Significance
To counteract diverse pathogens, vertebrates evolved adaptive immunity to generate diverse antibody repertoires through a B lymphocyte-specific somatic gene rearrangement process termed V(D)J recombination. Tight regulation of the V(D)J recombination process is vital to generating antibody diversity and preventing off-target activities that can predispose the oncogenic translocations. Recent studies have demonstrated V(D)J rearrangement is driven by cohesin-mediated chromatin loop extrusion, a process that establishes genomic loop domains by extruding chromatin, predominantly, between convergently oriented CTCF looping factor-binding elements (CBEs). By deleting and inverting CBEs within a critical antibody heavy chain gene locus developmental control region and a loop extrusion chromatin-anchor at the downstream end of this locus, we reveal how these elements developmentally contribute to generation of diverse antibody repertoires.
Keywords: V(D)J recombination, CTCF-binding elements (CBEs), CTCF, antibody repertoires, chromatin 3D structure
Abstract
Immunoglobulin heavy chain variable region exons are assembled in progenitor-B cells, from VH, D, and JH gene segments located in separate clusters across the Igh locus. RAG endonuclease initiates V(D)J recombination from a JH-based recombination center (RC). Cohesin-mediated extrusion of upstream chromatin past RC-bound RAG presents Ds for joining to JHs to form a DJH-RC. Igh has a provocative number and organization of CTCF-binding elements (CBEs) that can impede loop extrusion. Thus, Igh has two divergently oriented CBEs (CBE1 and CBE2) in the IGCR1 element between the VH and D/JH domains, over 100 CBEs across the VH domain convergent to CBE1, and 10 clustered 3′Igh-CBEs convergent to CBE2 and VH CBEs. IGCR1 CBEs segregate D/JH and VH domains by impeding loop extrusion-mediated RAG-scanning. Downregulation of WAPL, a cohesin unloader, in progenitor-B cells neutralizes CBEs, allowing DJH-RC-bound RAG to scan the VH domain and perform VH-to-DJH rearrangements. To elucidate potential roles of IGCR1-based CBEs and 3′Igh-CBEs in regulating RAG-scanning and elucidate the mechanism of the ordered transition from D-to-JH to VH-to-DJH recombination, we tested effects of inverting and/or deleting IGCR1 or 3′Igh-CBEs in mice and/or progenitor-B cell lines. These studies revealed that normal IGCR1 CBE orientation augments RAG-scanning impediment activity and suggest that 3′Igh-CBEs reinforce ability of the RC to function as a dynamic loop extrusion impediment to promote optimal RAG scanning activity. Finally, our findings indicate that ordered V(D)J recombination can be explained by a gradual WAPL downregulation mechanism in progenitor-B cells as opposed to a strict developmental switch.
Variable region exons that encode antigen-binding sites of antibodies are assembled in progenitor (“pro”)-B cells from germline VH, D, and JH gene segments (1). V(D)J recombination is initiated by RAG1/2 endonuclease (RAG) (2). RAG introduces DNA double-stranded breaks (DSBs) between VH, D, and JH coding segments and flanking recombination signal sequences (RSSs) (2). RSSs comprise a conserved heptamer, a spacer of 12 or 23 base pairs, and an AT-rich nonamer. To robustly initiate V(D)J recombination, RAG must bind and cleave a pairs of gene segments flanked by RSSs with complementary 12- and 23-bp spacers (termed 12-RSSs and 23-RSSs, respectively) (2). After RAG cleavage, 12/23-RSS matched gene segment ends and, separately, their corresponding RSS ends are fused by the classical nonhomologous end-joining (3). The mouse IgH locus (Igh) spans 2.7 megabases (Mbs) on chromosome 12 with over 100 VHs interspersed within a several Mb distal portion (1). This VH domain lies 100 kb upstream of a 50-kb region containing up to 13 Ds, with 4 JHs embedded within a 2-kb region just downstream of the most proximal D (DQ52) (1). RAG initiates Igh V(D)J recombination from a recombination center (RC) formed within highly transcribed chromatin that spans DQ52, the four JHs, and the intronic enhancer (iEμ) (4, 5). The VHs and JHs have 23RSSs and cannot be directly joined. Ds are flanked on either side by 12RSSs, allowing them to join to a downstream JH and an upstream VH to form a V(D)J exon (3). V(D)J recombination is developmentally ordered with Ds joined to a JH to form a DJH RC, after which VHs are joined to the upstream D12RSS of the DJH RC (3).
The CTCF chromatin looping factor binds target DNA sequences, termed CTCF-binding elements (“CBEs” or “CTCF sites”), in an orientation-specific manner (6, 7). In this regard, the numerous genomic CBEs, when in adjacent regions, can occur in the same, divergent, or in convergent orientations (8). The cohesin complex mediates extrusion of chromatin loops genome-wide, forming contact loops when extrusion in each direction reaches CTCF-bound CBEs that impede extrusion (9, 10). Such CBE-anchored chromatin loops occur most dominantly between CBEs in convergent orientation (8, 11), an orientation that forms the most stable anchor (9, 10). Convergent CBE orientation has been implicated in mediating physiological functions (12–17). However, CTCF-bound CBEs can impede loop extrusion regardless of orientation (18), and non-CBE-based impediments, for example highly transcribed chromatin, can impede extrusion and contribute to developmental or tissue-specific regulation of loop extrusion (18–24). The Igh contains numerous CBEs with remarkable relative orientation. The IGCR1 element, which lies just upstream of the most distal D segment (DFL16.1) has two divergently oriented CBEs, namely upstream CBE1 and downstream CBE2 (25). The long upstream VH- containing domain has over 100 CBEs, with most lying in convergent orientation to IGCR1 CBE1 (26). Ten consecutive CBEs, termed 3′Igh CBEs (27, 28) lie just downstream of Igh in convergent orientation to IGCR1 CBE2 and VH domain CBEs (25, 26). This organization has been proposed to have various functions in regulating V(D)J recombination (25, 29–31).
Cohesin-mediated loop extrusion provides the mechanistic underpinnings for RC-bound RAG to scan chromatin across the Igh locus for substrates (18, 19, 22, 24, 32). In pro-B cells, RC-bound RAG initiates scanning upon binding a JH-23RSS into one of its two active sites (1, 18, 24). For this scanning process, the active RC, which lacks CBEs, serves as a transcription-based dynamic downstream loop extrusion anchor, while IGCR1 serves as a CBE-based upstream anchor that terminates RAG scanning, preventing scanning from entering the VH-containing domain (18, 24, 33). The upstream orientation of the RAG-bound JH programs RAG scanning of upstream D-containing chromatin extruded past the RC (24). During RAG scanning of the D locus, only downstream D-12RSSs, convergently oriented to the JH23RSSs, are used, resulting in D-to-JH joining that deletes all sequences between the participating D and JH (24). Predominant utilization of RSSs, or cryptic RSSs, in convergent orientation to the initiating RC RSS is a mechanistic property of the linear RAG scanning process (22, 32).
While VHs-23RSSs are compatible for joining to D12-RSSs, the IGCR1 impedes access of VHs to the D locus during the D-to-JH rearrangement and, thereby, enforces ordered D-to-JH rearrangement before VH-to-DJH rearrangement in pro-B cells (18, 25, 32). Mutational inactivation of IGCR1 CBEs allows RC-bound RAG to scan directly into the proximal VH locus, causing the most proximal functional VH5-2 to robustly rearrange and dominate the VH repertoire, with little rearrangement of more distal VHs (18, 25, 32). The mechanism by which VH5-2 dominates rearrangement when IGCR1 CBEs are inactivated is based on its RSS-associated CBE that impedes extrusion past the RC, making it accessible for rearrangement (18). Indeed, dozens of the proximal VHs have RSS-associated CBEs. Thus, while deletion of the VH5-2 CBE results in a 50-fold reduction of VH5-2 rearrangement along with greatly reduced RC interaction, the next upstream VH becomes dominantly rearranged based on its RSS-associated CBE (18). Because dozens of the D-proximal VHs have RSS-associated CBEs, this portion of the VH locus is a major barrier to RAG scanning to further upstream VHs when IGCR1 CBEs are inactivated (18).
