Abstract
Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome with various comorbidities, multiple cardiac and extracardiac pathophysiologic abnormalities, and diverse phenotypic presentations. Since HFpEF is a heterogeneous disease with different phenotypes, individualized treatment is required. HFpEF with type 2 diabetes mellitus (T2DM) represents a specific phenotype of HFpEF, with about 45%-50% of HFpEF patients suffering from T2DM. Systemic inflammation associated with dysregulated glucose metabolism is a critical pathological mechanism of HFpEF with T2DM, which is intimately related to the expansion and dysfunction (inflammation and hypermetabolic activity) of epicardial adipose tissue (EAT). EAT is well established as a very active endocrine organ that can regulate the pathophysiological processes of HFpEF with T2DM through the paracrine and endocrine mechanisms. Therefore, suppressing abnormal EAT expansion may be a promising therapeutic strategy for HFpEF with T2DM. Although there is no treatment specifically for EAT, lifestyle management, bariatric surgery, and some pharmaceutical interventions (anti-cytokine drugs, statins, proprotein convertase subtilisin/kexin type 9 inhibitors, metformin, glucagon-like peptide-1 receptor agonists, and especially sodium-glucose cotransporter-2 inhibitors) have been shown to attenuate the inflammatory response or expansion of EAT. Importantly, these treatments may be beneficial in improving the clinical symptoms or prognosis of patients with HFpEF. Accordingly, well-designed randomized controlled trials are needed to validate the efficacy of current therapies. In addition, more novel and effective therapies targeting EAT are needed in the future.
Keywords: Epicardial adipose tissue, Heart failure with preserved ejection fraction, Type 2 diabetes mellitus, Inflammation, Anti-hyperglycemic drugs, Sodium-glucose cotransporter-2 inhibitors
Core Tip: Heart failure with preserved ejection fraction (HFpEF) is a heterogeneous syndrome requiring individualized treatment depending on phenotypic differences. HFpEF with type 2 diabetes mellitus is strongly associated with the expansion, inflammation, and hypermetabolic activity of epicardial adipose tissue (EAT). Thus, targeting EAT may be a promising therapeutic strategy for HFpEF with type 2 diabetes mellitus. Lifestyle management, bariatric surgery, and certain drugs may suppress the accumulation of EAT and improve the clinical symptoms and prognosis of HFpEF. More studies are required to validate the efficacy of current treatments and to develop new effective therapies.
INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF), a systemic and heterogeneous syndrome, is characterized by various comorbidities (mainly diabetes mellitus, hypertension, and metabolic syndrome), multiple cardiac and extracardiac pathophysiologic abnormalities, and diverse phenotypic presentations[1]. HFpEF is a growing public health challenge, which currently accounts for approximately half of HF cases, and its prevalence continues to rise due to an aging population and the increasing burden of comorbidities[2]. Additionally, HFpEF is associated with poor prognosis, with a 5-year mortality rate of up to 75%[3]. Standardized and effective interventions are lacking due to the complex pathophysiological underpinnings and clinical heterogeneity of HFpEF[4]. It may, however, be beneficial to halt disease progression and thus improve prognosis by providing individualized treatment based on phenotypic differences[4].
Type 2 diabetes mellitus (T2DM) is a substantial risk factor for the emergence and progression of HFpEF, and approximately 45%-50% of HFpEF cases suffer from T2DM, a specific phenotype of HFpEF[5,6]. Systemic inflammation related to glucose metabolism disorders is accepted as a critical pathological mechanism of HFpEF with T2DM, which is responsible for the expansion and dysfunction (inflammation and hypermetabolic activity) of epicardial adipose tissue (EAT)[7]. EAT, a metabolically active visceral fat depot, can regulate the pathophysiological processes of HFpEF with T2DM through the paracrine and endocrine mechanisms[8]. Thus, inhibiting the accumulation of EAT may be a promising therapeutic strategy for HFpEF with T2DM. At present, lifestyle management, bariatric surgery, and some medications may contribute to reducing the inflammation response or accumulation of EAT, despite the fact that there is no available treatment for EAT. Notably, these interventions may attenuate pathological changes and improve the prognosis in patients with HFpEF.
Currently, a comprehensive review is lacking discussing the pathogenesis of EAT-mediated HFpEF with T2DM and therapies to inhibit EAT expansion. In this review, we evaluated the role of EAT in the development of HFpEF with T2DM and discussed current therapies to attenuate EAT expansion as well as future therapeutic perspectives.
ANATOMY, PATHOLOGY AND PATHOPHYSIOLOGY OF EAT
Anatomy of EAT
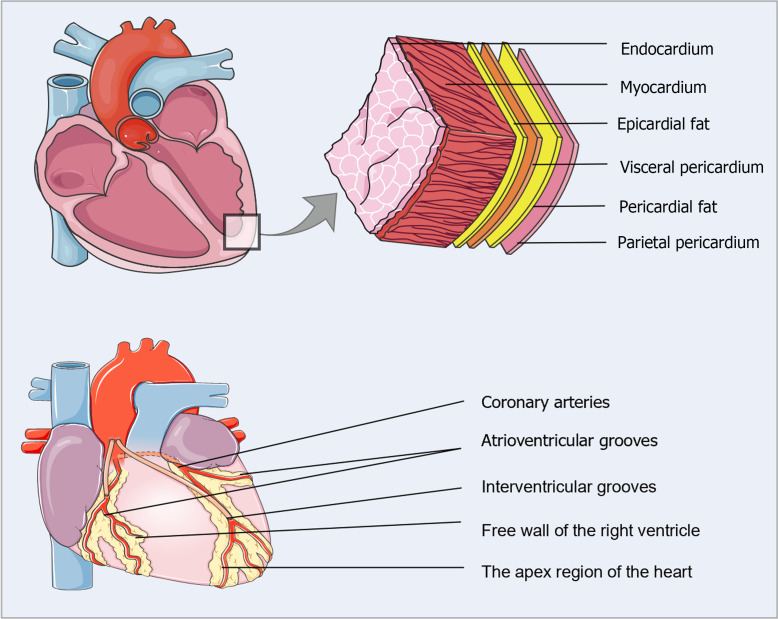
EAT represents the local visceral fat depot of the heart, located between the myocardium and the visceral pericardium[9] (Figure 1). Under healthy circumstances, EAT accounts for approximately 20% of the total heart weight and covers 80% of the cardiac surface[10,11]. In adults, EAT typically surrounds the coronary arteries and their major epicardial branches, mainly concentrated in the interventricular and atrioventricular grooves, with lesser amounts covering the atria, the free wall of the right ventricle, and the apex[9]. Interestingly, EAT is anatomically and functionally contiguous with the myocardium because of the shared microcirculation and the absence of muscle fascia, which may facilitate the local interaction of EAT with the myocardium and coronary arteries through vasocrine or paracrine cross-talk[12]. Microscopically, EAT consists typically of adipocytes specialized in energy storage but also includes inflammatory cells (mainly macrophages and mast cells), immune cells, stromovascular cells, and ganglia in normal adults. In pathological states, however, numerous inflammatory cell aggregates and abnormal expansion of the microvascular network are present in the EAT[13].
Figure 1.
Anatomical location of epicardial adipose tissue. Epicardial adipose tissue (EAT) is situated between the myocardium and the visceral pericardium. In normal adults, EAT usually accompanies the coronary arteries and their major epicardial branches, mainly concentrated in the interventricular and atrioventricular grooves, with lesser amounts covering the atria, the free wall of the right ventricle, and the apex.
Physiology of EAT
EAT acts as a shock absorber, protecting coronary arteries from excessive distortion and compression during the contraction of the adjacent myocardium[14]. EAT has a greater capacity to release and uptake free fatty acids (FFA) compared to other visceral fat depots. The myocardium metabolizes FFAs from the coronary arterial blood, which is shared with the contiguous EAT. FFA oxidation is responsible for almost 50%-70% of the energy production in the heart[15]. Accordingly, EAT might serve as a physiological buffer to protect the myocardium from excessive fatty acid levels and as a direct energy source to provide FFA under increased metabolic demand. Moreover, EAT expresses uncoupling protein-1 (UCP1), a thermogenic protein located in the inner membrane of mitochondria. UCP1 uncouples oxidative phosphorylation from ATP synthesis, ultimately dissipating energy as heat[16]. EAT might, therefore, provide direct heat to the myocardium and protect the heart under unfavorable hemodynamic conditions.
Pathophysiology of EAT
EAT has been widely established as a remarkably active endocrine organ that secretes various bioactive molecules, such as cytokines, adipokines, and chemokines, that can exert protective or detrimental effects depending on the local microenvironmental situation[17]. EAT can, therefore, locally modulate the adjacent myocardium and coronary arteries through the vasocrine or paracrine secretion of these bioactive molecules[12]. Physiologically, EAT mainly releases anti-inflammatory adipocytokines, such as adiponectin, adrenomedullin, omentin, and interleukin-10 (IL-10), which contribute to cardioprotection and anti-atherosclerosis[14]. In contrast, adipocytes enlarge and produce high quantities of FFAs under pathological conditions, triggering EAT expansion, localized hypoxia, and the infiltration of macrophages, ultimately resulting in a chronic inflammatory response[8]. Subsequently, numerous proinflammatory adipokines are produced and accumulated, including IL-6, tumor necrosis factor-alpha (TNF-α), monocyte chemotactic protein-1, leptin, resistin, and serglycin, which aggravate local inflammation, thereby affecting the heart and coronary arteries[12].
CONTRIBUTIONS OF EAT TO HFPEF WITH T2DM
EAT in the pathophysiology of HFpEF with T2DM
Dysregulated glucose metabolism is a fundamental clinical characteristic of T2DM and is strongly connected with the aberrant accumulation of EAT[18-20]. As reported in Table 1, EAT thickness over the right ventricular free wall, EAT volume, or EAT area were significantly higher in patients with impaired fasting glucose, insulin resistance, or T2DM than in control subjects[21-39]. A meta-analysis of nine studies by Li et al[40] confirmed a positive correlation between the presence of T2DM and EAT expansion. Eventually, increased EAT deposition interacts directly with the heart through mechanical and metabolic mechanisms, leading to myocardial fibrosis, cardiomyocyte stiffness, and left ventricular (LV) diastolic dysfunction, which are the essential pathological features of HFpEF (Figure 2).
Table 1.
