Abstract
As primary producers and ecosystem engineers, kelp (generally Order Laminariales) are ecologically important, and their decline could have far‐reaching consequences. Kelp are valuable in forming habitats for fish and invertebrates and are crucial for adaptation to climate change by creating coastal defenses and in providing key functions, such as carbon sequestration and food provision. Kelp are threatened by multiple stressors, such as climate change, over‐harvesting of predators, and pollution. In this opinion paper, we discuss how these stressors may interact to affect kelp, and how this varies under different contexts. We argue that more research that bridges kelp conservation and multiple stressor theory is needed and outline key questions that should be addressed as a priority. For instance, it is important to understand how previous exposure (either to earlier generations or life stages) determines responses to emerging stressors, and how responses in kelp scale up to alter food webs and ecosystem functioning. By increasing the temporal and biological complexity of kelp research in this way, we will improve our understanding allowing better predictions. This research is essential for the effective conservation and potential restoration of kelp in our rapidly changing world.
Keywords: conservation biology, global change, marine ecology, stressor interactions
Kelp are globally in decline due to human activity. Here, we give an overview of the role of multiple stressors, such as warming and pollution, and their interactions in driving these declines. We argue that further research is needed with greater biological and temporal realism to improve our understanding of the effects of multiple stressors on kelp for their effective conservation.
1. WHAT ARE KELP?
Kelp is a non‐taxonomic term that refers to ecologically important canopy‐forming large brown macroalgae, usually of the Order Laminariales (although other functionally similar macroalgae are sometimes included; Fraser, 2012), that inhabit hard substrata of the seafloor (Figure 1). Kelp play a key role in primary and secondary productivity through photosynthesis and the export of dissolved and particulate organic material in coastal ecosystems (Paine et al., 2021; Takao et al., 2015), where a large proportion of kelp production enters coastal food webs mainly as detritus (Krause‐Jensen et al., 2018; Krumhansl & Scheibling, 2011; Queirós et al., 2019; Steneck et al., 2002). Kelp form habitats for invertebrates and juvenile fish and also act as ecosystem engineers by altering water flow and sedimentation rates (Smale et al., 2013). The loss of kelp can drive disruptions in ecosystems (Filbee‐Dexter & Wernberg, 2018; Scherner et al., 2012), affecting the abundance and diversity of species, including economically important organisms, such as abalone (Kiyomoto et al., 2013). The kelp life cycle consists of two main life stages, the macroscopic diploid sporophyte and the microscopic haploid gametophyte (Figure 1). The sporophyte produces zoospores, which grow into gametophytes. Gametophytes produce gametes (through gametogenesis), which fuse to form zygotes, maturing into sporophytes (Hurd et al., 2014). Zoospores depend on lipid reserves as an energy source, while gametophytes and subsequent stages are supported by photosynthesis, thus zoospores use different biochemical mechanisms and are often sensitive to different levels and types of environmental stressors compared to other life stages (Leal et al., 2018).
FIGURE 1.

Examples of different kelp species at various life stages and locations; (a) Laminaria hyperboea in Ireland, (b) Macrocystis pyrifera forest in Tasmania, Australia, (c) Ecklonia radiata in Tasmania, Australia. Male and female gametophytes (d) and young sporophytes (e) of Macrocystis pyrifera. Pictures by Kenan Chan (a), Joanna Smart (b, c), and MS (d, e).
2. MULTIPLE STRESSORS IN KELP ECOSYSTEMS
As the human footprint on our planet continues to grow, most ecosystems are subject to multiple simultaneous stressors (Côté et al., 2016). Kelp are affected by stressors, such as rising sea temperature and marine heat waves, invasions by species introductions and range expansions, direct and indirect effects of over‐fishing, algal blooms, ocean acidification, loss of sea ice, and the resulting increase in ultraviolet exposure (Diehl et al., 2020; Donham et al., 2022; Filbee‐Dexter et al., 2019; Hollarsmith et al., 2020; Kroeker et al., 2017; Miranda et al., 2019; Shukla & Edwards, 2017; Starko et al., 2022). Kelp generally live in coastal habitats which are also exposed to stressors from land use including nutrient enrichment, toxins, and sedimentation (Wernberg et al., 2019). For example, near urban coasts, kelp are affected by toxins, such as copper from industrial waste and mine drainage, which decrease the rate of zoospore germination in Undaria pinnatifida, Macrocystis pyrifera, and Lessonia nigrescens (Contreras et al., 2007; Leal et al., 2018). Climate change is having negative effects by, for instance, causing reduced sea ice in polar regions, resulting in increased turbidity, and reduced light penetration. While in some cases shrinking ice caps may initially increase the substrata available for kelp settlement, it has been predicted that kelp growth, productivity, and vertical distribution will ultimately decline in these regions (Bonsell & Dunton, 2018; Filbee‐Dexter et al., 2019). Melting sea ice also locally reduces salinity, and Alaria esculenta, Saccharina latissima, and Laminaria solidungula have strong bleaching and high mortality in low salinities. In contrast, sporophytes of Laminaria digitata tolerate a range of salinities and may not be negatively affected (Karsten, 2007). Evidence also suggests that high levels of ultraviolet radiation can reduce growth by damaging biochemical processes in photosynthesis and denaturing DNA (Heinrich et al., 2015; Müller et al., 2008; Xiao et al., 2015).
These stressors, which operate from local to global scales, can interact to determine their combined cumulative effect (Figure 2; Falkenberg et al., 2012). Growing evidence suggests that the combined effect of two stressors is rarely additive (i.e., additive = the sum of their parts; Orr et al., 2020, 2022). Instead, stressor pairs frequently result in impacts which are more or less than the sum of their parts (i.e., non‐additive responses). These are known as synergistic and antagonistic stressor interactions, respectively (Jackson et al., 2021; Orr et al., 2020). Evidence suggests that climate warming can exacerbate the negative effects of acidification and pollutants. For instance, warming and acidification combine to inhibit gametophyte growth in Ecklonia stolonifera (Gao et al., 2019) and increase mortality of Macrocystis pyrifera zoospores (Gaitán‐Espitia et al., 2014), while warming and copper pollution interact synergistically to decrease the size of Macrocystis pyrifera gametophytes (but do not affect Undaria pinnatifida gametophytes; Leal et al., 2018). Warming also exacerbates damage caused by elevated ultraviolet radiation on sporophyte formation causing reduced egg release in Saccharina latissima and Laminaria digitata (Müller et al., 2008) and decreases in the zoospore germination rate in Alaria marginata (Fredersdorf et al., 2009). In contrast, increased temperature can mitigate the effects of some stressors. For example, warming reduced the negative effects of increased ultraviolet radiation in Alaria esculenta by promoting photosynthesis (Roleda, 2009). In comparison, other studies have found that warming exacerbates damage of elevated ultraviolet radiation on sporophyte formation, reduces egg release in Saccharina latissima and Laminaria digitata (Müller et al., 2008), and decreases the zoospore germination rate in Alaria marginata (Fredersdorf et al., 2009). Despite the growing understanding of the effects of multiple stressors on kelp (see Table S1) there are still large areas where further research is needed (Hoffman et al., 2003; Hollarsmith et al., 2020) to understand the growing list of context dependencies (i.e., variation in responses between species, locations, and life stages). Here, we outline knowledge gaps and key questions to help us understand how multiple stressors will affect kelp and shape the future global distribution of this important foundation species.
