Significance
Activation of H2O2 to selectively generate hydroxyl radical (·OH) with stronger oxidation performance is a challenging issue in current research. The cleavage pathway of H2O2 plays a crucial role in determining the selectivity of ·OH generation, with homolytic cleavage of H2O2 being more favorable for the selective generation of ·OH. Herein, the electronic structure of the Fe site was regulated by the introduction of a Cu dopant, which enhanced the adsorption and activation of H2O2 at the Fe site. The modification of the electron structure at the Fe site made the cleavage of H2O2 more inclined to homolytic cleavage, which improved the selectivity of •OH generation. Based on this, the efficient degradation of various organic pollutants can be realized.
Keywords: H2O2 photoactivation, electronic structure modulation, cleavage path, selective hydroxyl production, pollutant degradation
Abstract
Hydrogen peroxide (H2O2) is an important green oxidant in the field of sewage treatment, and how to improve its activation efficiency and generate free radicals with stronger oxidation performance is a key issue in current research. Herein, we synthesized a Cu-doped α-Fe2O3 catalyst (7% Cu–Fe2O3) for activation of H2O2 under visible light for degradation of organic pollutants. The introduction of a Cu dopant changed the d-band center of Fe closer to the Fermi level, which enhanced the adsorption and activation of the Fe site for H2O2, and the cleavage pathway of H2O2 changed from heterolytic cleavage to homolytic cleavage, thereby improving the selectivity of •OH generation. In addition, Cu doping also promoted the light absorption ability of α-Fe2O3 and the separation of hole–electron pairs, which enhanced its photocatalytic activities. Benefiting from the high selectivity of •OH, 7% Cu–Fe2O3 exhibited efficient degradation activities against ciprofloxacin, the degradation rate was 3.6 times as much as that of α-Fe2O3, and it had good degradation efficiency for a variety of organic pollutants.
The rapid rise in population and urbanization has led to a severe global issue of water pollution (1). As one of the greenest oxides, hydrogen peroxide (H2O2) plays an indispensable role in water pollution control (2). H2O2 can be activated in different ways to produce strongly oxidizing radicals for the degradation of organic pollutants, such as superoxide radicals (•O2−), hydroperoxyl radicals (•O2H), and hydroxyl radicals (•OH) (3, 4). However, due to the high activation energy of H2O2 molecules, the reaction kinetics of its activation are generally limited, which is the bottleneck problem limiting the use of H2O2 in the field of water treatment (5). The traditional homogeneous Fenton reaction relies on the oxidation and reduction of Fe2+/Fe3+ to activate H2O2 (Eqs. 1 and 2).
| [1] |
| [2] |
It is easy to form iron slime and cause excessive consumption of H2O2, which limits its application in wastewater treatment (6). In order to improve the activation efficiency of H2O2 and avoid the disadvantages of the traditional Fenton reaction, more and more studies have shifted the focus from homogeneous to heterogeneous systems (7). In particular, the heterogeneous photocatalytic activation of H2O2 using semiconductors as catalysts has broad research prospects. The photocatalytic reaction can provide photogenerated electrons for the activation of H2O2 and accelerate the cyclic reaction of metal ions (≡Mn + H2O2 → ≡Mn +1 + •OH + OH−, ≡Mn+1 + e−→ ≡Mn), which effectively improves the rate-limiting step of H2O2 activation and effectively avoids the secondary pollution caused by metal ion precipitation, with the advantages of high efficiency and green (8).
Being abundant, inexpensive, and nontoxic, Fe is one of the most prevalent elements in the earth's crust. Fe2O3, Fe3O4, and FeOOH are among several iron compounds that are abundant in nature and widely used for water treatment (9). Hematite (α-Fe2O3) is a semiconductor material that possesses several advantages, including high stability, environmental friendliness, and low cost. As a result, it has garnered considerable attention in the fields of photocatalysis and photo-Fenton (10). To enhance the photocatalytic and H2O2 activation activity of the catalyst, various strategies have been developed, including doping engineering (11), heterostructure construction (12), and crystal plane regulation (9). More importantly, the activation pathway of H2O2 may affect the degradation efficiency of the catalytic system by affecting the generation of free radicals (13). At present, the activation pathways of H2O2 mainly include heterolytic cleavage (Eq. 3) and homolytic cleavage (Eq. 4).
| [3] |
| [4] |
For the heterolytic cleavage pathway, the decomposition of H2O2 leads to O–H bond cleavage to generate *OOH and then through electron transfer to generate free radicals. This process tends to generate free radicals mainly including •HO2/•O2− and •OH (14–16). For the homolytic cleavage pathway, the direct breaking of the O–O bond in H2O2 produces two *OH intermediates, which are mainly formed into •OH through electron transport (3, 17). Considering the degradation efficiency of the catalytic system, we prefer the highly selective generation of •OH. This is due to the fact that •OH (+2.38 V vs. SHE) has a better oxidation capacity than •O2− (−0.33 V vs. SHE) for organic pollutants, especially for refractory organic pollutants (18, 19). Comparing the two cleavage pathways, the homolytic cleavage pathway seems to be more selective for the formation of •OH and thus more favorable for the degradation of organic pollutants. Kim et al. showed that •OH, •OOH, and •O2− can be generated by H2O2 cleavage, but the production of •OH is mainly through homolytic cleavage of H2O2 by heterogeneous catalysis (20). Xing et al. have demonstrated that the charge density of Fe3C in the Fe/Fe3C structure is moderate, which promotes the homolytic cleavage of H2O2 and the efficient generation of •OH. The high charge density of Fe0 in the Fe/Fe3C structure tends to promote the heterolytic cleavage of H2O2 to generate O2 with low oxidation capacity, resulting in the waste of H2O2 (21).
Herein, a Cu-doped α-Fe2O3 nanosheet (x% Cu–Fe2O3) was designed and synthesized to photoactivate H2O2 for the degradation of organic pollutants. Among them, the degradation rate of ciprofloxacin (CIP) by 7% Cu–Fe2O3 was 3.6 times that of the original α-Fe2O3. Furthermore, 7% Cu–Fe2O3 exhibited excellent degradation efficiency toward various organic pollutants. Combining experimental and theoretical research, we found that Cu doping alters the electronic structure of the Fe site, transforming the activation pathway of H2O2 from heterolytic cleavage to homolytic cleavage and improving the selectivity of •OH formation. Additionally, Cu dopants enhance the photocatalytic activity of α-Fe2O3 by changing its band structure, which promotes H2O2 activation. This work provides unique ideas and perspectives on the photoactivation mechanism of H2O2 for the highly selective generation of •OH by selecting the activation pathway of H2O2.
