Significance
Graphene nanoribbons (GNRs) carry unique characteristics due to their limited domain width and rich edge configurations. Yet, the capability of mass-producing them is the key to their wide range of applications. In this context, an inexpensive freezing–rolling–capillary compression strategy is developed, to rapidly yield GNRs at a kilogram scale. Intriguingly, the interlay spacing of GNRs can be readily tailored, representing a critical advance in the field of carbon nanomaterials. Consequently, an array of GNR nanocomposites containing heteroatoms; metal single atoms; and 0D, 1D, and 2D nanomaterials in-situ intercalated within the GNR matrix, are created, demonstrating their appealing utility in energy conversion and storage, including electrocatalysis, batteries, and supercapacitors, among other areas.
Keywords: graphene nanoribbon, rapid mass production, tunable interlayer spacing, functional nanofiller-dispersed GNR nanocomposites, energy conversion and storage
Abstract
Graphene nanoribbons (GNRs) are widely recognized as intriguing building blocks for high-performance electronics and catalysis owing to their unique width-dependent bandgap and ample lone pair electrons on both sides of GNR, respectively, over the graphene nanosheet counterpart. However, it remains challenging to mass-produce kilogram-scale GNRs to render their practical applications. More importantly, the ability to intercalate nanofillers of interest within GNR enables in-situ large-scale dispersion and retains structural stability and properties of nanofillers for enhanced energy conversion and storage. This, however, has yet to be largely explored. Herein, we report a rapid, low-cost freezing–rolling–capillary compression strategy to yield GNRs at a kilogram scale with tunable interlayer spacing for situating a set of functional nanomaterials for electrochemical energy conversion and storage. Specifically, GNRs are created by sequential freezing, rolling, and capillary compression of large-sized graphene oxide nanosheets in liquid nitrogen, followed by pyrolysis. The interlayer spacing of GNRs can be conveniently regulated by tuning the amount of nanofillers of different dimensions added. As such, heteroatoms; metal single atoms; and 0D, 1D, and 2D nanomaterials can be readily in-situ intercalated into the GNR matrix, producing a rich variety of functional nanofiller-dispersed GNR nanocomposites. They manifest promising performance in electrocatalysis, battery, and supercapacitor due to excellent electronic conductivity, catalytic activity, and structural stability of the resulting GNR nanocomposites. The freezing–rolling–capillary compression strategy is facile, robust, and generalizable. It renders the creation of versatile GNR-derived nanocomposites with adjustable interlay spacing of GNR, thereby underpinning future advances in electronics and clean energy applications.
Graphene sheets, graphene nanoribbons (GNRs), and three-dimensional (3D) graphene are a class of appealing nanomaterials for a myriad of practical applications owing to their exceptionally high mechanical stiffness and strength, outstanding electrical conductivity, and high optical transparency (1–3). GNRs, strips of graphene with mono- or oligo-layers and high length-to-width ratios, represent one of the most promising two-dimensional (2D) nanomaterials for next-generation nanoelectronics as GNRs possess a much enriched edge structure over graphene sheets. As such, it is more convenient to modulate their electronic structure by defects, doping, deformation, applied magnetics (or electric) field, adsorption, etc., thereby changing the properties of GNRs and rendering their applications in nanoelectronic devices (4–6). More importantly, they possess width-dependent electronic properties due to electron confinement within the limited dimension, resulting in semi-metal to semi-conductor transformation as the width decreases to below 10 nm (7, 8). Despite impressive advances in the synthesis of GNRs (e.g., longitudinal unzipping of carbon nanotubes (9), plasma etching of graphene sheets (10), epitaxial growth on patterned facet (11), electrospun polymeric nanofiber templating (12), and bottom-up molecular synthesis) (13–17), low-cost, large-scale production (>kilogram) of high-quality GNRs, a prerequisite for comprehensive investigation into their physicochemical properties and enabling their practical applications, has yet to be explored. More crucially, the ability to tune the interlayer spacing of GNR is key to tailoring the transport property of ionic species between GNRs in a variety of physicochemical processes (e.g., selective ion sieving (18), nanoconfined diffusive and electrokinetic ion transport (19), and optimized volumetric ion storage capacitance (20), as demonstrated in graphene sheets). It is notable that, owing to their compact and steady layer stacking structure, the interlayer distance regulation and thus inner surface functionalization of GNRs cannot be easily accessed, particularly for multilayered GNRs. Clearly, it remains a substantial challenge to open up GNR for introducing nanofillers.
Herein, we report a freezing–rolling–capillary compression route to mass-produce GNRs at a kilogram (i.e., >kg per batch) scale with tunable interlayer spacing that are capable of in-situ dispersing a set of nanofillers of interest in a rapid, low-cost, and controllable manner for high-performance electrochemical energy conversion and storage. The as-crafted GNRs attain an ultrahigh conductivity of 72,900 S/m, comparable to graphene nanosheets. Remarkably, the interlayer spacing of GNRs can by conveniently adjusted by the amount of introduced nanofillers functioning as the spacer during the freezing–rolling–capillary compression process. Consequently, a diversity of functional GNR nanocomposites composed of various nanofillers, including guest heteroatoms, single metal atoms, 0D SiO2 and molybdenum disulfide (MoS2) nanospheres, 1D polyaniline (PANi) nanorods, and 2D tin sulfide (SnS2) nanoflakes, in-situ intercalating into the GNR matrix are yielded. Such GNR-derived nanocomposites with uniform dispersion of nanofillers render effectively enhanced performances in electrocatalysis, battery, and supercapacitor as a result of their outstanding electronic conductivity, catalytic activity, and structural stability.