FIGURE 2.
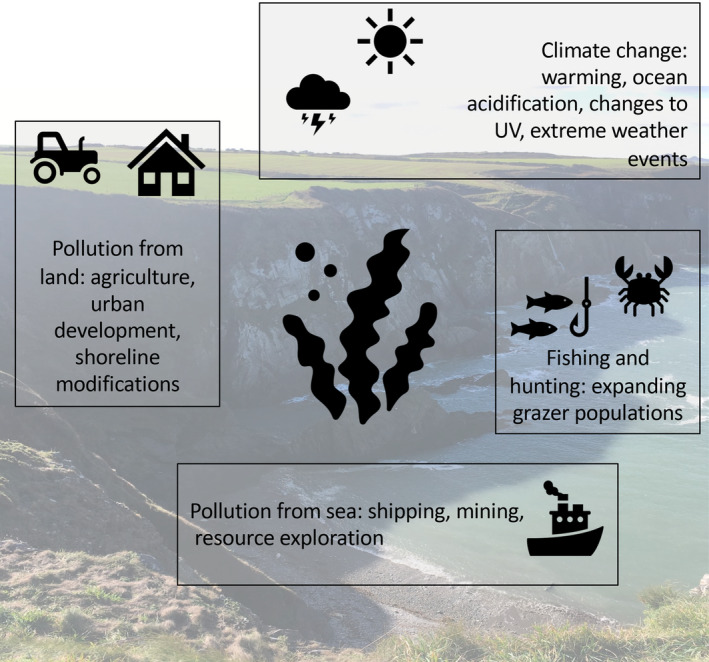
Schematic demonstrating the range of anthropogenic stressors that can affect kelp‐dominated systems. Local stressors include pollution (chemical and nutrient) from the land and sea and expanding grazer populations caused by human exploitation. Global stressors associated with climate change include acidification, changes to ultraviolet radiation, and temperature.
3. HOW DOES THE INTENSITY (OR SEVERITY) OF DIFFERENT STRESSORS AFFECT THE RESPONSE OF KELP POPULATIONS TO MULTIPLE STRESSORS?
Many field and experimental studies are limited in the resolution of the stressor gradient they use, which can cause problems when predicting impacts of stressors that vary in intensity over space and/or time (Vye et al., 2015). For instance, global sea temperatures have increased by 0.1°C per decade since the 1950s (IPCC, 2022), with rates of warming at least two times higher in the Arctic (IPCC, 2022). There is also some uncertainty in the level of warming that will be experienced over coming decades, with predictions ranging (on average) from 1.5 to 7°C (IPCC, 2022). Although some kelp, such as Saccharina latissima, have high thermal plasticity (Müller et al., 2008) and may have increased growth rate with warming, other species have reduced growth and productivity with increasing temperature (Table S1). This usually depends on the location, with populations at a latitude with temperatures near their thermal maxima experiencing reduced growth with increasing temperature, while the same species in a location with historical temperatures at the lower end of their thermal limits might experience higher growth rates with warming. However, warmer trailing edge kelp populations in the Northeast Atlantic have been shown to be more thermotolerant than cooler range center populations (King et al., 2018, 2019). The level of warming will also be important when determining the outcome of interactions with other stressors, and vice versa. For instance, with low nutrient concentrations, increasing temperature mitigates the reduced growth rates in juvenile S. japonica sporophytes (Gao et al., 2017) and juvenile Eisenia bicyclis sporophytes (Endo et al., 2017) but exacerbates the damage in juvenile Undaria pinnatifida sporophytes (Gao et al., 2013). In contrast, high nutrient concentrations ameliorate the negative impacts of warming in Macrocystis pyrifera (Fernández et al., 2020; Schmid et al., 2020). More research is needed over gradients of multiple stressor severity to make robust predictions about kelp responses to an uncertain future.
4. HOW DO MULTIPLE STRESSOR EFFECTS VARY OVER TIME IN KELP POPULATIONS?
Since kelp have distinct life stages, it is important to consider how combined stressor effects on one life stage influences the other stage (Gauci et al., 2022). When considering climate warming, the different life stages of kelp vary in tolerance to increased temperature, for example, Ecklonia cava sporophytes can tolerate a larger range than gametophytes (Takao et al., 2015). Similarly, ocean acidification can have contrasting effects on different life stages because of the different mechanisms used to maximize photosynthetic rates: both passive diffusion of CO2 as well as the carbon‐concentrating mechanism (Maberly et al., 1992). The use of both mechanisms could explain the lack of response to ocean acidification observed in sporophytes of many kelp species (Fernández et al., 2015; Gordillo et al., 2016; Hollarsmith et al., 2020; Iñiguez et al., 2016; Kang & Chung, 2018; Nunes et al., 2016). However, decreased survival and growth have been observed in Macrocystis pyrifera gametophytes under low pH (van der Loos et al., 2019). Zoospores do not rely on photosynthesis for growth; therefore, no effect of decreased pH on zoospore germination in Macrocystis pyrifera and Undaria pinnatifida has been detected (Leal et al., 2018; Roleda et al., 2012). Gauci et al. (2022) found that gametophyte exposure to warming reduced recruitment and thermal tolerance of juvenile sporophytes in Laminaria digitata. However, there is still an open question of how responses to multiple stressors at one life stage are transferred to the next. Here, amplified impacts may be caused by lagged responses to past events or accumulation of stress severity over time (Jackson et al., 2021), as seen in coral reefs (Hughes et al., 2019).
