Abstract
Aging-related hypoxia, oxidative stress, and inflammation pathophysiology are closely associated with human age-related carcinogenesis and chronic diseases. However, the connection between hypoxia and hormonal cell signaling pathways is unclear, but such human age-related comorbid diseases do coincide with the middle-aging period of declining sex hormonal signaling. This scoping review evaluates the relevant interdisciplinary evidence to assess the systems biology of function, regulation, and homeostasis in order to discern and decipher the etiology of the connection between hypoxia and hormonal signaling in human age-related comorbid diseases. The hypothesis charts the accumulating evidence to support the development of a hypoxic milieu and oxidative stress-inflammation pathophysiology in middle-aged individuals, as well as the induction of amyloidosis, autophagy, and epithelial-to-mesenchymal transition in aging-related degeneration. Taken together, this new approach and strategy can provide the clarity of concepts and patterns to determine the causes of declining vascularity hemodynamics (blood flow) and physiological oxygenation perfusion (oxygen bioavailability) in relation to oxygen homeostasis and vascularity that cause hypoxia (hypovascularity hypoxia). The middle-aging hypovascularity hypoxia hypothesis could provide the mechanistic interface connecting the endocrine, nitric oxide, and oxygen homeostasis signaling that is closely linked to the progressive conditions of degenerative hypertrophy, atrophy, fibrosis, and neoplasm. An in-depth understanding of these intrinsic biological processes of the developing middle-aged hypoxia could provide potential new strategies for time-dependent therapies in maintaining healthspan for healthy lifestyle aging, medical cost savings, and health system sustainability.
Keywords: healthspan, aging, hypoxia, oxidative stress, inflammation, nitric oxide, hypoxia-inducible factor, oxygen sensing
Introduction
Aging-related hypoxia, oxidative stress, and inflammation pathophysiology are closely associated with human age-related carcinogenesis and chronic diseases (Muz et al., 2015; Göbel et al., 2021; Mas-Bargues et al., 2021; Bouhamida et al., 2022; Gambini and Stromsnes, 2022; Leyane et al., 2022; Wei et al., 2022). However, the connection between hypoxia and hormonal cell signaling pathways is unclear (Yang et al., 2018; Tran et al., 2020; Jehanno et al., 2022), but such human age-related comorbid diseases do coincide with the middle-aging period of declining sex hormonal signaling (Horstman et al., 2012; Diamanti-Kandarakis et al., 2017; Khadilkar, 2019). The middle-aging period is between the fifth (40s) and before the seventh (60s) decade of life (Phua, 2021). Aged-related comorbid diseases (Franceschi et al., 2018; Laconi et al., 2020) are a global burden (Kocarnik et al., 2022) with consequences for future health system sustainability (Soerjomataram and Bray, 2021). In one study of cancer-related deaths in the United States, 90% of cancers were diagnosed in the aged group of those over 50 years (Siegel et al., 2022).
Current knowledge shows that age-related diseases in humans are complex and heterogeneous in nature. This scoping review (Munn et al., 2018; Sargeant and O’Connor, 2019) aims to evaluate the relevant interdisciplinary (Greenwald and Dunn, 2009; Okamura, 2019; Cherbuin et al., 2021) evidence that has been accrued to assess the systems biology (Wang et al., 2018; Batie et al., 2022; Zhang et al., 2022) of function, regulation, and homeostasis in order to discern and decipher the etiology of the connection between hypoxia and hormonal signaling in human age-related comorbid diseases. It requires a descriptive approach to provide a series of stepwise, evidence-based functional interactions between the interdisciplinary modules in constructing the hypothesis of hypovascularity hypoxia.
Altogether, this new approach and strategy (Brennan and Davey-Smith, 2022; Diokno, 2022; Zhang et al., 2022) can provide clarity of concepts and patterns to determine the causes of declining vascularity hemodynamics (blood flow) and physiological oxygenation perfusion (physoxia) related to oxygen homeostasis and vascularity that cause hypoxia (hypovascularity hypoxia).
The prostate aging degeneration hypothesis postulates that this triad of testosterone, vascular, and inflamm-aging results in the conjoining of nitric oxide downregulation and vascular/endothelial dysfunction and inflammation, leading to age-related dysfunctions of amyloidosis and autophagy within an evolutionary tumorigenesis microenvironment (Phua, 2021). The earlier author’s short text findings, published in the journal Medicines by MDPI, form the basis for the discussion on prostate aging degeneration, and the crosstalk between testosterone, vascular, inflamm-aging, p53, cellular senescence, amyloidosis and autophagy below.
This integrative scoping review (Munn et al., 2018; Sargeant and O’Connor, 2020) would evaluate the importance of vascular function from the prostate aging degeneration hypothesis (Phua, 2021) in unlocking the biological secrets of aging (Borrás, 2021) and for the non-mutagenic promoters in carcinogenesis (Brennan and Davey-Smith, 2022). Both disturbances of cellular oxygen homeostasis and their impact on the physiology of body functions (Tretter et al., 2021), as well as oxygen sensing that is being highjacked in cancer (Claesson-Welsh, 2020), are correlated.
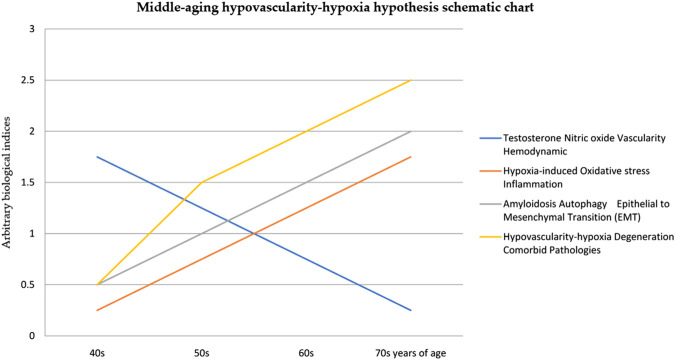
Middle-aging hypovascularity hypoxia hypothesis patterns
Fundamental cell signaling links
The middle-aging hypovascularity hypoxia hypothesis could provide us with insights into connecting various cell signaling pathways in understanding the etiology of hypoxia genesis and its downstream cellular pathophysiological effects. These are based on the three crucial cell signaling findings that can explain the development of hypovascularity hypoxia (Figure 1):
1. Endocrine signaling: testosterone or estrogen replacement therapy can effectively reverse the testosterone deprivation caused by orchiectomy in rats’ experiments with urethral hypovascularity (Yura et al., 2020; Gerbie et al., 2021), and hypogonadal status patients have been found to have urethral hypovascularity (Hofer and Morey, 2018).
2. Nitric oxide signaling: association of endothelial dysfunction and nitric oxide signaling in the pathogenesis of Alzheimer’s disease (Ahmed et al., 2022) and reduction of estrogen, which lowers nitric oxide bioavailability and induces amyloid deposition, have been observed (Cheboub et al., 2019).
3. Oxygen homeostasis: in a study of aging, the hypoxic response mediated by the hypoxia-inducible factor (HIF) at an environmental hypoxia of 15% oxygen for 6 weeks was associated with higher vascularity and was concluded to be the continuous, non-full-scale activation of the HIF pathway that appears to mediate protection against neurodegeneration (Ollonen et al., 2022). When cells have normal oxygen levels, the HIF is constantly degraded (Jaakkola et al., 2001; Berra et al., 2003; Voit and Sankaran, 2020) through the oxygen-sensing pathway in order to maintain oxygen homeostasis (Yang G. et al., 2020; Claesson-Welsh, 2020; Liao and Zhang, 2020; van Vliet et al., 2021a).
FIGURE 1.
Middle-aging hypoxia hypothesis.
Various aged-related chronic diseases, such as metabolic disorders, cardiovascular diseases, erectile dysfunction, cognition, and cancer, are associated with endocrine (Asih et al., 2017; Diamanti-Kandarakis et al., 2017; Foresta et al., 2017; Cai and Li, 2020; Cannarella et al., 2021; Jockers and Liu, 2021; Leisegang et al., 2021; Assar et al., 2022; Mazzilli et al., 2022; Romejko et al., 2022) and nitric oxide signaling (Radi, 2018; Carlström, 2021; Ledo et al., 2021; Mintz et al., 2021; Pourbagher-Shahri et al., 2021). Systemic microvascular ischemic endothelial dysfunction is a common condition associated with the pathogenesis of diseases (Andersson et al., 2017; Jalnapurkar et al., 2021; Balistreri, 2022), hypoxia (Chen et al., 2008; Jung et al., 2016; Chen et al., 2022a), and vascular remodeling (Yuan and Kevil, 2016; Rajendran P. et al., 2019; Huang et al., 2022). Loss of microvasculature (hypovascularity) implies a developing hypoxic milieu and suggests an important role for chronic hypoxia as an explanation for the progressive nature of fibrosis—the chronic hypoxia hypothesis (Fine and Norman, 2008). Hypoxia is one of the main causes of vascular remodeling (Huang et al., 2022), but it has not been investigated as direct links to the development of hypovascularity hypoxia (declining micro-vessel densities) (Huang and Giordano, 2008).
Physiological oxygenation perfusion
Oxygen homeostasis and its master regulator, the HIFs, are organizing principles for understanding metazoan evolution, ontology, physiology, and pathology (Semenza, 2010). Aging is accompanied by the development of systemic, gradually increasing hypoxia-related dysfunctions, which are a characteristic of many human diseases (Bowler and Ladomery, 2019; Dzhalilova and Makarova, 2022; Luo et al., 2022). Chronic (continuous, non-interrupted, and sustained) and cyclic (intermittent and transient) hypoxia, which are characterized by fluctuations in oxygen levels (Bader et al., 2020), have been linked to the development of human diseases and cancer (Saxena and Jolly, 2019; Chen et al., 2020; Liu et al., 2022). Chronic sustained hypoxia (CSH) seems to compromise the pulmonary circulation and carotid body stimulation to maintain oxygen levels, whereas the effects of chronic intermittent hypoxia (CIH) appear to be more targeted on the systemic circulation (Prieto-Lloret et al., 2021).
Currently, the experimental cellular oxygen bioavailability levels and findings are confusing and lack clarity in the literature. There are numerous publications showing a wide range of oxygen levels and applications: environmental hypoxia (15% O2) (Ollonen et al., 2022), non-physiological hyperoxia (21% O2) (Schumacher et al., 2022), normoxia/hypoxia/hyperoxia relativities (Tretter et al., 2020; Tretter, 2022), intermittent hyperoxia–hypoxia paradox (Hadanny and Efrati, 2020), normobaric oxygen paradox (Fratantonio et al., 2021), hyperoxia (100% O2)/hypoxia (12% O2) (Hommer et al., 2022), normoxia (>8.5% O2) (Liu et al., 2022), and “hyperoxic micro-oxygen factories” (Wang W. et al., 2022). This indicates a need to standardize definitions and understand the fluctuations of in vivo oxygen bioavailability levels in cellular physiology processes and toxicity (Tessema et al., 2021; Alva et al., 2022; Lius and Syafaah, 2022).
Therefore, due to the lack of standardized definitions, the findings of the hypoxic response mediated by the HIF at “environmental hypoxia” of 15% oxygen for 6 weeks in an aging mice study model, which was associated with higher vascularity (Ollonen et al., 2022) and “physiological hypoxia” at 7% oxygen in culture conditions showing an enhanced microvasculature formation in the laboratory kidney organoid (Schumacher et al., 2022), would need careful interpretation as “physoxia oxygenation perfusion.”
This review focuses on the actual physiological levels of oxygen exposure in normal human tissues in vivo (bioavailability) (Alva et al., 2022). A range of about 3%–7.4% oxygen (physoxia)would allow for the comparison of oxygen bioavailability levels between physoxia (5% O2), normoxia (20% O2), and hypoxia (1% O2) (McKeown, 2014). In addition, another term for physoxia is physioxia (Adebayo and Nakshatri, 2022; Alva et al., 2022), which has shown distinct key signaling network expression in laboratory cancer cells to recapitulate their physio-pathological status in the in vivo microenvironment (Kumar et al., 2022).
It is the hemodynamic (blood flow) in the microvasculature (microvascular/endothelial) perfusion network (Hesh et al., 2019; Schmid et al., 2019; Taylor and Bordoni, 2022) and not the content of oxygen in the blood that is the main physiological driver of in vivo tissue oxygenation perfusion by erythrocytes (Premont et al., 2020). Aging microvasculature (Kalaria and Hase, 2019; Graff et al., 2021) is associated with hypovascularity perfusion, which affects hemodynamics, oxygenation, and vascular remodeling, and is a cause of human diseases (Forsberg et al., 2018; Moeini et al., 2018; Dalby et al., 2019; Santamaría et al., 2020). The primary guarantor of tissue oxygenation is blood flow (hemodynamics) (Jacob et al., 2016), which would be affected by the developing microvasculature hypovascularity.
Nitric oxide–cyclic guanosine-monophosphate pathway—vascular function
The nitric oxide–cyclic guanosine 3′,5′-monophosphate (NO-cGMP) pathway (Garmaroudi et al., 2016; Mónica et al., 2016; Carlström, 2021) is central for maintaining and sustaining vasodilation (Böger and Hannemann, 2020), vasculature (Costa et al., 2021), and vascular function (Golshiri et al., 2020), as reduced nitric oxide bioavailability can cause endothelial dysfunction (Münzel et al., 2021; Boughaleb et al., 2022), vasoconstriction (Bank et al., 1994; Hannemann and Böger, 2022), and hypoxia (Reinero et al., 2021; Gajecki et al., 2022). Sex hormones, such as testosterone, are linked to the NO-cGMP pathway (Andric et al., 2010), indicating an interdependent relationship between testosterone (androgen) and nitric oxide levels (Hotta et al., 2019; Gur et al., 2020; Zabbarova et al., 2022), which can be related to fluctuations in oxygen perfusion bioavailability (Soni and Padwad, 2017; Nascimento-Filho et al., 2022). The endothelium is an endocrine organ (Stanek et al., 2018; Krüger-Genge et al., 2019) in the human vascular system (Chaudhry et al., 2022), forming the largest microvasculature endothelial surface area network and acting as the gatekeeper of vascular function (Hennigs et al., 2021; Boric and Figueroa, 2022; Howe et al., 2022) in microvasculature cellular communications (Clegg and Mac Gabhann, 2015; Reiterer and Branco, 2020). Such a microvascular dysfunction (endothelial dysfunction) is a common pathophysiological change that occurs in various diseases, such as type 2 diabetes, heart failure, dementia, and depression (Houben et al., 2017; Li W. et al., 2020). This provides a cross-talk between the testosterone–vascular–inflammation-aging triad (Phua, 2021) and nitric oxide signaling (Radi, 2018; Carlström, 2021; Ledo et al., 2021; Mintz et al., 2021; Pourbagher-Shahri et al., 2021).
Testosterone–vascular–inflamm-aging triad
The testosterone–vascular–inflamm-aging triad (Phua, 2021) is characterized by declining testosterone levels with age over 40 years (Gray et al., 1991; Araujo and Wittert, 2011) and testosterone regulating the NO-cGMP pathway (Andric et al., 2010; Hotta et al., 2019; Gur et al., 2020; Zabbarova et al., 2022). Testosterone deficiency is known to induce endothelial dysfunction (Hotta et al., 2019; Moreau, 2019; Babcock et al., 2022), decrease peri-urethral vascularity (hypovascularity) (Hofer et al., 2017), impair microvascular hyperemia (blood flow) (Corrigan et al., 2015), and reduce nitric oxide production (Vargas et al., 2007; Xiong et al., 2020). In turn, vascular aging is caused by endothelial dysfunction (Donato et al., 2018; Marchio et al., 2019; Rizzoni et al., 2019), which leads to lower peripheral vasodilation (Crecelius et al., 2010; Seals and Alexander, 2018; da Silva et al., 2022), and is correlated with reduced production of nitric oxide (Vanhoutte et al., 2017; Hotta et al., 2019). Inflamm-aging of chronic oxidative stress and inflammation pathophysiology (Phua, 2021) is part of vascular aging (Guzik and Touyz, 2017) and testosterone deficiency (Son et al., 2016; Kataoka et al., 2017; Rovira-Llopis et al., 2017; Babcock et al., 2022).
Nitric oxide signaling—vasodilation/vasoconstriction physoxia hemodynamics
Healthy tissue function, regulation, and homeostasis are dependent on the vascularity hemodynamic (microcirculation). The vascular endothelium and nitric oxide-mediated signaling govern the regulation of blood microcirculation (Tejero et al., 2019). Nitric oxide bioavailability and expression (Gantner et al., 2020; Akseh et al., 2021; Koukoulis et al., 2022) in signaling transduction (Lundberg and Weitzberg, 2022) through the NO-cGMP pathway (Golshiri et al., 2020) is an important biological aspect for nitric oxide signaling (Radi, 2018; Carlström, 2021; Ledo et al., 2021; Mintz et al., 2021; Pourbagher-Shahri et al., 2021) and the endogenous nitric oxide gasotransmitter (Yang et al., 2016; Nowaczyk et al., 2021) in cancer (Salihi et al., 2022), fibrosis (Chen et al., 2021b), and inflammation (Wang L. et al., 2022). Nitric oxide (NO) acts as a paracrine mediator of vasodilation (Freed and Gutterman, 2017), activating soluble guanylyl cyclase (sGC) in vascular smooth muscle cells and producing cyclic guanosine monophosphate (cGMP). It is this NO-sGC-cGMP signaling pathway that initiates relaxation of the vascular smooth muscle (vasodilation) and inhibits platelet aggregation in both the systemic and pulmonary circulations (Vanhoutte et al., 2017; Böger and Hannemann, 2020). In the systemic circulation, hypoxia results in local vasodilation, which has been shown to be brought about by stabilization of hypoxia-inducible factor-1α (HIF1α) and concomitant upregulation of endothelial nitric oxide synthase (Böger and Hannemann, 2020). In contrast, the physiological response to hypoxia in the pulmonary circulation is vasoconstriction (Böger and Hannemann, 2020). Nitric oxide-mediated activation of cyclic guanosine monophosphate (cGMP) signaling inhibits the acquisition of hypoxia-induced malignant phenotypes in tumor cells (Kim et al., 2020). Nitric oxide deficiency has been associated with the pathophysiological conditions of oxidative stress and inflammation (Shefa et al., 2017; Abdel-Zaher et al., 2021; Bayarri et al., 2021), which is similar to the testosterone–vascular–inflamm-aging triad (Phua, 2021).
Based on these facts, therapeutics that maintain and sustain nitric oxide bioavailability and expression (Gantner et al., 2020; Akseh et al., 2021; Koukoulis et al., 2022) through the NO-mediated cGMP pathway would indicate nitric oxide modulation of oxygen sensing (Berchner-Pfannschmidt et al., 2007; Hickok et al., 2013). Nitric oxide signaling donor/enhancer therapeutics (Andersson, 2018; Yang et al., 2021b; Lundberg and Weitzberg, 2022) would provide vasodilation hemodynamics and physoxia (physiological) oxygenation pharmacodynamics. Conversely, the therapeutics that cause nitric oxide downregulation would provide vasoconstriction hemodynamics and hypoxia pharmacodynamics. Such opposing therapeutic pharmacodynamic treatments showed diametrically opposed biological outcomes and side effects.
Nitric oxide in oxygen sensing: a new approach and strategy
Henceforth, modulation of nitric oxide in oxygen sensing (Berchner-Pfannschmidt et al., 2007; Hickok et al., 2013) is a new approach and strategy to understand “physoxia” (physiological) oxygenation (Lam et al., 2019; Mas-Bargues et al., 2019; Merkhan et al., 2021; Reiterer et al., 2022) perfusion in physoxia–NO-mediated rejuvenation–regeneration (Hachmo et al., 2020; Kamat et al., 2021; Rando and Jones, 2021) through the blood vasculature (Rodriguez et al., 2021). The physiological oxygen concentration is crucial for culturing stem cells for use in tissue engineering and regenerative medicine (Mas-Bargues et al., 2019), which can reduce the cytokine profiling of the human mesenchymal stem cell secretome (Merkhan et al., 2021). Nitric oxide-mediated vasodilation of vasculature hemodynamics (Freed and Gutterman, 2017; Böger and Hannemann, 2020) would provide necessary physoxia oxygenation perfusion for cells to constantly degrade the HIF (Jaakkola et al., 2001; Berra et al., 2003; Voit and Sankaran, 2020) through the oxygen-sensing pathway in order to maintain oxygen homeostasis (Yang G. et al., 2020; Claesson-Welsh, 2020; Liao and Zhang, 2020; van Vliet et al., 2021b). Therefore, such nitric oxide-enhanced oxygenation hemodynamic mechanisms (Dewhirst et al., 2005; Shu et al., 2015; Adams et al., 2021; Costa et al., 2021) can be seen as physoxia–NO-mediated reduction of hypoxia-induced oxidative stress and inflammation. NO is an important part of the host defense mechanism and play a protective role at the inflammatory site (Iwata et al., 2020).
Nitric oxide bioavailability and expression signaling
Androgens have been shown to modulate the effects of CIH on the brain (Snyder et al., 2018), correlate with metabolic, vascular, diabetic, and obesity parameters (Groti Antonič et al., 2020) and attenuate hypoxia-induced hypertension (Jiang et al., 2021). Testosterone has been shown to positively regulate functional human corpus cavernosum activities through inhibition of phosphodiesterase type-5 (PDE5) expression and the formation of cGMP and nitric oxide (Gur et al., 2020). Serum levels of testosterone are closely related to levels of endothelial nitric oxide levels (Akseh et al., 2021). It also possesses anti-oxidant (Mancini et al., 2008; Popp Marin et al., 2010; Mendell and MacLusky, 2019; Koukoulis et al., 2022) and anti-inflammatory (Aminuddin et al., 2019; Zhang et al., 2021b; Nasser et al., 2021; Rastrelli et al., 2022) pharmacodynamic properties. Testosterone replacement therapy is used to prevent type 2 diabetes (Haider et al., 2020; Wittert et al., 2021; Yeap and Wittert, 2022), erectile dysfunction (Canguven et al., 2016; Podlasek et al., 2016), and penile fibrosis (Montorsi and Oettel, 2005; Iacono et al., 2012) and to treat lower urinary tract symptoms (Ko et al., 2013; Yassin et al., 2014; Okada et al., 2018).
Testosterone has been shown to inhibit the expression of PDE5 (Gur et al., 2020). PDE5 is part of the NO-sGC-cGMP-PDE5 signaling pathway (Bajraktari et al., 2017; Gur et al., 2020), and its inhibition has been associated with potentiating cancer therapy (Muniyan et al., 2020), counteracting diabetic heart kinetics (Pofi et al., 2022), ameliorating heart failure through cGMP-dependent protein kinase (PKG) activation (Zhu et al., 2022), and supporting increased blood perfusion oxygenation of the vasculature (Giuliano et al., 2013). PDE5 inhibitors restore nitric oxide (Kalsi et al., 2005; Lee et al., 2022) and are selective vasodilators of the NO-cGMP signaling pathway (Aversa et al., 2019; Ahmed et al., 2021). PDE5 inhibitors have been shown to benefit vasculature oxygenation (Morelli et al., 2011; Michel et al., 2015), prevent ischemia–hypoxia (Saito et al., 2014; Zarifpour et al., 2015; Fujii et al., 2019), and reduce microvascular/endothelial dysfunction (Cellek et al., 2014; Ölmestig et al., 2020; Statsenko and Urologiia, 2021); they also reduce oxidative stress (Matsuo et al., 2020), inflammation (Vignozzi et al., 2013; Peixoto and Gomes, 2015), amyloids (Kang et al., 2022), and prostate weight (Kobayashi et al., 2022).
L-arginine/L-citrulline (Shatanawi et al., 2020; Wu G. et al., 2021; Bahadoran et al., 2021; Wijnands et al., 2021) and curcumin (Santos-Parker et al., 2017; Changal et al., 2020; Alidadi et al., 2021; Li K.-X. et al., 2022) are supplements that increase nitric oxide production through their respective L-arginine–nitric oxide pathways (Fleszar et al., 2019; Cziráki et al., 2020; Bahadoran et al., 2021). The increased bioavailability of nitric oxide improves vascular and endothelial function, which is beneficial for human health.
Anti-androgenic therapeutics
Androgen deprivation therapy (ADT) can cause side effects such as weight gain and emotional changes, and it increases the risk of cardiovascular disease, diabetes, and osteoporosis (MacLennan et al., 2023). The European Association of Urology guidelines recommend not offering neoadjuvant ADT before surgery for patients with prostate cancer (MacLennan et al., 2023). ADT increased markers of oxidative stress/inflammation and the serum levels of thromboxane A2 (TXA2), which is associated with cardiovascular risk (Álvarez-Maestro et al., 2021) and endothelial dysfunction (Gilbert et al., 2013; Teoh et al., 2022), similar to that seen in testosterone deficiency (Hotta et al., 2019; Moreau, 2019; Babcock et al., 2022).
Anti-androgenic therapeutics imply nitric oxide downregulation and are known to cause reductions in hemodynamics (Angrimani et al., 2020; Yoon et al., 2020), microvascular density (hypovascularity) (Hochberg et al., 2002; Donohue et al., 2005; Khwaja et al., 2016; Sun et al., 2018; Khera et al., 2020), and inflammation (Saylor et al., 2012; Hoogland et al., 2021; Nazha and Bilen, 2021). Finasteride-related anti-androgenic therapy is associated with an increased risk of higher-grade prostate cancer (Scailteux et al., 2019; Hu et al., 2020), erectile dysfunction (Fertig et al., 2017), and the post-finasteride syndrome of adverse side effects (Gupta M. A. et al., 2020; Diviccaro et al., 2020; Traish, 2020; Howell et al., 2021; Saengmearnuparp et al., 2021). Additionally, finasteride-related penile curvature/Peyronie’s disease is associated with the most adverse drug reaction reports (Schifano et al., 2022), and this Peyronie’s disease is associated with low testosterone (Askari et al., 2019), inflammation (Patel et al., 2020; Swislocki and Eisenberg, 2021), and fibrosis of the tunica albuginea (Chung et al., 2020; Segundo and Glina, 2020).
Translational imaging technologies and retinal microvasculature
Hypovascularity (Hofer and Morey, 2018; Yura et al., 2020; Gerbie et al., 2021) is the detectable loss of micro-vascularity, a geometric structure that serves as an interface hub for hemodynamics, oxygenation, and perfusion exchanges (Secomb, 2016; Pollock et al., 2022). Currently, the role of translational imaging technologies in interrogating the structural and functional status of the microcirculation for clinical applications in human diseases is being explored (Guerraty et al., 2021). There have been numerous retinal microvascular structural and blood flow studies that have shown an association with vascular aging (Wei et al., 2017; Orlov et al., 2019; Arnould et al., 2022; Gómez-Sánchez et al., 2022), sex hormones (Nuzzi et al., 2018; Feng et al., 2021; Aribas et al., 2022), and chronic diseases such as neurodegeneration/diabetic retinopathy (Marques et al., 2022), coronary artery disease (Zhong et al., 2022), type 2 diabetes (Kim H. et al., 2022), and hypertension (Zeng et al., 2022). Retinal microvasculature structures also responded to oxygen availability (Hommer et al., 2022), an aging reduction at >50 years (Abay et al., 2022), and to the anti-androgenic 5α-reductase inhibitor (Shin et al., 2020).
