Significance
Sleep loss is a public health epidemic which impairs mood and well-being of billions of people over the world. However, sleep deprivation also induces a rapid and effective mood improvement in a subset of patients with depressive disorder. The amygdala is a pivotal brain region affected by depression. Here, we show that one night of total sleep deprivation enhanced amygdala connectivity to the anterior cingulate cortex which correlated with better mood in both healthy and depressed individuals. This study highlights the key role of amygdala–cingulate circuit in bad mood regulation in both healthy and clinical populations. Our findings might have implications for the development of fast and unique antidepressant interventions.
Keywords: sleep deprivation, mood, antidepressant effect, amygdala, functional connectivity
Abstract
Sleep loss robustly disrupts mood and emotion regulation in healthy individuals but can have a transient antidepressant effect in a subset of patients with depression. The neural mechanisms underlying this paradoxical effect remain unclear. Previous studies suggest that the amygdala and dorsal nexus (DN) play key roles in depressive mood regulation. Here, we used functional MRI to examine associations between amygdala- and DN-related resting-state connectivity alterations and mood changes after one night of total sleep deprivation (TSD) in both healthy adults and patients with major depressive disorder using strictly controlled in-laboratory studies. Behavioral data showed that TSD increased negative mood in healthy participants but reduced depressive symptoms in 43% of patients. Imaging data showed that TSD enhanced both amygdala- and DN-related connectivity in healthy participants. Moreover, enhanced amygdala connectivity to the anterior cingulate cortex (ACC) after TSD associated with better mood in healthy participants and antidepressant effects in depressed patients. These findings support the key role of the amygdala–cingulate circuit in mood regulation in both healthy and depressed populations and suggest that rapid antidepressant treatment may target the enhancement of amygdala–ACC connectivity.
Sleep is an essential physiological need and humans typically spend around one-third of their lives sleeping. However, sleep loss due to extended work hours and/or leisure pursuits has become a common problem in modern society (1). Without sleep, mood and emotion modulation abilities become markedly disrupted. A number of studies have reported associations between sleep problems and mood disorders such as depression, with some suggesting that poor sleep can increase the risk of new-onset depression and/or exacerbate mood disturbance (2, 3).
Although sleep loss is associated with mood deficits in healthy individuals, about half of patients with a major depressive disorder (MDD) can experience transient improvement in depressive symptoms after sleep deprivation (4–7). However, the neural substrates of antidepressant effects caused by sleep deprivation remain elusive. A better understanding of the neural changes underlying the effects of sleep deprivation on mood may provide insights into the mechanisms underlying mood changes in MDD and ultimately contribute to antidepressant treatment and depression prevention.
The development of resting-state-functional magnetic resonance imaging (rs-fMRI) has advanced our understanding of functional brain organization in both healthy and clinical populations (8). A number of prior studies have demonstrated that sleep loss affects resting-state brain connectivity. Across these networks, sleep deprivation was associated with connectivity changes in a broad range of resting-state networks, including the default mode network (DMN); attention network; limbic network; and the auditory, visual, and motor networks (9–14). Although these studies have provided converging evidence suggesting that sleep loss triggers changes in the brain’s intrinsic connectivity profile, it remains unclear which network changes are specifically associated with changes in mood after sleep loss.
Numerous functional brain imaging studies in depressive patients have demonstrated alterations in the limbic regions, particularly the amygdala and anterior cingulate cortex (ACC), across the onset, progress, remission, and recurrence of depression (15, 16). Specifically, rs-fMRI studies have shown significantly decreased connectivity between the amygdala and ACC in patients with depression (17–24). Moreover, increased connectivity between the amygdala and ACC was observed in depressed patients after antidepressant treatments (20, 22, 23, 25, 26). Interestingly, increased connectivity between the amygdala and rostral ACC was also observed in healthy individuals with total sleep deprivation (TSD) (13). Taken together, these studies suggest that amygdala–ACC connectivity changes may be a potential mechanism for the rapid antidepressant treatment effects of sleep deprivation.
The dorsal nexus (DN) is a medial frontal brain region associated with altered functional connectivity in depression (27) that is also modulated in response to antidepressant therapies (28, 29). Increased functional connectivity between the DN and the right dorsolateral prefrontal cortex (R.dlPFC) has been reported in healthy individuals after sleep deprivation, leading to the hypothesis that DN–R.dlPFC connectivity increases may be another potential mechanism for the antidepressant treatment effects of TSD (30).
In this study, we sought to examine whether the previously reported amygdala-based or DN–R.dlPFC connectivity changes were associated with mood changes after one night of TSD in healthy controls and patients with MDD using strictly controlled in-laboratory sleep deprivation and fMRI protocols. In experiment 1, 38 healthy individuals completed mood rating scale and fMRI scans at baseline after normal sleep (BS), following one night of TSD, and after recovery sleep (RS). In experiment 2, 30 patients with MDD rated their mood states and completed fMRI scans at BS, following one night of TSD, and after RS. We hypothesized that amygdala-based and DN–R.dlPFC connectivity changes would be associated with mood alternations after TSD in both healthy individuals and depressed patients.
Results
Experiment 1: Demographic Details and Mood Changes after TSD in Healthy Individuals.
Fifty-four healthy participants were included in the present study. The TSD group (21 males/17 females, 33.5 ± 8.6 y) and the control group (8 males/8 females, 35.4 ± 9.5 y) did not differ significantly in terms of age, gender, race, education, or body mass index (P > 0.05; details are provided in SI Appendix, Table S1). In addition, the baseline levels of mood did not differ between groups (P > 0.05).
Participants underwent three rs-fMRI scanning sessions in a five-consecutive-day experiment in the laboratory (Fig. 1A). After a night of normal sleep on day 1, all participants had their first MRI scanning session in the morning of day 2 as the baseline. In the TSD group, participants had their second scanning session in the morning of day 3 after one night of TSD. After two nights of RS, participants had their third scanning session in the morning of day 5. Participants assigned to the control group slept every night and had their first, second, and third scanning sessions in the mornings of days 2, 3, and 5, respectively. All participants completed a 37-item shortened version of the Profile of Mood States (POMS-37) scale (31) every 2 h during days 2 to 5.