The timing of stressor events can also be important across generations, with historical exposure to stressors altering how organisms respond to current and future stress (Fernández et al., 2021; Jackson et al., 2021; Schmid et al. 2021). For instance, adaptation following stressor events can result in reduced impacts of similar future events on kelp populations (Filbee‐Dexter et al., 2020; Jackson et al., 2021). However, Filbee‐Dexter et al. (2020) found that kelps from degraded and healthy reefs were equally vulnerable to heatwaves. This suggests no effect of previous exposure, but much more research is needed to understand how different stressors interact over time, especially when species interactions are considered (Jackson et al., 2021).
5. HOW DO MULTIPLE STRESSORS SCALE UP TO ALTER KELP INTERACTIONS WITH OTHER SPECIES (AND ENTIRE KELP‐BASED FOOD WEBS)?
Although some kelp populations may survive and even increase productivity and resilience with anthropogenic stressors, many populations will decline in diversity and biomass, causing shifts to irreversible alternative states (Christie et al., 2019; Filbee‐Dexter & Wernberg, 2018; Kumagai et al., 2018; Miranda et al., 2019; Veenhof et al., 2022). In nutrient‐enriched and polluted waters, the green algae Ulva spp. may increase in abundance at the expense of canopy‐forming kelp (Martins et al., 2014; Russell et al., 2009; Scherner et al., 2012). Nutrient enrichment can also cause phytoplankton blooms, which decreases light penetration to kelp (Filbee‐Dexter et al., 2019), causing high mortality and decreasing growth rate, for example, in Undaria pinnatifida juvenile sporophytes (Gao et al., 2013). Alternatively, increased nutrient concentrations might increase kelp growth and resilience against competitive turf‐forming algae (Tamburello et al., 2019), but most of the evidence suggests a negative outcome for kelp. There is also evidence to suggest that over‐fishing of large predators can compound the effects of ocean warming via mechanisms such as range‐shifting grazers and marine heatwaves (Rogers‐Bennett & Catton, 2019; Vergés et al., 2016). However, we still know very little about how multiple stressors interact to alter complex food webs—a question that should be addressed (Kroeker et al., 2017).
Another important stressor in marine ecosystems is the loss of top predators (such as crabs from over‐harvesting or sea stars due to disease), which may lead to an increase in the abundance of herbivores, such as sea urchins, and subsequent over‐grazing of kelp (Gorra et al., 2022; Ling et al., 2009). For example, the hunting of otters in the 19th century reduced otter populations and led to an increase in sea urchin populations, and a subsequent decrease in kelp forest cover. The kelp population recovered after a hunting ban in 1911 with population densities maintained for decades until the decline of otter populations again in the 1990s due to increased predation by whales (Filbee‐Dexter & Scheibling, 2014; Paine, 1980). In Northern California, the dramatic decline in keystone predator population densities (such as the sunflower star, Pycnopodia helianthoides due to disease), also led to an increase in sea urchin population densities. Here, marine heat waves interacted synergistically with the loss of the sunflower star, resulting in a decline of the kelp Nereocystis luetkeana (McPherson et al., 2021). Ultimately, this can result in a shift from kelp forests to essentially barren grounds (Filbee‐Dexter & Scheibling, 2014; Smale et al., 2013), with implications for the entire food web. However, most studies on the effects of multiple stressors on kelp have focused on individuals at a single life stage and have been principally conducted in the laboratory, and thus may not represent the complexities of communities and ecosystems.
6. HOW DO THE IMPACTS OF MULTIPLE STRESSORS AFFECT THE FUNCTIONING OF KELP AND THE ECOSYSTEM SERVICES ASSOCIATED WITH THEM?
Kelp provide many important services for humans. Some kelp species are used for human consumption, play a role in mitigating climate change through carbon sequestration (Dolliver & O'Connor, 2022; Filbee‐Dexter & Wernberg, 2020; Gilson et al., 2021), or assist in climate adaptation by damping waves and protecting coasts (Smale et al., 2013; UNEP, 2023). Recent studies show that the economic value of kelp is at least three times higher than previously estimated (e.g., Northeast Atlantic kelp is valued at $71 k per hectare per year), which is driven primarily by fisheries production and nitrogen uptake (Eger et al., 2023). In a potential negative feedback loop, more regular enhanced wave action from climate change induced cyclones and storms has been shown to cause population losses (Perkol‐Finkel & Airoldi, 2010), but some kelp communities can recover quickly through frequent recruitment and high growth rates (Krumhansl et al., 2016). Other evidence suggests storms may promote kelp growth and increase kelp density, by reducing the diffusion boundary layer and increasing nutrient uptake, dislodging competing epiphytes, and increasing recruitment (e.g., in L. hyperborean; Pedersen et al., 2012).
Overall, knowledge on the interactive effect of multiple stressors on the functions and services provided by kelp is limited, with many studies restricted to population‐level responses in unrealistic laboratory trials (Bass et al., 2021; Smale et al., 2013). We need more experiments in complex, semi‐natural settings to fully understand how kelp responses scale up to alter ecosystem functions and services.
7. HOW WILL MULTIPLE STRESSORS IMPACT MITIGATION APPROACHES?
Despite variability in the responses of kelp to anthropogenic stressors, global kelp populations have already begun to decline since the 1980s, especially in the South Australian Gulfs, Tasmania, the Gulf of Maine, and the Arctic Sea (Johnson et al., 2011; Krumhansl et al., 2016; Perkol‐Finkel & Airoldi, 2010). Other kelp populations, however, have increased in the past, which may also be attributed to improved local management, such as reduction in local pollution and recovery of sea urchin predators on the west coast of Vancouver Island and Southern California Bight (Krumhansl et al., 2016). The larger variation in local trends compared with the global trend suggests that there is spatial variation in adaptations and resilience of kelp species to anthropogenic stressors (Smale et al., 2013). It also provides hope that management and restoration of kelp ecosystems could be successful with the control of only local stressors. For example, the experimental removal of sea urchins and limpets in eastern Australia led to an increase in the population size of E. radiata and Sargassum sinclairii after 3 months, compared to sites without the removal of these herbivores (Fletcher, 1987). Controlling or removing local stressors usually leads to such successful restoration outcomes when multiple stressor interactions are synergistic (Brown et al., 2013). Here, the removal of one stressor would result in improvements greater than expected additively. However, some evidence suggests the control of one stressor alone may not be sufficient for kelp restoration (Wilman, 2021). Where two stressors have a negative effect on kelp and interact antagonistically, substantial recovery of kelp may only be observed after the removal of both stressors. Indeed, the removal of only one stressor may even reduce ecosystem fitness further if the presence of the removed stressor mitigated the effects of other stressors (Brown et al., 2013). Therefore, the survival of kelp ecosystems may not be severely affected if a second stressor mitigates the damage from the first, but much more research is needed here as more nature recovery strategies are implemented (Eger et al., 2022).