Discussion
Middle-aging hypovascularity hypoxia hypothesis: cyclic/chronic hypoxic milieu
The middle-aging hypovascularity hypoxia hypothesis postulates that the consequences of middle-aging concomitant declining sex hormones (andropause/menopause) and nitric oxide signaling (Phua, 2021) lead to the steady loss of vascularity, hemodynamic changes, and an increasing cyclic/chronic hypoxic milieu (Fine and Norman, 2008) (Figure 1).
Loss of micro-vascularity (Hofer and Morey, 2018; Gerbie et al., 2021) implies an important role for cyclic/chronic hypoxic milieu (Fine and Norman, 2008) in regionalizing perfusion and contributing to the progression of age-related degenerative comorbid diseases in humans. Age-related declining sex hormones (andropause/menopause) are mediated by NO–oxygen sensing (Berchner-Pfannschmidt et al., 2007; Hickok et al., 2013), causing a hypoxic stress condition and dysregulation of the cellular biology of amyloidosis (Cheboub et al., 2019; Ahmed et al., 2022), which is counteracted by autophagy (Chuang et al., 2018; Wang and Le, 2019; Wang and Zhang, 2019), along with the epithelial-to-mesenchymal transition (EMT) (Byrne et al., 2016; Kim I. et al., 2022; Ribatti, 2022; Mohamed et al., 2023). A regionally restricted oxygen perfusion is a cyclic/chronic hypoxic environment that can lead to degenerative pathologies such as hypertrophy, atrophy, fibrosis, and neoplasm due to developing localized hypoxia (Figure 1). Examples of such a range of hypoxic degenerative pathologies can be seen in acute high-altitude hypoxia (hypobaric hypoxia) organ hypertrophy (Pena et al., 2022) and tissue edema (Mesentier-Louro et al., 2021). Others include androgen deprivation therapy promoting epithelial–mesenchymal transition (Byrne et al., 2016), cancer-associated fibroblasts (Kim I. et al., 2022), and prostate cancer progression metastasis (Mohamed et al., 2023).
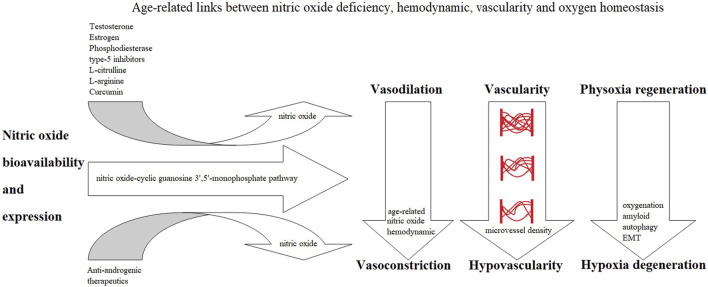
Nitric oxide-mediated hypovascularity: hypoxia-dependent processes
The prevailing narratives for prostate carcinogenesis progression are typically driven by an androgen-dependent process (Aurilio et al., 2020). However, the hypovascularity hypoxia hypothesis, which has accumulated evidence-based patterns, demonstrates an “androgen-induced nitric oxide-mediated hypovascularity hypoxia-dependent process.” Androgen (hormonal) and hypoxia signaling pathways are separate and independent (Tran et al., 2020). Nitric oxide (Dent et al., 2021) through cGMP (Friebe et al., 2020) provides the fundamental link to various NO-sGC-cGMP (Vanhoutte et al., 2017; Böger and Hannemann, 2020) and NO-sGC-cGMP-PDE5 (Bajraktari et al., 2017; Gur et al., 2020) cell signaling pathways to affect NO-mediated oxygen sensing (Berchner-Pfannschmidt et al., 2007; Hickok et al., 2013), affecting vascularity, hemodynamics, and oxygenation perfusion (Amdahl et al., 2019; Kapil et al., 2020) (Figure 2).
FIGURE 2.
Age-related links.
Adult prostate demonstrates remarkable regenerative capacity over multiple cycles of castration and androgen administration, suggesting the existence of an androgen-independent epithelial progenitor in benign prostatic hyperplasia and prostate cancer (Joseph et al., 2021). Androgen and estrogen receptors have been shown to intersect with the HIF/NF-kB signaling in prostate cancer (Russo et al., 2016), and hypoxia increases androgen receptor activity (Park et al., 2006) within a low-androgen environment (Mitani et al., 2011). Higher aggressiveness of prostate cancer correlates with testosterone deficiency (Neuzillet et al., 2019), and the progression of hormone-naïve prostate carcinomas correlates with a low number of vascular vessels (Smentoch et al., 2019). In a case study, superb microvascular imaging (SMI) identified poor internal blood flow in prostate stromal sarcoma (Ohashi et al., 2022).
Vascular aging hypovascularity niches
Oxygen plays a key role in cellular homeostasis, and physiological oxygen levels in various organs range between 2% and 9% in vivo, with the highest levels of 9% in the kidneys and the lowest of 0.5% in parts of the brain (Adebayo and Nakshatri, 2022). Hypovascularity (Yura et al., 2020; Gerbie et al., 2021), reduced microvascular density (Querfeld et al., 2020), and the partially preserved aging microvasculature (Lam et al., 2019) are evidence of decreasing vascularity, hemodynamic perfusion, and physiological oxygenation. Vascular aging, characterized by structural and functional alterations of the vascular wall, is a hallmark of aging (Scioli et al., 2014; Xu et al., 2017; Gkaliagkousi et al., 2022), and vascular endothelial cells can reshape their microenvironment, forming a “niche” (Lei et al., 2022). Decreased blood vessel density and endothelial cell subset dynamics occur during the aging of the endocrine system (J. Chen et al., 2021). In a mouse model of aging, the researchers used super-resolution ultrasound localization microscopy (ULM) and found significant decreases in blood velocity and significant increases in vascular tortuosity across all brain regions in the aged cohort (Lowerison et al., 2022).
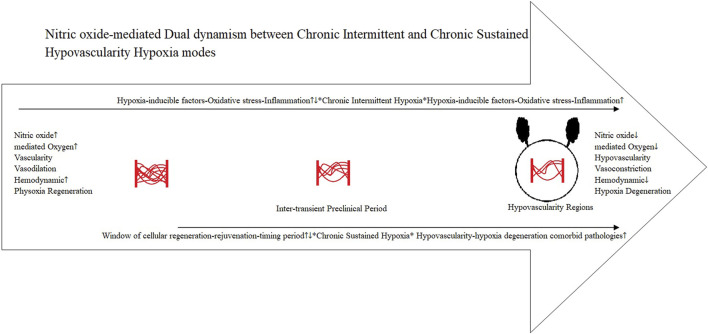
Such intimate vascular aging relationships are formed during the inter-transient preclinical (Krishnan et al., 2018; Younes et al., 2019; Adebayo and Nakshatri, 2022) period between the physoxia regeneration–hypoxia degeneration and adaptive (Pomatto et al., 2019a; Pomatto et al., 2019b) pathological (Lasne et al., 2006; Gilmore et al., 2021) homeostasis, which is time-dependent (Ming et al., 2013; Byrne et al., 2016; Rouverdo et al., 2023). The dual dynamics of observed CIH and CSH (Böger and Hannemann, 2020; Prieto-Lloret et al., 2021) form integration (local) and extension (region) between these two operating modes through the receding microvasculature network of interactions within the regions of cyclic/chronic hypoxia milieu in the tissues, glands, and organs niche (Minami et al., 2019; Jambusaria et al., 2020; Gifre-Renom et al., 2022; Xu et al., 2022) (Figure 3).
FIGURE 3.
Dual dynamism CIH–CSH.
The impaired tissue oxygen delivery is a major cause of organ damage and failure in critically ill patients, even when systemic parameters, including cardiac output and arterial hemoglobin saturation, are close to normal (Roy and Secomb, 2021). Assessments of microvascular function in organ systems are, therefore, crucial (Ku et al., 2021; Xu C. et al., 2022). The median microvascular density was reduced by 29% in skeletal muscle and 24% in the heart in animal models of chronic kidney disease and by 32% in human biopsy, autopsy, and imaging studies (Querfeld et al., 2020). Such a developing cyclic/chronic hypoxia milieu provided the chronic pathological trajectories in rheumatoid arthritis (Fearon et al., 2016; Sabi et al., 2022), cardiovascular (Li et al., 2020b; Godo et al., 2021; Cornuault et al., 2022), and diabetes (Catrina and Zheng, 2021) disorders.
Hypoxia pathological microenvironment niches
Earlier inter-transient preclinical period vascularity in the CIH-systemic circulation (Böger and Hannemann, 2020; Prieto-Lloret et al., 2021) could adequately respond to physoxia regeneration–rejuvenation (Hachmo et al., 2020; Kamat et al., 2021; Rando and Jones, 2021). However, localized cyclic/intermittent hypoxia (CIH) within a region is a potent proinflammatory stimulus in human diseases (Schaefer et al., 2017; Wilson et al., 2018; Korbecki et al., 2021). Thus, advancing the establishment of a late-stage dysfunctional vasculature through vascular remodeling (receding) (Ouarné et al., 2021; Ahmed et al., 2022; Ollonen et al., 2022), which would extend the “CSH compromised pulmonary circulation” region (Böger and Hannemann, 2020; Prieto-Lloret et al., 2021) into an integrated, developing, localized CSH self-perpetuating hypovascularity cyclic/chronic hypoxia pathological microenvironment niche (Carnero and Lleonart, 2016; Macharia et al., 2021; Frisbie et al., 2022) (Figure 3).
Pathological processes associated with hypoxia in age-related diseases are increasingly being recognized in chronic liver disease (Wan et al., 2022), chronic kidney disease (Wang B. et al., 2022), lower urinary tract symptoms—diabetes (Abler and Vezina, 2018), chronic prostatic disease (Luisetto et al., 2019), and prostate carcinogenesis (Deep and Panigrahia, 2015; Ashton and Bristow, 2020; Mohamed et al., 2023). Cyclic/intermittent hypoxia (CIH) induces a replication catastrophe, resulting in an increase in the activity of APOBEC3B (Bader et al., 2021). Hypoxia induces genomic instability through an increase in mutation frequency and enhanced replication stress, inhibiting DNA repair (Hassan Venkatesh et al., 2020; Kaplan and Glazer, 2020; Saitoh and Oda, 2021). Tumor initiation and progression are somatic evolutionary processes driven by the accumulation of genetic alterations (Lahouel et al., 2020; Zahir et al., 2020), and timing analyses suggest that driver mutations often precede diagnosis (preclinical) by many years, if not decades (Gerstung et al., 2020).
Window of cellular regeneration–rejuvenation: window of opportunity and timing hypothesis
Taken together, the critical inter-transient preclinical period (disease/cancer) of physoxia–NO-mediated hemodynamic (Lam et al., 2019; Mas-Bargues et al., 2019; Merkhan et al., 2021; Reiterer et al., 2022) window of cellular regeneration–rejuvenation (Hachmo et al., 2020; Kamat et al., 2021; Rando and Jones, 2021) can be associated with the window of opportunity and timing hypothesis (Figure 3). The window of opportunity and timing hypothesis is the critical timing of hormone therapy initiation with respect to age and/or the menopause (and andropause) transition, and optimal effects are evident with early initiation (Maki, 2013; Speth et al., 2018).
Current observational data show that menopause/hormonal replacement therapy (MPT/HRT) reduces “all-cause mortality” (Akter et al., 2022; Bluming, 2022; Hodis and Mack, 2022; The North American Menopause Society, 2022), decreases the risk of dementia among female patients with depression (Kim T.-Y. et al., 2022), lowers the risk of breast cancer (in Korea) (Baek et al., 2022), and reduces COVID-19 deaths (Sund et al., 2022). Testosterone replacement therapy (TRT) is recommended for late-onset hypogonadism in aging males over 65 years old (Nieschlag, 2020; Burte et al., 2021), but it is still a subject of controversy (Diokno, 2022; Mian et al., 2022). Regardless of testosterone levels, most symptomatic late-onset hypogonadism has been shown not to be correlated (Li et al., 2020c; Ishikawa et al., 2020; La Vignera et al., 2020; Tsuru et al., 2022).
Findings from a randomized clinical trial of testosterone therapy showed that it does not affect lower urinary tract symptoms, but it does improve markers of prostatitis in men with benign prostatic hyperplasia (Rastrelli et al., 2022). This can be reflective of the dual dynamism between the CIH and CSH modes (Böger and Hannemann, 2020; Prieto-Lloret et al., 2021), in which testosterone therapy is not effective on the lower urinary tract symptoms of the already established hypoxic pathological symptomatic localized CSH niche. Conversely, the partially preserved aging microvasculature would still provide the pro-vasculogenic CIH systemic effects (Lam et al., 2019) of testosterone therapy to improve markers of prostatitis in men with benign prostatic hyperplasia (Rastrelli et al., 2022) (Figure 3).
Epithelial–mesenchymal transition
EMT encompasses dynamic changes in cellular organization from epithelial-to-mesenchymal phenotypes, which leads to functional changes in cell migration and invasion, in a diverse range of physiological and pathological conditions (Yang J. et al., 2020). These changes occur during embryogenesis (type 1 EMT), wound healing, tissue regeneration, fibrosis (type 2 EMT) and in cancer, where they contribute to cell stemness (plasticity), drug resistance, immune escape, and metastasis (type 3 EMT) (Manfioletti and Fedele, 2022). EMT can be seen as a period of a bet-hedging—evolutionary cellular adaptation (Jolly et al., 2018; Capp and Thomas, 2022; Jain et al., 2022), which diversifies mesenchymal phenotypes (cancer stemness) and increases their survival (Mortezaee and Majidpoor, 2022) in changing hypoxia-induced pathophysiology (Lee et al., 2020; Papale et al., 2020; Nushtaeva et al., 2023; Zhang et al., 2023).
Hypoxia promotes the aggressiveness of prostate cancer by upregulating the expression of the EMT activator Zeb1 and SK3 channel (Bery et al., 2020). HIF-α promotes the migration and invasion of cancer-associated fibroblasts by miR-210 (Yang Q. et al., 2021). EMT regulators Twist, Slug, and Snail are associated with poor prostate cancer prognosis (Børretzen et al., 2021) and the transforming growth factor-β1 (TGF-β1) cytokine in the tumor microenvironment with autophagy induction (Jena et al., 2022).
Genetically modified mouse experimental models with enrichment of luminal progenitor cells in prostate inflammation, benign prostate hypertrophy, and prostate cancer and the intrinsic castration tolerance of these cells in resistance to androgen deprivation therapy suggest a role in carcinogenesis (Baures et al., 2022). Genomic analysis of benign prostatic hyperplasia implicates cellular re-landscaping in disease pathogenesis (Middleton et al., 2019), and 5-alpha reductase inhibitors (anti-androgenic) induce a prostate luminal to club cell transition in human benign prostatic hyperplasia (Joseph et al., 2022).
Mesenchymal-to-epithelial transition
EMT is reversal by the mesenchymal-to-epithelial transition (MET) (Yang J. et al., 2020; Manfioletti and Fedele, 2022), which can be synonymous to type 2 EMT, a reparative-associated fibrotic process in response to chronic inflammation (Marconi et al., 2021). The chief candidate for EMT reversal (regeneration/rejuvenation) would be the timely amelioration of hypoxia (López-Novoa and Nieto, 2009; Lee et al., 2020; Papale et al., 2020; Nushtaeva et al., 2023; Zhang et al., 2023) before the establishment of dysregulation–degeneration hypoxic cell biology (Phua, 2021; Rando and Jones, 2021) and cellular senescence (Welford and Giaccia, 2011; Barabutis et al., 2018; Lacroix et al., 2020; Otero-Albiol and Carnero, 2021). In a review article, new approaches to alleviating hypoxia through the modulation of the vascular state in the tumor microenvironment offer promise for ovarian cancer immunotherapeutic strategies (Klemba et al., 2020). Therefore, the reversal from EMT to MET can also be associated with the critical inter-transient preclinical (disease/cancer) period of time-dependent (Ming et al., 2013; Byrne et al., 2016; Rouverdo et al., 2023) physoxia–NO-mediated hemodynamic (Lam et al., 2019; Mas-Bargues et al., 2019; Merkhan et al., 2021; Reiterer et al., 2022) window of cellular regeneration–rejuvenation (Hachmo et al., 2020; Kamat et al., 2021; Rando and Jones, 2021) and the window of opportunity and timing hypothesis (Maki, 2013; Speth et al., 2018).
In vitro regenerative medicine experiments can provide evidence to support the favorable benefits of physoxia/physioxia oxygenation (Yttersian Sletta et al., 2017; Dennis et al., 2020; Dougherty et al., 2020; Dogan et al., 2022), growth factors (Wang et al., 2007; Zhang et al., 2021a; Kataoka et al., 2022), androgen (Bui et al., 2017; Nyquist et al., 2019), inflammation reduction (Shephard et al., 2022), and enhanced microvasculature (Schumacher et al., 2022). Aging reprograms the hematopoietic vascular niche to impede regeneration and promote fibrosis (Chen et al., 2021c) within blood vessel wall-associated tissue remodeling (Craig et al., 2022), and it also play a role in the association of cardiac fibroblasts and endothelial cells in myocarditis (Xuan et al., 2022).
Cellular senescence—autophagy
Cellular senescence persists during aging and promotes age-related pathologies through the pro-inflammatory senescence-associated secretory phenotype (SASP) (Covarrubias et al., 2020; van Vliet et al., 2021b), and its expression is dependent on oxygenation levels (van Vliet et al., 2021b). The contemporary aspects of age-related cellular senescence pathologies can also be the part of a crosstalk within the geroscience perspective in the characterization of the SASP as the “remodeling-associated secretory phenotype” (Gems and Kern, 2022), mechanisms of vascular aging (Ungvari et al., 2020), and in the role of aging endocrine diseases (Khosla et al., 2020).
The early protective role of wild-type p53 in suppressing inflammation and cancer is strongly associated with the regulation of important cellular activities of the cell cycle of senescence and apoptosis (Barabutis et al., 2018; Agupitan et al., 2020; Lacroix et al., 2020). The missense mutations in the TP53 gene are found most frequently across all cancer types and give rise to mutant p53 proteins that lose their tumor suppressive activities (Mantovani et al., 2019; Sabapathy and Lane, 2019; Stein et al., 2019).
Cellular senescence is a specialized form of growth arrest and plays a critical role in tumor suppression and aging, with autophagy being activated during the process of senescence (Yang et al., 2012; Rajendran S. et al., 2019; Tabibzadeh, 2023). Oxygen concentration can modulate cellular senescence and autophagy in human trophoblast cells (Seno et al., 2018).
Autophagy plays a role in early tumor suppression in terms of the cell regulation pathways and in their dysregulation in late stages, where they act as tumor promoters (Dower et al., 2018; Alvarez-Meythaler et al., 2020; Li H. et al., 2020; Lim and Murthy, 2020). Impaired autophagy predisposes individuals to age-related diseases, whereas interventions that stimulate autophagy often promote longevity (Leidal et al., 2018; Condello et al., 2019; Luo and Qin, 2019; Wong et al., 2020).
Penile rehabilitation oxygenation
Indeed, such a “physoxia-mediated” (oxygenation) hemodynamic is used for the early therapeutic effect of penile rehabilitation after prostatectomy (Marcu et al., 2020; Osadchiy et al., 2020; Nicolai et al., 2021). Regular erections (Montorsi and Oettel, 2005) are used to improve oxygenation and hemodynamics (reducing hypoxia and inflammation) (Welliver et al., 2014; Hadanny et al., 2018; Sen et al., 2020) and preserve the endothelial structure using PDE5 inhibitors (Elkamshoushi et al., 2021) to prevent penile fibrosis (El-Sakka and Yassin, 2010; Kaminsky et al., 2011). Long-term testosterone therapy improves long-term blood circulation of penile arteries, penile length and girth, erectile function, and nocturnal penile tumescence and duration (Canguven et al., 2016). Electrical penile erection stimulation in mice induced angiogenesis, cell survival, proliferation, and anti-fibrosis signaling pathways (Kwon et al., 2016).
Hypoxia-related carcinogenesis and chronic diseases
Both oxidative stress and inflammation are driven by hypoxia (Biddlestone et al., 2015; McGarry et al., 2018; Korbecki et al., 2021; Mesentier-Louro et al., 2021), which could explain why anti-oxidative therapies alone cannot restore cellular redox homeostasis (Tretter et al., 2021). Endothelial cell biology of functions and dysfunctions (Charreau, 2022) is an emerging approach to understanding microvascular endothelial heterogeneity and inflammation (Yang et al., 2021c; Rossi et al., 2022). Impaired endothelial function is thought to contribute to the increased cardiovascular risk (Craighead et al., 2020), vascular aging associated with atherosclerotic ischemic stroke (Koutsaliaris et al., 2022), and oxidative stress–inflammation in chronic kidney disease (Ebert et al., 2021). Aging is associated with chronic low-grade inflammation, cancer incidence, and mortality (Guerville et al., 2022), a physiological process mediated by numerous biological and genetic pathways, which are a driving force for all age-related diseases (Li et al., 2021). Cancer often arises in the context of an altered tissue microenvironment landscape (Laconi et al., 2021).
This pathophysiology of oxidative stress–inflammation induced by hypoxia is a response to humans’ exposure to acute high-altitude hypoxia (Malacrida et al., 2019; Mrakic-Sposta et al., 2022; Pham et al., 2022). Nature’s adaptive responses to nitric oxide emphasize the importance of nitric oxide’s vasodilator role in the native inhabitants living in high-altitude hypoxia environments (Qi-sheng et al., 2021; James et al., 2022). Genetically similar East African highlanders, the Amhara tribe, balance minimally elevated hemoglobin with a vasodilatory response to environmental hypoxia, whereas the Oromo tribe mainly relies on an elevated hemoglobin response (Cheong et al., 2017; Getu, 2022). Newborn llamas from the highland region have a reduced pulmonary vasoconstriction response to acute hypoxia due to an enhancement of NO pathways (Reyes et al., 2018). There is an association between 17β-estradiol receptors and nitric oxide signaling that augments the high-altitude adaptation of Ladakhi highlanders (Pooja et al., 2021). Lower mortality rates from cardiovascular diseases, diabetes, and cancers are seen in native highland residents (Thiersch and Swenson, 2018; Wander et al., 2020; Burtscher et al., 2021).
Hypoxia plays a critical role in shaping the genomic and evolutionary landscapes of cancer (Bhandari et al., 2020; Zhang X. et al., 2020), with a multifaceted interplay between hormones, growth factors, and hypoxia in a tumor microenvironment milieu (Lappano et al., 2022), including the HIFs (Satija et al., 2021; Sebestyén et al., 2021) and transforming growth factor (TGF-β) produced in the hypoxic, chronic inflammatory settings (Mortezaee and Majidpoor, 2022). Androgen deficiency/deprivation caused drastic endothelial dysfunction, resulting in reduced blood flow (ischemia/hypovascularity) to the prostate gland (Angrimani et al., 2020; Jin Cho and Pyo, 2020; Yoon et al., 2020), causing an ischemia–hypoxia stress tissue microenvironment (Thurmond et al., 2015; Byrne et al., 2016). Hypoxia stress caused the induction of amyloidosis-autophagy-EMT cell signaling interactions, beginning with amyloidosis (Cheboub et al., 2019; Phua, 2021) to allow cells to enter a dormant/resting stage (Audas et al., 2016; Mizejewski, 2017; Pavliukeviciene et al., 2019). Age-related amyloidoses (Rubel et al., 2020; Tasaki et al., 2021) are increasingly being discussed in the mainstream literature, including a range of organs: brain/Alzheimer’s (Shea et al., 2019; Schweighauser et al., 2022), renal amyloidosis (Gupta N. et al., 2020; Herrera, 2021; Gurung and Li, 2022), eyes/Alzheimer’s retinopathy (Mirzaei et al., 2020), type 2 diabetes/Alzheimer’s (Wang and Westermark, 2021), and cardiac amyloidosis (Garcia-Pavia et al., 2021; Hänselmann et al., 2022).
In turn, the amyloid protein aggregates are countered by autophagy and the ubiquitin proteasome system (Chuang et al., 2018; Wang and Le, 2019; Wang and Zhang, 2019). The availability of the HIFs in the hypoxia and inflammation pathophysiological states is primarily regulated post-translationally through the ubiquitin proteasome system (autophagy) (Günter et al., 2017; Cohen et al., 2019). HIFs enable cells to adapt to decreased oxygen bioavailability (Albanese et al., 2020; Hirota, 2020) with stochastic fluctuations of oxygen that will select for the bet-hedging (Warburg) phenotype (EMT) (Gravenmier et al., 2018). Under conditions of hypoxia, most eukaryotic cells can shift their primary metabolic strategy from oxidative phosphorylation to increased aerobic glycolysis, known as the Warburg effect (Elzakra and Kim, 2021; Kierans and Taylor, 2021).
Hypoxia in chronic kidney disease (Faivre et al., 2021; Wang B. et al., 2022) has been well studied and is used here to highlight the middle-aged (>50 years) (Ryu et al., 2019) hypovascularity (Fine and Norman, 2008; Evans et al., 2020; Querfeld et al., 2020) hypoxia (Faivre et al., 2021; Wang L. et al., 2022) pathological trajectories in linking the endocrine system (Zhao and Schooling, 2020; Li L. et al., 2022; Romejko et al., 2022), nitric oxide signaling, and oxygen homeostasis pathophysiology (Burmakin et al., 2021; Carlström, 2021; Xu M. et al., 2022; Edwards and Kurtcuoglu, 2022). Oxidative stress–inflammation pathophysiology (Ebert et al., 2021), amyloidosis (Ryu et al., 2019; Herrera, 2021; Gurung and Li, 2022), autophagy (Tang et al., 2020), and EMT (Chen et al., 2022b) would result in degenerative hypertrophy, fibrosis, atrophy, and neoplasm (Ryu et al., 2019; Pinto et al., 2021) comorbid conditions (Charles and Ferris, 2020). Moreover, PDE5 inhibitors can have beneficial renal protective effects by improving hemodynamics and reducing oxidative stress and inflammation (Georgiadis et al., 2020; Coskuner and Ozkan, 2021).