Epicardial adipose tissue expansion in patients with glucose metabolism disorders
|
Ref.
|
Participants, n
|
Amount of EAT in the observation group
|
Amount of EAT in the control group
|
P value
|
| EAT thickness (mm) measured by echocardiography thickness on the right ventricular free wall | ||||
| Baloglu et al[21], 2019 | T2DM patients: 128; healthy controls: 32 | 3.53 ± 0.79 | 4.64 ± 1.39 | < 0.001 |
| Akbas et al[22], 2014 | T2DM patients: 156; healthy controls: 50 | 4.66 ± 1.50 | 3.91 ± 1.60 | 0.005 |
| Chen et al[23], 2017 | T2DM patients: 167; healthy controls: 82 | 4.00 (3.00-5.00) | 2.00 (1.00-3.00) | < 0.001 |
| Philouze et al[24], 2017 | T2DM patients: 44; healthy controls: 35 | 6.40 ± 1.70 | 3.30 ± 1.10 | < 0.001 |
| Cetin et al[25], 2013 | T2DM patients: 139; age- and sex-matched controls: 40 | 6.00 ± 1.50 | 4.42 ± 1.00 | < 0.001 |
| Yafei et al[26], 2019 | T2DM patients: 76; age- and sex-matched controls: 30 | 6.23 ± 1.27 | 4.60 ± 1.03 | < 0.001 |
| Christensen et al[27], 2019 | T2DM patients: 770; age- and sex-matched controls: 234 | 4.60 ± 1.80 | 3.40 ± 1.20 | < 0.0001 |
| Wang et al[28], 2017 | T2DM with duration ≤ 10 yr: 35; T2DM with duration > 10 yr: 33 | 4.47 ± 1.90 | 5.45 ± 1.40 | < 0.05 |
| Altin et al[29], 2016 | Patients with IR: 113; age- and sex-matched controls: 112 | 7.34 ± 1.96 | 5.22 ± 1.75 | < 0.001 |
| Iacobellis et al[30], 2008 | Patients with IFG: 65; non-diabetic controls: 50 | Males: 8.00 ± 3.00 | 6.00 ± 2.00 | < 0.001 |
| Females: 7.10 ± 4.00 | 5.80 ± 3.00 | |||
| EAT volume (cm3) measured by computed tomography | ||||
| Wang et al[31], 2008 | T2DM patients: 49; non-diabetic controls: 78 | 166.1 ± 60.6 | 123.4 ± 41.8 | < 0.0001 |
| Akyürek et al[32], 2014 | T2DM patients: 93; non-diabetic controls: 85 | 40.1 ± 23.9 | 16.9 ± 7.7 | < 0.001 |
| Gullaksen et al[33], 2019 | T2DM patients: 44; non-diabetic controls: 59 | 119.0 ± 49.0 | 86.0 ± 40.0 | < 0.001 |
| Groves et al[34], 2014 | T2DM patients: 92; non-diabetic controls: 59 | 118.6 ± 43.0 | 70.0 ± 44.0 | < 0.0001 |
| Versteylen et al[35], 2012 | Patients with IFG: 118; non-diabetic controls: 209 | 92.0 ± 39.0 | 75.0 ± 34.0 | < 0.001 |
| EAT volume (cm3) or area (cm2) measured by cardiac magnetic resonance | ||||
| Huang et al[36], 2022 | T2DM with duration ≤ 5 yr: 56; T2DM with duration > 5 yr: 57 | 48.4 ± 13.4 cm3 | 58.4 ± 17.3 cm3 | < 0.001 |
| Evin et al[37], 2016 | T2DM patients: 20; healthy controls: 19 | 135.0 ± 31.0 cm3 | 90.0 ± 30.0 cm3 | < 0.001 |
| Al-Talabany et al[38], 2018 | T2DM patients: 54; non-diabetic controls: 29 | 13.5 ± 3.5 cm2 | 11.8 ± 4.1 cm2 | < 0.05 |
| Rado et al[39], 2019 | Prediabetes patients: 100; healthy controls: 200 | 9.2 cm2 | 7.7 cm2 | < 0.001 |
EAT: Epicardial adipose tissue; IFG: Impaired fasting glucose; IR: Insulin resistance; T2DM: Type 2 diabetes mellitus.
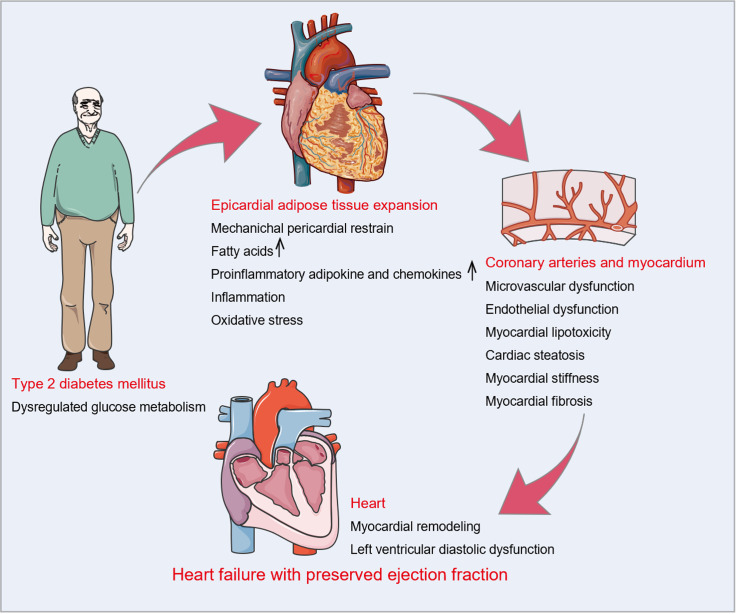
Figure 2.
Epicardial adipose tissue in the pathophysiology of heart failure with preserved ejection fraction with type 2 diabetes mellitus. Dysregulated glucose metabolism is intimately related to the expansion of epicardial adipose tissue (EAT). Increased EAT deposition interacts directly with the heart through mechanical and metabolic mechanisms. Mechanically, EAT expansion may directly contribute to pericardial restrain, resulting in left ventricular (LV) diastolic dysfunction. Metabolically, EAT enlargement is linked to the buildup of free fatty acids, which may induce myocardial lipotoxicity and cardiac steatosis. Simultaneously, hypertrophic adipocytes and activated macrophages secrete numerous proinflammatory adipocytokines and chemokines in EAT. Subsequent local inflammation, excessive oxidative stress, microvascular and endothelial dysfunction, and myocardial stiffness and fibrosis ultimately lead to LV remodeling and diastolic dysfunction.
In terms of machinery, increased EAT occupies a large space in the cardiac fossa and applies a compressive contact force on the heart, resulting in pericardial restrain, increased ventricular filling pressures, and LV diastolic dysfunction. A meta-analysis of 11 studies showed that increasing EAT was independently associated with LV diastolic dysfunction even after adjusting for age, sex, and measures of adiposity[41]. In patients with T2DM, Christensen et al[27] and Song et al[42] substantiated the deleterious effect of increased EAT on LV global longitudinal strain and LV diastolic function assessed by peak velocity during early diastole (E)/peak velocity during atrial contraction (A) ratio, early diastolic mitral annular velocity (e’), and E/e’ ratio.
In terms of metabolism, EAT enlargement is linked to the buildup of FFAs and lipid metabolites[43], which induce myocardial lipotoxicity and in turn contribute to excessive oxidative stress, endoplasmic reticulum stress, and mitochondrial dysfunction, ultimately causing LV diastolic dysfunction[44]. Furthermore, excessive cardiomyocyte lipid deposits may lead to cardiac steatosis, which has been demonstrated to be an early marker of diabetic heart disease and is independently associated with LV diastolic function[45-47]. Simultaneously, hypertrophic adipocytes and activated macrophages exhibit increased production of proinflammatory adipocytokines and chemokines in EAT. These proinflammatory factors cause local inflammation, excessive oxidative stress, microvascular and endothelial dysfunction, and extracellular matrix deposition through vasocrine or paracrine mechanisms, resulting in cardiomyocyte stiffness, myocardial fibrosis, and subsequent LV diastolic dysfunction[8,9].
Relationship between increased EAT and clinical characteristics of HFpEF
As shown in Table 2, EAT expansion is closely related to severe pathologic changes, clinical manifestations, and long-term prognosis in individuals with HFpEF[48-55]. According to research by van Woerden et al[48] and Pugliese et al[54], enlarged EAT is linked to increased plasma myocardial injury markers. Wang et al[49] found that the EAT volume was positively correlated with elevated inflammatory markers (C-reactive protein), LV hypertrophy (LV mass index), and LV diastolic dysfunction (E/e’ ratio and tricuspid regurgitation velocity). Venkateshvaran et al[50] confirmed that higher EAT was linked not only to LV hypertrophy and diastolic dysfunction but also to endothelial dysfunction. Koepp et al[51] showed that thickened EAT was associated with elevated cardiac filling pressures, pulmonary hypertension, and pericardial constraint. Additionally, some studies have confirmed that increased EAT may lead to decreased exercise tolerance or quality of life[50-54]. Importantly, EAT thickening was correlated with a 1.12-fold increased risk of the composite endpoint of death and HF hospitalization after 21 mo of follow-up, according to Pugliese et al[54]. After 24 mo of follow-up, van Woerden et al[55] confirmed that EAT expansion increased the risk of all-cause mortality, HF hospitalization, and the composite endpoint.
Table 2.
Relationship between increased epicardial adipose tissue and clinical characteristics of heart failure with preserved ejection fraction
|
Ref.
|
Participants, n
|
Imaging method
|
Relationship between increased EAT and clinical characteristics of HFpEF
|
||
|
Pathological changes
|
Clinical manifestations
|
Prognosis
|
|||
| van Woerden et al[48], 2018 | 64 HF patients with LVEF > 40% | CMR | Myocardial injury: increased creatine kinase-MB and TnT | Decreased quality of life (KCCQ score) | |
| Wang et al[49], 2022 | 53 HF patients with LVEF > 50% | CMR | Inflammation: increased CRP; LV hypertrophy: increased LVmass index; LV diastolic dysfunction: increased E/e' and tricuspid regurgitation velocity | ||
| Venkateshvaran et al[50], 2022 | 182 HF patients with LVEF > 50% | Echo | Inflammation; endothelial dysfunction; LV hypertrophy: increased LV septal wall thickness; LV diastolic dysfunction: increased E peak deceleration time | Decreased quality of life (KCCQ score) | |
| Koepp et al[51], 2020 | 169 HF patients with LVEF > 50% | Echo | Increased cardiac filling pressures, pulmonary hypertension, and pericardial restraint | Decreased exercise capacity (VO2, AVO2 diff) | |
| Haykowsky et al[52], 2018 | 100 HF patients with LVEF > 50% | CMR | Decreased exercise capacity (VO2, 6-min walk test, leg power) | ||
| Gorter et al[53], 2020 | 75 HF patients with LVEF > 45% | Echo | Decreased exercise capacity (VO2) | ||
| Pugliese et al[54], 2021 | 188 HF patients with LVEF > 50% | Echo | Myocardial injury: increased TnT; inflammation: increased CRP | Decreased exercise capacity (peak VO2 and AVO2 diff) | Increased risk of the composite endpoint of HF hospitalization and cardiovascular deaths |
| van Woerden et al[55], 2022 | 105 HF patients with LVEF > 40% | CMR | Increased risk of HF hospitalization, all-cause death, and the composite endpoint | ||
AVO2 diff: Non-invasive arterial-venous oxygen content difference; CMR: Cardiac magnetic resonance; CRP: C-reactive protein; EAT: Epicardial adipose tissue; Echo: Echocardiography; E/e': Peak velocity during early diastole/early diastolic mitral annular velocity; HF: Heart failure; HFpEF: Heart failure with preserved ejection fraction; KCCQ: Kansas City cardiomyopathy questionnaire; LV: Left ventricular; LVEF: Left ventricular ejection fraction; MB: Myocardial band; TnT: Troponin T; VO2: Peak oxygen consumption.