8. CONCLUSION: WHAT DOES THE FUTURE LOOK LIKE FOR KELP?
As sea temperature rises, some kelp begin to approach the edge of their range and experience range contractions (Smale et al., 2013; Vye et al., 2015; Vergés et al., 2016), while temperate‐adapted algae expand into polar regions and outcompete kelp which are less well‐adapted to higher temperatures (Filbee‐Dexter et al., 2019). For example, the distribution of suitable habitats for Ecklonia cava is predicted to decrease to between 15% and 45% of current distributions by 2100, and it could become extinct with increased herbivore grazing (Takao et al., 2015). In contrast, some species of Arctic kelp have high optimal temperatures, higher than those predicted to occur with climate change (Filbee‐Dexter et al., 2019), suggesting that an increase in sea temperature may be beneficial to the growth and productivity of some stages in some kelp species. This was shown in Spitsbergen, where the increasing temperature between 1996 and 2014 increased total biomass and stability of the ecosystem (Paar et al., 2019). In parts of Alaska, the species composition of kelp communities has also remained relatively static between 1978 and 2012 despite increasing temperature (Filbee‐Dexter et al., 2019). Although the loss of Arctic sea ice will decrease salinity and increase turbidity locally, Arctic rocky shores may present a large potential for the future niches of kelp as they expand northwards (Filbee‐Dexter et al., 2019; Paar et al., 2019).
The context dependencies in kelp responses to multiple stressors could be further understood using advances in multiple stressor theory, with key areas outlined above. Overall, an increase in complexity (biological and temporal) is needed, alongside a focus on mitigation strategies. Here, the multifaceted concept of stressor similarity could be used to predict the outcome of restoration plans (Orr et al., 2022). There is hope that increasing awareness will remove some of the local stressors on marine ecosystems, such as by reducing coastal pollution and over‐harvesting of predators (Steneck et al., 2002). However, evidence suggests that climate tipping points are dangerously close (Lenton et al., 2019) and that global stressors such as increasing temperature, loss of Arctic sea ice, and ocean acidification may already be irreversible. Population declines will be inevitable, but some kelp species will adapt to anthropogenic stressors, increasing growth and reproduction. However, this may be constrained if some life stages are inhibited. As the effects of stressors on all life stages are still largely unknown for most kelp species, future research must focus on understanding these impacts for effective kelp conservation.
AUTHOR CONTRIBUTIONS
Brigitte Wear: Conceptualization (lead); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); validation (equal); visualization (equal); writing – original draft (lead). Nessa E. O'Connor: Investigation (equal); validation (equal); writing – review and editing (equal). Matthias J. Schmid: Investigation (equal); validation (equal); visualization (equal); writing – review and editing (equal). Michelle C. Jackson: Data curation (equal); investigation (equal); methodology (equal); project administration (equal); supervision (lead); validation (equal); visualization (equal); writing – review and editing (lead).
ACKNOWLEDGEMENTS
This work was supported by the Department of Biology at the University of Oxford and Science Foundation Ireland's President of Ireland Future Research Leaders Programme: [18/FR/6198] “Beyond biofuel: Advanced seaweed cultivation for biodiscovery and climate change mitigation”. MS was also funded through a European Commission Marie Sklodowska‐Curie Actions Postdoctoral Fellowship (Project 101066815 ‐ ASPIRE).
Supporting information
Table S1
Wear, B. , O’Connor, N. E. , Schmid, M. J. , & Jackson, M. C. (2023). What does the future look like for kelp when facing multiple stressors? Ecology and Evolution, 13, e10203. 10.1002/ece3.10203
DATA AVAILABILITY STATEMENT
No data are associated with this paper.
REFERENCES
- Bass, A. , Wernberg, T. , Thomsen, M. , & Smale, D. (2021). Another decade of marine climate change experiments: Trends, progress and knowledge gaps. Frontiers in Marine Science, 8, 714462. [Google Scholar]
- Bonsell, C. , & Dunton, K. H. (2018). Long‐term patterns of benthic irradiance and kelp production in the central Beaufort Sea reveal implications of warming for Arctic inner shelves. Progress in Oceanography, 162, 160–170. [Google Scholar]
- Brown, C. J. , Saunders, M. I. , Possingham, H. P. , & Richardson, A. J. (2013). Managing for interactions between local and global stressors of ecosystems. PLoS One, 8, 1–10. 10.1371/journal.pone.0065765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, H. , Andersen, G. S. , Bekkby, T. , Fagerli, C. W. , Gitmark, J. K. , Gundersen, H. , & Rinde, E. (2019). Shifts between sugar kelp and turf algae in Norway: Regime shifts or fluctuations between different opportunistic seaweed species? Frontiers in Marine Science, 6, 1–10. 10.3389/fmars.2019.00072 36817748 [DOI] [Google Scholar]
- Contreras, L. , Medina, M. H. , Andrade, S. , Oppliger, V. , & Correa, J. A. (2007). Effects of copper on early developmental stages of Lessonia nigrescens Bory (Phaeophyceae). Environmental Pollution, 145, 75–83. [DOI] [PubMed] [Google Scholar]
- Côté, I. M. , Darling, E. S. , & Brown, C. J. (2016). Interactions among ecosystem stressors and their importance in conservation. Proceedings of the Royal Society B: Biological Sciences, 283, 1–9. 10.1098/rspb.2015.2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl, N. , Karsten, U. , & Bischof, K. (2020). Impacts of combined temperature and salinity stress on the endemic Arctic brown seaweed Laminaria solidungula . Polar Biology, 43, 647–656. [Google Scholar]