Middle-aging sex hormone—endogenous nitric oxide-mediated oxygen homeostasis
Maintenance of cellular oxygen homeostasis during sex hormone–nitric oxide downregulation and hemodynamic reduction due to hypovascularity is the key physiological challenge during middle age (Kumar and Choi, 2015; Gravenmier et al., 2018; Janaszak-Jasiecka et al., 2021; Assar et al., 2022; Xu C. et al., 2022). When “physoxia” oxygen delivery is disrupted by microvascular hypovascularity (ischemia–hypoxia), it triggers intrinsic adaptive biological processes to facilitate heterogeneous cell survival in the hypoxia-degenerative environment (Feil et al., 2022; Wicks and Semenza, 2022; Yang et al., 2022). This indicates a critical juncture of timing when this window of “cellular regeneration–rejuvenation–timing” is made possible when oxygen levels are restored to normal “physoxia” conditions (Dogan et al., 2021, 2022; Merkhan et al., 2021).
The aim of early intervention is to prevent physiological oxygen deprivation (hypoxia) related to aging (Fine and Norman, 2008; Kumar and Choi, 2015; Claesson-Welsh, 2020; Wei et al., 2022). Androgens have the potential to prevent age-related impairment in ischemia-induced neovascularization (Lam et al., 2019; Gerbie et al., 2021) and could provide hemodynamic physoxia oxygenation to continually act on HIF degradation through the oxygen-sensing pathway (Jaakkola et al., 2001; Berra et al., 2003; Strowitzki et al., 2019; Voit and Sankaran, 2020).
Human nitric oxide production decreases with age, losing 50% by age 40 and 85% by age 65 (Gerhard et al., 1996; Taddei et al., 2001). This preclinical period coincides with the science of nitric oxide in all chronic diseases being associated with decreased blood flow to the affected organ, resulting in increased inflammation, oxidative stress, and immune dysfunction (Bryan et al., 2023). Lack of nitric oxide bioavailability in post-menopausal women is well documented (Novensà et al., 2011; Fredette et al., 2018; Somani et al., 2019), and menopause/hormonal replacement therapy (MPT/HRT) can replace nitric oxide (Best et al., 1998; Cicinelli et al., 1999; Bednarek-Tupikowska et al., 2008). The estrogen replacement therapy has been shown to reduce oxidative stress (Bellanti et al., 2013; Unfer et al., 2015; Borrás et al., 2021), reduce oxidative stress/inflammation (Vural et al., 2006; Georgiadou and Sbarouni, 2009; Jee et al., 2021; Estrada-Cruz et al., 2022), and improve hemodynamics (Redberg et al., 2000; Light et al., 2001; Deschênes et al., 2010). Similarly, testosterone replacement therapy can reduce oxidative stress (Mancini et al., 2008; Popp Marin et al., 2010; Mendell and MacLusky, 2019; Koukoulis et al., 2022) and inflammation (Bianchi, 2019; Mohamad et al., 2019; Rastrelli et al., 2019) and improve hemodynamics (Efesoy et al., 2018; König et al., 2019; Cipriani et al., 2021). This indicates that sex hormones can reduce hypoxia-induced oxidative stress and inflammation during the critical window of cellular regeneration, rejuvenation, and timing period through physoxia–NO-mediated hemodynamic oxygenation. In cancer, the two-concentration (biphasic) hypothesis of nitric oxide has determined that low levels of nitric oxide are cancer promoting, while high levels of nitric oxide are protective against cancer (Soni et al., 2020).
Comorbidities in the middle-aged group (35–59 years) of ischemic heart disease were less severe than those of the older age group (60–69 years) (Zhou et al., 2022), with the first chronic condition developing in the 50s or 60s (Zhu et al., 2018). Data analysis showed a reduction in cardiovascular disease and breast cancer in women aged under 60 years who were on hormone replacement therapy, as seen in the Women’s Health Initiative Trial (Langer, 2017; Lobo, 2017; El Khoudary et al., 2020). Women who were BRCA1/BRCA2 mutation carriers and were under 45 years of age and who received risk-reducing salpingo-oophorectomy and hormonal replacement treatment did not affect their breast cancer rates (Michaelson-Cohen et al., 2021). Furthermore, premenopausal women are better protected against cardiac hypertrophy (Wu et al., 2020). Sleep quality and the accumulation of cortical amyloid-β are associated in post-menopausal women from the Kronos Early Estrogen Prevention Study (Zeydan et al., 2021) and the long preclinical period (approx. 10–15 years) prior to symptomatic Alzheimer disease onset (Younes et al., 2019; Elman et al., 2020; Elman-Shina and Efrati, 2022).
Prostate cancer incidence is rare for those under 50 years of age, increasing to 1 in 52 by age 59 and to more than 1 in 2 at age 65 or older (Rosario and Rosario, 2022). Higher testosterone levels are associated with smaller prostate size (Xia et al., 2021), and younger age testosterone replacement therapy leads to prostate stabilization (Zhang Q. et al., 2020). In prostate cancer patients, low serum testosterone has been found to be associated with androgen receptor expression (Schatzl et al., 2002; Husain et al., 2016; Feng and He, 2019; Hashmi et al., 2019).
Nitric oxide physiology and the nitrate–nitrite–nitric oxide pathway
Nitric oxide is a strong vasodilatory and anti-inflammatory signaling molecule that plays diverse roles in maintaining vascular homeostasis (Cyr et al., 2020), biological functions (Gantner et al., 2020), and in carcinogenesis (Mintz et al., 2021). This new strategy, as a physoxia–NO-mediated mechanism, allows for a causal relationship (Kiani et al., 2022) to be understood in the aftermath of sex hormone–nitric oxide downregulation with the non-canonical pathways for nitric oxide synthesis in the body, known as the nitrate–nitrite–nitric oxide pathway (Kapil et al., 2020; Mintz et al., 2021).
Findings are consistent with observational reports linking dietary nitric oxide sources to beneficial health outcomes associated with the Mediterranean diet (Martínez-González et al., 2015; Shannon et al., 2021), dietary spermidine (Wu et al., 2022), and longevity (healthspan) in Blue Zone populations (Vasto et al., 2014; Nieddu et al., 2020). The Mediterranean diet increases serum nitric oxide (Shannon et al., 2018; Mohajeri and Cicero, 2023), improves endothelial function (Shannon et al., 2020; Fatima et al., 2023), is anti-oxidant (Gantenbein and Kanaka-Gantenbein, 2021; Karbasi et al., 2023), and is anti-inflammatory (Tsigalou et al., 2020; Soda et al., 2021). Beetroot juice as a dietary nitrate supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud’s phenomenon (Shepherd et al., 2019).
Conclusion
Both these hypotheses—the prostate aging degeneration (Phua, 2021) and middle-aging hypovascularity hypoxia—provide complimentary evidence supporting the importance of time-dependent maintenance of vascular function and vascularity hemodynamics, respectively.
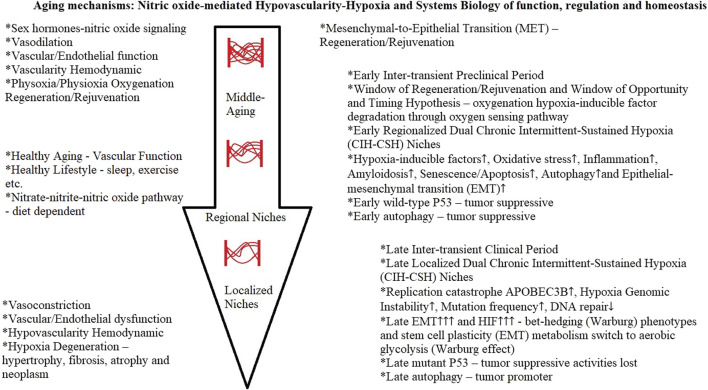
In a Mendelian randomization analysis study, the evidence suggested that menopause accelerates the epigenetic aging of blood (Levine et al., 2016). Hypoxia is one of the common characteristics of cancer (Liao et al., 2023), and the hypovascularity hypoxia hypothesis provided evidence of an early hypoxia milieu genesis during middle-age nitric oxide-mediated vascular aging. Cancer hypoxia is one of the most important hallmarks of cancer; it affects gene expression, metabolism, and ultimately, tumor biology-related processes (Sebestyén et al., 2021). All in all, this scoping review can provide the clarity of concepts and patterns to determine the aging mechanisms as a consequence of nitric oxide-mediated hypovascularity hypoxia development, which affects the early and late downstream stages of systems biology of function, regulation, and homeostasis (Figure 4).
FIGURE 4.
Aging mechanisms.
Nitric oxide is necessary for maintaining and sustaining physiological oxygenation during the critical windows of cellular regeneration–rejuvenation and the timing hypothesis of hormone therapy. Physiological concentrations of testosterone significantly increased nitric oxide production (Campelo et al., 2012), and baseline testosterone levels predict body composition and metabolic response to TRT (Deepika et al., 2022) and support the prostate safety of TRT in newly diagnosed men with hypogonadism (Debruyne et al., 2017).
A data-driven generative model suggests a mechanistic explanation for why the selective fitness advantage (bet-hedging) introduced by specific driver genes is tissue-dependent in a tumorigenesis timeline (Lahouel et al., 2020). Hypovascularity hypoxia constitutes the mechanistic interface of a self-perpetuating hypovascularity cyclic/chronic hypoxia dual dynamism CIH–CSH modes (Böger and Hannemann, 2020; Prieto-Lloret et al., 2021) within the pathological tumorigenesis microenvironment niche (Schiffer et al., 2018) (Figure 3).
Endogenous nitric oxide bioavailability and expression is the key gasotransmitter of the NO-cGMP pathway (Liu et al., 2017; Krishnan et al., 2018; Friebe et al., 2020; Feil et al., 2022; Kang et al., 2022). Nitric oxide is involved in the regulation of vasodilation, platelet aggregation, inflammation, hypoxic adaptation, and oxidative stress (Gajecki et al., 2022). Therefore, this insidious early age-related menopause/andropause endogenous nitric oxide-mediated hypovascularity hypoxia development needs further investigation. Emerging genomic evidence from population and experimental studies points to an important role for non-mutagenic promoters in driving cancer incidence rates, and new approaches and research strategies are needed to break this impasse (Brennan and Davey-Smith, 2022). This can provide the needed answers to this important question regarding healthspan for healthy lifestyle aging, cost savings in medical care, and sustainability of the health system.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Ethical review and approval was not required for the animal study because Review article Frontiers to ascertain.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflicts of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abay R. N., Akdeniz G. Ş., Katipoğlu Z., Kerimoğlu H. (2022). Normative data assessment of age-related changes in macular and optic nerve head vessel density using optical coherence tomography angiography. Photodiagnosis Photodyn. Ther. 37, 102624. 10.1016/j.pdpdt.2021.102624 [DOI] [PubMed] [Google Scholar]
- Abdel-Zaher A. O., Abd-Ellatief R. B., Aboulhagag N. A., Farghaly H. S. M., Al-Wasei F. M. M. (2021). The potential relationship between gasotransmitters and oxidative stress, inflammation and apoptosis in lead-induced hepatotoxicity in rats. Tissue & cell 71, 101511. 10.1016/j.tice.2021.101511 [DOI] [PubMed] [Google Scholar]
- Abler L. L., Vezina C. M. (2018). Links between lower urinary tract symptoms, intermittent hypoxia and diabetes: Causes or cures? Respir. physiology Neurobiol. 256, 87–96. 10.1016/j.resp.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. A., Uryash A., Lopez J. R., Sackner M. A. (2021). The endothelium as a therapeutic Target in diabetes: A narrative review and perspective. Front. physiology 12, 638491. 10.3389/fphys.2021.638491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebayo A. K., Nakshatri H. (2022). Modeling preclinical cancer studies under physioxia to enhance clinical translation. Cancer Res. 82 (23), 4313–4321. 10.1158/0008-5472.CAN-22-2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agupitan A. D., Neeson P., Williams S., Howitt J., Haupt S., Haupt Y. (2020). P53: A guardian of Immunity Becomes its Saboteur through mutation. Int. J. Mol. Sci. 21 (10), 3452. 10.3390/ijms21103452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Jing Y., Mockett B. G., Zhang H., Abraham W. C., Liu P. (2022). Partial endothelial nitric oxide synthase deficiency Exacerbates cognitive Deficit and amyloid pathology in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. Int. J. Mol. Sci. 23 (13), 7316. 10.3390/ijms23137316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W. S., Geethakumari A. M., Biswas K. H. (2021). Phosphodiesterase 5 (PDE5): Structure-function regulation and therapeutic applications of inhibitors. Biomed. Pharmacother. = Biomedecine Pharmacother. 134, 111128. 10.1016/j.biopha.2020.111128 [DOI] [PubMed] [Google Scholar]
- Akseh S., Karimi M. A., Safaie N., Valizadeh A., Rahmanpour D., Pezeshkian M., et al. (2021). The serum levels of testosterone in coronary artery disease patients; relation to NO, eNOS, endothelin-1, and disease severity. Hormone Mol. Biol. Clin. investigation 43 (1), 55–61. 10.1515/hmbci-2021-0026 [DOI] [PubMed] [Google Scholar]
- Akter N., Kulinskaya E., Steel N., Bakbergenuly I. (2022). The effect of hormone replacement therapy on the survival of UK women: A retrospective cohort study 1984-2017. BJOG Int. J. obstetrics Gynaecol. 129 (6), 994–1003. 10.1111/1471-0528.17008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese A., Daly L. A., Mennerich D., Kietzmann T., Sée V. (2020). The role of hypoxia-inducible factor post-translational modifications in regulating its Localisation, stability, and activity. Int. J. Mol. Sci. 22 (1), 268. 10.3390/ijms22010268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alidadi M., Liberale L., Montecucco F., Majeed M., Al-Rasadi K., Banach M., et al. (2021). Protective effects of curcumin on endothelium: An updated review. Adv. Exp. Med. Biol. 1291, 103–119. 10.1007/978-3-030-56153-6_6 [DOI] [PubMed] [Google Scholar]
- Alva R., Gardner G. L., Liang P., Stuart J. A. (2022). Supraphysiological oxygen levels in Mammalian cell culture: Current state and future perspectives. Cells 11 (19), 3123. 10.3390/cells11193123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Maestro M., Eguibar A., Chanca P., Klett-Mingo M., Gómez Rivas J., Buño-Soto A., et al. (2021). Androgen deprivation therapy in patients with prostate cancer increases serum levels of thromboxane A(2): Cardiovascular implications. Front. Cardiovasc. Med. 8, 653126. 10.3389/fcvm.2021.653126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Meythaler J. G., Garcia-Mayea Y., Mir C., Kondoh H., Lleonart M. E. (2020). Autophagy Takes center stage as a possible cancer hallmark. Front. Oncol. 10, 586069. 10.3389/fonc.2020.586069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdahl M. B., DeMartino A. W., Gladwin M. T. (2019). Inorganic nitrite bioactivation and role in physiological signaling and therapeutics. Biol. Chem. 401 (1), 201–211. 10.1515/hsz-2019-0349 [DOI] [PubMed] [Google Scholar]
- Aminuddin A., Salamt N., Ahmad Fuad A. F., Chin K. Y., Ugusman A., Soelaiman I. N., et al. (2019). Vascular dysfunction among Malaysian men with increased BMI: An indication of Synergistic effect of free testosterone and inflammation. Med. Kaunas. Lith. 55 (9), 575. 10.3390/medicina55090575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K.-E. (2018). PDE5 inhibitors - pharmacology and clinical applications 20 years after sildenafil discovery. Br. J. Pharmacol. 175 (13), 2554–2565. 10.1111/bph.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson K. E., Boedtkjer D. B., Forman A. (2017). The link between vascular dysfunction, bladder ischemia, and aging bladder dysfunction. Ther. Adv. Urology 9 (1), 11–27. 10.1177/1756287216675778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric S. A., Janjic M. M., Stojkov N. J., Kostic T. S. (2010). Testosterone-induced modulation of nitric oxide-cGMP signaling pathway and androgenesis in the rat Leydig cells. Biol. reproduction 83 (3), 434–442. 10.1095/biolreprod.110.083626 [DOI] [PubMed] [Google Scholar]
- Angrimani D. S. R., Francischini M. C. P., Brito M. M., Vannucchi C. I. (2020). Prostatic hyperplasia: Vascularization, hemodynamic and hormonal analysis of dogs treated with finasteride or orchiectomy. PloS one 15 (6), e0234714. 10.1371/journal.pone.0234714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A. B., Wittert G. A. (2011). Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metabolism 25 (2), 303–319. 10.1016/j.beem.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribas E., Ahmadizar F., Mutlu U., Ikram M. K., Bos D., Laven J. S. E., et al. (2022). Sex steroids and markers of micro- and macrovascular damage among women and men from the general population. Eur. J. Prev. Cardiol. 29 (9), 1322–1330. 10.1093/eurjpc/zwaa031 [DOI] [PubMed] [Google Scholar]
- Arnould L., Guenancia C., Binquet C., Delcourt C., Chiquet C., Daien V., et al. (2022). Retinal vascular network: Changes with aging and systemic vascular disease (cardiac and cerebral). J. francais d’ophtalmologie 45 (1), 104–118. 10.1016/j.jfo.2021.09.004 [DOI] [PubMed] [Google Scholar]
- Ashton J., Bristow R. (2020). Bad neighbours: Hypoxia and genomic instability in prostate cancer. Br. J. radiology 93 (1115), 20200087. 10.1259/bjr.20200087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asih P. R., Tegg M. L., Sohrabi H., Carruthers M., Gandy S. E., Saad F., et al. (2017). Multiple mechanisms linking type 2 diabetes and Alzheimer’s disease: Testosterone as a modifier. J. Alzheimer’s Dis. JAD 59 (2), 445–466. 10.3233/JAD-161259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askari M., Mohamad Mirjalili S. A., Bozorg M., Azizi R., Namiranian N. (2019). The prevalence of Peyronie’s disease in diabetic patients -2018- Yazd. Diabetes & metabolic syndrome 13 (1), 604–607. 10.1016/j.dsx.2018.11.039 [DOI] [PubMed] [Google Scholar]
- Assar M. E., Angulo J., García-Rojo E., Sevilleja-Ortiz A., García-Gómez B., Fernández A., et al. (2022). Early manifestation of aging-related vascular dysfunction in human penile vasculature-A potential explanation for the role of erectile dysfunction as a harbinger of systemic vascular disease. GeroScience 44 (1), 485–501. 10.1007/s11357-021-00507-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audas T. E., Audas D. E., Jacob M. D., Ho J. J. D., Khacho M., Wang M., et al. (2016). Adaptation to Stressors by systemic protein Amyloidogenesis. Dev. Cell 39 (2), 155–168. 10.1016/j.devcel.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurilio G., Cimadamore A., Mazzucchelli R., Lopez-Beltran A., Verri E., Scarpelli M., et al. (2020). Androgen receptor signaling pathway in prostate cancer: From genetics to clinical applications. Cells 9 (12), 2653. 10.3390/cells9122653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa A., Duca Y., Condorelli R. A., Calogero A. E., La Vignera S. (2019). Androgen deficiency and phosphodiesterase type 5 expression changes in aging Male: Therapeutic implications. Front. Endocrinol. 10, 225. 10.3389/fendo.2019.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock M. C., DuBose L. E., Witten T. L., Stauffer B. L., Hildreth K. L., Schwartz R. S., et al. (2022). Oxidative stress and inflammation are associated with age-related endothelial dysfunction in men with low testosterone. J. Clin. Endocrinol. metabolism 107 (2), e500–e514. 10.1210/clinem/dgab715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S. B., Dewhirst M. W., Hammond E. M. (2020). Cyclic hypoxia: An update on its characteristics, methods to Measure it and biological implications in cancer. Cancers 13 (1), 23. 10.3390/cancers13010023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader S. B., Ma T. S., Simpson C. J., Liang J., Maezono S. E. B., Olcina M. M., et al. (2021). Replication catastrophe induced by cyclic hypoxia leads to increased APOBEC3B activity. Nucleic acids Res. 49 (13), 7492–7506. 10.1093/nar/gkab551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J. K., Kim H. I., Kang M. J., Seon K. E., Kim E. H., Seo S. K. (2022). Relationship between the type of hormone replacement therapy and incidence of breast cancer in Korea. Climacteric J. Int. Menopause Soc. 25, 516–522. 10.1080/13697137.2022.2077096 [DOI] [PubMed] [Google Scholar]
- Bahadoran Z., Mirmiran P., Kashfi K., Ghasemi A. (2021). Endogenous flux of nitric oxide: Citrulline is preferred to Arginine. Acta physiol. Oxf. Engl. 231 (3), e13572. 10.1111/apha.13572 [DOI] [PubMed] [Google Scholar]
- Bajraktari G., Burhenne J., Bugert P., Haefeli W. E., Weiss J. (2017). Cyclic guanosine monophosphate modulates accumulation of phosphodiesterase 5 inhibitors in human platelets. Biochem. Pharmacol. 145, 54–63. 10.1016/j.bcp.2017.08.026 [DOI] [PubMed] [Google Scholar]
- Balistreri C. R. (2022). Promising strategies for preserving adult endothelium health and reversing its dysfunction: From Liquid biopsy to new omics technologies and Noninvasive circulating biomarkers. Int. J. Mol. Sci. 23 (14), 7548. 10.3390/ijms23147548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Khan G. A. (1994). Mechanism of vasoconstriction induced by chronic inhibition of nitric oxide in rats. Hypertension 24 (3), 322–328. 10.1161/01.hyp.24.3.322 [DOI] [PubMed] [Google Scholar]
- Barabutis N., Schally A. V., Siejka A. (2018). P53, GHRH, inflammation and cancer. EBioMedicine 37, 557–562. 10.1016/j.ebiom.2018.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batie M., Kenneth N. S., Rocha S. (2022). Systems approaches to understand oxygen sensing: How multi-omics has driven advances in understanding oxygen-based signalling. Biochem. J. 479 (3), 245–257. 10.1042/BCJ20210554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baures M., Dariane C., Tika E., Puig Lombardi E., Barry Delongchamps N., Blanpain C., et al. (2022). Prostate luminal progenitor cells: From mouse to human, from health to disease. Nat. Rev. Urol. 19 (4), 201–218. 10.1038/s41585-021-00561-2 [DOI] [PubMed] [Google Scholar]
- Bayarri M. A., Milara J., Estornut C., Cortijo J. (2021). Nitric oxide system and Bronchial Epithelium: More than a Barrier. Front. physiology 12, 687381. 10.3389/fphys.2021.687381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek-Tupikowska G., Tworowska-Bardzinska U., Tupikowski K. (2008). Effects of estrogen and estrogen-progesteron on serum nitric oxide metabolite concentrations in post-menopausal women. J. Endocrinol. investigation 31 (10), 877–881. 10.1007/BF03346435 [DOI] [PubMed] [Google Scholar]
- Bellanti F., Matteo M., Rollo T., De Rosario F., Greco P., Vendemiale G., et al. (2013). Sex hormones modulate circulating antioxidant enzymes: Impact of estrogen therapy. Redox Biol. 1 (1), 340–346. 10.1016/j.redox.2013.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchner-Pfannschmidt U., Yamac H., Trinidad B., Fandrey J. (2007). Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J. Biol. Chem. 282 (3), 1788–1796. 10.1074/jbc.M607065200 [DOI] [PubMed] [Google Scholar]
- Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. (2003). HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 22 (16), 4082–4090. 10.1093/emboj/cdg392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bery F., Figiel S., Kouba S., Fontaine D., Guéguinou M., Potier-Cartereau M., et al. (2020). Hypoxia promotes prostate cancer aggressiveness by upregulating EMT-activator Zeb1 and SK3 channel expression. Int. J. Mol. Sci. 21 (13), 4786. 10.3390/ijms21134786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best P. J., Berger P. B., Miller V. M., Lerman A. (1998). The effect of estrogen replacement therapy on plasma nitric oxide and endothelin-1 levels in postmenopausal women. Ann. Intern. Med. 128 (4), 285–288. 10.7326/0003-4819-128-4-199802150-00006 [DOI] [PubMed] [Google Scholar]
- Bhandari V., Li C. H., Bristow R. G., Boutros P. C. PCAWG Consortium (2020). Divergent mutational processes distinguish hypoxic and normoxic tumours. Nat. Commun. 11 (1), 737. 10.1038/s41467-019-14052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi V. E. (2019). The anti-inflammatory effects of testosterone. J. Endocr. Soc. 3 (1), 91–107. 10.1210/js.2018-00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddlestone J., Bandarra D., Rocha S. (2015). The role of hypoxia in inflammatory disease (review). Int. J. Mol. Med. 35 (4), 859–869. 10.3892/ijmm.2015.2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluming A. Z. (2022). Hormone replacement therapy after breast cancer: It is time. Cancer J. (Sudbury, Mass.) 28 (3), 183–190. 10.1097/PPO.0000000000000595 [DOI] [PubMed] [Google Scholar]
- Böger R., Hannemann J. (2020). Dual role of the L-arginine-ADMA-NO pathway in systemic hypoxic vasodilation and pulmonary hypoxic vasoconstriction. Pulm. Circ. 10 (2), 2045894020918850. 10.1177/2045894020918850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric M. P., Figueroa X. F. (2022). Editorial: Cell communication in vascular biology, volume II. Front. physiology 13, 903056. 10.3389/fphys.2022.903056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás C., Ferrando M., Inglés M., Gambini J., Lopez-Grueso R., Edo R., et al. (2021). Estrogen replacement therapy induces antioxidant and longevity-related genes in women after medically induced menopause. Oxidative Med. Cell. Longev. 2021, 8101615. 10.1155/2021/8101615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás C. (2021). The challenge of unlocking the biological secrets of aging. Front. aging 2, 676573. 10.3389/fragi.2021.676573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børretzen A., Gravdal K., Haukaas S. A., Mannelqvist M., Beisland C., Akslen L. A., et al. (2021). The epithelial-mesenchymal transition regulators Twist, Slug, and Snail are associated with aggressive tumour features and poor outcome in prostate cancer patients. J. pathology. Clin. Res. 7 (3), 253–270. 10.1002/cjp2.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughaleb H., Lobysheva I., Dei Zotti F., Balligand J. L., Montiel V. (2022). Biological assessment of the NO-dependent endothelial function. Mol. (Basel, Switz. 27 (22), 7921. 10.3390/molecules27227921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhamida E., Morciano G., Perrone M., Kahsay A. E., Della Sala M., Wieckowski M. R., et al. (2022). The interplay of hypoxia signaling on mitochondrial dysfunction and inflammation in cardiovascular diseases and cancer: From molecular mechanisms to therapeutic approaches. Biology 11 (2), 300. 10.3390/biology11020300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler E., Ladomery M. R. (2019). Working with hypoxia. Methods Mol. Biol. Clift. N.J.) 1990, 109–133. 10.1007/978-1-4939-9463-2_10 [DOI] [PubMed] [Google Scholar]
