The study attempts to compare the efficacy of standard-course and short-course therapy for urinary tract infections in children 2 months to 10 years old.
Key Points
Question
What is the efficacy of short-course vs standard-course therapy for children with urinary tract infection?
Finding
In this randomized clinical trial of 664 children, those children assigned to standard-course therapy had lower rates of treatment failure than children assigned to short-course therapy, but the rate in both treatment groups was low.
Meaning
The low failure rate in children receiving short-course therapy suggests that it could be considered as a reasonable treatment option.
Abstract
Importance
There is a paucity of pediatric-specific comparative data to guide duration of therapy recommendations in children with urinary tract infection (UTI).
Objective
To compare the efficacy of standard-course and short-course therapy for children with UTI.
Design, Setting, Participants
The Short Course Therapy for Urinary Tract Infections (SCOUT) randomized clinical noninferiority trial took place at outpatient clinics and emergency departments at 2 children’s hospitals from May 2012, through, August 2019. Data were analyzed from January 2020, through, February 2023. Participants included children aged 2 months to 10 years with UTI exhibiting clinical improvement after 5 days of antimicrobials.
Intervention
Another 5 days of antimicrobials (standard-course therapy) or 5 days of placebo (short-course therapy).
Main Outcome Measures
The primary outcome, treatment failure, was defined as symptomatic UTI at or before the first follow-up visit (day 11 to 14). Secondary outcomes included UTI after the first follow-up visit, asymptomatic bacteriuria, positive urine culture, and gastrointestinal colonization with resistant organisms.
Results
Analysis for the primary outcome included 664 randomized children (639 female [96%]; median age, 4 years). Among children evaluable for the primary outcome, 2 of 328 assigned to standard-course (0.6%) and 14 of 336 assigned to short-course (4.2%) had a treatment failure (absolute difference of 3.6% with upper bound 95% CI of 5.5.%). Children receiving short-course therapy were more likely to have asymptomatic bacteriuria or a positive urine culture at or by the first follow-up visit. There were no differences between groups in rates of UTI after the first follow-up visit, incidence of adverse events, or incidence of gastrointestinal colonization with resistant organisms.
Conclusions and Relevance
In this randomized clinical trial, children assigned to standard-course therapy had lower rates of treatment failure than children assigned to short-course therapy. However, the low failure rate of short-course therapy suggests that it could be considered as a reasonable option for children exhibiting clinical improvement after 5 days of antimicrobial treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT01595529
Introduction
In adult patients with urinary tract infection (UTI), short courses of antimicrobial therapy (3 to 7 days) have long been the standard of care.1 Although some pediatric data suggest that shorter durations of antimicrobials are effective in children,2,3,4,5 these data are limited and contradictory. Longer durations of antimicrobial therapy are still endorsed by the American Academy of Pediatrics UTI guidelines for children younger than 24 months.6 Additional pediatric-specific comparative data are needed to guide recommendations for optimal durations of therapy.
We aimed to determine if, among children exhibiting clinical improvement 5 days after starting antimicrobial therapy, halting antimicrobial therapy (short-course therapy) resulted in the similar rates of treatment failure (symptomatic UTI) as continuing antimicrobial therapy for another 5 days (standard-course therapy). We also aimed to evaluate the impact of treatment duration on several secondary outcomes, including UTI after the first follow-up visit, asymptomatic bacteriuria, positive urine culture, presence of adverse events, and gastrointestinal colonization with resistant organisms.
Methods
Eligibility and Enrollment
This multicenter, randomized, double-blind, placebo-controlled, noninferiority clinical trial evaluated short-course (5 days) vs standard-course (10 days) oral antimicrobial therapy for children exhibiting clinical improvement after the first 5 days of antimicrobial therapy. Children 2 months to 10 years of age diagnosed with a UTI (defined below) and prescribed 1 of 5 frequently used antimicrobials (amoxicillin-clavulanate, cefixime, cefdinir, cephalexin, or trimethoprim-sulfamethoxazole) by a health care professional associated with Children’s Hospital of Philadelphia or the UPMC Children’s Hospital of Pittsburgh were eligible for enrollment. Children were recruited from primary care offices, emergency departments, and inpatient wards. The institutional review boards at both institutions approved the study. Interested parents provided written consent for their child to participate in the study. Assent was obtained from developmentally capable children.