Results and Discussion
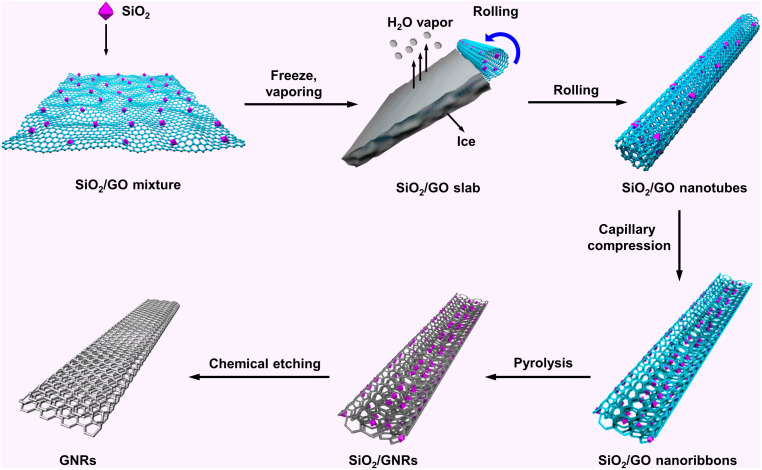
Fig. 1 depicts the freezing–rolling–capillary compression route to mass-produce GNRs. First, large-sized, monolayer graphene oxide (GO) nanosheets (SI Appendix, Fig. S1A) with an average size of 20 μm (SI Appendix, Fig. S1B) were chosen as the precursor matrix. The commercial SiO2 colloidal sol was first selected as a representative nanofiller and homogeneously mixed with the GO nanosheets. The mixture was then frozen in liquid nitrogen. Subsequently, it was placed in a lyophilizer and freeze-dried at 223 K at a barometric pressure of ~10 Pa (second panel in Fig. 1A; see Materials and Methods in SI Appendix). During the freeze-drying process, the vaporization of the ice slab can induce the self-rolling of GO sheets, forming the curled SiO2/GO nanocomposites with a tubular morphology (third panel; Fig. 1). To further depict the rolling process, a schematic for illustrating the rolling mechanism is shown in SI Appendix, Fig. S2. As the GO sheets are ultralight, the rolled nanotubes cannot be physically self-supported. Similar to hollow bowl–like carbon nanospheres induced by the capillary compression of hollow carbon nanospheres when the shell is too thin to provide sufficient mechanical support (21), the tubular topology of SiO2/GO was prone to collapse as a result of the soft graphene layer. The van der Waals interaction between different SiO2/GO layers promoted this tendency, thereby yielding ribbon-shaped SiO2/GO nanocomposites (fourth panel; Fig. 1). High-temperature pyrolysis in Ar/H2 (95:5 by volume) flow followed by etching with hydrofluoric acid to remove the oxygen-containing groups and the residual SiO2 nanoparticles, respectively, yielded GNRs with the engineered interlayer spacing (last panel; Fig. 1).
Fig. 1.
Schematic illustration of the freezing–rolling–capillary compression route to GNRs.
Fig. 2 A–C shows the SEM images of as-prepared GNRs, where the ribbon-shaped morphology is clearly evident, confirming the successful topological transformation from planar GO nanosheets to ribbon-like nanostructures of GNR. A close-up SEM image of GNRs (Fig. 2C) reveals their nanoribbon-shaped structure. The GNRs have an average width of 500 nm (SI Appendix, Fig. S3). A large length-to-width ratio was found (Fig. 2 A and B). Similar to that of the reduced GO nanosheets (SI Appendix, Fig. S4), a wrinkled morphology was formed on the surface of GNR (Fig. 2C), which can be ascribed to chemical reduction and capillary compression of the rolled GNRs that inevitably result in the shrinkage of planar carbon layers. TEM image of GNR shows a planar structure with an excellent uniformity (Fig. 2D). The atomic force microscopy image also shows a ribbon-like nanostructure of GNR (SI Appendix, Fig. S5) with a thickness of ~30 nm derived from the wrinkled morphology. In addition, the GNR achieves a high specific surface area of 532 m2/g with an average pore size distribution of 4 nm (SI Appendix, Fig. S6). Raman spectroscopy (633-nm laser excitation) was used to further evaluate the quality of GNRs (Fig. 2E). The relative intensity ratios of the D (1,325 cm−1) and G bands (1,590 cm−1) (ID/IG) are 0.82 and 1.01 for GNR and GO sheets, respectively (SI Appendix, Fig. S7). The decreased ID/IG value of GNRs compared to that of GO sheets indicates that the conjugated structure of carbon planes was recovered during chemical reduction with hydrazine hydrate. The existence of 2D band of GNRs further confirms a relatively high graphitization degree (22). The intensity mappings of the D, G, and 2D bands agree well with the surface morphology of GNRs (SI Appendix, Fig. S8). Subsequently, the field effect transistor (FET; Fig. 2F) was assembled to evaluate the electrical property of GNRs. Fig. 2G shows a representative scanning electron microscopy (SEM) image of a GNR FET, where a single GNR is bridged over the channel between a source electrode and a drain electrode. Fig. 2H displays an I–V characteristic of GNR FET at different voltages under ambient condition. The average electrical conductivity of GNR on SiO2 substrate was measured to be 72,900 S/m (from 10 similar devices). Fig. 2I presents the source–drain current (Ids) of GNR FET as a function of gate voltage at VD = 1 V. The drain current shows no obvious change during the gate voltage sweeping from −40 V to 40 V, confirming the highly conductive metallic/semi-metallic feature of the as-synthesized GNRs.
Fig. 2.
(A–C) Representative SEM images of GNRs at different magnifications. (D) TEM image of a GNR. (E) Raman spectrum of GNRs. (F) Schematic of the GNR FET with an SiO2/Si back gate. (G) SEM image of the GNR FET. (H) Electrical characteristics of the GNR FET at zero gate bias. (I) Source–drain current of the GNR FET as a function of gate voltage (VD = 1 V).