CURRENT INTERVENTIONS TARGETING EAT AND FUTURE THERAPEUTIC PERSPECTIVES IN HFPEF WITH T2DM
EAT plays an important role in the development and progression of HFpEF with T2DM and is strongly associated with an increased risk of adverse outcomes. Therefore, alleviating EAT expansion may be a promising therapeutic strategy. Although no treatment is available specifically for EAT, lifestyle management, bariatric surgery, and medications (Table 3) including anti-hyperlipidemia, anti-cytokines, and anti-hyperglycemia have been demonstrated to reduce the inflammation response or expansion of EAT and appear to be beneficial for HFpEF (Figure 3).
Table 3.
Pharmacological interventions targeting epicardial adipose tissue
|
Ref.
|
Imaging method
|
Participants, n
|
Intervention method and duration
|
Change of EAT
|
Other findings
|
| Park et al[74], 2010 | Echo | 145 coronary artery stenosis patients | Atorvastatin: n = 82, 20 mg/d; simvastatin: n = 63, 10 mg/d; for 6-8 mo | Atorvastatin decreased EAT thickness (0.47 ± 0.65 mm) more than simvastatin (EAT 0.12 ± 0.52 mm, P = 0.001) | Decreased TC, TG, and LDL-C |
| Soucek et al[75], 2015 | CT | 38 atrial fibrillation patients | Atorvastatin: 80 mg/d, for 3 mo | EAT volume decreased from 86.9 (64.1-124.8) mL to 92.3 (62.0- 133.3) mL (P < 0.05) | Decreased CRP, TC, and LDL-C |
| Alexopoulos et al[76], 2013 | CT | 420 hyperlipidemic post-menopausal women | Atorvastatin: n = 194, 80 mg/d; pravastatin: n = 226, 40 mg/d; for 12 mo | Atorvastatin decreased EAT volume (3.38%) more than pravastatin (0.83%, P = 0.025) | Decreased TC, TG, and LDL-C |
| Rivas Galvez et al[78], 2020 | Echo | 41 patients treated with PCSK9 inhibitors | Evolocumab: n = 16; alirocumab: n = 8; twice in 6 mo | EAT thickness decreased by 20.39% (P = 0.0001). | Decreased BMI, TC, and LDL-C |
| Iacobellis et al[82], 2017 | Echo | 41 patients T2DM | Metformin: 500 mg-1000 mg, twice daily, for 6 mo | EAT thickness changed from 7.4 ± 1.6 mm to 7.5 ± 1.5 mm and 6.9 ± 1.3 mm at 3 and 6 mo, respectively | Decreased BMI |
| Ziyrek et al[83], 2019 | Echo | 40 T2DM patients | Metformin: 1000 mg, twice daily, for 3 mo | EAT thickness decreased from 5.07 ± 1.33 mm to 4.76 ± 1.32 mm (P < 0.001) | |
| Iacobellis et al[84], 2020 | Echo | 51 T2DM patients | Metformin: 500 mg-1000 mg, twice daily, for 6 mo | EAT thickness decreased from 8.0 ± 2.5 mm to 7.4 ± 2.5 mm and 7.5 ± 2.4 mm at 3 and 6 mo, respectively (compared with baseline P < 0.016) | |
| Moody et al[90], 2014 | CMR | 12 T2DM patients | Pioglitazone: 15 mg/d, for 2 wk, then increase to 45 mg/d, for 22 wk | EAT area decreased from 15.3 ± 3.9 cm2 to 14.0 ± 3.9 cm2 (P = 0.03) | Decreased paracardial adipose tissue; improved left ventricular diastolic function |
| Lima-Martínez et al[94], 2015 | Echo | 26 T2DM patients | Combination of sitagliptin (50 mg) and metformin (1000 mg), twice daily, for 24 wk | EAT thickness reduction of 15% (P = 0.001) | |
| van Eyk et al[99], 2019 | CMR | 22 T2DM patients | Liraglutide: 0.6 mg/d gradually increased to 1.8 mg/d in 2 wk, for 26 wk | EAT area reduction of 0 ± 2 cm2 | Decreased visceral fat volume |
| Bizino et al[100], 2020 | CMR | 23 T2DM patients | Liraglutide: 0.6 mg/d gradually increased to 1.8 mg/d in 2 wk, 26 wk | EAT area reduction of 1.1 ± 6.0 cm2 | Decreased body weight and subcutaneous fat |
| Iacobellis et al[82], 2017 | Echo | 54 T2DM patients | Combination of liraglutide (increased to 1.8 mg/once daily) and metformin (1000 mg, twice daily), for 12 wk | EAT thickness reduction of 29% and 36% at 3 and 6 mo, respectively | Decreased BMI and HbA1c |
| Zhao et al[101], 2021 | Echo | 21 T2DM patients | Liraglutide: 0.6 mg/d gradually increased to 1.2 mg/d in 3-5 d, for 3 mo | EAT decreased from 5.00 (5.0-7.0) mm to 3.95 ± 1.43 mm (P < 0.001) | Decreased weight, HbA1c, TC, TG, and LDL-C |
| Dutour et al[102], 2016 | CMR | 22 T2DM patients | Exenatide: 5 mg twice daily, for 4 wk, then increase to 10 mg twice daily, for 22 wk | EAT volume reduction of 8.8 ± 2.1% | Decreased weight, HbA1c, and hepatic triglyceride content |
| Morano et al[103], 2015 | Echo | 25 T2DM patients | Combination of exenatide (5 mg twice daily, for 1 mo, and then increase to 10 mg twice daily, for 2 mo) and liraglutide (1.2 mg/d), for 3 mo | EAT thickness decreased from 9.4 ± 1.6 mm to 8.0 ± 1.9 mm (P = 0.003) | Decreased MRI; improved renal resistive index |
| Iacobellis et al[104], 2020 | Echo | 6 T2DM patients | Semaglutide: n = 30, 1 mg weekly; dulaglutide: n = 30, 1.5 mg weekly; for 12 wk | EAT thickness reduction of 20% in both semaglutide and dulaglutide groups | Decreased BMI and HbA1c |
| Requena et al[108], 2021 | CMR | 84 non-diabetic patients with HFrEF | Empagliflozin: 10 mg/d, for 6 mo | EAT volume reduction of 5.14 mL, P < 0.05 | Decreasing subcutaneous fat and matrix volume |
| Ardahanlı et al[109], 2021 | Echo | 37 T2DM patients | Empagliflozin: 10 mg/d, for 6 mo | EAT thickness decreased from 7.6 ± 1.7 mm to 6.7 ± 1.3 mm (P < 0.001) | Decreased BMI, waist circumference, HbA1c, uric acid, systolic and diastolic blood pressure, and carotid intima-media thickness |
| Iacobellis et al[84], 2020 | Echo | 51 T2DM patients | Combination of dapagliflozin (5 to 10 mg/d) and metformin (500 to 1000 mg, twice daily), for 24 mo | EAT thickness decreased by 15% from baseline to 12 wk and 20% after 24 wk (compared with baseline P < 0.01) | Decreased weight and HbA1c |
| Sato et al[110], 2018 | CT | 20 T2DM patients | Dapagliflozin: 10 mg/d, for 6 mo | EAT volume reduction of 16.4 ± 8.3 mL (P < 0.05) | Decreased HbA1c, TNF-α, TG, insulin resistance, and left atrial dimension |
| Sato et al[111], 2020 | CT | 18 T2DM patients with coronary artery disease | Dapagliflozin: 5 mg/d, for 6 mo | EAT volume reduction of 15.2 ± 12.8 mL (P < 0.05) | Decreased HbA1c, TNF-α, and insulin resistance |
| Braha et al[112], 2021 | CT | 52 T2DM patients | Dapagliflozin: 10 mg/d, for 6 mo | EAT volume reduction of 17.1% (P < 0.001) | Decreased BMI, triglyceride glucose index, and HbA1c |
| Yagi et al[113], 2017 | Echo | 13 T2DM patients | Canagliflozin: 100 mg/d, for 6 mo | EAT thickness decreased from 9.3 ± 2.5 to 8.1 ± 2.3 mm (P < 0.01) and to 7.3 ± 2.0 mm (P < 0.001) at 3 mo and 6 mo, respectively | Decreased BMI |
| Fukuda et al[114], 2017 | CMR | 9 T2DM patients | Ipragliflozin: 50 mg/d, 12 wk | EAT volume decreased from 102 (79-126) mL to 89 (66-109) mL (P = 0.008) | Decreased weight, BMI, HbA1c, TG, leptin, fasting plasma glucose, and insulin resistance |
| Bouchi et al[115], 2017 | CMR | 19 T2DM patients | Luseogliflozin: 2.5-5.0 mg/d for 12 wk | EAT volume decreased from 117 (96-136) mL to 111 (88-134) mL (P = 0.048) | Decreased weight, BMI, systolic and diastolic blood pressure, HbA1c, fasting plasma glucose, insulin resistance, and CRP |
| Gaborit et al[116], 2021 | CMR | 26 T2DM patients | Empagliflozin: 10 mg/d, 12 wk | EAT volume decreased from 108.5 ± 31.8 mL to 106.9 ± 31.8 mL (P = 0.09) | Decreased BMI, TG, HbA1c, fasting blood glucose, liver fat content, and visceral fat volume |
BMI: Body mass index; CMR: Cardiovascular magnetic resonance; CRP: C-reactive protein; CT: Computed tomography; EAT: Epicardial adipose tissue; Echo: Echocardiography; HbA1c: Glycosylated hemoglobin; HFrEF: Heart failure with reduced ejection fraction; LDL-C: Low-density lipoprotein cholesterol; MRI: Magnetic resonance imaging; PCSK9: Proprotein convertase subtilisin/kexin type 9; T2DM: Type 2 diabetes mellitus; TC: Total cholesterol; TG: Triglycerides; TNF-α: Tumor necrosis factor-α.
