Abstract
Introduction
Patients with systemic lupus erythematous were vulnerable to severe coronavirus disease 2019 infection and the negative impact of disrupted healthcare delivery. Telemedicine has been a popular alternative to standard in-person care during the pandemic despite the lack of evidence.
Methods
This was a 1-year pragmatic randomized-controlled trial. Patients followed at the lupus nephritis clinic were randomized to either telemedicine or standard follow-up in a 1:1 ratio. Patients in the telemedicine group were followed up via videoconferencing. Standard follow-up group patients continued conventional in-person outpatient care. The primary outcome of the study was the proportion of patients in low disease activity after 1 year. Secondary outcomes included cost-of-illness, safety, and various patient-reported outcomes.
Results
From 6/2020 to 12/2021, 144 patients were randomized and 141 patients (telemedicine: 70, standard follow-up: 71) completed the study. At 1 year, 80.0% and 80.2% of the patients in the telemedicine group and standard follow-up group were in lupus low disease activity state or complete remission, respectively (p = 0.967). Systemic lupus erythematous disease activity indices, number of flares and frequency of follow-ups were also similar. There were no differences in the cost-of-illness, quality of life or mental health scores. However, significantly more patients in the telemedicine group (41.4% vs 5.6%; p < 0.001) required switch of mode of follow-up and higher proportion of them had hospitalization during the study period (32.9% vs 15.5%; p = 0.016). Being in the telemedicine group or not in low disease activity at baseline were the independent predictors of hospitalization (odds ratio: 2.6; 95% confidence interval: 1.1–6.1, odds ratio: 2.7, 95% confidence interval: 1.1–6.7, respectively) in the post hoc analysis.
Conclusions
In patients with systemic lupus erythematous, telemedicine predominant follow-up resulted in similar 1-year disease control compared to standard care. However, it needed to be complemented by in-person visits, especially in patients with unstable disease.
Keywords: Coronavirus disease 2019, systemic lupus erythematosus, telehealth, telemedicine
Introduction
Patients with systemic lupus erythematosus (SLE) are at increased risk of severe coronavirus disease 2019 (COVID-19) infection due to the underlying immune dysregulation, comorbidities, and use of immunosuppressive medications.1,2 During the outbreak, these vulnerable patients faced the difficult choice between COVID-19 infection risk during a clinic visit and postponing the needed care. The management of their disease might also be affected by the disruption in healthcare delivery. An apparent alternative would be to adopt telemedicine (TM) or telehealth, the use of telecommunication technologies to provide medical information and services, to maintain medical care while minimizing exposure.3,4
Despite being widely implemented during the pandemic, evidence supporting the use of TM in rheumatology has been limited. 5 A systematic review in 2017 concluded that there was no good evidence supporting the use of TM for managing autoimmune rheumatic diseases due to the high risk of bias of the published studies. 6 In a subsequent randomized controlled trial (RCT) in 2018, TM follow-up (FU) could achieve similar disease control as conventional care in rheumatoid arthritis patients with low disease activity or in remission. 7 Two studies conducted during COVID-19 outbreak reported favorable acceptance of TM as the mode of care in patients with connective tissue diseases.8,9 However, there is no RCT-evaluating TM FU in patients with SLE.
We hypothesize that TM is an effective and safe mode of healthcare delivery for maintaining stable SLE disease control during the COVID-19 pandemic. Thus, we conducted a one-year RCT comparing Telemedicine and standard in-person FU in patients with SLE (Tele-SLE). We primarily aimed to evaluate the effectiveness of TM delivered care in maintaining disease activity control compared to conventional in-person outpatient FU in patients with SLE via a pragmatic trial. The secondary objectives were to compare the patient-reported outcomes (PROs), cost-of-illness, and safety between the two modes of healthcare delivery. The 6-month results of the study focusing on patient satisfaction has been previously reported. 10
Patients and methods
Study design and patients
This was a 1-year, single-center, open-label, pragmatic RCT conducted at a regional hospital in Hong Kong. From May to December 2020, consecutive patients ≥ 18-year old with SLE according to the 2019 EULAR/ACR classification criteria followed up at the lupus nephritis clinic were invited by the investigators to participate in the study. 11 Patients or their carers needed to possess the technology for conducting a TM consultation (a smartphone, tablet or computer with audio and video capabilities and internet connection). Patients were excluded if they were pregnant, incapable of answering a questionnaire, or unwilling to attend blood and urine tests. Participants were randomized 1:1 to either TM (TM group) or standard FU (SF group) using a computer-generated random number sequence. Baseline clinical characteristics were collected before randomization and allocation concealment was ensured by the use of sequentially numbered, opaque, sealed envelopes. The study was approved by the local ethical committee and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
