMT1 alter its catalytic activity and decrease N-terminal trimethylation. Protein Sci. 2017;26:1639–1652. doi: 10.1002/pro.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackie B.D., Chen D., Dong G., Dong C., Parker H., Schaner Tooley C.E., et al. Selective peptidomimetic inhibitors of NTMT1/2: rational design, synthesis, characterization, and crystallographic studies. J. Med. Chem. 2020;63:9512–9522. doi: 10.1021/acs.jmedchem.0c00689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen D., Dong G., Deng Y., Noinaj N., Huang R. Structure-based discovery of cell-potent peptidomimetic inhibitors for protein N-terminal methyltransferase 1. ACS Med. Chem. Lett. 2021;12:485–493. doi: 10.1021/acsmedchemlett.1c00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D., Dong G., Noinaj N., Huang R. Discovery of bisubstrate inhibitors for protein N-terminal methyltransferase 1. J. Med. Chem. 2019;62:3773–3779. doi: 10.1021/acs.jmedchem.9b00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tooley J.G., Schaner Tooley C.E. New roles for old modifications: emerging roles of N-terminal post-translational modifications in development and disease. Protein Sci. 2014;23:1641–1649. doi: 10.1002/pro.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrossian T.C., Clarke S.G. Uncovering the human methyltransferasome. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wlodarski T., Kutner J., Towpik J., Knizewski L., Rychlewski L., Kudlicki A., et al. Comprehensive structural and substrate specificity classification of the Saccharomyces cerevisiae methyltransferome. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrossian T., Clarke S. Bioinformatic identification of novel methyltransferases. Epigenomics. 2009;1:163–175. doi: 10.2217/epi.09.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz J.E., Dlakić M., Clarke S. Automated identification of putative methyltransferases from genomic open reading frames. Mol. Cell. Proteomics. 2003;2:525–540. doi: 10.1074/mcp.M300037-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Clarke S.G. Protein methylation at the surface and buried deep: thinking outside the histone box. Trends Biochem. Sci. 2013;38:243–252. doi: 10.1016/j.tibs.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jakobsson M.E., Małecki J.M., Halabelian L., Nilges B.S., Pinto R., Kudithipudi S., et al. The dual methyltransferase METTL13 targets N terminus and Lys55 of eEF1A and modulates codon-specific translation rates. Nat. Commun. 2018;9:3411. doi: 10.1038/s41467-018-05646-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamey J.J., Winter D.L., Yagoub D., Overall C.M., Hart-Smith G., Wilkins M.R. Novel N-terminal and lysine methyltransferases that target translation elongation factor 1A in yeast and human. Mol. Cell. Proteomics. 2016;15:164–176. doi: 10.1074/mcp.M115.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar-Garea A., Forne I., Vetter I., Kremmer E., Thomae A., Imhof A. Developmental regulation of N-terminal H2B methylation in Drosophila melanogaster. Nucleic Acids Res. 2012;40:1536–1549. doi: 10.1093/nar/gkr935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong C., Mao Y., Tempel W., Qin S., Li L., Loppnau P., et al. Structural basis for substrate recognition by the human N-terminal methyltransferase 1. Genes Dev. 2015;29:2343–2348. doi: 10.1101/gad.270611.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engel S.R., Wong E.D., Nash R.S., Aleksander S., Alexander M., Douglass E., et al. New data and collaborations at the Saccharomyces genome database: updated reference genome, alleles, and the alliance of genome resources. Genetics. 2022;220:iyab224. doi: 10.1093/genetics/iyab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sathyan K.M., Fachinetti D., Foltz D.R. α-amino trimethylation of CENP-A by NRMT is required for full recruitment of the centromere. Nat. Commun. 2017;8 doi: 10.1038/ncomms14678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petkowski J.J., Schaner Tooley C.E., Anderson L.C., Shumilin I.A., Balsbaugh J.L., Shabanowitz J., et al. Substrate specificity of mammalian N-terminal α-amino methyltransferase. Biochemistry. 