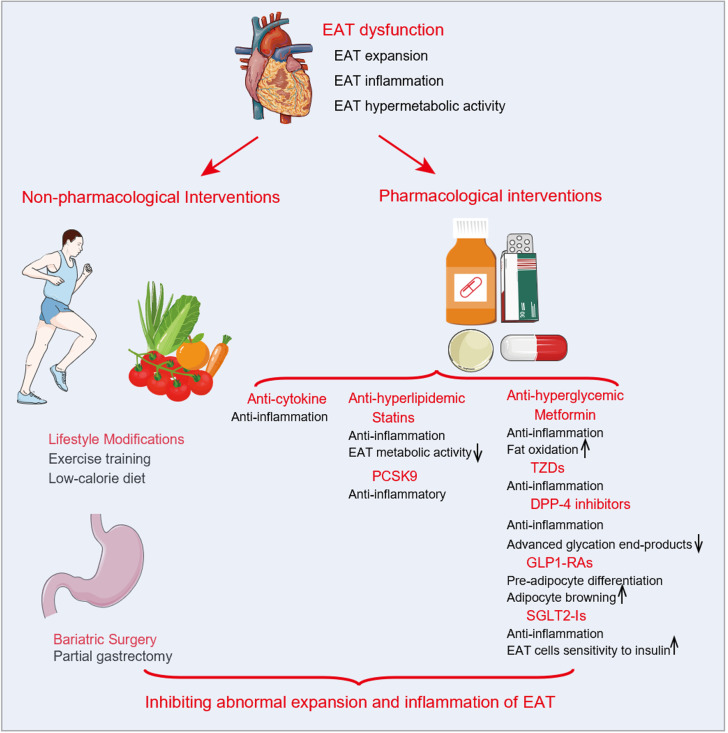
Figure 3.
Current interventions targeting epicardial adipose tissue and possible mechanisms. Current interventions targeting epicardial adipose tissue (EAT) reported in the literature include non-pharmacological interventions (lifestyle management and bariatric surgery) and pharmacological interventions related to anti-cytokines, anti-hyperlipidemia, and anti-hyperglycemia. By increasing fat oxidation or sensitivity to insulin and inhibiting inflammation or hypermetabolic activity, these interventions may prevent abnormal expansion and inflammation of EAT. EAT: Epicardial adipose tissue; DPP-4: Dipeptidyl peptidase 4; GLP1-RAs: Glucagon-like peptide-1 receptor agonists; PCSK9: Proprotein convertase subtilisin/kexin type 9; SGLT2-Is: Sodium-glucose cotransporter 2 inhibitors; TZDs: Thiazolidinediones.
Non-pharmacological interventions
In diabetic and obese patients, lifestyle modifications (including a low-calorie diet and exercise training) and bariatric surgery can reduce EAT levels. Twenty severely obese patients were shown to have a 32% reduction in EAT thickness and alleviation in LV hypertrophy and diastolic dysfunction after 6 mo of calorie restriction with moderate exercise[56]. Serrano-Ferrer et al[57] confirmed that exercise training significantly reduced EAT thickness and serum TNF-α, increased lipocalin, and improved LV myocardial strain and strain rate. A study by Honkala et al[58] reported that 2 wk of continuous exercise training resulted in decreased EAT volume and myocardial triglyceride levels and improved aerobic exercise tolerance and insulin sensitivity in 16 patients with T2DM. A meta-analysis including five studies confirmed that exercise training reduced epicardial fat deposition[59].
Several studies have reported that bariatric surgery substantially reduces the accumulation of EAT in patients[60-64]. Gaborit et al[62] found a 27% reduction in EAT volume in obese patients at a 6-mo follow-up after bariatric surgery. In addition, individuals with HFpEF appear to benefit from lifestyle changes and bariatric surgery in terms of improved microvascular and endothelial dysfunction, left ventricular remodeling and diastolic dysfunction, exercise tolerance, and quality of life[65-68]. Thus, lifestyle modification and bariatric surgery may alleviate the abnormal expansion of EAT in HFpEF patients with obesity and T2DM and improve LV diastolic function and clinical symptoms. Nevertheless, further research is required to determine whether it can improve the prognosis of patients.
Pharmacological interventions
Anti-cytokine drugs: Inflammation is an essential driver of abnormal EAT expansion. Theoretically, anti-cytokine drugs (anti-IL-1 and anti-IL-6, etc) can interfere with the pathophysiological process of EAT expansion and may eventually decrease EAT accumulation. Unfortunately, there are no relevant studies to confirm this. Furthermore, anti-cytokine drugs, particularly IL-1 blockade, have shown cardioprotective effects in many cardiovascular diseases[69]. Nevertheless, few clinical studies have examined their effects on HFpEF, and the results are inconsistent. The D-HART trial showed that a 14-d intervention with anakinra, an IL-1 blocker, significantly reduced the systemic inflammatory response and improved aerobic exercise capacity in individuals with HFpEF (n = 12)[70]. Contrarily, the D-HART 2 trial found that anakinra intervention for 12 d failed to improve exercise capacity in patients with HFpEF (n = 21)[71]. Therefore, whether anti-cytokine drugs reduce EAT deposition has not been confirmed in clinical investigations, and their role in HFpEF with T2DM requires validation in standardized randomized controlled trials.
Anti-hyperlipidemic drugs: Statins are 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors that can significantly reduce endogenous cholesterol production by inhibiting the rate-limiting enzyme in cholesterol synthesis[72]. As the anti-inflammatory effects have been established, researchers have begun to explore the role of statins in EAT in the last decade. According to Parisi et al[73], statin therapy dramatically decreased EAT thickness and EAT-secreted inflammatory mediators in individuals with aortic stenosis. In patients who successfully underwent percutaneous coronary intervention, Park et al[74] demonstrated that atorvastatin (20 mg/d) reduced EAT thickness more significantly than simvastatin/ezetimibe (10/10 mg/d). Soucek et al[75] confirmed that substantial reductions in EAT were associated with intensive atorvastatin therapy (80 mg/d) in atrial fibrillation patients undergoing pulmonary vein isolation. A study by Alexopoulos et al[76] showed that intensive treatment (ator-vastatin, 80 mg/d) was more successful in inducing EAT reduction than moderate-intensity treatment (pravastatin, 40 mg/d) in hyperlipidemic post-menopausal women.
Furthermore, proprotein convertase subtilisin/kexin type 9 (PCSK9), part of the EAT secretome, is involved in EAT-induced inflammation[77]. Therefore, PCSK9 inhibitors, a new class of lipid-lowering drugs, may inhibit the abnormal expansion of EAT. A non-randomized cohort of 24 patients reported a 20.39% reduction in EAT thickness after 6 mo of PCSK9 inhibitor treatment (evolocumab or alirocumab)[78]. In recent years, statin therapy has been reported to considerably reduce mortality in patients with HFpEF, possibly associated with a reduction in the inflammatory response or accumulation of EAT[79,80]. Thus, hypolipidemic medicines may attenuate aberrant EAT expansion and be advantageous in diabetic HFpEF, and well-designed randomized controlled trials are still needed to validate this.
Anti-hyperglycemic drugs: Metformin, an oral anti-hyperglycemic drug for patients with T2DM, lowers blood glucose levels by decreasing hepatic glucose production (gluconeogenesis) and improves insulin sensitivity by increasing peripheral glucose uptake and utilization[81]. In recent years, several studies have begun to explore its impacts on EAT, as its positive effects on reducing body weight and fat composition have been revealed. Iacobellis et al[82] showed that metformin treatment (500-1000 mg, twice daily) for 3-6 mo failed to reduce EAT thickness in patients with T2DM. In contrast, Ziyrek et al[83] found a significant reduction of EAT thickness after 3 mo of metformin monotherapy (1000 mg, twice daily) in individuals with T2DM. After increasing the sample size, Iacobellis et al[84] also discovered that metformin slightly reduced EAT thickness. Additionally, metformin treatment decreased mortality in HFpEF patients and improved LV hypertrophy and diastolic dysfunction[85,86]. Unfortunately, studies on the effects of metformin on EAT accumulation are scarce and controversial, and future research is needed to generate robust evidence.
Thiazolidinediones (TZDs), which are peroxisome proliferator-activated receptor gamma (PPAR- γ) agonists, can enhance insulin sensitivity by activating peroxisome proliferator-activated receptor gamma[87]. As a result, it reduces the secretion of proinflammatory cytokines in the visceral fat depots and thereby can inhibit the abnormal enlargement of EAT[88]. Pioglitazone, a member of TZDs, was shown to significantly reduce EAT inflammatory markers (IL-6, TNF-α, resistin, and matrix metalloproteinase-9) and increase adiponectin in patients with coronary artery disease and metabolic syndrome[89]. According to Moody et al[90], pioglitazone treatment was linked to a 9% reduction in EAT area and improvement in LV diastolic function in patients with T2DM, and there was a significant negative correlation between EAT and LV diastolic function. However, TZDs may cause serious cardiovascular adverse effects, especially HF[91,92]. As a result, the clinical use of TZDs in the treatment of HFpEF is limited due to their potential to exacerbate HF.
Dipeptidyl peptidase 4 (DPP-4) inhibitors improve glucose-dependent insulin secretion by increasing bioactive incretins, which inhibit glucagon release and then promote insulin production to decrease blood glucose levels[93]. Only a single-group pre-post study by Lima-Martínez et al[94] showed that 26 overweight patients with T2DM had a 15% reduction in EAT thickness after 6 mo of treatment with a combination of metformin and sitagliptin, a DPP-4 inhibitor. Unfortunately, there is a lack of research on regulating EAT using DPP-4 inhibitors alone. Therefore, relevant studies still need to support whether DPP-4 inhibitors can reduce EAT accumulation. In addition, it is controversial whether an increased risk of HF is associated with DPP-4 inhibitors[95].