We excluded children with any of the following: recovery of a second uropathogen (more than 104 colony forming units [CFU] per mL for samples collected by suprapubic aspiration or catheterization or more than 5 × 104 CFU per mL for samples collected by clean catch) at the time of diagnosis, hospitalization for bacteremia, admission to the intensive care unit; urine culture yielding a pathogen resistant to the initially prescribed antimicrobial, catheter-associated UTI, history of UTI within 30 days, phenylketonuria; congenital or anatomic abnormalities of the genitourinary tract other than known grade I to II vesicoureteral reflux, duplicated collecting systems, or hydronephrosis; previous genitourinary tract surgery (except circumcision), inability to tolerate oral medications; presence of an immunocompromising condition; type I hypersensitivity or anaphylactic reaction to study products; previous enrollment in the study; enrollment in another therapeutic drug study (excluding vaccine studies); gestational age of less than 36 weeks (only for children younger than 2 years of age at enrollment); or not available for follow-up visits.
Study Procedures
We contacted potentially eligible families between 2 and 5 days after the patient was diagnosed with a UTI (day 1) to determine interest in participation in the study and to assess if the patient was improving clinically. For children that appeared to be improving and whose caregiver was interested in participation, we arranged an in-person visit (at the child’s home or at an affiliated clinic) on days 2 to 5 (enrollment visit). Children were considered to have clinical improvement if they were afebrile (no documented temperature 38 °C or higher within 24 hours of the visit) and free of any symptoms of UTI (see definition of UTI below). At the enrollment visit, we administered a symptom questionnaire, measured the child’s temperature, and performed a targeted assessment for suprapubic, abdominal, or flank tenderness, and confirmed that the child met all eligibility criteria. When feasible, a stool sample for the assessment of bacterial colonization was collected at the enrollment visit. For children who had their enrollment visits on days 2 to 4, clinical improvement was reconfirmed on day 5 or on the morning of day 6 by telephone.
After consent, participants were randomized (1:1) to receive either an additional 5 days of the prescribed antimicrobial (standard-course therapy) or 5 days of matching placebo (short-course therapy). Randomization was stratified according to the presence or absence of fever and according to the antimicrobial initially prescribed. All children started their randomized study therapy on day 6 and continued through day 10; those starting study therapy in the evening of day 6 were advised to complete their last dose of study product on the morning of day 11.
To evaluate clinical outcomes, we performed 2 in-person visits on days 11 to 14 and days 24 to 30. At each visit, we administered a symptom questionnaire, asked if there was any interim diagnostic testing for a UTI, measured the child’s temperature, performed a targeted assessment for suprapubic, abdominal, or flank tenderness, and collected stool samples. A urine sample was also collected at the day 11 to 14 visit. Urine was only collected at the day 24 to 30 visit if symptoms of UTI were reported at the time of the visit or when urine collection had failed on the day 11 to 14 visit. During follow-up visits, we used perineal bags to collect samples from non–toilet-trained asymptomatic children, bladder catheterization or clean catch for non–toilet-trained symptomatic children, or clean catch for toilet-trained children. We called families on day 38 to 44 to determine whether UTI or adverse events had occurred since the day 24 to 30 visit.
Outcomes
The primary outcome, treatment failure, was defined as occurrence of UTI (defined below) between day 6 and the day 11 to 14 visit. Secondary outcomes included: (1) UTI at any time after the day 11 to 14 visit, (2) asymptomatic bacteriuria (defined below) at the day 11 to 14 visit, (3) gastrointestinal colonization at or before the day 24 to 30 visit with antimicrobial-resistant Escherichia coli or Klebsiella pneumoniae (eTable in Supplement 1) not present at the enrollment visit, (4) clinical symptoms of UTI between day 6 and the day 11 to 14 visit, and (5) positive urine culture (defined below) between day 6 and the day 11 to 14 visit. We also compared the proportion of children with any reported adverse events in each group between days 6 and 38 to 44.