Interventions
Patients randomized to receive TM FU were scheduled for a real-time video consultation via video-conferencing software ZOOM (Zoom Video Communications Inc, California, US). Patients in the SF group received standard in-person outpatient care. In both groups, medication titration was based on a shared decision between the patients and treating clinicians aiming at achieving complete remission or Lupus Low Disease Activity State (LLDAS) according to the international management guideline. 12 Complete remission was defined as absence of clinical activity with no use of systemic glucocorticoids (GC), and immunosuppressants (IS); and LLDAS as systemic lupus erythematosus disease activity index 2000 (SLEDAI-2k) ≤ 4, no activity in any major organ, no new disease activity features, physician global assessment (PGA) ≤ 1 with GC ≤7.5 mg of prednisone daily and well tolerated IS.13,14 In-person clinic consultations could be arranged as requested by the clinicians or patients in the TM group. Similarly, TM consultations could be arranged as required in the SF group. The frequency of visits was based on clinical judgements, as well as mutual decisions of the attending clinicians and patients. Prior to each consultation, all patients needed to have pre-ordered blood (complete blood count, liver and renal function tests, c3, c4 and anti-dsDNA) and 24-hour urine total protein checked. Blood pressure and body weight measurements were done prior to each consultation at the clinic for the SF group or at home for the TM group. All consultations, either virtual or physical, were performed by clinicians with more than 5 years of experience (HS, CCS, and LST). Both the rheumatologists and patients had no previous experience with virtual consultations. Prescribed medications could be collected in person by the patients or their designated representatives at the hospital or community pharmacies.
Study outcomes
SLE disease activity at each consultation was assessed by SLEDAI-2k and PGA. Disease flares were captured with the SELENA flare index. 15 All participants were asked to complete the LupusQoL, Health Assessment Questionnaire (HAQ) and Hospital Anxiety and Depression Scale (HADS). LupusQoL is a disease-targeted PRO measure that is developed for SLE patients. HAQ covers various common daily activities to assess disability. HADS was developed to assess anxiety and depression in medical patients. The Chinese versions of the above tools have all been studied in patients with rheumatic diseases.16–18 The participants were also asked to complete a questionnaire on employment outcomes and out-of-pocket expenses at the end of the study. 19 Both direct and indirect costs of illness were assessed. Direct costs collected consisted of all costs of private hospital/clinic facilities (including costs of visits, medications, investigations, and hospitalizations) as public health care services were largely free-of-charge; and patients’ out-of-pocket expenses for health products, non-traditional therapies (hydrotherapy, acupuncture, and massage), aid devices, transportation fee to the health care providers, private household helper, and adaptation to houses. Indirect costs represented the productivity loss due to SLE, which included sick leave, unemployment, and days off from household work or daily activities. Participants were asked to rate post-consultation satisfaction. The responses were assigned a value of 0–4 (strongly disagree to strongly agree), with a higher score indicating that the respondent was satisfied with the FU and a 2 indicating a neutral response (Supplemental Figure 1).
The primary endpoint of the study was the percentage of patients in complete remission or LLDAS at the end of the study. Secondary endpoints included: (a) the lupusQoL, HAQ and HADS scores; (b) direct and indirect costs of illness; (c) number of FU visits and hospitalization during the study period; (d) incidence of COVID-19 infection in one year; and (e) patient overall satisfaction score.
Statistical analysis
To test for equality, considering a difference less than 20% was of no clinical importance, and the percentage of patients in remission or LLDAS was 65% after one year in both groups, the required sample size in each group to achieve an 80% power at 95% confidence was 70.
For each group, baseline demographic and clinical characteristics were reported as mean values with 95%CIs for continuous variables and as numbers and percentages for categorical variables. The groups were be compared by chi-square test and student t-test where appropriate. We used an intention-to-treat approach to analyze the outcomes of the randomized patients. The outcomes in the TM and SF groups were compared by chi-squared test or Fisher's exact test and Student's t-test where appropriate. Within-group changes from baseline to the last FU were analyzed by paired Student's t-test. Multivariate regression models were used to adjust for the baseline differences between the two groups if any. Results with p-value of less than 0.05 were regarded as significant. Statistical analyses were performed using the Statistics Package for Social Sciences (IBM SPSS Statistics Version 26 [IBM, Armonk, New York, USA]).