Results
Structure and Characterization of Catalysts.
The synthesis process of Cu-doped α-Fe2O3 was shown in Fig. 1A. A series of catalysts with different Cu content (denoted as x% Cu–Fe2O3, x is the molar ratio of Cu doping) were synthesized by hydrothermal method by adjusting the amount of metal salt added. The actual doping amount of the samples was determined by inductively coupled plasma mass spectrometry (ICP-MS), which was a little different from the theoretical doping amount (SI Appendix, Table S1). Of note, 7% Cu–Fe2O3 was taken as the representative sample for study because it had the best performance. Transmission electron microscopy (TEM) revealed that the α-Fe2O3 nanosheets had a hexagonal shape with a transverse dimension of approximately 250 nm. Cu doping changed the original regular hexagonal structure of α-Fe2O3 nanosheets into disc-shaped nanosheets. The lattice spacing of α-Fe2O3 and 7% Cu–Fe2O3, as measured by TEM, was approximately 0.246 nm and 0.251 nm, respectively, corresponding to the (110) crystal plane (Fig. 1 B and D). This indicates that Cu doping does not change the exposed crystal plane of the catalyst, and lattice expansion may be due to the fact that the ionic radius of the doped element (0.77/0.73 Å) is greater than that of Fe3+ (0.55 Å). The element mapping indicated that the Cu element was successfully doped and evenly distributed (Fig. 1 C and E). The X-ray diffraction (XRD) peaks of α-Fe2O3 match the standard card 33-0664, and those of 7% Cu–Fe2O3 generally agree with it, indicating that Cu doping has no significant effect on the crystal structure of α-Fe2O3 (Fig. 1F). Furthermore, the XRD pattern of 7% Cu–Fe2O3 does not exhibit any characteristic peak of CuO/Cu2O, suggesting that Cu is doped into the lattice of α-Fe2O3 instead of forming a composite structure with CuO or Cu2O. The Fourier transform infrared (FT-IR) spectroscopy (Fig. 1G) showed that the peaks at 466, 546, and 772 cm−1 correspond to the Fe-O stretching vibration mode in α-Fe2O3. These peaks in 7% Cu–Fe2O3 slightly decrease, possibly due to the effect of Cu doping on the Fe-O stretching vibration (22).
Fig. 1.
(A) Schematic diagram of synthesis of x% Cu–Fe2O3. (B) TEM images and high-resolution TEM images of α-Fe2O3. (C) High-angle annular dark field image and element mapping image of α-Fe2O3. (D) TEM images and high-resolution TEM images of 7% Cu–Fe2O3. (E) High-angle annular dark field image and element mapping image of 7% Cu–Fe2O3. (F) XRD pattern and (G) FT-IR pattern of α-Fe2O3 and 7% Cu–Fe2O3. (H) The Fe 2p high-resolution XPS spectra of α-Fe2O3 and 7% Cu–Fe2O3. (I) The Cu 2p high-resolution XPS spectrum of 7% Cu–Fe2O3.
Raman scattering is highly sensitive to lattice disorder. Therefore, the broadening and intensity reduction of the Raman peak of α-Fe2O3 incorporated with Cu can be attributed to the disordered substitution of Cu (SI Appendix, Fig. S1) (23). The X-ray photoelectron spectroscopy (XPS) was used to determine the influence of Cu doping on the electronic structure and chemical state of α-Fe2O3 and to reveal the catalytic advantage of 7% Cu–Fe2O3. High-resolution spectra of Fe and Cu were calibrated at 284.8 eV using the C 1s XPS high-resolution spectrum (SI Appendix, Fig. S2). The effect of Cu doping on electron density around Fe was revealed by analyzing the high-resolution XPS spectra of Fe 2p (Fig. 1H). For α-Fe2O3, the characteristic peaks near 710.2 eV and 723.5 eV were attributed to Fe 2p3/2 and Fe 2p1/2, respectively, and there were two distinct satellite peaks at 718.5 eV and 732.5 eV, indicating that Fe (III) is the main content on the catalyst surface (24). After Cu doping, the two fitting peaks of Fe 2p3/2 moved toward the direction of low binding energy and the peak intensity decreases, which meant that Cu doping changed the chemical state of Fe. This may be due to the electrical interaction between Cu and Fe induced by Cu doping leading to electron transfer from Cu to Fe, thus increasing the electron cloud density around Fe. In addition, the decrease of the satellite peak intensity of 7% Cu–Fe2O3 also indicated that the electronic interaction between Fe and Cu reduced the surface Fe (III) content (25). The Cu 2p high-resolution XPS spectrum (Fig. 1I) revealed that the main valence states of Cu in 7% Cu–Fe2O3 are Cu (II) (935.1 eV) and Cu (I) /Cu (932.6 eV) (26). The Cu Auger LMM spectrum was collected due to the characteristic peaks of both metallic copper and copper oxides appeared in the range of 932.5 to 932.6 eV, which made it difficult to distinguish. The Cu Auger LMM spectrum (SI Appendix, Fig. S3) revealed the presence of Cu (I) and Cu (II) but not metallic copper (27). The surface area of α-Fe2O3 and 7% Cu–Fe2O3 is 18.43 m2/g and 16.12 m2/g, respectively. This suggested that the specific surface area is not a major factor contributing to the catalytic reaction (SI Appendix, Fig. S4 A and B and Table S2) (28). The thickness and surface potential of the synthesized materials were studied by atomic force microscopy and Kelvin probe force microscopy (KPFM). The results indicated that the thickness of the catalyst is basically unchanged before and after doping, which is about 6 to 7 nm. It is gratifying that the KPFM test results showed that the surface potential of the catalyst was significantly enhanced and fluctuated after Cu doping, which will be beneficial to the transport and transfer of photogenerated electrons (SI Appendix, Fig. S5).
Catalytic Degradation of the 7% Cu–Fe2O3/H2O2/Vis System.
