Abstract
A 47-year-old man presented with visual loss in the right eye 8 h after the first dose of a coronavirus disease 2019 (COVID-19) vaccine developed by Pfizer/BioNTech (BNT162b2). The best corrected visual acuity (BCVA) was 20/200. Fundus examination showed dilated and tortuous retinal veins at the posterior pole, retinal hemorrhages throughout the fundus, and macular edema. Fluorescein angiography showed multiple hypofluorescent spots that appeared to be fluorescent block due to retinal hemorrhages and hyperfluorescent leakage from the retinal veins. The eye was diagnosed with central retinal vein occlusion (CRVO). For the treatment of macular edema, intravitreal injection of aflibercept (IVA) was administered and treated with one plus pro re nata regimen. Five IVAs were performed over a 10-month follow-up period, with resolution of macular edema, and the BCVA recovered to 20/20. The patient was young and had no history of diabetes mellitus, hypertension, or atherosclerotic diseases, and his blood tests showed no abnormal findings. Both antigen test and polymerase chain reaction test for COVID-19 were negative, and the antibody test was positive due to vaccination. The development of CRVO in this patient may have been related to COVID-19 vaccination, and the appropriate IVA treatment resulted in a good visual prognosis.
Keywords: Central retinal vein occlusion, Vaccine, COVID-19, mRNA
Introduction
Since late 2019, the coronavirus disease 2019 (COVID-19) has caused widespread mortality globally and is known to cause thromboembolic complications [1]. Although microangiopathic retinal changes have been reported in patients with COVID-19, it is unclear whether they are associated with viral infection or prolonged hypoxemia [2, 3]. Reports have shown the development of retinal vein occlusion (RVO) in patients with COVID-19 [3–7], which is postulated to occur due to hypercoagulability, inflammation, and microvascular alterations [8]. Furthermore, RVO cases after COVID-19 vaccination have been reported [8–10]. Here, we report a case of central RVO (CRVO) in a healthy young patient immediately after COVID-19 vaccination with an mRNA-based Pfizer/BioNTech vaccine, BNT162b2.
Case Report
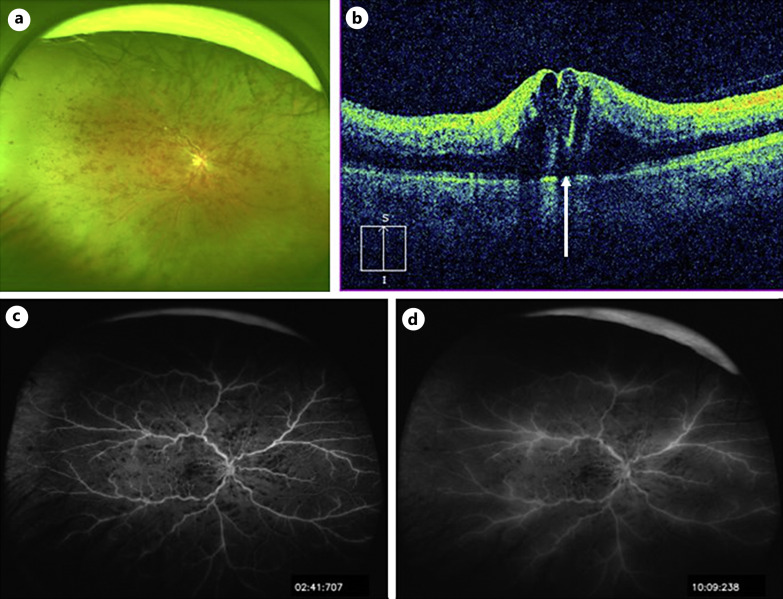
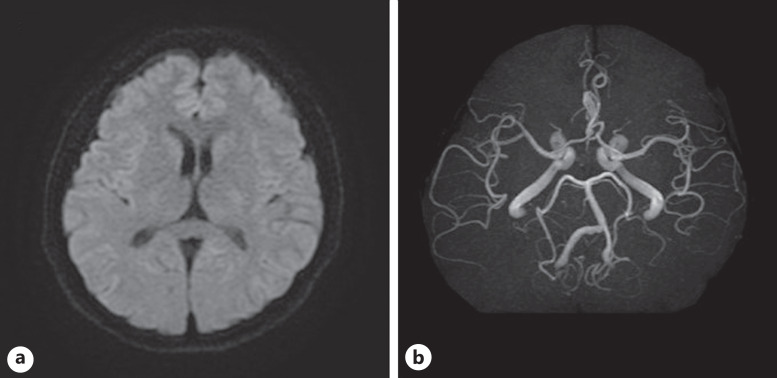
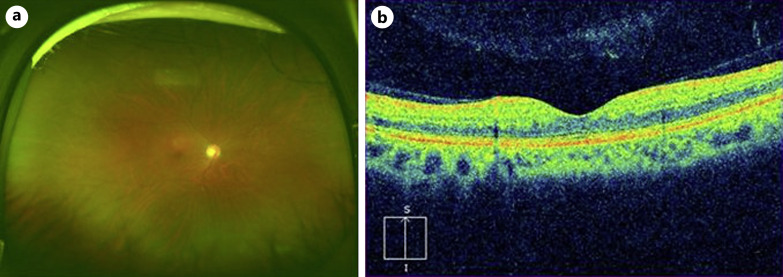