It is important to note that the SiO2 colloidal sol played a critical role in forming GNRs. The SEM images of SiO2/GO nanocomposite prepared by adding different amount of SiO2 sol into a 10 mL of GO solution are shown in SI Appendix, Fig. S9. Clearly, in the absence of SiO2 colloidal sol, pristine GO sheets were constrainedly rolled after freeze-drying; however, the formed graphene sheets are not uniform with a large width (SI Appendix, Fig. S9A). The ribbons were formed when the amount of SiO2 sol was increased to 0.5 mL, yet the majority of the as-produced SiO2/GO still exists in the sheet form (SI Appendix, Fig. S9B). In contrast, increasing the amount of SiO2 sol to 2.0 mL yielded a wrapped SiO2 layer that was too thick to render the freeze-drying-induced rolling of the SiO2/GO nanocomposites (SI Appendix, Fig. S9C). Consequently, 1 mL SiO2 sol was identified to be an optimal amount for producing uniform GNRs. These results clearly suggest that a moderate amount of introduced SiO2 sol is conducive to create high-quality GNRs. The GNRs possess a great structural stability after being chemically reduced, as evidenced by the intact morphology after ultrasonication in hexane (SI Appendix, Fig. S10).
As noted above, the production of GNRs can be readily scaled up to ~1 kg by employing an industrial food lyophilizer for the freeze-drying treatment of SiO2/GO nanocomposites. The optical paragraph and SEM images of such mass-produced GNRs are shown in SI Appendix, Fig. S11, where the same nanoribbon-like morphology was seen (SI Appendix, Fig. S11 B and C). Clearly, the GNRs can be conveniently produced at a kg scale via a rapid, low-cost freezing–rolling–capillary compression process.
It is well documented that freezing in liquid nitrogen creates large spacing between the overlapped GO layers, enabling them to accommodate versatile nanofillers and guest molecules (18, 23). In sharp contrast, it remains challenging to open up and tailor the interlayer spacing in GNRs as the layers are tightly stacked together. In our work, the interlayer spacing of as-prepared GNRs, however, can be facilely tuned by adjusting the amount of SiO2 colloidal sol nanoparticles introduced. The inserted SiO2 nanoparticles function as not only spacer but also mechanical support between each GO layer. As such, the stacked graphene layers can be effectively separated, leading to expanded interlayer spacing after chemical removal of the incorporated SiO2 nanoparticles. Fig. 3 A–C shows the TEM images of SiO2/GNRs nanocomposites (after pyrolysis yet prior to hydrofluoric acid (HF) etching) prepared with a different amount of SiO2 nanoparticles introduced (i.e., 0.5, 1.0, and 1.6 mL, respectively), clearly displaying the nanoribbon-shaped morphology (SI Appendix, Figs. S12–S14), where the SiO2 nanoparticles were effectively wrapped by the GNRs without any dissociated aggregates. After etching away SiO2 nanoparticles by HF, GNRs were successfully yielded, as evidenced by the TEM images in Fig. 3 D–F. Fig. 3G schematically details the capillary compression of the rolled GNRs, followed by pyrolysis and HF etching to remove SiO2, yielding GNRs with tunable interlayer spacing. The interlayer spacing of GNRs regulated by the amount of SiO2 nanoparticles added was examined by X-ray diffraction (XRD). Fig. 3H shows the XRD patterns of characteristic (002) diffraction peaks of GNRs prepared with the addition of different amount of SiO2 sol nanoparticles. The GNRs were denoted according to the amount of SiO2 nanoparticles (functioning as spacer) introduced [i.e., GNRs (0.5 mL SiO2), GNRs (1.0 mL SiO2), and GNRs (1.6 mL SiO2)]. The GNRs (0.5 mL SiO2), GNRs (1.0 mL SiO2), and GNRs (1.6 mL SiO2) display board diffraction peaks located at 24.5°, 16.3°, and 9.8°, respectively, corresponding to the interlayer distances of 3.63, 5.41, and 9.04 Å. In contrast, the GNRs prepared from pristine GO nanosheets without SiO2 sol display a diffraction location of 25.7° with an interlayer spacing of 3.46 Å, close to those of pristine graphite (26.1° and 3.34 Å, respectively), as shown in Fig. 3H. SI Appendix, Fig. S15, depicts the tailoring of interlayer spacing of GNRs rendered by different amounts of the SiO2 nanofillers intercalated.
Fig. 3.
(A–C) TEM images of SiO2/GNRs nanocomposites prepared with the introduction of (A) 0.5, (B) 1.0, and (C) 1.6 mL of SiO2 sol nanoparticles. (D–F) The corresponding TEM images of GNRs in (A–C) after chemical reduction (i.e., pyrolysis) and removal of SiO2 sol nanoparticles. (G) Schematic illustration of the tunable interlayer distance within a GNR regulated by adding a proper amount of SiO2 sol nanoparticles to intercalate GNR. (H) XRD patterns of raw graphite, GNRs prepared without any SiO2, and GNRs with intercalation of 0.5, 1.0, and 1.6 mL SiO2 sol nanoparticles.
Hybridizing GNRs with nanofillers of interest is of key importance for their applications in energy conversion and storage. Notably, conventional routes to GNR nanocomposites are plagued by synthesizing GNRs first followed by subsequent introduction of functional nanofillers. As a result, nanofillers are only situated on the surface of GNR. In this context, the ability to in-situ sandwich nanofillers between the adjacent layers of GNR opens up a means of creating various functional GNR nanocomposites. On the basis of the XRD study as discussed above, it is clear that the interlayer distance within a GNR can be facilely regulated by the amount of nanofiller (i.e., spacer) introduced, suggesting the effectiveness of freezing–rolling–capillary compression strategy in yielding nanoparticle-hybridized GNR nanocomposites with tunable interlayer gaps, thereby rendering them with great potential for a broad range of applications as demonstrated below.