Glucagon-like peptide-1 receptor agonists (GLP1-RAs) comprise a novel anti-diabetic drug class that maintains glucose homeostasis by stimulating glucose-dependent insulin secretion, suppressing glucagon release, and inhibiting gastric emptying[96]. Previous studies reported the presence of GLP-1R in EAT with mRNA and protein expression, and targeting GLP-1R in EAT can reduce local adi-pogenesis, enhance fat utilization, and drive brown fat differentiation[97,98]. According to research by van Eyk et al[99] and Bizino et al[100], liraglutide reduced visceral or subcutaneous fat but failed to reduce EAT accumulation in T2DM. Five investigations, however, demonstrated that liraglutide[82,101-103], exenatide[102,103], semaglutide[104], and dulaglutide[104] not only significantly decreased EAT deposition but also improved glycolipid metabolism disorders. A meta-analysis performed by Berg et al[105] confirmed that GLP1-RAs suppressed the abnormal accumulation of EAT. Moreover, liraglutide treatment has been shown to improve LV stiffness and diastolic dysfunction and reduce mortality in HFpEF patients[106]. As a result, GLP1-RAs can inhibit abnormal EAT expansion and may be beneficial for HFpEF. However, further research on this subject is still necessary due to the small numbers of both studies and subjects.
Sodium-glucose cotransporter 2 inhibitors (SGLT2-Is), the newly developed anti-hyperglycemic agents, bind to the SGLT2 transporter in the proximal tubule of the kidney and then promote the urinary excretion of glucose by preventing the reabsorption of glucose[96]. In recent years, SGLT2-Is have been found to play an essential role in mediating anti-inflammatory effects, and therefore its role in regulating EAT has gained significant attention. In individuals undergoing cardiac surgery, Diaz Díaz-Rodríguez et al[107] demonstrated the expression of SGLT2 in EAT and that dapagliflozin promoted the differentiation of EAT cells and decreased the release of proinflammatory chemokines in in vitro assays. Multiple clinical studies have demonstrated that SGLT2-Is (empagliflozin[108,109], dapagliflozin[84,110-112], canagliflozin[113], ipragliflozin[114], luseogliflozin[115]) can dramatically decrease EAT deposition, improve glucolipid metabolism, and reduce inflammatory responses. Conversely, only one study by Gaborit et al[116] indicated that empagliflozin failed to reduce EAT volume in patients with T2DM.
A meta-analysis conducted by Masson et al[117] confirmed that SGLT2-Is could significantly reduce EAT accumulation and improve glucolipid metabolism. Interestingly, Requena-Ibáñez et al[108] reported that empagliflozin could reduce EAT volume in patients with non-diabetic HFrEF. According to Yagi et al[113], canagliflozin reduced EAT thickness independent of lowering blood glucose. Thus, SGLT2-Is play an essential role in inhibiting EAT accumulation, possibly independent of glycemic control. Moreover, the current studies confirmed that SGLT2-Is exerts direct pleiotropic effects on the myocardium of HFpEF model animals through multiple mechanisms, such as reducing inflammation, suppressing oxidative stress, and improving cardiac structural and functional dysfunction (myocardial hypertrophy, stiffness fibrosis, and LV diastolic dysfunction)[118-121]. Clinically, SGLT2-Is (em-pagliflozin and dapagliflozin) have been confirmed to improve exercise tolerance[122] and quality of life in HFpEF patients[123,124] and lower the risk of cardiovascular death or HF hospitalization[125-127]. Consequently, SGLT2-Is exhibit significant prevention of abnormal EAT expansion and positive therapeutic effects in HFpEF, which warrants further clinical validation.
SUMMARY AND FUTURE PERSPECTIVES
T2DM can be one of the essential drivers of the occurrence and development of HFpEF and is associated with a worse prognosis of HFpEF. Systemic inflammation associated with glucose metabolism disorders is a crucial pathological mechanism for HFpEF with T2DM, which is associated with the expansion and dysfunction of EAT. EAT is a facilitator of the pathophysiological process of HFpEF, which may promote inflammation, oxidative stress, myocardial steatosis, and myocardial fibrosis via vasocrine or paracrine mechanisms, ultimately contributing to LV remodeling and diastolic dysfunction. Accordingly, inhibition of the expansion of EAT may be an attractive therapeutic intervention for HFpEF with T2DM.
Currently, lifestyle management, bariatric surgery, and certain medications related to anti-cytokines, anti-hyperlipidemia, and anti-hyperglycemia can help to alleviate the inflammation and or accumulation of EAT and reduce clinical symptoms or improve long-term prognosis in patients with HFpEF. Nevertheless, the specific mechanisms by which these drugs inhibit EAT expansion remain to be further explored, and clinical studies on their use in HFpEF with T2DM are lacking. As a result, relevant foundational research and well-designed randomized controlled trials are needed to elucidate the pharmacological mechanisms and efficacy of current interventions. Another critical aspect is to develop new methods to suppress the inflammation or expansion of EAT. Concomitantly, it is essential to thoroughly investigate the mechanisms of abnormal accumulation of EAT so that more novel and effective therapies targeting EAT will become available.
CONCLUSION
In the development of HFpEF with T2DM, the expansion and dysfunction of EAT exert an essential role. Through vasocrine or paracrine pathways, abnormal EAT accumulation may lead to inflammation, oxidative stress, myocardial steatosis, and myocardial fibrosis, resulting in LV remodeling and diastolic dysfunction, which are essential features of HFpEF. Therefore, targeting EAT may be a prospective therapeutic intervention for HFpEF with T2DM. At present, lifestyle management, bariatric surgery, and pharmaceutical interventions may help alleviate the expansion of EAT and improve the clinical manifestations or prognoses of HFpEF patients. Nonetheless, well-designed randomized controlled studies are required to confirm the efficacy of existing treatments. Moreover, it is hoped that more novel and effective therapies targeting EAT will become available in the future.
Footnotes
Conflict-of-interest statement: There are no conflicts of interest associated with the senior author or coauthor who contributed their efforts to this manuscript.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: December 28, 2022
First decision: February 8, 2023
Article in press: April 24, 2023
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eiras S, Spain; Papazafiropoulou A, Greece; Xu L, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Cai YX
Contributor Information
Yu-Jiao Shi, Department of Cardiovascular Medicine, Xiyuan Hospital, Chinese Academy of Traditional Chinese Medicine, Beijing 100091, China.
Guo-Ju Dong, Department of Cardiovascular Medicine, Xiyuan Hospital, Chinese Academy of Traditional Chinese Medicine, Beijing 100091, China. 13691393589@163.com.
Ming Guo, Department of Cardiovascular Medicine, Xiyuan Hospital, Chinese Academy of Traditional Chinese Medicine, Beijing 100091, China.
References
- 1.Pfeffer MA, Shah AM, Borlaug BA. Heart Failure With Preserved Ejection Fraction In Perspective. Circ Res . 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol . 2017;14:591–602. doi: 10.1038/nrcardio.2017.65. [DOI] [PubMed] [Google Scholar]
- 3.Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol . 2017;70:2476–2486. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- 4.Omote K, Verbrugge FH, Borlaug BA. Heart Failure with Preserved Ejection Fraction: Mechanisms and Treatment Strategies. Annu Rev Med . 2022;73:321–337. doi: 10.1146/annurev-med-042220-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype-Specific Treatment of Heart Failure With Preserved Ejection Fraction: A Multiorgan Roadmap. Circulation . 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anker SD, Butler J, Filippatos G, Shahzeb Khan M, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Seronde MF, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M EMPEROR-Preserved Trial Committees and Investigators. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur J Heart Fail . 2020;22:2383–2392. doi: 10.1002/ejhf.2064. [DOI] [PubMed] [Google Scholar]
- 7.Packer M. Disease-treatment interactions in the management of patients with obesity and diabetes who have atrial fibrillation: the potential mediating influence of epicardial adipose tissue. Cardiovasc Diabetol . 2019;18:121. doi: 10.1186/s12933-019-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony SR, Guarnieri AR, Gozdiff A, Helsley RN, Phillip Owens A, Tranter M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin Sci (Lond) . 2019;133:2329–2344. doi: 10.1042/CS20190578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol . 2022;19:593–606. doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV, Bordi C. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol . 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Shirani J, Berezowski K, Roberts WC. Quantitative measurement of normal and excessive (cor adiposum) subepicardial adipose tissue, its clinical significance, and its effect on electrocardiographic QRS voltage. Am J Cardiol . 1995;76:414–418. doi: 10.1016/s0002-9149(99)80116-7. [DOI] [PubMed] [Google Scholar]
- 12.Couselo-Seijas M, Rodríguez-Mañero M, González-Juanatey JR, Eiras S. Updates on epicardial adipose tissue mechanisms on atrial fibrillation. Obes Rev . 2021;22:e13277. doi: 10.1111/obr.13277. [DOI] [PubMed] [Google Scholar]
- 13.Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab . 2011;22:450–457. doi: 10.1016/j.tem.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev . 2007;8:253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 15.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013–1022. [PubMed] [Google Scholar]
- 16.Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab . 2013;98:E1448–E1455. doi: 10.1210/jc.2013-1265. [DOI] [PubMed] [Google Scholar]
- 17.Gaborit B, Sengenes C, Ancel P, Jacquier A, Dutour A. Role of Epicardial Adipose Tissue in Health and Disease: A Matter of Fat? Compr Physiol . 2017;7:1051–1082. doi: 10.1002/cphy.c160034. [DOI] [PubMed] [Google Scholar]