A 47-year-old man had visual impairment in his right eye 8 h after a first dose of COVID-19 vaccination (BNT162b2). Two days later, the patient visited a nearby clinic, where he was diagnosed with CRVO in the right eye and referred to our hospital 4 days after the onset. The best corrected visual acuity (BCVA) in the right eye was 20/200. Slit lamp examination revealed no abnormalities in the anterior segments and clear ocular media, but the fundus examination showed retinal hemorrhages throughout the fundus, dilated and tortuous retinal veins, and macular edema (Fig. 1a, b). Optical coherence tomography (OCT) showed cystoid macular edema and small serous retinal detachment (Fig. 1b). Fluorescein angiograms showed multiple hypofluorescent spots that appeared to be fluorescent block due to retinal hemorrhages and hyperfluorescent leakage from the retinal veins (Fig. 1c, d). The eye was diagnosed with non-ischemic CRVO. The patient had no history of diabetes mellitus, hypertension, or atherosclerotic diseases. The results of laboratory tests are shown in Table 1. The triglyceride level was high at 256 mg/dL, the activated partial thromboplastin time was mildly shortened (30.6 s), and protein C was higher than normal (177%), but the other values were otherwise within normal range (Table 1). Electrocardiography showed no evidence of ischemic changes or heart failure. Magnetic resonance (MR) imaging of the brain showed no evidence of cerebral infarction and head MR angiography showed no abnormal findings such as cerebral aneurysm (Fig. 2a, b). The patient had no history of COVID-19 infection, or the COVID-19 antigen and polymerase chain reaction tests were negative. The COVID-19 S-antibody result performed 7 weeks after the vaccination showed an increase (372 AU/mL) because of the vaccination. The patient was treated by intravitreal injection of aflibercept (IVA) (EYLEA®, Regeneron Pharmaceuticals, Inc., Tarrytown, NY) 2 weeks after the onset, followed by IVA in a pro re nata regimen. Five IVAs were performed over a 10-month follow-up period, with resolution of macular edema and retinal hemorrhages (Fig. 3a, b), and the BCVA recovered to 20/20.
Fig. 1.
Baseline findings. a An ultra-widefield fundus photograph taken with Optos California (Nikon Corporation, Tokyo, Japan). Multiple retinal hemorrhages are observed throughout the fundus, and retinal veins are tortuous and dilated. b Vertical section of optical coherence tomography (OCT) image taken with Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA). Cystoid macular edema and small serous retinal detachment (arrow) are observed. c, d Ultra-widefield fluorescein angiograms taken with Optos California (c early phase, d late phase). Multiple hypofluorescent spots that appeared to be fluorescent block due to retinal hemorrhages (c, d) and hyperfluorescent leakage from the retinal veins are observed (d).
Table 1.