- Brennan P., Davey-Smith G. (2022). Identifying novel causes of cancers to enhance cancer prevention: New strategies are needed. J. Natl. Cancer Inst. 114 (3), 353–360. 10.1093/jnci/djab204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan N. S., Ahmed S., Lefer D. J., Hord N., von Schwarz E. R. (2023). Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action. Nitric oxide Biol. Chem. 132, 1–7. 10.1016/j.niox.2023.01.003 [DOI] [PubMed] [Google Scholar]
- Bui A. T., Huang M. E., Havard M., Laurent-Tchenio F., Dautry F., Tchenio T. (2017). Transient exposure to androgens induces a remarkable self-sustained quiescent state in dispersed prostate cancer cells. Cell cycleGeorget. Tex.) 16 (9), 879–893. 10.1080/15384101.2017.1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmakin M., Fasching A., Kobayashi H., Urrutia A. A., Damdimopoulos A., Palm F., et al. (2021). Pharmacological HIF-PHD inhibition reduces renovascular resistance and increases glomerular filtration by stimulating nitric oxide generation. Acta physiol. Oxf. Engl. 233 (1), e13668. 10.1111/apha.13668 [DOI] [PubMed] [Google Scholar]
- Burte C., Lejeune H., Faix A., Desvaux P., Almont T., Cuzin B., et al. (2021). Practical recommendations for the management of testosterone deficiency. Progres en urologie J. de l’Association francaise d’urologie de la Soc. francaise d’urologie 31 (8–9), 458–476. 10.1016/j.purol.2020.09.026 [DOI] [PubMed] [Google Scholar]
- Burtscher J., Millet G. P., Burtscher M. (2021). Does living at moderate altitudes in Austria affect mortality rates of various causes? An ecological study. BMJ open 11 (6), e048520. 10.1136/bmjopen-2020-048520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne N. M., Nesbitt H., Ming L., McKeown S. R., Worthington J., McKenna D. J. (2016). Androgen deprivation in LNCaP prostate tumour xenografts induces vascular changes and hypoxic stress, resulting in promotion of epithelial-to-mesenchymal transition. Br. J. cancer 114 (6), 659–668. 10.1038/bjc.2016.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z., Li H. (2020). An updated review: Androgens and cognitive impairment in older men. Front. Endocrinol. 11, 586909. 10.3389/fendo.2020.586909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campelo A. E., Cutini P. H., Massheimer V. L. (2012). Testosterone modulates platelet aggregation and endothelial cell growth through nitric oxide pathway. J. Endocrinol. 213 (1), 77–87. 10.1530/JOE-11-0441 [DOI] [PubMed] [Google Scholar]
- Canguven O., Talib R. A., El-Ansari W., Shamsoddini A., Salman M., Al-Ansari A. (2016). RigiScan data under long-term testosterone therapy: Improving long-term blood circulation of penile arteries, penile length and girth, erectile function, and nocturnal penile tumescence and duration. aging male official J. Int. Soc. Study Aging Male 19 (4), 215–220. 10.1080/13685538.2016.1230602 [DOI] [PubMed] [Google Scholar]
- Cannarella R., Condorelli R. A., Barbagallo F., La Vignera S., Calogero A. E. (2021). Endocrinology of the aging prostate: Current concepts. Front. Endocrinol. 12, 554078. 10.3389/fendo.2021.554078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capp J.-P., Thomas F. (2022). From developmental to atavistic bet-hedging: How cancer cells pervert the exploitation of random single-cell phenotypic fluctuations. BioEssays news Rev. Mol. Cell. Dev. Biol. 44, e2200048. 10.1002/bies.202200048 [DOI] [PubMed] [Google Scholar]
- Carlström M. (2021). Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17 (9), 575–590. 10.1038/s41581-021-00429-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A., Lleonart M. (2016). The hypoxic microenvironment: A determinant of cancer stem cell evolution. BioEssays news Rev. Mol. Cell. Dev. Biol. 38, S65–S74. 10.1002/bies.201670911 [DOI] [PubMed] [Google Scholar]
- Catrina S.-B., Zheng X. (2021). Hypoxia and hypoxia-inducible factors in diabetes and its complications. Diabetologia 64 (4), 709–716. 10.1007/s00125-021-05380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellek S., Cameron N. E., Cotter M. A., Fry C. H., Ilo D. (2014). Microvascular dysfunction and efficacy of PDE5 inhibitors in BPH-LUTS. Nat. Rev. Urol. Engl. 11, 231–241. 10.1038/nrurol.2014.53 [DOI] [PubMed] [Google Scholar]
- Changal K. H., Khan M. S., Bashir R., Sheikh M. A. (2020). Curcumin Preparations can improve flow-mediated Dilation and endothelial function: A meta-analysis. Complementary Med. Res. 27 (4), 272–281. 10.1159/000506180 [DOI] [PubMed] [Google Scholar]
- Charles C., Ferris A. H. (2020). Chronic kidney disease. Prim. care 47 (4), 585–595. 10.1016/j.pop.2020.08.001 [DOI] [PubMed] [Google Scholar]
- Charreau B. (2022). Advances in endothelial cell biology: From knowledge to control. Int. J. Mol. Sci. 23, 6403. 10.3390/ijms23126403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry R., Miao J. H., Rehman A. (2022). Physiology, cardiovascular. Treasure Island: StatPearls Publishing. [PubMed] [Google Scholar]
- Cheboub A., Regouat N., Djidjik R., Slimani A., Hadj-Bekkouche F. (2019). Short-term aromatase inhibition induces prostatic alterations in adult wistar rat: A biochemical, histopathological and immunohistochemical study. Acta Histochem. 121 (8), 151441. 10.1016/j.acthis.2019.151441 [DOI] [PubMed] [Google Scholar]
- Chen J., Lippo L., Labella R., Tan S. L., Marsden B. D., Dustin M. L., et al. (2021). Decreased blood vessel density and endothelial cell subset dynamics during ageing of the endocrine system. EMBO J. 40 (1), e105242. 10.15252/embj.2020105242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Pittman R. N., Popel A. S. (2008). Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxidants redox Signal. 10 (7), 1185–1198. 10.1089/ars.2007.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.-S., Chiu W. T., Hsu P. L., Lin S. C., Peng I. C., Wang C. Y., et al. (2020). Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 27 (1), 63. 10.1186/s12929-020-00658-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., He Y., Zhao S., He X., Xue D. (2022a). Hypoxic/ischemic inflammation, MicroRNAs and δ-Opioid receptors: Hypoxia/Ischemia-Sensitive versus-Insensitive organs. Front. aging Neurosci. 14, 847374. 10.3389/fnagi.2022.847374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Pu Q., Ma Y., Zhang H., Ye T., Zhao C., et al. (2021b). Aging reprograms the hematopoietic-vascular niche to impede regeneration and promote fibrosis. Cell metab. 33 (2), 395–410.e4. 10.1016/j.cmet.2020.11.019 [DOI] [PubMed] [Google Scholar]
- Chen Y., Yuan S., Cao Y., Kong G., Jiang F., Li Y., et al. (2021c). Gasotransmitters: Potential therapeutic molecules of fibrotic diseases. Oxidative Med. Cell. Longev. 2021, 3206982. 10.1155/2021/3206982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zou H., Lu H., Xiang H., Chen S. (2022b). Research progress of endothelial-mesenchymal transition in diabetic kidney disease. J. Cell. Mol. Med. 26 (12), 3313–3322. 10.1111/jcmm.17356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong H. I., Janocha A. J., Monocello L. T., Garchar A. C., Gebremedhin A., Erzurum S. C., et al. (2017). Alternative hematological and vascular adaptive responses to high-altitude hypoxia in East African highlanders. Am. J. physiology. Lung Cell. Mol. physiology 312 (2), L172–L177. 10.1152/ajplung.00451.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N., Iijima K., Kalula S., Malhotra R., Rasmussen L. J., Chan A., et al. (2021). Societal need for interdisciplinary ageing research: An International alliance of research Universities "ageing, longevity and health" Stream (IARU-ALH) position statement. Biomed. hub 6, 42–47. 10.1159/000513513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang E., Hori A. M., Hesketh C. D., Shorter J. (2018). Amyloid assembly and disassembly. J. Cell Sci. 131 (8), jcs189928. 10.1242/jcs.189928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P. H., Han T. M., Rudnik B., Das A. K. (2020). Peyronie’s disease: What do we know and how do we treat it? Can. J. urology 27 (S3), 11–19. [PubMed] [Google Scholar]
- Cicinelli E., Ignarro L. J., Matteo M. G., Galantino P., Schonauer L. M., Falco N. (1999). Effects of estrogen replacement therapy on plasma levels of nitric oxide in postmenopausal women. Am. J. obstetrics Gynecol. 180, 334–339. 10.1016/s0002-9378(99)70209-7 [DOI] [PubMed] [Google Scholar]
- Cipriani S., Maseroli E., Di Stasi V., Scavello I., Todisco T., Rastrelli G., et al. (2021). Effects of testosterone treatment on clitoral haemodynamics in women with sexual dysfunction. J. Endocrinol. investigation 44 (12), 2765–2776. 10.1007/s40618-021-01598-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson-Welsh L. (2020). Oxygen sensing; a stunningly elegant molecular machinery highjacked in cancer. Upsala J. Med. Sci. 125 (3), 205–210. 10.1080/03009734.2020.1769231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg L. E., Mac Gabhann F. (2015). Systems biology of the microvasculature. Integr. Biol. quantitative Biosci. nano macro 7 (5), 498–512. 10.1039/c4ib00296b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M., Amir S., Golan M., Ben-Neriah Y., Mabjeesh N. J. (2019). β-TrCP upregulates HIF-1 in prostate cancer cells. Prostate 79 (4), 403–413. 10.1002/pros.23746 [DOI] [PubMed] [Google Scholar]
- Condello M., Pellegrini E., Caraglia M., Meschini S. (2019). Targeting autophagy to Overcome human diseases. Int. J. Mol. Sci. 20 (3), 725. 10.3390/ijms20030725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornuault L., Rouault P., Duplàa C., Couffinhal T., Renault M. A. (2022). Endothelial dysfunction in heart failure with preserved Ejection Fraction: What are the experimental Proofs? Front. physiology 13, 906272. 10.3389/fphys.2022.906272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan F. E., 3rd, Al Mheid I., Eapen D. J., Hayek S. S., Sher S., Martin G. S., et al. (2015). Low testosterone in men predicts impaired arterial elasticity and microvascular function. Int. J. Cardiol. 194, 94–99. 10.1016/j.ijcard.2015.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskuner E. R., Ozkan B. (2021). Reno-protective effects of Phosphodiesterase 5 inhibitors. Clin. Exp. Nephrol. 25 (6), 585–597. 10.1007/s10157-021-02051-6 [DOI] [PubMed] [Google Scholar]
- Costa T. J., Barros P. R., Arce C., Santos J. D., da Silva-Neto J., Egea G., et al. (2021). The homeostatic role of hydrogen peroxide, superoxide anion and nitric oxide in the vasculature. Free Radic. Biol. Med. 162, 615–635. 10.1016/j.freeradbiomed.2020.11.021 [DOI] [PubMed] [Google Scholar]
- Covarrubias A. J., Kale A., Perrone R., Lopez-Dominguez J. A., Pisco A. O., Kasler H. G., et al. (2020). Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages. Nat. Metab. 2 (11), 1265–1283. 10.1038/s42255-020-00305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D. J., James A. W., Wang Y., Tavian M., Crisan M., Péault B. M. (2022). Blood vessel resident human stem cells in health and disease. Stem cells Transl. Med. 11 (1), 35–43. 10.1093/stcltm/szab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craighead D. H., Freeberg K. A., Seals D. R. (2020). Vascular endothelial function in Midlife/older adults classified according to 2017 American College of Cardiology/American heart association blood Pressure guidelines. J. Am. Heart Assoc. 9 (17), e016625. 10.1161/JAHA.120.016625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius A. R., Kirby B. S., Voyles W. F., Dinenno F. A. (2010). Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am. J. Physiology - Heart Circulatory Physiology 299 (5), H1633–H1641. 10.1152/ajpheart.00614.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr A. R., Huckaby L. V., Shiva S. S., Zuckerbraun B. S. (2020). Nitric oxide and endothelial dysfunction. Crit. care Clin. 36 (2), 307–321. 10.1016/j.ccc.2019.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cziráki A., Lenkey Z., Sulyok E., Szokodi I., Koller A. (2020). L-Arginine-Nitric oxide-Asymmetric Dimethylarginine pathway and the coronary circulation: Translation of basic science results to clinical Practice. Front. Pharmacol. 11, 569914. 10.3389/fphar.2020.569914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva F. C., de Araújo B. J., Cordeiro C. S., Arruda V. M., Faria B. Q., Guerra J. F. D. C., et al. (2022). Endothelial dysfunction due to the inhibition of the synthesis of nitric oxide: Proposal and characterization of an in vitro cellular model. Front. physiology 13, 978378. 10.3389/fphys.2022.978378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby R. B., Eskildsen S. F., Videbech P., Frandsen J., Mouridsen K., Sørensen L., et al. (2019). Oxygenation differs among white matter hyperintensities, intersected fiber tracts and unaffected white matter. Brain Commun. 1 (1), fcz033. 10.1093/braincomms/fcz033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debruyne F. M. J., Behre H. M., Roehrborn C. G., Maggi M., Wu F. C. W., Schröder F. H., et al. (2017). Testosterone treatment is not associated with increased risk of prostate cancer or worsening of lower urinary tract symptoms: Prostate health outcomes in the registry of hypogonadism in men. BJU Int. 119 (2), 216–224. 10.1111/bju.13578 [DOI] [PubMed] [Google Scholar]
- Deep G., Panigrahia G. K. (2015). Hypoxia-induced signaling promotes prostate cancer progression: Exosomes role as messenger of hypoxic response in tumor microenvironment. Crit. Rev. Oncog. 20 (5–6), 419–434. 10.1615/CritRevOncog.v20.i5-6.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepika F., Ballato E., Colleluori G., Aguirre L., Chen R., Qualls C., et al. (2022). Baseline testosterone predicts body composition and metabolic response to testosterone therapy. Front. Endocrinol. 13, 915309. 10.3389/fendo.2022.915309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J. E., Whitney G. A., Rai J., Fernandes R. J., Kean T. J. (2020). Physioxia stimulates Extracellular Matrix deposition and increases Mechanical properties of human Chondrocyte-derived tissue-Engineered Cartilage. Front. Bioeng. Biotechnol. 8, 590743. 10.3389/fbioe.2020.590743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent M. R., DeMartino A. W., Tejero J., Gladwin M. T. (2021). Endogenous Hemoprotein-dependent signaling pathways of nitric oxide and nitrite. Inorg. Chem. 60 (21), 15918–15940. 10.1021/acs.inorgchem.1c01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M. C., Descovich D., Moreau M., Granger L., Kuchel G. A., Mikkola T. S., et al. (2010). Postmenopausal hormone therapy increases retinal blood flow and protects the retinal nerve fiber layer. Investigative Ophthalmol. Vis. Sci. 51 (5), 2587–2600. 10.1167/iovs.09-3710 [DOI] [PubMed] [Google Scholar]
- Dewhirst M., Gustavo S., Hughes H. (2005). Adding nitric oxide to hemoglobin found to enhance oxygenation. Oncol. Times 27 (12), 17. 10.1097/01.COT.0000302898.46679.6d [DOI] [Google Scholar]
- Diamanti-Kandarakis E., Dattilo M., Macut D., Duntas L., Gonos E. S., Goulis D. G., et al. (2017). Mechanisms in ENDOCRINOLOGY: Aging and anti-aging: A Combo-Endocrinology overview. Eur. J. Endocrinol. 176 (6), R283–R308. 10.1530/EJE-16-1061 [DOI] [PubMed] [Google Scholar]
- Diokno A. C. (2022). The role of testosterone in men’s health: Is it time for a new approach. Int. urology Nephrol. 54, 2767. 10.1007/s11255-022-03292-4 [DOI] [PubMed] [Google Scholar]
- Diviccaro S., Melcangi R. C., Giatti S. (2020). Post-finasteride syndrome: An emerging clinical problem. Neurobiol. stress 12, 100209. 10.1016/j.ynstr.2019.100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan F., Aljumaily R. M. K., Kitchen M., Forsyth N. R. (2021). DNMT3B is an oxygen-Sensitive De Novo Methylase in human mesenchymal stem cells. Cells 10 (5), 1032. 10.3390/cells10051032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan F., Aljumaily R. M. K., Kitchen M., Forsyth N. R. (2022). Physoxia Influences global and gene-specific Methylation in Pluripotent stem cells. Int. J. Mol. Sci. 23 (10), 5854. 10.3390/ijms23105854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato A. J., Machin D. R., Lesniewski L. A. (2018). Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circulation Res. 123 (7), 825–848. 10.1161/CIRCRESAHA.118.312563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue J. F., Hayne D., Karnik U., Thomas D. R., Foster M. C. (2005). Randomized, placebo-controlled trial showing that finasteride reduces prostatic vascularity rapidly within 2 weeks. BJU Int. 96 (9), 1319–1322. 10.1111/j.1464-410X.2005.05849.x [DOI] [PubMed] [Google Scholar]
- Dougherty J. A., Patel N., Kumar N., Rao S. G., Angelos M. G., Singh H., et al. (2020). Human cardiac progenitor cells enhance Exosome Release and promote angiogenesis under physoxia. Front. cell Dev. Biol. 8, 130. 10.3389/fcell.2020.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower C. M., Wills C. A., Frisch S. M., Wang H. G. (2018). Mechanisms and context underlying the role of autophagy in cancer metastasis. Autophagy 14 (7), 1110–1128. 10.1080/15548627.2018.1450020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhalilova D. S., Makarova O. V. (2022). The role of hypoxia-inducible factor in the mechanisms of aging. Biochem. Biokhimiia 87 (9), 995–1014. 10.1134/S0006297922090115 [DOI] [PubMed] [Google Scholar]
- Ebert T., Neytchev O., Witasp A., Kublickiene K., Stenvinkel P., Shiels P. G. (2021). Inflammation and oxidative stress in chronic kidney disease and Dialysis patients. Antioxidants redox Signal. 35 (17), 1426–1448. 10.1089/ars.2020.8184 [DOI] [PubMed] [Google Scholar]
- Edwards A., Kurtcuoglu V. (2022). Renal blood flow and oxygenation. Pflugers Archiv Eur. J. physiology 474 (8), 759–770. 10.1007/s00424-022-02690-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efesoy O., Çayan S., Akbay E. (2018). The effect of testosterone replacement therapy on penile hemodynamics in hypogonadal men with erectile dysfunction, having Veno-Occlusive dysfunction. Am. J. men’s health 12 (3), 634–638. 10.1177/1557988318754931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoudary S. R., Aggarwal B., Beckie T. M., Hodis H. N., Johnson A. E., Langer R. D., et al. (2020). Menopause transition and cardiovascular disease risk: Implications for timing of early prevention: A scientific statement from the American heart association. Circulation 142 (25), e506–e532. 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- El-Sakka A. I., Yassin A. A. (2010). Amelioration of penile fibrosis: Myth or reality. J. Androl. 31 (4), 324–335. 10.2164/jandrol.109.008730 [DOI] [PubMed] [Google Scholar]
- Elkamshoushi A. M., Badae N. M., Kabary M. G., Omar S. I. (2021). Evaluation of daily avanafil efficacy in improving the endothelial function in Egyptian males with erectile dysfunction. Andrologia 53 (1), e13833. 10.1111/and.13833 [DOI] [PubMed] [Google Scholar]
- Elman J. A., Panizzon M. S., Gustavson D. E., Franz C. E., Sanderson-Cimino M. E., Lyons M. J., et al. (2020). Amyloid-β Positivity predicts cognitive decline but cognition predicts progression to amyloid-β Positivity. Biol. psychiatry 87 (9), 819–828. 10.1016/j.biopsych.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman-Shina K., Efrati S. (2022). Ischemia as a common trigger for Alzheimer’s disease. Front. aging Neurosci. 14, 1012779. 10.3389/fnagi.2022.1012779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzakra N., Kim Y. (2021). HIF-1α metabolic pathways in human cancer. Adv. Exp. Med. Biol. 1280, 243–260. 10.1007/978-3-030-51652-9_17 [DOI] [PubMed] [Google Scholar]
- Estrada-Cruz N. A., Manuel-Apolinar L., Segura-Uribe J. J., Almanza-Pérez J. C., Fortis-Barrera Á., Orozco-Suárez S., et al. (2022). Short-term administration of tibolone reduces inflammation and oxidative stress in the hippocampus of ovariectomized rats fed high-fat and high-fructose. Nutr. Neurosci. 26, 275–289. 10.1080/1028415X.2022.2046964 [DOI] [PubMed] [Google Scholar]
- Evans R. G., Smith D. W., Lee C. J., Ngo J. P., Gardiner B. S. (2020). What Makes the kidney Susceptible to hypoxia? Anat. Rec. 303 (10), 2544–2552. 10.1002/ar.24260 [DOI] [PubMed] [Google Scholar]
- Faivre A., Scholz C. C., de Seigneux S. (2021). Hypoxia in chronic kidney disease: Towards a paradigm shift? Nephrol. dialysis, Transplant. official Publ. Eur. Dialysis Transpl. Assoc. - Eur. Ren. Assoc. 36 (10), 1782–1790. 10.1093/ndt/gfaa091 [DOI] [PubMed] [Google Scholar]
- Fatima K., Rashid A. M., Memon U. A. A., Fatima S. S., Javaid S. S., Shahid O., et al. (2023). Mediterranean diet and its effect on endothelial function: A meta-analysis and systematic review. Ir. J. Med. Sci. 192 (1), 105–113. 10.1007/s11845-022-02944-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon U., Canavan M., Biniecka M., Veale D. J. (2016). Hypoxia, mitochondrial dysfunction and synovial invasiveness in rheumatoid arthritis. Nat. Rev. Rheumatol. 12 (7), 385–397. 10.1038/nrrheum.2016.69 [DOI] [PubMed] [Google Scholar]
- Feil R., Lehners M., Stehle D., Feil S. (2022). Visualising and understanding cGMP signals in the cardiovascular system. Br. J. Pharmacol. 179 (11), 2394–2412. 10.1111/bph.15500 [DOI] [PubMed] [Google Scholar]
- Feng Q., He B. (2019). Androgen receptor signaling in the development of castration-Resistant prostate cancer. Front. Oncol. 9, 858. 10.3389/fonc.2019.00858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z., Wang G., Xia H., Li M., Liang G., Dong T., et al. (2021). Macular vascular geometry changes with sex and age in healthy subjects: A Fundus Photography study. Front. Med. 8, 778346. 10.3389/fmed.2021.778346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fertig R. M., Gamret A. C., Darwin E., Gaudi S. (2017). Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatology online J. 23 (11), 13030. 10.5070/d32311037240 [DOI] [PubMed] [Google Scholar]
- Fine L. G., Norman J. T. (2008). Chronic hypoxia as a mechanism of progression of chronic kidney diseases: From hypothesis to novel therapeutics. Kidney Int. 74 (7), 867–872. 10.1038/ki.2008.350 [DOI] [PubMed] [Google Scholar]
- Fleszar M. G., Wiśniewski J., Zboch M., Diakowska D., Gamian A., Krzystek-Korpacka M. (2019). Targeted metabolomic analysis of nitric oxide/L-arginine pathway metabolites in dementia: Association with pathology, severity, and structural brain changes. Sci. Rep. 9 (1), 13764. 10.1038/s41598-019-50205-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresta C., Ferlin A., Lenzi A., Montorsi P. Italian Study Group on Cardiometabolic Andrology (2017). The great opportunity of the andrological patient: Cardiovascular and metabolic risk assessment and prevention. Andrology 5 (3), 408–413. 10.1111/andr.12342 [DOI] [PubMed] [Google Scholar]
- Forsberg K. M. E., Zhang Y., Reiners J., Ander M., Niedermayer A., Fang L., et al. (2018). Endothelial damage, vascular bagging and remodeling of the microvascular bed in human microangiopathy with deep white matter lesions. Acta neuropathol. Commun. 6 (1), 128. 10.1186/s40478-018-0632-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Morsiani C., Conte M., Santoro A., Grignolio A., et al. (2018). The Continuum of aging and age-related diseases: Common mechanisms but Different rates. Front. Med. 5, 61. 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratantonio D., Virgili F., Zucchi A., Lambrechts K., Latronico T., Lafère P., et al. (2021). Increasing oxygen partial Pressures induce a distinct transcriptional response in human PBMC: A pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 22 (1), 458. 10.3390/ijms22010458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredette N. C., Meyer M. R., Prossnitz E. R. (2018). Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J. steroid Biochem. Mol. Biol. 176, 65–72. 10.1016/j.jsbmb.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed J. K., Gutterman D. D. (2017). Communication is key: Mechanisms of Intercellular signaling in vasodilation. J. Cardiovasc. Pharmacol. 69 (5), 264–272. 10.1097/FJC.0000000000000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A., Sandner P., Schmidtko A. (2020). cGMP: a unique 2nd messenger molecule - recent developments in cGMP research and development. Naunyn-Schmiedeberg’s archives Pharmacol. 393 (2), 287–302. 10.1007/s00210-019-01779-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbie L., Buckanovich R. J., Coffman L. (2022). Carcinoma-associated mesenchymal stem/stromal cells: Architects of the pro-tumorigenic tumor microenvironment. Stem cells Dayt. Ohio) 40 (8), 705–715. 10.1093/stmcls/sxac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Yamashita S., Hayashi N., Goto T., Koyama J., Sato T., et al. (2019). Phosphodiesterase type 5 inhibitor attenuates chronic ischemia-induced prostatic hyperplasia in a rat model. Prostate 79 (5), 536–543. 10.1002/pros.23759 [DOI] [PubMed] [Google Scholar]
- Gajecki D., Gawryś J., Szahidewicz-Krupska E., Doroszko A. (2022). Role of erythrocytes in nitric oxide metabolism and paracrine regulation of endothelial function. Antioxidants (Basel, Switz. 11 (5), 943. 10.3390/antiox11050943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambini J., Stromsnes K. (2022). Oxidative stress and inflammation: From mechanisms to therapeutic approaches. Biomedicines. 10, 753. 10.3390/biomedicines10040753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantenbein K. V., Kanaka-Gantenbein C. (2021). Mediterranean diet as an antioxidant: The impact on metabolic health and Overall Wellbeing. Nutrients 13 (6), 1951. 10.3390/nu13061951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner B. N., LaFond K. M., Bonini M. G. (2020). Nitric oxide in cellular adaptation and disease. Redox Biol. 34, 101550. 10.1016/j.redox.2020.101550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Pavia P., Rapezzi C., Adler Y., Arad M., Basso C., Brucato A., et al. (2021). Diagnosis and treatment of cardiac amyloidosis: A position statement of the ESC Working group on myocardial and Pericardial diseases. Eur. heart J. 42 (16), 1554–1568. 10.1093/eurheartj/ehab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmaroudi F. S., Handy D. E., Liu Y. Y., Loscalzo J. (2016). Systems pharmacology and Rational Polypharmacy: Nitric oxide-cyclic GMP signaling pathway as an Illustrative example and Derivation of the general case. PLoS Comput. Biol. 12 (3), e1004822. 10.1371/journal.pcbi.1004822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D., Kern C. C. (2022). Is “cellular senescence” a misnomer? GeroScience 44 (5), 2461–2469. 10.1007/s11357-022-00652-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis G., Zisis I. E., Docea A. O., Tsarouhas K., Fragkiadoulaki I., Mavridis C., et al. (2020). Current concepts on the Reno-protective effects of phosphodiesterase 5 inhibitors in acute kidney Injury: Systematic Search and review. J. Clin. Med. 9 (5), 1284. 10.3390/jcm9051284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadou P., Sbarouni E. (2009). Effect of hormone replacement therapy on inflammatory biomarkers. Adv. Clin. Chem. 47, 59–93. 10.1016/s0065-2423(09)47003-3 [DOI] [PubMed] [Google Scholar]