Definitions
We defined UTI by the presence of all the following 3 criteria: (1) 1 or more of the following signs or symptoms of UTI: fever (defined as temperature of at least 38 °C); pain in the suprapubic, abdominal, or flank area; urinary urgency, frequency, or hesitancy; dysuria in children 2 years or older or in children younger than 2 years of age; and poor feeding or vomiting; (2) pyuria, defined as 10 or more white blood cells per cubic millimeter (uncentrifuged specimen) or 5 or more white blood cells/high-powered field (centrifuged specimen) or leukocyte esterase more than or equal to trace on dipstick urinalysis; and (3) a positive urine culture defined by growth of a single uropathogen at counts 5 × 104 or higher CFU per mL (suprapubic aspiration or catheterized specimen) or 105 or higher CFU per mL (clean voided specimen).7
The outcome of asymptomatic bacteriuria was defined as presence of a positive urine culture for a uropathogen, using the same colony count thresholds above and without regard to pyuria, but in the absence of symptoms attributable to UTI. The outcome of positive urine culture was defined as presence of a uropathogen, using the same colony count thresholds above, in a child not meeting criteria for a UTI (either due the lack of symptoms or pyuria).
Antimicrobials and Placebos
Participants were randomized to receive 5 more days of the antimicrobial agent they were prescribed by their health care professional or a matching placebo. Patients that were prescribed cefdinir by their health care professional received either cefixime or placebo during the days 6 to 10 intervention period. Amoxicillin-clavulanate was dosed by amoxicillin component at 80 to 100 mg/kg per day divided twice daily, trimethoprim-sulfamethoxazole at 8 mg/kg per day, trimethoprim divided twice daily, cefixime at 8 mg/kg per day in 1 dose, and cephalexin at 50 mg/kg per day in 3 divided doses. For each antimicrobial, a matching placebo was dosed at the same volume calculated for active treatment. For concealment, liquid placebo was formulated for the same appearance (color and texture), flavor, and consistency as active study drug and provided in matching bottles. Trial participants and their parents, investigators, and trial personnel remained unaware of treatment assignment.
Statistical Methods
Anticipating that short-course therapy was not likely to be superior to a standard-course therapy, we designed this study as a noninferiority trial.8 We decided a priori that a difference in absolute rates of treatment failure of more than 5% would be clinically meaningful and thus used this as our noninferiority margin. Our power and sample estimates were based on the assumption that treatment failure would be observed in 5% of children receiving standard-course therapy9 and no more than 10% of children receiving short-course therapy. Based on these assumptions, this 1-sided noninferiority analysis would require 326 evaluable participants per treatment group to achieve 90% power with significance level of 0.05.
Primary analysis followed intent-to-treat principles and included all children who received at least 1 dose of study product and were evaluated for treatment failure at or before the day 11 to 14 visit. Specifically, the primary noninferiority analysis evaluated whether there was greater than a 5% increase in the absolute risk of treatment failure in children assigned to short-course therapy compared with children assigned to standard-course therapy.
For the primary analysis, we used a 1-sided Fisher exact test to evaluate whether the upper bound of the CI for the absolute risk difference exceeded 5%. For secondary outcomes, we used 2-sided Fisher exact tests to assess whether these outcomes differed in the treatment groups. We compared the proportion of adverse events between treatment groups using a 2-sided χ2 test. For the primary outcome, we tested treatment-by-subpopulation interactions using likelihood ratio tests. For the primary outcome, we also present results of a per-protocol analysis (eMethods 1 in Supplement 1). For all analyses, we used a significance level of .05.
Post Hoc Analyses
We calculated the number needed to treat for each significant outcome. While the primary outcome, treatment failure, was defined as occurrence of UTI between day 6 and the day 11 to 14 visit, children in the standard-course group were potentially receiving antibiotics during 5 of the 9 days during which the outcome could occur. To assess whether detection bias10 could have affected our results, we conducted a post hoc analysis in which both groups had 9 days after cessation of antibiotics during which the primary outcome could occur (days 6 to 14 for short course and days 11 to 18 for standard course).