Results
From June 2020, a total of 144 patients with SLE were randomized (TM: 72, SF: 72) and 3 patients self-withdrew from the study. At the end of the study (December 2021), 70 patients in the TM group and 71 patients in the SF completed one-year FU (Figure 1). All patients were Chinese. The mean age was 44.5 ± 11.4 years and there was a female predominance of 90.8%. The median time from initial SLE diagnosis to randomization was 168 (range: 1–528) months. The majority of the patients had biopsy-proven class III, IV or V lupus nephritis (87.2%) and were on prednisolone (89.4%) with a median daily dose of 5 (range: 0–35) mg. A significant proportion of the patients (73.8%) were on antimalarials. Many of them (68.1%) were on additional IS with the commonest being mycophenolate mofetil (43.2%). At the beginning of the study, while 66.0% of the patients were in LLDAS, none were in complete disease remission. There were no baseline differences, including demographics, SLEDAI-2k (TM: 3.8 ± 2.3, SF: 3.2 ± 2.2, p = 0.13), PGA (TM: 0.62 ± 0.65, SF: 0.46 ± 0.59, p = 0.13) and SLE damage index (TM: 1.1 ± 1.3, SF: 0.8 ± 1.1, p = 0.10), between the two groups (Table 1).
Figure 1.
Trial profile. TM: telemedicine; SF: standard follow-up.
Table 1.
Baseline demographic and clinical characteristics of the patients in the intention-to-treat population.
| All participants (N = 141) | Standard group (N = 71) | Telemedicine group (N = 70) | |
|---|---|---|---|
| Age, years | 44.5 ± 11.4 | 44.5 ± 11.3 | 44.4 + 11.6 |
| Female sex | 128 (90.8) | 64 (90.1) | 64 (91.4) |
| Chinese | 141 (100) | 71 (100) | 70 (100) |
| Median time from diagnosis to randomization, months | 168 (range: 1–528) | 144 (range: 1–432) | 198 (range: 6–528) |
| Biopsy-proven LN class III/IV/V | 123 (87.2%) | 60 (84.5) | 63 (90.0) |
| SLEDAI-2k | 3.5 ± 2.3 | 3.2 ± 2.2 | 3.8 ± 2.3 |
| PGA score | 0.54 ± 0.62 | 0.46 ± 0.59 | 0.62 ± 0.65 |
| SLE damage index | 1.0 ± 1.2 | 0.8 ± 1.1 | 1.1 ± 1.3 |
| LLDAS | 93 (66.0) | 49 (69.0) | 44 (62.9) |
| Complete remission | 0 (0) | 0 (0) | 0 (0) |
| Prednisolone use | 126 (89.4) | 60 (84.5) | 66 (94.3) |
| Median prednisolone dosage, mg/day | 5.0 (range: 0–35.0) | 5.0 (range: 0–35.0) | 5.0 (range: 0–35.0) |
| Immunosuppressive agent use | 97 (68.1) | 48 (67.6) | 49 (70.0) |
| Antimalarial use | 104 (73.8) | 55 (77.5) | 49 (70.0) |
Data are reported as mean ± SD or number (%) unless stated otherwise. SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; SLE: systemic lupus erythematous; PGA: physician global assessment; LLDAS: lupus low disease activity state; SDI: Systemic Lupus International Collaborating Clinics/American College of Rheumatology (SLICC/ACR) Damage Index.
The results with respect to the primary and major secondary outcomes are provided in Table 2. At one year, 80.0% and 80.2% of the patients in the TM group and SF group were in LLDAS or complete remission, respectively (p = 0.967). SLE disease activity indices including SLEDAI-2k, PGA, proteinuria amount and serum anti-ds-DNA level remained similar between the two groups. There was also no difference in the mean daily prednisolone dose (TM: 4.82 ± 2.83 mg, SF: 4.55 ± 3.16 mg, p = 0.601) at the end of the study comparing the two groups. Within the study period, 28 (40%) patients in the TM group and 21 (29.6%) patients in the SF group had disease flare (p = 0.20). There were no differences in the lupusQoL and HADS scores between the two groups at the end of the study. A slightly higher HAQ score was noted in the TM group (TM: 0.28 ± 0.49, SF: 0.14 ± 0.31, p = 0.035). The PGA significantly improved in the TM group (baseline: 0.62 ± 0.65, end of FU: 0.40 ± 0.50, p = 0.014), whereas the HAQ score dropped in the SF group (baseline: 0.20 ± 0.44, end of FU: 0.14 ± 0.31, p = 0.049). Otherwise, there was no other significant change in the disease activity or PRO parameters within the two groups from baseline to the end of the study. There was also no significant change in the mean daily prednisolone dose in both groups.
Table 2.