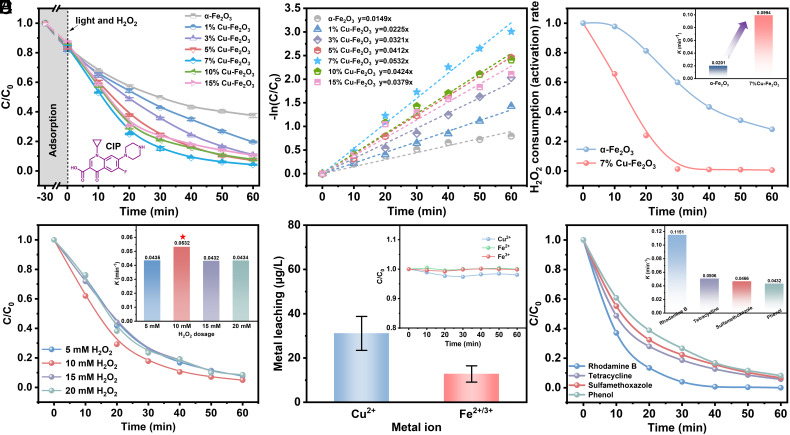
The degradation efficiency of Cu- doped α-Fe2O3 photoactivated H2O2 on pollutants was investigated using 10 mg/L CIP solution as the probe pollutant. Benefiting from the excellent ability of Cu-doped α-Fe2O3 to photoactivate H2O2, the degradation efficiency of CIP was significantly improved compared to that of α-Fe2O3. Among them, 7% Cu–Fe2O3 exhibited the best degradation effect on CIP, with a degradation rate of 95.8% achieved within 60 min (Fig. 2A). The pseudo-first-order kinetic constant (k) reached 0.0532 min−1, which is about 3.6 times that of α-Fe2O3 (Fig. 2B). This result is superior to that of catalysts reported in some previous studies (SI Appendix, Table S3). To elucidate that the degradation process is dominated by the photoactivation of H2O2 by the catalyst, a series of experiments were conducted. First, the adsorption or removal of CIP by the catalyst under dark conditions was investigated. The concentration of CIP did not decrease significantly after the catalyst reacted with CIP for 30 min under dark conditions, indicating that the adsorption or degradation of CIP by the photocatalyst was not obvious in the absence of visible light irradiation (SI Appendix, Fig. S6). Second, the degradation of CIP by α-Fe2O3 and 7% Cu–Fe2O3 in the case of visible light irradiation alone or H2O2 addition alone was explored (SI Appendix, Fig. S7). α-Fe2O3 and 7% Cu–Fe2O3 had weak degradation effect on CIP under pure visible light irradiation, and the degradation efficiency of 7% Cu–Fe2O3 on CIP was slightly higher than that of α-Fe2O3. In the presence of H2O2 alone, the results showed that the degradation of CIP by the catalysts was extremely weak and almost negligible, which indicated that α-Fe2O3 and 7% Cu–Fe2O3 could not directly activate H2O2 effectively. The above experimental results indicated that the degradation of CIP is due to the photoactivation of H2O2 to generate active species. The H2O2 consumption experiment showed that the consumption ability of 7% Cu–Fe2O3 to H2O2 was significantly higher than that of α-Fe2O3 (Fig. 2C, the inset showed that the consumption rate was significantly increased), indicating that the photoactivation ability of 7% Cu–Fe2O3 to H2O2 was significantly improved. In addition, the optimal dosage of H2O2 in the catalytic system was studied (Fig. 2D). The results showed that when the concentration of H2O2 in the catalytic system was 10 mM, the degradation efficiency of CIP was the highest. When the concentration of H2O2 in the system exceeded 10 mM, the degradation efficiency of CIP decreased, which may be due to the excessive H2O2 will react with •OH as follows: H2O2 + •OH = HO2• + H2O, thus consuming •OH (29).
Fig. 2.
(A) The degradation efficiency of CIP (10 mg/L) by the prepared samples photoactivated H2O2. (B) Pseudo-first-order kinetic curves of the degradation efficiency of CIP by the prepared samples photoactivated H2O2. (C) The consumption of H2O2 by α-Fe2O3/H2O2/Vis and 7% Cu–Fe2O3/H2O2/Vis catalytic systems. (D) Effect of H2O2 dosage on the degradation of CIP by the 7% Cu–Fe2O3/H2O2/Vis catalytic system. (E) Leakage of metal ions in the 7% Cu–Fe2O3/H2O2/Vis catalytic system and degradation of CIP by homogeneous ions of the same concentration. (F) Degradation of different organic pollutants by the 7% Cu–Fe2O3/H2O2/Vis catalytic system. Reaction conditions: [CIP] = [RhB] = [TC] = [SMZ] = [Phenol] = 10 mg/L; [catalyst] = 0.2 g/L; [H2O2] = 10 mM; illumination conditions: 300 W Xe lamp; [Temp] = 20 °C; initial pH = 6.8.
To ensure that the treated water was not contaminated and the catalytic reaction was not affected by metal ion leakage from the catalytic material, we measured the metal ion leakage using ICP-MS (Fig. 2E). The results showed that the leakage of metal ions of 7% Cu–Fe2O3 was quite low during the reaction. The leakage amount of Cu ions was about 31.12 μg/L, and that of Fe ions was about 12.79 μg/L, which is much lower than that of China drinking water standard (The limits of Cu and Fe in GB5749-2022 are 1.0 mg/L and 0.3 mg/L, respectively) (30). Homogeneous Cu2+, Fe2+, and Fe3+ were separately added at the same concentration as the catalyst leaching to the catalytic system (H2O2/Vis) to eliminate the influence of the leakage ions on the catalytic reaction (Inset of Fig. 2E). The results showed that the above homogeneous ions had minimal impact on the degradation of CIP, which indicated that the CIP was degraded by 7% Cu–Fe2O3 photoactivated H2O2. In addition, 7% Cu–Fe2O3 has degradation effect on a variety of organic compounds, including rhodamine, tetracycline, sulfamethoxazole, and phenol (Fig. 2F), indicating that the catalytic system has good oxidative degradation ability.