Summary of hematological workup (abnormal results in bold)
| Test | Result | Normal values |
|---|---|---|
| Hemoglobin, g/L | 15.2 | 13.7–16.8 |
| Hematocrit, % | 42.3 | 40.7–50.1 |
| Platelet count (109/L) | 260 | 158–348 |
| White cell count (109/L) | 6.6 | 3.3–8.6 |
| Triglycerides, mg/dL | 256 H | 40–234 |
| Low-density lipoprotein cholesterol | 73 | 65–163 |
| Prothrombin time (INR) | 1.0 | 0.9–1.1 |
| Activated partial thromboplastin time, s | 30.6 L | 35–50 |
| D-dimer, µg/mL | 0.3 | <1.0 |
| C-reactive protein, mg/L | 0.01 | <0.14 |
| Erythrocyte sedimentation rate, mm/h | 3.7 | 2–10 |
| eGFR, mL/min/1.73 m2 | 92 | >90 |
| HbA1c, % | 5.5 | 4.9–6.0 |
| HIV | Negative | Negative |
| Anti-cardiolipin IgG Ab, U/mL | 4 | <10 |
| ACE, U/L | 7.9 | 7–25 |
| Protein C, % | 177 H | 70–140 |
| Protein S, % | 71.4 | 60–150 |
| Antithrombin III activity, % | 103 | 80–130 |
| SARS-CoV-2 screeningPCR | Negative | Negative |
| COVID-19 antigen | Negative | Negative |
| Anti-SARS-CoV-2 IgG Ab, AU/mL | 372 H | <49 |
H values in bold letters are values above the normal range, and L values in bold letters are values below the normal range. INR, international normalized ratio; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; IgG, immunoglobulin G, Ab, antibody; ACE, angiotensin-converting enzyme; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction.
Fig. 2.
Baseline findings. a A diffusion-weighted image of brain magnetic resonance imaging. No abnormal findings such as cerebral infarction are seen. b Head magnetic resonance angiography scan. No abnormal findings such as cerebral aneurysm are seen.
Fig. 3.
Findings 10 months after the onset. a An ultra-widefield fundus photograph taken with Optos California (Nikon Corporation, Tokyo, Japan). Retinal hemorrhages almost resolved. b Vertical section of optical coherence tomography (OCT) image taken with Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA). Cystoid macular edema and serous retinal detachment disappear.
Discussion
RVO is caused by thrombosis in the retinal vein resulting from compression by atherosclerotic changes in the adjacent retinal artery [11–13] and is often associated with age, hypertension, diabetes mellitus, hyperlipidemia, atherosclerotic diseases, and glaucoma [11–13]. RVO is particularly likely to occur in the elderly, and its occurrence in young patients is uncommon [11, 12]. Therefore, when RVO develops in younger patients, the cause of disease should be investigated by checking medical history, family history, oral medications, smoking history, and blood sampling [11, 12]. In the current case, the patient was younger than 50 years, and there were no abnormalities in his medical history or family history that should be noted, nor was there any history of smoking or oral medication. Not only them, but there were no abnormal findings in the blood sample. Actually, it turned out that the patient had received the first dose of mRNA-based COVID-19 vaccine (BNT162b2 developed by Pfizer/BioNTech) 8 h before the visual impairment. Recently, a series of cases of RVO after COVID-19 infection or COVID-19 vaccination have been reported [3–10, 14], although the direct causal relationship has not been proven. These events have been attributed to hypercoagulability, inflammation, and microvascular alterations [8]. There has been a report of various intraocular inflammations after COVID-19 vaccination [15], as well as a report of patient with papillophlebitis [16], which is an uncommon disease considered as a clinical variant of CRVO that is typically seen in healthy young adults [16]. However, in the current case, papillophlebitis was ruled out because there was no marked fluorescent leakage from the optic disc on fluorescein angiogram, as usually seen in papillophlebitis. In the current case, there were no symptoms specific for COVID-19 infection, or both COVID-19 antigen test and polymerase chain reaction were negative, so COVID-19 infection itself was also ruled out. The COVID-19 antibody test performed approximately 7 weeks after the COVID-19 vaccination showed an increase due to the vaccination. Vascular occlusion has been reported as a complication after COVID-19 vaccination, particularly associated with ChAdOx1 (Oxford/AstraZeneca) [17], and both ChAdOx1 and BNT162b2 have been reported for the development of RVO [14]. The vaccine-induced adverse events after administration of the adenovirus-based SARS-Cov-2 vaccines such as ChAdOx1 vaccine have been described as vaccine-induced immune thrombotic thrombocytopenia syndrome or thrombosis with thrombocytopenia syndrome resulting in a venous or arterial thrombosis, including cerebral venous sinus thrombosis and thrombocytopenia [17]. Regarding mRNA-based SARS-CoV-2 vaccines such as BNT162b2, mRNA vaccine contains polyethylene glycol (PEG) [14]. Pegylated interferon-associated retinopathy is a well-known condition [14, 18]. Pegylation of polypeptides such as interferon with PEG improves pharmacodynamic and pharmacokinetic profiles, but PEG has been associated with development of RVO [14].