Primary and key secondary outcomes in the intention-to-treat population.
| Standard group (N = 71) | Telemedicine group (N = 70) | P-value | |
|---|---|---|---|
| Primary endpoint | |||
| Proportion of patients in complete remission or LLDAS | 80.0% | 80.2% | 0.967 |
| Proportion of patients in complete remission | 2.8% | 0% | 0.496 |
| Key secondary outcomes | |||
| SLEDAI-2k | 2.8 ± 2.6 | 3.3 ± 2.3 | 0.243 |
| PGA | 0.36 ± 0.46 | 0.40 ± 0.50 | 0.639 |
| Proteinuria, g/day | 0.37 ± 0.46 | 0.44 ± 0.67 | 0.431 |
| Serum anti-ds-DNA | 174.5 ± 201.7 | 221.2 ± 227.0 | 0.199 |
| Prednisolone dosage, mg/day | 4.55 ± 3.16 | 4.82 ± 2.83 | 0.601 |
| Proportion of patients with flare | 29.6% | 40.0% | 0.200 |
| HADS | |||
| Anxiety scale | 5.8 ± 4.0 | 5.6 ± 4.3 | 0.770 |
| Depression scale | 5.3 ± 3.9 | 5.3 ± 4.2 | 0.989 |
| Lupus QoL score for Physical health Pain Planning Intimate relationship Burden to others Emotional health Body image Fatigue |
82.4 ± 18.2 83.1 ± 20.1 86.5 ± 18.2 76.1 ± 28.9 79.7 ± 19.0 81.8 ± 17.3 79.5 ± 23.8 77.1 ± 20.4 |
77.6 ± 21.3 80.9 ± 17.9 82.7 ± 21.3 74.0 ± 25.4 73.6 ± 22.9 81.5 ± 18.9 79.6 ± 21.4 75.1 ± 20.7 |
0.160 0.500 0.261 0.727 0.086 0.910 0.993 0.562 |
| HAQ | 0.14 ± 0.31 | 0.28 ± 0.49 | 0.035 |
| Overall patient satisfaction | 3.2 ± 0.8 | 3.4 ± 0.6 | 0.030 |
| Mean indirect costs of illness, HKD | 26,681 | 12,016 | 0.200 |
| Out-of-pocket costs for health care services, HKD | 12,297 | 13,547 | 0.830 |
| Total number of FU (physical or TM) | 5.7 ± 1.7 | 6.0 ± 2.0 | 0.400 |
| Proportion of patients with switch of FU mode | 5.6% | 41.4% | <0.001 |
| Proportion of patients requiring hospitalization | 15.5% | 32.9% | 0.016 |
| Incidence of COVID-19 | 0% | 0% | n/a |
Data are reported as mean ± SD or number (%). LLDAS: lupus low disease activity state; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000; PGA: physician global assessment; HADS: Hospital Anxiety and Depression Scale; HAQ: Health Assessment Questionnaire Disability Index; FU: follow-up.
Although the mean indirect costs of illness were numerically higher in the SF group (HKD26,681 vs HKD12,016, p = 0.20), the out-of-pocket costs for health care services other than those provided by the public sector were similar between the two groups (TM: HKD13,547 vs SF: HKD12,297, p = 0.83) in the study period. The total number of FU (physical or TM) was similar in the two groups (TM: 6.0 ± 2.0, SF: 5.7 ± 1.7, p = 0.40). However, significantly more patients in the TM group (29/70, 41.4% vs 4/71, 5.6%; p < 0.001) required a switch of mode of FU (reasons listed in Supplemental Table 1); and 18 (24.7%) patients in the TM group were followed-up physically in the last consultation. The proportion of patients requiring hospitalization during the study period was also higher in the TM group (TM: 23/70, 32.9% vs 11/71, 15.5%; p = 0.016) (causes listed in Supplemental Table 2). In the post hoc analysis, patients who were hospitalized were more likely to be in the TM group, had more active disease with higher damage scores, and tended to be on higher dose of GC (Table 3). After adjusting for damage score, prednisolone dosage and age, being in the TM group (OR 2.6, 95% CI 1.1–6.1) or not being in LLDAS at baseline (OR 2.8, 95%CI 1.2–9.7) were the independent predictors of hospitalization (dependent variable) in the multivariate logistic regression model. None of the participants had COVID-19 infection throughout the study.
Table 3.
Baseline characteristics of the patients with and without hospitalization in one year.
| Patients without hospitalization (N = 107) | Patients with hospitalization (N = 34) | P-value | |
|---|---|---|---|
| Age, years | 44.0 ± 11.4 | 46.1 ± 11.3 | 0.345 |
| Female sex | 95 (88.8) | 33 (97.1) | 0.189 |
| Time from diagnosis to randomization, months | 180 ± 107 | 180 ± 117 | 0.99 |
| Biopsy-proven LN class III/IV/V | 92 (86.0) | 31 (91.2) | 1.000 |
| SLE damage index | 0.8 ± 1.2 | 1.4 ± 1.3 | 0.029 |
| SLEDAI-2k | 3.2 ± 2.2 | 4.3 ± 2.3 | 0.014 |
| PGA score | 0.54 ± 0.49 | 0.91 ± 0.82 | 0.002 |
| Proteinuria, g/day | 0.36 ± 0.53 | 0.82 ± 0.82 | 0.004 |
| Serum anti-ds-DNA | 194 ± 197 | 219 ± 231 | 0.529 |
| Mean prednisolone dosage, mg/day | 4.8 ± 3.6 | 7.2 ± 7.6 | 0.083 |
| Immunosuppressive agent use | 69 (64.5) | 27 (79.4) | 0.139 |
| Randomized to TM group | 47 (43.9) | 23 (67.6) | 0.016 |
Data are reported as mean ± SD or number (%). LN: lupus nephritis; SLEDAI-2K: systemic lupus erythematosus disease activity index 2000; PGA: physician global assessment; TM: telemedicine.