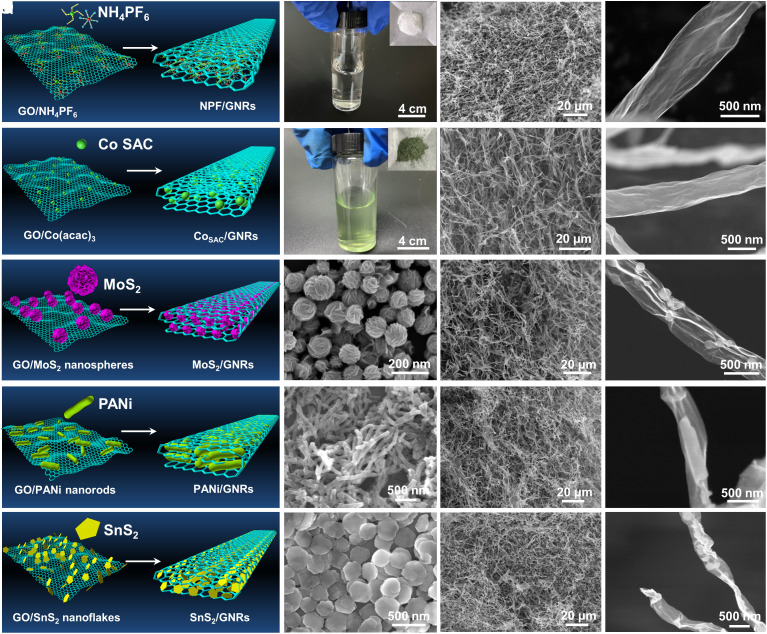
By capitalizing on the freezing–rolling–capillary compression strategy (Fig. 1), we in-situ produced a gamut of functional nanofiller-intercalated GNR nanocomposites. The nanofillers include N, P, and F heteroatoms; metal single atoms; 0D MoS2 nanospheres; 1D PANi nanofibers; and 2D SnS2 nanoflakes. The respective GNR nanocomposites are schematically illustrated in Fig. 4 A, E, I, M, and Q. Briefly, various nanofillers in the form of either precursors (ammonium hexafluorophosphate [NH4PF6; SI Appendix, Fig. S16) and Co(acac)3] or original nanomaterials (MoS2, PANi, and SnS2) were first mixed with pristine large-sized GO nanosheets. The as-obtained NH4PF6/GO, Co(acac)3/GO, MoS2/GO, PANi/GO, and SnS2/GO nanocomposites were then treated with freeze-drying, rolling, and capillary compression, followed by chemical reduction with N2H4·H2O (for PANi/GO sample) and pyrolysis to yield GNR-based nanocomposites. Hereafter, they are denoted NPF/GNRs, CoSAC/GNRs, MoS2/GNRs, PANi/GNRs, and SnS2/GNRs, respectively, where nitrogen, phosphorus and fluorine doped (NPF) and CoSAC refer to nitrogen (N), phosphorus (P), and fluorine (F) heteroatoms and Co single-atom catalyst (SAC), respectively (Materials and Methods in SI Appendix).
Fig. 4.
(A) Schematic of route to NPF/GNRs; (B) optical micrographs of NH4PF6 powder and solution; (C and D) SEM images of the as-prepared NPF/GNRs. (E) Schematic of route to CoSAC/GNRs; (F) optical micrographs of Co(acac)3 powder and solution; (G and H) SEM images of the as-prepared CoSAC/GNRs. (I) Schematic of route to MoS2/GNRs; SEM images of (J) MoS2 nanospheres and (K and L) the as-prepared MoS2/GNRs. (M) Schematic of route to PANi/GNRs; SEM images of (N) PANi nanorods and (O and P) the as-prepared PANi/GNRs. (Q) Schematic of route to SnS2/GNRs; SEM images of (R) SnS2 nanoflakes and (S and T) the as-prepared SnS2/GNRs.
In what follows, we discuss sequentially the crafting of each GNR-based nanocomposite noted above. First, N, P, and F codoped GNRs (NPF/GNRs) were created as metal-free electrocatalysts (Fig. 4A). Specifically, the NH4PF6 powders (used as small-molecule dopants) were dissolved in DI water (Fig. 4B) and mixed with the GO aqueous solution under vigorous stirring and sonication. The freezing–rolling–capillary compression followed by high-temperature pyrolysis was carried out to produce NPF/GNRs. A representative SEM image at low magnification confirms the success in creating NPF/GNRs with a ribbon-shaped morphology (Fig. 4C), within which a wrinkle structure with a smooth surface was seen in the close-up SEM image (Fig. 4D). The XRD pattern of NPF/GNRs displays a main diffraction peak at 26.1° (SI Appendix, Fig. S17), consistent with the reported heteroatom-doped graphene (24, 25). The elemental mapping analysis of NPF/GNRs shows uniform distribution of C, N, P, and F elements (SI Appendix, Fig. S18), indicating a homogeneous doping of GNRs with N, P, and F heteroatoms. The survey X-ray photoelectron spectroscopy (XPS) spectrum shows the characteristic peaks of C, N, P, and F (SI Appendix, Fig. S19), suggesting the codoping of N, P, and F. The presence of O 1s peak can be ascribed to residual oxygen-containing groups of GO sheets or physicochemically adsorbed oxygen (23). The N 1s XPS spectrum (SI Appendix, Fig. S19C) fitted to yield four peaks centered at 391.1, 400.3, 401.2, and 402.7 eV can be attributed to the pyridinic, pyrrolic, graphitic, and oxidized nitrogen, respectively, where the pyridinic and graphitic nitrogen are dominant. The P–C and P–O bonds are coexisted in the P 2p XPS spectrum (SI Appendix, Fig. S19D). The high-resolution F 1s spectrum features ionic (685.2 eV) and semi-ionic (687.8 eV) C–F bonds (SI Appendix, Fig. S19E). Taken together, it is clear that the N, P, and F were codoped within NPF/GNRs, which could alter the electronic structure and spatial distribution of surface species and defects, thereby leading to intriguing electrocatalytic activities.