Fluorescent in situ hybridization and chromosome conformation capture (3C)-based studies revealed the VH domain to undergo large-scale contraction in pro-B cells (34–42). VH locus contraction was proposed to bring distal VHs into proximity with the DJH RC for recombinational access (43). Recent studies revealed that locus contraction is mediated by loop extrusion, which extends across the several Mb VH locus due to approximately fourfold downregulation of the WAPL cohesin unloading factor in pro-B cells (44). In this context, depletion of WAPL in nonlymphoid cells extends genome-wide loop extrusion by increasing cohesin density, allowing it to bypass CBEs and potentially other impediments (45–47). WAPL downregulation in pro-B cells reduces loop extrusion impediment activity of IGCR1 CBEs, proximal VH-associated CBEs, and others, allowing RAG scanning from a DJH RC to extend linearly across the VH domain (22). Abelson murine leukemia virus-transformed pro-B cell lines (“v-Abl lines”) can be viably arrested in the G1-cell cycle phase in which V(D)J recombination occurs (48). Introduction of RAG into G1-arrested RAG-deficient v-Abl lines activates robust RAG scanning across the D domain (19, 22, 24). However, scanning is impeded at IGCR1, and there is little VH-to-DJH joining (19, 22, 24). Inactivation of IGCR1 CBEs leads to dominant rearrangement of VH5-2 in v-Abl lines due to its RSS-associated CBE (18). In this regard, v-Abl lines have high WAPL levels and do not neutralize VH locus CBEs (19, 22). Neutralization of CBE impediments by depletion of CTCF or WAPL extends cohesin loop extrusion and RAG-scanning past IGCR1 and proximal VHs to the most distal VHs (19, 22). Thus, v-Abl pro-B cell studies have provided substantial mechanistic insights into VH-locus contraction and the RAG chromatin scanning process (1).
Despite recent advances in elucidating functions of Igh CBEs during V(D)J recombination, many questions remain. While an early study indicated that IGCR1 CBEs act synergistically to segregate the VH domain from the D/JH domain (49), the question of whether orientation of these CBEs is critical to their function remained open. Likewise, 3′Igh CBEs confine Igh class switch recombination (CSR) activity to the Igh (50). However, their roles in V(D)J recombination have not been resolved (1). Finally, a long-standing question is how the developmental transition from D-to-JH joining to VH-to-DJH joining is regulated. While a role for the DJH intermediate in signaling the transition (51, 52) has been considered, such a transition could, in theory, be mediated by gradual WAPL-downregulation during pro-B cell development (1). We now describe studies that address these questions.
Results
Role of IGCR1 CBEs in D-to-JH and Proximal VH-to-DJH Rearrangements.
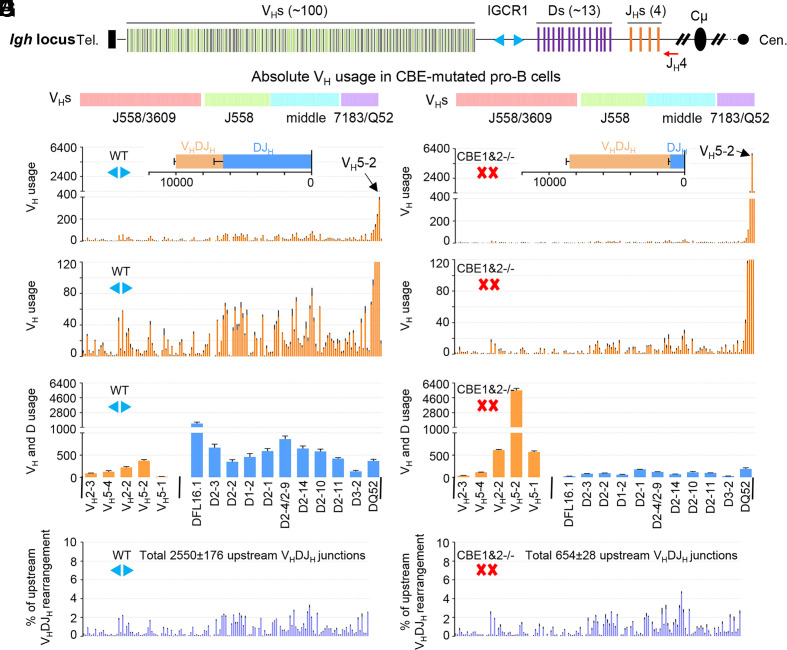
To gain insight into factors that determine primary bone marrow (BM) pro-B cell VH repertoires, we assessed effects of IGCR1 CBE1 and CBE2 inactivation via high-resolution HTGTS-V(D)J-Seq (24). For these assays, we purified B220+CD43highIgM− pro-B cells from BM of wild-type (WT) 129SV controls and previously generated IGCR1/CBE1&2−/− mice (25). We performed HTGTS-V(D)J-Seq on DNA from these samples using a JH4 bait primer to compare their levels of D-to-JH and VH-to-DJH rearrangements (Fig. 1A and SI Appendix, Table S1) (32). A JH4 bait primer was used for HTGTS-V(D)J-Seq analyses in this study to eliminate potential confounding effects of rearrangements within extrachromosomal deletion products (24). For these analyses, individual peaks found in HTGTS libraries can be normalized as a fraction of total HTGTS reads for a given experiment, which reveals absolute V(D)J levels of each rearranging gene segment. Alternatively, peaks can be normalized as a fraction of total recovered junctions across a given locus or section of a locus to reveal the relative utilization of a given gene segments to each other as a percentage of all junctions in the analyzed region (SI Appendix, Fig. S1). Such analyses are useful for examining effects of potential regulatory element mutations. For example, finding a decrease in total reads between two samples in the absence of differences in the junction profile, reflects decreased RAG or RC activity without changes in long range scanning patterns (22).
Fig. 1.
Role of IGCR1/CBE1 and CBE2 in D-to-JH and proximal VH-to-DJH rearrangements. (A) Schematic of the murine Igh locus showing VHs, Ds, JHs, CHs, and IGCR1. The red arrow indicates the JH4 coding end (CE) bait primer. (B–E) Utilization of VHs across the entire Igh locus in WT (B and C) and CBE1&2−/− (D and E) pro-B cells. The VHDJH and DJH junctions are shown in the Insets (n = 3 mice, mean±SEM; all HTGTS libraries are normalized to 78,091 total reads; see SI Appendix, Table S1). (F and G) Proximal VH usage in VHDJH junctions and D usage DJH junctions of WT (F) and CBE1&2−/− (G) pro-B cells (n = 3 mice, mean ± SEM). (H and I) Relative percentage of upstream VHs beyond the five most proximal VHs normalized to the indicated VHDJH junction number in WT (H) and CBE1&2−/− (I) pro-B cells. Upstream VHs junctions are extracted from the data of B and D (n = 3 mice, mean ± SEM).