2012;51:5942–5950. doi: 10.1021/bi300278f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nevitt C., Tooley J.G., Schaner Tooley C.E. N-terminal acetylation and methylation differentially affect the function of MYL9. Biochem. J. 2018;475:3201–3219. doi: 10.1042/BCJ20180638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petkowski J.J., Bonsignore L.A., Tooley J.G., Wilkey D.W., Merchant M.L., Macara I.G., et al. NRMT2 is an N-terminal monomethylase that primes for its homologue NRMT1. Biochem. J. 2013;456:453–462. doi: 10.1042/BJ20131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong C., Dong G., Li L., Zhu L., Tempel W., Liu Y., et al. An asparagine/glycine switch governs product specificity of human N-terminal methyltransferase NTMT2. Commun. Biol. 2018;1:1–9. doi: 10.1038/s42003-018-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faughn J.D., Dean W.L., Tooley C.E.S. The N-terminal methyltransferase homologs NRMT1 and NRMT2 exhibit novel regulation of activity through heterotrimer formation: interactions between NRMT1 and NRMT2. Protein Sci. 2018;27:1585–1599. doi: 10.1002/pro.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., Kuang Z., Xue L., Yue J., Khan M.H., Zhu Z., et al. Structural basis for peptide binding of α-N terminal methyltransferase from Saccharomyces cerevisiae. Crystallogr. Rep. 2021;66:1316–1321. [Google Scholar]
- 46.Jakobsson M.E. Structure, activity and function of the dual protein lysine and protein N-terminal methyltransferase METTL13. Life (Basel) 2021;11:1121. doi: 10.3390/life11111121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demirci H., Gregory S.T., Dahlberg A.E., Jogl G. Multiple-site trimethylation of ribosomal protein L11 by the PrmA methyltransferase. Structure. 2008;16:1059–1066. doi: 10.1016/j.str.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cameron D.M., Gregory S.T., Thompson J., Suh M.-J., Limbach P.A., Dahlberg A.E. Thermus thermophilus L11 methyltransferase, PrmA, is dispensable for growth and preferentially modifies free ribosomal protein L11 prior to ribosome assembly. J. Bacteriol. 2004;186:5819–5825. doi: 10.1128/JB.186.17.5819-5825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demirci H., Gregory S.T., Dahlberg A.E., Jogl G. Recognition of ribosomal protein L11 by the protein trimethyltransferase PrmA. EMBO J. 2007;26:567–577. doi: 10.1038/sj.emboj.7601508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mazzoleni M., Figuet S., Martin-Laffon J., Mininno M., Gilgen A., Leroux M., et al. Dual targeting of the protein methyltransferase PrmA contributes to both chloroplastic and mitochondrial ribosomal protein L11 methylation in arabidopsis. Plant Cell Physiol. 2015;56:1697–1710. doi: 10.1093/pcp/pcv098. [DOI] [PubMed] [Google Scholar]
- 51.Vanet A., Plumbridge J.A., Guérin M.-F., Alix J.-H. Ribosomal protein methylation in Escherichia coli: the gene prmA, encoding the ribosomal protein L11 methyltransferase, is dispensable. Mol. Microbiol. 1994;14:947–958. doi: 10.1111/j.1365-2958.1994.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 52.Hu Y., Comjean A., Rodiger J., Liu Y., Gao Y., Chung V., et al. FlyRNAi.org—the database of the Drosophila RNAi screening center and transgenic RNAi project: 2021 update. Nucleic Acids Res. 2021;49:D908–D915. doi: 10.1093/nar/gkaa936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang J., Al-Ouran R., Hu Y., Kim S.-Y., Wan Y.-W., Wangler M.F., et al. MARRVEL: integration of human and model organism genetic resources to facilitate functional annotation of the human genome. Am. J. Hum. Genet. 2017;100:843–853. doi: 10.1016/j.ajhg.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bradley D. The evolution of post-translational modifications. Curr. Opin. Genet. Dev. 2022;76 doi: 10.1016/j.gde.2022.101956. [DOI] [PubMed] [Google Scholar]