- 18.Smith U, Kahn BB. Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids. J Intern Med . 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J Clin Endocrinol Metab . 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 20.Burgeiro A, Fuhrmann A, Cherian S, Espinoza D, Jarak I, Carvalho RA, Loureiro M, Patrício M, Antunes M, Carvalho E. Glucose uptake and lipid metabolism are impaired in epicardial adipose tissue from heart failure patients with or without diabetes. Am J Physiol Endocrinol Metab . 2016;310:E550–E564. doi: 10.1152/ajpendo.00384.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baloglu I, Turkmen K, Selcuk NY, Tonbul HZ, Ozcicek A, Hamur H, Iyısoy S, Akbas EM. The Relationship Between Visceral Adiposity Index and Epicardial Adipose Tissue in Patients with Type 2 Diabetes Mellitus. Exp Clin Endocrinol Diabetes . 2021;129:390–395. doi: 10.1055/a-0892-4290. [DOI] [PubMed] [Google Scholar]
- 22.Akbas EM, Hamur H, Demirtas L, Bakirci EM, Ozcicek A, Ozcicek F, Kuyrukluyildiz U, Turkmen K. Predictors of epicardial adipose tissue in patients with type 2 diabetes mellitus. Diabetol Metab Syndr . 2014;6:55. doi: 10.1186/1758-5996-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Wu W, Wang L, Shi Y, Shen F, Gu X, Jia Z. Association Between 25-Hydroxyvitamin D and Epicardial Adipose Tissue in Chinese Non-Obese Patients with Type 2 Diabetes. Med Sci Monit . 2017;23:4304–4311. doi: 10.12659/MSM.904755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Philouze C, Obert P, Nottin S, Benamor A, Barthez O, Aboukhoudir F. Dobutamine Stress Echocardiography Unmasks Early Left Ventricular Dysfunction in Asymptomatic Patients with Uncomplicated Type 2 Diabetes: A Comprehensive Two-Dimensional Speckle-Tracking Imaging Study. J Am Soc Echocardiogr . 2018;31:587–597. doi: 10.1016/j.echo.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Cetin M, Cakici M, Polat M, Suner A, Zencir C, Ardic I. Relation of epicardial fat thickness with carotid intima-media thickness in patients with type 2 diabetes mellitus. Int J Endocrinol . 2013;2013:769175. doi: 10.1155/2013/769175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yafei S, Elsewy F, Youssef E, Ayman M, Elshafei M, Abayazeed R. Echocardiographic association of epicardial fat with carotid intima-media thickness in patients with type 2 diabetes. Diab Vasc Dis Res . 2019;16:378–384. doi: 10.1177/1479164119827602. [DOI] [PubMed] [Google Scholar]
- 27.Christensen RH, Hansen CS, von Scholten BJ, Jensen MT, Pedersen BK, Schnohr P, Vilsbøll T, Rossing P, Jørgensen PG. Epicardial and pericardial adipose tissues are associated with reduced diastolic and systolic function in type 2 diabetes. Diabetes Obes Metab . 2019;21:2006–2011. doi: 10.1111/dom.13758. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Zhang Y, Liu W, Su B. Evaluation of Epicardial Adipose Tissue in Patients of Type 2 Diabetes Mellitus by Echocardiography and its Correlation with Intimal Medial Thickness of Carotid Artery. Exp Clin Endocrinol Diabetes . 2017;125:598–602. doi: 10.1055/s-0042-114035. [DOI] [PubMed] [Google Scholar]
- 29.Altin C, Sade LE, Gezmis E, Yilmaz M, Ozen N, Muderrisoglu H. Assessment of epicardial adipose tissue and carotid/femoral intima media thickness in insulin resistance. J Cardiol . 2017;69:843–850. doi: 10.1016/j.jjcc.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, Barbaro G, Gerstein HC. Relationship of epicardial fat thickness and fasting glucose. Int J Cardiol . 2008;128:424–426. doi: 10.1016/j.ijcard.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 31.Wang CP, Hsu HL, Hung WC, Yu TH, Chen YH, Chiu CA, Lu LF, Chung FM, Shin SJ, Lee YJ. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin Endocrinol (Oxf) . 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 32.Akyürek Ö, Efe D, Kaya Z. Thoracic periaortic adipose tissue in relation to cardiovascular risk in type 2 diabetes mellitus. Wien Klin Wochenschr . 2014;126:767–773. doi: 10.1007/s00508-014-0611-8. [DOI] [PubMed] [Google Scholar]
- 33.Gullaksen S, Funck KL, Laugesen E, Hansen TK, Dey D, Poulsen PL. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc Dis Res . 2019;16:328–336. doi: 10.1177/1479164119825761. [DOI] [PubMed] [Google Scholar]
- 34.Groves EM, Erande AS, Le C, Salcedo J, Hoang KC, Kumar S, Mohar DS, Saremi F, Im J, Agrawal Y, Nadeswaran P, Naderi N, Malik S. Comparison of epicardial adipose tissue volume and coronary artery disease severity in asymptomatic adults with versus without diabetes mellitus. Am J Cardiol . 2014;114:686–691. doi: 10.1016/j.amjcard.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Versteylen MO, Takx RA, Joosen IA, Nelemans PJ, Das M, Crijns HJ, Hofstra L, Leiner T. Epicardial adipose tissue volume as a predictor for coronary artery disease in diabetic, impaired fasting glucose, and non-diabetic patients presenting with chest pain. Eur Heart J Cardiovasc Imaging . 2012;13:517–523. doi: 10.1093/ehjci/jes024. [DOI] [PubMed] [Google Scholar]
- 36.Huang S, Li Y, Jiang L, Ren Y, Wang J, Shi K, Yan WF, Qian WL, Yang ZG. Impact of Type 2 Diabetes Mellitus on Epicardial Adipose Tissue and Myocardial Microcirculation by MRI in Postmenopausal Women. J Magn Reson Imaging . 2022;56:1404–1413. doi: 10.1002/jmri.28121. [DOI] [PubMed] [Google Scholar]
- 37.Evin M, Broadhouse KM, Callaghan FM, McGrath RT, Glastras S, Kozor R, Hocking SL, Lamy J, Redheuil A, Kachenoura N, Fulcher GR, Figtree GA, Grieve SM. Impact of obesity and epicardial fat on early left atrial dysfunction assessed by cardiac MRI strain analysis. Cardiovasc Diabetol . 2016;15:164. doi: 10.1186/s12933-016-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Talabany S, Mordi I, Graeme Houston J, Colhoun HM, Weir-McCall JR, Matthew SZ, Looker HC, Levin D, Belch JJF, Dove F, Khan F, Lang CC. Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord . 2018;18:31. doi: 10.1186/s12872-018-0770-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rado SD, Lorbeer R, Gatidis S, Machann J, Storz C, Nikolaou K, Rathmann W, Hoffmann U, Peters A, Bamberg F, Schlett CL. MRI-based assessment and characterization of epicardial and paracardial fat depots in the context of impaired glucose metabolism and subclinical left-ventricular alterations. Br J Radiol . 2019;92:20180562. doi: 10.1259/bjr.20180562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Liu B, Li Y, Jing X, Deng S, Yan Y, She Q. Epicardial fat tissue in patients with diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019;18:3. doi: 10.1186/s12933-019-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nerlekar N, Muthalaly RG, Wong N, Thakur U, Wong DTL, Brown AJ, Marwick TH. Association of Volumetric Epicardial Adipose Tissue Quantification and Cardiac Structure and Function. J Am Heart Assoc . 2018;7:e009975. doi: 10.1161/JAHA.118.009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song XT, Wang SK, Zhang PY, Fan L, Rui YF. Association between epicardial adipose tissue and left ventricular function in type 2 diabetes mellitus: Assessment using two-dimensional speckle tracking echocardiography. J Diabetes Complications . 2022;36:108167. doi: 10.1016/j.jdiacomp.2022.108167. [DOI] [PubMed] [Google Scholar]
- 43.Kankaanpää M, Lehto HR, Pärkkä JP, Komu M, Viljanen A, Ferrannini E, Knuuti J, Nuutila P, Parkkola R, Iozzo P. Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab . 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 44.Leggat J, Bidault G, Vidal-Puig A. Lipotoxicity: a driver of heart failure with preserved ejection fraction? Clin Sci (Lond) . 2021;135:2265–2283. doi: 10.1042/CS20210127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, Romijn JA, de Roos A, Lamb HJ. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol . 2008;52:1793–1799. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 46.Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, Buss SJ, Steen H, Schnackenburg B, Bierhaus A, Nawroth PP, Katus HA. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging . 2012;35:804–811. doi: 10.1002/jmri.22879. [DOI] [PubMed] [Google Scholar]
- 47.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, Levine BD, Raskin P, Victor RG, Szczepaniak LS. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation . 2007;116:1170–1175. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 48.van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail . 2018;20:1559–1566. doi: 10.1002/ejhf.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Butcher SC, Kuneman JH, Lustosa RP, Fortuni F, Ajmone Marsan N, Knuuti J, Bax JJ, Delgado V. The Quantity of Epicardial Adipose Tissue in Patients Having Ablation for Atrial Fibrillation With and Without Heart Failure. Am J Cardiol . 2022;172:54–61. doi: 10.1016/j.amjcard.2022.02.021. [DOI] [PubMed] [Google Scholar]
- 50.Venkateshvaran A, Faxen UL, Hage C, Michaëlsson E, Svedlund S, Saraste A, Beussink-Nelson L, Fermer ML, Gan LM, Tromp J, Lam CSP, Shah SJ, Lund LH. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: insights from the PROMIS-HFpEF study. Eur J Heart Fail. 2022;24:2251–2260. doi: 10.1002/ejhf.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and Functional Impact of Epicardial Adipose Tissue in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail . 2020;8:657–666. doi: 10.1016/j.jchf.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW. Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC Heart Fail . 2018;6:640–649. doi: 10.1016/j.jchf.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorter TM, van Woerden G, Rienstra M, Dickinson MG, Hummel YM, Voors AA, Hoendermis ES, van Veldhuisen DJ. Epicardial Adipose Tissue and Invasive Hemodynamics in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail . 2020;8:667–676. doi: 10.1016/j.jchf.2020.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Pugliese NR, Paneni F, Mazzola M, De Biase N, Del Punta L, Gargani L, Mengozzi A, Virdis A, Nesti L, Taddei S, Flammer A, Borlaug BA, Ruschitzka F, Masi S. Impact of epicardial adipose tissue on cardiovascular haemodynamics, metabolic profile, and prognosis in heart failure. Eur J Heart Fail . 2021;23:1858–1871. doi: 10.1002/ejhf.2337. [DOI] [PubMed] [Google Scholar]