WAPL downregulation in mouse pro-B cells (22, 44) allows RAG to scan the entire VH locus, which results in highly reproducible utilization of the different VHs (Fig. 1 B and C). However, in WT pro-B cells, VH5-2 (“VH81X”) and three immediately upstream VHs with RSS-associated CBEs are much more highly utilized than any VHs further upstream (Fig. 1 B and C). In such steady-state BM pro-B cell populations, DJH rearranged alleles are more prevalent than VH(D)JH rearranged alleles (Fig. 1 B, Inset), likely reflecting steady-state distributions within pro-B cells entering the compartment and successively generating DJH and VH-to-DJH rearrangements before leaving the compartment. Inactivation of IGCR1 by mutational inactivation of CBE1 and CBE2 (18, 25, 32), allows VH5-2 to dominate the VHDJH repertoire (Fig. 1 D and E). Moreover, the great majority of VH5-2 rearrangements are nonproductive (SI Appendix, Fig. S2 and Table S2), consistent with selection against productive VH5-2 rearrangements (53). This finding also confirms that dominant VH5-2 rearrangements in pro-B cells do not result from cellular selection (25). Rearrangement frequencies of more distal VHs are dramatically decreased upon IGCR1 inactivation (Compare Fig. 1 B and C with Fig. 1 D and E; also see SI Appendix, Table S1). Strikingly, however, in CBE1&2−/− pro-B cell populations as compared to WT pro-B cell populations, the absolute level of VHDJH rearrangements is greatly increased with the vast majority utilizing VH5-2, while the absolute level of DJH rearrangements and upstream VH rearrangements is, correspondingly, decreased (Fig. 1 E–G and SI Appendix, Table S1).
The above findings support the notion that, in the absence of IGCR1 CBE activity in normal pro-B cells, RAG scanning continues between the DJH RC and VH5-2, which allows VH5-2 to dominate VH-to-DJH rearrangements due to its robust CBE-mediated interaction with the DJH RC (18). Moreover, the finding that the increased frequency of VH-to-DJH rearrangements in the steady-state CBE1&2−/− pro-B results almost totally from increased VH5-2 rearrangements indicates that these dominant rearrangements occur at the D-to-JH scanning stage before sufficient WAPL downregulation neutralizes proximal VH-RSS-associated CBE impediments to allow upstream scanning. These high-resolution HTGTS-V(D)J-seq studies also revealed another notable finding. Despite the dramatically reduced levels of upstream VH rearrangements in CBE1&2−/− pro-B cells, the upstream VHs beyond the several most proximal VHs, relative to each other, have rearrangement junction patterns (i.e. relative levels compared to each other), that were nearly identical to those of WT pro-B cells (Fig. 1, Compare panels H and I). Thus, VH5-2, and to a lesser extent immediately upstream VHs, dominate initial rearrangements in the absence of IGCR1 CBE activity, and, in doing so, terminate most RAG upstream scanning. However, RAG scanning that does proceed beyond VH5-2 continues through the remainder of the VH locus, with similar VH usage patterns as those of WT cells. Overall, these findings indicate that, while most VH5-2 rearrangements in CBE1&2−/− pro-B cells occur before WAPL downregulation, a small fraction of CBE1&2−/− pro-B that do not form VH5-2 rearrangements on one or both Igh alleles undergo upstream VH-to-DJH recombination events at normal frequencies when WAPL-downregulation reaches appropriate levels.
Role of IGCR1/CBE1 and CBE2 in Regulating RAG Scanning into the VH Locus.
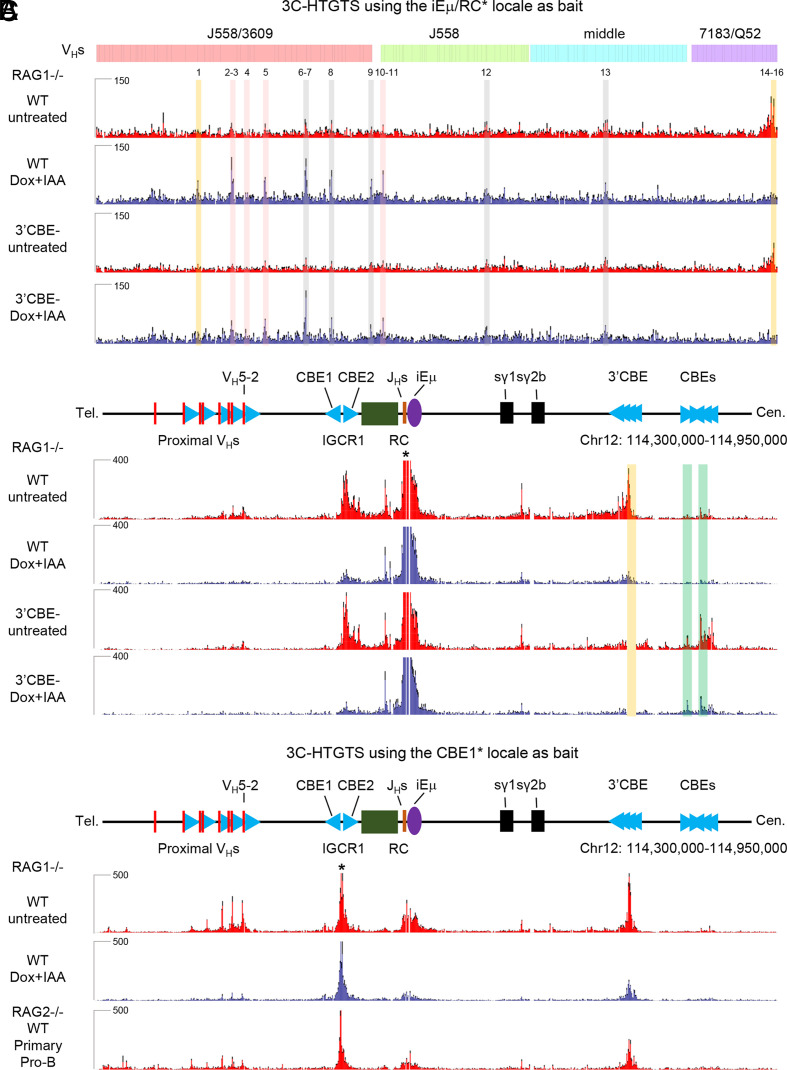
To further assess potential mechanisms by which IGCR1 CBE impediment activity is modulated to promote RAG scanning of the VH domain, we applied the highly sensitive 3C-HTGTS chromatin interaction assay to explore interactions of the RC-based iEμ enhancer element with upstream and downstream Igh locus chromatin domains in WT rag2−/− and IGCR1/CBE1&2−/−rag2−/− cultured pro-B cells derived from the corresponding mouse lines. RAG-deficient cells must be used for such assays to eliminate confounding effects of V(D)J recombination events on such interactions (18, 19, 22, 24). These studies revealed that the iEμ/RC interacts robustly with 15 highly focused regions across the 2.4 Mb VH locus in WT rag2−/−pro-B cells (Fig. 2A and SI Appendix, Fig. S3) (19, 22). Among the most robust of these iEμ/RC interacting peaks are peaks associated with robustly transcribed PAX5-activated intergenic repeat (PAIR) elements (38, 54, 55) in the J558/3609, and J558 VH-containing regions in the distal portion of the VH locus (Fig. 2A and SI Appendix, Fig. S3; Peaks 1, 4, 6, 8–10). RC interactions with transcribed PAIR element-associated sequences are considered a hallmark of loop extrusion-mediated VH locus contraction in pro-B cells (38). In this regard, locus contraction results from an approximately fourfold developmental downregulation of WAPL in pro-B cells (44), which at least partially neutralizes IGCR1-CBEs, proximal VH CBEs, and likely other VH locus CBEs, and potentially transcription-associated impediments to RC-based RAG linear scanning (22, 44). Although VH5-2 and proximal VHs are the most dominantly utilized VHs in normal pro-B cells (Fig. 1B), they show only low-level interactions with the RC in RAG2-deficient WT pro-B cells at steady state (Fig. 2 A and B), consistent with most CBE-based interactions being diminished by WAPL-downregulation in a large fraction of the pro-B cells (22). In this regard, some WT rag2−/− pro-B cells retain interactions between the RC and IGCR1(Fig. 2B, upper track), indicating that some cells in the population have not fully down-regulated WAPL and/or that IGCR1 impediment activity is not completely neutralized by physiological levels of WAPL downregulation (Fig. 2B; upper track).