- 55.Baeza J., Smallegan M.J., Denu J.M. Mechanisms and dynamics of protein acetylation in mitochondria. Trends Biochem. Sci. 2016;41:231–244. doi: 10.1016/j.tibs.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy E.D., Michnick S.W., Landry C.R. Protein abundance is key to distinguish promiscuous from functional phosphorylation based on evolutionary information. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2594–2606. doi: 10.1098/rstb.2012.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beltrao P., Bork P., Krogan N.J., Noort V. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013;9:714. doi: 10.1002/msb.201304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minguez P., Parca L., Diella F., Mende D.R., Kumar R., Helmer-Citterich M., et al. Deciphering a global network of functionally associated post-translational modifications. Mol. Syst. Biol. 2012;8:599. doi: 10.1038/msb.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leutert M., Entwisle S.W., Villén J. Decoding post-translational modification crosstalk with proteomics. Mol. Cell. Proteomics. 2021;20 doi: 10.1016/j.mcpro.2021.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen S.-J., Melnykov A., Varshavsky A. Evolution of substrates and components of the Pro/N-degron pathway. Biochemistry. 2020;59:582–593. doi: 10.1021/acs.biochem.9b00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conner M.M., Parker H.V., Falcone D.R., Chung G., Schaner Tooley C.E. Novel regulation of the transcription factor ZHX2 by N-terminal methylation. Transcription. 2022;13:1–15. doi: 10.1080/21541264.2022.2079184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia K., Huang G., Wu W., Shrestha R., Wu B., Xiong Y., et al. In vivo methylation of OLA1 revealed by activity-based target profiling of NTMT1. Chem. Sci. 2019;10:8094–8099. doi: 10.1039/c9sc02550b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen T., Muratore T.L., Schaner-Tooley C.E., Shabanowitz J., Hunt D.F., Macara I.G. N-terminal α-methylation of RCC1 is necessary for stable chromatin association and normal mitosis. Nat. Cell Biol. 2007;9:596–603. doi: 10.1038/ncb1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore W.J., Zhang C., Clarke P.R. Targeting of RCC1 to chromosomes is required for proper mitotic spindle assembly in human cells. Curr. Biol. 2002;12:1442–1447. doi: 10.1016/s0960-9822(02)01076-x. [DOI] [PubMed] [Google Scholar]
- 65.Hao Y., Macara I.G. Regulation of chromatin binding by a conformational switch in the tail of the ran exchange factor RCC1. J. Cell Biol. 2008;182:827–836. doi: 10.1083/jcb.200803110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hitakomate E., Hood F.E., Sanderson H.S., Clarke P.R. The methylated N-terminal tail of RCC1 is required for stabilisation of its interaction with chromatin by ran in live cells. BMC Cell Biol. 2010;11:43. doi: 10.1186/1471-2121-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bailey A.O., Panchenko T., Sathyan K.M., Petkowski J.J., Pai P.-J., Bai D.L., et al. Posttranslational modification of CENP-A influences the conformation of centromeric chromatin. Proc. Natl. Acad. Sci. U. S. A. 2013;110:11827–11832. doi: 10.1073/pnas.1300325110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai X., Otake K., You C., Cai Q., Wang Z., Masumoto H., et al. Identification of novel α-n-methylation of CENP-B that regulates its binding to the centromeric DNA. J. Proteome Res. 2013;12:4167–4175. doi: 10.1021/pr400498y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bergmüller E., Gehrig P.M., Gruissem W. Characterization of post-translational modifications of histone H2B-variants isolated from Arabidopsis thaliana. J. Proteome Res. 2007;6:3655–3668. doi: 10.1021/pr0702159. [DOI] [PubMed] [Google Scholar]
- 70.Medzihradszky K.F., Zhang X., Chalkley R.J., Guan S., McFarland M.A., Chalmers M.J., et al. Characterization of Tetrahymena histone H2B variants and posttranslational populations by electron capture dissociation (ECD) fourier transform ion cyclotron mass spectrometry (FT-ICR MS) Mol. Cell. Proteomics. 2004;3:872–886. doi: 10.1074/mcp.M400041-MCP200. [DOI] [PubMed] [Google Scholar]
- 71.Desrosiers R., Tanguay R.M. Methylation of Drosophila histones at proline, lysine, and arginine residues during heat shock. J. Biol. Chem. 1988;263:4686–4692. [PubMed] [Google Scholar]
- 72.Strickland M.S., Strickland W.N., Holt C. The histone H2B from the sperm cell of the starfish Marthasterias glacialis. Eur. J. Biochem. 1980;106:541–548. doi: 10.1111/j.1432-1033.1980.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu T., Hozumi K., Horiike S., Nunomura K., Ikegami S., Takao T., et al. A covalently crosslinked histone. Nature. 1996;380:32. doi: 10.1038/380032a0. [DOI] [PubMed] [Google Scholar]
