Abstract
Retinal ganglion cells (RGCs) expanding from the retina to the brain are primary victims of neurodegeneration in glaucoma, a leading cause of blindness; however, the neighboring astroglia survive the glaucoma-related stress and promote neuroinflammation. In light of diverse functions of caspase-8 in apoptosis, cell survival, and inflammation, this study investigated the importance of caspase-8 in different fates of glaucomatous RGCs and astroglia using two experimental approaches in parallel. In the first approach, cell type-specific responses of RGCs and astroglia to a caspase-8 cleavage-inhibiting pharmacological treatment were studied in rat eyes with or without experimentally induced glaucoma. The second approach utilized an experimental model of glaucoma in mice in which astroglial caspase-8 was conditionally deleted by cre/lox. Findings of these experiments revealed cell type-specific distinct processes that regulate caspase-8 functions in experimental glaucoma, which are involved in inducing the apoptosis of RGCs and promoting the survival and inflammatory responses of astroglia. Deletion of caspase-8 in astroglia protected RGCs against glia-driven inflammatory injury, while the inhibition of caspase-8 cleavage inhibited apoptosis in RGCs themselves. Various caspase-8 functions impacting both RGC apoptosis and astroglia-driven neuroinflammation may suggest the multi-target potential of caspase-8 regulation to provide neuroprotection and immunomodulation in glaucoma.
Keywords: Astroglia, Caspase-8, Glaucoma, Neurodegeneration, Neuroinflammation
1. Introduction
In glaucoma, a leading cause of irreversible blindness, apoptosis of RGC somas in the retina, degeneration of distal and proximal axons in the optic nerve, and loss of synapses at dendrites and axon terminals lead to progressive loss of visual function (Syc-Mazurek and Libby, 2019). Interplaying compartmentalized processes are implicated in the glaucomatous degeneration of RGCs at different subcellular regions. While RGCs are primary victims of glaucomatous neurodegeneration, the neighboring glia survive the glaucoma-related stress and promote widespread inflammatory responses. Glia-driven neuroinflammation is viewed to create an additional damaging force on RGC somas, axons, and synapses (Baris and Tezel, 2019; Mac Nair and Nickells, 2015; Tezel, 2013; Williams et al., 2017). Although improved molecular understanding is critical to develop neuroprotective and immunomodulatory treatments for glaucoma, current understanding of the molecular regulation of neurodegenerative and neuroinflammatory processes is incomplete.
Despite some topographic and temporal variations, both astroglia and microglia profoundly respond to glaucoma-related tissue stress and neuron injury by morphological, molecular, and functional alterations (Lye-Barthel et al., 2013; Seitz et al., 2013). However, among multiple glial subtypes, astroglia is particularly important due to their rapidly produced, broadly manifest, and long lasting responses with widespread impacts on neuron survival in glaucoma. These features make the astroglia excellent candidates for testing as an immunomodulatory treatment target for neuroprotection. Indeed, many recent studies focusing on astroglia have supported their critical roles in the phenotype driving innate and adaptive immune responses with neurodestructive consequences in glaucoma. It is best exemplified by tumor necrosis factor-alpha (TNF-α), a major pro-inflammatory and pro-apoptotic cytokine secreted by reactive astroglia in glaucoma (Tezel et al., 2001; Yan et al., 2000). Among the dead receptors, pro-inflammatory activities of TNF receptor-1 (TNFR1) are considerably stronger (Tezel et al., 2007; Yang et al., 2011), and TNF-α through the TNFR1 signaling induces RGC apoptosis, oligodendrocyte death, and axon degeneration (Nakazawa et al., 2006; Tezel and Wax, 2000; Tezel et al., 2004). Recent studies of human donor eyes with glaucoma (Yang et al., 2011) and animal models (Tezel et al., 2012) have pointed to astroglial NF-κB as a common transcriptional regulator of multiple inflammation pathways linked to glaucomatous neurodegeneration, including TNFR signaling, toll-like receptor (TLR) signaling, and inflammasome activation (Luo et al., 2010; Tezel et al., 2012; Yang et al., 2011). In a more recent study, transgenic inhibition of astroglial NF-κB has provided immunomodulation and neuroprotection in experimental mouse glaucoma (Yang et al., 2020).