The past decade has witnessed rapid development of SACs supported on carbon matrix for electrocatalysis (24, 26). Notably, carbon matrix is required to possess excellent electrical conductivity to provide an unobstructed pathway for electron transfer. In this context, the GNRs were used as a loading substrate for preparing cobalt SACs (CoSAC/GNRs, Fig. 4E) in our study. Typically, 1,10-phenanthroline (SI Appendix, Fig. S20) and cobalt(III) acetylacetonate [Co(acac)3; Fig. 4F and SI Appendix, Fig. S21] were used as the anchoring ligand and metal precursor of CoSAC, respectively. The XRD pattern of the as-prepared CoSAC/GNRs shows a main diffraction peak at about 26.1° without discernible diffraction peaks of cobalt metal or oxides (SI Appendix, Fig. S22). The EDS mapping shows the uniform dispersion of the introduced N heteroatom and Co species (SI Appendix, Fig. S23). The SEM images at low (Fig. 4G) and high (Fig. 4H) magnifications display the ribbon morphology of CoSAC/GNRs. The XPS was performed to identify the chemical states of N and Co in CoSAC/GNRs. The survey XPS spectrum shows a tiny peak of Co, indicating that a fractional amount of Co atoms existed along with N and C in the sample (SI Appendix, Fig. S24A). In the C 1s XPS spectrum, some residual oxygen-containing groups derived from the precursor GO nanosheets were observed (SI Appendix, Fig. S24B). The four peaks at 399.1, 400.6, 401.7, and 403.5 eV in the N 1s XPS spectrum can be indexed to pyridinic, pyrrolic, graphitic N, and oxidized N species, respectively (SI Appendix, Fig. S24C). The two peaks at 781.2 and 796.8 eV in the Co 2p XPS spectrum can be attributed to metallic Co 2p3/2 and Co 2p1/2, respectively (SI Appendix, Fig. S24D).
In addition to heteroatoms and metal single atoms, nanofillers of different dimensions can also be conveniently incorporated within GNRs. Fig. 4I depicts the intercalation of 0D MoS2 nanospheres within GNR. The SEM images of MoS2 nanospheres at different magnifications are shown in Fig. 4J and SI Appendix, Fig. S25. The XRD pattern of MoS2 nanospheres shows the characteristic diffraction peaks (SI Appendix, Fig. S26), which can be well indexed to the hexagonal phase of MoS2 (JCPDS No. 75-1539) (26, 27). The SEM image of the as-prepared MoS2/GNRs at low magnification is shown in Fig. 4K. Interestingly, the MoS2 nanospheres wrapped between the interlayers of GNR are clearly evident in Fig. 4L, where the spherical morphology of MoS2 was well retained. Moreover, 1D PANi nanorods can be placed between the interlayers of GNR (Fig. 4M). The success in preparing 1D PANi nanorods was substantiated by SEM (Fig. 4N and SI Appendix, Fig. S27) and XRD (SI Appendix, Fig. S28). The SEM images of the as-prepared PANi/GNRs at low (Fig. 4O) and high (Fig. 4P) magnifications reveal that PANi nanorods were encapsulated by GNR in PANi/GNRs nanocomposites. Furthermore, as shown in Fig. 4Q, 2D SnS2 nanoflakes (Fig. 4R and SI Appendix, Fig. S29) can be sandwiched within the GNR interlayers. The XRD pattern shows diffraction peaks at 14.9, 28.5, 32.3, and 50.3°, corresponding to the (001), (100), (101), and (110) facets of SnS2 (JCPDS No. 23-0677) (28, 29), respectively (SI Appendix, Fig. S30). The SEM imaging at low (Fig. 4S) and high (Fig. 4T) magnifications as well as the corresponding EDS mapping corroborates the successful crafting of SnS2/GNRs containing the embedded 2D SnS2 nanoflakes (SI Appendix, Fig. S31). For comparison, all these five functional nanomaterials with unrolled morphologies were also developed, including N-, P-, F-doped graphene sheets (NPF/G, SI Appendix, Fig. S32), Co single atoms loaded on the graphene sheets (CoSAC/G, SI Appendix, Fig. S33), MoS2-embellished graphene sheets (MoS2/G, SI Appendix, Fig. S34), PANi anchored on graphene sheets (PANi/G, SI Appendix, Fig. S35), and SnS2-modified graphene sheets (SnS2/G, SI Appendix, Fig. S36).
We now turn our attention to exploit the GNR-based functional nanocomposites above for a set of energy conversion and storage devices. First, NPF/GNRs were employed as metal-free electrocatalysts for electrochemical oxygen reduction reaction (ORR) in a three-electrode system (SI Appendix, Fig. S37). The cyclic voltammogram (CV) measurements were conducted in N2- and O2-saturated 0.1 M KOH solution, where a substantial current reduction was discerned in O2-saturated solution (SI Appendix, Fig. S37A), indicating the high electrocatalytic ability of NPF/GNRs. SI Appendix, Fig. S37B, compares the linear sweep voltammetry (LSV) curves of pristine GNRs, NPF/GNRs, NPF/G, and commercial Pt/C. The NPF/GNRs display a much higher ORR activity than that of pristine GNRs, reflecting the more positive onset potential, half-wave potential, and much higher limiting diffusion current. Moreover, the onset potential and half-wave potential of NPF/GNRs are 0.89 and 0.79 V (vs. reversible hydrogen electrode (RHE)), respectively, comparable to those of the Pt/C catalyst. In addition, it can also be detected that the half-wave potential of NPF/GNRs (0.79 V vs. RHE) is more positive than that of NPF/G sample (0.80 V vs. RHE), which can be ascribed to the fact that ribbon-shaped NPF/GNRs are more prone to form connected conductive networks that further contribute to the electrocatalytic oxygen reduction. The polarization curves of NPF/GNRs at different rotating rates (from 400 to 2,500 rpm) were recorded (SI Appendix, Fig. S37C), from which the Koutecky–Levich (K–L) plots can be constructed (SI Appendix, Fig. S37D), yielding the number of transferred electrons of approximately 4, thus revealing a four-electron process for ORR.