- Gerbie E. Y., Bury M. I., Chan Y. Y., Morey A. F., Sharma A. K., Hofer M. D. (2021). Testosterone and estrogen repletion in a hypogonadal environment improves post-operative angiogenesis. Urology 152, 9–e1. [DOI] [PubMed] [Google Scholar]
- Gerhard M., Roddy M. A., Creager S. J., Creager M. A. (1996). Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension 27 (4), 849–853. 10.1161/01.hyp.27.4.849 [DOI] [PubMed] [Google Scholar]
- Gerstung M., Jolly C., Leshchiner I., Dentro S. C., Gonzalez S., Rosebrock D., et al. (2020). The evolutionary history of 2,658 cancers. Nature 578 (7793), 122–128. 10.1038/s41586-019-1907-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getu A. (2022). Ethiopian native Highlander’s adaptation to chronic high-altitude hypoxia. BioMed Res. Int. 2022, 5749382. 10.1155/2022/5749382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifre-Renom L., Daems M., Luttun A., Jones E. A. V. (2022). Organ-specific endothelial cell Differentiation and impact of microenvironmental Cues on endothelial heterogeneity. Int. J. Mol. Sci. 23 (3), 1477. 10.3390/ijms23031477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert S. E., Tew G. A., Bourke L., Winter E. M., Rosario D. J. (2013). Assessment of endothelial dysfunction by flow-mediated dilatation in men on long-term androgen deprivation therapy for prostate cancer. Exp. Physiol. 98 (9), 1401–1410. 10.1113/expphysiol.2013.073353 [DOI] [PubMed] [Google Scholar]
- Gilmore A. C., Flaherty S. J., Somasundaram V., Scheiblin D. A., Lockett S. J., Wink D. A., et al. (2021). An in vitro tumorigenesis model based on live-cell-generated oxygen and nutrient gradients. Commun. Biol. 4 (1), 477. 10.1038/s42003-021-01954-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F., Ückert S., Maggi M., Birder L., Kissel J., Viktrup L. (2013). The mechanism of action of phosphodiesterase type 5 inhibitors in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Eur. Urol. 63 (3), 506–516. 10.1016/j.eururo.2012.09.006 [DOI] [PubMed] [Google Scholar]
- Gkaliagkousi E., Lazaridis A., Dogan S., Fraenkel E., Tuna B. G., Mozos I., et al. (2022). Theories and molecular Basis of vascular aging: A review of the literature from VascAgeNet group on pathophysiological mechanisms of vascular aging. Int. J. Mol. Sci. 23 (15), 8672. 10.3390/ijms23158672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel A., Dell'Endice S., Jaschke N., Pählig S., Shahid A., Hofbauer L. C., et al. (2021). The role of inflammation in breast and prostate cancer metastasis to bone. Int. J. Mol. Sci. 22 (10), 5078. 10.3390/ijms22105078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godo S., Takahashi J., Yasuda S., Shimokawa H. (2021). Role of inflammation in coronary Epicardial and microvascular dysfunction. Eur. Cardiol. 16, e13. 10.15420/ecr.2020.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golshiri K., Ataei Ataabadi E., Portilla Fernandez E. C., Jan Danser A. H., Roks A. J. M. (2020). The importance of the nitric oxide-cGMP pathway in age-related cardiovascular disease: Focus on phosphodiesterase-1 and soluble guanylate cyclase. Basic & Clin. Pharmacol. Toxicol. 127 (2), 67–80. 10.1111/bcpt.13319 [DOI] [PubMed] [Google Scholar]
- Gómez-Sánchez L., Gómez-Sánchez M., Patino-Alonso C., Recio-Rodríguez J. I., González-Sánchez J., Agudo-Conde C., et al. (2022). Retinal blood vessel calibre and vascular ageing in a general Spanish population: A EVA study. Eur. J. Clin. investigation 52 (2), e13684. 10.1111/eci.13684 [DOI] [PubMed] [Google Scholar]
- Graff B. J., Payne S. J., El-Bouri W. K. (2021). The ageing brain: Investigating the role of age in changes to the human cerebral microvasculature with an in silico model. Front. aging Neurosci. 13, 632521. 10.3389/fnagi.2021.632521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravenmier C. A., Siddique M., Gatenby R. A. (2018). Adaptation to stochastic temporal Variations in Intratumoral blood flow: The Warburg effect as a bet hedging strategy. Bull. Math. Biol. 80 (5), 954–970. 10.1007/s11538-017-0261-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray A., Feldman H. A., McKinlay J. B., Longcope C. (1991). Age, disease, and changing sex hormone levels in middle-aged men: Results of the Massachusetts male aging study. J. Clin. Endocrinol. Metabolism 73 (5), 1016–1025. 10.1210/jcem-73-5-1016 [DOI] [PubMed] [Google Scholar]
- Greenwald P., Dunn B. K. (2009). Do we make optimal use of the potential of cancer prevention? Recent results cancer Res. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 181, 3–17. 10.1007/978-3-540-69297-3_1 [DOI] [PubMed] [Google Scholar]
- Groti Antonič K., Antonič B., Žuran I., Pfeifer M. (2020). Testosterone treatment longer than 1 year shows more effects on functional hypogonadism and related metabolic, vascular, diabetic and obesity parameters (results of the 2-year clinical trial). aging male official J. Int. Soc. Study Aging Male 23 (5), 1–13. 10.1080/13685538.2020.1793132 [DOI] [PubMed] [Google Scholar]
- Guerraty M., Bhargava A., Senarathna J., Mendelson A. A., Pathak A. P. (2021). Advances in translational imaging of the microcirculation. Microcirculation 28 (3), e12683. 10.1111/micc.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerville F., Bourdel-Marchasson I., Déchanet-Merville J., Pellegrin I., Soubeyran P., Appay V., et al. (2022). Does inflammation contribute to cancer incidence and mortality during aging? A Conceptual review. Cancers 14 (7), 1622. 10.3390/cancers14071622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günter J., Ruiz-Serrano A., Pickel C., Wenger R. H., Scholz C. C. (2017). The functional interplay between the HIF pathway and the ubiquitin system - more than a one-way road. Exp. cell Res. 356 (2), 152–159. 10.1016/j.yexcr.2017.03.027 [DOI] [PubMed] [Google Scholar]
- Gupta M. A., Vujcic B., Gupta A. K. (2020a). Finasteride Use is associated with higher Odds of Obstructive sleep Apnea: Results from the US Food and drug administration adverse events reporting system. Skinmed 18 (3), 146–150. [PubMed] [Google Scholar]
- Gupta N., Kaur H., Wajid S. (2020b). Renal amyloidosis: An update on diagnosis and pathogenesis. Protoplasma 257 (5), 1259–1276. 10.1007/s00709-020-01513-0 [DOI] [PubMed] [Google Scholar]
- Gur S., Alzweri L., Yilmaz-Oral D., Kaya-Sezginer E., Abdel-Mageed A. B., Dick B., et al. (2020). Testosterone positively regulates functional responses and nitric oxide expression in the isolated human corpus cavernosum. Andrology 8, 1824. 10.1111/andr.12866 [DOI] [PubMed] [Google Scholar]
- Gurung R., Li T. (2022). Renal amyloidosis: Presentation, diagnosis, and management. Am. J. Med. 135, S38–S43. 10.1016/j.amjmed.2022.01.003 [DOI] [PubMed] [Google Scholar]
- Guzik T. J., Touyz R. M. (2017). Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70 (4), 660–667. 10.1161/HYPERTENSIONAHA.117.07802 [DOI] [PubMed] [Google Scholar]
- Hachmo Y., Hadanny A., Abu Hamed R., Daniel-Kotovsky M., Catalogna M., Fishlev G., et al. (2020). Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cells: A prospective trial. Aging 12 (22), 22445–22456. 10.18632/aging.202188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadanny A., Efrati S. (2020). The hyperoxic-hypoxic paradox. Biomolecules 10 (6), 958. 10.3390/biom10060958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadanny A., Lang E., Copel L., Meir O., Bechor Y., Fishlev G., et al. (2018). Hyperbaric oxygen can induce angiogenesis and recover erectile function. Int. J. Impot. Res. 30 (6), 292–299. 10.1038/s41443-018-0023-9 [DOI] [PubMed] [Google Scholar]
- Haider K. S., Haider A., Saad F., Doros G., Hanefeld M., Dhindsa S., et al. (2020). Remission of type 2 diabetes following long-term treatment with injectable testosterone undecanoate in patients with hypogonadism and type 2 diabetes: 11-year data from a real-world registry study. Diabetes, Obes. metabolism 22 (11), 2055–2068. 10.1111/dom.14122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann J., Böger R. (2022). Dysregulation of the nitric oxide/Dimethylarginine pathway in hypoxic pulmonary vasoconstriction-molecular mechanisms and clinical significance. Front. Med. 9, 835481. 10.3389/fmed.2022.835481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänselmann A., Berliner D., Bauersachs J., Bavendiek U. 2022. Cardiac amyloidosis—Interdisciplinary approach to diagnosis and therapy. Herz, 47 ( 4), pp.324–331. 10.1007/s00059-022-05122-w [DOI] [PubMed] [Google Scholar]
- Hashmi A. A., Mudassir G., Irfan M., Hussain Z. F., Hashmi S. K., Asif H., et al. (2019). Prognostic significance of high androgen receptor expression in prostatic Acinar Adenocarcinoma. Asian Pac. J. cancer Prev. APJCP 20 (3), 893–896. 10.31557/APJCP.2019.20.3.893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan Venkatesh G., Bravo P., Shaaban Moustafa Elsayed W., Amirtharaj F., Wojtas B., Abou Khouzam R., et al. (2020). Hypoxia increases mutational load of breast cancer cells through frameshift mutations. Oncoimmunology 9 (1), 1750750. 10.1080/2162402X.2020.1750750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennigs J. K., Matuszcak C., Trepel M., Körbelin J. (2021). Vascular endothelial cells: Heterogeneity and targeting approaches. Cells 10 (10), 2712. 10.3390/cells10102712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera G. A. (2021). Renal amyloidosis: Pathogenesis. Ultrastruct. Pathol. 45 (4–5), 267–275. 10.1080/01913123.2021.1972065 [DOI] [PubMed] [Google Scholar]
- Hesh C. A., Qiu Y., Lam W. A. (2019). Vascularized Microfluidics and the blood-endothelium interface. Micromachines 11 (1), 18. 10.3390/mi11010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok J. R., Vasudevan D., Jablonski K., Thomas D. D. (2013). Oxygen dependence of nitric oxide-mediated signaling. Redox Biol. 1 (1), 203–209. 10.1016/j.redox.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K. (2020). Basic biology of hypoxic responses mediated by the transcription factor HIFs and its implication for medicine. Biomedicines 8 (2), 32. 10.3390/biomedicines8020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D. A., Basillote J. B., Armenakas N. A., Vasovic L., Shevchuk M., Pareek G., et al. (2002). Decreased suburethral prostatic microvessel density in finasteride treated prostates: A possible mechanism for reduced bleeding in benign prostatic hyperplasia. J. urology 167 (4), 1731–1733. 10.1097/00005392-200204000-00034 [DOI] [PubMed] [Google Scholar]
- Hodis H. N., Mack W. J. (2022). Menopausal hormone replacement therapy and reduction of all-cause mortality and cardiovascular disease: It is about time and timing. Cancer J. (Sudbury, Mass.) 28 (3), 208–223. 10.1097/PPO.0000000000000591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. D., Kapur P., Cordon B. H., Hamoun F., Russell D., Scott J. M., et al. (2017). Low testosterone levels result in decreased Periurethral vascularity via an androgen receptor-mediated process: Pilot study in urethral Stricture tissue. Urology 105, 175–180. 10.1016/j.urology.2017.02.037 [DOI] [PubMed] [Google Scholar]
- Hofer M. D., Morey A. F. (2018). Role of androgens for urethral homeostasis. Transl. Androl. urology 7 (4), 521–525. 10.21037/tau.2018.02.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer N., Kallab M., Sim Y. C., Lee A. X., Chua J., Tan B., et al. (2022). Effect of hyperoxia and hypoxia on retinal vascular parameters assessed with optical coherence tomography angiography. Acta Ophthalmol. 100 (6), e1272–e1279. 10.1111/aos.15077 [DOI] [PubMed] [Google Scholar]
- Hoogland A. I., Jim H. S. L., Gonzalez B. D., Small B. J., Gilvary D., Breen E. C., et al. (2021). Systemic inflammation and symptomatology in patients with prostate cancer treated with androgen deprivation therapy: Preliminary findings. Cancer 127 (9), 1476–1482. 10.1002/cncr.33397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstman A. M., Dillon E. L., Urban R. J., Sheffield-Moore M. (2012). The role of androgens and estrogens on healthy aging and longevity. journals gerontology. Ser. A, Biol. Sci. Med. Sci. 67 (11), 1140–1152. 10.1093/gerona/gls068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta Y., Kataoka T., Kimura K. (2019). Testosterone deficiency and endothelial dysfunction: Nitric oxide, Asymmetric Dimethylarginine, and endothelial progenitor cells. Sex. Med. Rev. 7 (4), 661–668. 10.1016/j.sxmr.2019.02.005 [DOI] [PubMed] [Google Scholar]
- Houben A. J. H. M., Martens R. J. H., Stehouwer C. D. A. (2017). Assessing microvascular function in humans from a chronic disease perspective. J. Am. Soc. Nephrol. JASN 28 (12), 3461–3472. 10.1681/ASN.2017020157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K. L., Cybulsky M., Fish J. E. (2022). The endothelium as a hub for cellular communication in Atherogenesis: Is there Directionality to the message? Front. Cardiovasc. Med. 9, 888390. 10.3389/fcvm.2022.888390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S., Song W., Pastuszak A., Khera M. (2021). Differential gene expression in post-finasteride syndrome patients. J. Sex. Med. 18 (9), 1479–1490. 10.1016/j.jsxm.2021.05.009 [DOI] [PubMed] [Google Scholar]
- Hu X., Wang Y. H., Yang Z. Q., Shao Y. X., Yang W. X., Li X. (2020). Association of 5-alpha-reductase inhibitor and prostate cancer incidence and mortality: A meta-analysis. Transl. Androl. urology 9 (6), 2519–2532. 10.21037/tau-20-843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Akgün E. E., Mehmood K., Zhang H., Tang Z., Li Y. (2022). Mechanism of hypoxia-mediated smooth muscle cell proliferation leading to vascular remodeling. BioMed Res. Int. 2022, 3959845. 10.1155/2022/3959845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Giordano F. J. (2008). Chapter 13. Oxygen as a direct and indirect biological determinant in the vasculature. Methods Enzym. 444, 285–304. 10.1016/S0076-6879(08)02813-9 [DOI] [PubMed] [Google Scholar]
- Husain I., Shukla S., Soni P., Husain N. (2016). Role of androgen receptor in prostatic neoplasia versus hyperplasia. J. cancer Res. Ther. 12 (1), 112–116. 10.4103/0973-1482.151429 [DOI] [PubMed] [Google Scholar]
- Iacono F., Prezioso D., Ruffo A., Illiano E., Romis L., Di Lauro G., et al. (2012). Testosterone deficiency causes penile fibrosis and organic erectile dysfunction in aging men. Evaluating association among Age, TDS and ED. BMC Surg. 12, S24. 10.1186/1471-2482-12-S1-S24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Tsujimura A., Miyoshi M., Miyoshi Y., Ogasa T., Hiramatsu I., et al. (2020). Endocrinological and symptomatic characteristics of patients with late-onset hypogonadism classified by functional categories based on testosterone and luteinizing hormone levels. Int. J. urology official J. Jpn. Urological Assoc. 27 (9), 767–774. 10.1111/iju.14296 [DOI] [PubMed] [Google Scholar]
- Iwata M., Inoue T., Asai Y., Hori K., Fujiwara M., Matsuo S., et al. (2020). The protective role of localized nitric oxide production during inflammation may be mediated by the heme oxygenase-1/carbon monoxide pathway. Biochem. biophysics Rep. 23, 100790. 10.1016/j.bbrep.2020.100790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Sci. (New York, N.Y.) 292 (5516), 468–472. 10.1126/science.1059796 [DOI] [PubMed] [Google Scholar]
- Jacob M., Chappell D., Becker B. F. (2016). Regulation of blood flow and volume exchange across the microcirculation. Crit. care (London, Engl. 20 (1), 319. 10.1186/s13054-016-1485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain P., Duddu A. S., Jolly M. K. (2022). Stochastic population dynamics of cancer stemness and adaptive response to therapies. Essays Biochem. 66 (4), 387–398. 10.1042/EBC20220038 [DOI] [PubMed] [Google Scholar]
- Jalnapurkar S., Landes S., Wei J., Mehta P. K., Shufelt C., Minissian M., et al. (2021). Coronary endothelial dysfunction appears to be a manifestation of a systemic process: A report from the Women’s ischemia syndrome evaluation - coronary vascular dysfunction (WISE-CVD) study. PloS one 16 (9), e0257184. 10.1371/journal.pone.0257184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambusaria A., Hong Z., Zhang L., Srivastava S., Jana A., Toth P. T., et al. (2020). Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife 9, e51413. 10.7554/eLife.51413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. Y., Amy L. N., Erica C. H., Wanjun G., Joe A., Esteban A. M., et al. (2022). Time domains of hypoxia responses and –omics insights. Front. Physiol. 13, 885295. 10.3389/fphys.2022.885295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janaszak-Jasiecka A., Siekierzycka A., Płoska A., Dobrucki I. T., Kalinowski L. (2021). Endothelial dysfunction driven by hypoxia-the influence of oxygen deficiency on NO bioavailability. Biomolecules 11 (7), 982. 10.3390/biom11070982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee D., Park S. H., Hwang H. S., Kim H. S., Kim M. S., Kim E. C. (2021). Effects of hormone replacement therapy on lens opacity, serum inflammatory cytokines, and antioxidant levels. Ann. Med. 53 (1), 707–714. 10.1080/07853890.2021.1928275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jehanno C., Le Goff P., Habauzit D., Le Page Y., Lecomte S., Lecluze E., et al. (2022). Hypoxia and ERα transcriptional crosstalk is associated with endocrine resistance in breast cancer. Cancers 14 (19), 4934. 10.3390/cancers14194934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena B. C., Das C. K., Banerjee I., Bharadwaj D., Majumder R., Das S., et al. (2022). TGF-β1 induced autophagy in cancer associated fibroblasts during hypoxia contributes EMT and glycolysis via MCT4 upregulation. Exp. cell Res. 417 (1), 113195. 10.1016/j.yexcr.2022.113195 [DOI] [PubMed] [Google Scholar]
- Jiang S., Chen G., Yang Z., Wang D., Lu Y., Zhu L., et al. (2021). Testosterone attenuates hypoxia-induced hypertension by affecting NRF1-mediated transcriptional regulation of ET-1 and ACE. Hypertens. Res. official J. Jpn. Soc. Hypertens. 44 (11), 1395–1405. 10.1038/s41440-021-00703-4 [DOI] [PubMed] [Google Scholar]
- Jin Cho W., Pyo J. S. (2020). Immunohistochemical analysis of the impact of ischemic change in benign prostatic hyperplasia. Pathology Res. Pract. 216 (1), 152694. 10.1016/j.prp.2019.152694 [DOI] [PubMed] [Google Scholar]
- Jockers R., Liu J. (2021). Editorial: Endocrinology in cancer and aging. Front. Endocrinol. 12, 722929. 10.3389/fendo.2021.722929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly M. K., Kulkarni P., Weninger K., Orban J., Levine H. (2018). Phenotypic plasticity, bet-hedging, and androgen independence in prostate cancer: Role of non-genetic heterogeneity. Front. Oncol. 8, 50. 10.3389/fonc.2018.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. B., Henry G. H., Malewska A., Reese J. C., Mauck R. J., Gahan J. C., et al. (2022). 5-Alpha reductase inhibitors induce a prostate luminal to club cell transition in human benign prostatic hyperplasia. J. pathology 256 (4), 427–441. 10.1002/path.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. B., Turco A. E., Vezina C. M., Strand D. W. (2021). Progenitors in prostate development and disease. Dev. Biol. 473, 50–58. 10.1016/j.ydbio.2020.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C., Jung F., Kelm M. (2016). The microcirculation in hypoxia: The center of the battlefield for oxygen. Clin. Hemorheol. Microcirc. 63, 169–172. 10.3233/CH-1663301 [DOI] [PubMed] [Google Scholar]
- Kalaria R. N., Hase Y. (2019). Neurovascular ageing and age-related diseases. Sub-cellular Biochem. 91, 477–499. 10.1007/978-981-13-3681-2_17 [DOI] [PubMed] [Google Scholar]
- Kalsi J. S., Ralph D. J., Thomas P., Bellringer J., Minhas S., Kell P. D., et al. (2005). A nitric oxide-releasing PDE5 inhibitor relaxes human corpus cavernosum in the absence of endogenous nitric oxide. J. Sex. Med. 2 (1), 53–57. 10.1111/j.1743-6109.2005.20105.x [DOI] [PubMed] [Google Scholar]
- Kamat S. M., Mendelsohn A. R., Larrick J. W. (2021). Rejuvenation through oxygen, more or less. Rejuvenation Res. 24 (2), 158–163. 10.1089/rej.2021.0014 [DOI] [PubMed] [Google Scholar]
- Kaminsky A., Sperling H., Popken G. (2011). Primary and secondary prevention of erectile dysfunction. Der Urol. Ausg. A 50 (10), 1265–1268. 10.1007/s00120-011-2620-9 [DOI] [PubMed] [Google Scholar]
- Kang B. W., Kim F., Cho J. Y., Kim S., Rhee J., Choung J. J. (2022). Phosphodiesterase 5 inhibitor mirodenafil ameliorates Alzheimer-like pathology and symptoms by multimodal actions. Alzheimer’s Res. Ther. 14 (1), 92. 10.1186/s13195-022-01034-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapil V., Khambata R. S., Jones D. A., Rathod K., Primus C., Massimo G., et al. (2020). The Noncanonical pathway for in vivo nitric oxide generation: The nitrate-nitrite-nitric oxide pathway. Pharmacol. Rev. 72 (3), 692–766. 10.1124/pr.120.019240 [DOI] [PubMed] [Google Scholar]
- Kaplan A. R., Glazer P. M. (2020). Impact of hypoxia on DNA repair and genome integrity. Mutagenesis 35 (1), 61–68. 10.1093/mutage/gez019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbasi S., Mohamadian M., Naseri M., Khorasanchi Z., Zarban A., Bahrami A., et al. (2023). A Mediterranean diet is associated with improved total antioxidant content of human breast milk and infant urine. Nutr. J. 22 (1), 11. 10.1186/s12937-023-00841-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Hotta Y., Maeda Y., Kimura K. (2017). Testosterone deficiency causes endothelial dysfunction via elevation of Asymmetric Dimethylarginine and oxidative stress in castrated rats. J. Sex. Med. 14 (12), 1540–1548. 10.1016/j.jsxm.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Kataoka T., Ito H., Mori T., Hotta Y., Sanagawa A., Maeda Y., et al. (2022). Testosterone improved erectile function by upregulating transcriptional expression of growth factors in late androgen replacement therapy model rats. Int. J. Impot. Res. 10.1038/s41443-022-00627-8 [DOI] [PubMed] [Google Scholar]
- Khadilkar S. S. (2019). Post-reproductive health: Window of opportunity for preventing Comorbidities. J. obstetrics Gynaecol. India 69, 1–5. 10.1007/s13224-019-01202-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera M., Than J. K., Anaissie J., Antar A., Song W., Losso B., et al. (2020). Penile vascular abnormalities in young men with persistent side effects after finasteride use for the treatment of androgenic alopecia. Transl. Androl. urology 9 (3), 1201–1209. 10.21037/tau.2020.03.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Farr J. N., Tchkonia T., Kirkland J. L. (2020). The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 16 (5), 263–275. 10.1038/s41574-020-0335-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja M. A., Nawaz G., Muhammad S., Jamil M. I., Faisal M., Akhter S. (2016). The effect of two Weeks Preoperative finasteride therapy in reducing prostate vascularity. J. Coll. Physicians Surgeons--Pakistan JCPSP 26 (3), 213–215. 10.2016/JCPSP.213215 [DOI] [PubMed] [Google Scholar]
- Kiani A. K., Bonetti G., Medori M. C., Caruso P., Manganotti P., Fioretti F., et al. (2022). Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 63, E239–E245. 10.15167/2421-4248/jpmh2022.63.2S3.2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierans S. J., Taylor C. T. (2021). Regulation of glycolysis by the hypoxia-inducible factor (HIF): Implications for cellular physiology. J. physiology 599 (1), 23–37. 10.1113/JP280572 [DOI] [PubMed] [Google Scholar]
- Kim H., Yoo J., Han K., Lee D. Y., Fava M., Mischoulon D., et al. (2022a). Hormone therapy and the decreased risk of dementia in women with depression: A population-based cohort study. Alzheimer’s Res. Ther. 14 (1), 83. 10.1186/s13195-022-01026-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I., Choi S., Yoo S., Lee M. (2022b). Cancer-associated fibroblasts in the hypoxic tumor microenvironment. Cancers 14 (14), 3321. 10.3390/cancers14143321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Barsoum I. B., Loh H., Paré J. F., Siemens D. R., Graham C. H. (2020). Inhibition of hypoxia-inducible factor 1α accumulation by glyceryl trinitrate and cyclic guanosine monophosphate. Biosci. Rep. 40 (1), BSR20192345. 10.1042/BSR20192345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T. Y., Song Y. Y., Na Y. J., Lee Y. H., Kim J. Y., Lee M. W. (2022c). The impairment of the deep vascular complex in prolonged type 2 diabetes patients without clinical diabetic retinopathy. PloS one 17 (6), e0269182. 10.1371/journal.pone.0269182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemba A., Bodnar L., Was H., Brodaczewska K. K., Wcislo G., Szczylik C. A., et al. (2020). Hypoxia-mediated decrease of ovarian cancer cells reaction to treatment: Significance for Chemo- and Immunotherapies. Int. J. Mol. Sci. 21 (24), 9492. 10.3390/ijms21249492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y. H., Moon D. G., Moon K. H. (2013). Testosterone replacement alone for testosterone deficiency syndrome improves moderate lower urinary tract symptoms: One Year Follow-up. World J. Men’s Health 31 (1), 47–52. 10.5534/wjmh.2013.31.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Zha X., Nagase K., Inamura S., Taga M., Aoki Y., et al. (2022). Phosphodiesterase 5 inhibitor suppresses prostate weight increase in type 2 diabetic rats. Life Sci. 298, 120504. 10.1016/j.lfs.2022.120504 [DOI] [PubMed] [Google Scholar]