In this study, we sought to determine the importance of caspase-8 in different fates of RGCs and astroglia in glaucoma. What captured our attention on caspase-8 is multifold. Besides the intrinsic mitochondrial pathway, RGC apoptosis in glaucoma involves the extrinsic pathway activated after binding of dead receptors, including TNFR1 (Tezel et al., 2001; Tezel and Wax, 2000) and Fas (Krishnan et al., 2019). The caspase 8 that is the initiator caspase of extrinsic apoptosis pathway is activated in RGCs undergoing apoptosis in the glaucomatous human retina (Yang et al., 2011) and animal models (Huang et al., 2005; McKinnon et al., 2002a; Tezel et al., 2012). Although various caspases have been targeted to inhibit RGC apoptosis in different models of RGC injury (Tezel and Wax, 2000, 1999; Tezel and Yang, 2004), only one study investigated the effects of non-specific caspase inhibition in an experimental model of glaucoma, which promoted neuron survival in ocular hypertensive rat eyes (McKinnon et al., 2002b). Multiple studies have also tested the effects of various drugs on different caspases to determine treatment effects on RGC apoptosis. However, when considering the caspase-8, its specific importance for RGC apoptosis in glaucoma has remained unstudied. Even more stimulating of our interest on caspase-8 is the growing knowledge of its non-apoptotic functions. It has been increasingly recognized that caspase-8 has two opposing biological functions. Enzymatic activity of caspase-8 can promote cell death through the extrinsic pathway of apoptosis (evident in glaucomatous RGCs (McKinnon et al., 2002a, Tezel and Wax, 2000)). In contrast, catalytic activity of caspase-8 can promote cell survival and inflammation (Burguillos et al., 2011; Oberst et al., 2011) through NF-κB activation (evident in glaucomatous glia (Tezel et al., 2012, Yang et al., 2011, Yang et al., 2020)). Apparently, engagement of dead receptors and TLRs (both are up-regulated on glaucomatous astroglia (Luo et al., 2010, Tezel et al., 2001, Yan et al., 2000)) can activate caspase-8-mediated inflammation, while suppression of caspase-8 functions induces necroptosis, a necrosis-like programmed cell death (He et al., 2009; Zhang et al., 2009).
Recently recognized roles of caspase-8 in cell survival and inflammation, in addition to its previously known roles in apoptosis (Tummers and Green, 2017), led us to hypothesize that caspase-8 may function differently in glaucomatous RGCs and astroglia and play critical roles in different fates of these cell types in glaucoma. In order to test this hypothesis and to dissect the roles of cleavage-dependent versus cleavage-independent functions of caspase-8 in RGC apoptosis and glia-driven neuroinflammation, we used two experimental approaches in parallel. In the first approach, we studied the cell type-specific responses of RGCs and astroglia to a caspase-8 cleavage-inhibiting pharmacological treatment in rat eyes with experimentally induced glaucoma. In the second approach, we utilized the cre recombinase (cre)/loxP system to conditionally delete caspase-8 in astroglia for further analysis of caspase-8 regulation in astroglia-driven neuroinflammation in ocular hypertensive mice. Findings of these experiments revealed cell type-specific distinct processes that regulate caspase-8 functions in experimental glaucoma, such as inducing apoptosis in RGCs and promoting cell survival and inflammatory responses in astroglia. Caspase-8 deletion in astroglia protected RGCs against the neurodegenerative outcomes of astroglia-driven inflammation in experimental glaucoma, while the inhibition of caspase-8 cleavage led to decreased apoptosis in RGCs themselves. The cell type-specific regulation of caspase-8 functions that impacts both RGC apoptosis and astroglia-driven neuroinflammation stimulate further work to value the multi-target potential of caspase-8 regulation to develop treatments for neuroprotection and immunomodulation in glaucoma.
2. Materials and methods
2.1. Animals
All experiments with animals were approved by the Institutional Animal Care and Use Committee, and all procedures complied with the tenets of the ARVO statement for the use of animals in ophthalmic and vision research.