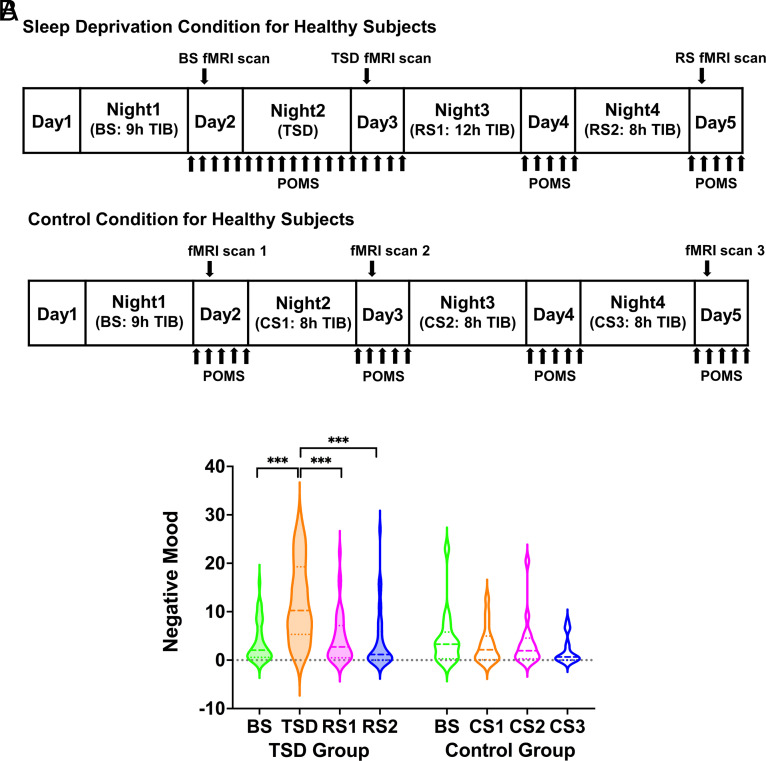
Fig. 1.
(A) Protocol of experiment 1. The first scanning session took place in the morning of day 2 after 9 h BS. Participants were then randomized to either a TSD or a control condition. In the TSD condition, participants with one night of TSD had the second scanning session in the morning of day 3. After two nights of RS, participants had the third scanning session in the morning of day 5. In the control condition, participants with ≥8 h CS every night had the second and third scanning sessions in the morning of days 3 and 5, respectively. All participants completed the POMS scale every 2 h during day 2 to 5. (B) POMS-negative mood score across the four consecutive days for the TSD group and the control group. Negative mood worsened significantly following one night of TSD compared to BS and improved significantly after one night of RS. The dashed lines represent the median values, and the dotted lines represent the quartiles. TIB: time in bed; BS: baseline sleep; TSD: total sleep deprivation; RS: recovery sleep; CS: control sleep; POMS: Profile of Mood States. ***P < 0.001.
To examine whether negative or positive mood differed across days in the TSD group, we conducted one-way repeated-measures analyses of variance (ANOVA) and post-hoc tests. Negative mood and positive mood significantly worsened following one night of TSD compared to BS (P < 0.05, Fig. 1B and SI Appendix, Fig. S1). After one night of RS, both mood states improved compared to after TSD (P < 0.05) and returned to BS (P > 0.05).
The same analyses were applied to the control group. No significant differences in mood states across days were found in the control group (P > 0.05, Fig. 1B and SI Appendix, Fig. S1).
Increased Amygdala–ACC Connectivity Was Associated with Less Mood Worsening after TSD in Healthy Individuals.
Amygdala-based functional connectivity across the whole brain was used to evaluate differences between TSD and BS in the TSD group. We found significant increases in amygdala connectivity to bilateral ACC (Fig. 2 A and B and SI Appendix, Table S2) after TSD compared to BS. The ACC cluster survived whole-brain cluster-level family-wise error (FWE) correction at P < 0.001 and voxel-level FWE correction at P < 0.05. We also found significant decreases in amygdala connectivity to the left parietal lobule and right precentral/postcentral gyrus (SI Appendix, Table S2). The strength of amygdala connectivity to the above regions after TSD differed significantly from BS and RS (Fig. 2B and SI Appendix, Fig. S2 A and B). Amygdala connectivity with these regions did not differ among BS, control sleep 1, and control sleep 3 in the control group (Fig. 2B and SI Appendix, Fig. S2 A and B).
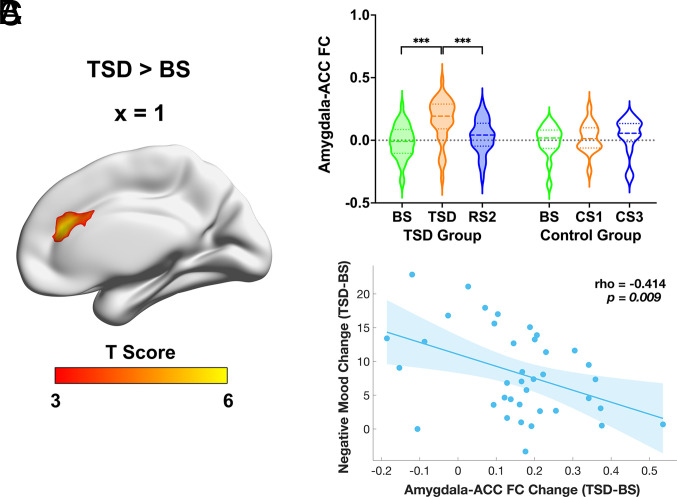
Fig. 2.
(A) Increased FC of the amygdala with ACC following TSD relative to BS in the healthy TSD group. Paired t tests were performed to examine the differences in FC of amygdala with all other voxels in the brain between BS and TSD, with age, gender, and head motion as covariates. The threshold was set at whole-brain FWE-corrected P < 0.001 at a cluster level, with cluster size larger than 30 voxels. (B) FC between the amygdala and ACC increased significantly after TSD, while it decreased significantly after RS2 in the TSD group. There were no significant amygdala–ACC connectivity changes among corresponding days in the control group. The dashed lines represent the median values, and the dotted lines represent the quartiles. (C) The scatter plot shows a significant correlation between increased amygdala–ACC connectivity and reduced mood worsening from baseline to TSD while controlling for age, gender, head motion, and baseline negative mood (rho = −0.414, P < 0.01). FC: functional connectivity; ACC: anterior cingulate cortex; BS: baseline sleep; TSD: total sleep deprivation; RS: recovery sleep; CS: control sleep; FWE: family-wise error. ***P < 0.001.
We computed partial correlations to estimate the association between amygdala-related connectivity changes and negative mood changes pre- to post-TSD, with age, gender, head motion, and baseline negative mood as covariates. We found a significant correlation between amygdala–ACC connectivity increases and less mood worsening (i.e., better mood) (rho = −0.414, P < 0.01; Fig. 2C). However, mood worsening did not correlate significantly with changes in amygdala connectivity with the other brain regions (i.e., left parietal lobule and right precentral/postcentral gyrus) (respective rho = −0.163 and 0.046, P > 0.05; SI Appendix, Fig. S2 C and D).