- Kocarnik J. M., Compton K., Dean F. E., Fu W., Gaw B. L., Harvey J. D., et al. (2022). Cancer incidence, mortality, Years of life Lost, Years lived with Disability, and Disability-Adjusted life Years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol. 8 (3), 420–444. 10.1001/jamaoncol.2021.6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König C. S., Balabani S., Hackett G. I., Strange R. C., Ramachandran S. (2019). Testosterone therapy: An assessment of the clinical consequences of changes in Hematocrit and blood flow characteristics. Sex. Med. Rev. 7 (4), 650–660. 10.1016/j.sxmr.2019.01.003 [DOI] [PubMed] [Google Scholar]
- Korbecki J., Kojder K., Kapczuk P., Kupnicka P., Gawrońska-Szklarz B., Gutowska I., et al. (2021). The effect of hypoxia on the expression of CXC Chemokines and CXC Chemokine receptors-A review of literature. Int. J. Mol. Sci. 22 (2), 843. 10.3390/ijms22020843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukoulis G. N., Filiponi M., Gougoura S., Befani C., Liakos P., Bargiota Α. (2022). Testosterone and dihydrotestosterone modulate the redox homeostasis of endothelium. Cell Biol. Int. 46 (4), 660–670. 10.1002/cbin.11768 [DOI] [PubMed] [Google Scholar]
- Koutsaliaris I. K., Moschonas I. C., Pechlivani L. M., Tsouka A. N., Tselepis A. D. (2022). Inflammation, oxidative stress, vascular aging and atherosclerotic ischemic stroke. Curr. Med. Chem. 29 (34), 5496–5509. 10.2174/0929867328666210921161711 [DOI] [PubMed] [Google Scholar]
- Krishnan S. M., Kraehling J. R., Eitner F., Bénardeau A., Sandner P. (2018). The impact of the nitric oxide (NO)/Soluble guanylyl cyclase (sGC) signaling Cascade on kidney health and disease: A preclinical perspective. Int. J. Mol. Sci. 19 (6), 1712. 10.3390/ijms19061712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger-Genge A., Blocki A., Franke R. P., Jung F. (2019). Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 20 (18), 4411. 10.3390/ijms20184411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M.-C., Kober F., Lai Y. C., Pohlmann A., Qadri F., Bader M., et al. (2021). Cardiovascular magnetic resonance detects microvascular dysfunction in a mouse model of hypertrophic cardiomyopathy. J. Cardiovasc. magnetic Reson. official J. Soc. Cardiovasc. Magnetic Reson. 23 (1), 63. 10.1186/s12968-021-00754-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B., Adebayo A. K., Prasad M., Capitano M. L., Wang R., Bhat-Nakshatri P., et al. (2022). Tumor collection/processing under physioxia uncovers highly relevant signaling networks and drug sensitivity. Sci. Adv. 8 (2), eabh3375. 10.1126/sciadv.abh3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Choi D.-K. (2015). Hypoxia inducible factor pathway and physiological adaptation: A cell survival pathway. Mediat. Inflamm. 2015, 584758. 10.1155/2015/584758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon M.-H., Park S. H., Song K. M., Ghatak K., Limanjaya A., Ryu D. S., et al. (2016). Penile erection induces angiogenic, survival, and antifibrotic signals: Molecular events associated with penile erection induced by cavernous nerve stimulation in mice. Int. J. urology official J. Jpn. Urological Assoc. 23 (7), 614–622. 10.1111/iju.13105 [DOI] [PubMed] [Google Scholar]
- La Vignera S., Condorelli R. A., Calogero A. E., Cannarella R., Mongioì L. M., Duca Y., et al. (2020). Symptomatic late-onset hypogonadism but normal total testosterone: The importance of testosterone annual decrease velocity. Ann. Transl. Med. 8, 163. 10.21037/atm.2019.11.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi E., Cheri S., Fanti M., Marongiu F. (2021). Aging and cancer: The Waning of community Bonds. Cells 10 (9), 2269. 10.3390/cells10092269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi E., Marongiu F., DeGregori J. (2020). Cancer as a disease of old age: Changing mutational and microenvironmental landscapes. Br. J. cancer 122 (7), 943–952. 10.1038/s41416-019-0721-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix M., Riscal R., Arena G., Linares L. K., Le Cam L. (2020). Metabolic functions of the tumor suppressor p53: Implications in normal physiology, metabolic disorders, and cancer. Mol. Metab. 33, 2–22. 10.1016/j.molmet.2019.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahouel K., Younes L., Danilova L., Giardiello F. M., Hruban R. H., Groopman J., et al. (2020). Revisiting the tumorigenesis timeline with a data-driven generative model. Proc. Natl. Acad. Sci. U. S. A. 117 (2), 857–864. 10.1073/pnas.1914589117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam Y. T., Lecce L., Yuen S. C., Wise S. G., Handelsman D. J., Karas R. H., et al. (2019). Androgens ameliorate impaired ischemia-induced neovascularization due to aging in male mice. Endocrinology 160 (5), 1137–1149. 10.1210/en.2018-00951 [DOI] [PubMed] [Google Scholar]
- Langer R. D. (2017). The evidence base for HRT: What can we believe. Climacteric 20 (2), 91–96. 10.1080/13697137.2017.1280251 [DOI] [PubMed] [Google Scholar]
- Lappano R., Todd L. A., Stanic M., Cai Q., Maggiolini M., Marincola F., et al. (2022). Multifaceted interplay between hormones, growth factors and hypoxia in the tumor microenvironment. Cancers 14 (3), 539. 10.3390/cancers14030539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasne D., Jude B., Susen S. (2006). From normal to pathological hemostasis. Can. J. Anaesth. = J. Can. d’anesthesie 53, S2–S11. 10.1007/BF03022247 [DOI] [PubMed] [Google Scholar]
- Ledo A., Lourenço C. F., Cadenas E., Barbosa R. M., Laranjinha J. (2021). The bioactivity of neuronal-derived nitric oxide in aging and neurodegeneration: Switching signaling to degeneration. Free Radic. Biol. Med. 162, 500–513. 10.1016/j.freeradbiomed.2020.11.005 [DOI] [PubMed] [Google Scholar]
- Lee M.-K., Lee J. H., Sohn S. Y., Lee S. Y., Jeong T. Y., Kim S. C. (2022). Effect of low-dose tadalafil once daily on glycemic control in patients with type 2 diabetes and erectile dysfunction: A randomized, double-blind, placebo-controlled pilot study. Diabetology metabolic syndrome 14 (1), 56. 10.1186/s13098-022-00825-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Chandel N. S., Simon M. C. (2020). Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. cell Biol. 21 (5), 268–283. 10.1038/s41580-020-0227-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z., Hu X., Wu Y., Fu L., Lai S., Lin J., et al. (2022). The role and mechanism of the vascular endothelial niche in diseases: A review. Front. physiology 13, 863265. 10.3389/fphys.2022.863265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidal A. M., Levine B., Debnath J. (2018). Autophagy and the cell biology of age-related disease. Nat. cell Biol. 20 (12), 1338–1348. 10.1038/s41556-018-0235-8 [DOI] [PubMed] [Google Scholar]
- Leisegang K., Roychoudhury S., Slama P., Finelli R. (2021). The mechanisms and management of age-related oxidative stress in male hypogonadism associated with non-communicable chronic disease. Antioxidants (Basel, Switz. 10 (11), 1834. 10.3390/antiox10111834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. E., Lu A. T., Chen B. H., Hernandez D. G., Singleton A. B., Ferrucci L., et al. (2016). Menopause accelerates biological aging. Proc. Natl. Acad. Sci. U. S. A. 113 (33), 9327–9332. 10.1073/pnas.1604558113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyane T. S., Jere S. W., Houreld N. N. (2022). Oxidative stress in ageing and chronic degenerative pathologies: Molecular mechanisms involved in counteracting oxidative stress and chronic inflammation. Int. J. Mol. Sci. 23 (13), 7273. 10.3390/ijms23137273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Gu Y., Shang X., Zhou Y., Zhang H., Zuo L., et al. (2020d). Decreased testosterone secretion index and free testosterone level with multiple symptoms for late-onset hypogonadism identification: A nationwide multicenter study with 5980 aging males in China. Aging 12 (24), 26012–26028. 10.18632/aging.202227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.-X., Wang Z. C., Machuki J. O., Li M. Z., Wu Y. J., Niu M. K., et al. (2022a). Benefits of curcumin in the vasculature: A therapeutic candidate for vascular remodeling in arterial hypertension and pulmonary arterial hypertension? Front. physiology 13, 848867. 10.3389/fphys.2022.848867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Ju H., Jin H., Chen H., Sun M., Zhou Z. (2022b). Low testosterone level and risk of adverse clinical events among male patients with chronic kidney disease: A systematic review and meta-analysis of cohort studies. J. Healthc. Eng. 2022, 3630429. 10.1155/2022/3630429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Schram M. T., Sörensen B. M., van Agtmaal M. J. M., Berendschot T. T. J. M., Webers C. A. B., et al. (2020a). Microvascular phenotyping in the Maastricht study: Design and main findings, 2010-2018. Am. J. Epidemiol. 189 (9), 873–884. 10.1093/aje/kwaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., He S., Ma B. (2020c). Autophagy and autophagy-related proteins in cancer. Mol. Cancer 19 (1), 12. 10.1186/s12943-020-1138-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Zhang Q., Nasser M. I., Xu L., Zhang X., Zhu P., et al. (2020b). Oxygen homeostasis and cardiovascular disease: A role for HIF? Biomed. Pharmacother. = Biomedecine Pharmacother. 128, 110338. 10.1016/j.biopha.2020.110338 [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang Z., Ren Y., Wang Y., Fang J., Yue H., et al. (2021). Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 22 (2), 165–187. 10.1007/s10522-021-09910-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Liu X., Zhang C., Zhang Q. (2023). Tumor hypoxia: From basic knowledge to therapeutic implications. Seminars cancer Biol. 88, 172–186. 10.1016/j.semcancer.2022.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Zhang Q. (2020). Understanding the oxygen-sensing pathway and its therapeutic implications in diseases. Am. J. pathology 190 (8), 1584–1595. 10.1016/j.ajpath.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light K. C., Hinderliter A. L., West S. G., Grewen K. M., Steege J. F., Sherwood A., et al. (2001). Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. J. Hypertens. 19 (2), 269–278. 10.1097/00004872-200102000-00014 [DOI] [PubMed] [Google Scholar]
- Lim J., Murthy A. (2020). Targeting autophagy to treat cancer: Challenges and Opportunities. Front. Pharmacol. 11, 590344. 10.3389/fphar.2020.590344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Ni W., Zhang J., Wang G., Li F., Ren W. (2017). Administration of curcumin protects kidney Tubules against renal ischemia-Reperfusion Injury (RIRI) by modulating nitric oxide (NO) signaling pathway. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol. 44 (1), 401–411. 10.1159/000484920 [DOI] [PubMed] [Google Scholar]
- Liu Q., Palmgren V. A. C., Danen E. H., Le Dévédec S. E. (2022). Acute vs. chronic vs. intermittent hypoxia in breast cancer: A review on its application in in vitro research. Mol. Biol. Rep. 49 (11), 10961–10973. 10.1007/s11033-022-07802-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lius E. E., Syafaah I. (2022). Hyperoxia in the management of respiratory failure: A literature review. Ann. Med. Surg. 81 (81), 104393. 10.1016/j.amsu.2022.104393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo R. A. (2017). Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 13 (4), 220–231. 10.1038/nrendo.2016.164 [DOI] [PubMed] [Google Scholar]
- López-Novoa J. M., Nieto M. A. (2009). Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. EMBO Mol. Med. 1 (6–7), 303–314. 10.1002/emmm.200900043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowerison M. R., Sekaran N. V. C., Zhang W., Dong Z., Chen X., Llano D. A., et al. (2022). Aging-related cerebral microvascular changes visualized using ultrasound localization microscopy in the living mouse. Sci. Rep. 12 (1), 619. 10.1038/s41598-021-04712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisetto M., Ahmadabadi B. N., Mashori G. R., Hamid G. A. (2019). The association between hypoxia, chronic ischemia and alters prostate structure and progress of chronic prostatic disease. Archives Pharm. Pharm. Sci. 3 (1), 042–078. 10.29328/journal.apps.1001016 [DOI] [Google Scholar]
- Lundberg J. O., Weitzberg E. (2022). Nitric oxide signaling in health and disease. Cell 185 (16), 2853–2878. 10.1016/j.cell.2022.06.010 [DOI] [PubMed] [Google Scholar]
- Luo L., Qin Z.-H. (2019). Autophagy, aging, and longevity. Adv. Exp. Med. Biol. 1206, 509–525. 10.1007/978-981-15-0602-4_24 [DOI] [PubMed] [Google Scholar]
- Luo Z., Tian M., Yang G., Tan Q., Chen Y., Zhang Q., et al. (2022). Hypoxia signaling in human health and diseases: Implications and prospects for therapeutics. Signal Transduct. Target. Ther. 7 (1), 218. 10.1038/s41392-022-01080-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macharia L. W., Muriithi W., Heming C. P., Nyaga D. K., Aran V., Mureithi M. W., et al. (2021). The genotypic and phenotypic impact of hypoxia microenvironment on glioblastoma cell lines. BMC cancer 21 (1), 1248. 10.1186/s12885-021-08978-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan S., Azevedo N., Duncan E., Dunsmore J., Fullwood L., Lumen N., et al. (2023). Mapping European association of Urology guideline Practice across Europe: An Audit of androgen deprivation therapy Use before prostate cancer surgery in 6598 cases in 187 Hospitals across 31 European Countries. Eur. Urol. 83, 393. 10.1016/j.eururo.2022.12.031 [DOI] [PubMed] [Google Scholar]
- Maki P. M. (2013). Critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause (New York, N.Y.) 20 (6), 695–709. 10.1097/GME.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacrida S., Giannella A., Ceolotto G., Reggiani C., Vezzoli A., Mrakic-Sposta S., et al. (2019). Transcription factors regulation in human peripheral white blood cells during hypobaric hypoxia exposure: An in-vivo experimental study. Sci. Rep. 9 (1), 9901. 10.1038/s41598-019-46391-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini A., Leone E., Festa R., Grande G., Silvestrini A., de Marinis L., et al. (2008). Effects of testosterone on antioxidant systems in male secondary hypogonadism. J. Androl. 29 (6), 622–629. 10.2164/jandrol.107.004838 [DOI] [PubMed] [Google Scholar]
- Manfioletti G., Fedele M. (2022). Epithelial-mesenchymal transition (EMT) 2021. Int. J. Mol. Sci. 23, 5848. 10.3390/ijms23105848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F., Collavin L., Del Sal G. (2019). Mutant p53 as a guardian of the cancer cell. Cell death Differ. 26 (2), 199–212. 10.1038/s41418-018-0246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchio P., Guerra-Ojeda S., Vila J. M., Aldasoro M., Victor V. M., Mauricio M. D. (2019). Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxidative Med. Cell. Longev. 2019, 1–32. 10.1155/2019/8563845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi G. D., Fonticoli L., Rajan T. S., Pierdomenico S. D., Trubiani O., Pizzicannella J., et al. (2021). Epithelial-mesenchymal transition (EMT): The type-2 EMT in wound healing, tissue regeneration and organ fibrosis. Cells 10 (7), 1587. 10.3390/cells10071587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu D. R., Iorga L., Diaconu C. C., Spinu A. D., Mischianu D., Bratu O. G. (2020). Benefits of erectile function recovery programs after radical prostatectomy (Review). Exp. Ther. Med. 20 (3), 2406–2410. 10.3892/etm.2020.8934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques I. P., Ferreira S., Santos T., Madeira M. H., Santos A. R., Mendes L., et al. (2022). Association between neurodegeneration and macular perfusion in the progression of diabetic retinopathy: A 3-year longitudinal study. Ophthalmol. J. Int. d’ophtalmologie. Int. J. Ophthalmol. Zeitschrift fur Augenheilkunde 245 (4), 335–341. 10.1159/000522527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-González M. A., Salas-Salvadó J., Estruch R., Corella D., Fitó M., Ros E. PREDIMED INVESTIGATORS (2015). Benefits of the Mediterranean diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 58 (1), 50–60. 10.1016/j.pcad.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Mas-Bargues C., Alique M., Barrús-Ortiz M. T., Borrás C., Rodrigues-Díez R. (2021). Exploring new Kingdoms: The role of Extracellular Vesicles in Oxi-inflamm-aging related to Cardiorenal syndrome. Antioxidants (Basel, Switz. 11 (1), 78. 10.3390/antiox11010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas-Bargues C., Sanz-Ros J., Román-Domínguez A., Inglés M., Gimeno-Mallench L., El Alami M., et al. (2019). Relevance of oxygen concentration in stem cell culture for regenerative medicine. Int. J. Mol. Sci. 20 (5), 1195. 10.3390/ijms20051195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Miyata Y., Araki K., Mukae Y., Otsubo A., Ohba K., et al. (2020). Efficacy of tadalafil therapy and changes in oxidative stress levels in male patients with lower urinary tract symptoms and Overactive bladder. Low. Urin. Tract. symptoms 12 (1), 47–53. 10.1111/luts.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzilli R., Zamponi V., Olana S., Mikovic N., Cimadomo D., Defeudis G., et al. (2022). Erectile dysfunction as a marker of endocrine and glycemic disorders. J. Endocrinol. investigation 45 (8), 1527–1534. 10.1007/s40618-022-01788-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry T., Biniecka M., Veale D. J., Fearon U. (2018). Hypoxia, oxidative stress and inflammation. Free Radic. Biol. Med. 125, 15–24. 10.1016/j.freeradbiomed.2018.03.042 [DOI] [PubMed] [Google Scholar]
- McKeown S. R. (2014). Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. radiology 87 (1035), 20130676. 10.1259/bjr.20130676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell A. L., MacLusky N. J. (2019). The testosterone metabolite 3α-androstanediol inhibits oxidative stress-induced ERK phosphorylation and neurotoxicity in SH-SY5Y cells through an MKP3/DUSP6-dependent mechanism. Neurosci. Lett. 696, 60–66. 10.1016/j.neulet.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Merkhan M. M., Shephard M. T., Forsyth N. R. (2021). Physoxia alters human mesenchymal stem cell secretome. J. tissue Eng. 12, 20417314211056132. 10.1177/20417314211056132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesentier-Louro L. A., Rangel B., Stell L., Shariati M. A., Dalal R., Nathan A., et al. (2021). Hypoxia-induced inflammation: Profiling the first 24-hour posthypoxic plasma and central nervous system changes. PloS one 16 (3), e0246681. 10.1371/journal.pone.0246681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian A. H., Yang D. Y., Kohler T. S. (2022). Current management and Controversies Surrounding andropause. Urologic Clin. N. Am. 49 (4), 583–592. 10.1016/j.ucl.2022.07.003 [DOI] [PubMed] [Google Scholar]
- Michaelson-Cohen R., Gabizon-Peretz S., Armon S., Srebnik-Moshe N., Mor P., Tomer A., et al. (2021). Breast cancer risk and hormone replacement therapy among BRCA carriers after risk-reducing salpingo-oophorectomy. Eur. J. cancer 148, 95–102. 10.1016/j.ejca.2021.02.007 [DOI] [PubMed] [Google Scholar]
- Michel M. C., Chess-Williams R., Hegde S. S. (2015). Are blood vessels a target to treat lower urinary tract dysfunction? Germany: Naunyn-Schmiedeberg’s archives of pharmacology. [DOI] [PubMed] [Google Scholar]
- Middleton L. W., Shen Z., Varma S., Pollack A. S., Gong X., Zhu S., et al. (2019). Genomic analysis of benign prostatic hyperplasia implicates cellular re-landscaping in disease pathogenesis. JCI insight 5 (12), e129749. 10.1172/jci.insight.129749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami T., Muramatsu M., Kume T. (2019). Organ/tissue-specific vascular endothelial cell heterogeneity in health and disease. Biol. Pharm. Bull. 42 (10), 1609–1619. 10.1248/bpb.b19-00531 [DOI] [PubMed] [Google Scholar]
- Ming L., Byrne N. M., Camac S. N., Mitchell C. A., Ward C., Waugh D. J., et al. (2013). Androgen deprivation results in time-dependent hypoxia in LNCaP prostate tumours: Informed scheduling of the bioreductive drug AQ4N improves treatment response. Int. J. cancer 132 (6), 1323–1332. 10.1002/ijc.27796 [DOI] [PubMed] [Google Scholar]
- Mintz J., Vedenko A., Rosete O., Shah K., Goldstein G., Hare J. M., et al. (2021). Current advances of nitric oxide in cancer and Anticancer therapeutics. Vaccines 9 (2), 94. 10.3390/vaccines9020094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei N., Shi H., Oviatt M., Doustar J., Rentsendorj A., Fuchs D. T., et al. (2020). Alzheimer’s retinopathy: Seeing disease in the eyes. Front. Neurosci. 14, 921. 10.3389/fnins.2020.00921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani T., Yamaji R., Higashimura Y., Harada N., Nakano Y., Inui H. (2011). Hypoxia enhances transcriptional activity of androgen receptor through hypoxia-inducible factor-1α in a low androgen environment. J. steroid Biochem. Mol. Biol. 123 (1–2), 58–64. 10.1016/j.jsbmb.2010.10.009 [DOI] [PubMed] [Google Scholar]
- Mizejewski G. (2017). Breast cancer and amyloid bodies: Is there a role for amyloidosis in cancer-cell dormancy. Breast Cancer Targets Ther. 9, 287–291. 10.2147/BCTT.S131394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeini M., Lu X., Avti P. K., Damseh R., Bélanger S., Picard F., et al. (2018). Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci. Rep. 8 (1), 8219. 10.1038/s41598-018-26543-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri M., Cicero A. F. G. (2023). Adherence to the Mediterranean diet association with serum levels of nitric oxide, Prostacyclin, and thromboxane B(2) among Prinzmetal Angina patients and healthy Persons. Nutrients 15 (3), 738. 10.3390/nu15030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad N. V., Wong S. K., Wan Hasan W. N., Jolly J. J., Nur-Farhana M. F., Ima-Nirwana S., et al. (2019). The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22 (2), 129–140. 10.1080/13685538.2018.1482487 [DOI] [PubMed] [Google Scholar]
- Mohamed O. A., Tesen H. S., Hany M., Sherif A., Abdelwahab M. M., Elnaggar M. H. (2023). The role of hypoxia on prostate cancer progression and metastasis. Mol. Biol. Rep. 50, 3873–3884. 10.1007/s11033-023-08251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mónica F. Z., Bian K., Murad F. (2016). The endothelium-dependent nitric oxide–cGMP pathway. Adv. Pharmacol. 77, 1–27. 10.1016/bs.apha.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Montorsi F., Oettel M. (2005). Testosterone and sleep-related erections: An overview. J. Sex. Med. 2 (6), 771–784. 10.1111/j.1743-6109.2005.00095.x [DOI] [PubMed] [Google Scholar]
- Moreau K. L. (2019). Modulatory influence of sex hormones on vascular aging. Am. J. physiology. Heart circulatory physiology 316 (3), H522. 10.1152/ajpheart.00745.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A., Sarchielli E., Comeglio P., Filippi S., Mancina R., Gacci M., et al. (2011). Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J. Sex. Med. 8 (10), 2746–2760. 10.1111/j.1743-6109.2011.02416.x [DOI] [PubMed] [Google Scholar]
- Mortezaee K., Majidpoor J. (2022). Key promoters of tumor hallmarks. Int. J. Clin. Oncol. 27 (1), 45–58. 10.1007/s10147-021-02074-9 [DOI] [PubMed] [Google Scholar]
- Mortezaee K., Majidpoor J., Kharazinejad E. (2022). Epithelial-mesenchymal transition in cancer stemness and heterogeneity: Updated. Med. Oncol. N. Lond. Engl. 39 (12), 193. 10.1007/s12032-022-01801-0 [DOI] [PubMed] [Google Scholar]
- Mrakic-Sposta S., Biagini D., Bondi D., Pietrangelo T., Vezzoli A., Lomonaco T., et al. (2022). OxInflammation at high altitudes: A Proof of concept from the Himalayas. Antioxidants (Basel, Switz. 11 (2), 368. 10.3390/antiox11020368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyan S., Rachagani S., Parte S., Halder S., Seshacharyulu P., Kshirsagar P., et al. (2020). Sildenafil Potentiates the therapeutic efficacy of Docetaxel in advanced prostate cancer by stimulating NO-cGMP signaling. Clin. cancer Res. official J. Am. Assoc. Cancer Res. 26 (21), 5720–5734. 10.1158/1078-0432.CCR-20-1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn Z., Peters M. D. J., Stern C., Tufanaru C., McArthur A., Aromataris E. (2018). Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18 (1), 143. 10.1186/s12874-018-0611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel T., Templin C., Cammann V. L., Hahad O. (2021). Takotsubo Syndrome: Impact of endothelial dysfunction and oxidative stress. Free Radic. Biol. Med. 169, 216–223. 10.1016/j.freeradbiomed.2021.03.033 [DOI] [PubMed] [Google Scholar]
- Muz B., de la Puente P., Azab F., Azab A. K. (2015). The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia Auckl. N.Z. 3, 83–92. 10.2147/HP.S93413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Filho C. H. V., Glinos A. T., Jang Y., Goloni-Bertollo E. M., Castilho R. M., Squarize C. H. (2022). From tissue physoxia to cancer hypoxia, cost-effective methods to study tissue-specific O(2) levels in cellular biology. Int. J. Mol. Sci. 23 (10), 5633. 10.3390/ijms23105633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser S. A., Afify E. A., Kobeissy F., Hamam B., Eid A. H., El-Mas M. M. (2021). Inflammatory Basis of atherosclerosis: Modulation by sex hormones. Curr. Pharm. Des. 27 (18), 2099–2111. 10.2174/1381612827666210122142811 [DOI] [PubMed] [Google Scholar]
- Nazha B., Bilen M. A. (2021). Circulating interleukin 6, androgen deprivation therapy, and fatigue in prostate cancer: Is inflammation the link. Cancer 127, 1371. 10.1002/cncr.33398 [DOI] [PubMed] [Google Scholar]