- 74.Kmiecik D., Belaiche D., Sautiere P., Loucheux-Lefebvre M.-H., Kerckaert J.-P. Complete sequence of Sipunculus nudus erythrocyte histone H2B and its gene. Identification of an N,N-dimethylproline residue at the amino-terminus. Eur. J. Biochem. 1991;198:275–283. doi: 10.1111/j.1432-1033.1991.tb16012.x. [DOI] [PubMed] [Google Scholar]
- 75.Balasingam N., Brandon H.E., Ross J.A., Wieden H.-J., Thakor N. Cellular roles of the human Obg-like ATPase 1 (hOLA1) and its YchF homologs. Biochem. Cell Biol. 2020;98:1–11. doi: 10.1139/bcb-2018-0353. [DOI] [PubMed] [Google Scholar]
- 76.Cai Q., Fu L., Wang Z., Gan N., Dai X., Wang Y. α-N-methylation of damaged DNA-binding protein 2 (DDB2) and its function in nucleotide excision repair. J. Biol. Chem. 2014;289:16046–16056. doi: 10.1074/jbc.M114.558510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beck C., Boehler C., Guirouilh Barbat J., Bonnet M.-E., Illuzzi G., Ronde P., et al. PARP3 affects the relative contribution of homologous recombination and nonhomologous end-joining pathways. Nucleic Acids Res. 2014;42:5616–5632. doi: 10.1093/nar/gku174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dai X., Rulten S.L., You C., Caldecott K.W., Wang Y. Identification and functional characterizations of N-terminal α-N-methylation and phosphorylation of serine 461 in human poly(ADP-ribose) polymerase 3. J. Proteome Res. 2015;14:2575–2582. doi: 10.1021/acs.jproteome.5b00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meng F., Du Y., Miller L.M., Patrie S.M., Robinson D.E., Kelleher N.L. Molecular-level description of proteins from Saccharomyces cerevisiae using quadrupole FT hybrid mass spectrometry for top down proteomics. Anal. Chem. 2004;76:2852–2858. doi: 10.1021/ac0354903. [DOI] [PubMed] [Google Scholar]
- 80.Webb K.J., Laganowsky A., Whitelegge J.P., Clarke S.G. Identification of two SET domain proteins required for methylation of lysine residues in yeast ribosomal protein Rpl42ab. J. Biol. Chem. 2008;283:35561–35568. doi: 10.1074/jbc.M806006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sadaie M., Shinmyozu K., Nakayama J. A conserved SET domain methyltransferase, Set11, modifies ribosomal protein Rpl12 in fission yeast. J. Biol. Chem. 2008;283:7185–7195. doi: 10.1074/jbc.M709429200. [DOI] [PubMed] [Google Scholar]
- 82.Kamp R.M., Srinivasa B.R., Von Knoblauch K., Subramanian A.R. Occurrence of a methylated protein in chloroplast ribosomes. Biochemistry. 1987;26:5866–5870. [Google Scholar]
- 83.Alamgir M., Eroukova V., Jessulat M., Xu J., Golshani A. Chemical-genetic profile analysis in yeast suggests that a previously uncharacterized open reading frame, YBR261C, affects protein synthesis. BMC Genomics. 2008;9:583. doi: 10.1186/1471-2164-9-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu S., Hausmann S., Carlson S.M., Fuentes M.E., Francis J.W., Pillai R., et al. METTL13 methylation of eEF1A increases translational output to promote tumorigenesis. Cell. 2019;176:491–504.e21. doi: 10.1016/j.cell.2018.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bar-Nun S., Glickman M.H. Proteasomal AAA-ATPases: structure and function. Biochim. Biophys. Acta. 2012;1823:67–82. doi: 10.1016/j.bbamcr.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Bard J.A.M., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and function of the 26S proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura Y., Kurata Y., Ishikawa A., Okayama A., Kamita M., Hirano H. N-terminal methylation of proteasome subunit Rpt1 in yeast. Proteomics. 2013;13:3167–3174. doi: 10.1002/pmic.201300207. [DOI] [PubMed] [Google Scholar]
- 88.Tsai C., Aslam K., Drendel H.M., Asiago J.M., Goode K.M., Paul L.N., et al. Hsp31 is a stress response chaperone that intervenes in the protein misfolding process. J. Biol. Chem. 2015;290:24816–24834. doi: 10.1074/jbc.M115.678367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ariga H., Iguchi-Ariga S.M.M. Advances in Experimental Medicine and Biology. Springer Singapore; Singapore: 2017. DJ-1/PARK7 protein: parkinson’s disease, cancer and oxidative stress-induced diseases; pp. 5–24. [Google Scholar]