Increased DN–R.dlPFC Connectivity Did Not Correlate with Mood Worsening after TSD in Healthy Individuals.
To examine whether DN–R.dlPFC connectivity differed across the three scanning sessions in the TSD group, we conducted one-way repeated-measures ANOVA and post-hoc tests while controlling for head motion. DN connectivity increased significantly with previously reported (30) right middle frontal gyrus (R.MFG, P < 0.05) but not superior frontal gyrus (R.SFG, P > 0.05) after TSD compared to BS, while connectivity of both pairings decreased significantly after RS (P < 0.05, Fig. 3 B and C). The same analyses were applied to the control group. Neither DN–R.MFG nor DN–R.SFG connectivity differed significantly across the three scanning sessions in the control group (Fig. 3 B and C).
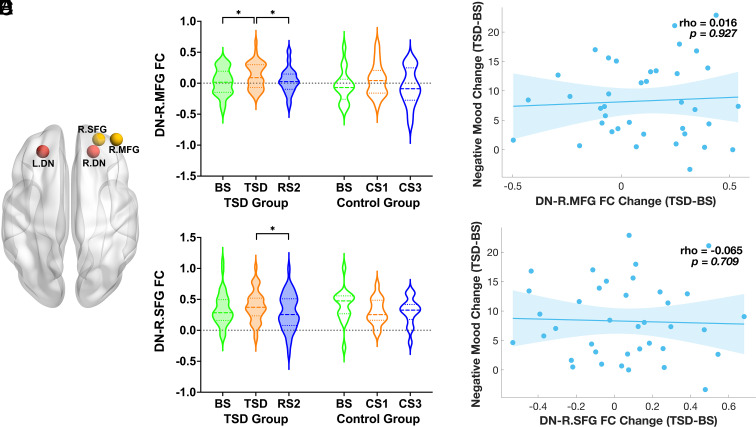
Fig. 3.
(A) Location of bilateral DN seed (red), R.MFG and R.SFG (yellow) regions of interest. DN connectivity with (B) R.MFG and (C) R.SFG increased after TSD, while decreased following RS2 in the TSD group. There were no significant DN–R.MFG/SFG connectivity changes among corresponding days in the control group. The dashed lines represent the median values, and the dotted lines represent the quartiles. After TSD, neither (D) DN–R.MFG nor (E) DN–R.SFG connectivity increases correlated significantly with mood worsening while controlling for age, gender, head motion, and baseline negative mood (respective rho = 0.016 and −0.065, P > 0.05). DN: dorsal nexus; R.MFG: right middle frontal gyrus; R.SFG: right superior frontal gyrus; FC: functional connectivity; BS: baseline sleep; TSD: total sleep deprivation; RS: recovery sleep; CS: control sleep. *P < 0.05.
Next, we computed partial correlations to estimate the association between DN–R.dlPFC connectivity changes and negative mood changes pre- to post-TSD, with age, gender, head motion, and baseline negative mood as covariates. No significant correlations were found between DN–R.dlPFC connectivity increases and mood worsening in the TSD group (respective rho = 0.016 and −0.065, P > 0.05, Fig. 3 D and E).
Experiment 2: Demographic Details and Improved Depressive Mood after TSD in Patients with MDD.
Thirty depressed participants were included in the data analyses (7 males/23 females, 34 ± 9.9 y). Similar to the protocol of experiment 1, all participants underwent three rs-fMRI scanning sessions in a five-consecutive-day experiment in the laboratory (SI Appendix, Fig. S3A). After two nights of normal sleep on day 1 and day 2, participants had their first MRI scanning session in the morning of day 3 as the baseline. After one night of TSD, participants had their second scanning session in the morning of day 4. After one night of RS, participants had their third scanning session in the morning of day 5. All participants completed the depressive mood assessment during days 3 to 5.
Thirteen out of 30 (43%) depressed participants experienced mood improvement, and the remaining 17 participants experienced mood worsening or no change after one night of TSD (Fig. 4A).
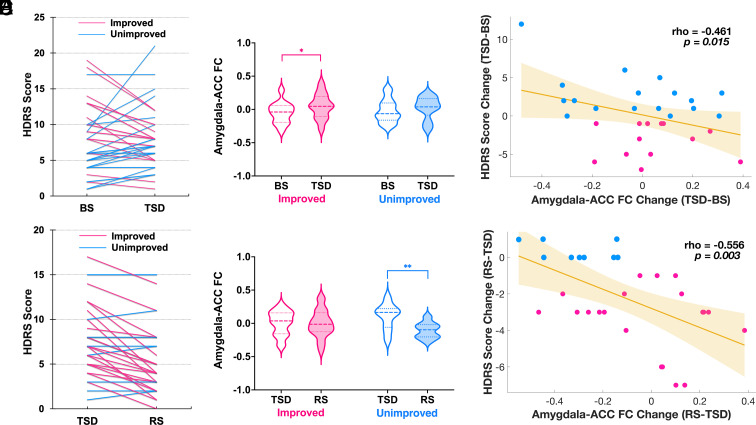
Fig. 4.
(A) Thirteen out of 30 depressed participants experienced mood improvement (i.e., decreases of HDRS score) after TSD compared to BS, and the remaining seventeen participants experienced mood nonimprovement (i.e., worsening or no change). (B) Comparison of amygdala–ACC FC between BS and TSD in improved mood group (pink) and unimproved mood group (blue). Amygdala–ACC FC increases after TSD were evident in patients with improved mood (P < 0.05), while the increases in patients with unimproved mood did not reach statistical significance. The dashed lines represent the median values, and the dotted lines represent the quartiles. (C) Scatter plot showing a significant correlation between increased amygdala–ACC FC and improved depressive mood from BS to TSD while controlling for age, gender, head motion, and baseline depressive mood (rho = −0.461, P < 0.05). (D) Twenty out of 29 patients experienced mood improvement after RS, rather than returning to depressed status. (E) Comparison of amygdala–ACC FC between TSD and RS in improved mood group (pink) and unimproved mood group (blue). Amygdala–ACC FC decreases after RS were evident in patients with unimproved mood (P < 0.01), while the decreases in patients with improved mood did not reach statistical significance. The dashed lines represent the median values, and the dotted lines represent the quartiles. (F) The scatter plot shows a significant correlation between decreased amygdala–ACC FC and mood worsening from TSD to RS while controlling for age, gender, and head motion (rho = −0.556, P < 0.01). BS: baseline sleep; TSD: total sleep deprivation; RS: recovery sleep; ACC: anterior cingulate cortex; FC: functional connectivity; HDRS: Hamilton Rating Scale for Depression; Pink lines and dots: improved mood; Blue lines and dots: unimproved mood. *P < 0.05, **P < 0.01.