Results
Study Population
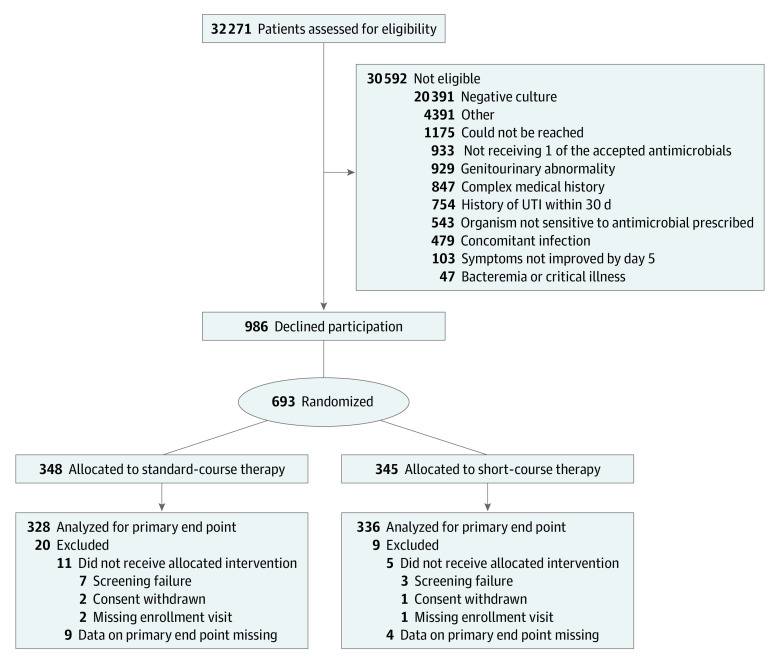
A total of 32 271 children were screened, 1679 were eligible and 693 were randomized (Figure). Of note, only 103 children were excluded because their symptoms had not improved by day 5 (Figure). Of the 693 children randomized, 16 did not receive at least 1 dose of the allocated treatment and an additional 13 did not contribute data toward the primary outcome; as such, the analysis for the primary outcome includes 664 children.
Figure. Enrollment, Randomization, and Follow-up of Children in the Trial.
UTI indicates urinary tract infection.
Most randomized participants were White (64%), non-Hispanic (91%), and afebrile at the time of initial diagnosis (62%). The median age of children enrolled was 4 years. Demographic and clinical characteristics were similar between treatment groups (Table 1).
Table 1. Selected Demographic and Clinical Characteristics of Children With Data on Primary Outcome According to Treatment Groupa.
| Characteristic | Children, No. (%) | ||
|---|---|---|---|
| Standard-course therapy (n = 328) | Short-course therapy (n = 336) | All children (n = 664) | |
| Age group | |||
| 2-23 mo | 60 (18) | 64 (19) | 124 (19) |
| 2-6 y | 183 (46) | 202 (60) | 385 (58) |
| 7-10 y | 85 (26) | 70 (21) | 155 (23) |
| Sex | |||
| Female | 316 (96) | 323 (96) | 639 (96) |
| Male | 12 (4) | 13 (4) | 25 (4) |
| Raceb | |||
| Black | 73 (22) | 83 (25) | 156 (23) |
| Multiracial | 23 (7) | 25 (7) | 48 (7) |
| White | 217 (66) | 210 (63) | 427 (64) |
| Otherc | 14 (4) | 18 (5) | 32 (5) |
| Unknown | 1 (0) | 0 (0) | 1 (0) |
| Ethnicityb | |||
| Hispanic | 33 (10) | 27 (8) | 60 (9) |
| Non-Hispanic | 295 (90) | 309 (92) | 604 (91) |
| Febrile at presentation | |||
| Yes | 122 (37) | 128 (38) | 250 (38)d |
| No | 206 (63) | 208 (62) | 414 (62) |
| Medication | |||
| Amoxicillin-clavulanate | 4 (1) | 2 (1) | 6 (1) |
| Cefdinir | 183 (56) | 185 (55) | 368 (55) |
| Cefixime | 2 (1) | 1 (0) | 3 (0) |
| Cephalexin | 103 (31) | 112 (33) | 215 (32) |
| Trimethoprim-sulfamethoxazole | 36 (11) | 36 (11) | 72 (11) |
| Study site | |||
| Children’s Hospital of Philadelphia | 143 (44) | 152 (45) | 295 (44) |
| UPMC Children’s Hospital of Pittsburgh | 185 (56) | 184 (55) | 369 (56) |
| Infecting organism | |||
| Escherichia coli | 296 (90) | 301 (90) | 597 (90) |
| Proteus mirabilis | 20 (6) | 22 (7) | 42 (6) |
| Klebsiella pneumoniae | 5 (2) | 4 (1) | 9 (1) |
| Other | 7 (2) | 9 (3) | 16 (2) |
| Method of urine collection | |||
| Clean voided | 257 (78) | 268 (80) | 525 (79) |
| Catheterization | 70 (21) | 65 (19) | 135 (20) |
| Unknown | 1 (0) | 3 (1) | 4 (1) |
| Received 1-time dose of intravenous or intramuscular antimicrobial | |||