Fig. 2.
Role of IGCR1/CBE1 and CBE2 in regulating RAG scanning into the VH locus. (A) 3C-HTGTS signal counts of all VHs in WT (red) and CBE1&2−/− (blue) RAG2-deficient pro-B cells baiting from iEμ/RC (*). Each library was normalized to 160,314 total junctions (n = 3 mice, mean ± SEM). 18 peaks across VHs region are called by MACS2 pipeline and highlighted in gray (peaks 1–9, 12–15 are called in both conditions), orange (peaks 10–11 are called only in WT), green (peaks 16–18 are called only in CBE1&2−/−) (SI Appendix, Fig. S3). (B) Zoom-in 3C-HTGTS profiles of Igh locus from proximal VHS to 3′Igh CBEs.
Notably, CBE1&2−/−rag2−/− cultured pro-B cells exhibit greatly increased RC interaction with proximal VH5-2 and the 3 proximal VHs immediately upstream, which, based on prior studies (18), is dependent on their RSS-associated CBEs (Fig. 2 A and B and SI Appendix, Fig. S3; Peaks 16–18). Strikingly, nearly all major further upstream interaction peaks were also present in chromatin from CBE1&2−/−rag2−/− pro-B cells, mostly at similar relative levels to those in WT rag2−/− pro-B cells (Fig. 2A). Given that the chromatin interactions are investigated in RAG-deficient cells and upstream interactions are not impacted by proximal VH-to DJH recombination events, it is not unexpected that a large number of IGCR1/CBE-mutated pro-B cells would have robust interactions between the RC to the upstream VHs after developmental WAPL downregulation. As previously described (18), the RC also robustly interacts downstream with the transcribed enhancer-like element between Cγ1 and Cγ2b (38) and with the 3′Igh CBEs in pro-B cells (Fig. 2B, upper track); these interactions were not altered by IGCR1 CBE1 and CBE2 deletion (Fig. 2B, lower track).
Influence of IGCR1 CBEs and Their Orientation on VH-Utilization in pro-B Cells.
We have previously generated CBE1−/− and CBE2−/− mice (49). To assess whether orientation of IGCR1/CBEs is critical for Igh V(D)J recombination control, we replaced CBE1 or CBE2 with their inverted sequences to generate a CBE1inv or CBE2inv allele in mouse 129SV ES cells (SI Appendix, Fig. S4 A and B) (25, 49) (Methods). We employed HTGTS-V(D)J-Seq to assay JH4-based utilization of the various VHs in pro-B populations harboring IGCR1 CBE deletion or inversion mutations (Fig. 3). CBE1−/− pro-B cell populations have markedly increased VH5-2 rearrangements and markedly decreased rearrangements of more distal VHs, with the degree of increases and decreases modestly, but significantly, less than those observed for CBE1&2−/− pro-B cells (Fig. 3 A and E and SI Appendix, Table S1). In contrast, CBE2−/− pro-B cells had very modestly increased VH5-2 rearrangements (2.5-fold; Fig. 3 B and E) and normal patterns of distal VH rearrangements (Fig. 3 B and E and SI Appendix, Table S1). These findings indicate that CBE1 and CBE2 cooperatively provide the full impact of IGCR1 scanning impediment activities and unequivocally demonstrate that CBE1 plays a much more dominant role. CBE1inv/inv BM pro-B cells also have significantly increased levels of proximal VH5-2 utilization relative to WT; but to a much lower extent than CBE1−/− pro-B cells, indicating that ability of CBE1 to impede RAG scanning is dampened, but not abrogated, when inverted (Fig. 3 C and E and SI Appendix, Table S1). In contrast, CBE2inv/inv pro-B cells were very similar to WT pro-B cells with respect to utilization of VH5-2 and upstream VHs (Fig. 3 D and E and SI Appendix, Table S1). Consistent with our findings for IGCR1/CBE1&2−/− pro-B cells (Fig. 1), the rearrangement pattern of upstream VHs, relative to each other, was not markedly impacted by CBE1 or CBE2 deletions or inversions (SI Appendix, Fig. S5 A–F and Table S1).
Fig. 3.
The mutation of IGCR1/CBEs alters VHs utilization in pro-B cells. (A–D) Each panel shows the utilization of VHs across the entire Igh locus in indicated IGCR1/CBE-mutated pro-B cells. The VHDJH and DJH junctions are shown in Insets (n = 3 mice, mean ± SEM; All HTGTS libraries are normalized to 78,091 total reads; see SI Appendix, Table S1). (E) VHs usage in WT and indicated IGCR1/CBE-mutated pro-B cells (n = 3 mice, mean ± SEM).
We performed 3C-HTGTS on RAG-deficient IGCR1/WT, IGCR1/CBE1&2−/−, IGCR1/CBE1inv/inv and IGCR1/CBE2inv/inv v-Abl lines with bait primers to the iEμ in the RC (SI Appendix, Fig. S6A) and VH5-2 (SI Appendix, Fig. S6B) locales. In CBE1−/− pro-B cell populations, we observed robust, albeit somewhat diminished, interactions between the iEμ/RC bait and the proximal VH-CBEs compared to interactions in CBE1&2−/− pro-B cells (SI Appendix, Fig. S6A, CBE1−/− vs. CBE1&2−/−). In CBE2−/− pro-B cells, we observed a modest increase in the interactions between the iEμ/RC bait and the proximal VH-CBEs (SI Appendix, Fig. S6A, CBE2−/− vs. CBE1&2−/−). These findings indicate that IGCR1 CBEs play a cooperative role in impeding loop extrusion-mediated proximal VH-CBEs and RC interactions and, again, that CBE1 has a more dominant role. In contrast, when CBE1 or CBE2 was inverted, interactions between proximal VH-CBEs and iEμ/RC showed only very modest changes compared to those when IGCR1 CBEs are in normal orientation (SI Appendix, Fig. S6 A and B).
Influence of 3′Igh CBEs on D-to-JH and VH-to-DJH Rearrangement Patterns and Levels.
As mentioned above, v-Abl pro-B cell lines provide a very useful system for studies of RAG chromatin scanning process (1). In particular, the ability to study RAG scanning in a system in which WAPL can be completely depleted allows experiments to test the proposal that 3′Igh CBEs reinforce the loop anchor provided by the RC when juxtaposed via loop extrusion (1). To investigate potential contributions of 3′Igh CBEs on RAG scanning, we deleted all 3′CBEs in RAG1-deficient C57BL/6 WAPL-degron v-Abl cells that contain a single copy of the Igh locus (22). For analyses, WT or 3′CBE-deleted (3′CBE−) WAPL-degron v-Abl cells were arrested in G1 and then left untreated or treated with Dox plus IAA to degrade WAPL (22). Subsequent introduction of RAG into untreated WT WAPL-degron v-Abl cells activated V(D)J recombination leading to robust D-to-JH recombination, very low-level VH5-2 to DJH recombination, and extremely low-level upstream VH-to-DJH recombination (Fig. 4A and SI Appendix, Table S3), as v-Abl lines have high WAPL levels and RAG scanning is impeded at IGCR1 (22). Dox plus IAA treatment completely depletes WAPL in these G1-arrested v-Abl lines, with little effect on viability (22). Introduction of RAG into WAPL-depleted WT v-Abl cells activated D-to-JH rearrangement, but the level of DJH rearrangements was reduced 6.5-fold compared to that of untreated cells (Fig. 4I and SI Appendix, Table S3). This reduction was associated with dramatically decreased distal DFL16.1-JH and, to a lesser degree, most other DJH absolute rearrangement levels (Fig. 4 M and N). Notably, however, DQ52 rearrangement levels showed little change (Fig. 4M).