Increased Amygdala–ACC Connectivity Was Associated with Mood Improvement after TSD in Patients with MDD.
FMRI analysis for experiment 2 focused on connectivity changes identified in experiment 1. We performed a 2 (sleep condition: BS vs. TSD) × 2 (mood change: improved vs. unimproved) mixed-factorial ANOVA and observed a significant increase of amygdala–ACC connectivity after TSD in depressed participants with improved mood (P < 0.05; Fig. 4B), but found no difference in depressed participants with unimproved mood. Connectivity of DN–R.MFG and DN–R.SFG also increased but did not reach statistical significance in depressed participants with either improved or unimproved mood (SI Appendix, Fig. S3 B and C).
Next, we computed partial correlations to estimate associations between connectivity (i.e., DN–R.SFG, DN–R.MFG, and amygdala–ACC) changes and depressive mood changes pre- to post-TSD, with age, gender, head motion, and baseline depressive mood as covariates. Improved depressive mood was correlated with amygdala–ACC connectivity increases (rho = −0.461, P < 0.05, Fig. 4C), but not connectivity increases of DN–R.MFG or DN–R.SFG (respective rho = −0.074 and 0.218, P > 0.05; SI Appendix, Fig. S3 D and E).
Increased Amygdala–ACC Connectivity Was Associated with Mood Improvement from TSD to RS in Patients with MDD.
Data from 29 depressed participants were available for the RS analyses (7 males/22 females, 34.3 ± 9.9 y). Twenty out of 29 (69%) participants experienced mood improvement, and the remaining nine participants experienced mood worsening or no change after one night of RS (Fig. 4D).
Based on the significant correlational findings between amygdala–ACC connectivity and depressive mood pre- to post-TSD, we performed a two (sleep condition: TSD vs. RS) × two (mood change: improved vs. unimproved) mixed-factorial ANOVA and found a significant decrease of amygdala–ACC connectivity in patients with unimproved mood after RS compared to TSD (Fig. 4E, P < 0.01), while the decrease did not reach statistical significance in patients with improved mood.
We then computed partial correlations to estimate the association between amygdala–ACC connectivity changes and depressive mood changes after RS, with age, gender, and head motion as covariates. There was a significant correlation between increased amygdala–ACC connectivity and improved depressive mood from TSD to RS (Fig. 4F, rho = −0.556, P < 0.01). However, no such correlation was found in healthy participants from TSD to RS.
Discussion
In this study, we evaluated changes in mood and functional connectivity networks after TSD in both healthy participants and patients with MDD. Consistent with previous studies, we found a worsening of mood in healthy participants while an improvement in mood in a subset of MDD patients after TSD. After one night of TSD, both amygdala–ACC and DN–R.dlPFC resting-state connectivity increased in healthy participants. Of note, amygdala–ACC connectivity increased significantly in patients with improved mood but not unimproved mood. However, only increased amygdala–ACC connectivity but not DN–R.dlPFC connectivity associated significantly with better mood in both healthy participants and patients with MDD. This association remained significant after RS in patients with MDD. Our findings suggest that amygdala–ACC network connectivity may reflect the neural resilience to mood disruption after sleep loss and thus may be a potential target for antidepressant interventions. This study evaluates mood and functional connectivity due to TSD in both healthy and depressed participants. A strength of this study was the use of a strictly controlled in-laboratory TSD protocol including both serial MRI scanning and mood ratings.
Consistent with previous studies (13, 30), we found increased resting-state connectivity of amygdala–ACC, DN–R.MFG, and DN–R.SFG after TSD. Among these brain networks, only increased connectivity between the amygdala and ACC was associated with better mood, which generalized to both healthy and depressed individuals. The amygdala has a well-documented role in the processing of emotionally salient information (32), while the ACC is thought to monitor and regulate emotional conflicts through a top-down pathway (33, 34). The increased amygdala–ACC connectivity following TSD may reflect enhanced cortical regulation over limbic mood disturbance (35). The ACC region defined functionally in the current study is located in the genual part of ACC (BA32) and overlaps largely with the genual ACC region identified by pioneering work from Wu and colleagues (36, 37) using fluorodeoxyglucose-positron emission tomography (FDG-PET) to compare depressed responders to TSD with depressed nonresponders. Specifically, responders’ overactivation of genual ACC at baseline was normalized after TSD, whereas nonresponders and normal controls showed no change in genual ACC activity. The genual ACC, connected to the amygdala, has been suggested to be of particular importance in the control of mood (38).
A previous study has suggested that the DN is a key node mediating the hyper-connectivity between the affective network (AN), DMN, and cognitive control network in patients with MDD and may underlie the concurrent occurrence of heterogeneous symptoms in depression such as excessive self-focus, affective dysregulation, hyperarousal, and impaired cognition (39). Additional studies further reported that SSRI antidepressant medication (28) and ketamine (29) reduced DN-related connectivity with regions in the DMN and AN. It has been speculated that DN–R.dlPFC connectivity increases might underlie the antidepressant effects of TSD (30). However, while we observed DN–R.dlPFC connectivity increases in depressed individuals after TSD, we did not find significant associations between increased DN–R.dlPFC connectivity and improved mood in either healthy or depressed individuals, suggesting that DN connectivity might not be the neural substrates of mood changes after sleep deprivation.
While interindividual differences in vulnerability to SD-associated cognitive impairments have been reported (40–42), the neural mechanisms underlying this phenotypic vulnerability are not well characterized. One study categorized individuals as vulnerable or resilient to SD based on impairments in working memory, finding that individuals classified as vulnerable showed altered activity in the prefrontal cortex, posterior parietal cortex, and motor area, with no changes in the “resilient” group (43). Another study found response-specific differences in brain activation after SD between groups classified by inhibitory efficiency (44). Notably, our study suggests that interindividual variability in the neural response to SD is associated with mood changes. In the current study, increases in amygdala–ACC connectivity after TSD were associated with less mood disruption in healthy participants and better mood improvement in depressed patients. As noted in the introduction, increased amygdala–ACC connectivity has been associated with mood improvement after antidepressant treatments with selective serotonin reuptake inhibitors or cognitive behavioral therapy (22, 25, 26). Our findings of increased amygdala–ACC functional connectivity correlating with mood improvement in MDD patients after TSD suggest that amygdala–cingulate connectivity change may be a common neural pathway of different antidepressant interventions, even though the effects of TSD are short lived.