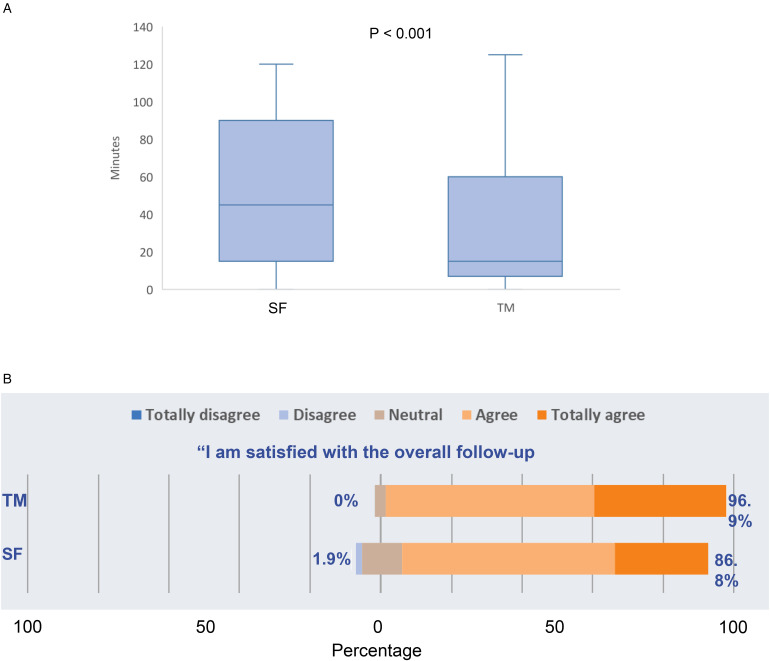
The waiting time from entering the clinic waiting room (virtual or real) to seeing the doctors was much shorter in the TM group (16.2 ± 22.8 vs 63.0 ± 35.6 min, p < 0.001) (Figure 2A). The overall patient satisfaction score about the FU process was significantly higher in the TM group (3.4 ± 0.6 vs 3.2 ± 0.8, p = 0.03) (Figure 2B). At the end of the study, 67.9% of the overall participants agreed (versus 15.0% who did not agree) to use TM as a mode of future FU.
Figure 2.
(A) Mean waiting time between entering the clinic waiting room (virtual or real) and seeing a rheumatologist. Data for each group are presented as box plots: The line within boxes correspond to median; the range between the lower (Q1) and upper (Q3) bounds of the boxes is the IQR. Whiskers represent scores outside IQR and ends in maximum and minimum. Data were analyzed using Mann-Whitney-U test. (B) Satisfaction scores of patients who used telemedicine compared to standard follow-up. The response is shown as a percentage with positive responses on the right. The neutral category was removed when calculating percentages. TM, telemedicine, SF, standard follow-up; IQR: interquartile range.
Discussion
Due to obvious logistic advantages, remote care by TM has been widely used during the pandemic. A survey conducted in 35 European countries from May to June 2020 showed that the majority of face-to-face rheumatology consultations were converted into TM consultations. 20 Evidence supporting the efficacious, safe, and cost-effective use of TM in autoimmune rheumatic diseases is urgently needed. 21 Tele-SLE was the first RCT comparing TM versus standard in-person FU in patients with SLE. We found that in a pragmatic clinical setting, the disease activity control at one year in patients randomized to TM care was similar to those in the conventional physical consultation group. There were also no differences in quality of life, psychological outcomes, and patient-reported cost-of-illness between the two groups. However, patients in the TM group or with unstable baseline disease had an increased risk of hospitalization during the one-year study period.