| Yes | 3 (1) | 3 (1) | 6 (1) |
| No | 325 (99) | 333 (99) | 658 (99) |
| Symptoms at presentation (age >2 y) | |||
| Suprapubic pain/tenderness | 11 (3) | 22 (7) | 33 (5) |
| Abdominal pain/tenderness | 93 (28) | 95 (28) | 188 (28) |
| Flank pain/tenderness | 22 (7) | 16 (5) | 38 (6) |
| Urinary urgency | 74 (23) | 73 (22) | 147 (22) |
| Urinary frequency | 108 (33) | 109 (32) | 217 (33) |
| Urinary hesitancy | 25 (8) | 21 (6) | 46 (7) |
| Symptoms at presentation (age <2 y) | |||
| Poor feeding | 21 (6) | 22 (7) | 43 (6) |
| Vomiting | 22 (7) | 22 (7) | 44 (7) |
There were no significant differences in characteristics between the 2 treatment groups.
Race and ethnicity were reported by the parents.
Includes American Indian or Alaska native, Asian, Native Hawaiian, or other Pacific Islander.
A total of 94% of children aged 2 to 23 months were febrile.
Primary Outcome
A total of 2 children assigned to standard-course therapy (0.6%) and 14 children assigned to short-course therapy (4.2%) (Table 2) met criteria for treatment failure. The upper limit of the CI for the absolute difference (ie, 5.5%) exceeded the prespecified 5% margin we had required for noninferiority. Treatment failure was not related to age, fever, prescribed antimicrobial therapy, or study site. A total of 1 child assigned to standard-course therapy (0.3%) and 6 children assigned to short-course therapy (1.8%) had a febrile UTI between day 6 and the day 11 to 14 visit. In the per protocol analysis (eMethods 2 in Supplement 1), differences in the proportion of children with treatment failure between treatment groups were smaller.
Table 2. Primary and Secondary Outcomes.
| Characteristics | Children, No./total No. (%) | P value | Difference of proportions, % (95% CI) | No. needed to treata | ||
|---|---|---|---|---|---|---|
| Standard-course therapy | Short-course therapy | All children | ||||
| Primary outcome—treatment failure | ||||||
| UTI between day 6 and day 11-14 visit | 2/328 (0.6) | 14/336 (4.2) | 16/664 (2.4) | <.01 | 3.6 (≤5.5)b | 28 |
| Secondary outcomes | ||||||
| UTI after day 11-14 visit | 12/326 (3.7) | 13/322 (4.0) | 25/648 (3.9) | .97 | 0.4 (−2.6 to 3.3) | c |
| Asymptomatic bacteriuria at day 11-14 visit | 11/328 (3.4) | 32/336 (9.5) | 40/664 (6.0) | <.01 | 6.2 (2.5-9.9) | 17 |
| UTI symptoms day 6 through day 11-14 visit | 30/328 (9.1) | 41/336 (12.2) | 71/664 (10.7) | .25 | 3.1 (−1.6 to 7.7) | c |
| Positive urine culture day 6 though day 11-14 visitd | 15/328 (4.6) | 42/336 (12.5) | 47/664 (7.1) | <.01 | 7.9 (3.7-12.1) | 13 |
| Stool antimicrobial resistance at day 24-30 visite | 23/298 (7.7) | 28/310 (9.0) | 51/608 (8.4) | .66 | 1.3 (−3.1 to 5.7) | c |
Abbreviation: UTI, urinary tract infection.