Second, the CoSAC/GNRs nanocomposites were also employed electrocatalysts for hydrogen evolution reaction (HER) (Fig. 5A). The presence of Co single atoms was confirmed by high-angle annular dark-field-scanning transmission electron microscopy (HAADF-STEM; Fig. 5B), emerging as the white dots due to the different Z contrast of Co, N, and C atoms. The Co content in CoSAC/GNRs determined by inductively coupled plasma-optical emission spectroscopy is 1.4 wt%. The atomic structure of CoSAC/GNRs was examined by X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS). Fig. 5C displays the XANES of Co K-edge against control samples of Co foil and CoO. Both the intensity and position of the fingerprint peak (7,729 eV) of CoSAC/GNRs are located between the Co foil and CoO, suggesting that the Co atoms in CoSAC/GNRs are in a positive valence state. Moreover, the CoSAC/GNRs show a preedge peak (7,711 eV), attributing to a fingerprint of Co–N4 square planar structures. The EXAFS spectroscopy reveals local environment evolutions of the single Co site (30), where only one main peak at 1.5 Å, corresponding to the Co–N first coordination shell, was detected and no Co–Co peak (2.2 Å) and Co–O peak (1.7 Å) were seen (Fig. 5D). The Co K-edge EXAFS of the first shell in R space can be well fitted with the Co–N scattering paths (Fig. 5E; where the Inset shows the corresponding fitted atomic structure model). The fitted results of EXAFS data are shown in SI Appendix, Table S1. Taken together, the Co atoms are coordinated with four nitrogen atoms in CoSAC/GNRs. Fig. 5F compares the HER polarization curves of pristine GNRs, CoSAC/GNRs, CoSAC/G, and commercial Pt/C, where a good HER catalytic activity of CoSAC/GNRs, comparable to commercial Pt/C, was observed, as summarized in SI Appendix, Figs. S38 and S39 [i.e., the overpotentials (η) of pristine GNRs (387 mV), CoSAC/GNRs (67 mV), CoSAC/G (75 mV), and Pt/C (59 mV) at the current density of 5 mA cm−2 in SI Appendix, Fig. S38, and the Tafel slope of pristine GNRs (174 mV/dec), CoSAC/GNRs (48 mV/dec), CoSAC/G (50 mV/dec), and Pt/C (44 mV/dec) in SI Appendix, Fig. S39A]. After a 3,000-cycle accelerated durability test in acidic solution, the polarization curve shows negligible change (SI Appendix, Fig. S39B). Clearly, the as-crafted CoSAC/GNRs nanocomposites represent the promising electrocatalyst toward water splitting.
Fig. 5.
(A) Schematic of HER promoted by CoSAC/GNRs electrocatalysts. (B) Aberration-corrected HAADF-STEM image of CoSAC/GNRs. (C) Co K-edge XANES spectra and (D) the corresponding Fourier transform (FT) of the K-edge for CoO, CoSAC/GNRs, and Co foil. (E) The corresponding EXAFS R space-fitting curves of CoSAC/GNRs, where the schematic atomic model of CoSAC/GNRs is shown as an Inset. (F) The HER polarization curves of pristine GNRs, CoSAC/GNRs, CoSAC/G, and commercial Pt/C electrodes. (G) Schematic depicting a LIB with the MoS2/GNRs anode. (H) SEM image and the corresponding EDS mapping of the MoS2/GNRs anode. (I) CV curves of MoS2/GNRs electrode at a scanning rate of 0.1 mV/s. (J) Discharge–charge curves of pure GNRs, MoS2/G, and MoS2/GNRs at a current density of 100 mA/g. (K) Long-term cycling performance of MoS2/GNR electrode. (L) A blue LED powered by a coin-cell LIB with the MoS2/GNR anode.
Third, a lithium ion battery (LIB) by capitalizing on the as-prepared MoS2/GNRs as the anode was assembled (Fig. 5G). The SEM image and EDS mapping clearly show the entrapping of MoS2 nanospheres within GNR (Fig. 5H). The MoS2/GNR anode in LIB displays the characteristic lithium ion storage peaks at 1.54 and 2.25 V in the anodic scan, and 1.90, 1.20, and 0.45 V in the cathodic scan (Fig. 5I). The corresponding voltage plateaus in the charge–discharge curves were also detected (Fig. 5J), consistent with the respective peaks on the CV curves. A high specific capacity of 1,210 mAh/g at 0.1 A/g was achieved by the MoS2/GNRs electrode, which is much higher than that of pure GNRs (145) and MoS2/G (1,090 mAh/g) anodes. All results above substantiate the good lithium storage capability of the MoS2/GNRs electrode. Moreover, MoS2/GNRs also exhibit an excellent cycling performance at a high current density of 1 A/g with the specific capacity of 572 mAh/g initially and 465 mAh/g at the 500th cycle (Fig. 5K). Comparatively, MoS2/G nanomaterial shows a high initial specific capacity of 531 mAh/g at 1 A/g, yet a severe fading occurred as the specific capacity was decreased to 211 mAh/g after 500 cycles. This excellent cycling performance of MoS2/GNRs over the poor result of MoS2/G was due to the wrapping effect of GNRs for the active MoS2 nanospheres, which can buffer the volume expansion to avoid structural collapse of MoS2 during long-range electrochemical process. A blue-light-emitting diode (LED) (Fig. 4L) and an electric fan (SI Appendix, Fig. S40; even after 500 cycles) can be powered by the MoS2/GNRs-anode LIB. As such, MoS2/GNRs stand out as a promising anode material for LIBs with favorable lithium ion storage performance owing to the uniform dispersion of MoS2 nanospheres within GNRs.