The clinical care of patients with SLE has been heavily jeopardized by the COVID-19 pandemic. In a questionnaire-based study from the SMILE Lupus Cohort in Italy, about two-third of patients had missed appointments secondary to healthcare delivery disruption. 22 An online survey from the INSPIRE Lupus Cohort in India reported worsening of disease in 25% of patients, likely contributed by difficulty in scheduling hospital visits as a result of the lockdown restrictions. 23
We demonstrated in the current study that TM could be a feasible alternative to physical FU in patients with SLE. An observational study done in Singapore during the pandemic also showed the disease activity at the next visit and the corticosteroid dosages prescribed were similar between teleconsultations and physical FUs in SLE patients. 24 In a large American survey done in 2021 of over 6000 patients with autoimmune rheumatic diseases (15% with SLE), patients who had TM visits were at lower risk of stopping medications than patients who had neither an office nor a TM visit (OR 0.67, 95%CI: 0.51–0.88). 25
The fear of infection and interruption of medical care had negative repercussion on lupus patients’ mental as well as general health. A questionnaire-based study in 405 patients with SLE using Depression, Anxiety and Stress Scales (DASS-21) found that stress, anxiety, and depression were moderate to severe in 12.3%, 38.7%, and 27.7% of the respondents in May 2020. 26 In another study of 63 patients with SLE done during the pandemic, 47.5% and 48.3% of the questionnaire respondents reported an increase in anxiety and depression respectively, and over 40% scored worse in measures of pain interference, fatigue and cognitive abilities. 27 A French nation-wide survey with 536 SLE respondents revealed that the main reported difficulties during the pandemic were issues regarding access to medical care (25.4%), loss of employment (24.4%) and financial difficulties (11%). 28 The comparable psycho-social outcomes of SLE patients in the TM and SF groups shown in our study are reassuring that virtual care could address the major concerns of the patients during the pandemic without increasing costs.
It should however be noted that patients in the TM group had more hospitalizations and a significant proportion of them required conversion to in-person consultation during the study period. These could off-set the conceived benefits of TM. Although many of the hospitalizations did not appear to be related to SLE diseases, the major reasons for switching to physical visits were perceived unstable disease and occurrence of new symptoms. It may reflect the lack of confidence of either patients or clinicians in virtual assessment accuracy. In fact, a survey in 2021 revealed that 93% of the clinicians and 86% of the patients with autoimmune rheumatic diseases (32% with SLE) rated TM as worse than physical consultations in terms of assessment accuracy. 29 It was found in a recent prospective study in patients with inflammatory rheumatic diseases that the most significant treatment decision discordance between virtual and physical consultations was GC tapering in patients with SLE. 30 A retrospective study done during the pandemic also reported a higher likelihood of additional face-to-face appointment after TM consultation in patients with lupus in a rheumatology unit. 31 It appeared that lupus patients with unstable disease control could not be optimally managed by TM alone, as not being in LLDAS was a predictor of hospitalization in our study. During the COVID-19 outbreak or when the health facilities are overstretched, a hybrid mode of FU with TM complemented by in-person visits when necessary may be helpful. TM can also be integrated into the conventional system for managing patients with stable SLE to cope with the increasing disease prevalence and workforce limitations beyond the pandemic.32,33 Further research on validation of disease-specific PRO, adaptation of the physical examination to virtual consultations, and development of innovative technologies allowing remote monitoring of clinical conditions is encouraged. Appropriate training for healthcare providers is also vital to improve the efficacy and sustainability of telehealth.34,35
There are several limitations. First, the enrollment of patients accepting TM care only and the lack of blinding may introduce bias. In fact, a significant proportion of patients refused participating in the trial at screening, although we found no difference in the baseline clinical characteristics between the study subjects and those who refused TM (data not shown). About 10% of screened patients were excluded due to a lack of equipment. The issue of digital literacy potentially exacerbating the health care disparity should be addressed. 36 Second, the predominance of patients with nephritis may limit the generalizability of the results. Monitoring of proteinuria is the fundamental element of disease activity assessment in nephritis patients, whereas physical examination may be more essential in patients with mainly cutaneous and musculoskeletal manifestations. On the other hand, the lack of urinary sediment examination might lead to under-estimation of disease activity in the TM group. Third, as a significant proportion of the sampled patients already experienced one of the defining components of the study outcome at baseline, i.e., LLDAS, the power to detect a difference between the groups was reduced. The cross-sectional endpoints also might not represent the lupus disease control over time, and the more realistic definition of remission was published only after the commencement of the study. 37 Lastly, due to the low transmission rate in Hong Kong during the study period, the hypothesis of reducing the risk of COVID-19 infection by remote care could not be properly tested. Our results should be interpreted in the context of the local outbreak situation and anti-endemic measures which could affect the patient's preference for TM.
Conclusion
In the first trial comparing remote and in-person care during the COVID-19 pandemic, SLE patients in the TM group had similar 1-year disease activity control and better satisfaction versus those in the standard FU group. There were no significant differences in PROs and cost-of-illness. However, a significant proportion of patients cared for by TM required in-person visits or were hospitalized during the study period. The results of the study suggest that TM-delivered care could help minimize exposure to SARS-CoV-2, while maintaining disease control and psychosocial well-being during the pandemic, but should be supplemented by physical visits as required, particularly in SLE patients with unstable disease.