To explore the causes of pollutant degradation, a series of scavengers were introduced into the reaction system to identify the active species that degrade pollutants (31). The addition of mannitol and AgNO3 inhibited the degradation efficiency of CIP most obviously (SI Appendix, Fig. S8). The results indicated that •OH plays a significant role in the degradation of CIP by photoactivation of H2O2, and electron transfer facilitates the generation of free radicals. The electron paramagnetic resonance (EPR) test provided direct evidence for photoactivation of H2O2 to produce reactive oxygen species by α-Fe2O3 and 7% Cu–Fe2O3 so as to explore the reason for the improvement of pollutant degradation efficiency after Cu doping (32). The •OH was detected using 5,5-dimethyl-1-pyrroline N-oxide (DMPO) as the capture agent and water as the solvent. The •OH signals (four peaks with a peak intensity of 1:2:2:1) appeared in both α-Fe2O3 and 7% Cu–Fe2O3 reaction systems (Fig. 3A), whereas the relative intensity of the signal of 7% Cu–Fe2O3 was much stronger than that of α-Fe2O3, indicating that much more •OH was produced in the 7% Cu–Fe2O3 reaction system than the α-Fe2O3 reaction system (7). The •O2− was determined using DMPO as the capture agent and methanol as the solvent. Both α-Fe2O3 and 7% Cu–Fe2O3 reaction systems showed a six-peak •O2− signal (Fig. 3B), and the signal intensity of α-Fe2O3 and 7% Cu–Fe2O3 was basically the same (28). In addition, the presence of 1O2 was not found in either reaction system (Fig. 3C). The •O2− and •OH were quantified using 0.05 mM p-nitro blue tetrazolium chloride (NBT) and 0.5 mM terephthalic acid (33, 34). The amount of •O2− produced by the α-Fe2O3 catalytic system was approximately 143.79 μM, while that of the 7% Cu–Fe2O3 catalytic system was 155.27 μM within 60 min, showing little difference in •O2− production, which is consistent with the results obtained from EPR analysis (Fig. 3D). For the •OH, the yield of the α-Fe2O3 catalytic system is about 0.06 mM, and that of the 7% Cu–Fe2O3 catalytic system is significantly higher, reaching 4.01 mM, which is also consistent with the EPR results (Fig. 3E). As can be seen from the yield chart, the yield of •OH was greatly increased in the first 20 min of the reaction, which was highly similar to the degradation process of CIP, indicating that •OH played an extremely important role in the degradation process. The selective calculation results of free radical formation indicate that the selectivity of •OH generated by photoactivation of H2O2 is significantly enhanced after Cu-doped α-Fe2O3 (Fig. 3F). The selectivity of α-Fe2O3 photoactivated H2O2 for generating •O2− and •OH was found to be 55.9% and 44.1%, respectively, whereas the selectivity of 7% Cu–Fe2O3 was 3.7% and 96.3%, respectively. Notably, the selectivity for •OH formation was significantly improved after Cu doping. This enhancement may be attributed to changes in the activity and electron cloud density of the α-Fe2O3 surface sites induced by Cu doping, which in turn altered the decomposition pathway of H2O2, facilitating the generation of •OH. These findings are of great interest for further research.
Fig. 3.
EPR spectra of (A) •OH, (B) •O2−, and (C) 1O2 of α-Fe2O3/H2O2/Vis and 7% Cu–Fe2O3/H2O2/Vis catalytic systems. The •O2− (D) and •OH (E) quantification of α-Fe2O3/H2O2/Vis and 7% Cu–Fe2O3/H2O2/Vis catalytic systems. (F) Selectivity of α-Fe2O3/H2O2/Vis and 7% Cu–Fe2O3/H2O2/Vis catalytic systems for •OH and •O2− generation.
Mechanism of H2O2 Activation Enhancement and •OH Formation Selective Elevation.
Density functional theory (DFT) calculations were used to investigate the effect of Cu doping on the electronic structure of α-Fe2O3 and the activation path of H2O2 in an attempt to reveal the mechanism that the yield and selectivity of •OH increase significantly. Based on our TEM study results, the synthesized catalyst mainly exposed (110) crystal planes, so we established the catalyst structure model with exposed (110) crystal planes (SI Appendix, Fig. S9). First, the adsorption of H2O2 on the catalyst surface was studied to determine the adsorption site and configuration of H2O2. There are two main adsorption configurations of H2O2 on α-Fe2O3 and 7% Cu–Fe2O3, namely end-on adsorption and side-on adsorption (35). As shown in Fig. 4 A and B, H2O2 tends to be adsorbed on Fe sites of α-Fe2O3 and 7% Cu–Fe2O3 in end-on mode, and the adsorption energy is −0.76 eV and −0.81 eV, respectively. These results indicate that Cu doping does not change the adsorption site and adsorption configuration of H2O2 and thus may promote adsorption by affecting the electronic structure of the Fe sites. The results of energy band structure (SI Appendix, Fig. S10) and partial density of states (PDOS) (SI Appendix, Fig. S11) calculations supported our conjecture that Cu doping leads to an upward shift of the d-band center of Fe (from −1.37 eV for α-Fe2O3 to −1.35 eV for 7% Cu–Fe2O3) closer to the Fermi energy level, enhancing the adsorption of H2O2 by the Fe site and the catalytic activity of the Fe sites.
Fig. 4.
Adsorption energy for different adsorption configurations and activation energy of different cleavage pathways of H2O2 on (A) α-Fe2O3 and (B) 7% Cu–Fe2O3. Charge differential density calculation and Bader charge calculation of H2O2 adsorbed on (C) α-Fe2O3 and (D) 7% Cu–Fe2O3. (E) Plot of optimal photoactivation pathways of α-Fe2O3 and 7% Cu–Fe2O3 to H2O2. In situ Raman spectroscopy of (F) α-Fe2O3/H2O2/Vis and (G) 7% Cu–Fe2O3/H2O2/Vis catalytic systems. Cu LMM spectra of catalysts of the 7% Cu–Fe2O3/H2O2/Vis catalytic system at different reaction times, including (H) 0 min, (I) 10 min, (J) 30 min, and (K) 60 min.
In addition, the calculated results of the charge density difference are not difficult to see that H2O2 gains more electrons at the Fe adsorption site (activation site) after Cu doping, and the calculated Bader charge transfer increases from 0.090 e− to 0.141 e− (Fig. 4 C and D). This indicates that Cu doping promotes the transfer of electrons from the catalyst to H2O2, which is conducive to the adsorption of H2O2 and the subsequent activation cleavage. It is worth noting that although Cu doping does not change the adsorption configuration of H2O2 on the catalyst surface, it is found that Cu doping affects the cleavage pathway of H2O2 by calculating the free energy of generating H2O2 cleavage intermediates. For α-Fe2O3, the activation cleavage of H2O2 is more likely to be heterolytic cleavage (H2O2 → *H + *OOH) due to its lower energy barrier. On the contrary, for 7% Cu–Fe2O3, H2O2 is more inclined to homolytic cleavage (H2O2 → *OH + *OH). The heterolytic cleavage of H2O2 at the active site of α-Fe2O3 is thermodynamically possible but faces a large activation energy barrier (ΔE = +0.54 eV). In contrast, H2O2 on 7% Cu–Fe2O3 can be homolytic cleavage without obstacle to form *OH intermediate (ΔE = −0.32 eV), and this reaction occurs spontaneously (Fig. 4E). The above calculation results show that Cu doping not only changes the electronic structure of the adsorption site to promote the adsorption of H2O2 but more importantly changes the activation path of H2O2. The homolytic cleavage of H2O2 is more favorable to improve the yield and selectivity of •OH, which is due to the fact that the *OOH intermediate produced by the heterolytic cleavage of H2O2 is easy to produce O2 and •O2− with relatively weak oxidation capacity, thus causing the waste of H2O2 (18, 21, 36).