- 90.Bankapalli K., Saladi S., Awadia S.S., Goswami A.V., Samaddar M., D’Silva P. Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae. J. Biol. Chem. 2015;290:26491–26507. doi: 10.1074/jbc.M115.673624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen P., Paschoal Sobreira T.J., Hall M.C., Hazbun T.R. Discovering the N-terminal methylome by repurposing of proteomic datasets. J. Proteome Res. 2021;20:4231–4247. doi: 10.1021/acs.jproteome.1c00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Newell-Litwa K.A., Horwitz R., Lamers M.L. Non-muscle myosin II in disease: mechanisms and therapeutic opportunities. Dis. Model. Mech. 2015;8:1495–1515. doi: 10.1242/dmm.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Coluccio L.M. Advances in Experimental Medicine and Biology. Springer International Publishing; Cham: 2020. Myosins: a superfamily of molecular motors; pp. 199–231. [Google Scholar]
- 94.Hamey J.J., Wilkins M.R. The protein methylation network in yeast: a landmark in completeness for a eukaryotic post-translational modification. Proc Natl Acad Sci U S A. 2023;120 doi: 10.1073/pnas.2215431120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uhlén M., Fagerberg L., Hallström B.M., Lindskog C., Oksvold P., Mardinoglu A., et al. Tissue-based map of the human proteome. Science. 2015;347 doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 96.Hong A.R., Kim K., Lee J.Y., Yang J.-Y., Kim J.H., Shin C.S., et al. Transformation of mature osteoblasts into bone lining cells and RNA sequencing-based transcriptome profiling of mouse bone during mechanical unloading. Endocrinol. Metab. (Seoul) 2020;35:456–469. doi: 10.3803/EnM.2020.35.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaiser S., Park Y.-K., Franklin J.L., Halberg R.B., Yu M., Jessen W.J., et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabates-Bellver J., Van der Flier L.G., de Palo M., Cattaneo E., Maake C., Rehrauer H., et al. Transcriptome profile of human colorectal adenomas. Mol. Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 99.Brune V., Tiacci E., Pfeil I., Döring C., Eckerle S., van Noesel C.J.M., et al. Origin and pathogenesis of nodular lymphocyte–predominant Hodgkin lymphoma as revealed by global gene expression analysis. J. Exp. Med. 2008;205:2251–2268. doi: 10.1084/jem.20080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sperger J.M., Chen X., Draper J.S., Antosiewicz J.E., Chon C.H., Jones S.B., et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13350–13355. doi: 10.1073/pnas.2235735100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Finak G., Bertos N., Pepin F., Sadekova S., Souleimanova M., Zhao H., et al. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 2008;14:518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 102.Ertel A., Dean J.L., Rui H., Liu C., Witkiewicz A., Knudsen K.E., et al. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–4163. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rizzolio F., Lucchetti C., Caligiuri I., Marchesi I., Caputo M., Klein-Szanto A.J., et al. Retinoblastoma tumor-suppressor protein phosphorylation and inactivation depend on direct interaction with Pin1. Cell Death Differ. 2012;19:1152–1161. doi: 10.1038/cdd.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Catlin J.P., Marziali L.N., Rein B., Yan Z., Feltri M.L., Schaner Tooley C.E. Age-related neurodegeneration and cognitive impairments of NRMT1 knockout mice are preceded by misregulation of RB and abnormal neural stem cell development. Cell Death Dis. 2021;12:1014. doi: 10.1038/s41419-021-04316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z., Zhang L., Liu D., Yang Z., Xuan D., Zhang Y. Knockdown of NRMT enhances sensitivity of retinoblastoma cells to cisplatin through upregulation of the CENPA/Myc/Bcl2 axis. Cell Death Discov. 2022;8:14. doi: 10.1038/s41420-021-00622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J., Song H., Chen C., Chen L., Dai Y., Sun P.-H., et al. Methyltransferase-like protein 11A promotes migration of cervical cancer cells via up-regulating ELK3. Pharmacol. Res. 2021;172 doi: 10.1016/j.phrs.2021.105814. [DOI] [PubMed] [Google Scholar]