- 55.van Woerden G, van Veldhuisen DJ, Manintveld OC, van Empel VPM, Willems TP, de Boer RA, Rienstra M, Westenbrink BD, Gorter TM. Epicardial Adipose Tissue and Outcome in Heart Failure With Mid-Range and Preserved Ejection Fraction. Circ Heart Fail . 2022;15:e009238. doi: 10.1161/CIRCHEARTFAILURE.121.009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) . 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 57.Serrano-Ferrer J, Crendal E, Walther G, Vinet A, Dutheil F, Naughton G, Lesourd B, Chapier R, Courteix D, Obert P. Effects of lifestyle intervention on left ventricular regional myocardial function in metabolic syndrome patients from the RESOLVE randomized trial. Metabolism . 2016;65:1350–1360. doi: 10.1016/j.metabol.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Honkala SM, Motiani KK, Eskelinen JJ, Savolainen A, Saunavaara V, Virtanen KA, Löyttyniemi E, Kapanen J, Knuuti J, Kalliokoski KK, Hannukainen JC. Exercise Training Reduces Intrathoracic Fat Regardless of Defective Glucose Tolerance. Med Sci Sports Exerc . 2017;49:1313–1322. doi: 10.1249/MSS.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Launbo N, Zobel EH, von Scholten BJ, Faerch K, Jørgensen PG, Christensen RH. Targeting epicardial adipose tissue with exercise, diet, bariatric surgery or pharmaceutical interventions: A systematic review and meta-analysis. Obes Rev . 2021;22:e13136. doi: 10.1111/obr.13136. [DOI] [PubMed] [Google Scholar]
- 60.Hunt SC, Davidson LE, Adams TD, Ranson L, McKinlay RD, Simper SC, Litwin SE. Associations of Visceral, Subcutaneous, Epicardial, and Liver Fat with Metabolic Disorders up to 14 Years After Weight Loss Surgery. Metab Syndr Relat Disord . 2021;19:83–92. doi: 10.1089/met.2020.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu FZ, Huang YL, Wu CC, Wang YC, Pan HJ, Huang CK, Yeh LR, Wu MT. Differential Effects of Bariatric Surgery Versus Exercise on Excessive Visceral Fat Deposits. Medicine (Baltimore) . 2016;95:e2616. doi: 10.1097/MD.0000000000002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gaborit B, Jacquier A, Kober F, Abdesselam I, Cuisset T, Boullu-Ciocca S, Emungania O, Alessi MC, Clément K, Bernard M, Dutour A. Effects of bariatric surgery on cardiac ectopic fat: lesser decrease in epicardial fat compared to visceral fat loss and no change in myocardial triglyceride content. J Am Coll Cardiol . 2012;60:1381–1389. doi: 10.1016/j.jacc.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Salman AA, Salman MA, Soliman A, Youssef A, Labib S, Helmy MY, Marie MA, Shawkat M, Mostafa A, Tourky MS, Sarhan MD, Qassem MG, Shaaban HE, Omar MG, Abouelregal TE. Changes of epicardial fat thickness after laparoscopic sleeve gastrectomy: a prospective study. Ann Med . 2021;53:523–530. doi: 10.1080/07853890.2021.1903072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willens HJ, Byers P, Chirinos JA, Labrador E, Hare JM, de Marchena E. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am J Cardiol . 2007;99:1242–1245. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 65.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA . 2016;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail . 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angadi SS, Mookadam F, Lee CD, Tucker WJ, Haykowsky MJ, Gaesser GA. High-intensity interval training vs. moderate-intensity continuous exercise training in heart failure with preserved ejection fraction: a pilot study. J Appl Physiol (1985) . 2015;119:753–758. doi: 10.1152/japplphysiol.00518.2014. [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal R, Harling L, Efthimiou E, Darzi A, Athanasiou T, Ashrafian H. The Effects of Bariatric Surgery on Cardiac Structure and Function: a Systematic Review of Cardiac Imaging Outcomes. Obes Surg . 2016;26:1030–1040. doi: 10.1007/s11695-015-1866-5. [DOI] [PubMed] [Google Scholar]
- 69.Szekely Y, Arbel Y. A Review of Interleukin-1 in Heart Disease: Where Do We Stand Today? Cardiol Ther . 2018;7:25–44. doi: 10.1007/s40119-018-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Tassell BW, Arena R, Biondi-Zoccai G, Canada JM, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A. Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study) Am J Cardiol . 2014;113:321–327. doi: 10.1016/j.amjcard.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Tassell BW, Trankle CR, Canada JM, Carbone S, Buckley L, Kadariya D, Del Buono MG, Billingsley H, Wohlford G, Viscusi M, Oddi-Erdle C, Abouzaki NA, Dixon D, Biondi-Zoccai G, Arena R, Abbate A. IL-1 Blockade in Patients With Heart Failure With Preserved Ejection Fraction. Circ Heart Fail . 2018;11:e005036. doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science . 2001;292:1160–1164. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
- 73.Parisi V, Petraglia L, D'Esposito V, Cabaro S, Rengo G, Caruso A, Grimaldi MG, Baldascino F, De Bellis A, Vitale D, Formisano R, Ferro A, Paolillo S, Davin L, Lancellotti P, Formisano P, Perrone Filardi P, Ferrara N, Leosco D. Statin therapy modulates thickness and inflammatory profile of human epicardial adipose tissue. Int J Cardiol . 2019;274:326–330. doi: 10.1016/j.ijcard.2018.06.106. [DOI] [PubMed] [Google Scholar]
- 74.Park JH, Park YS, Kim YJ, Lee IS, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J Cardiovasc Ultrasound . 2010;18:121–126. doi: 10.4250/jcu.2010.18.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soucek F, Covassin N, Singh P, Ruzek L, Kara T, Suleiman M, Lerman A, Koestler C, Friedman PA, Lopez-Jimenez F, Somers VK. Effects of Atorvastatin (80 mg) Therapy on Quantity of Epicardial Adipose Tissue in Patients Undergoing Pulmonary Vein Isolation for Atrial Fibrillation. Am J Cardiol . 2015;116:1443–1446. doi: 10.1016/j.amjcard.2015.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alexopoulos N, Melek BH, Arepalli CD, Hartlage GR, Chen Z, Kim S, Stillman AE, Raggi P. Effect of intensive versus moderate lipid-lowering therapy on epicardial adipose tissue in hyperlipidemic post-menopausal women: a substudy of the BELLES trial (Beyond Endorsed Lipid Lowering with EBT Scanning) J Am Coll Cardiol . 2013;61:1956–1961. doi: 10.1016/j.jacc.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 77.Dozio E, Ruscica M, Vianello E, Macchi C, Sitzia C, Schmitz G, Tacchini L, Corsi Romanelli MM. PCSK9 Expression in Epicardial Adipose Tissue: Molecular Association with Local Tissue Inflammation. Mediators Inflamm . 2020;2020:1348913. doi: 10.1155/2020/1348913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivas Galvez RE, Morales Portano JD, Trujillo Cortes R, Gomez Alvarez EB, Sanchez Cubias SM, Zelaya SM. Reduction of epicardial adipose tissue thickness with PCSK9 inhibitors. Eur Heart J . 2020;41:ehaa946.3008. [Google Scholar]
- 79.Alehagen U, Benson L, Edner M, Dahlström U, Lund LH. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ Heart Fail . 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 80.Fukuta H, Goto T, Wakami K, Ohte N. The effect of statins on mortality in heart failure with preserved ejection fraction: a meta-analysis of propensity score analyses. Int J Cardiol . 2016;214:301–306. doi: 10.1016/j.ijcard.2016.03.186. [DOI] [PubMed] [Google Scholar]
- 81.LaMoia TE, Shulman GI. Cellular and Molecular Mechanisms of Metformin Action. Endocr Rev . 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iacobellis G, Mohseni M, Bianco SD, Banga PK. Liraglutide causes large and rapid epicardial fat reduction. Obesity (Silver Spring) . 2017;25:311–316. doi: 10.1002/oby.21718. [DOI] [PubMed] [Google Scholar]
- 83.Ziyrek M, Kahraman S, Ozdemir E, Dogan A. Metformin monotherapy significantly decreases epicardial adipose tissue thickness in newly diagnosed type 2 diabetes patients. Rev Port Cardiol (Engl Ed) . 2019;38:419–423. doi: 10.1016/j.repc.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 84.Iacobellis G, Gra-Menendez S. Effects of Dapagliflozin on Epicardial Fat Thickness in Patients with Type 2 Diabetes and Obesity. Obesity (Silver Spring) . 2020;28:1068–1074. doi: 10.1002/oby.22798. [DOI] [PubMed] [Google Scholar]
- 85.Gu J, Yin ZF, Zhang JF, Wang CQ. Association between long-term prescription of metformin and the progression of heart failure with preserved ejection fraction in patients with type 2 diabetes mellitus and hypertension. Int J Cardiol . 2020;306:140–145. doi: 10.1016/j.ijcard.2019.11.087. [DOI] [PubMed] [Google Scholar]
- 86.Halabi A, Sen J, Huynh Q, Marwick TH. Metformin treatment in heart failure with preserved ejection fraction: a systematic review and meta-regression analysis. Cardiovasc Diabetol . 2020;19:124. doi: 10.1186/s12933-020-01100-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab . 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 88.Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol Metab . 2014;25:293–302. doi: 10.1016/j.tem.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grosso AF, de Oliveira SF, Higuchi Mde L, Favarato D, Dallan LA, da Luz PL. Synergistic anti-inflammatory effect: simvastatin and pioglitazone reduce inflammatory markers of plasma and epicardial adipose tissue of coronary patients with metabolic syndrome. Diabetol Metab Syndr . 2014;6:47. doi: 10.1186/1758-5996-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moody AJ, Molina-Wilkins M, Clarke GD, Merovci A, Solis-Herrera C, Cersosimo E, Chilton RJ, Iozzo P, Gastaldelli A, Abdul-Ghani M, DeFronzo RA. Pioglitazone reduces epicardial fat and improves diastolic function in patients with type 2 diabetes. Diabetes Obes Metab. 2023;25:426–434. doi: 10.1111/dom.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lago RM, Singh PP, Nesto RW. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet . 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 92.Nassif M, Kosiborod M. Effect of glucose-lowering therapies on heart failure. Nat Rev Cardiol . 2018;15:282–291. doi: 10.1038/nrcardio.2017.211. [DOI] [PubMed] [Google Scholar]
- 93.Deacon CF. Dipeptidyl peptidase 4 inhibitors in the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol . 2020;16:642–653. doi: 10.1038/s41574-020-0399-8. [DOI] [PubMed] [Google Scholar]
- 94.Lima-Martínez MM, Paoli M, Rodney M, Balladares N, Contreras M, D'Marco L, Iacobellis G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: a pilot study. Endocrine . 2016;51:448–455. doi: 10.1007/s12020-015-0710-y. [DOI] [PubMed] [Google Scholar]