- Dolliver, J. , & O'Connor, N. (2022). Whole system analysis is required to determine the fate of macroalgal carbon: A systematic review. Journal of Phycology, 58, 364–376. 10.1111/jpy.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham, E. M. , Strope, L. T. , Hamilton, S. L. , & Kroeker, K. J. (2022). Coupled changes in pH, temperature, and dissolved oxygen impact the physiology and ecology of herbivorous kelp forest grazers. Global Change Biology, 28, 3023–3039. [DOI] [PubMed] [Google Scholar]
- Eger, A. M. , Layton, C. , McHugh, T. A. , Gleason, M. , & Eddy, N. (2022). Kelp restoration guidebook: Lessons learned from kelp projects around the world. The Nature Conservancy. [Google Scholar]
- Eger, A. M. , Marzinelli, E. M. , Beas‐Luna, R. , Blain, C. O. , Blamey, L. K. , Byrnes, J. E. , Carnell, P. E. , Choi, C. G. , Hessing‐Lewis, M. , Kim, K. Y. , & Kumagai, N. H. (2023). The value of ecosystem services in global marine kelp forests. Nature Communications, 14, 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, H. , Suehiro, K. , Gao, X. , & Agatsuma, Y. (2017). Interactive effects of elevated summer temperature, nutrient availability, and irradiance on growth and chemical compositions of juvenile kelp, Eisenia bicyclis . Phycological Research, 65, 118–126. 10.1111/pre.12170 [DOI] [Google Scholar]
- Falkenberg, L. J. , Russell, B. D. , & Connell, S. D. (2012). Stability of strong species interactions resist the synergistic effects of local and global pollution in kelp forests. PLoS One, 7, e33841. 10.1371/journal.pone.0033841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, P. A. , Gaitán‐Espitia, J. D. , Leal, P. P. , Schmid, M. , Revill, A. T. , & Hurd, C. L. (2020). Nitrogen sufficiency enhances thermal tolerance in habitat‐ forming kelp: Implications for acclimation under thermal stress. Nature Scientific Reports, 10, 1–12. 10.1038/s41598-020-60104-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, P. A. , Navarro, J. M. , Camus, C. , Torres, R. , & Buschmann, A. H. (2021). Effect of environmental history on the habitat‐forming kelp Macrocystis pyrifera responses to ocean acidification and warming: A physiological and molecular approach. Scientific Reports, 11, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández, P. A. , Roleda, M. Y. , & Hurd, C. L. (2015). Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynthesis Research, 124, 293–304. 10.1007/s11120-015-0138-5 [DOI] [PubMed] [Google Scholar]
- Filbee‐Dexter, K. , & Scheibling, R. E. (2014). Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series, 495, 1–25. 10.3354/meps10573 [DOI] [Google Scholar]
- Filbee‐Dexter, K. , & Wernberg, T. (2018). Rise of turfs: A new battlefront for globally declining kelp forests. Bioscience, 68, 64–76. 10.1093/biosci/bix147 [DOI] [Google Scholar]
- Filbee‐Dexter, K. , & Wernberg, T. (2020). Substantial blue carbon in overlooked Australian kelp forests. Scientific Reports, 10, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee‐Dexter, K. , Wernberg, T. , Fredriksen, S. , Norderhaug, K. M. , & Pedersen, M. F. (2019). Arctic kelp forests: Diversity, resilience and future. Global and Planetary Change, 172, 1–14. 10.1016/j.gloplacha.2018.09.005 [DOI] [Google Scholar]
- Filbee‐Dexter, K. , Wernberg, T. , Grace, S. P. , Thormar, J. , Fredriksen, S. , Narvaez, C. N. , Feehan, C. J. , & Norderhaug, K. M. (2020). Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports, 10, 13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher, W. J. (1987). Interactions among subtidal Australian Sea urchins, gastropods, and algae: Effects of experimental removals. Ecological Monographs, 57, 89–109. [Google Scholar]
- Fraser, C. I. (2012). Is bull‐kelp kelp? The role of common names in science. New Zealand Journal of Marine and Freshwater Research, 46, 279–284. [Google Scholar]
- Fredersdorf, J. , Müller, R. , Becker, S. , Wiencke, C. , & Bischof, K. (2009). Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia, 160, 483–492. 10.1007/s00442-009-1326-9 [DOI] [PubMed] [Google Scholar]
- Gaitán‐Espitia, J. D. , Hancock, J. R. , Padilla‐Gamiño, J. L. , Rivest, E. B. , Blanchette, C. A. , Reed, D. C. , & Hofmann, G. E. (2014). Interactive effects of elevated temperature and pCO2 on early‐life‐history stages of the giant kelp Macrocystis pyrifera . Journal of Experimental Marine Biology and Ecology, 457, 51–58. 10.1016/j.jembe.2014.03.018 [DOI] [Google Scholar]
- Gao, X. , Choi, H. G. , Park, S. K. , Kim, J. H. , Yu, O. H. , & Nam, K. W. (2019). Sporophytic photosynthesis and gametophytic growth of the kelp Ecklonia stolonifera affected by ocean acidification and warming. Aquaculture Research, 50, 856–861. 10.1111/are.13957 [DOI] [Google Scholar]
- Gao, X. , Endo, H. , Nagaki, M. , & Agatsuma, Y. (2017). Interactive effects of nutrient availability and temperature on growth and survival of different size classes of Saccharina japonica (Laminariales, Phaeophyceae). Phycologia, 56, 253–260. 10.2216/16-91.1 [DOI] [Google Scholar]
- Gao, X. , Endo, H. , Taniguchi, K. , & Agatsuma, Y. (2013). Combined effects of seawater temperature and nutrient condition on growth and survival of juvenile sporophytes of the kelp Undaria pinnatifida (Laminariales; Phaeophyta) cultivated in northern Honshu, Japan. Journal of Applied Phycology, 25, 269–275. 10.1007/s10811-012-9861-x [DOI] [Google Scholar]
- Gauci, C. , Bartsch, I. , Martins, N. , & Liesner, D. (2022). Cold thermal priming of Laminaria digitata (Laminariales, Phaeophyceae) gametophytes enhances gametogenesis and thermal performance of sporophytes. Frontiers in Marine Science, 9, 1–12. 10.3389/fmars.2022.862923 35450130 [DOI] [Google Scholar]