In situ Raman spectroscopy was used to identify the decomposition intermediates, which provided direct evidence for the decomposition path of H2O2. The key to distinguish the two decomposition modes of H2O2 is the identification of the two decomposition intermediates *OOH and *OH. As shown in Fig. 4 F and G, the peak near 878 cm−1 is the O–O stretching vibration of H2O2 (37). In the α-Fe2O3/H2O2/Vis catalytic system, as the reaction time goes on, it is not difficult to see that a clear Raman peak appears near 614 cm−1 (Fig. 4F), which should be attributed to the stretching vibration of Fe–O in Fe–OOH (38, 39). In the catalytic system of 7% Cu–Fe2O3/H2O2/Vis, since Cu doping did not change the adsorption configuration of H2O2, the Raman peak at 614 cm−1 was attributed to the telescopic vibration of Fe–O in Fe–*H2O2 (Fe–OOH). Encouragingly, a Raman peak has emerged near 563 cm−1, which is attributed to tensile vibrations of Fe–O in Fe–OH (Fig. 4G) (40, 41). The results demonstrate that in the 7% Cu–Fe2O3/H2O2/Vis system, H2O2 molecules undergo photoactivated homolytic cleavage (*HO–*OH) to form *OH, which differs from the α-Fe2O3/H2O2/Vis system. This suggests that the introduction of Cu dopants alters the cleavage pathway of H2O2, promoting the selective generation of •OH. The analytical results obtained from Raman spectroscopy are in agreement with the DFT calculations.
The Cu Auger LMM spectra at different reaction times (0 min, 10 min, 30 min, and 60 min) provided evidence for the involvement of Cu sites in H2O2 photoactivation. Before the reaction proceeds, the area ratio of Cu (II) is higher than Cu (I) in the Cu Auger LMM spectrum. This is due to the fact that electrons around Cu are enriched around Fe after Cu doping into α-Fe2O3; hence, the Cu mainly exists in the form of Cu (II) in the catalyst (Fig. 4H). After 10 min of reaction, a large amount of Cu (II) is converted into Cu (I), which is because Cu dopants can be used as trapping centers of photogenerated electrons, leading to electron enrichment around Cu sites (Eqs. 5 and 6) (Fig. 4I). As the reaction progresses, the amount of Cu (II) increases, and the transfer of electrons from Cu (I) to Fe promotes the reduction of Fe (III) to Fe (II) (Eq. 7) (Fig. 4 J and K). At the end of the reaction, the valence state of Cu returns to a state similar to that before the reaction began. In this way, Cu is driven by photogenerated electrons to realize the valence state cycle of Cu, thus continuously providing electrons to the Fe site to produce Fe (II) for H2O2 activation (Eq. 8). A mechanistic diagram of the reaction process is presented in Supporting Information (SI Appendix, Fig. S12).
| [5] |
| [6] |
| [7] |
| [8] |
To clarify another important reason for the enhanced performance of 7% Cu–Fe2O3 photoactivated H2O2, the photocatalytic properties of α-Fe2O3 and 7% Cu–Fe2O3 were investigated. First of all, the light absorption capacity (SI Appendix, Fig. S13) and band gap (SI Appendix, Fig. S14) of the sample obtained were analyzed by UV-vis diffuse reflectance spectroscopy. The optical absorption edge of α-Fe2O3 extends from 647.3 nm to 683.6 nm of 7% Cu–Fe2O3, and the band gap narrows from 1.97 eV of α-Fe2O3 to 1.90 eV of 7% Cu–Fe2O3, which indicates that Cu doping promotes the light absorption ability of the catalyst. Valence band (VB) XPS spectra determined VB of 1.07 eV and 1.24 eV for α-Fe2O3 and 7% Cu–Fe2O3, respectively (SI Appendix, Fig. S15). The above experimental results are in good agreement with the calculated results of band structure and PDOS. The carrier separation efficiency and charge transfer ability of the catalyst were evaluated by transient photocurrent (SI Appendix, Fig. S16) and electrochemical impedance spectroscopy (SI Appendix, Fig. S17). Compared with α-Fe2O3, 7% Cu–Fe2O3 has stronger photocurrent and smaller electrochemical impedance, which indicates that 7% Cu–Fe2O3 has better carrier separation and charge transfer ability, which will be beneficial to photoactivation of H2O2. The separation efficiency of the electron–hole pairs and the lifetime of the photogenerated charge of the catalyst were evaluated by steady-state photoluminescence (PL) (SI Appendix, Fig. S18) and time-resolved PL (SI Appendix, Fig. S19) experiments. The PL intensity of 7% Cu–Fe2O3 is significantly reduced compared with that of α-Fe2O3, which means that the recombination of electrons and holes is effectively inhibited. Compared with α-Fe2O3, 7% Cu–Fe2O3 has a shorter PL average lifetime, which indicates that 7% Cu–Fe2O3 has a more convenient charge separation and transfer channel.
Degradation Mechanisms of Organic Pollutants and Application Prospect of Catalysts.