Another potential explanation for the interindividual differences in vulnerability/resilience to TSD may lie in the duration of the rapid eye movement (REM) sleep. Major depression has been associated with abnormalities in REM sleep, in particular a reduced latency to REM sleep after sleep onset (45, 46). Excess REM sleep would diminish noradrenaline, resulting in decreased binding to the ɑ-2 receptor in the medial frontal lobes (47, 48), comprised of the ACC and the medial prefrontal cortex (mPFC). As a result, there would be impaired top-down control of the amygdala (49). Thus, mood deterioration observed in MDD may be driven by excess REM sleep which impairs ACC/mPFC–amygdala emotional function (2). The absence of REM sleep with TSD may improve top-down control of the amygdala to improve ACC/mPFC–amygdala emotional function, resulting in an antidepressant effect. Combined with our study, the evidence indicates potential treatments for depression by enhancing amygdala–ACC connectivity.
Our study has some limitations. First, the sample size of depressed patients was smaller than that of healthy participants, albeit larger than that used in previous studies (25–27). Therefore, these findings should be viewed as preliminary and need to be replicated in a larger cohort of depressed patients. Second, our sample included more female than male depressed patients. This may reflect the fact that MDD is more prevalent in females compared to males (50, 51). A considerably larger sample size would be needed to evaluate gender differences in the association between functional connectivity changes and mood changes after sleep loss in MDD. Third, the protocols in study 1 and study 2 were slightly different. Study 1 had no adaptation night and two nights of RS, while study 2 had an adaptation night and only one night of RS. The association between increased amygdala–ACC connectivity and improved mood remained significant after RS in patients with MDD but not in healthy individuals. It is possible that the second night of RS in healthy individuals could have impacted recovery imaging findings, though the focus of the current study is TSD rather than RS. Fourth, the correlational analyses that we performed between functional connectivity metrics and mood ratings could not provide evidence of causality. Future studies could combine sleep deprivation with neuromodulation techniques (e.g., transcranial magnetic stimulation and transcranial direct current stimulation) to test causal circuit–based mechanisms of mood changes by inducing appropriate changes in the identified brain networks. In addition, despite conducting multiple experiments to validate our findings, accurately extracting signals from small brain structures, such as the amygdala, remains challenging due to potential partial volume effects, which might impact the interpretation of our results. Hence, there is a need for future studies, particularly those employing advanced MRI image techniques (e.g., seven Tesla MRI) that offer improved signal-to-noise ratio and higher spatial resolution to confirm our findings further. Finally, this study focused on resting-state connectivity and did not examine task-related brain activation or connectivity changes after sleep deprivation such as during the processing of negative stimuli (52–54). Future studies should combine both resting-state and task-based fMRI to obtain more comprehensive understanding of the antidepressant effects of sleep deprivation on brain function and mood.
In summary, this study suggests that enhanced connectivity in the amygdala–ACC circuitry may be a common neural network signature underlying the paradoxical mood changes in response to sleep deprivation in healthy individuals and patients with MDD. Our findings support the key role of amygdala–ACC coupling as a potential explanation for the rapid antidepressant response in MDD patients and effective regulation of bad mood in healthy individuals after sleep loss.
Materials and methods
Participants.
In experiment 1, 75 healthy participants were recruited and randomized to either the TSD or control group. Four participants withdrew, resulting in 54 subjects in the TSD group and 17 subjects in the control group. After excluding subjects who showed drowsiness (n = 7 in TSD group) or excessive head motion (n = 9 in TSD group; n = 1 in control group) during scans following BS, TSD, or RS, 38 TSD subjects (21 males/17 females, 33.5 ± 8.6 y) and 16 controls (8 males/8 females, 35.4 ± 9.5 y) were included in the final sample. In experiment 2, 38 patients with MDD were recruited, but two of them were excluded for not finishing imaging scans or mood ratings. We also excluded patients for drowsiness (n = 4) or excessive head motion (n = 2), which resulted in 30 MDD patients in the final sample (7 males/23 females, 34 ± 9.9 y). Depression was defined as a score ≥18 on the 17-item version of Hamilton Rating Scale for Depression (HDRS-17) (55, 56). Patients were free of all antidepressant medications for 2 wk (4 wk for fluoxetine) prior to starting the study. Patients who had a history of unipolar features or comorbid anxiety disorder were included. Normality was established on the basis of a clinical history and interview, blood and urine tests, and a physical examination.
Inclusion criteria for all participants were as follows: 1) age between 21 and 50 y; 2) body mass index within 15% of normal; 3) no shift work, transmeridian travel, or irregular sleep/wake routine in the past 60 d; 4) stable, normally timed sleep–wake cycle as established by interview, 1-week daily sleep log, and 1-wk wrist actigraphy evidence of sleep defined by: a. habitual nocturnal sleep duration between 6 h and 9 h; b. habitual morning awakening between 06:00 and 08:00; and c. no evidence of habitual napping; 5) no sleep disorder (e.g., narcolepsy, apnea) other than insomnia, as established by sleep history, actigraphy, pulse oximetry, and pre-SD sleep testing; 6) no history of psychiatric disorders such as bipolar disorder and schizophrenia (other than MDD for the patient group), neurological disorders (e.g., epilepsy), infectious diseases (e.g., HIV infection, urinary tract infection), disorders of the gastrointestinal system (e.g., peptic ulcer, inflammatory bowel disease), autoimmune disorders (e.g., systematic lupus erythematosus, rheumatoid arthritis, fibromyalgia), neoplastic diseases, or endocrine/metabolic diseases (e.g., thyroid disease, diabetes); 7) no history of alcohol or drug abuse in the past year based on the SCID and urine toxicology screen; 8) not a current smoker; 9) no acute or chronic, debilitating medical conditions such as hypertension, fever, or thyroid disease; 10) no concurrent medication; 11) no caffeine intake one week before the laboratory study; 12) no metallic implants, pacemakers, mechanical heart valve, tattoos, or history of working in metal workshops; 13) not claustrophobic, or intolerant of the scanner environment; and 14) able to comprehend English (given that all scales were in this language) and provide informed consent.
The study was approved by the Institutional Review Board of the University of Pennsylvania and was carried out in accordance with the Declaration of Helsinki. All participants provided a written informed consent before enrollment and were compensated for participation.
Overall Design.
The two experiments in this study were performed in the laboratory at the Clinical Translational Research Center at the Hospital of the University of Pennsylvania for five consecutive days. All participants underwent three rs-fMRI scanning sessions.