Fourth, the utility of PANi/GNRs nanocomposites as the electrode material for supercapacitors with ultrafast charge–discharge capability was demonstrated. The galvanostatic charge–discharge curves (SI Appendix, Fig. S41A) demonstrate that PANi/GNRs achieved a faradaic pseudo-capacitance property with a high specific capacitance of 832 F/g at a current density of 5 A/g, which is superior to the results of PANi/G (734 F/g) and pure GNRs (92 F/g). The CV curves of PANi/GNRs tested in a three-electrode system over a potential window of −0.2 to 0.8 V vs. saturated calomel electrode at different scan rates of 10, 20, 50, 100, and 200 mV/s are shown in SI Appendix, Fig. S41B. The peaks at 0.1 and 0.5 V can be attributed to the transition between luecoemeraldine/emeraldine and the redox of hydroxide-amino-terminated oligoanilines, respectively (31). The CV curves remain unchanged even at a high scan rate of 200 mV/s, implying the fast redox rate capability of PANi/GNRs. Moreover, a high capacity retention of 91% was seen after 5,000 cycles (SI Appendix, Fig. S41C), suggesting the excellent cycling stability of PANi/GNR nanocomposites as a result of effective intercalating of PANi nanorods in GNRs. However, the PANi/G nanomaterial undergoes a rapid decay, which can be ascribed to the absence of GNR wrapping to accommodate large volume expansion/contraction of the active PANi during the electrochemical reaction. These structural evolutions eventually lead to structural collapse inside the electrode material, resulting in the fading of its specific capacities.
Finally, sodium ion batteries (SIBs) were also assembled with the SnS2/GNRs nanocomposites as the anode material. Galvanostatic discharge–charge profiles tested at 100 mA/g exhibit specific capacities of 486, 379, and 141 mAh/g for GRN, SnS2/G, and SnS2/GNR electrodes (SI Appendix, Fig. S42A), respectively. The CV curves of SnS2/GNRs electrode present a broad cathodic peak at 1.52 V derived from sodium intercalation into 2D SnS2 layers (SI Appendix, Fig. S42B). The peak at 0.54 V can be indexed to the Na–Sn alloying process (29, 32). In the anodic sweep, the predominant oxidative peak at 1.25 V can be assigned to the desodiation reaction of NaxSn. At a high current density of 1 A/g, the SnS2/GNRs nanocomposites achieve a 96% retention of its initial specific capacity (288 mAh/g) after 500 cycles (SI Appendix, Fig. S42C). However, SnS2/G nanomaterial with SnS2 nanoflakes dispersed on the surface of graphene sheets shows an apparently decreased capacity. A much lower capacity retention of 40% was realized for SnS2/G, which was resulted from the structural difference that SnS2 nanoflakes can be integrally wrapped in the layer spacing of GNRs. The intriguing sodium storage performance of SnS2/GNRs can be ascribed to the uniformly dispersed SnS2 nanoflakes within GNR as well as the high conductivity and aspect ratio of the GNR matrix that promotes the charge transport in the SnS2/GNRs electrode.
Conclusion
In summary, we developed a simple freezing–rolling–capillary compression strategy to render a kilogram (kg)-scale synthesis of high-quality GNRs and GNR-derived nanocomposites with nanofillers of interest intercalating between the layers within GNR. The as-produced GNRs possess excellent electronic properties and structural integrity. It is worth noting that the interlayer spacing of GNR can be well regulated via capillary compression, depending on the amount of nanofillers intercalated. A series of nanofillers, including heteroatoms; metal single atoms; and 0D, 1D, and 2D nanomaterials, can be uniformly incorporated into the GNR interlayers during in-situ freezing–rolling–capillary compression process, yielding a rich variety of GNR-derived nanocomposites. These functional nanocomposites manifest enhanced performance in electrocatalysis, batteries, and supercapacitors over graphene nanosheet–based counterparts, substantiating the synergy of the conductive, interconnected GNR matrix (for concealing nanofillers) and the inserted functional nanofillers. Notably, the hallmarks of our freezing–rolling–capillary compression strategy are threefold. First, it produces a kg-scale technologically important GNR rapidly and expensively. The production could be further scaled up to >10 kg, depending on the accessibility of the number and volume of mechanical lyophilizers for freeze-drying process. Second, it affords, an effective means of tailoring the interlayer spacing within GNR. Third, diverse nanofillers of interest can be conveniently dispersed within the GNR interlayers for applications in energy conversion and storage. Our study highlights the robustness of freezing–rolling–capillary compression approach in mass-producing GNRs with tunable dimension, spacing, and situated nanofillers, which is unattainable by conventional routes, thereby opening up an avenue for the expanded fundamental and practical applications of GNRs.
Materials and Methods
The synthetic strategy and experimental section of this work are provided in SI Appendix. These methods contain the used chemical reagents and the synthesis of pure GNRs, NPF/GNRs, CoSAC/GNRs, pure MoS2 nanospheres, SnS2 nanoflakes, PANi nanorods, MoS2/GNRs, SnS2/GNRs, and PANi/GNRs. Detailed electrochemical measurements and structural characterizations are also included.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We are grateful to the financial supports by the National Key R&D Program of China (2017YFA0208200), the National Natural Science Foundation of China (22022505 and 21872069), the Fundamental Research Funds for the Central Universities (020514380266, 020514380272, and 020514380274), the Scientific and Technological Innovation Special Fund for Carbon Peak and Carbon Neutrality of Jiangsu Province (BK20220008), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (19KJA460004), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Natural Science Foundation of Xuzhou City (KC21283).