Benefiting from the highly selective formation of hydroxyl radicals, 7% Cu–Fe2O3 can degrade target pollutants efficiently, but the conversion intermediates generated in the degradation process may be potential environmental pollutants. Molecular electrostatic potential surface (MEPs) of pollutants can be calculated to predict the attack sites of free radicals. MEPs of pollutants can clearly reflect the electrophilic attack zone and nucleophilic attack zone of molecules. Electrophilic reactivity is indicated by negative potentials (red areas) and nucleophilic reactivity is indicated by positive potentials (blue areas). The •OH is a typical strong electrophilic free radical (42), and the electrophilic attack region of CIP is mainly distributed in the carboxyl, hydroxyl, and piperazine ring regions (Fig. 5A). Therefore, the attack degradation of CIP by •OH begins in these regions. In order to understand CIP degradation process in the 7% Cu–Fe2O3/H2O2/Vis system, the intermediate products of CIP degradation process were analyzed by high-performance liquid chromatography and mass spectrometry (HPLC-MS). According to the CIP degradation intermediates identified by HPLC-MS analysis, three possible CIP degradation pathways were proposed (Fig. 5B). Both degradation pathways I and II first oxidized CIP to intermediates (C1) with an m/z of 362.14 through hydroxyl addition and fluorine substitution. In pathway I, C1 underwent dehydrogenation and hydroxylation to form CI1, which was then subjected to decarboxylation and ring opening, resulting in a reduction in the molecular weight of the pollutant (43). Pathway II mainly involves the attack of •OH on the piperazine ring, leading to the opening of the ring structure. Piperazine is gradually oxidized, producing the CII3 product with an m/z of 263.24 (44). Pathway III mainly involves the opening of the piperazine ring through decarboxylation and hydroxylation to facilitate further oxidation. Ultimately, the molecular weight of the main intermediates decreases continuously through •OH oxidation, culminating in mineralization into CO2 and H2O. The possible degradation pathways analyzed by HPLC-MS revealed that the primary sites where the contaminants were attacked by •OH were consistent with the results calculated by MEPs, so the analyzed degradation paths have high reliability.
Fig. 5.
(A) Molecular electrostatic potential surface (MEPs) of CIP. (B) Degradation pathway of CIP by the 7% Cu–Fe2O3/H2O2/Vis catalytic system. (C) A simple device to simulate the practical application of the 7% Cu–Fe2O3/H2O2/Vis catalytic system.
Assessing the toxicity and potential harm of degradation intermediates is critical to evaluate the effectiveness of pollutant degradation. To evaluate the toxicity of CIP and its degradation intermediates, toxicity evaluation software was employed, using Daphnia magna LC50 (48 h), Fathead minnow LC50 (96 h), developmental toxicity value, and mutagenicity value as evaluation criteria. The toxicity of most intermediates to Daphnia magna and Fathead minnow is significantly reduced, with only CIII5 to Daphnia magna and CI4 to Fathead minnow showing greater toxicity than CIP (SI Appendix, Fig. S20 A and B). Regarding the developmental toxicity value, all intermediates except CII2 have smaller values. Regarding the mutagenicity value, the values of all intermediates except CII2 and CIII4 are reduced. Overall, the toxicity of CIP is effectively reduced after degradation using the 7% Cu–Fe2O3/H2O2/Vis catalytic system (SI Appendix, Fig. S21 A and B).
The reusability and stability of catalysts are particularly important for practical water treatment applications. To investigate the reusability of the 7% Cu–Fe2O3 catalyst, five successive CIP degradation experiments were conducted. After 5 cycles, the CIP degradation efficiency of the 7% Cu–Fe2O3 catalyst remained close to 90%, indicating that the prepared catalyst has good cycling performance of Cu and Fe ions and good stability (SI Appendix, Fig. S22). The XRD and Fe 2p XPS high-resolution patterns before and after the reaction showed no significant changes (SI Appendix, Figs. S23 and S24), indicating that the catalyst has good stability and is suitable for actual water treatment processes. The presence of organic matter or anions in actual polluted wastewater may compete with pollutants for adsorption sites or consume free radicals, which can affect the degradation efficiency of the system. To test the applicability of the 7% Cu–Fe2O3 catalyst in practical applications, we added humic acid (HA), HCO3−, and Cl− to the system to simulate the actual water environment and investigate their effect on the degradation of CIP. The addition of organic matter or anions had little effect on the degradation efficiency of the 7% Cu–Fe2O3/H2O2/Vis catalytic system, indicating its practical application potential (SI Appendix, Fig. S25).
The highly efficient degradation effect of the 7% Cu–Fe2O3/H2O2/Vis catalytic system and the need for its future practical application urge us to explore further its treatment potential. Considering the good dispersion of 7% Cu–Fe2O3 in solution, a polyvinylidene fluoride membrane was used as the catalyst carrier to improve its repeatability and avoid secondary pollution. The preparation of 7% Cu–Fe2O3 membrane was done through vacuum filtration, and the catalyst was firmly attached to the surface of the membrane, ensuring that it would not detach during the catalytic reaction (SI Appendix, Fig. S26). To verify the performance of the 7% Cu–Fe2O3 membrane, an experimental device was built as shown in Fig. 5C. The device used secondary effluent from Nankai University Sewage Treatment Plant (Tianjin, China) as a solvent, and RhB was added as an indicator for catalytic degradation. Remarkably, the RhB solution achieved complete decolorization removal in the end effluent after passing through the designed catalytic membrane device. This suggests that the 7% Cu–Fe2O3 catalyst has great potential for future practical applications.
Conclusions
In summary, we synthesized the 7% Cu–Fe2O3 photocatalyst which can activate H2O2 to selectively generate •OH and has a great degradation effect on various organic pollutants. TEM results showed that the Cu dopant was successfully introduced. The results of quantitative experiments on EPR and free radical showed that the selectivity of •OH generation in the catalytic system of 7% Cu–Fe2O3/H2O2/Vis was significantly improved. The calculation results of PDOS and band structure indicated that Cu doping changed the electronic structure of the Fe site, making the center of the d-band of Fe moved up closer to the Fermi level. Charge differential density and Bader charge transfer calculation results showed that the introduction of Cu promoted the electron transfer of 7% Cu–Fe2O3 to H2O2 adsorbed on the surface and thus promoted the activation of H2O2. Gibbs free energy calculation and in situ Raman spectroscopy manifested that the cleavage pathway of H2O2 changed from heterolytic cleavage to homolytic cleavage after Cu doping, which was conducive to the highly selective generation of •OH. In addition, the introduction of the Cu dopant changed the photoelectric properties of α-Fe2O3 and enhanced its photocatalytic activity, and Cu acted as a capture center for photogenerated electrons to transfer electrons to Fe sites and promoted valence cycling of Fe. Based on the above reasons, 7% Cu–Fe2O3 photoactivated H2O2 to degrade CIP significantly. Our work proved that the change of H2O2 cleavage path can effectively enhance the selective formation of •OH, providing a unique perspective and idea for doping engineering to enhance the Fenton-like reactivity mechanism.
Materials and Methods
Preparation of the Catalyst.