- 107.Jiang X., Liu B., Nie Z., Duan L., Xiong Q., Jin Z., et al. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target. Ther. 2021;6:1–16. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gu J., Zhan Y., Zhuo L., Zhang Q., Li G., Li Q., et al. Biological functions of m6A methyltransferases. Cell Biosci. 2021;11:15. doi: 10.1186/s13578-020-00513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Y., Zhang W., Shen F., Yang X., Liu H., Dai S., et al. YTH domain proteins: a family of m6A readers in cancer progression. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.629560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu K., Yang Y., Feng G.-H., Sun B.-F., Chen J.-Q., Li Y.-F., et al. Mettl3-mediated m6A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–1114. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H., Xu J., Lazarovici P., Quirion R., Zheng W. cAMP response element-binding protein (CREB): a possible signaling molecule link in the pathophysiology of schizophrenia. Front. Mol. Neurosci. 2018;11:255. doi: 10.3389/fnmol.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Parker H.V., Schaner Tooley C.E. Opposing regulation of the Nα-trimethylase METTL11A by its family members METTL11B and METTL13. J. Biol. Chem. 2023;299 doi: 10.1016/j.jbc.2023.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conner M.M., Schaner Tooley C.E. Three’s a crowd – why did three N-terminal methyltransferases evolve for one job? J. Cell Sci. 2023;136:jcs260424. doi: 10.1242/jcs.260424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson S.L., Mao Y., Zhang G., Hanjra P., Peterson D.L., Huang R. Kinetic mechanism of protein N-terminal methyltransferase 1. J. Biol. Chem. 2015;290:11601–11610. doi: 10.1074/jbc.M114.626846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang G., Richardson S.L., Mao Y., Huang R. Design, synthesis, and kinetic analysis of potent protein N-terminal methyltransferase 1 inhibitors. Org. Biomol. Chem. 2015;13:4149–4154. doi: 10.1039/c5ob00120j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen D., Dong C., Dong G., Srinivasan K., Min J., Noinaj N., et al. Probing the plasticity in the active site of protein N-terminal methyltransferase 1 using bisubstrate analogues. J. Med. Chem. 2020;63:8419–8431. doi: 10.1021/acs.jmedchem.0c00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen D., Meng Y., Yu D., Noinaj N., Cheng X., Huang R. Chemoproteomic study uncovers HemK2/KMT9 as a new target for NTMT1 bisubstrate inhibitors. ACS Chem. Biol. 2021;16:1234–1242. doi: 10.1021/acschembio.1c00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dong G., Iyamu I.D., Vilseck J.Z., Chen D., Huang R. Improved cell-potent and selective peptidomimetic inhibitors of protein N-terminal methyltransferase 1. Molecules. 2022;27:1381. doi: 10.3390/molecules27041381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dong G., Deng Y., Yasgar A., Yadav R., Talley D., Zakharov A.V., et al. Venglustat inhibits protein N-terminal methyltransferase 1 in a substrate-competitive manner. J. Med. Chem. 2022;65:12334–12345. doi: 10.1021/acs.jmedchem.2c01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng Y., Dong G., Meng Y., Noinaj N., Huang R. Structure–activity relationship studies of venglustat on NTMT1 inhibition. J. Med. Chem. 2023;66:1601–1615. doi: 10.1021/acs.jmedchem.2c01854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Q., Wu W., Jia K., Qi G., Sun X.S., Li P. Design and characterization of PROTAC degraders specific to protein N-terminal methyltransferase 1. Eur. J. Med. Chem. 2022;244 doi: 10.1016/j.ejmech.2022.114830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lienhard G.E. Non-functional phosphorylations? Trends Biochem. Sci. 2008;33:351–352. doi: 10.1016/j.tibs.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 123.Hamey J.J., Separovich R.J., Wilkins M.R. MT-MAMS: protein methyltransferase motif analysis by mass spectrometry. J. Proteome Res. 2018;17:3485–3491. doi: 10.1021/acs.jproteome.8b00396. [DOI] [PubMed] [Google Scholar]
- 124.Low T.Y., Syafruddin S.E., Mohtar M.A., Vellaichamy A., A Rahman N.S., Pung Y.-F., et al. Recent progress in mass spectrometry-based strategies for elucidating protein–protein interactions. Cell. Mol. Life Sci. 2021;78:5325–5339. doi: 10.1007/s00018-021-03856-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salas D., Stacey R.G., Akinlaja M., Foster L.J. Next-generation interactomics: considerations for the use of co-elution to measure protein interaction networks. Mol. Cell. Proteomics. 2020;19:1–10. doi: 10.1074/mcp.R119.001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ju S., Kwon Y., Kim J.-M., Park D., Lee S., Lee J.-W., et al. iNrich, rapid and robust method to enrich N-terminal proteome in a highly multiplexed platform. Anal. Chem. 2020;92:6462–6469. doi: 10.1021/acs.analchem.9b05653. [DOI] [PubMed] [Google Scholar]