Fig. 4.
Role of 3′Igh CBEs in RC activity during loop extrusion. (A–D) Utilization of VHs across the entire Igh locus in WT (A and B) and 3′CBE− (C and D) v-Abl cells with or without Dox/IAA treatments. The VHDJH and DJH junctions are shown in Insets. (n = 3 repeats from three independent clones, mean ± SEM; all HTGTS libraries are normalized to 1,964,102 total reads; see SI Appendix, Table S3). (E–H) Relative percentage of VHs utilization normalized to the indicated VHDJH junction number (n = 3 repeats, mean ± SEM; percentages are plotted from the data of Fig. 4 A–D). (I and J) Absolute level of VHDJH and DJH rearrangements in WT and 3′CBE− lines (n = 3 repeats, mean ± SEM; t test, P < 0.01, **). (K and L) Relative percentages of VHDJH and DJH normalized to untreated or treated WT conditions. (M and N) Absolute (M) and relative (N) D usage in DJH rearrangements in untreated and WAPL-depleted WT v-Abl cells (relative percentage was normalized to 106,936 DJH junctions in untreated or 16,565 DJH junctions in WAPL-depleted cells; see SI Appendix, Table S3).
WAPL-depletion also led to RAG-scanning and utilization of VHs across the 2.4 Mb VH locus (Fig. 4 A and B and SI Appendix, Table S3) as expected (22). The absolute level of VH DJH rearrangements in WAPL-depleted WT v-Abl cells was similar to that of the low level of proximal VH rearrangements in untreated cells; but represented a 5.3-fold increase with respect to their fraction of DJH rearrangements (Fig. 4 I and J and Discussion). In both untreated and WAPL-depleted 3′CBE− lines, we observed a further 25% decrease in DJH rearrangement levels from their baseline levels. We observed a similar 25% decrease in VHDJH rearrangements in untreated 3′CBE− lines and a nearly 50% decrease in WAPL-depleted 3′CBE lines (compare Fig. 4 panels B and D, Fig. 4 I–L). Despite the further reduction of VHDJH rearrangement levels in WAPL-depleted 3′CBE− v-Abl cells, their relative VH usage pattern was very similar to that of WAPL-depleted WT lines (compare Fig. 4, panel F and H).
We performed 3C-HTGTS in RAG-deficient WT and 3′CBE− v-Abl cells baiting from iEμ/RC (Fig. 5 A and B and SI Appendix, Figs. S7 B and C and S8). These studies revealed, as previously described (22), that complete depletion of WAPL substantially increased a number of interaction peaks in the J558/3609, J558 and middle VH regions (Fig. 5A and SI Appendix, Fig. S8 Dox+IAA vs untreated; Peaks 1–13). Many of the sequences that contribute to these peaks were highly transcribed including the well-characterized PAIR elements (Fig. 5A and SI Appendix, Fig. S8; Peaks 1–8). In contrast, peaks in the proximal 7183/Q52 region that are dominant in untreated v-Abl cells are mainly associated with proximal VH RSS-CBEs and were significantly diminished by WAPL depletion (Fig. 5A and SI Appendix, Fig. S8, Dox+IAA vs untreated; Peaks 14–16). Notably, in 3′CBE− cells, these same major interaction peaks, including those associated with proximal VHRSS-CBEs in untreated and those associated with the upstream VH regions in treated and untreated cells, remain robust and largely correspond to those in the same locations as in the WT line (Fig. 5A and SI Appendix, Fig. S8, 3′CBE− vs. WT). While the intensity of some peaks in the untreated or WAPL-depleted 3′CBE− v-Abl cells were somewhat diminished compared those of the untreated or WAPL-depleted WT v-Abl cells, when viewed at high resolution they are clearly still associated with same transcriptional or CBE impediments (Fig. 5A and SI Appendix, Fig. S8). Upon the deletion of 3′Igh CBEs, multiple CBEs downstream of the 3′Igh CBEs appear to gain robust interactions with the iEμ/RC (Fig. 5B). Finally, WAPL-depletion also substantially diminished interactions of IGCR1 CBE1 with both CBE-based (proximal VHs and 3′Igh CBEs and transcription-based (RC and γ1-γ2b enhancer) loop extrusion impediments (Fig. 5C).
Fig. 5.
3C-HTGTS profiles at Igh locus bating from RC in WT and 3′CBE− WAPL-degron v-Abl cells. (A) 3C-HTGTS signal counts at VHs domains of WT and 3′CBE− RAG1-deficient v-Abl lines baiting from iEμ/RC (*) with (red) or without (blue) Dox/IAA treatment. Each library was normalized to 160,314 total junctions (n = 3 repeats from three independent clones, mean ± SEM). Sixteen peaks across VHs region are called by MACS2 pipeline and highlighted in gray (peak 1, 6–9, 12–13 are called in WAPL-depleted WT v-Abl cells and WT pro-B cells), red (peaks 2–5, 10–11 are called in WAPL-depleted v-Abl cells), orange (peak1 is called only in WAPL-depleted WT v-Abl cells), and green (peaks are called in 3′CBE− v-Abl cells); peaks 14–16 are present in untreated WT and 3′CBE− v-Abl lines (SI Appendix, Fig. S8). (B) Zoom-in 3C-HTGTS profiles of Igh locus from proximal VHS to 3′Igh CBEs. (C) 3C-HTGTS signal counts of Igh locus from proximal VHs to 3′Igh CBEs in RAG-deficient WT v-Abl cells (red), WAPL-depleted v-Abl cells (blue) and pro-B cells (red) baiting from IGCR1/CBE1 (*). Each library was normalized to 112,525 total junctions (n = 3 repeats from three independent clones or 3 mice, mean ± SEM).
Discussion
HTGTS-V(D)J-Seq analyses provided a deep analysis of VH repertoires in primary WT and IGCR1/CBE1&2−/− pro-B cells (Fig. 1). In addition, 3C-HTGTS-Seq analyses of iEμ/RC interactions across the Igh locus in RAG2-deficient primary WT and IGCR1/CBE1&2−/− pro-B cells complemented the HTGTS-V(D)J-Seq findings to reveal a likely mechanism by which the ordered transition from D-to-JH versus VH-to-DJH rearrangement is regulated (Fig. 2). Our overall findings indicate that this developmental transition can be explained in the context of gradual WAPL downregulation in pro-B cells (Outlined in SI Appendix, Fig. S9). Our findings indicate that robust D-to-JH rearrangements occur in WT pro-B cells before WAPL is sufficiently down-regulated to allow scanning to pass IGCR1 or proximal VH-associated CBEs. In CBE1&2−/− pro-B cells, robust iEμ/RC interactions with proximal VHRSS-associated CBEs promote their robust rearrangements and suppress scanning to upstream VHs. Our finding that low-level rearrangements of upstream VHs in CBE1&2−/− pro-B cells have normal RAG-scanning patterns is consistent with these rearrangements occurring after WAPL-downregulation in the cells that have not formed proximal VHDJH rearrangements on both alleles. In CBE1&2−/−rag2−/− pro-B cells, lack of V(D)J recombination upon WAPL downregulation allows the iEμ/RC to scan into the upstream VH domain where it reaches normal impediments in a substantial fraction of the cells. In this context, the relatively robust contribution of VH5-2 and immediately upstream VHs to the WT pro-B repertoire indicates that these VHs are dominantly utilized in normal pro-B cells until WAPL levels are sufficiently down-regulated Finally, in support of this model, proximal VHs are poorly utilized in v-Abl cells in which RAG is introduced after complete WAPL-depletion (22) (Fig. 4).