FeCl3·6H2O (0.0025 mol, 0.6825 g) and CuCl2·2H2O in different molar ratios were dispersed in 1.75 mL water and 25 mL ethanol mixture under magnetic stirring, and the mixture was evenly mixed after stirring for 10 min. To the solution, 2 g CH3COONa was added, and the stirring was continued for 30 min. Then, the mixture was transferred to a 50-mL capacity Teflon-lined stainless steel reactor and kept in the oven at 180 °C for 12 h. After the reactor is cooled to room temperature, the red product is separated by centrifugation, washed several times with distilled water and countless ethanol by suction filtration method, and dried in the oven at 60 °C for more than 6 h to obtain dry dark red powder. The Cu-doped α-Fe2O3 is denoted as x% Cu–Fe2O3 according to the molar ratio of Cu doping. Six samples with doping ratios of 1%, 3%, 5%, 7%, 10%, and 15% were synthesized, among which 7% Cu–Fe2O3 had the best degradation effect. The synthesis of α-Fe2O3 is basically the same as that of x% Cu–Fe2O3, except that CuCl2·2H2O is not added.
H2O2 Photoactivation Degradation Experiment.
In a representative experiment of photoactivation of H2O2, 20 mg of the catalyst was added to a 100-mL configuration of aqueous pollutant solution and sonicated to disperse it evenly. First, the suspension was stirred for 30 min to ensure that the adsorption equilibrium was established. Second, 100 μL of H2O2 (30% wt) solution was added to the suspension, and a 300-W xenon lamp equipped with a 420-nm cutoff filter was used as the light source. At certain irradiation intervals, 3 mL of the suspension was extracted, and its degradation was measured after filtration. CIP and degraded intermediates were determined by high-performance liquid chromatography–mass spectrometry (HPLC-MS, Orbitrap Fusion, Thermo, USA) with the eluent consisting of 0.1% formic acid and acetonitrile at a flow rate of 0.2 mL/min.
Quantitative Experiments of Free Radicals.
Determination of free •O2− concentration.
Yellow nitroblue tetrazolium (NBT, with the reduction potential of −0.05 V) was reduced by •O2− (with the reduction potential of −0.33 V) to blue formazan, and 1 mol NBT can react with 4 mol •O2. We quantified the concentration of generated •O2− in the suspension by recording the residual concentration of NBT on a UV-vis spectrophotometer (maximum absorbance at 259 nm).
Determination of free •OH concentration.
Free •OH were determined via PL according to the reaction of terephthalic acid with hydroxyl radicals that produces 2-OH-terephthalic acid with a fluorescence peak located at 426 nm after excitation at 312 nm. Measurement of fluorescence intensity was performed on a FluoroMax-P spectrophotometer.
Computational Details.
We carried out all the DFT calculations in the Vienna ab initio simulation (VASP5.4.4) code. The exchange–correlation is simulated with PBE functional, and the ion–electron interactions were described by the PAW method. The vdWs interaction was included by using the empirical DFT-D3 method. The α-Fe2O3 (110) and 7% Cu–Fe2O3 (110) surface was used to catalyze the reduction of H2O2. Atoms in the upper two layers of the surface are allowed to move freely while the bottom two layers are fixed to simulate the surface of the structure. The Monkhorst–Pack grid mesh–based Brillouin zone k-points are set as 2 × 2 × 1 for all periodic structure with the cutoff energy of 450 eV. The convergence criteria are set as 0.01 eV A−1 and 10−5 eV in force and energy, respectively. The free energy calculation of species adsorption (ΔG) is based on the following model: ΔG = ΔE + ΔEZPE + ΔH0→T – TΔS. Herein, ΔE, ΔEZPE, and ΔS, respectively, represent the changes of electronic energy, zero-point energy, and change in entropy. The ΔH0→T refers to the change in enthalpy when heating from 0 K to T K.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
This work is financially supported by the Ministry of Science and Technology of People’s Republic of China as a key R&D project (grant no. 2019YFC1804104), the National Natural Science Foundation of China as a Shandong Joint Fund Project (grant no. U1906222) and the Tianjin Science and Technology Bureau as a key science and technology supporting project (grant no. S19ZC60133).
Author contributions
H.Z. and Q.Z. designed research; H.Z. and R.Z. performed research; R.Z. contributed new reagents/analytic tools; H.Z., R.Z., and P.W. analyzed data; and H.Z., P.W., and Q.Z. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Zhou Q., et al. , Generating dual active species by triple-atom-sites through peroxymonosulfate activation for treating micropollutants in complex water. Proc. Natl. Acad. Sci. U.S.A. 120, e2300085120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodges B. C., Cates E. L., Kim J. H., Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 13, 642–650 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Zhu L., et al. , Designing 3D-MoS2 sponge as excellent cocatalysts in advanced oxidation processes for pollutant control. Angew. Chem. Int. Ed. Engl. 59, 13968–13976 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Xu J., et al. , Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 4, 233–241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller C. J., et al. , Kinetic analysis of H2O2 activation by an iron(III) complex in water reveals a nonhomolytic generation pathway to an iron(IV)oxo complex. ACS Catal. 11, 787–799 (2021). [Google Scholar]
- 6.Su L., et al. , Regulating local electron density of iron single sites by introducing nitrogen vacancies for efficient photo-Fenton process. Angew. Chem. Int. Ed. Engl. 60, 21261–21266 (2021). [DOI] [PubMed] [Google Scholar]
- 7.Zhan S., et al. , Efficient Fenton-like process for pollutant removal in eectron-rich/poor reaction sites induced by surface oxygen vacancy over cobalt–zinc oxides. Environ. Sci. Technol. 54, 8333–8343 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Nie X., et al. , Highly efficient adsorption and catalytic degradation of ciprofloxacin by a novel heterogeneous Fenton catalyst of hexapod-like pyrite nanosheets mineral clusters. Appl. Catal. B 300, 120734 (2022). [Google Scholar]
- 9.Zong M., et al. , Facet-dependent photodegradation of methylene blue by hematite nanoplates in visible light. Environ. Sci. Technol. 55, 677–688 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Bykova E., et al. , Structural complexity of simple Fe2O3 at high pressures and temperatures. Nat. Commun. 7, 10661 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian M., et al. , Electronic structure, optical properties, and photoelectrochemical activity of Sn-doped Fe2O3 thin films. J. Phys. Chem. C 124, 12548–12558 (2020). [Google Scholar]