Supplemental Material
Supplemental material, sj-docx-1-jtt-10.1177_1357633X231181714 for Telemedicine for follow-up of systemic lupus erythematosus during the 2019 coronavirus pandemic: A pragmatic randomized controlled trial by Ho So, Evelyn Chow, Isaac T Cheng, Sze-Lok Lau, Tena K Li, Cheuk-Chun Szeto and Lai-Shan Tam in Journal of Telemedicine and Telecare
Acknowledgements
The authors would like to express our gratitude to all medical staff, research assistants and participating patients. We would also like to thank the University of Central Lancashire & East Lancashire Hospitals NHS Trust for granting us permission to use the LupusQoL questionnaire.
Footnotes
Authors’ contributions: HS, CCS, and LST designed the trial. HS, EC, ITC, SLL, and TKL collected the study data. HS, EC, ITC, SLL, and TKL performed the data analysis. HS, CCS, and LST wrote the manuscript. All authors critically reviewed the manuscript for important intellectual content.
Availability of data and material: Data are available upon request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: This study was approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee, No. 2020-0254.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Hong Kong College of Physicians Young Investigator Research Grant (grant number 2020).
Informed consent: Written informed consent was obtained from all study participants.
Trial registration number: NCT04368299
ORCID iD: Ho So https://orcid.org/0000-0001-7113-9390
Supplemental material: Supplemental material for this article is available online.
References
- 1.Cordtz R, Kristensen S, Dalgaard LPH, et al. Incidence of COVID-19 hospitalisation in patients with systemic lupus erythematosus: A nationwide cohort study from Denmark. J Clin Med 2021; 10: 3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugarte-Gil MF, Alarcón GS, Izadi Z, et al. Characteristics associated with poor COVID-19 outcomes in individuals with systemic lupus erythematosus: Data from the COVID-19 global rheumatology alliance. Ann Rheum Dis 2022; 81: 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis 2020; 79: 851–858. [DOI] [PubMed] [Google Scholar]
- 4.Mikuls TR, Johnson SR, Fraenkel L, et al. American college of rheumatology guidance for the management of rheumatic disease in adult patients during the COVID-19 pandemic: Version 1. Arthritis Rheumatol 2020; 72: 1241–1251. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed S, Grainger R, Santosa A, et al. APLAR recommendations on the practice of telemedicine in rheumatology. Int J Rheum Dis 2022; 25: 247–258. [DOI] [PubMed] [Google Scholar]
- 6.McDougall JA, Ferucci ED, Glover J, et al. Telerheumatology: A systematic review. Arthritis Care Res (Hoboken) 2017; 69: 1546–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Thurah A, Stengaard-Pedersen K, Axelsen M, et al. Tele-health followup strategy for tight control of disease activity in rheumatoid arthritis: Results of a randomized controlled trial. Arthritis Care Res (Hoboken) 2018; 70: 353–360. [DOI] [PubMed] [Google Scholar]
- 8.Cavagna L, Zanframundo G, Codullo V, et al. Telemedicine in rheumatology: A reliable approach beyond the pandemic. Rheumatology (Oxford) 2021; 60: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.So H, Szeto CC, Tam LS. Patient acceptance of using telemedicine for follow-up of lupus nephritis in the COVID-19 outbreak. Ann Rheum Dis 2021; 80: e97. [DOI] [PubMed] [Google Scholar]
- 10.So H, Chow E, Cheng IT, et al. Use of telemedicine for follow-up of lupus nephritis in the COVID-19 outbreak: The 6-month results of a randomized controlled trial. Lupus 2022; 31: 488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aringer M, Costenbader K, Daikh D, et al. 2019 European league against rheumatism/American college of rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019; 71: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 13.Franklyn K, Lau CS, Navarra SV, et al. Definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis 2016; 75: 1615–1621. [DOI] [PubMed] [Google Scholar]
- 14.Drenkard C, Villa AR, García-Padilla C, et al. Remission of systemic lupus erythematosus. Medicine-Baltimore 1996; 75: 88–98. [DOI] [PubMed] [Google Scholar]
- 15.Petri M, Buyon J, Kim M. Classification and definition of major flares in SLE clinical trials. Lupus 1999; 8: 685–691. [DOI] [PubMed] [Google Scholar]
- 16.Wang SL, Wu B, Leng L, et al. Validity of LupusQoL-China for the assessment of health related quality of life in Chinese patients with systemic lupus erythematosus. PLoS One 2013; 8: e63795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu H, Luan L, Yang K, et al. Psychometric validation of Chinese health assessment questionnaire for use in rheumatoid arthritis patients in China. Int J Rheum Dis 2017; 20: 1987–1992. [DOI] [PubMed] [Google Scholar]