In the current case, the patient had visual impairment in the right eye 8 h after COVID-19 vaccination, confirming the development of CRVO. The BCVA in the right eye was 20/200 at the initial visit to our hospital, but prompt treatment with intravitreal injection of anti-vascular endothelial growth factor (VEGF) agents resulted in a good visual prognosis. The patient required 5 IVAs within 10 months after the onset of CRVO, but the macular edema resolved and the BCVA improved to 20/20. No recurrence of macular edema has been observed since then. Endo et al. [9] assumed that inflammation was involved in the development of RVO after vaccination and first used intravitreal injection of dexamethasone. However, they reported that the patient’s BCVA continued to deteriorate afterward, so they administered an intravitreal dose of bevacizumab, which improved the BCVA [9]. In the current case, good BCVA improvement was achieved with administration of intravitreal dose of anti-VEGF agent alone, without administration of steroids. Intravitreal injections of steroids have complications such as increased intraocular pressure [19, 20] and cataracts [21], so it is best not to use them if we can avoid using them.
The highlights of this case report, which are different from other reports [8–10], are as follows: (1) the onset of the disease occurred very early, about 8 h after the vaccination; (2) the patient’s BCVA improved to 20/20 with prompt diagnosis and appropriate treatment, even though it was quite poor (20/200) at the initial visit to our hospital; (3) we could follow up the patient for more than 10 months after the onset of the disease; and (4) any steroids were not used, but only intravitreal anti-VEGF agent treatment was used, which resulted in a good improvement in the BCVA.
In summary, we report a young and healthy patient who developed CRVO immediately after the first dose of COVID-19 vaccination. Although the direct relationship between the vaccination and the development of CRVO is unknown, vaccination-induced onset was suspected. Prompt diagnosis and appropriate treatment resulted in a good visual prognosis. Since use of COVID-19 vaccination is expected to continue, cases of RVO after vaccination are likely to occur in the future. This report should provide very useful information for both clinicians and patients regarding the diagnosis and treatment of RVO after COVID-19 vaccination in future practice. However, this is a case report of a single case. A detailed study with a large number of cases is needed in the future. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000530697).
Statement of Ethics
Written informed consent for publication was obtained from the patient for publication of the details of his medical case and any accompanying images. Ethical approval is not required for this study in accordance with local guidelines.
Conflict of Interest Statement
The authors declare that they have no conflicts of interests.
Funding Sources
There is no funding source.
Author Contributions
Kiyona Ishiguro and Yuya Esaki made substantial contributions to the acquisition of the data. Kiyona Ishiguro and Yoshio Hirano wrote the manuscript. Kiyona Ishiguro, Yoshio Hirano, Yuya Esaki, and Tsutomu Yasukawa reviewed and revised the manuscript.