Our current studies confirm unequivocally that CBE1 and CBE2 function synergistically to provide the full RAG scanning impediment activity of IGCR1 and that CBE1 provides the major portion of this activity (Fig. 3). We have previously shown that the linear scanning process from the JH or DJH recombination centers is strongly impeded by CTCF bound IGCR1 CBEs until they are neutralized by WAPL downregulation (19, 22). Moreover, based on CTCF depletion studies, we found CBE1 retains bound CTCF under conditions in which CBE2 completely loses bound CTCF, which implies CBE1 more strongly binds CTCF (19). Such stronger CTCF-binding activity may form a basis for the stronger RAG-scanning impediment activity of CBE1 versus CBE2. Finally, reminiscent of the effects of VH5-2 RSS-associated CBE inversion as compared to complete inactivation on proximal VH5-2 rearrangements (18), normal orientation of IGCR1 CBEs is required to provide physiological levels of RAG-scanning impediment activity, but both retain substantial activity when inverted (Fig. 3).
Our findings on the impact of WAPL-depletion on chromatin interactions and RAG scanning activity support a model in which 3′Igh CBEs reinforce RC activity during Igh V(D)J recombination (SI Appendix, Fig. S10A) (1). In WT v-Abl cells with high WAPL expression, the RC robustly interacts with the downstream γ1-γ2b enhancer and the 3′Igh CBEs, which, as proposed (1), could reinforce its loop-extrusion impediment activity (Fig. 5B). Complete WAPL depletion in v-Abl cells substantially diminishes downstream RC interactions (Fig. 5B). In addition, interactions of the IGCR1/CBE1 with the RC, as well as with the γ1-γ2b enhancer and 3′Igh CBEs, are also greatly diminished in WAPL-depleted v-Abl cells (Fig. 5C), consistent with WAPL-depletion diminishing transcription-based RC impediment activity. In contrast, in WT pro-B cells, in which WAPL levels are modestly reduced (44), these interactions are relatively robust (Fig. 2B). We propose that the 6.5-fold decrease in DJH rearrangements in WAPL-depleted v-Abl cells results from decreased RC activity (Figs. 4I and 5B), as previously proposed for a similar reduction in Vκ-to-Jκ rearrangements upon WAPL depletion in this v-Abl line (22). Notably, WAPL-depletion reduced rearrangement levels of all Ds, other than DQ52, leading to DQ52 contributing more substantially to residual DJH rearrangements (Fig. 4N). A similar overall trend was obtained when previously reported data that employed JH 1-4 baits (22) were analyzed for absolute levels, as well as relative percentages (SI Appendix, Fig. S7 D and E and Table S4). We propose that DQ52 recombination, after WAPL depletion, may be less affected, because it accesses RAG by diffusion (versus scanning) from its RC location (24). Finally, diverse VHDJH rearrangements in WAPL-depleted cells occurred at a similarly low, absolute level to those of proximal VHs in untreated cells. However, VH rearrangements in WAPL-depleted cells contributed to a 5.3-fold increase in the proportion of VHDJH/DJH rearrangements (Fig. 4 I and J). This latter finding may reflect increased levels of VHDJH recombination in WAPL-depleted v-Abl cells compensating for reduced RC activity (Fig. 4). However, the net effect is that overall V(D)J recombination levels are much lower in WAPL-depleted v-Abl lines than in BM pro-B cells as reported (22).
Deletion of 3′Igh CBEs decreased DJH and VHDJH rearrangement levels in both untreated and WAPL-depleted v-Abl cells. Yet, despite the nearly 50% decrease in V(D)J junctions in WAPL-depleted 3′CBE− versus WAPL depleted WT v-Abl cells, their VH utilization patterns across the VH locus were nearly identical. These findings support the proposal that the 3′Igh CBEs help maintain RC activity by reinforcing its impediment activity for the RAG scanning process (SI Appendix, Fig. S10) (1). We note that the extent to which the 3′Igh CBEs reinforce RC activity may be compensated, in its absence, by interactions of the RC with downstream CBEs; it is also notable that these downstream CBE interactions are diminished by complete WAPL downregulation (Fig. 5B). Such compensatory activity of downstream CBEs, in the absence of 3′Igh CBEs, was also implicated in the context of Igh class switch recombination (50). Finally, normal pro-B cells do not completely down-regulate WAPL levels (44), which may contribute to preserving 3′Igh CBEs/RC interactions and RC activity in these cells. In this regard, WT and 3′CBE− pro-B cells have indistinguishable Igh V(D)J recombination patterns (22).
Comparison of the RC-interacting peaks from 129SV pro-B cells (Fig. 2A), and C57BL/6 v-Abl cells (Fig. 5A) shows that a number of the peaks C57BL/6 Peaks 6–9, 12–13 (Fig. 5A) are shared, while others are unique due to the significant differences in the VH loci in these two strains. Major peaks in J558/3609, distal VHs region, are often associated with PAIR elements (Peaks 1, 4, 6, 8–10 in Fig. 2A and peaks 1-8 in Fig. 5A), while other peaks in J558/3609, J558, and middle VH regions (Peaks 2, 3, 5, 7, 11–15 in Fig. 2A and peaks 9–13 in Fig. 5A) are associated with transcription or CBE-binding motifs. These observations support the notion that when WAPL is down-regulated, upon the neutralization of IGCR1, various transcription sites and CBEs still form sufficiently active loop extrusion impediments to promote interactions with the RC during RAG scanning of upstream VHs locus sequences.
Methods
Mice.
Wild-type 129SV mice were purchased from Charles River Laboratories International. RAG2-deficient mice in 129SV background were purchased from Taconic. All animal experiments were performed under protocols approved by the Institutional Animal Care and Use Committee of Boston Children’s Hospital.
Generation of IGCR1 CBE-Inversion Mice.
A previously described pLNTK targeting vector (49) containing inversion mutations of the 20-bp CBE1 and corresponding upstream activating sequence (WT sequence: 5′-TGCTTCCCCCTTGTGGCCATGAGCATTACTGCA-3′; inverted: 5′-TGCAGTAATGCTCATGGCCACAAGGGGGAAGCA-3′); or the 19-bp CBE2 (WT sequence: 5′-TCTCCACAAGAGGGCAGAA-3′; inverted sequence: 5′-TTCTGCCCTCTTGTGGAGA-3′) sites within IGCR1 were electroporated into TC1 ES cells. Successfully targeted clones with CBE1 or CBE2 inversion integration were assessed by Southern blot analyses using StuI-digested (13.9-kb untargeted; 10-kb targeted) or SpeI-digested (16.3-kb untargeted; 12.7-kb targeted) genomic DNA with appropriate probes. Two independently targeted clones containing each inversion mutation were subjected to adenovirus mediated Cre deletion to remove the NeoR gene, karyotyped, and injected for germline transmission. Homozygous mice were generated through breeding and genotype was confirmed by PCR genotyping (Primer sequences are listed in SI Appendix, Table S5).
Generation of v-Abl Cell Lines.
The WT v-Abl-kinase-transformed pro-B cell line (v-Abl pro-B cells) was derived by retroviral infection of BM pro-B cells derived from rag2−/− mice as described (48). IGCR1-mutated RAG2-deficient v-Abl lines were established by breeding each IGCR1 mutant mice with rag2−/− germline mice to generate RAG2-deficient homozygous IGCR1-mutated mice (i.e., IGCR1/CBE1−/−rag2−/−), and deriving v-Abl lines as described (48). All these mutations were confirmed by PCR genotyping. 3′Igh CBE deletion in single Igh WAPL-degron v-Abl (22) were generated by designed sgRNAs and screened by PCR. The sequence of all sgRNAs and oligos used is listed in SI Appendix, Table S5.