- Gilson, A. R. , Smale, D. A. , & O'Connor, N. (2021). Ocean warming and species range shifts affect rates of ecosystem functioning by altering consumer–resource interactions. Ecology, 102, e03341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordillo, F. J. L. , Carmona, R. , Viñegla, B. , Wiencke, C. , & Jiménez, C. (2016). Effects of simultaneous increase in temperature and ocean acidification on biochemical composition and photosynthetic performance of common macroalgae from Kongsfjorden (Svalbard). Polar Biology, 39, 1993–2007. 10.1007/s00300-016-1897-y [DOI] [Google Scholar]
- Gorra, T. R. , Garcia, S. C. , Langhans, M. R. , Hoshijima, U. , Estes, J. A. , Raimondi, P. T. , Tinker, M. T. , Kenner, M. C. , & Kroeker, K. J. (2022). Southeast Alaskan kelp forests: Inferences of process from large‐scale patterns of variation in space and time. Proceedings of the Royal Society B: Biological Sciences, 289, 20211697. 10.1098/rspb.2021.1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich, S. , Valentin, K. , Frickenhaus, S. , & Wiencke, C. (2015). Temperature and light interactively modulate gene expression in Saccharina latissima (Phaeophyceae). Journal of Phycology, 51, 93–108. 10.1111/jpy.12255 [DOI] [PubMed] [Google Scholar]
- Hoffman, J. R. , Hansen, L. J. , & Klinger, T. (2003). Interactions between UV radiation and temperature limit inferences from single‐factor experiments. Journal of Phycology, 39, 268–272. [Google Scholar]
- Hollarsmith, J. A. , Buschmann, A. H. , Camus, C. , & Grosholz, E. D. (2020). Varying reproductive success under ocean warming and acidification across giant kelp (Macrocystis pyrifera) populations. Journal of Experimental Marine Biology and Ecology, 522, 151247. [Google Scholar]
- Hughes, T. P. , Kerry, J. T. , Connolly, S. R. , Baird, A. H. , Eakin, C. M. , Heron, S. F. , Hoey, A. S. , Hoogenboom, M. O. , Jacobson, M. , Liu, G. , & Pratchett, M. S. (2019). Ecological memory modifies the cumulative impact of recurrent climate extremes. Nature Climate Change, 9, 40–43. [Google Scholar]
- Hurd, C. L. , Harrison, P. J. , Bischof, K. , & Lobban, C. S. (2014). Seaweed ecology and physiology (2nd ed.). Cambridge University Press. [Google Scholar]
- Iñiguez, C. , Carmona, R. , Lorenzo, M. R. , Niell, F. X. , Wiencke, C. , & Gordillo, F. J. L. (2016). Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula . Marine Biology, 163, 1–18. 10.1007/s00227-016-3024-6 [DOI] [Google Scholar]
- IPCC . (2022). Climate Change 2022: Impacts, adaptation, and vulnerability. In Pörtner H.‐O., Roberts D. C., Tignor M., Poloczanska E. S., Mintenbeck K., Alegría A., Craig M., Langsdorf S., Löschke S., Möller V., Okem A., & Rama B. (Eds.), Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. [Google Scholar]
- Jackson, M. C. , Pawar, S. , & Woodward, G. (2021). The temporal dynamics of multiple stressor effects: From individuals to ecosystems. Trends in Ecology and Evolution, 36, 402–410. 10.1016/j.tree.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Johnson, C. R. , Banks, S. C. , Barrett, N. S. , Cazassus, F. , Dunstan, P. K. , Edgar, G. J. , Frusher, S. D. , Gardner, C. , Haddon, M. , Helidoniotis, F. , Hill, K. L. , Holbrook, N. J. , Hosie, G. W. , Last, P. R. , Ling, S. D. , Melbourne‐Thomas, J. , Miller, K. , Pecl, G. T. , Richardson, A. J. , … Taw, N. (2011). Climate change cascades: Shifts in oceanography, species' ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology, 400, 17–32. 10.1016/j.jembe.2011.02.032 [DOI] [Google Scholar]
- Kang, J. W. , & Chung, I. K. (2018). The interactive effects of elevated CO2 and ammonium enrichment on the physiological performances of Saccharina japonica (Laminariales, Phaeophyta). Ocean Science Journal, 53, 487–497. [Google Scholar]
- Karsten, U. (2007). Research note: Salinity tolerance of Arctic kelps from Spitsbergen. Phycological Research, 55, 257–262. 10.1111/j.1440-1835.2007.00468.x [DOI] [Google Scholar]
- King, N. G. , McKeown, N. J. , Smale, D. A. , Wilcockson, D. C. , Hoelters, L. , Groves, E. A. , Stamp, T. , & Moore, P. J. (2019). Evidence for different thermal ecotypes in range Centre and trailing edge kelp populations. Journal of Experimental Marine Biology and Ecology, 514–515, 10–17. 10.1016/j.jembe.2019.03.004 [DOI] [Google Scholar]
- King, N. G. , Wilcockson, D. C. , Webster, R. , Smale, D. A. , Hoelters, L. S. , & Moore, P. J. (2018). Cumulative stress restricts niche filling potential of habitat‐forming kelps in a future climate. Functional Ecology, 32, 288–299. 10.1111/1365-2435.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyomoto, S. , Tagawa, M. , Nakamura, Y. , Horii, T. , Watanabe, S. , Tozawa, T. , Yatsuya, K. , Yoshimura, T. , & Tamaki, A. (2013). Decrease of abalone resources with disappearance of macroalgal beds around the Ojika Islands, Nagasaki, southwestern Japan. Journal of Shellfish Research, 32, 51–58. [Google Scholar]
- Krause‐Jensen, D. , Lavery, P. , Serrano, O. , Marba, N. , Masque, P. , & Duarte, C. M. (2018). Sequestration of macroalgal carbon: The elephant in the blue carbon room. Biology Letters, 14, 20180236. 10.1098/rsbl.2018.0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker, K. J. , Kordas, R. L. , & Harley, C. D. G. (2017). Embracing interactions in ocean acidification research: Confronting multiple stressor scenarios and context dependence. Biology Letters, 13, 20160802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumhansl, K. A. , Okamoto, D. K. , Rassweiler, A. , Novak, M. , Bolton, J. J. , Cavanaugh, K. C. , Connell, S. D. , Johnson, C. R. , Konar, B. , Ling, S. D. , Micheli, F. , Norderhaug, K. M. , Pérez‐Matus, A. , Sousa‐Pinto, I. , Reed, D. C. , Salomon, A. K. , Shears, N. T. , Wernberg, T. , Anderson, R. J. , … Byrnes, J. E. K. (2016). Global patterns of kelp forest change over the past half‐century. Proceedings of the National Academy of Sciences of the United States of America, 113, 13785–13790. 10.1073/pnas.1606102113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumhansl, K. A. , & Scheibling, R. E. (2011). Detrital production in Nova Scotian kelp beds: Patterns and processes. Marine Ecology Progress Series, 421, 67–82. 10.3354/meps08905 [DOI] [Google Scholar]