Two to 6-month-old Brown Norway rats and the mouse lines described below (including both sexes) were studied. All animals were housed in 12 h light/dark cycle and received standard rodent chow and water ad libitum. The mouse line with astroglia-targeting caspase-8 deletion was generated by breeding the caspase-8f/f (provided by Drs. Stan Krajewski, Sanford-Burnham Medical Research Institute, and Razqallah Hakem, University of Toronto) (Krajewska et al., 2011; Salmena et al., 2003) into glial fibrillary acidic protein (GFAP)-cre/ERT2 (Zhang et al., 1996) (stock no. 012849; The Jackson Laboratory, Bar Harbor, ME). All of the utilized mouse lines were on a C57BL/6 J background, and cross breeding of floxed and cre lines was followed by crossing of the offsprings with caspase-8f/f.
For conditional recombination, tamoxifen (dissolved in corn oil) was given to crossbreds by intraperitoneal injection (5 mg/40 g mouse) once a day for 5 consecutive days. Controls including wild-type for cre (wild-type for the floxed allele with/without cre) received similar tamoxifen injections. Additional controls for tamoxifen treatment included the littermates given oil vehicle only. Tamoxifen injections were started a week before the experimental induction of ocular hypertension as described below. A PCR-based method with primers detecting cre (5′-GCC AGT CTA GCC CAC TCC TT-3′, and 5′-TCC CTG AAC ATG TCC ATC AG-3′) and caspase-8flox alleles (5′-GGCCTTCCTGAGTACTGTCACCTGT-3′ and 5′-CCAGGAAAAGATTTGTGTCTAGC-3′) verified the genotype.
2.2. Induction of experimental glaucoma
Ocular hypertension was experimentally induced by microbead injections into anterior chamber (Sappington et al., 2010) by applying a modification as previously described (Cone et al., 2010) for more consistent ocular hypertension and greater axonal loss. Similar to our previous studies (Yang et al., 2016; Yang et al., 2020), we injected 4 μl of polystyrene microbeads (a mixture of 6 μm and 1 μm beads) followed by 1 μl viscoelastic (10 mg/ml sodium hyaluronate) into the anterior chamber of mouse eyes using a Hamilton syringe (Hamilton Company, Reno, NV). In rats, 7 μl of 15 μm polystyrene microbeads, followed by 3 μl viscoelastic, were injected. Injections were repeated at week 4 to maintain ocular hypertension. Fellow eyes were similarly injected with an equivalent volume of physiologic saline.
This commonly utilized microbead occlusion model allowed us to experimentally induce ocular hypertension in GFAP/caspase-8 and control mice. It was also advantageous to study inflammatory outcomes of glaucoma (Yang et al., 2016; Yang et al., 2020), because neuroinflammation develops as a consequence of induced ocular hypertension in this model as opposed to pre-existing inflammation (as evident in hereditary DBA/2 J glaucoma).
Intraocular pressure was measured before and after injections and twice weekly thereafter using a TonoLab rebound tonometer (TioLat, Helsinki, Finland) in isoflurane-anesthetized mice and awake rats. We calculated the intraocular pressure-time integral for each animal to minimize the influence of intraocular pressure variability among animals (by first integrating the mean intraocular pressure over time in ocular hypertensive eye, then subtracting the intraocular pressure-time integral from that in normotensive fellow eye), and excluded those with an intraocular pressure increase above 40 mmHg.
2.3. Pharmacological treatment
For pharmacological inhibition of caspase-8 cleavage in rat eyes, a cell-permeable selective inhibitor peptide, z-IETD-fmk (Sigma-Aldrich; St. Louis, MO; catalog number: C1230) was injected into the vitreous cavity (4 μl of 200 μM solution). By considering the estimated vitreous volume in rats (~13–20 μl) (Dureau et al., 2001), the vitreous concentration of z-IETD-fmk was ~50 μM. The utilized treatment dose was chosen based on in vitro and in vivo testing and the known dose/response curve in rats (Shabanzadeh et al., 2015; Tezel and Wax, 1999; Tezel and Yang, 2004). An equal volume of vehicle (0.2% DMSO in PBS) was similarly injected into control eyes. Intraocular injections were applied at days 0, 14, and 28 after microbead/viscoelastic injections, and the experimental period was ended at 6 weeks. Although z-IETD-fmk is an irreversible inhibitor of caspase-8 cleavage (with an expected half-life in the vitreous humor of ~1 day (Dureau et al., 2001, Koh et al., 2004, Yemisci et al., 2012), due to asynchrony of cellular responses to experimental glaucoma, its injection was repeated by optimizing for a realistic balance between the desired treatment response and the number of intravitreal injections in rat eyes. A pulled glass microneedle (100 μm tip) attached to a 10 μl Hamilton syringe (Hamilton Company) was used for intravitreal injections. For consecutive injections, the needle tip was inserted into different quadrants of the superior hemisphere through the sclera posterior to the limbus.