In experiment 1, participants arrived at the laboratory in the afternoon of day 1 and were provided 9 h time in bed (TIB) from 21:30 to 06:30. A baseline scan took place in the morning of day 2. The participants were then randomized to either a TSD or a control condition. For the TSD condition, the participants were not allowed any sleep during night 2 and underwent the second scan in the morning of day 3. After RS for two consecutive nights on day 3 (12 h TIB, from 18:30 to 06:30) and day 4 (8 h TIB, from 22:30 to 06:30), the third scan took place in the morning of day 5. For the control condition, participants were allowed 8 h TIB every night on day 2, day 3, and day 4 (from 22:30 to 06:30) and underwent the second and third scans in the morning of days 3 and 5, respectively.
Participants completed 30-min neurobehavioral test batteries every 2 h during the protocol when they were awake and not participating in fMRI scans during days 2 to 5. The test battery included the Forward and Backward Digit Span Task, the Psychomotor Vigilance Task, the Digit Symbol Substitution Task, the Karolinska Sleepiness Scale, and the POMS scale.
In experiment 2, participants came to the laboratory at about 17:00 on day 1 and were allowed 9 h TIB (from 22:00 to 07:00) on night 1 and night 2. The participants finished the HDRS-NOW for depressive mood assessment after breakfast on days 2 to 5. On day 3, participants underwent the first scan in the morning and were not allowed any sleep during night 3. In the morning of day 4, the participants underwent the second scan and were allowed 12 h TIB (from 19:00 to 07:00) on night 4 for recovery from the 36 h period of TSD. All participants underwent the third scan in the morning of day 5.
For both experiments 1 and 2, the scanning time for the three scanning sessions was kept constant (experiment 1: from 07:00 to 10:00; experiment 2: from 08:00 to 10:00) to avoid potential time-of-day differences. Participants were instructed to lie still in the scanner with their eyes looking at a crosshair on the screen, thinking of nothing in particular during the scan. A MR-comparable video camera was used to monitor participants’ eyes to confirm that they did not fall asleep in the scanner.
Participants stayed in the lab throughout the study and were monitored by trained staff at all times. The participants were provided meals and snacks at regular, prespecified hours. Caffeine, alcohol, and exercises were prohibited on the experimental days, while reading, television, video games, and other sedentary activities were permitted between test bouts (completed on a computer). Participants wore a wrist actigraphy and ambulatory electroencephalography and electrocardiography recording equipment for 24 h intervals. The light levels were kept constant at <50 lx during scheduled wakefulness and <1 lx during scheduled sleep periods. Ambient temperature was maintained at 22 °C to 24 °C.
Functional MRI Data Acquisition, Preprocessing, and Analysis.
All MRIs for both experiments were performed in a 3T Siemens Trio system (Siemens Medical Systems, Erlangen, Germany) with an eight-channel head coil array. A multiband gradient-echo EPI sequence was used for resting-state blood oxygen level–dependent (BOLD) fMRI data acquisition with the following parameters: TR = 2 s, TE = 24 ms, flip angle = 90°, FOV = 220 × 220 mm, matrix = 64 × 64 × 36, slice thickness = 4 mm, 36 interleaved slices with no gap. A total of 210 images were acquired within 7 min’ scanning for each participant. After functional scans, a high-resolution (0.8 × 0.8 × 0.8 mm) T1-weighted anatomical scan was conducted using a standard 3D magnetization-prepared rapid-acquisition gradient echo sequence for structural reference.
Imaging data preprocessing was performed using the Statistical Parametric Mapping software (SPM 12, Wellcome Department of Cognitive Neurology, UK) and the DPABI V6.2 toolbox (57) implemented in Matlab 2021a (Math Works, Natick, MA, USA). The workflow of the preprocessing is summarized as follows: i) removal of the ten initial volumes of the BOLD signals to achieve signal stabilization; ii) realignment of functional images for motion correction; iii) slice timing correction; iv) exclusion of participants (n = 9 for TSD group and n = 1 for control group in experiment 1; n = 2 in experiment 2) with high levels of gross motion (i.e., >0.3 mm mean framewise displacement). Data volumes that were associated with more than 0.3 mm mean framewise displacement were censored (i.e., despiking) to reduce the influence of motion artifacts; v) removal of nine confounding signals (including six head motion parameters and signals from the global mean, white matter and cerebral spinal fluid) as well as the temporal derivative, quadratic term, and temporal derivatives of each quadratic term (24 regressors total); vi) coregistration of functional images to their respective T1 images; vii) alignment of the coregistered images to the standard Montreal Neurological Institute (MNI) space (i.e., normalization); viii) removal of linear trends; ix) temporal filtering of time series between 0.01 and 0.08 Hz to reduce low-frequency drift and physiological high-frequency respiratory and cardiac noise; and x) image smoothing using an isotropic Gaussian kernel with a full width at a half-maximum of 8 mm. The combination of iv) and v) has been shown to be a good strategy to remove motion-related variance in studies of functional connectivity (58).
DN–R.dlPFC Functional Connectivity Changes Following TSD.
The regions of interest (ROIs) for the (DN; Talairach coordinates: left: −24, 35, 28, right: 18, 34, 29) and two areas in the R.dlPFC [i.e., right middle frontal gyrus (R.MFG; Talairach coordinates: 38, 46, 18) and right superior frontal gyrus (R.SFG; Talairach coordinates: 23, 46, 18)] were defined as spheres with a radius of 6 mm centered at specific coordinates according to previous works (27, 30). The bilateral DN seed combining the left and right DN seeds was created using SPM. After converting these Talairach coordinates into MNI coordinates, an interregional (ROI to ROI) correlation analysis was performed to investigate the functional connectivity between DN and these two ROIs. The individual correlation matrix was then transformed to a z-score matrix by applying Fisher’s r-to-z transformation {z = 0.5 Ln [(1 + r)/(1 − r)]}. Since head motion (i.e., mean framewise displacement) was greater after TSD than that of BS (P < 0.01) and RS P < 0.05) in both healthy TSD subjects and depressed patients, we regressed out head motion metrics (i.e., mean framewise displacement) from these z-transformed correlation coefficients to get unstandardized residuals for subsequent analyses. DN–R.dlPFC connectivity changes following TSD were computed by subtracting residuals for correlation coefficients (z) after BS from that after TSD. In experiment 1, DN–R.dlPFC connectivity differences among the three scanning sessions were examined using one-way repeated-measures ANOVA and post-hoc tests [Statistical Package for the Social Sciences (SPSS), version 25, IBM, Chicago, IL]. In experiment 2, DN–R.dlPFC connectivity differences between patients with different mood states, and between different sleep states, as well as the sleep x mood interaction effect, were examined by performing a two (sleep condition: BS vs. TSD) × two (mood change: improved vs. unimproved) mixed-factorial ANOVA.
Amygdala-Based Functional Connectivity Changes Following TSD.