Number of children that would need to be treated with standard-course therapy to prevent 1 treatment failure. Confidence interval not presented because, for some outcomes, upper bound is unbounded. This analysis was added post hoc.
One-sided confidence interval, therefore only upper bound of is shown; by definition, lower bound is −100.
Number needed to treat not presented because difference in proportions was not significant.
With or without symptoms.
Defined by recovery of antimicrobial-resistant Escherichia coli or Klebsiella pneumoniae from the stool of children in whom these resistant organisms were not present at enrollment.
Secondary Outcomes
Children receiving short-course therapy were more likely to have asymptomatic bacteriuria (absolute risk difference, 6.2%; 95% CI, 2.5%-9.9%) or a positive urine culture (absolute risk difference, 7.9%; 95% CI, 3.7%-12.1%) at or before the day 11 to 14 visit (Table 2). No other statistically significant differences were apparent between treatment groups for the other secondary outcomes assessed (Table 2). Rates of adverse events were similar in the 2 treatment groups (Table 3).
Table 3. Adverse Events According to Study Group.
| Characteristic | Children, No. (%) | P value | ||
|---|---|---|---|---|
| Standard-course therapy (n = 328) | Short-course therapy (n = 336) | All children (n = 664) | ||
| Participants with adverse events | 155 (47.3) | 147 (43.8) | 302 (45.5) | .10 |
| Most frequently reported adverse events | ||||
| Diarrhea | 43 (13.1) | 34 (10.1) | 77 (11.6) | .41 |
| Cough | 21 (6.4) | 26 (7.7) | 47 (7.1) | .28 |
| Pyrexia | 17 (5.2) | 18 (5.4) | 35 (5.3) | .60 |
| Vomiting | 11 (3.4) | 20 (6.0) | 31 (4.7) | 1.00 |
| Rhinorrhea | 13 (4.0) | 16 (4.8) | 29 (4.4) | .16 |
| Other | 115 (35.1) | 111 (33.0) | 226 (34.0) | .75 |
| Severity of adverse event | ||||
| Mild | 107 (32.6) | 117 (34.8) | 224 (33.7) | .64 |
| Moderate | 47 (14.3) | 26 (7.7) | 73 (11.0) | .61 |
| Severe | 1 (0.3) | 4 (1.2) | 5 (0.8) | .37 |
| Serious | 2 (0.6) | 4 (1.2) | 6 (0.9)a | .69 |
Of these, only 1 (pyelonephritis in the short-course group on day 12) was deemed possibly associated with study treatment.
Post Hoc Analyses
The number needed to treat for standard-course therapy was 28, meaning that 28 children would need to be treated with an additional 5 days of antimicrobials to prevent 1 treatment failure (Table 2). The number needed to treat to prevent 1 febrile treatment failure was 67. A total of 9 of 328 children assigned to standard-course therapy (2.7%) and 14 of 336 children assigned to short-course therapy (4.2%) had a UTI within 9 days of study product discontinuation (difference in proportions of 1.4% with upper bound of the 95% CI of 4.2; P = .32).
Discussion
In this noninferiority trial involving children aged 2 months to 10 years diagnosed with symptomatic UTI who had exhibited clinical improvement after the first 5 days of antimicrobials, standard-course therapy was associated with lower rates of treatment failure compared with short-course therapy (0.6% vs 4.2%, respectively). Among secondary outcomes, standard-course therapy was associated with lower rates of asymptomatic bacteriuria and positive urine culture at the day 11 to 14 visit. No differences between treatment groups were apparent with regards to rates of UTI after the day 11 to 14 visit, symptoms of UTI, adverse events, or the emergence of antimicrobial resistance in gastrointestinal strains of Es coli or K pneumoniae.