- Neuzillet Y., Raynaud J. P., Dreyfus J. F., Radulescu C., Rouanne M., Schneider M., et al. (2019). Aggressiveness of localized prostate cancer: The key Value of testosterone deficiency evaluated by both total and bioavailable testosterone: AndroCan study results. Hormones cancer 10 (1), 36–44. 10.1007/s12672-018-0351-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai M., Urkmez A., Sarikaya S., Fode M., Falcone M., Albersen M., et al. (2021). Penile rehabilitation and treatment Options for erectile dysfunction following radical prostatectomy and Radiotherapy: A systematic review. Front. Surg. 8, 636974. 10.3389/fsurg.2021.636974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieddu A., Vindas L., Errigo A., Vindas J., Pes G. M., Dore M. P. (2020). Dietary Habits, Anthropometric features and daily Performance in two independent long-Lived populations from Nicoya peninsula (Costa Rica) and Ogliastra (Sardinia). Nutrients 12 (6), 1621. 10.3390/nu12061621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieschlag E. (2020). Late-onset hypogonadism: A concept comes of age. Andrology 8 (6), 1506–1511. 10.1111/andr.12719 [DOI] [PubMed] [Google Scholar]
- Novensà L., Novella S., Medina P., Segarra G., Castillo N., Heras M., et al. (2011). Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERα/ERβ balance in female mice. PloS one 6 (9), e25335. 10.1371/journal.pone.0025335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowaczyk A., Kowalska M., Nowaczyk J., Grześk G. (2021). Carbon monoxide and nitric oxide as Examples of the youngest Class of Transmitters. Int. J. Mol. Sci. 22 (11), 6029. 10.3390/ijms22116029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nushtaeva A., Ermakov M., Abdurakhmanova M., Troitskaya O., Belovezhets T., Varlamov M., et al. (2023). Gradually reprograms breast cancer fibroblasts into pro-tumorigenic cells via mesenchymal-epithelial transition. Int. J. Mol. Sci. 24 (3), 2494. 10.3390/ijms24032494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzi R., Scalabrin S., Becco A., Panzica G. (2018). Gonadal hormones and retinal disorders: A review. Front. Endocrinol. 9, 66. 10.3389/fendo.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyquist M. D., Corella A., Mohamad O., Coleman I., Kaipainen A., Kuppers D. A., et al. (2019). Molecular determinants of response to high-dose androgen therapy in prostate cancer. JCI insight 4 (19), e129715. 10.1172/jci.insight.129715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M., Shiraishi T., Fujihara A., Yamada T., Ueda T., Hongo F., et al. (2022). Detection of relatively poor but definitive blood supply in prostate stromal sarcoma using transrectal ultrasonography with superb microvascular imaging. Int. cancer Conf. J. 11, 215–218. 10.1007/s13691-022-00552-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., Miyake H., Ishida T., Sumii K., Enatsu N., Chiba K., et al. (2018). Improved lower urinary tract symptoms associated with testosterone replacement therapy in Japanese men with late-onset hypogonadism. Am. J. Men’s Health 12 (5), 1403–1408. 10.1177/1557988316652843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura K. (2019). Interdisciplinarity revisited: Evidence for research impact and dynamism. Palgrave Commun. 5 (1), 141. 10.1057/s41599-019-0352-4 [DOI] [Google Scholar]
- Ollonen T., Kurkela M., Laitakari A., Sakko S., Koivisto H., Myllyharju J., et al. (2022). Activation of the hypoxia response protects mice from amyloid-β accumulation. Cell. Mol. life Sci. CMLS 79 (8), 432. 10.1007/s00018-022-04460-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ölmestig J., Marlet I. R., Hansen R. H., Rehman S., Krawcyk R. S., Rostrup E., et al. (2020). Tadalafil may improve cerebral perfusion in small-vessel occlusion stroke-a pilot study. Brain Commun. 2 (1), fcaa020. 10.1093/braincomms/fcaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov N. V., Coletta C., van Asten F., Qian Y., Ding J., AlGhatrif M., et al. (2019). Age-related changes of the retinal microvasculature. PloS one 14 (5), e0215916. 10.1371/journal.pone.0215916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osadchiy V., Eleswarapu S. V., Mills S. A., Pollard M. E., Reiter R. E., Mills J. N. (2020). Efficacy of a preprostatectomy multi-modal penile rehabilitation regimen on recovery of postoperative erectile function. Int. J. Impot. Res. 32 (3), 323–328. 10.1038/s41443-019-0187-y [DOI] [PubMed] [Google Scholar]
- Otero-Albiol D., Carnero A. (2021). Cellular senescence or stemness: Hypoxia flips the coin. J. Exp. Clin. cancer Res. CR 40 (1), 243. 10.1186/s13046-021-02035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouarné M., Pena A., Franco C. A. (2021). From remodeling to quiescence: The transformation of the vascular network. Cells Dev. 168, 203735. 10.1016/j.cdev.2021.203735 [DOI] [PubMed] [Google Scholar]
- Papale M., Buccarelli M., Mollinari C., Russo M. A., Pallini R., Ricci-Vitiani L., et al. (2020). Hypoxia, inflammation and Necrosis as determinants of glioblastoma cancer stem cells progression. Int. J. Mol. Sci. 21 (8), 2660. 10.3390/ijms21082660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-Y., Kim Y. J., Gao A. C., Mohler J. L., Onate S. A., Hidalgo A. A., et al. (2006). Hypoxia increases androgen receptor activity in prostate cancer cells. Cancer Res. 66 (10), 5121–5129. 10.1158/0008-5472.CAN-05-1341 [DOI] [PubMed] [Google Scholar]
- Patel D. P., Christensen M. B., Hotaling J. M., Pastuszak A. W. (2020). A review of inflammation and fibrosis: Implications for the pathogenesis of Peyronie’s disease. World J. urology 38 (2), 253–261. 10.1007/s00345-019-02815-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavliukeviciene B., Zentelyte A., Jankunec M., Valiuliene G., Talaikis M., Navakauskiene R., et al. (2019). Amyloid β oligomers inhibit growth of human cancer cells. PloS one 14 (9), e0221563. 10.1371/journal.pone.0221563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto C. A., Gomes F. O. D. S. (2015). The role of phosphodiesterase-5 inhibitors in prostatic inflammation: A review. J. Inflamm. Lond. Engl. 12, 54. 10.1186/s12950-015-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena E., El Alam S., Siques P., Brito J. (2022). Oxidative stress and diseases associated with high-altitude exposure. Antioxidants (Basel, Switz. 11 (2), 267. 10.3390/antiox11020267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham K., Frost S., Parikh K., Puvvula N., Oeung B., Heinrich E. C. (2022). Inflammatory gene expression during acute high-altitude exposure. J. physiology 600 (18), 4169–4186. 10.1113/JP282772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phua T. J. (2021). The etiology and pathophysiology genesis of benign prostatic hyperplasia and prostate cancer: A new perspective. Med. (Basel, Switz. 8 (6), 30. 10.3390/medicines8060030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto P. C., Rönnau C., Burchardt M., Wolff I. (2021). Kidney cancer and chronic kidney disease: Too close for Comfort. Biomedicines 9 (12), 1761. 10.3390/biomedicines9121761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooja , Sharma V., Sharma M., Varshney R., Kumar B., Sethy N. K. (2021). Association between 17β-estradiol receptors and nitric oxide signaling augments high-altitude adaptation of Ladakhi highlanders. High Alt. Med. Biol. 22 (2), 174–183. 10.1089/ham.2020.0187 [DOI] [PubMed] [Google Scholar]
- Podlasek C. A., Mulhall J., Davies K., Wingard C. J., Hannan J. L., Bivalacqua T. J., et al. (2016). Translational perspective on the role of testosterone in sexual function and dysfunction. J. Sex. Med. 13 (8), 1183–1198. 10.1016/j.jsxm.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pofi R., Giannetta E., Feola T., Galea N., Barbagallo F., Campolo F., et al. (2022). Sex-specific effects of daily tadalafil on diabetic heart kinetics in RECOGITO, a randomized, double-blind, placebo-controlled trial. Sci. Transl. Med. 14 (649), eabl8503. 10.1126/scitranslmed.abl8503 [DOI] [PubMed] [Google Scholar]
- Pollock J. D., Murray I., Bordes S., Makaryus A. N. (2023). “Physiology, cardiovascular hemodynamics,” in StatPearls [internet]. Treasure island (FL) (StatPearls Publishing; ), 13. [PubMed] [Google Scholar]
- Pomatto L. C. D., Sun P. Y., Davies K. J. A. (2019b). To adapt or not to adapt: Consequences of declining Adaptive Homeostasis and Proteostasis with age. Mech. ageing Dev. 177, 80–87. 10.1016/j.mad.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto L. C. D., Sun P. Y., Yu K., Gullapalli S., Bwiza C. P., Sisliyan C., et al. (2019a). Limitations to adaptive homeostasis in an hyperoxia-induced model of accelerated ageing. Redox Biol. 24, 101194. 10.1016/j.redox.2019.101194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp Marin D., Bolin A. P., dos Santos R. d. C. M., Curi R., Otton R. (2010). Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem. Funct. 28 (5), 394–402. 10.1002/cbf.1669 [DOI] [PubMed] [Google Scholar]
- Pourbagher-Shahri A. M., Farkhondeh T., Talebi M., Kopustinskiene D. M., Samarghandian S., Bernatoniene J. (2021). An overview of NO signaling pathways in aging. Mol. (Basel, Switz. 26 (15), 4533. 10.3390/molecules26154533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont R. T., Reynolds J. D., Zhang R., Stamler J. S. (2020). Role of nitric oxide carried by hemoglobin in cardiovascular physiology: Developments on a three-Gas respiratory cycle. Circulation Res. 126 (1), 129–158. 10.1161/CIRCRESAHA.119.315626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Lloret J., Olea E., Gordillo-Cano A., Docio I., Obeso A., Gomez-Niño A., et al. (2021). Maladaptive pulmonary vascular responses to chronic sustained and chronic intermittent hypoxia in rat. Antioxidants 11 (1), 54. 10.3390/antiox11010054antiox11010054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi-sheng W. U., Cui-ping Y. A. N. G., Yong-bin C. H. E. N. (2021). A review of high-altitude hypoxia adaptation and hypoxic solid tumor. J. Sichuan Univ. 52 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfeld U., Mak R. H., Pries A. R. (2020). Microvascular disease in chronic kidney disease: The base of the iceberg in cardiovascular comorbidity. Clin. Sci. 134 (12), 1333–1356. 10.1042/CS20200279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. (2018). Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U. S. A. 115 (23), 5839–5848. 10.1073/pnas.1804932115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran P., Alzahrani A. M., Hanieh H. N., Kumar S. A., Ben Ammar R., Rengarajan T., et al. (2019a). Autophagy and senescence: A new insight in selected human diseases. J. Cell. physiology 234 (12), 21485–21492. 10.1002/jcp.28895 [DOI] [PubMed] [Google Scholar]
- Rajendran S., Shen X., Glawe J., Kolluru G. K., Kevil C. G. (2019b). Nitric oxide and hydrogen Sulfide regulation of ischemic vascular growth and remodeling. Compr. Physiol. 9 (3), 1213–1247. 10.1002/cphy.c180026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando T. A., Jones D. L. (2021). Regeneration, rejuvenation, and replacement: Turning Back the Clock on tissue aging. Cold Spring Harb. Perspect. Biol. 13 (9), a040907. 10.1101/cshperspect.a040907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrelli G., Cipriani S., Lotti F., Cellai I., Comeglio P., Filippi S., et al. (2022). Testosterone does not affect lower urinary tract symptoms while improving markers of prostatitis in men with benign prostatic hyperplasia: A randomized clinical trial. J. Endocrinol. investigation 45 (7), 1413–1425. 10.1007/s40618-022-01776-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrelli G., Cipriani S., Lotti F., Cellai I., Comeglio P., Boddi V., et al. (2019). “May. Testosterone replacement therapy is able to reduce prostate inflammation in men with BPH, metabolic syndrome and hypogonadism: Preliminary results from a randomized placebo-controlled clinical trial,” in Endocrine abstracts (Bioscientifica; ), 63. [Google Scholar]
- Redberg R. F., Nishino M., McElhinney D. B., Dae M. W., Botvinick E. H. (2000). Long-term estrogen replacement therapy is associated with improved exercise capacity in postmenopausal women without known coronary artery disease. Am. heart J. 139 (4), 739–744. 10.1016/s0002-8703(00)90058-9 [DOI] [PubMed] [Google Scholar]
- Reinero M., Beghetti M., Tozzi P., Segesser L. K. v., Samaja M., Milano G. (2021). Nitric oxide-cGMP pathway modulation in an experimental model of hypoxic pulmonary hypertension. J. Cardiovasc. Pharmacol. Ther. 26 (6), 665–676. 10.1177/10742484211014162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer M., Branco C. M. (2020). Endothelial cells and organ function: Applications and implications of understanding unique and reciprocal remodelling. FEBS J. 287 (6), 1088–1100. 10.1111/febs.15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiterer M., Eakin A., Johnson R. S., Branco C. M. (2022). Hyperoxia Reprogrammes microvascular endothelial cell response to hypoxia in an organ-specific Manner. Cells 11 (16), 2469. 10.3390/cells11162469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R. V., Díaz M., Ebensperger G., Herrera E. A., Quezada S. A., Hernandez I., et al. (2018). The role of nitric oxide in the cardiopulmonary response to hypoxia in highland and lowland newborn llamas. J. physiology 596 (23), 5907–5923. 10.1113/JP274340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. (2022). ‘Epithelial endothelial transition and endothelial mesenchymal transition. Int. J. Dev. Biol. 10.1387/ijdb.210234dr [DOI] [PubMed] [Google Scholar]
- Rizzoni D., Rizzoni M., Nardin M., Chiarini G., Agabiti-Rosei C., Aggiusti C., et al. (2019). Vascular aging and disease of the small vessels. High blood Press. Cardiovasc. Prev. official J. Italian Soc. Hypertens. 26 (3), 183–189. 10.1007/s40292-019-00320-w [DOI] [PubMed] [Google Scholar]
- Rodriguez D., Watts D., Gaete D., Sormendi S., Wielockx B. (2021). Hypoxia pathway proteins and their impact on the blood vasculature. Int. J. Mol. Sci. 22 (17), 9191. 10.3390/ijms22179191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romejko K., Rymarz A., Sadownik H., Niemczyk S. (2022). Testosterone deficiency as one of the major endocrine disorders in chronic kidney disease. Nutrients 14 (16), 3438. 10.3390/nu14163438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario E., Rosario D. J. (2022). Localized prostate cancer. Treasure Island: StatPearls. [PubMed] [Google Scholar]
- Rossi M. T., Langston J. C., Singh N., Merali C., Yang Q., Merali S., et al. (2022). Molecular Framework of mouse endothelial cell dysfunction during inflammation: A Proteomics approach. Int. J. Mol. Sci. 23 (15), 8399. 10.3390/ijms23158399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouver W., do N., Gonçalves L. T., Giesen J. A. S., Santos da Costa C., Merlo E., et al. (2023). Sex hormones and vascular reactivity: A temporal evaluation in resistance arteries of male rats. J. Mol. Endocrinol. 70 (1), e220147. 10.1530/JME-22-0147 [DOI] [PubMed] [Google Scholar]
- Rovira-Llopis S., Bañuls C., de Marañon A. M., Diaz-Morales N., Jover A., Garzon S., et al. (2017). Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 108, 155–162. 10.1016/j.freeradbiomed.2017.03.029 [DOI] [PubMed] [Google Scholar]
- Roy T. K., Secomb T. W. (2021). Effects of impaired microvascular flow regulation on metabolism-perfusion matching and organ function. Microcirculation 28 (3), e12673. 10.1111/micc.12673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubel M. S., Fedotov S. A., Grizel A. V., Sopova J. V., Malikova O. A., Chernoff Y. O., et al. (2020). Functional Mammalian amyloids and amyloid-like proteins. Life (Basel, Switz. 10 (9), 156. 10.3390/life10090156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M. A., Ravenna L., Pellegrini L., Petrangeli E., Salvatori L., Magrone T., et al. (2016). Hypoxia and inflammation in prostate cancer progression. Cross-Talk with androgen and estrogen receptors and cancer stem cells. Endocr. metabolic immune Disord. drug targets 16 (4), 235–248. 10.2174/1871530316666161130160144 [DOI] [PubMed] [Google Scholar]
- Ryu J., Ryu H., Kim S., Chin H. J., Na K. Y., Chae D. W., et al. (2019). Comparison of cancer prevalence between patients with glomerulonephritis and the general population at the time of kidney biopsy. PloS one 14 (10), e0224024. 10.1371/journal.pone.0224024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabapathy K., Lane D. P. (2019). Understanding p53 functions through p53 antibodies. J. Mol. cell Biol. 11 (4), 317–329. 10.1093/jmcb/mjz010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabi E. M., Singh A., Althafar Z. M., Behl T., Sehgal A., Singh S., et al. (2022). Elucidating the role of hypoxia-inducible factor in rheumatoid arthritis. Inflammopharmacology 30 (3), 737–748. 10.1007/s10787-022-00974-4 [DOI] [PubMed] [Google Scholar]
- Saengmearnuparp T., Lojanapiwat B., Chattipakorn N., Chattipakorn S. (2021). The connection of 5-alpha reductase inhibitors to the development of depression. Biomed. Pharmacother. = Biomedecine Pharmacother. 143, 112100. 10.1016/j.biopha.2021.112100 [DOI] [PubMed] [Google Scholar]
- Saito M., Tsounapi P., Oikawa R., Shimizu S., Honda M., Sejima T., et al. (2014). Prostatic ischemia induces ventral prostatic hyperplasia in the SHR; possible mechanism of development of BPH. Sci. Rep. 4, 3822. 10.1038/srep03822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh T., Oda T. (2021). DNA damage response in multiple Myeloma: The role of the tumor microenvironment. Cancers 13 (3), 504. 10.3390/cancers13030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihi A., Al-Naqshabandi M. A., Khudhur Z. O., Housein Z., Hama H. A., Abdullah R. M., et al. (2022). Gasotransmitters in the tumor microenvironment: Impacts on cancer chemotherapy (Review). Mol. Med. Rep. 26 (1), 233. 10.3892/mmr.2022.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaría R., González-Álvarez M., Delgado R., Esteban S., Arroyo A. G. (2020). Remodeling of the microvasculature: May the blood flow Be with You. Front. physiology 11, 586852. 10.3389/fphys.2020.586852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Parker J. R., Strahler T. R., Bassett C. J., Bispham N. Z., Chonchol M. B., Seals D. R. (2017). Curcumin supplementation improves vascular endothelial function in healthy middle-aged and older adults by increasing nitric oxide bioavailability and reducing oxidative stress. Aging 9 (1), 187–208. 10.18632/aging.101149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant J. M., O’Connor A. M. (2020). Scoping reviews, systematic reviews, and meta-analysis: Applications in Veterinary medicine. Front. veterinary Sci. 7, 11. 10.3389/fvets.2020.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satija S., Kaur H., Tambuwala M. M., Sharma P., Vyas M., Khurana N., et al. (2021). Hypoxia-inducible factor (HIF): Fuel for cancer progression. Curr. Mol. Pharmacol. 14 (3), 321–332. 10.2174/1874467214666210120154929 [DOI] [PubMed] [Google Scholar]
- Saxena K., Jolly M. K. (2019). Acute vs. Chronic vs. Cyclic hypoxia: Their Differential dynamics, molecular mechanisms, and effects on tumor progression. Biomolecules 9 (8), 339. 10.3390/biom9080339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor P. J., Kozak K. R., Smith M. R., Ancukiewicz M. A., Efstathiou J. A., Zietman A. L., et al. (2012). Changes in biomarkers of inflammation and angiogenesis during androgen deprivation therapy for prostate cancer. Oncol. 17 (2), 212–219. 10.1634/theoncologist.2011-0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scailteux L.-M., Rioux-Leclercq N., Vincendeau S., Balusson F., Nowak E., Oger E. Network of Pathologists in Brittany (2019). Use of 5α-reductase inhibitors for benign prostate hypertrophy and risk of high grade prostate cancer: A French population-based study. BJU Int. 123 (2), 293–299. 10.1111/bju.14495 [DOI] [PubMed] [Google Scholar]
- Schaefer E., Wu W., Mark C., Yang A., DiGiacomo E., Carlton-Smith C., et al. (2017). Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol. Commun. 1 (4), 326–337. 10.1002/hep4.1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzl G., Madersbacher S., Gsur A., Preyer M., Haidinger G., Haitel A., et al. (2002). Association of polymorphisms within androgen receptor, 5alpha-reductase, and PSA genes with prostate volume, clinical parameters, and endocrine status in elderly men. Prostate 52 (2), 130–138. 10.1002/pros.10101 [DOI] [PubMed] [Google Scholar]
- Schifano N., Capogrosso P., Boeri L., Fallara G., Chiappini S., Rewhorn M., et al. (2022). Are finasteride-related penile curvature/Peyronie’s disease adverse event reports worthy of further clinical investigation? Disproportionality analysis based on both the Food and drug administration (FDA) and the European medicines Agency (EMA) pharmacov. Int. J. Impot. Res. 10.1038/s41443-022-00568-2 [DOI] [PubMed] [Google Scholar]
- Schiffer D., Annovazzi L., Casalone C., Corona C., Mellai M. (2018). Glioblastoma: Microenvironment and niche concept. Cancers 11 (1), 5. 10.3390/cancers11010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid F., Barrett M. J. P., Obrist D., Weber B., Jenny P. (2019). Red blood cells stabilize flow in brain microvascular networks. PLoS Comput. Biol. 15 (8), e1007231. 10.1371/journal.pcbi.1007231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A., Roumans N., Rademakers T., Joris V., Eischen-Loges M. J., van Griensven M., et al. (2022). Enhanced microvasculature formation and patterning in iPSC-derived kidney organoids cultured in physiological hypoxia. Front. Bioeng. Biotechnol. 10, 860138. 10.3389/fbioe.2022.860138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighauser M., Arseni D., Bacioglu M., Huang M., Lövestam S., Shi Y., et al. (2022). Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature 605 (7909), 310–314. 10.1038/s41586-022-04650-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scioli M. G., Bielli A., Arcuri G., Ferlosio A., Orlandi A. (2014). Ageing and microvasculature. Vasc. cell 6, 19. 10.1186/2045-824X-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. R., Alexander L. M. (2018). Vascular aging. J. Appl. physiology 125 (6), 1841–1842. 10.1152/japplphysiol.00448.2018 [DOI] [PubMed] [Google Scholar]
- Sebestyén A., Kopper L., Dankó T., Tímár J. (2021). Hypoxia signaling in cancer: From Basics to clinical Practice. Pathology Oncol. Res. POR 27, 1609802. 10.3389/pore.2021.1609802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secomb T. W. (2016). Hemodynamics. Compr. Physiol. 6 (2), 975–1003. 10.1002/cphy.c150038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segundo A., Glina S. (2020). Prevalence, risk factors, and erectile dysfunction associated with Peyronie’s disease among men Seeking Urological care. Sex. Med. 8 (2), 230–236. 10.1016/j.esxm.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza G. L. (2010). Oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2 (3), 336–361. 10.1002/wsbm.69 [DOI] [PubMed] [Google Scholar]
- Sen V., Sahin M. O., Irer B., Koc E., Yildiz G. (2020). The impact of hyperbaric oxygen therapy on erectile functions and serum testosterone levels in patients with erectile dysfunction. aging male official J. Int. Soc. Study Aging Male 23 (1), 66–70. 10.1080/13685538.2019.1578740 [DOI] [PubMed] [Google Scholar]
- Seno K., Tanikawa N., Takahashi H., Ohkuchi A., Suzuki H., Matsubara S., et al. (2018). Oxygen concentration modulates cellular senescence and autophagy in human trophoblast cells. Am. J. reproductive Immunol. 79 (6), e12826. 10.1111/aji.12826 [DOI] [PubMed] [Google Scholar]
- Shannon O. M., Ashor A. W., Scialo F., Saretzki G., Martin-Ruiz C., Lara J., et al. (2021). Mediterranean diet and the hallmarks of ageing. Eur. J. Clin. Nutr. 75 (8), 1176–1192. 10.1038/s41430-020-00841-x [DOI] [PubMed] [Google Scholar]
- Shannon O. M., Stephan B. C., Minihane A. M., Mathers J. C., Siervo M. (2018). Nitric oxide boosting effects of the mediterranean diet: A potential mechanism of action. Journals Gerontology Ser. A 73 (7), 902–904. 10.1093/gerona/gly087pp. [DOI] [PubMed] [Google Scholar]
- Shannon O. M., Mendes I., Köchl C., Mazidi M., Ashor A. W., Rubele S., et al. (2020). Mediterranean diet increases endothelial function in adults: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. 150 (5), 1151–1159. 10.1093/jn/nxaa002 [DOI] [PubMed] [Google Scholar]
- Shatanawi A., Momani M. S., Al-Aqtash R., Hamdan M. H., Gharaibeh M. N. (2020). L-citrulline supplementation increases plasma nitric oxide levels and reduces Arginase activity in patients with type 2 diabetes. Front. Pharmacol. 11, 584669. 10.3389/fphar.2020.584669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea D., Hsu C. C., Bi T. M., Paranjapye N., Childers M. C., Cochran J., et al. (2019). α-Sheet secondary structure in amyloid β-peptide drives aggregation and toxicity in Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 116 (18), 8895–8900. 10.1073/pnas.1820585116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefa U., Yeo S. G., Kim M. S., Song I. O., Jung J., Jeong N. Y., et al. (2017). Role of gasotransmitters in oxidative Stresses, Neuroinflammation, and neuronal repair. BioMed Res. Int. 2017, 1689341. 10.1155/2017/1689341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard M. T., Merkhan M. M., Forsyth N. R. (2022). Human mesenchymal stem cell secretome driven T cell Immunomodulation is IL-10 dependent. Int. J. Mol. Sci. 23 (21), 13596. 10.3390/ijms232113596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd A. I., Costello J. T., Bailey S. J., Bishop N., Wadley A. J., Young-Min S., et al. (2019). ‘“Beet” the cold: Beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud’s phenomenon. J. Appl. physiology 127 (5), 1478–1490. 10.1152/japplphysiol.00292.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y. K., Lee G. W., Kang S. W., Kim S. J., Kim A. Y. (2020). Macular abnormalities associated with 5α-reductase inhibitor. JAMA Ophthalmol. 138 (7), 732–739. 10.1001/jamaophthalmol.2020.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X., Keller T. C. S., Begandt D., Butcher J. T., Biwer L., Keller A. S., et al. (2015). Endothelial nitric oxide synthase in the microcirculation. Cell. Mol. life Sci. CMLS 72 (23), 4561–4575. 10.1007/s00018-015-2021-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A. (2022). Cancer statistics, 2022. CA a cancer J. Clin. 72 (1), 7–33. 10.3322/caac.21708 [DOI] [PubMed] [Google Scholar]