Author contributions
Y.Y., Mingkai Liu, Z.J., and Z. Lin conceived the idea of this study; F.L., Y.H., and Z.Q. contributed to sample synthesis and characterization; B.W., Q.M., and Minjie Liu helped with the electrochemical performance measurements; Y.Y., Y.H., Z.Q., and R.Z. performed the EXAFS measurements and contributed to the related data fitting and analysis; Z.F., J.Z., X.M., Z. Li, and S.Z. provided valuable suggestions to the experiments; F.L., Y.Y., Mingkai Liu, Z.J., and Z. Lin wrote the manuscript; and all authors discussed the results.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Yan Yan, Email: yanyan@jsnu.edu.cn.
Mingkai Liu, Email: liumingkai@jsnu.edu.cn.
Zhong Jin, Email: zhongjin@nju.edu.cn.
Zhiqun Lin, Email: z.lin@nus.edu.sg.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Sun Z., Fang S., Hu Y. H., 3D graphene materials: From understanding to design and synthesis control. Chem. Rev. 120, 10336–10453 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Cheng C., Li S., Thomas A., Kotov N. A., Haag R., Functional graphene nanomaterials based architectures: Biointeractions, fabrications, and emerging biological applications. Chem. Rev. 117, 1826–1914 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Chang L., Hu Y. H., Breakthroughs in designing commercial-level mass-loading graphene electrodes for electrochemical double-layer capacitors. Matter 1, 596–620 (2019). [Google Scholar]
- 4.Kolmer M., et al. , Rational synthesis of atomically precise graphene nanoribbons directly on metal oxide surfaces. Science 369, 571–575 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Rizzo D. J., et al. , Inducing metallicity in graphene nanoribbons via zero-mode superlattices. Science 369, 1597–1603 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Wei D., et al. , Scalable synthesis of few-layer graphene ribbons with controlled morphologies by a template method and their applications in nanoelectromechanical switches. J. Am. Chem. Soc. 131, 11147–11154 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Higginbotham A. L., Kosynkin D. V., Sinitskii A., Sun Z., Tour J. M., Lower-defect graphene oxide nanoribbons from multiwalled carbon nanotubes. ACS Nano 4, 2059–2069 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Chen C., et al. , Sub-10-nm graphene nanoribbons with atomically smooth edges from squashed carbon nanotubes. Nat. Electron. 4, 653–663 (2021). [Google Scholar]
- 9.Kosynkin D. V., et al. , Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458, 872–876 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Jin Z., et al. , Metallized DNA nanolithography for encoding and transferring spatial information for graphene patterning. Nat. Commun. 4, 1663 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Sprinkle M., et al. , Scalable templated growth of graphene nanoribbons on SiC. Nat. Nanotechnol. 5, 727–731 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Liu N., et al. , Large-scale production of graphene nanoribbons from electrospun polymers. J. Am. Chem. Soc. 136, 17284–17291 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Jolly A., Miao D., Daigle M., Morin J.-F., Emerging bottom-up strategies for the synthesis of graphene nanoribbons and related structures. Angew. Chem. Int. Ed. 59, 4624–4633 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Cai J., et al. , Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Nguyen G. D., et al. , Atomically precise graphene nanoribbon heterojunctions from a single molecular precursor. Nat. Nanotechnol. 12, 1077–1082 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.-C., et al. , Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol. 10, 156–160 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Talirz L., Ruffieux P., Fasel R., On-surface synthesis of atomically precise graphene nanoribbons. Adv. Mater. 28, 6222–6231 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Abraham J., et al. , Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12, 546–550 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Cheng C., et al. , Ion transport in complex layered graphene-based membranes with tuneable interlayer spacing. Sci. Adv. 2, e1501272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z., et al. , Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage. Nat. Energy 5, 160–168 (2020). [Google Scholar]
- 21.Fei R., et al. , In situ hard-template synthesis of hollow bowl-like carbon: A potential versatile platform for sodium and zinc ion capacitors. Adv. Energy Mater. 10, 2002741 (2020). [Google Scholar]
- 22.Yu W. J., et al. , Synthesis of edge-closed graphene ribbons with enhanced conductivity. ACS Nano 4, 5480–5486 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Chen L., et al. , Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Xiong Y., et al. , Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Zhao L., et al. , Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation. Appl. Catal. B-Environ. 231, 224–233 (2018). [Google Scholar]
- 26.Yan Y., et al. , Robust wrinkled MoS2/N-C bifunctional electrocatalysts interfaced with single Fe atoms for wearable zinc-air batteries. Proc. Natl. Acad. Sci. U.S.A. 118, e2110036118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S., Chowdari B. V. R., Wen Z., Jin J., Yang J., Constructing highly oriented configuration by few-layer MoS2: Toward high-performance lithium-ion batteries and hydrogen evolution reactions. ACS Nano 9, 12464–12472 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Shown I., et al. , Carbon-doped SnS2 nanostructure as a high-efficiency solar fuel catalyst under visible light. Nat. Commun. 9, 169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z., et al. , Highly reversible sodiation/desodiation from a carbon-sandwiched SnS2 nanosheet anode for sodium ion batteries. Nano Lett. 20, 3844–3851 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Wang P., et al. , Atomically dispersed cobalt catalyst anchored on nitrogen-doped carbon nanosheets for lithium-oxygen batteries. Nat. Commun. 11, 1576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou K., et al. , A hydrogel of ultrathin pure polyaniline nanofibers: Oxidant-templating preparation and supercapacitor application. ACS Nano 12, 5888–5894 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y., et al. , Sandwich-like SnS2/Graphene/SnS2 with expanded interlayer distance as high-rate lithium/sodium-ion battery anode materials. ACS Nano 13, 9100–9111 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.