To our knowledge, our study represents the largest trial to date comparing outcomes in children randomized to standard-course vs short-course antimicrobial therapy. While previous trials, taken together, suggest that 1 day of antimicrobial therapy is not as effective as standard-course therapy for UTI,2 there is limited pediatric evidence from previously completed trials that compare other shorter durations of therapy (ie, 2 to 4 days) vs standard-course therapy. Of the 10 randomized trials performed to date comparing 2 to 4 days of antimicrobials to longer durations,3 most (9 of 10) included less than 100 evaluable children and many suffered from methodological limitations. For example, the 1 study11 that included more than 100 children (174 evaluable girls) and compared 3 vs 10 days of sulfamethizole was limited by the inclusion of children with asymptomatic bacteriuria, use of a positive culture as the only outcome of interest, and lack of blinding.
We had expected that children with fever at the time of the initial diagnosis would be more likely to have short-course therapy fail. Instead, we found that the absolute difference in treatment failure between study arms was similar in the subgroups of children with and without fever at the time of diagnosis; however, since the trial was not powered to detect treatment effect heterogeneity within subgroups, the results of the subgroup analysis should be not considered as definitive.
Children randomized to short-course therapy were more likely to have asymptomatic bacteriuria or a positive urine culture at or before the day 11 to 14 visit. The importance of these findings is unclear. The presence of bacteria in the urine is not necessarily clinically relevant if the child continues to improve and does not go on to have a recurrent infection. The fact that rates of UTI after day 11 to 14 visit were similar suggests that differences in bacteriuria or a positive urine culture at or before the day 11 to 14 visit did not contribute to development of UTIs after the first follow-up visit. Children in the antimicrobial group may have been less likely to have these outcomes simply because the day 11 to 14 visit was so close to the last dose of antimicrobial (ie, last dose of study product could have been taken on the same day as the day 11 visit). A longer period between the last dose of study product and the first follow-up visit might have mitigated the between-group differences observed in these secondary outcomes. Of note, the protocol allowed us to use perineal bags to collect urine from asymptomatic children presenting for follow-up. If the culture from a bag sample was positive, we categorized these as cases of asymptomatic bacteriuria. While this may have inflated the number of children with asymptomatic bacteriuria, it likely did not bias our results given that the treatment groups were balanced with regards to age.
Most children whose therapy failed were afebrile and, accordingly, are not at risk for kidney scarring. Given that 67 children need to be treated with standard-course therapy to prevent 1 febrile UTI and given that scarring occurs in approximately 1 in 7 children with febrile UTI,12,13,14 approximately 469 children would need to be treated to prevent 1 child from developing kidney scarring.15
Strengths and Limitations
Our trial has several strengths: a large, diverse population of children; enrollment of a sizeable proportion of children with fever at presentation (38%); use of stringent diagnostic criteria for UTI; enrollment of children treated with various antimicrobials; monitoring for targeted antimicrobial resistance in stool commensals; and modest attrition. Limitations stem from slight imbalance between treatment groups in number of children excluded from the primary analysis, reduced power for subgroup analyses, assessment of emergence of antimicrobial resistance only for E coli and K pneumoniae strains, lack of detailed data on the societal costs and benefits of each treatment strategy, absence of data on adherence to the originally prescribed antimicrobials on days 1 to 5, lack of strain-level data on recovered uropathogens, and lack of data on outcomes, such as kidney scarring.