HTGTS-V(D)J-seq and Data Analyses.
Pro-B cells used in HTGTS-V(D)J-seq experiments were purified from WT or IGCR1-mutated mice as described (53). 2ug pro-B cell genomic DNA were used to generate each library. The sequence of the JH4 coding end primer (129SV background) used to generate HTGTS-V(D)J-seq libraries is listed in SI Appendix, Table S5. HTGTS-V(D)J-seq libraries were prepared as described (24). HTGTS-V(D)J-seq libraries were sequenced using paired-end 300-bp sequencing on a Mi-Seq (Illumina) machine. The WT 129SV pro-B cell data shown in Fig. 1 and SI Appendix, Figs. S1 and S2 were extracted from a prior publication (GSM2183881- GSM2183883) (53). All libraries were normalized to total reads (junctions+germine reads) or junctions across a given locus, and the VHDJH and DJH junctions are described in SI Appendix, Tables S1 and S2. When normalized to total reads, all libraires were normalized to the smallest libraries from the same batch of experiments. The number of normalized reads or junctions is indicated in the figure and figure legends. D usage from the VHDJH joins was analyzed via the VDJ_annotation pipeline. Productive and nonproductive VHDJH joins were analyzed via VDJ_productivity_annotation pipeline (Data, Materials, and Software Availability).
RAG recombination and treatment of WAPL-degron v-Abl cells was performed as described (22), and the JH4 coding end primer (C57BL/6 background) used to generate HTGTS-V(D)J-seq libraries. All libraries were normalized to total reads or junctions, and the VHDJH and DJH junctions are described in SI Appendix, Table S3. D usage from the VHDJH joins was analyzed by the VDJ_annotation pipeline.
3C-HTGTS and Data Analyses.
RAG2-deficient pro-B cells for 3C-HTGTS were purified and cultured as described (19). Cycling or G1-arrested RAG2-deficient v-Abl pro-B cells for 3C-HTGTS were prepared as described (18). Treatment of WAPL-degron v-Abl cells was performed as described (22). 3C-HTGTS was performed as described (18). Briefly, 10 million cells were cross-linked with 2% (v/v) formaldehyde for 10 min at RT. Cells were lysed in 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 1% Triton X-100 and protease inhibitors (Roche, #11836153001). Nuclei were digested with 700 units of NlaIII (NEB, #R0125) restriction enzyme at 37°C overnight, followed by ligation (T4 DNA ligase NEB M0202L) at 16°C overnight. Cross-links were reversed and samples were treated with Proteinase K (Roche, #03115852001) and RNase A (Invitrogen, #8003089) prior to DNA precipitation. 3C-HTGTS libraries were generated using LAM-HTGTS (56), and primers are listed in SI Appendix, Table S5.
3C-HTGTS libraries were sequenced using paired-end 150-bp sequencing on a Next-seq550 (Illumina) or paired-end 300-bp sequencing on a Mi-Seq (Illumina) machine. Data were processed as described previously (18). In addition, the PCR artificial junctions at Chr12: 114,692,680 in SI Appendix, Fig. S6 A were removed from the total junctions. The junctions from Chr12 were extracted and counted for normalization. All 3C-libraires were normalized to the smallest libraries from the same batch of experiments. The number of normalized junctions is indicated in the figure legends. For peak analysis, 3C-HTGTS profiles were analyzed by MACS2 pipeline to call robust interaction peaks (macs2 bdgpeakcall -c20 -l400 -g1000 was used for pro-B 3C-HTGTS in Fig. 2 and macs2 bdgpeakcall -c30 -l400 -g1000 was used for v-Abl 3C-HTGTS in Fig. 5). The peaks that showed >twofold intensity change were annotated as unique peaks of indicated condition. CBE motifs were called by the MEME-FIMO scanning CTCF motif (MA0139.1 in JASPAR database) (57) and validated by previously published ChIP-seq data (19, 22).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank members of the Alt laboratory for stimulating discussions. This work was supported by the NIH Grant R01AI020047 to F.W.A. and Grant F31-AI117920 to S.G.L.; Z.B. and H.-Q.D. were supported in part by Cancer Research Institute Irvington Fellowships. F.W.A. is an investigator of the Howard Hughes Medical Institute.
Author contributions
Z.L., L.Z., S.G.L., Z.B., and F.W.A. designed research; Z.L., L.Z., S.G.L., Y.Z., H.-Q.D., and Z.B. performed research; Z.L., S.G.L., C.G., and Z.B. contributed new reagents/analytic tools; Z.L., A.Y.Y., S.G.L., Z.B., and F.W.A. analyzed data; and Z.L., L.Z., Z.B., and F.W.A. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: C.M., University of California, San Diego; and A.N., National Cancer Institute.
Contributor Information
Zhuoyi Liang, Email: zhuoyi.liang@childrens.harvard.edu.
Zhaoqing Ba, Email: bazhaoqing@nibs.ac.cn.
Frederick W. Alt, Email: alt@enders.tch.harvard.edu.
Data, Materials, and Software Availability
3C-HTGTS and HTGTS-V(D)J-seq data were processed through published pipelines (http://robinmeyers.github.io/transloc_pipeline/) as described (18). D usage in VHDJH joins was processed via a custom pipeline (https://github.com/Yyx2626/VDJ_annotation/) (19). Productive and non-productive junctions were processed via another pipeline (https://github.com/Yyx2626/VDJ_annotation/) (19). HTGTS-V(D)J-seq, 3C-HTGTS and GRO-seq sequencing data reported in this study are available through GEO (GSE230605) (58). All study data are included in the article and/or SI Appendix. Previously published data were used for this work [GSE151910 (22) and GSE821126 (53)].