For experiment 1, a seed-based analysis was performed to investigate functional connectivity between bilateral amygdala and the rest of the brain. The amygdala seed was defined by a bilateral amygdala gray matter mask from an automated anatomical labeling region of interest library (59). Mean BOLD time series from the amygdala seed were extracted and correlated with time series of all other voxels within the brain to create whole-brain Pearson’s correlation coefficient maps. Fisher’s r-to-z transformation {z = 0.5 Ln [(1 + r)/(1 − r)]} was then applied on these maps to improve the normality of the correlation coefficients. The z-transformed correlation maps were then entered into a group-level analysis using paired t tests to examine functional connectivity differences between BS and TSD, with age, gender, and head motion metrics as covariates. The threshold was set at whole-brain FWE-corrected P < 0.001 at a cluster level, with cluster size larger than 30 voxels. Clusters showing significant connectivity differences were then defined as ROIs. Correlation coefficients (z) between amygdala seed and these ROIs were computed by extracting time series from the z-transformed correlation maps. We regressed out head motion metrics from these z-transformed correlation coefficients to get unstandardized residuals for subsequent analyses.
For experiment 2, the bilateral amygdala seed was defined using the same method as previously stated. The ACC was defined by drawing a sphere centered at MNI coordinate (0, 21, 36) with a 6-mm radius. Based on findings of experiment 1, we computed and z-transformed correlation coefficients between bilateral amygdala and ACC in experiment 2. We then regressed out head motion metrics from these z-transformed correlation coefficients to get unstandardized residuals for subsequent analyses. The main effects of sleep and mood, and their interaction effect on amygdala–ACC connectivity, were examined by performing a two (sleep condition: BS vs. TSD and TSD vs. RS, respectively) × two (mood change: improved vs. unimproved) mixed-factorial ANOVA. For both experiments 1 and 2, functional connectivity changes between amygdala and ROIs from BS to TSD were computed by subtracting residuals for correlation coefficients (z) after BS from that after TSD. From TSD to RS, functional connectivity changes between amygdala and ROIs were computed by subtracting residuals after TSD from that after RS.
Mood Score Acquisition and Statistical Analysis Following TSD.
In experiment 1, the POMS-37 scale measured mood on six subscales, including tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, confusion- bewilderment, and vigor-activity (31). Healthy participants were instructed to rate 37 adjectives on a five-point scale (0 = not at all, 4 = extremely). The total negative mood score was acquired by summing the former five subscales, and the vigor subscale score represented the positive mood score (60, 61). Averaged negative mood score for each day was used in the following analyses. Negative mood changes following TSD were computed by subtracting the negative mood score after BS from that after TSD. Negative mood changes following RS were computed by subtracting the negative mood score after TSD from that after RS. Mood differences across the four consecutive days (day 2, day 3, day 4, day 5) were examined by performing one-way repeated-measures ANOVA and post-hoc tests (SPSS 25, IBM, Chicago, IL, USA).
In experiment 2, as has been a standard practice in prior studies of the antidepressant effects of SD (62), a modified version of the HDRS-17 (called the HDRS-NOW) that removes items 4, 5, 6, and 16 was used each morning because the time frame for these items was not appropriate for daily ratings. Depressive mood differences between BS and TSD were investigated by performing paired t tests. Depressive mood changes following TSD were computed by subtracting the depressive mood score after BS from that after TSD. Depressive mood changes following RS were computed by subtracting the depressive mood score after TSD from that after RS.
Correlations between Functional Connectivity Changes and Mood Changes.
Spearman’s correlation analyses were performed to examine the associations between functional connectivity changes and negative/depressive mood changes from BS to TSD while controlling for age, gender, head motion, and baseline negative/depressive mood (SPSS 25, IBM, Chicago, IL, USA). For correlation analyses between functional connectivity changes and mood changes from TSD to RS, baseline mood was not included as a covariate.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This research was supported in part by the grants from the NIH (NIH R01 HL102119, R01 MH107571, R01 NS113889, and R01 CTRC UL1RR024134), and a pilot grant from the Institute for Aging of the University of Pennsylvania. The funders had no role in the study design, data collection and analysis, data interpretation, writing of the manuscript, or the decision to submit the article for publication.
Author contributions
P.G., M.Y., D.F.D., and H.R. designed research; Y.C., P.G., T.M., P.Q., H.L., Z.F., S.X., J.R.G., H.B., and H.R. performed research; Y.C., M.Y., J.R., and Xiaocui Zhang analyzed data; and Y.C., P.G., M.Y., Y.D., J.R., H.S., J.X., E.B., N.G., M.B., M.E.T., Y.I.S., J.A.D., Xiaochu Zhang, and H.R. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Resting-state fMRI and behavioral data have been deposited in Open Science Framework (https://osf.io/ubhpx/) (63).