2.4. Pattern electroretinography
In order to assess RGC function, pattern electroretinography (PERG) responses were recorded using a dual PERG system (Jörvec, Miami, FL) as previously described (Chou et al., 2014; Saleh et al., 2007; Yang et al., 2020). Right and left eye responses to black-white reversing gratings (0.05 cycles/degree, and 100% contrast) were extracted using a snout electrode, and the waveforms were retrieved using an asynchronous averaging method (including at least 1800 responses per eye). The PERG amplitude was evaluated peak-to-trough. Electrophysiological recordings were performed in a masked fashion.
2.5. Caspase-8 activity assay
A fluorometric assay kit was used to measure caspase-8 bioactivity (Abcam, Cambridge, MA; catalog number: ab39534) according to the manufacturer’s instructions. This assay is based on detection and quantification of the fluorescence of the cleaved substrate, Ile-Glu-Thr-Asp (IETD)-AFC. Samples were run in triplicate for fluorescence measurement in a 96-well plate.
2.6. Optic nerve axon counting
Similar to our previous studies (Yang et al., 2016; Yang et al., 2020), 1 μm-thick cross-sections of the optic nerve embedded in epoxy resin were used for axon quantification. Toluidine blue-stained sections were imaged as non-overlapping tiles using Zeiss AxioObserver.Z1 microscope and the Zen software (Carl Zeiss, Thornwood, NY) for axon counts representing the entire area of cross-sections. Nerve outlines were manually traced on mosaics of images, and the size and shape parameters were used to exclude intervening glia, myelin debris, and highly degenerated axons. Axon counting was performed in a masked fashion, and the axon loss was determined by the ratio of axon counts in ocular hypertensive eye to normotensive fellow eye.
2.7. RGC counting
RGCs were counted in whole mounts of the retina after immunolabeling for a marker protein, RNA-binding protein with multiple splicing (RBPMS, 1:200; GeneTex, Irvine, CA; catalog number: GTX118619), as previously described (Yang et al., 2020). Nonoverlapping tile images of the whole mounted retina were acquired for a depth of 0–30 μm as z stacks (with 5 μm step size) at a magnification of 10 on a laser scanning confocal microscope (Red A1, Nikon Ti Eclipse; Nikon, Melville, NY) using the NIS-elements AR5 software (Nikon). The images collapsed into two dimensional images to generate maximum intensity projections were used for RGC counting similar to that described for axon counting. In addition to RBPMS immunolabeling, an additional criterion included a minimal soma size of 10 μm to eliminate dying or phagocytized RGCs, as we previously described (Yang et al., 2016; Yang et al., 2020). The researcher counting RGCs was masked to the experimental group of samples. The RGC loss was expressed as the ratio of RBPMS-labeled RGC soma counts in ocular hypertensive to fellow eye.
2.8. Tissue immunolabeling
Retinas, and 6-μm thick histological sections of the paraffin-embedded optic nerve head tissues, were immunolabeled using specific antibodies, as previously described (Yang et al., 2016; Yang et al., 2020). Briefly, the dissected retinas were fixed in 4% paraformaldehyde for 1 h at room temperature. After rinsing in PBS, a blocking step included 1 h incubation in 1% BSA (Sigma-Aldrich) and 0.3% Triton X-100 (ThermoFisher Scientific; Waltham, MA) in PBS. The following incubation with primary antibodies at 4 °C overnight utilized monoclonal antibodies to GFAP (1:500; Abcam; catalog number: ab68428 and ab10062) or TNF-α (1:500; Abcam, catalog number: ab109322), and phosphorylation site-specific antibodies to receptor-interacting protein kinase-3 (RIPK3) [phospho T231 + S232] (1:500; Abcam, catalog number: ab222320) or mixed lineage kinase domain like pseudokinase (MLKL) [S345] (1:500; Abcam, catalog number: ab196436). Secondary antibody incubation used Alexa Fluor dye-labeled secondary antibodies (1:1000; ThermoFisher). The primary antibody was replaced with serum for negative control, or an inappropriate secondary antibody was used to determine species specificity. In addition, a Click-iT™ TUNEL imaging assay kit (ThermoFisher; catalog number: C10245) was used for detection of apoptotic cells. Labeled retinas were mounted on glass slides, and images were collected using the laser scanning confocal microscope (Red A1, Nikon Ti Eclipse; Nikon).