- 95.Li L, Li S, Deng K, Liu J, Vandvik PO, Zhao P, Zhang L, Shen J, Bala MM, Sohani ZN, Wong E, Busse JW, Ebrahim S, Malaga G, Rios LP, Wang Y, Chen Q, Guyatt GH, Sun X. Dipeptidyl peptidase-4 inhibitors and risk of heart failure in type 2 diabetes: systematic review and meta-analysis of randomised and observational studies. BMJ . 2016;352:i610. doi: 10.1136/bmj.i610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Palmer SC, Tendal B, Mustafa RA, Vandvik PO, Li S, Hao Q, Tunnicliffe D, Ruospo M, Natale P, Saglimbene V, Nicolucci A, Johnson DW, Tonelli M, Rossi MC, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque LI, Lloyd A, Ahmad N, Liu Y, Tiv S, Millard T, Gagliardi L, Kolanu N, Barmanray RD, McMorrow R, Raygoza Cortez AK, White H, Chen X, Zhou X, Liu J, Rodríguez AF, González-Colmenero AD, Wang Y, Li L, Sutanto S, Solis RC, Díaz González-Colmenero F, Rodriguez-Gutierrez R, Walsh M, Guyatt G, Strippoli GFM. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ . 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dozio E, Vianello E, Malavazos AE, Tacchini L, Schmitz G, Iacobellis G, Corsi Romanelli MM. Epicardial adipose tissue GLP-1 receptor is associated with genes involved in fatty acid oxidation and white-to-brown fat differentiation: A target to modulate cardiovascular risk? Int J Cardiol. 2019;292:218–224. doi: 10.1016/j.ijcard.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 98.Iacobellis G, Camarena V, Sant DW, Wang G. Human Epicardial Fat Expresses Glucagon-Like Peptide 1 and 2 Receptors Genes. Horm Metab Res . 2017;49:625–630. doi: 10.1055/s-0043-109563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Eyk HJ, Paiman EHM, Bizino MB, de Heer P, Geelhoed-Duijvestijn PH, Kharagjitsingh AV, Smit JWA, Lamb HJ, Rensen PCN, Jazet IM. A double-blind, placebo-controlled, randomised trial to assess the effect of liraglutide on ectopic fat accumulation in South Asian type 2 diabetes patients. Cardiovasc Diabetol. 2019;18:87. doi: 10.1186/s12933-019-0890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bizino MB, Jazet IM, de Heer P, van Eyk HJ, Dekkers IA, Rensen PCN, Paiman EHM, Lamb HJ, Smit JW. Placebo-controlled randomised trial with liraglutide on magnetic resonance endpoints in individuals with type 2 diabetes: a pre-specified secondary study on ectopic fat accumulation. Diabetologia . 2020;63:65–74. doi: 10.1007/s00125-019-05021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao N, Wang X, Wang Y, Yao J, Shi C, Du J, Bai R. The Effect of Liraglutide on Epicardial Adipose Tissue in Type 2 Diabetes. J Diabetes Res . 2021;2021:5578216. doi: 10.1155/2021/5578216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dutour A, Abdesselam I, Ancel P, Kober F, Mrad G, Darmon P, Ronsin O, Pradel V, Lesavre N, Martin JC, Jacquier A, Lefur Y, Bernard M, Gaborit B. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: a prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes Metab . 2016;18:882–891. doi: 10.1111/dom.12680. [DOI] [PubMed] [Google Scholar]
- 103.Morano S, Romagnoli E, Filardi T, Nieddu L, Mandosi E, Fallarino M, Turinese I, Dagostino MP, Lenzi A, Carnevale V. Short-term effects of glucagon-like peptide 1 (GLP-1) receptor agonists on fat distribution in patients with type 2 diabetes mellitus: an ultrasonography study. Acta Diabetol . 2015;52:727–732. doi: 10.1007/s00592-014-0710-z. [DOI] [PubMed] [Google Scholar]
- 104.Iacobellis G, Villasante Fricke AC. Effects of Semaglutide Versus Dulaglutide on Epicardial Fat Thickness in Subjects with Type 2 Diabetes and Obesity. J Endocr Soc . 2020;4:bvz042. doi: 10.1210/jendso/bvz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berg G, Barchuk M, Lobo M, Nogueira JP. Effect of glucagon-like peptide-1 (GLP-1) analogues on epicardial adipose tissue: A meta-analysis. Diabetes Metab Syndr . 2022;16:102562. doi: 10.1016/j.dsx.2022.102562. [DOI] [PubMed] [Google Scholar]
- 106.Nguyen TD, Shingu Y, Amorim PA, Schenkl C, Schwarzer M, Doenst T. GLP-1 Improves Diastolic Function and Survival in Heart Failure with Preserved Ejection Fraction. J Cardiovasc Transl Res . 2018;11:259–267. doi: 10.1007/s12265-018-9795-z. [DOI] [PubMed] [Google Scholar]
- 107.Díaz-Rodríguez E, Agra RM, Fernández ÁL, Adrio B, García-Caballero T, González-Juanatey JR, Eiras S. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovasc Res . 2018;114:336–346. doi: 10.1093/cvr/cvx186. [DOI] [PubMed] [Google Scholar]
- 108.Requena-Ibáñez JA, Santos-Gallego CG, Rodriguez-Cordero A, Vargas-Delgado AP, Mancini D, Sartori S, Atallah-Lajam F, Giannarelli C, Macaluso F, Lala A, Sanz J, Fuster V, Badimon JJ. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: From the EMPA-TROPISM Study. JACC Heart Fail . 2021;9:578–589. doi: 10.1016/j.jchf.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 109.Ardahanlı İ, Aslan R, Çelik M, Akgün O, Akyüz O. Effects of Empagliflozin on Carotid Intima-Media Thickness and Epicardial Fat Tissue Volume in Patients with Type-2 Diabetes Mellitus. Lokman Hekim Health Sci. 2021;1:74–80. [Google Scholar]
- 110.Sato T, Aizawa Y, Yuasa S, Kishi S, Fuse K, Fujita S, Ikeda Y, Kitazawa H, Takahashi M, Sato M, Okabe M. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol . 2018;17:6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato T, Aizawa Y, Yuasa S, Fujita S, Ikeda Y, Okabe M. The Effect of Dapagliflozin Treatment on Epicardial Adipose Tissue Volume and P-Wave Indices: An Ad-hoc Analysis of The Previous Randomized Clinical Trial. J Atheroscler Thromb . 2020;27:1348–1358. doi: 10.5551/jat.48009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Braha A, Albai A, Timar B, Cipu D, Vasiluță L, Potre O, Timar R. Predictors of Epicardial Fat Volume Decrease after Dapagliflozin Treatment in Patients with Type 2 Diabetes. Medicina (Kaunas) . 2021;58 doi: 10.3390/medicina58010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yagi S, Hirata Y, Ise T, Kusunose K, Yamada H, Fukuda D, Salim HM, Maimaituxun G, Nishio S, Takagawa Y, Hama S, Matsuura T, Yamaguchi K, Tobiume T, Soeki T, Wakatsuki T, Aihara KI, Akaike M, Shimabukuro M, Sata M. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr . 2017;9:78. doi: 10.1186/s13098-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fukuda T, Bouchi R, Terashima M, Sasahara Y, Asakawa M, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Ipragliflozin Reduces Epicardial Fat Accumulation in Non-Obese Type 2 Diabetic Patients with Visceral Obesity: A Pilot Study. Diabetes Ther . 2017;8:851–861. doi: 10.1007/s13300-017-0279-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bouchi R, Terashima M, Sasahara Y, Asakawa M, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, Izumiyama H, Hashimoto K, Yoshimoto T, Ogawa Y. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovasc Diabetol . 2017;16:32. doi: 10.1186/s12933-017-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gaborit B, Ancel P, Abdullah AE, Maurice F, Abdesselam I, Calen A, Soghomonian A, Houssays M, Varlet I, Eisinger M, Lasbleiz A, Peiretti F, Bornet CE, Lefur Y, Pini L, Rapacchi S, Bernard M, Resseguier N, Darmon P, Kober F, Dutour A. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovasc Diabetol . 2021;20:57. doi: 10.1186/s12933-021-01237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masson W, Lavalle-Cobo A, Nogueira JP. Effect of SGLT2-Inhibitors on Epicardial Adipose Tissue: A Meta-Analysis. Cells . 2021;10 doi: 10.3390/cells10082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kolijn D, Pabel S, Tian Y, Lódi M, Herwig M, Carrizzo A, Zhazykbayeva S, Kovács Á, Fülöp GÁ, Falcão-Pires I, Reusch PH, Linthout SV, Papp Z, van Heerebeek L, Vecchione C, Maier LS, Ciccarelli M, Tschöpe C, Mügge A, Bagi Z, Sossalla S, Hamdani N. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc Res . 2021;117:495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 119.Pabel S, Wagner S, Bollenberg H, Bengel P, Kovács Á, Schach C, Tirilomis P, Mustroph J, Renner A, Gummert J, Fischer T, Van Linthout S, Tschöpe C, Streckfuss-Bömeke K, Hasenfuss G, Maier LS, Hamdani N, Sossalla S. Empagliflozin directly improves diastolic function in human heart failure. Eur J Heart Fail. 2018;20:1690–1700. doi: 10.1002/ejhf.1328. [DOI] [PubMed] [Google Scholar]
- 120.Cappetta D, De Angelis A, Ciuffreda LP, Coppini R, Cozzolino A, Miccichè A, Dell'Aversana C, D'Amario D, Cianflone E, Scavone C, Santini L, Palandri C, Naviglio S, Crea F, Rota M, Altucci L, Rossi F, Capuano A, Urbanek K, Berrino L. Amelioration of diastolic dysfunction by dapagliflozin in a non-diabetic model involves coronary endothelium. Pharmacol Res . 2020;157:104781. doi: 10.1016/j.phrs.2020.104781. [DOI] [PubMed] [Google Scholar]
- 121.He L, Ma S, Zuo Q, Zhang G, Wang Z, Zhang T, Zhai J, Guo Y. An Effective Sodium-Dependent Glucose Transporter 2 Inhibition, Canagliflozin, Prevents Development of Hypertensive Heart Failure in Dahl Salt-Sensitive Rats. Front Pharmacol . 2022;13:856386. doi: 10.3389/fphar.2022.856386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, Lamba S, Sharma K, Khan SS, Chandra L, Gordon RA, Ryan JJ, Chaudhry SP, Joseph SM, Chow CH, Kanwar MK, Pursley M, Siraj ES, Lewis GD, Clemson BS, Fong M, Kosiborod MN. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med . 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, Ponikowski P, Shah SJ, Senni M, Vedin O, Verma S, Peil B, Pocock SJ, Zannad F, Packer M, Anker SD. Empagliflozin, Health Status, and Quality of Life in Patients With Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation . 2022;145:184–193. doi: 10.1161/CIRCULATIONAHA.121.057812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN, Januzzi JL. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med . 2022;28:809–813. doi: 10.1038/s41591-022-01703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huynh K. Empagliflozin improves clinical outcomes for HFpEF in EMPEROR-Preserved. Nat Rev Cardiol . 2021;18:737. doi: 10.1038/s41569-021-00627-z. [DOI] [PubMed] [Google Scholar]
- 126.Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam CSP, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs CJW, Comin-Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer-Gamba MA, Al Habeeb W, Han Y, Cabrera Honorio JW, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva JFK, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderäng U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM DELIVER Trial Committees and Investigators. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N Engl J Med . 2022;387:1089–1098. doi: 10.1056/NEJMoa2206286. [DOI] [PubMed] [Google Scholar]
- 127.Zhao L, Guo W, Huang W, Wang L, Huang S. Benefit of sodium-glucose cotransporter-2 inhibitors on survival outcome is related to the type of heart failure: A meta-analysis. Diabetes Res Clin Pract . 2022;187:109871. doi: 10.1016/j.diabres.2022.109871. [DOI] [PubMed] [Google Scholar]