Supporting Information
References
- 1.Lockley S. W., et al. , Effect of reducing interns’ weekly work hours on sleep and attentional failures. N. Engl. J. Med. 351, 1829–1837 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Goldstein A. N., Walker M. P., The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 10, 679–708 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krause A. J., et al. , The sleep-deprived human brain. Nat. Rev. Neurosci. 18, 404–418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boland E. M., et al. , Meta-analysis of the antidepressant effects of acute sleep deprivation. J. Clin. Psychiatry 78, e1020–e1034 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Wu J. C., Bunney W. E., The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am. J. Psychiatry 147, 14–21 (1990). [DOI] [PubMed] [Google Scholar]
- 6.Hemmeter U.-M., Hemmeter-Spernal J., Krieg J.-C., Sleep deprivation in depression. Expert Rev. Neurother. 10, 1101–1115 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Wirz-Justice A., Van den Hoofdakker R. H., Sleep deprivation in depression: What do we know, where do we go? Biol. Psychiatry 46, 445–453 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Woodward N. D., Cascio C. J., Resting-state functional connectivity in psychiatric disorders. JAMA Psychiatry 72, 743–744 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Havas J. A., Parimal S., Soon C. S., Chee M. W. L., Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. Neuroimage 59, 1745–1751 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Wang Y., Liu H., Hitchman G., Lei X., Module number of default mode network: Inter-subject variability and effects of sleep deprivation. Brain Res. 1596, 69–78 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Yeo B. T. T., Tandi J., Chee M. W. L., Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage 111, 147–158 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Shao Y., et al. , Decreased thalamocortical functional connectivity after 36 hours of total sleep deprivation: Evidence from resting state fMRI. PLoS One 8, e78830 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao Y., et al. , Altered resting-state amygdala functional connectivity after 36 hours of total sleep deprivation. PLoS One 9, e112222 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann T., et al. , The brain functional connectome is robustly altered by lack of sleep. Neuroimage 127, 324–332 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray E. A., Wise S. P., Drevets W. C., Localization of dysfunction in major depressive disorder: Prefrontal cortex and amygdala. Biol. Psychiatry 69, e43–e54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzagalli D. A., Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology 36, 183–206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand A., et al. , Activity and connectivity of brain mood regulating circuit in depression: A functional magnetic resonance study. Biol. Psychiatry 57, 1079–1088 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Anand A., Li Y., Wang Y., Lowe M. J., Dzemidzic M., Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. Neuroimaging 171, 189–198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lui S., et al. , Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry 168, 642–648 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Chen C.-H., et al. , Functional coupling of the amygdala in depressed patients treated with antidepressant medication. Neuropsychopharmacology 33, 1909–1918 (2008). [DOI] [PubMed] [Google Scholar]
- 21.Satterthwaite T. D., et al. , Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivity. Mol. Psychiatry 21, 894–902 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straub J., et al. , Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. J. Affect. Disord. 209, 135–139 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y., et al. , Functional impairment-based segmentation of anterior cingulate cortex in depression and its relationship with treatment effects. Hum. Brain Mapp. 42, 4035–4047 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Q., He Y., Depression, neuroimaging and connectomics: A selective overview. Biol. Psychiatry 77, 223–235 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Anand A., et al. , Antidepressant effect on connectivity of the mood-regulating circuit: An fMRI study. Neuropsychopharmacology 30, 1334–1344 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Anand A., Li Y., Wang Y., Gardner K., Lowe M. J., Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An fMRI study. J. Neuropsychiatry Clin. Neurosci. 19, 274–282 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheline Y. I., Price J. L., Yan Z., Mintun M. A., Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. U.S.A. 107, 11020–11025 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCabe C., et al. , SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol. Psychiatry 16, 592–594 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Scheidegger M., et al. , Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7, e44799 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosch O. G., et al. , Sleep deprivation increases dorsal nexus connectivity to the dorsolateral prefrontal cortex in humans. Proc. Natl. Acad. Sci. U.S.A. 110, 19597–19602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shacham S., A shortened version of the profile of mood states. J. Pers. Assess. 47, 305–306 (1983). [DOI] [PubMed] [Google Scholar]
- 32.Zald D. H., The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 41, 88–123 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Kerns J. G., Anterior cingulate conflict monitoring and adjustments in control. Science 303, 1023–1026 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Etkin A., Egner T., Kalisch R., Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray R. D., Zald D. H., Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 36, 479–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu J. C., Chad J., Buchsbaum M. S., Bunney E., Effect of sleep deprivation on brain metabolism of depressed patients. Am. J. Psychiatry 149, 538–543 (1992). [DOI] [PubMed] [Google Scholar]
- 37.Wu J., et al. , Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am. J. Psychiatry 156, 1149–1158 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Drevets W. C., Savitz J., Trimble M., The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 13, 663–681 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norbury R., Mannie Z., Cowen P. J., Imaging vulnerability for depression. Mol. Psychiatry 16, 1067–1068 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Tkachenko O., Dinges D. F., Interindividual variability in neurobehavioral response to sleep loss: A comprehensive review. Neurosci. Biobehav. Rev. 89, 29–48 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Brieva T. E., Casale C. E., Yamazaki E. M., Antler C. A., Goel N., Cognitive throughput and working memory raw scores consistently differentiate resilient and vulnerable groups to sleep loss. Sleep 44, zsab197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casale C. E., Yamazaki E. M., Brieva T. E., Antler C. A., Goel N., Raw scores on subjective sleepiness, fatigue, and vigor metrics consistently define resilience and vulnerability to sleep loss. Sleep 45, zsab228 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mu Q., et al. , Decreased cortical response to verbal working memory following sleep deprivation. Sleep 28, 55–67 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Chuah Y. M. L., Venkatraman V., Dinges D. F., Chee M. W. L., The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J. Neurosci. 26, 7156–7162 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Armitage R., Sleep and circadian rhythms in mood disorders. Acta Psychiatr. Scand. Suppl. 115, 104–115 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D., REM sleep dysregulation in depression: State of the art. Sleep Med. Rev. 17, 377–390 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Aston-Jones G., Cohen J. D., An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Blier P., Briley M., The noradrenergic symptom cluster: Clinical expression and neuropharmacology. Neuropsychiatr. Dis. Treat. 7, 15–20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramos B. P., Arnsten A. F. T., Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 113, 523–536 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hankin B. L., Abramson L. Y., Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychol. Bull. 127, 773–96 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Parker G., Brotchie H., Gender differences in depression. Int. Rev. psychiatry 22, 429–436 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Chuah L. Y. M., et al. , Sleep deprivation and interference by emotional distracters. Sleep 33, 1305–1313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoo S., Gujar N., Hu P., Jolesz F. A., Walker M. P., The human emotional brain without sleep–a prefrontal-amygdala disconnect. Curr. Biol. 17, 1–8 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Motomura Y., et al. , Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One 8, e56578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamilton M., A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams J. B. W., A structured interview guide for the hamilton depression rating scale. Arch. Gen. Psychiatry 45, 742–747 (1988). [DOI] [PubMed] [Google Scholar]
- 57.Yan C. G., Di Wang X., Zuo X. N., Zang Y. F., DPABI: Data processing analysis for (Resting-State) brain imaging. Neuroinformatics 14, 339–351 (2016). [DOI] [PubMed] [Google Scholar]
- 58.Ciric R., et al. , Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 154, 174–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tzourio-Mazoyer N., et al. , Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Novita Indra E., Sukarmin Y., Swasta Budayati E., Widiyanto M., Mood state profile as overtraining predictors: Considering gender and two different class types. Adv. Soc. Sci. Educ. Humanit. Res. 278, 534–537 (2019). [Google Scholar]
- 61.Aghababa A., et al. , Effects of COVID-19 on physical activity and mood in the middle-aged people: Concerns and strategies. Turkish J. Sport. Med. 57, 38–43 (2022). [Google Scholar]
- 62.Leibenluft E., Moul D. E., Schwartz P. J., Madden P. A., Wehr T. A., A clinical trial of sleep deprivation in combination with antidepressant medication. Psychiatry Res. 46, 213–227 (1993). [DOI] [PubMed] [Google Scholar]
- 63.Chai Y., et al. , Enhanced amygdala-cingulate connectivity associates with better mood in both healthy and depressive individuals after sleep deprivation. Open Science Framework. https://osf.io/ubhpx/. Accessed 9 May 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Resting-state fMRI and behavioral data have been deposited in Open Science Framework (https://osf.io/ubhpx/) (63).