Funding Statement
There is no funding source.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–47. 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller CG, Kim BJ. Central retinal vein occlusion in a 46-year-old man with COVID-19: case report and review of the literature. Case Rep Ophthalmol. 2021;12(2):646–52. 10.1159/000517417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yahalomi T, Pikkel J, Arnon R, Pessach Y. Central retinal vein occlusion in a young healthy COVID-19 patient: a case report. Am J Ophthalmol Case Rep. 2020;20:100992. 10.1016/j.ajoc.2020.100992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gaba WH, Ahmed D, Al Nuaimi RK, Dhanhani AA, Eatamadi H. Bilateral central retinal vein occlusion in a 40-year-old man with severe coronavirus disease 2019 (COVID-19) pneumonia. Am J Case Rep. 2020;21:e927691. 10.12659/AJCR.927691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheth JU, Narayanan R, Goyal J, Goyal V. Retinal vein occlusion in COVID-19: a novel entity. Indian J Ophthalmol. 2020;68(10):2291–3. 10.4103/ijo.IJO_2380_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walinjkar JA, Makhija SC, Sharma HR, Morekar SR, Natarajan S. Central retinal vein occlusion with COVID-19 infection as the presumptive etiology. Indian J Ophthalmol. 2020;68(11):2572–4. 10.4103/ijo.IJO_2575_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pur DR, Catherine Danielle Bursztyn LL, Iordanous Y. Branch retinal vein occlusion in a healthy young man following mRNA COVID-19 vaccination. Am J Ophthalmol Case Rep. 2022;26:101445. 10.1016/j.ajoc.2022.101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Endo B, Bahamon S, Martínez-Pulgarín DF. Central retinal vein occlusion after mRNA SARS-CoV-2 vaccination: a case report. Indian J Ophthalmol. 2021;69(10):2865–6. 10.4103/ijo.IJO_1477_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takacs A, Ecsedy M, Nagy ZZ. Possible COVID-19 MRNA vaccine-induced case of unilateral central retinal vein occlusion. Ocul Immunol Inflamm. 2022;1:1–6. 10.1080/09273948.2022.2094811. [DOI] [PubMed] [Google Scholar]
- 11. Fong ACO, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol. 1993;37(6):393–417. 10.1016/0039-6257(93)90138-w. [DOI] [PubMed] [Google Scholar]
- 12. Liu Q, Lahey JM, Karlen R, Stewart JM. Laboratory evaluation of hypercoagulable states in patients with central retinal vein occlusion who are less than 56 years of age. Retina. 2018;38(6):1175–9. 10.1097/IAE.0000000000001661. [DOI] [PubMed] [Google Scholar]
- 13. Hirano Y, Suzuki N, Tomiyasu T, Kurobe R, Yasuda Y, Esaki Y, et al. Multimodal imaging of microvascular abnormalities in retinal vein occlusion. J Clin Med. 2021;10(3):405. 10.3390/jcm10030405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park HS, Byun Y, Byeon SH, Kim SS, Kim YJ, Lee CS. Retinal hemorrhage after SARS-CoV-2 vaccination. J Clin Med. 2021;10(23):5705. 10.3390/jcm10235705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C; COVID-19 Vaccination Ocular Inflammatory Events Study Group . Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12(1):4. 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Insausti-García A, Reche-Sainz JA, Ruiz-Arranz C, López Vázquez Á, Ferro-Osuna M. Papillophlebitis in a COVID-19 patient: inflammation and hypercoagulable state. Eur J Ophthalmol. 2022;32(1):NP168–72. 10.1177/1120672120947591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simpson CR, Shi T, Vasileiou E, Katikireddi SV, Kerr S, Moore E, et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat Med. 2021;27(7):1290–7. 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulman JA, Liang C, Kooragayala LM, King J. Posterior segment complications in patients with hepatitis C treated with interferon and ribavirin. Ophthalmology. 2003;110(2):437–42. 10.1016/S0161-6420(02)01741-4. [DOI] [PubMed] [Google Scholar]
- 19. Inatani M, Iwao K, Kawaji T, Hirano Y, Ogura Y, Hirooka K, et al. Intraocular pressure elevation after injection of triamcinolone acetonide: a multicenter retrospective case-control study. Am J Ophthalmol. 2008;145(4):676–81. 10.1016/j.ajo.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 20. Hirano Y, Ito T, Nozaki M, Yasukawa T, Sakurai E, Yoshida M, et al. Intraocular pressure elevation following triamcinolone acetonide administration as related to administration routes. Jpn J Ophthalmol. 2009;53(5):519–22. 10.1007/s10384-009-0692-5. [DOI] [PubMed] [Google Scholar]
- 21. Challa JK, Gillies MC, Penfold PL, Gyory JF, Hunyor AB, Billson FA. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998;26(4):277–81. 10.1111/j.1442-9071.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.