- Kumagai, N. H. , García Molinos, J. , Yamano, H. , Takao, S. , Fujii, M. , & Yamanaka, Y. (2018). Ocean currents and herbivory drive macroalgae‐to‐coral community shift under climate warming. Proceedings of the National Academy of Sciences of the United States of America, 115, 8990–8995. 10.1073/pnas.1716826115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal, P. P. , Hurd, C. L. , Sander, S. G. , Armstrong, E. , Fernández, P. A. , Suhrhoff, T. J. , & Roleda, M. Y. (2018). Copper pollution exacerbates the effects of ocean acidification and warming on kelp microscopic early life stages. Scientific Reports, 8, 1–13. 10.1038/s41598-018-32899-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenton, T. M. , Rockström, J. , Gaffney, O. , Rahmstorf, S. , Richardson, K. , Steffen, W. , & Schellnhuber, H. J. (2019). Climate tipping points – Too risky to bet against. Nature, 575, 592–595. [DOI] [PubMed] [Google Scholar]
- Ling, S. D. , Johnson, C. R. , Frusher, S. D. , & Ridgway, K. (2009). Overfishing reduces resilience of kelp beds to climate‐driven catastrophic phase shift. Proceedings of the National Academy of Sciences of the United States of America, 106, 22341–22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly, S. C. , Raven, J. A. , & Johnston, A. M. (1992). Discrimination between 12C and 13C by marine plants. Oecologia, 91, 481–492. [DOI] [PubMed] [Google Scholar]
- Martins, C. D. L. , Lhullier, C. , Ramlov, F. , Simonassi, J. C. , Gouvea, L. P. , Noernberg, M. , Maraschin, M. , Colepicolo, P. , Hall‐Spencer, J. M. , & Horta, P. A. (2014). Seaweed chemical diversity: An additional and efficient tool for coastal evaluation. Journal of Applied Phycology, 26, 2037–2045. 10.1007/s10811-014-0361-z [DOI] [Google Scholar]
- McPherson, M. L. , Finger, D. J. I. , Houskeeper, H. F. , Bell, T. W. , Carr, M. H. , Rogers‐Bennett, L. , & Kudela, R. M. (2021). Large‐scale shift in the structure of a kelp forest ecosystem co‐occurs with an epizootic and marine heatwave. Communications Biology, 4, 1–9. 10.1038/s42003-021-01827-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda, R. J. , Coleman, M. A. , Tagliafico, A. , Rangel, M. S. , Mamo, L. T. , Barros, F. , & Kelaher, B. P. (2019). Invasion‐mediated effects on marine trophic interactions in a changing climate: Positive feedbacks favour kelp persistence. Proceedings of the Royal Society B: Biological Sciences, 286, 20182866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, R. , Wiencke, C. , & Bischof, K. (2008). Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the Arctic and North Sea. Climate Research, 37, 203–213. 10.3354/cr00762 [DOI] [Google Scholar]
- Nunes, J. , Mccoy, S. J. , Findlay, H. S. , Hopkins, F. E. , Kitidis, V. , Rayner, L. , Widdicombe, S. , & Queiro, A. M. (2016). Two intertidal, non‐calcifying macroalgae (Palmaria palmata and Saccharina latissima) show complex and variable responses to short‐term CO2 acidification. ICES Journal of Marine Science, 73, 887–896. [Google Scholar]
- Orr, J. A. , Rillig, M. C. , & Jackson, M. C. (2022). Similarity of anthropogenic stressors is multifaceted and scale dependent. Natural Sciences, 2, 1–8. 10.1002/ntls.20210076 [DOI] [Google Scholar]
- Orr, J. A. , Vinebrooke, R. D. , Jackson, M. C. , Kroeker, K. J. , Kordas, R. L. , Mantyka‐Pringle, C. , van den Brink, P. J. , de Laender, F. , Stoks, R. , Holmstrup, M. , Matthaei, C. D. , Monk, W. A. , Penk, M. R. , Leuzinger, S. , Schäfer, R. B. , & Piggott, J. J. (2020). Towards a unified study of multiple stressors: Divisions and common goals across research disciplines. Proceedings of the Royal Society B: Biological Sciences, 287, 20200421. 10.1098/rspb.2020.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paar, M. , de la Vega, C. , Horn, S. , Asmus, R. , & Asmus, H. (2019). Kelp belt ecosystem response to a changing environment in Kongsfjorden (Spitsbergen). Ocean and Coastal Management, 167, 60–77. 10.1016/j.ocecoaman.2018.09.003 [DOI] [Google Scholar]
- Paine, E. R. , Schmid, M. , Boyd, P. W. , Diaz‐Pulido, G. , & Hurd, C. L. (2021). Rate and fate of dissolved organic carbon release by seaweeds: A missing link in the coastal ocean carbon cycle. Journal of Phycology, 57, 1375–1391. [DOI] [PubMed] [Google Scholar]
- Paine, R. T. (1980). Food webs: Linkage, interaction strength and community infrastructure. Journal of Animal Ecology, 49, 667–685. 10.2307/4220 [DOI] [Google Scholar]
- Pedersen, M. F. , Nejrup, L. B. , Fredriksen, S. , Christie, H. , & Norderhaug, K. M. (2012). Effects of wave exposure on population structure, demography, biomass and productivity of the kelp Laminaria hyperborea . Marine Ecology Progress Series, 451, 45–60. 10.3354/meps09594 [DOI] [Google Scholar]
- Perkol‐Finkel, S. , & Airoldi, L. (2010). Loss and recovery potential of marine habitats: An experimental study of factors maintaining resilience in subtidal algal forests at the Adriatic Sea. PLoS One, 5, 10791–10802. 10.1371/journal.pone.0010791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirós, A. M. , Stephens, N. , Widdicombe, S. , Tait, K. , McCoy, S. J. , Ingels, J. , Rühl, S. , Airs, R. , Beesley, A. , Carnovale, G. , Cazenave, P. , Dashfield, S. , Hua, E. , Jones, M. , Lindeque, P. , McNeill, C. L. , Nunes, J. , Parry, H. , Pascoe, C. , … Somerfield, P. J. (2019). Connected macroalgal‐sediment systems: Blue carbon and food webs in the deep coastal ocean. Ecological Monographs, 89, 1–21. 10.1002/ecm.1366 [DOI] [Google Scholar]