- Smentoch J., Szade J., Żaczek A. J., Eltze E., Semjonow A., Brandt B., et al. (2019). Low numbers of vascular vessels correlate to progression in Hormone-Naïve prostate carcinomas undergoing radical prostatectomy. Cancers 11 (9), 1356. 10.3390/cancers11091356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder B., Duong P., Trieu J., Cunningham R. L. (2018). Androgens modulate chronic intermittent hypoxia effects on brain and behavior. Hormones Behav. 106, 62–73. 10.1016/j.yhbeh.2018.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soda K., Uemura T., Sanayama H., Igarashi K., Fukui T. (2021). Polyamine-rich diet Elevates blood spermine levels and inhibits pro-inflammatory status: An interventional study. Med. Sci. (Basel, Switz. 9 (2), 22. 10.3390/medsci9020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerjomataram I., Bray F. (2021). Planning for tomorrow: Global cancer incidence and the role of prevention 2020-2070. Nat. Rev. Clin. Oncol. 18 (10), 663–672. 10.1038/s41571-021-00514-z [DOI] [PubMed] [Google Scholar]
- Somani Y. B., Pawelczyk J. A., De Souza M. J., Kris-Etherton P. M., Proctor D. N. (2019). Aging women and their endothelium: Probing the relative role of estrogen on vasodilator function. Am. J. physiology. Heart circulatory physiology 317 (2), H395. 10.1152/ajpheart.00430.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son S.-W., Lee J. S., Kim H. G., Kim D. W., Ahn Y. C., Son C. G. (2016). Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. J. Neurochem. 136 (1), 106–117. 10.1111/jnc.13371 [DOI] [PubMed] [Google Scholar]
- Soni S., Padwad Y. S. (2017). HIF-1 in cancer therapy: Two decade long story of a transcription factor. Acta Oncol. Stockh. Swed. 56 (4), 503–515. 10.1080/0284186X.2017.1301680 [DOI] [PubMed] [Google Scholar]
- Soni Y., Softness K., Arora H., Ramasamy R. (2020). The Yin Yang role of nitric oxide in prostate cancer. Am. J. Men’s Health 14 (1), 1557988320903191. 10.1177/1557988320903191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth R. C., D'Ambra M., Ji H., Sandberg K. (2018). A heartfelt message, estrogen replacement therapy: Use it or lose it. Am. J. physiology. Heart circulatory physiology 315 (6), H1765–H1778. 10.1152/ajpheart.00041.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek A., Fazeli B., Bartuś S., Sutkowska E. (2018). The role of endothelium in physiological and pathological states: New data. BioMed Res. Int. 2018, 1098039. 10.1155/2018/1098039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statsenko M. E., Turkina S. V., Tyschenko I. A., Kosivtsova M. A., Kostromeev S. A. (2021). Effect of Tadalafil SZ on endothelial function in patients with erectile dysfunction. Urol. Mosc. Russ. 1999) 1_2021 (1), 50–54. 10.18565/urology.2021.1.50-54 [DOI] [PubMed] [Google Scholar]
- Stein Y., Rotter V., Aloni-Grinstein R. (2019). Gain-of-Function mutant p53: All the Roads lead to tumorigenesis. Int. J. Mol. Sci. 20 (24), 6197. 10.3390/ijms20246197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowitzki M. J., Cummins E. P., Taylor C. T. (2019). Protein hydroxylation by hypoxia-inducible factor (HIF) Hydroxylases: Unique or ubiquitous? Cells 8 (5), 384. 10.3390/cells8050384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Wang Y. C., Guo K., Du J., Zhou H. J., Ren A. J. (2018). Finasteride inhibits microvascular density and VEGF expression in the seminal vesicle of rats. Zhonghua nan ke xue = Natl. J. Androl. 24 (5), 387–392. [PubMed] [Google Scholar]
- Sund M., Fonseca-Rodríguez O., Josefsson A., Welen K., Fors Connolly A. M. (2022). Association between pharmaceutical modulation of oestrogen in postmenopausal women in Sweden and death due to COVID-19: A cohort study. BMJ open 12 (2), e053032. 10.1136/bmjopen-2021-053032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swislocki A. L. M., Eisenberg M. L. (2021). Peyronie disease as a marker of inflammation-is there Hope on the Horizon? Am. J. Med. 134 (10), 1218–1223. 10.1016/j.amjmed.2021.06.015 [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. (2023). Role of autophagy in aging: The good, the bad, and the ugly. Aging cell 22 (1), e13753. 10.1111/acel.13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S., Virdis A., Ghiadoni L., Salvetti G., Bernini G., Magagna A., et al. (2001). Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38 (2), 274–279. 10.1161/01.hyp.38.2.274 [DOI] [PubMed] [Google Scholar]
- Tang C., Livingston M. J., Liu Z., Dong Z. (2020). Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 16 (9), 489–508. 10.1038/s41581-020-0309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaki M., Lavatelli F., Obici L., Obayashi K., Miyamoto T., Merlini G., et al. (2021). Age-related amyloidosis outside the brain: A state-of-the-art review. Ageing Res. Rev. 70, 101388. 10.1016/j.arr.2021.101388 [DOI] [PubMed] [Google Scholar]
- Taylor A. M., Bordoni B. (2022). Histology, blood vascular system. Treasure Island: StatPearls Publishing. [PubMed] [Google Scholar]
- Tejero J., Shiva S., Gladwin M. T. (2019). Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol. Rev. 99 (1), 311–379. 10.1152/physrev.00036.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh J. Y.-C., Tian X. Y., Wong C. Y. P., Lau C. W., Cheng C. K., Tang V. W. L., et al. (2022). Endothelial dysfunction after androgen deprivation therapy and the possible underlying mechanisms. Prostate 82 (1), 13–25. 10.1002/pros.24244 [DOI] [PubMed] [Google Scholar]
- Tessema B., Sack U., Serebrovska Z., König B., Egorov E. (2021). Effects of hyperoxia on aging biomarkers: A systematic review. Front. aging 2, 783144. 10.3389/fragi.2021.783144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The North American Menopause Society (2022). The 2022 hormone therapy position statement of the North American Menopause Society. Menopause 29 (7), 767–794. 10.1097/GME.0000000000002028 [DOI] [PubMed] [Google Scholar]
- Thiersch M., Swenson E. R. (2018). High altitude and cancer mortality. High Alt. Med. Biol. 19 (2), 116–123. 10.1089/ham.2017.0061 [DOI] [PubMed] [Google Scholar]
- Thurmond P., Yang J. H., Li Y., Lerner L. B., Azadzoi K. M. (2015). Structural modifications of the prostate in hypoxia, oxidative stress, and chronic ischemia. Korean J. Urology 56 (3), 187–196. 10.4111/kju.2015.56.3.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish A. M. (2020). Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil. Steril. 113 (1), 21–50. 10.1016/j.fertnstert.2019.11.030 [DOI] [PubMed] [Google Scholar]
- Tran M. G. B., Bibby B. A. S., Yang L., Lo F., Warren A. Y., Shukla D., et al. (2020). Independence of HIF1a and androgen signaling pathways in prostate cancer. BMC cancer 20 (1), 469. 10.1186/s12885-020-06890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Hochreiter B., Zach M. L., Krenn K., Klein K. U. (2021). Understanding cellular redox homeostasis: A challenge for precision medicine. Int. J. Mol. Sci. 23 (1), 106. 10.3390/ijms23010106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V. (2022). Special Issue: Cellular oxygen homeostasis. Int. J. Mol. Sci. 23, 4505. 10.3390/ijms23094505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V., Zach M. L., Böhme S., Ullrich R., Markstaller K., Klein K. U. (2020). Investigating disturbances of oxygen homeostasis: From cellular mechanisms to the clinical Practice. Front. physiology 11, 947. 10.3389/fphys.2020.00947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigalou C., Konstantinidis T., Paraschaki A., Stavropoulou E., Voidarou C., Bezirtzoglou E. (2020). Mediterranean diet as a Tool to Combat inflammation and chronic diseases. An overview. Biomedicines 8 (7), 201. 10.3390/biomedicines8070201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuru T., Tsujimura A., Mizushima K., Kurosawa M., Kure A., Uesaka Y., et al. (2023). International Prostate Symptom Score and quality of life index for lower urinary tract symptoms are associated with aging males symptoms rating scale for late-onset hypogonadism symptoms. World J. Men's Health 41 (1), 101–109. 10.5534/wjmh.210171pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unfer T. C., Figueiredo C. G., Zanchi M. M., Maurer L. H., Kemerich D. M., Duarte M. M. F., et al. (2015). Estrogen plus progestin increase superoxide dismutase and total antioxidant capacity in postmenopausal women. Climacteric J. Int. Menopause Soc. 18 (3), 379–388. 10.3109/13697137.2014.964669 [DOI] [PubMed] [Google Scholar]
- Ungvari Z., Tarantini S., Sorond F., Merkely B., Csiszar A. (2020). Mechanisms of vascular aging, A geroscience perspective: JACC focus Seminar. J. Am. Coll. Cardiol. 75 (8), 931–941. 10.1016/j.jacc.2019.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet T., Casciaro F., Demaria M. (2021b). To breathe or not to breathe: Understanding how oxygen sensing contributes to age-related phenotypes. Ageing Res. Rev. 67, 101267. 10.1016/j.arr.2021.101267 [DOI] [PubMed] [Google Scholar]
- van Vliet T., Varela-Eirin M., Wang B., Borghesan M., Brandenburg S. M., Franzin R., et al. (2021a). Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol. Cell. 81 (9), 2041–2052.e6. 10.1016/j.molcel.2021.03.018 [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Shimokawa H., Feletou M., Tang E. H. C. (2017). Endothelial dysfunction and vascular disease – A 30th anniversary update. Acta Physiol. 219 (1), 22–96. 10.1111/apha.12646 [DOI] [PubMed] [Google Scholar]
- Vargas F., Moreno J. M., Wangensteen R., Rodríguez-Gómez I., García-Estañ J. (2007). The endocrine system in chronic nitric oxide deficiency. Eur. J. Endocrinol. 156 (1), 1–12. 10.1530/eje.1.02314 [DOI] [PubMed] [Google Scholar]
- Vasto S., Barera A., Rizzo C., Di Carlo M., Caruso C., Panotopoulos G. (2014). Mediterranean diet and longevity: An example of nutraceuticals? Curr. Vasc. Pharmacol. 12 (5), 735–738. 10.2174/1570161111666131219111818 [DOI] [PubMed] [Google Scholar]
- Vignozzi L., Gacci M., Cellai I., Morelli A., Maneschi E., Comeglio P., et al. (2013). PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate 73 (13), 1391–1402. 10.1002/pros.22686 [DOI] [PubMed] [Google Scholar]
- Voit R. A., Sankaran V. G. (2020). Stabilizing HIF to ameliorate anemia. Cell 180 (1), 6. 10.1016/j.cell.2019.12.010 [DOI] [PubMed] [Google Scholar]
- Vural P., Akgul C., Canbaz M. (2006). Effects of hormone replacement therapy on plasma pro-inflammatory and anti-inflammatory cytokines and some bone turnover markers in postmenopausal women. Pharmacol. Res. 54 (4), 298–302. 10.1016/j.phrs.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Wan Y., Li X., Slevin E., Harrison K., Li T., Zhang Y., et al. (2022). Endothelial dysfunction in pathological processes of chronic liver disease during aging. FASEB J. 36 (1), e22125. 10.1096/fj.202101426R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander K., Su M., Mattison P. M., Sum C. Y., Witt C. C., Shenk M. K., et al. (2020). High-altitude adaptations mitigate risk for hypertension and diabetes-associated anemia. Am. J. Phys. Anthropol. 172 (2), 156–164. 10.1002/ajpa.24032 [DOI] [PubMed] [Google Scholar]
- Wang B., Li Z. L., Zhang Y. L., Wen Y., Gao Y. M., Liu B. C. (2022a). Hypoxia and chronic kidney disease. EBioMedicine 77, 103942. 10.1016/j.ebiom.2022.103942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G.-M., Kovalenko B., Huang Y., Moscatelli D. (2007). Vascular endothelial growth factor and angiopoietin are required for prostate regeneration. Prostate 67 (5), 485–499. 10.1002/pros.20534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Xie X., Ke B., Huang W., Jiang X., He G. (2022b). Recent advances on endogenous gasotransmitters in inflammatory dermatological disorders. J. Adv. Res. 38, 261–274. 10.1016/j.jare.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.-S., Oldham W. M., Maron B. A., Loscalzo J. (2018). Systems biology approaches to redox metabolism in stress and disease states. Antioxidants redox Signal. 29 (10), 953–972. 10.1089/ars.2017.7256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zheng H., Jiang J., Li Z., Jiang D., Shi X., et al. (2022c). Engineering micro oxygen factories to slow tumour progression via hyperoxic microenvironments. Nat. Commun. 13 (1), 4495. 10.1038/s41467-022-32066-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Le W.-D. (2019). Autophagy and ubiquitin-proteasome system. Adv. Exp. Med. Biol. 1206, 527–550. 10.1007/978-981-15-0602-4_25 [DOI] [PubMed] [Google Scholar]
- Wang Y., Westermark G. T. (2021). The amyloid forming Peptides islet amyloid Polypeptide and amyloid β Interact at the molecular level. Int. J. Mol. Sci. 22 (20), 11153. 10.3390/ijms222011153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang H. (2019). Regulation of autophagy by mTOR signaling pathway. Adv. Exp. Med. Biol. 1206, 67–83. 10.1007/978-981-15-0602-4_3 [DOI] [PubMed] [Google Scholar]
- Wei Y., Giunta S., Xia S. (2022). Hypoxia in aging and aging-related diseases: Mechanism and therapeutic strategies. Int. J. Mol. Sci. 23 (15), 8165. 10.3390/ijms23158165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Jiang H., Shi Y., Qu D., Gregori G., Zheng F., et al. (2017). Age-related alterations in the retinal microvasculature, microcirculation, and Microstructure. Investigative Ophthalmol. Vis. Sci. 58 (9), 3804–3817. 10.1167/iovs.17-21460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford S. M., Giaccia A. J. (2011). Hypoxia and senescence: The impact of oxygenation on tumor suppression. Mol. cancer Res. MCR 9 (5), 538–544. 10.1158/1541-7786.MCR-11-0065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R. C. J., Mechlin C., Goodwin B., Alukal J. P., McCullough A. R. (2014). A pilot study to determine penile oxygen saturation before and after vacuum therapy in patients with erectile dysfunction after radical prostatectomy. J. Sex. Med. 11 (4), 1071–1077. 10.1111/jsm.12445 [DOI] [PubMed] [Google Scholar]
- Wicks E. E., Semenza G. L. (2022). Hypoxia-inducible factors: Cancer progression and clinical translation. J. Clin. investigation 132 (11), e159839. 10.1172/JCI159839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnands K. A., Meesters D. M., Vandendriessche B., Briedé J. J., van Eijk H. M., Brouckaert P., et al. (2021). Microcirculatory function during endotoxemia—a functional citrulline-arginine-NO pathway and NOS3 complex is essential to maintain the microcirculation. Int. J. Mol. Sci. 22 (21), 11940. 10.3390/ijms222111940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. N., Anderson M., Snyder B., Duong P., Trieu J., Schreihofer D. A., et al. (2018). Chronic intermittent hypoxia induces hormonal and male sexual behavioral changes: Hypoxia as an advancer of aging. Physiology Behav. 189, 64–73. 10.1016/j.physbeh.2018.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittert G., Bracken K., Robledo K. P., Grossmann M., Yeap B. B., Handelsman D. J., et al. (2021). Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): A randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. lancet Diabetes & Endocrinol. 9 (1), 32–45. 10.1016/S2213-8587(20)30367-3 [DOI] [PubMed] [Google Scholar]
- Wong S. Q., Kumar A. V., Mills J., Lapierre L. R. (2020). Autophagy in aging and longevity. Hum. Genet. 139 (3), 277–290. 10.1007/s00439-019-02031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Meininger C. J., McNeal C. J., Bazer F. W., Rhoads J. M. 2021. Role of L-arginine in nitric oxide synthesis and health in humans. Amino Acids Nutr. Health Amino Acids Gene Expr., 1332, metabolic regulation, and exercising performance, pp.167–187. 10.1007/978-3-030-74180-8_10 [DOI] [PubMed] [Google Scholar]
- Wu H., Wang J., Jiang H., Liu X., Sun X., Chen Y., et al. (2022). The association of dietary spermidine with all-cause mortality and CVD mortality: The US National Health and Nutrition Examination Survey, 2003 to 2014. Frontiers in Public Health, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Dai F., Li C., Zou Y. (2020). Gender Differences in cardiac hypertrophy. J. Cardiovasc. Transl. Res. 13 (1), 73–84. 10.1007/s12265-019-09907-z [DOI] [PubMed] [Google Scholar]
- Xia B. W., Zhao S. C., Chen Z. P., Chen C., Liu T. S., Yang F., et al. (2021). Relationship between serum total testosterone and prostate volume in aging men. Sci. Rep. 11 (1), 14122–14127. 10.1038/s41598-021-93728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Kong X., Jiang J., Yang Z., Jiang R. (2020). Low androgen status inhibits erectile function by inducing eNOS uncoupling in rat corpus cavernosum. Andrology 8 (6), 1875–1883. 10.1111/andr.12844 [DOI] [PubMed] [Google Scholar]
- Xu C., Sellke F. W., Abid M. R. (2022a). Assessments of microvascular function in organ systems. Am. J. physiology. Heart circulatory physiology 322 (6), H891–H905. 10.1152/ajpheart.00589.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Lichtenberger F. B., Erdoǧan C., Lai E., Persson P. B., Patzak A., et al. (2022b). Nitric oxide signalling in descending vasa recta after hypoxia/Re-oxygenation. Int. J. Mol. Sci. 23 (13), 7016. 10.3390/ijms23137016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Wang B., Ren C., Hu J., Greenberg D. A., Chen T., et al. (2017). Age-related impairment of vascular structure and functions. Aging Dis. 8 (5), 590–610. 10.14336/AD.2017.0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Y., Chen C., Wen Z., Wang D. W. (2022). The roles of cardiac fibroblasts and endothelial cells in myocarditis. Front. Cardiovasc. Med. 9, 882027. 10.3389/fcvm.2022.882027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Sener A., Ji Y., Pei Y., Pluth M. D. (2016). Gasotransmitters in biology and medicine: Molecular mechanisms and drug targets. Oxidative Med. Cell. Longev. 2016, 4627308. 10.1155/2016/4627308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Shi R., Zhang Q. (2020a). Hypoxia and oxygen-sensing signaling in gene regulation and cancer progression. Int. J. Mol. Sci. 21 (21), 8162. 10.3390/ijms21218162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Antin P., Berx G., Blanpain C., Brabletz T., Bronner M., et al. (2020b). Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. cell Biol. 21 (6), 341–352. 10.1038/s41580-020-0237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Harris A. L., Davidoff A. M. (2018). Hypoxia and hormone-mediated pathways Converge at the Histone Demethylase KDM4B in cancer. Int. J. Mol. Sci. 19 (1), 240. 10.3390/ijms19010240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M. Y., Lin P. M., Liu Y. C., Hsiao H. H., Yang W. C., Hsu J. F., et al. (2012). Induction of cellular senescence by doxorubicin is associated with upregulated miR-375 and induction of autophagy in K562 cells. PloS one 7(5), e37205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Wijerathne H., Langston J. C., Kiani M. F., Kilpatrick L. E. (2021a). Emerging approaches to understanding microvascular endothelial heterogeneity: A Roadmap for developing anti-inflammatory therapeutics. Int. J. Mol. Sci. 22 (15), 7770. 10.3390/ijms22157770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Song C., Gao M., Wang S., Yu H., Li Y. (2022). Effects of smoking on the retina of patients with dry age-related macular degeneration by optical coherence tomography angiography. BMC Ophthalmol., 22 ( 1), p.315, 10.1186/s12886-022-02525-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gu J., Li X., Xue C., Ba L., Gao Y., et al. (2021b). HIF-1α promotes the migration and invasion of cancer-associated fibroblasts by miR-210. Aging Dis. 12 (7), 1794–1807. 10.14336/AD.2021.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Huang Z., Li L.-L. (2021c). Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 13 (2), 444–459. 10.1039/d0nr07484e [DOI] [PubMed] [Google Scholar]
- Yassin D.-J., El Douaihy Y., Yassin A. A., Kashanian J., Shabsigh R., Hammerer P. G. (2014). Lower urinary tract symptoms improve with testosterone replacement therapy in men with late-onset hypogonadism: 5-year prospective, observational and longitudinal registry study. World J. urology 32 (4), 1049–1054. 10.1007/s00345-013-1187-z [DOI] [PubMed] [Google Scholar]
- Yeap B. B., Wittert G. A. (2022). Testosterone, diabetes risk, and diabetes prevention in men. Endocrinol. metabolism Clin. N. Am. 51 (1), 157–172. 10.1016/j.ecl.2021.11.004 [DOI] [PubMed] [Google Scholar]
- Yoon S., Alfajaro M. M., Cho K. O., Choi U. S., Je H., Jung J., et al. (2020). Perfusion change in benign prostatic hyperplasia before and after castration in a canine model: Contrast enhanced ultrasonography and CT perfusion study. Theriogenology 156, 97–106. 10.1016/j.theriogenology.2020.06.026 [DOI] [PubMed] [Google Scholar]
- Younes L., Albert M., Moghekar A., Soldan A., Pettigrew C., Miller M. I. (2019). Identifying Changepoints in biomarkers during the preclinical phase of Alzheimer’s disease. Front. aging Neurosci. 11, 74. 10.3389/fnagi.2019.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yttersian Sletta K., Tveitarås M. K., Lu N., Engelsen A. S. T., Reed R. K., Garmann-Johnsen A., et al. (2017). Oxygen-dependent regulation of tumor growth and metastasis in human breast cancer xenografts. PloS one 12 (8), e0183254. 10.1371/journal.pone.0183254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Kevil C. G. (2016). Nitric oxide and hydrogen Sulfide regulation of ischemic vascular remodeling. Microcirculation 23 (2), 134–145. 10.1111/micc.12248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura E. M., Bury M. I., Chan Y., Morey A. F., Sharma A. K., Hofer M. D. (2020). Reversing urethral hypovascularity through testosterone and estrogen supplementation. Urology 146, 242–247. 10.1016/j.urology.2020.06.103 [DOI] [PubMed] [Google Scholar]
- Zabbarova I. V., Ikeda Y., Kozlowski M. G., Tyagi P., Birder L. A., Chakrabarty B., et al. (2022). Benign prostatic hyperplasia/obstruction ameliorated using a soluble guanylate cyclase activator. J. Pathology 256 (4), 442–454. 10.1002/path.5859pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahir N., Sun R., Gallahan D., Gatenby R. A., Curtis C. (2020). Characterizing the ecological and evolutionary dynamics of cancer. Nat. Genet. 52 (8), 759–767. 10.1038/s41588-020-0668-4 [DOI] [PubMed] [Google Scholar]
- Zarifpour M., Nomiya M., Sawada N., Andersson K. E. (2015). Protective effect of tadalafil on the functional and structural changes of the rat ventral prostate caused by chronic pelvic ischemia. Prostate 75 (3), 233–241. 10.1002/pros.22909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng R., Garg I., Bannai D., Kasetty M., Katz R., Park J. Y., et al. (2022). Retinal microvasculature and vasoreactivity changes in hypertension using optical coherence tomography-angiography. Graefe's Archive Clin. Exp. Ophthalmol. 260 (11), 3505–3515. 10.1007/s00417-022-05706-6pp. [DOI] [PubMed] [Google Scholar]
- Zeydan B., Lowe V. J., Tosakulwong N., Lesnick T. G., Senjem M. L., Jack C. R., Jr, et al. (2021). Sleep quality and cortical amyloid-β deposition in postmenopausal women of the Kronos early estrogen prevention study. Neuroreport 32 (4), 326–331. 10.1097/WNR.0000000000001592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Huang R., Hu H., Yu L., Tang Q., Tao Y., et al. (2020a). Integrative analysis of hypoxia-associated Signature in Pan-cancer. iScience 23 (9), 101460. 10.1016/j.isci.2020.101460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Yang J., Sun Y., Song J., Gao D., Huang S., et al. (2023). Interleukin-6 and hypoxia synergistically promote EMT-mediated invasion in epithelial ovarian cancer via the IL-6/STAT3/HIF-1α feedback loop. Anal. Cell. Pathol., 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhao H., Horney J., Johnson N., Saad F., Haider K. S., et al. (2021). Testosterone deficiency, long-term testosterone therapy, and inflammation. J. Cardiovasc. Pharmacol. Ther. 26 (6), 638–647. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang H., Hao Z. (2021b). A numerical bone regeneration model incorporating angiogenesis, considering oxygen-induced secretion of vascular endothelial growth factor and vascular remodeling. J. biomechanics 127, 110656. 10.1016/j.jbiomech.2021.110656 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhong Y., Saad F., Haider K. S., Haider A., Clendenin A. G., et al. (2020b). Testosterone therapy may reduce prostate cancer risk due to testosterone deficiency at a young age via stabilizing serum testosterone levels. aging male official J. Int. Soc. Study Aging Male 23 (2), 112–118. 10.1080/13685538.2019.1578739 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang H., Oliveira R. H. M., Zhao C., Popel A. S. (2022). Systems biology of angiogenesis signaling: Computational models and omics. WIREs Mech. Dis. 14 (4), e1550. 10.1002/wsbm.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J. V., Schooling C. M. (2020). The role of testosterone in chronic kidney disease and kidney function in men and women: A bi-directional Mendelian randomization study in the UK Biobank. BMC Med. 18 (1), 122. 10.1186/s12916-020-01594-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P., Li Z., Lin Y., Peng Q., Huang M., Jiang L., et al. (2022). Retinal microvasculature impairments in patients with coronary artery disease: An optical coherence tomography angiography study. Acta Ophthalmol. 100 (2), 225–233. 10.1111/aos.14806 [DOI] [PubMed] [Google Scholar]
- Zhou D., Wang L., Ding S., Shen M., Qiu H. (2022). Phenotypic disease network analysis to identify comorbidity patterns in hospitalized patients with ischemic heart disease using large-scale administrative data. Healthc. 10 (1), 80. 10.3390/healthcare10010080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Ueda K., Hashimoto M., Zhang M., Sasaki M., Kariya T., et al. (2022). The mitochondrial regulator PGC1α is induced by cGMP–PKG signaling and mediates the protective effects of phosphodiesterase 5 inhibition in heart failure. FEBS Lett. 596 (1), 17–28. 10.1002/1873-3468.14228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Heng B. H., Teow K. L. (2018). Lifetime trajectory simulation of chronic disease progression and comorbidity development. J. Biomed. Inf. 88, 29–36. 10.1016/j.jbi.2018.11.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.