Conclusion
This trial failed to show that short-course therapy was statistically noninferior to standard-course therapy using a 5% noninferiority margin. However, given that (1) treatment failure occurred infrequently in the short-course group (4.2%), (2) in a post hoc analysis, rates of UTI within 9 days of stopping antimicrobial therapy in those receiving short-course and standard-course therapy were similar (4.2% vs 2.7%, respectively, P = .32), and (3) a large number of children needed to be treated with standard-course therapy to prevent 1 child from developing kidney scarring, all suggest that short-course therapy could be considered as a reasonable option for children exhibiting clinical improvement after 5 days of antimicrobial treatment.
eMethods1. Per protocol analysis
eTable. Primary outcomes and subgroup analysis in the per protocol population
eMethods 2. Microbiological methods for processing stool samples
Trial protocol 1
Trial protocol 2
Data sharing statement
References
- 1.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 2.Keren R, Chan E. A meta-analysis of randomized, controlled trials comparing short- and long-course antibiotic therapy for urinary tract infections in children. Pediatrics. 2002;109(5):E70-E0. doi: 10.1542/peds.109.5.e70 [DOI] [PubMed] [Google Scholar]
- 3.Michael M, Hodson EM, Craig JC, Martin S, Moyer VA. Short compared with standard duration of antibiotic treatment for urinary tract infection: a systematic review of randomised controlled trials. Arch Dis Child. 2002;87(2):118-123. doi: 10.1136/adc.87.2.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran D, Muchant DG, Aronoff SC. Short-course versus conventional length antimicrobial therapy for uncomplicated lower urinary tract infections in children: a meta-analysis of 1279 patients. J Pediatr. 2001;139(1):93-99. doi: 10.1067/mpd.2001.114698 [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald A, Mori R, Lakhanpaul M, Tullus K. Antibiotics for treating lower urinary tract infection in children. Cochrane Database Syst Rev. 2012;(8):CD006857. doi: 10.1002/14651858.CD006857.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts KB; Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330 [DOI] [PubMed] [Google Scholar]
- 7.Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Pyuria and bacteriuria in urine specimens obtained by catheter from young children with fever. J Pediatr. 1994;124(4):513-519. doi: 10.1016/S0022-3476(05)83127-0 [DOI] [PubMed] [Google Scholar]
- 8.D’Agostino RB Sr, Massaro JM, Sullivan LM. Non-inferiority trials: design concepts and issues—the encounters of academic consultants in statistics. Stat Med. 2003;22(2):169-186. doi: 10.1002/sim.1425 [DOI] [PubMed] [Google Scholar]
- 9.Hoberman A, Wald ER, Hickey RW, et al. Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics. 1999;104(1 Pt 1):79-86. doi: 10.1542/peds.104.1.79 [DOI] [PubMed] [Google Scholar]
- 10.Phillips MR, Kaiser P, Thabane L, Bhandari M, Chaudhary V; Retina Evidence Trials InterNational Alliance (R.E.T.I.N.A.) Study Group . Risk of bias: why measure it, and how? Eye (Lond). 2022;36(2):346-348. doi: 10.1038/s41433-021-01759-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Short-term treatment of acute urinary tract infection in girls. Copenhagen study group of urinary tract infections in children. Scand J Infect Dis. 1991;23(2):213-20. doi: 10.3109/00365549109023403 [DOI] [PubMed] [Google Scholar]
- 12.Shaikh N, Craig JC, Rovers MM, et al. Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr. 2014;168(10):893-900. doi: 10.1001/jamapediatrics.2014.637 [DOI] [PubMed] [Google Scholar]
- 13.Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126(6):1084-1091. doi: 10.1542/peds.2010-0685 [DOI] [PubMed] [Google Scholar]
- 14.Shaikh N, Shope TR, Hoberman A, et al. Corticosteroids to prevent kidney scarring in children with a febrile urinary tract infection: a randomized trial. Pediatr Nephrol. 2020;35(11):2113-2120. doi: 10.1007/s00467-020-04622-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaikh N, Morone NE, Lopez J, et al. Does this child have a urinary tract infection? JAMA. 2007;298(24):2895-2904. doi: 10.1001/jama.298.24.2895 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods1. Per protocol analysis
eTable. Primary outcomes and subgroup analysis in the per protocol population
eMethods 2. Microbiological methods for processing stool samples
Trial protocol 1
Trial protocol 2
Data sharing statement