- Rogers‐Bennett, L. , & Catton, C. A. (2019). Marine heat wave and multiple stressors tip bull kelp forest to sea urchin barrens. Scientific Reports, 9, 15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roleda, M. Y. (2009). Photosynthetic response of Arctic kelp zoospores exposed to radiation and thermal stress. Photochemical & Photobiological Sciences, 8, 1302–1312. 10.1039/b901098j [DOI] [PubMed] [Google Scholar]
- Roleda, M. Y. , Morris, J. N. , McGraw, C. M. , & Hurd, C. L. (2012). Ocean acidification and seaweed reproduction: Increased CO2 ameliorates the negative effect of lowered pH on meiospore germination in the giant kelp Macrocystis pyrifera (Laminariales, Phaeophyceae). Global Change Biology, 18, 854–864. 10.1111/j.1365-2486.2011.02594.x [DOI] [Google Scholar]
- Russell, B. D. , Thompson, J. A. I. , Falkenberg, L. J. , & Connell, S. D. (2009). Synergistic effects of climate change and local stressors: CO2 and nutrient‐driven change in subtidal rocky habitats. Global Change Biology, 15, 2153–2162. [Google Scholar]
- Scherner, F. , Bonomi Barufi, J. , & Horta, P. A. (2012). Photosynthetic response of two seaweed species along an urban pollution gradient: Evidence of selection of pollution‐tolerant species. Marine Pollution Bulletin, 64, 2380–2390. 10.1016/j.marpolbul.2012.08.012 [DOI] [PubMed] [Google Scholar]
- Schmid, M. , Fernández, P. A. , Gaitán‐Espitia, J. D. , Virtue, P. , Leal, P. P. , Revill, A. T. , Nichols, P. D. , & Hurd, C. L. (2020). Stress due to low nitrate availability reduces the biochemical acclimation potential of the giant kelp Macrocystis pyrifera to high temperature. Algal Research, 47, 101895. 10.1016/j.algal.2020.101895 [DOI] [Google Scholar]
- Schmid, M. , Guihéneuf, F. , Nitschke, U. , & Stengel, D. B. (2021). Acclimation potential and biochemical response of four temperate macroalgae to light and future seasonal temperature scenarios. Algal Research, 54, 102190. [Google Scholar]
- Shukla, P. , & Edwards, M. S. (2017). Elevated pCO2 is less detrimental than increased temperature to early development of the giant kelp, Macrocystis pyrifera (Phaeophyceae, Laminariales). Phycologia, 56, 638–648. 10.2216/16-120.1 [DOI] [Google Scholar]
- Smale, D. A. , Burrows, M. T. , Moore, P. , O'Connor, N. , & Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: A northeast Atlantic perspective. Ecology and Evolution, 3, 4016–4038. 10.1002/ece3.774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starko, S. , Neufeld, C. J. , Gendall, L. , Timmer, B. , Campbell, L. , Yakimishyn, J. , Druehl, L. , & Baum, J. K. (2022). Microclimate predicts kelp forest extinction in the face of direct and indirect marine heatwave effects. Ecological Applications, 32, e2673. [DOI] [PubMed] [Google Scholar]
- Steneck, R. S. , Graham, M. H. , Bourque, B. J. , Corbett, D. , Erlandson, J. M. , Estes, J. A. , & Tegner, M. J. (2002). Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environmental Conservation, 29, 436–459. 10.1017/S0376892902000322 [DOI] [Google Scholar]
- Takao, S. , Kumagai, N. H. , Yamano, H. , Fujii, M. , & Yamanaka, Y. (2015). Projecting the impacts of rising seawater temperatures on the distribution of seaweeds around Japan under multiple climate change scenarios. Ecology and Evolution, 5, 213–223. 10.1002/ece3.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburello, L. , Ravaglioli, C. , Mori, G. , Nuccio, C. , & Bulleri, F. (2019). Enhanced nutrient loading and herbivory do not depress the resilience of subtidal canopy forests in Mediterranean oligotrophic waters. Marine Environmental Research, 149, 7–17. 10.1016/j.marenvres.2019.05.015 [DOI] [PubMed] [Google Scholar]
- United Nations Environment Programme, & Norwegian Blue Forests Network . (2023). Into the blue: Securing a sustainable future for kelp forests . https://wedocs.unep.org/20.500.11822/42255
- van der Loos, L. M. , Schmid, M. , Leal, P. P. , McGraw, C. M. , Britton, D. , Revill, A. T. , Virtue, P. , Nichols, P. D. , & Hurd, C. L. (2019). Responses of macroalgae to CO2 enrichment cannot be inferred solely from their inorganic carbon uptake strategy. Ecology and Evolution, 9, 125–140. 10.1002/ece3.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenhof, R. J. , Dworjanyn, S. A. , Champion, C. , & Coleman, M. A. (2022). Grazing and recovery of kelp gametophytes under ocean warming. Frontiers in Marine Science, 9, 866136. [Google Scholar]
- Vergés, A. , Doropoulos, C. , Malcolm, H. A. , Skye, M. , Garcia‐Pizá, M. , Marzinelli, E. M. , Campbell, A. H. , Ballesteros, E. , Hoey, A. S. , Vila‐Concejo, A. , & Bozec, Y. M. (2016). Long‐term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences of the United States of America, 113, 13791–13796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vye, S. R. , Emmerson, M. C. , Arenas, F. , Dick, J. T. A. , & O'Connor, N. E. (2015). Stressor intensity determines antagonistic interactions between species invasion and multiple stressor effects on ecosystem functioning. Oikos, 124, 1005–1012. 10.1111/oik.01583 [DOI] [Google Scholar]
- Wernberg, T. , Krumhansl, K. , Filbee‐Dexter, K. , & Pedersen, M. F. (2019). Status and trends for the world's kelp forests. In Sheppard C. (Ed.), World seas: An environmental evaluation (pp. 57–78). Elsevier. [Google Scholar]
- Wilman, E. A. (2021). Kelp forests: Catastrophes, resilience, and management. Frontiers in Ecology and Evolution, 9, 674792. 10.3389/fevo.2021.674792 [DOI] [Google Scholar]
- Xiao, X. , De Bettignies, T. , Olsen, Y. S. , Agusti, S. , & Duarte, C. M. (2015). Sensitivity and acclimation of three canopy‐ forming seaweeds to UVB radiation and warming. PLoS One, 10, e0143031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
No data are associated with this paper.