For analysis of astroglial morphology, confocal images of the GFAP-labeled retinal whole mounts were analyzed as we previously described (Yang et al., 2020). The imaging parameters analyzed included the mean pixel intensity of GFAP immunolabeling across each image (expressed as GFAP intensity that reflects the GFAP expression level of astroglia) and the number of GFAP+ pixels divided by the total number of pixels in each image (expressed as GFAP coverage that reflects the size and density of individual cells).
2.9. Enzyme-linked immunosorbent assay (ELISA)
Protein lysates of the retina were obtained by homogenization in a lysis buffer (50 mM HEPES-KOH pH 8.0, 100 mM KCl, 2 mM EDTA, 0.10% NP-40, 2 mM dithiothreitol, 10% glycerol, supplemented with protease and phosphatase inhibitors) as previously described. (Yang et al., 2016; Yang et al., 2020) After protein concentration was determined using a colorimetric Bradford protein assay (BioRad, Hercules, CA), a set of cytokines/chemokines was profiled, as previously described (Yang et al., 2016; Yang et al., 2020), using a Multi-Analyte ELISArray kit (Qiagen, Valencia, CA) with detection sensitivity at low pg/ml levels. In addition to triplicated wells of experimental samples, arrays included negative controls, and the positive and nonspecific binding controls provided by the kit. After calculation of concentrations from a standard curve, and values were corrected for protein concentration.
2.10. Quantitative Western blot analysis
Enriched samples of RGCs and astroglia were isolated from the dissociated cell mixtures obtained from retina samples using the two-step immunomagnetic cell selection methodology as we previously described (Tezel et al., 2010; Tezel et al., 2012; Yang et al., 2020). After depletion of macrophage/microglia in the first step of immunomagnetic cell selection, RGCs and astroglia were selected from cell mixtures by incubation with magnetic beads bound to a monoclonal antibody to Thy1.1 (1:10; ThermoFisher, catalog number: 14–0900-81) or astrocyte cell surface antigen-2 (ACSA-2) (1:10; Miltenyl Biotech, catalog number: 130–099-138), respectively. The cell type-specific protein samples utilized in this study were pooled from the experimental eyes matched for the cumulative intraocular pressure exposure (~30 rats and 20 mice per group).
Immunoblot membranes were probed overnight with antibodies to procaspase-8 and cleaved subunit (1:1000; Novus Biologicals, Centennial, CO, catalog number: NBP1-05123) and cellular FLICE-like inhibitory protein (cFLIP; Novus Biologicals, 1:500, catalog number: NBP2-37359). In addition, primary antibodies included phosphorylation site-specific antibodies to NF-κB subunit, p65 [phospho-Ser536] (1:500; Abcam, catalog number: ab86299) or c-Jun N-terminal kinase-1 (JNK1) [phospho-T183] (1:500; Abcam, catalog number: ab47337). Additional primary antibodies included phosphorylation site-specific antibodies to RIPK3 [phospho T231 + S232] (1:500; Abcam, catalog number: ab222320) or MLKL [S345] (1:500; Abcam, catalog number: ab196436). After secondary antibody incubation with infrared dye-labeled secondary antibodies (1:10,000; Li-Cor, Lincoln, NE) for 1 h, proteins were visualized and quantified using Odyssey CLx Infrared Imaging system (Li-Cor). For internal loading and transferring control, a total protein stain (Revert™ 700, Li-Cor) was used prior to primary antibody incubation. In addition, a β-actin antibody (1:1000; ThermoFisher, catalog number: MA5-15739, or Abcam, catalog number: ab179467) was mixed with the primary antibody. Band intensities were normalized to β-actin, and the glaucoma-related fold change in protein expression was calculated.
2.11. Statistical analysis
Statistical analysis of the experimental data was carried out in a masked fashion using a software (SigmaPlot, version 12.5; Systat Software, Inc., San Jose, CA). Comparison of group differences used the one-way analysis of variance (ANOVA) followed by the pairwise multiple comparison test of Holm-Sidak, or the Kruskal-Wallis one-way ANOVA on ranks followed by the Tukey test. A P value of less than 0.05 was considered statistically significant. Data are presented as mean ± SD along with the P values.