- 127.Holmes W.M., Mannakee B.K., Gutenkunst R.N., Serio T.R. Loss of amino-terminal acetylation suppresses a prion phenotype by modulating global protein folding. Nat. Commun. 2014;5:4383. doi: 10.1038/ncomms5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Armache J.-P., Jarasch A., Anger A.M., Villa E., Becker T., Bhushan S., et al. Cryo-EM structure and rRNA model of a translating eukaryotic 80S ribosome at 5.5-Å resolution. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19748–19753. doi: 10.1073/pnas.1009999107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tanaka Y., Nureki O., Kurumizaka H., Fukai S., Kawaguchi S., Ikuta M., et al. Crystal structure of the CENP-B protein–DNA complex: the DNA-binding domains of CENP-B induce kinks in the CENP-B box DNA. EMBO J. 2001;20:6612–6618. doi: 10.1093/emboj/20.23.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tachiwana H., Kagawa W., Shiga T., Osakabe A., Miya Y., Saito K., et al. Crystal structure of the human centromeric nucleosome containing CENP-A. Nature. 2011;476:232–235. doi: 10.1038/nature10258. [DOI] [PubMed] [Google Scholar]
- 131.Hassler M., Singh S., Yue W.W., Luczynski M., Lakbir R., Sanchez-Sanchez F., et al. Crystal structure of the retinoblastoma protein N domain provides insight into tumor suppression, ligand interaction, and holoprotein architecture. Mol. Cell. 2007;28:371–385. doi: 10.1016/j.molcel.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yeh J.I., Levine A.S., Du S., Chinte U., Ghodke H., Wang H., et al. Damaged DNA induced UV-damaged DNA-binding protein (UV-DDB) dimerization and its roles in chromatinized DNA repair. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E2737–E2746. doi: 10.1073/pnas.1110067109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alamo L., Ware J.S., Pinto A., Gillilan R.E., Seidman J.G., Seidman C.E., et al. Effects of myosin variants on interacting-heads motif explain distinct hypertrophic and dilated cardiomyopathy phenotypes. Elife. 2017;6 doi: 10.7554/eLife.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koller-Eichhorn R., Marquardt T., Gail R., Wittinghofer A., Kostrewa D., Kutay U., et al. Human OLA1 defines an ATPase subfamily in the Obg damily of GTP-binding proteins. J. Biol. Chem. 2007;282:19928–19937. doi: 10.1074/jbc.M700541200. [DOI] [PubMed] [Google Scholar]
- 135.Sankhala R.S., Lokareddy R.K., Begum S., Pumroy R.A., Gillilan R.E., Cingolani G. Three-dimensional context rather than NLS amino acid sequence determines importin α subtype specificity for RCC1. Nat. Commun. 2017;8:979. doi: 10.1038/s41467-017-01057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Varshavsky A. The N-end rule pathway and regulation by proteolysis: the N-end rule pathway. Protein Sci. 2011;20:1298–1345. doi: 10.1002/pro.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen S.-J., Wu X., Wadas B., Oh J.-H., Varshavsky A. An N-end rule pathway that recognizes proline and destroys gluconeogenic enzymes. Science. 2017;355 doi: 10.1126/science.aal3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dong C., Chen S.-J., Melnykov A., Weirich S., Sun K., Jeltsch A., et al. Recognition of nonproline N-terminal residues by the Pro/N-degron pathway. Proc. Natl. Acad. Sci. U. S. A. 2020;117:14158–14167. doi: 10.1073/pnas.2007085117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chen S.-J., Kim L., Song H.K., Varshavsky A. Aminopeptidases trim Xaa-Pro proteins, initiating their degradation by the Pro/N-degron pathway. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2115430118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fujimoto H., Higuchi M., Koike M., Ode H., Pinak M., Bunta J.K., et al. A possible overestimation of the effect of acetylation on lysine residues in KQ mutant analysis. J. Comput. Chem. 2012;33:239–246. doi: 10.1002/jcc.21956. [DOI] [PubMed] [Google Scholar]