- 18.Mok CC, Chan KL, Ho LY. Association of depressive/anxiety symptoms with quality of life and work ability in patients with systemic lupus erythematosus. Clin Exp Rheumatol 2016; 34: 389–395. [PubMed] [Google Scholar]
- 19.Zhu TY, Tam LS, Leung YY, et al. Socioeconomic burden of psoriatic arthritis in Hong Kong: Direct and indirect costs and the influence of disease pattern. J Rheumatol 2010; 37: 1214–1220. [DOI] [PubMed] [Google Scholar]
- 20.Dejaco C, Alunno A, Bijlsma JW, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: A survey among EULAR countries. Ann Rheum Dis 2021; 80: 518–526. [DOI] [PubMed] [Google Scholar]
- 21.de Thurah A, Bosch P, Marques A, et al. 2022 EULAR points to consider for remote care in rheumatic and musculoskeletal diseases. Ann Rheum Dis 2022; 81: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez GA, Argolini LM, Bellocchi C, et al. Impact of the COVID-19 pandemic in patients with systemic lupus erythematosus throughout one year. Clin Immunol 2021; 231: 108845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathi M, Singh P, Bi HP, et al. Impact of the COVID-19 pandemic on patients with systemic lupus erythematosus: Observations from an Indian inception cohort. Lupus 2021; 30: 158–164. [DOI] [PubMed] [Google Scholar]
- 24.Au Eong JTW, Lateef A, Liang S, et al. Impact of teleconsultation on subsequent disease activity and flares in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2022; 61: 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George MD, Baker JF, Banerjee S, et al. Social distancing, health care disruptions, telemedicine use, and treatment interruption during the COVID-19 pandemic in patients with or without autoimmune rheumatic disease. ACR Open Rheumatology 2021; 3: 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tee CA, Salido EO, Reyes PWC, et al. Psychological state and associated factors during the 2019 coronavirus disease (COVID-19) pandemic among Filipinos with rheumatoid arthritis or systemic lupus erythematosus. Open Access Rheumatol 2020; 12: 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasturi S, Price LL, Paushkin V, et al. Impact of the first wave of the COVID-19 pandemic on systemic lupus erythematosus patients: Results from a multi-center prospective cohort. Lupus 2021; 30: 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scherlinger M, Zein N, Gottenberg J-E, et al. Difficulties and psychological impact of the SARS-CoV-2 pandemic in patients with systemic lupus erythematosus: A nationwide patient association study. Healthcare 2022; 10: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloan M, Lever E, Harwood R, et al. Telemedicine in rheumatology: A mixed methods study exploring acceptability, preferences and experiences among patients and clinicians. Rheumatology (Oxford) 2022; 61: 2262–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piga M, Floris A, Congia M, et al. Telemedicine in rheumatology: High specificity and sensitivity of follow-up virtual video consultations during COVID-19 pandemic. Rheumatology 2021; 61: 1795–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu W, De Silva T, Eades L, et al. The impact of telerheumatology and COVID-19 on outcomes in a tertiary rheumatology service: A retrospective audit. Rheumatology 2021; 60: 3478–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte-García A, Hocaoglu M, Valenzuela-Almada M, et al. Rising incidence and prevalence of systemic lupus erythematosus: A population-based study over four decades. Ann Rheum Dis 2022; 81: 1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger J, Putrik P, Buttgereit F, et al. Workforce requirements in rheumatology: A systematic literature review informing the development of a workforce prediction risk of bias tool and the EULAR points to consider. RMD Open 2018; 4: e000756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith AC, Thomas E, Snoswell CL, et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare 2020; 26: 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas EE, Haydon HM, Mehrotra A, et al. Building on the momentum: Sustaining telehealth beyond COVID-19. J Telemed Telecare 2022; 28: 301–308. [DOI] [PubMed] [Google Scholar]
- 36.Gallegos-Rejas VM, Thomas EE, Kelly JT, et al. A multi-stakeholder approach is needed to reduce the digital divide and encourage equitable access to telehealth. J Telemed Telecare 2023; 29: 73–78. [DOI] [PubMed] [Google Scholar]
- 37.van Vollenhoven RF, Bertsias G, Doria A, et al. 2021 DORIS definition of remission in SLE: Final recommendations from an international task force. Lupus Sci Med 2021; 8: e000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jtt-10.1177_1357633X231181714 for Telemedicine for follow-up of systemic lupus erythematosus during the 2019 coronavirus pandemic: A pragmatic randomized controlled trial by Ho So, Evelyn Chow, Isaac T Cheng, Sze-Lok Lau, Tena K Li, Cheuk-Chun Szeto and Lai-Shan Tam in Journal of Telemedicine and Telecare