3. Results
3.1. Cell type-specific responses to caspase 8 cleavage-inhibiting treatment in ocular hypertensive rat eyes
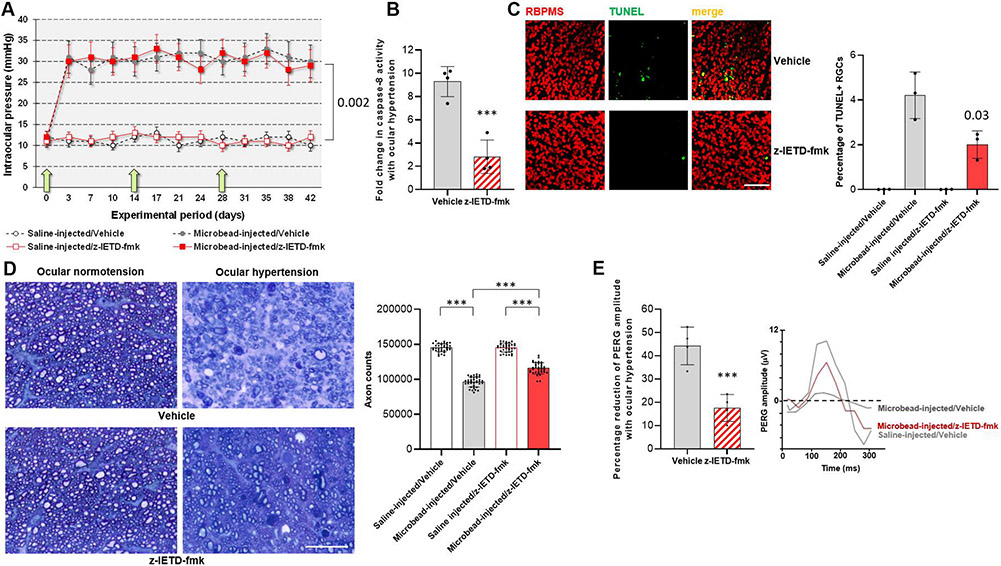
In order to determine cell type-specific functions of caspase-8, our initial experimental approach analyzed RGC and astroglia responses to a caspase-8 cleavage-inhibiting pharmacological treatment (z-IETD-fmk) in rat eyes with or without experimental glaucoma. Microbead injections into rat eyes induced a moderate increase in intraocular pressure, which was maintained through the experimental period of 6 weeks. However, physiological saline-injected control eyes presented a steady level of intraocular pressure below 12 mmHg (P = 0.002). The magnitude and duration of intraocular pressure elevation (and the extent of neuron loss) was similar to previous studies of the same experimental model (Sappington et al., 2010). Fig. 1A shows the intraocular pressure curve in experimental groups with or without z-IETD-fmk treatment. Note that the z-IETD-fmk treatment, or similar injections of vehicle, did not affect the intraocular pressure curves (P = 0.56).
Fig. 1. Experimental modeling of ocular hypertension-induced glaucoma in rats and pharmacological inhibition of caspase-8 cleavage.
A. Intraocular pressure curves through the 6-weeks experimental period. Green arrows show the time points for z-IETD-fmk injections. Microbead injections resulted in a significant increase in intraocular pressure (P = 0.002) that did not change with z-IETD-fmk treatment or injections of vehicle only (P = 0.56; n > 40/group). B. Caspase-8 activity assay detected a significant decrease in z-IETD-fmk treated retinas (***P < 0.001; n > 4/group). C. TUNEL labeling (green) in retinal whole mounts showed a significant decrease (P = 0.03) in the number RBPMS-labeled RGCs (red) in z-IETD-fmk-injected ocular hypertensive eyes than vehicle-injected ocular hypertensive controls (n > 4/group; scale bar, 100 μm). D. Axon counts in optic nerve cross-sections indicated approximately 40% protection against ocular hypertension-induced axon loss with z-IETD-fmk treatment (***P < 0.001; n > 36/group; scale bar, 10 μm). E. The PERG amplitude was also preserved in z-IETD-fmk-treated ocular hypertensive eyes compared to vehicle-injected ocular hypertensive controls (n > 4/group). Data are presented as mean ± SD, P values were obtained using a one-way ANOVA.