- 12.Zhou L., et al. , Highly efficient photo-Fenton degradation of methyl orange facilitated by slow light effect and hierarchical porous structure of Fe2O3-SiO2 photonic crystals. Appl. Catal. B 237, 1160–1167 (2018). [Google Scholar]
- 13.Cheaib K., et al. , Selective formation of an FeIVO or an FeIIIOOH intermediate from iron(II) and H2O2: Controlled heterolytic versus homolytic oxygen–oxygen bond cleavage by the second coordination sphere. Angew. Chem. Int. Ed. Engl. 58, 854–858 (2019). [DOI] [PubMed] [Google Scholar]
- 14.He J., Yang X., Men B., Wang D., Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 39, 97–109 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Plauck A., Stangland E. E., Dumesic J. A., Mavrikakis M., Active sites and mechanisms for H2O2 decomposition over Pd catalysts. Proc. Natl. Acad. Sci. U.S.A. 113, E1973–E1982 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W., et al. , Visible light-induced marine bacterial inactivation in seawater by an in situ photo-Fenton system without additional oxidants: Implications for ballast water sterilization. ACS EST Water 1, 1483–1494 (2021). [Google Scholar]
- 17.Fu H., et al. , Axial coordination tuning Fe single-atom catalysts for boosting H2O2 activation. Appl. Catal. B 321, 122012 (2023). [Google Scholar]
- 18.Ling C., et al. , Atomic-layered Cu5 nanoclusters on FeS2 with dual catalytic sites for efficient and selective H2O2 activation. Angew. Chem. Int. Ed. Engl. 61, e202200670 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Yang X., Xu X., Xu J., Han Y., Iron oxychloride (FeOCl): An efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 135, 16058–16061 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Kim M., et al. , Metal-organic framework-derived ZrO2 on N/S-doped porous carbons for mechanistic and kinetic inspection of catalytic H2O2 homolysis. Carbon 203, 630–649 (2023). [Google Scholar]
- 21.Xing Y., et al. , Fe/Fe3C boosts H2O2 utilization for methane conversion overwhelming O2 generation. Angew. Chem. Int. Ed. Engl. 60, 8889–8895 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Zhao B., Huang X., Ding Y., Bi Y., Bias-free solar-driven syngas production: A Fe2O3 photoanode featuring single-atom cobalt integrated with a silver-palladium cathode. Angew. Chem. Int. Ed. Engl. 62, e202213067 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Jin H., et al. , Oxygen vacancy promoted heterogeneous Fenton-like degradation of ofloxacin at pH 3.2–9.0 by Cu substituted magnetic Fe3O4@FeOOH nanocomposite. Environ. Sci. Technol. 51, 12699–12706 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Song G., et al. , High conversion to aromatics via CO2-FT over a CO-reduced Cu-Fe2O3 catalyst integrated with HZSM-5. ACS Catal. 10, 11268–11279 (2020). [Google Scholar]
- 25.Dong Q., et al. , Activating Cu/Fe2O3 nanoislands rooted on N-rich porous carbon nanosheets via the Mott-Schottky effect for rechargeable Zn-air battery. Chem. Eng. J. 442, 136128 (2022). [Google Scholar]
- 26.Wang P., et al. , Atomic insights for optimum and excess doping in photocatalysis: A case study of few-layer Cu-ZnIn2S4. Adv. Funct. Mater. 29, 1807013 (2019). [Google Scholar]
- 27.Liu P., Hensen E. J. M., Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 135, 14032–14035 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Zhan H., et al. , Photocatalytic O2 activation and reactive oxygen species evolution by surface B-N bond for organic pollutants degradation. Appl. Catal. B 310, 121329 (2022). [Google Scholar]
- 29.Sun G., et al. , Bulk-to-nano regulation of layered metal oxide gears H2O2 activation pathway for its stoichiometric utilization in selective oxidation reaction. Appl. Catal. B 313, 121461 (2022). [Google Scholar]
- 30.Wang Y., Han X., Liu Y., Removal of carbon monoxide from simulated flue gas using two new Fenton systems: Mechanism and kinetics. Environ. Sci. Technol. 53, 10387–10397 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Qin Y., et al. , Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: A critical review. Water Res. 137, 130–143 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q., Ma S., Zhan S., Superior photocatalytic disinfection effect of Ag-3D ordered mesoporous CeO2 under visible light. Appl. Catal. B 224, 27–37 (2018). [Google Scholar]
- 33.Zhou Z., et al. , Boosting the activation of molecular oxygen and the degradation of tetracycline over high loading Ag single atomic catalyst. Water Res. 201, 117314 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Xie L., et al. , Pauling-type adsorption of O2 induced electrocatalytic singlet oxygen production on N-CuO for organic pollutants degradation. Nat. Commun. 13, 5560 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Z., et al. , Amorphous nickel oxides supported on carbon nanosheets as high-performance catalysts for electrochemical synthesis of hydrogen peroxide. ACS Catal. 12, 5911–5920 (2022). [Google Scholar]
- 36.Nosaka Y., Nosaka A. Y., Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Wang H., et al. , Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 332, 572–581 (2018). [Google Scholar]
- 38.Ho R. Y. N., Roelfes G., Feringa B. L., Que L., Raman evidence for a weakened O−O bond in mononuclear low-spin iron(III)−hydroperoxides. J. Am. Chem. Soc. 121, 264–265 (1999). [Google Scholar]
- 39.Das B., et al. , Self pH regulated iron(II) catalyst for radical free oxidation of benzyl alcohols. App. Catal. A 589, 117292 (2020). [Google Scholar]
- 40.Xu X., et al. , Revealing *OOH key intermediates and regulating H2O2 photoactivation by surface relaxation of Fenton-like catalysts. Proc. Natl. Acad. Sci. U.S.A. 119, e2205562119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H. Q., et al. , Unmasking the critical role of the ordering degree of bimetallic nanocatalysts on oxygen reduction reaction by in situ raman spectroscopy. Angew. Chem. Int. Ed. Engl. 61, e202117834 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Zhang H., et al. , Different reaction mechanisms of SO4•− and •OH with organic compound interpreted at molecular orbital level in Co(II)/peroxymonosulfate catalytic activation system. Water Res. 229, 119392 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Li W., et al. , Highly efficient activation of peroxymonosulfate by cobalt sulfide hollow nanospheres for fast ciprofloxacin degradation. J. Hazard. Mater. 389, 121856 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Chen F., et al. , Catalytic degradation of ciprofloxacin by a visible-light-assisted peroxymonosulfate activation system: Performance and mechanism. Water Res. 173, 115559 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.