Supporting Information
References
- 1.Zhang Y., Zhang X., Dai H.-Q., Hu H., Alt F. W., The role of chromatin loop extrusion in antibody diversification. Nat. Rev. Immunol. 9, 1–17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C., Zhang Y., Liu C. C., Schatz D. G., Structural insights into the evolution of the RAG recombinase. Nat. Rev. Immunol. 22, 353–370 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Alt F. W., Zhang Y., Meng F.-L., Guo C., Schwer B., Mechanisms of programmed DNA lesions and genomic instability in the immune system. Cell 152, 417–429 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji Y., et al. , The in vivo pattern of binding of RAG1 and RAG2 to antigen receptor loci. Cell 141, 419–431 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng G., Schatz D. G., Regulation and evolution of the RAG recombinase. Adv. Immunol. 128, 1–39 (2015). [DOI] [PubMed] [Google Scholar]
- 6.MacPherson M. J., Sadowski P. D., The CTCF insulator protein forms an unusual DNA structure. BMC Mol. Biol. 11, 101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakahashi H., et al. , A genome-wide map of CTCF multivalency redefines the CTCF code. Cell Rep. 3, 1678–1689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao S. S. P., et al. , A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fudenberg G., et al. , Formation of chromosomal domains by loop extrusion. Cell Rep. 15, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanborn A. L., et al. , Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. U.S.A. 112, E6456–E6465 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vietri Rudan M., et al. , Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Rep. 10, 1297–1309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraft K., et al. , Serial genomic inversions induce tissue-specific architectural stripes, gene misexpression and congenital malformations. Nat. Cell Biol. 21, 305–310 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Hsieh T.-H.S., et al. , Resolving the 3D landscape of transcription-linked mammalian chromatin folding. Mol. Cell 78, 539–553.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y., et al. , CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell 162, 900–910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Wit E., et al. , CTCF binding polarity determines chromatin looping. Mol. Cell 60, 676–684 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Jia Z., et al. , Tandem CTCF sites function as insulators to balance spatial chromatin contacts and topological enhancer-promoter selection. Genome Biol. 21, 75 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruiz-Velasco M., et al. , CTCF-mediated chromatin loops between promoter and gene body regulate alternative splicing across individuals. Cell Syst. 5, 628–637.e6 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Jain S., Ba Z., Zhang Y., Dai H.-Q., Alt F. W., CTCF-binding elements mediate accessibility of RAG substrates during chromatin scanning. Cell 174, 102–116.e14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ba Z., et al. , CTCF orchestrates long-range cohesin-driven V(D)J recombinational scanning. Nature 586, 305–310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banigan E. J., et al. , Transcription shapes 3D chromatin organization by interacting with loop extrusion. Proc. Natl. Acad. Sci. U.S.A. 120, e2210480120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brandão H. B., et al. , RNA polymerases as moving barriers to condensin loop extrusion. Proc. Natl. Acad. Sci. U.S.A. 116, 20489–20499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai H.-Q., et al. , Loop extrusion mediates physiological Igh locus contraction for RAG scanning. Nature 590, 338–343 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neguembor M. V., et al. , Transcription-mediated supercoiling regulates genome folding and loop formation. Mol. Cell 81, 3065–3081.e12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., et al. , The fundamental role of chromatin loop extrusion in physiological V(D)J recombination. Nature 573, 600–604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo C., et al. , CTCF-binding elements mediate control of V(D)J recombination. Nature 477, 424–430 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degner S. C., Wong T. P., Jankevicius G., Feeney A. J., Cutting edge: Developmental stage-specific recruitment of cohesin to CTCF sites throughout immunoglobulin loci during B lymphocyte development. J. Immunol. 182, 44–48 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birshtein B., The role of CTCF binding sites in the 3′ immunoglobulin heavy chain regulatory region. Front. Genet. 3, 251 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrett F. E., et al. , Chromatin architecture near a potential 3′ End of the Igh locus involves modular regulation of histone modifications during B-cell development and in vivo occupancy at CTCF sites. Mol. Cell Biol. 25, 1511–1525 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bossen C., Mansson R., Murre C., Chromatin topology and the regulation of antigen receptor assembly. Annu. Rev. Immunol. 30, 337–356 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Proudhon C., Hao B., Raviram R., Chaumeil J., Skok J. A., Long-range regulation of V(D)J recombination. Adv. Immunol. 128, 123–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vian L., et al. , The energetics and physiological impact of cohesin extrusion. Cell 173, 1165–1178.e20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu J., et al. , Chromosomal loop domains direct the recombination of antigen receptor genes. Cell 163, 947–959 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin S. G., Ba Z., Alt F. W., Zhang Y., RAG chromatin scanning during V(D)J recombination and chromatin loop extrusion are related processes in Advances in Immunology (Elsevier, 2018), pp. 93–135. [DOI] [PubMed] [Google Scholar]
- 34.Benner C., Isoda T., Murre C., New roles for DNA cytosine modification, eRNA, anchors, and superanchors in developing B cell progenitors. Proc. Natl. Acad. Sci. U.S.A. 112, 12776–12781 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuxa M., et al. , Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 18, 411–422 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jhunjhunwala S., et al. , The 3D structure of the immunoglobulin heavy-chain locus: Implications for long-range genomic interactions. Cell 133, 265–279 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kosak S. T., et al. , Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296, 158–162 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Medvedovic J., et al. , Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity 39, 229–244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montefiori L., et al. , Extremely long-range chromatin loops link topological domains to facilitate a diverse antibody repertoire. Cell Rep. 14, 896–906 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roldán E., et al. , Locus “decontraction” and centromeric recruitment contribute to allelic exclusion of the immunoglobulin heavy-chain gene. Nat. Immunol. 6, 31–41 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rother M. B., et al. , Nuclear positioning rather than contraction controls ordered rearrangements of immunoglobulin loci. Nucleic Acids Res. 44, 175–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sayegh C., Jhunjhunwala S., Riblet R., Murre C., Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells. Genes Dev. 19, 322–327 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas J. S., Zhang Y., Dudko O. K., Murre C., 3D trajectories adopted by coding and regulatory DNA elements: First-passage times for genomic interactions. Cell 158, 339–352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill L., et al. , Wapl repression by Pax5 promotes V gene recombination by Igh loop extrusion. Nature 584, 142–147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gassler J., et al. , A mechanism of cohesin-dependent loop extrusion organizes zygotic genome architecture. EMBO J. 36, 3600–3618 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haarhuis J. H. I., et al. , The cohesin release factor WAPL restricts chromatin loop extension. Cell 169, 693–707.e14 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wutz G., et al. , Topologically associating domains and chromatin loops depend on cohesin and are regulated by CTCF, WAPL, and PDS5 proteins. EMBO J. 36, 3573–3599 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bredemeyer A. L., et al. , ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature 442, 466–470 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Lin S. G., Guo C., Su A., Zhang Y., Alt F. W., CTCF-binding elements 1 and 2 in the Igh intergenic control region cooperatively regulate V(D)J recombination. Proc. Natl. Acad. Sci. U.S.A. 112, 1815–1820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X., Yoon H. S., Chapdelaine-Williams A. M., Kyritsis N., Alt F. W., Physiological role of the 3′IgH CBEs super-anchor in antibody class switching. Proc. Natl. Acad. Sci. U.S.A. 118, e2024392118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alt F. W., et al. , Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 3, 1209–1219 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumari G., Sen R., Chapter two–Chromatin interactions in the control of immunoglobulin heavy chain gene assembly in Advances in Immunology, Molecular Mechanisms that Orchestrate the Assembly of Antigen Receptor Loci, Murre C., Ed. (Academic Press, 2015), pp. 41–92. [DOI] [PubMed] [Google Scholar]
- 53.Lin S. G., et al. , Highly sensitive and unbiased approach for elucidating antibody repertoires. Proc. Natl. Acad. Sci. U.S.A. 113, 7846–7851 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebert A., et al. , The distal VH gene cluster of the Igh locus contains distinct regulatory elements with Pax5 transcription factor-dependent activity in Pro-B cells. Immunity 34, 175–187 (2011). [DOI] [PubMed] [Google Scholar]
- 55.Verma-Gaur J., et al. , Noncoding transcription within the Igh distal VH region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc. Natl. Acad. Sci. U.S.A. 109, 17004–17009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frock R. L., et al. , Genome-wide detection of DNA double-stranded breaks induced by engineered nucleases. Nat. Biotechnol. 33, 179–186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bailey T. L., Johnson J., Grant C. E., Noble W. S., The MEME suite. Nucleic Acids Res. 43, W39–W49 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye A. Y., Contribution of IGCR1 and 3′ CBE super anchor to developmental regulation of Igh V(D)J recombination. Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE230605. Deposited 25 April 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
3C-HTGTS and HTGTS-V(D)J-seq data were processed through published pipelines (http://robinmeyers.github.io/transloc_pipeline/) as described (18). D usage in VHDJH joins was processed via a custom pipeline (https://github.com/Yyx2626/VDJ_annotation/) (19). Productive and non-productive junctions were processed via another pipeline (https://github.com/Yyx2626/VDJ_annotation/) (19). HTGTS-V(D)J-seq, 3C-HTGTS and GRO-seq sequencing data reported in this study are available through GEO (GSE230605) (58). All study data are included in the article and/or SI Appendix. Previously published data were used for this work [GSE151910 (22) and GSE821126 (53)].