When we assayed caspase-8 activity in retina samples, we detected >four-fold decrease in ocular hypertension-induced caspase-8 activity in z-IETD-fmk-injected eyes relative to controls that were injected with vehicle only (P < 0.001; Fig. 1B). TUNEL labeling in retinal whole mounts also showed decreased number of apoptotic RGCs in z-IETD-fmk-injected ocular hypertensive eyes than vehicle-injected ocular hypertensive controls (P = 0.03; Fig. 1C).
As presented in Fig. 1D, comparison of axon counts in optic nerve cross-sections detected approximately 40% protection against ocular hypertension-induced axon loss (80% axon survival in the z-IETD-fmk group versus 66% axon survival in the vehicle group) with z-IETD-fmk-treatment (P < 0.001). Similarly, the RGC function assessed by PERG responses was preserved in z-IETD-treated ocular hypertensive eyes compared to the vehicle-injected ocular hypertensive controls (P < 0.001; Fig. 1E).
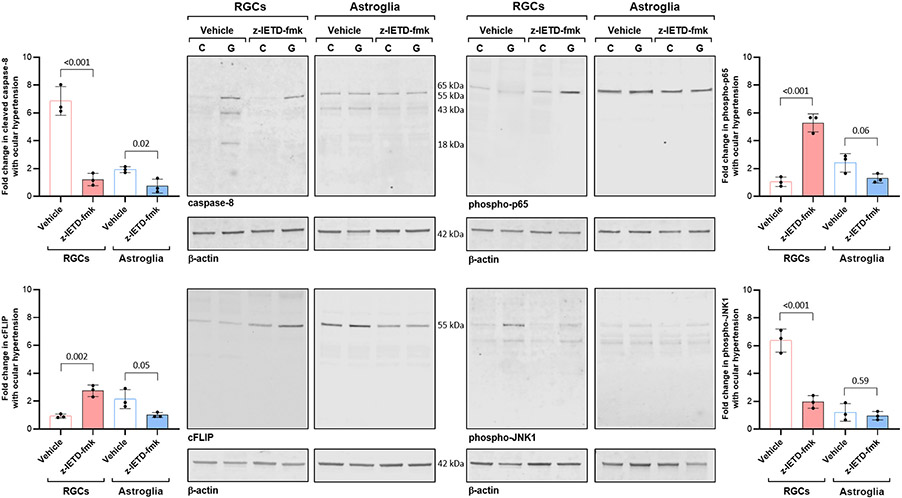
We next isolated RGC and astroglia proteins from these rat retinas to analyze cell type-specific responses to inhibition of caspase-8 cleavage. As shown in Fig. 2, immunoblots of RGC and astroglia proteins detected caspase-8 expression in both cell types. While the full-length (55 kDa) inactive proenzyme was expressed in astroglia, cleaved caspase-8 (both the partially cleaved p43 subunit and the fully processed p18 active enzyme) was detected in ocular hypertensive RGCs. However, RGCs isolated from z-IETD-fmk-treated ocular hypertensive eyes presented procaspase-8 expression, but the cleaved caspase-8 was no longer detectable. Besides inactive zymogen, the partially processed p43 subunit (which might result from the cleavage by cFLIPL (Krueger et al., 2001)) was detectable in astroglia samples isolated from vehicle-injected eyes, which was also decreased with z-IETD-fmk treatment. The apoptotic p18 subunit of caspase-8 was not detectable in astroglia samples.
Fig. 2. RGC and astroglia responses to z-IETD-fmk treatment.
RGC and astroglia proteins were isolated from rat eyes with experimental glaucoma (G) or normotensive controls (C) that were injected with z-IETD-fmk or vehicle only. Immunoblots were probed with antibodies to caspase-8, phospho-p65, cFLIP, or phospho-JNK1 (n > 3/group). Data are presented as mean ± SD, P values (obtained using a one-way ANOVA) for the statistical comparison of experimental groups are given in each graph.
In contrast to caspase-8 expression pattern, astroglia exhibited high expression of cFLIP (55 kDa cFLIPL isoform (Salvesen and Walsh, 2014, Ueffing et al., 2008)), while the cFLIP immunoreactivity was faint in RGCs, which was increased with z-IETD-fmk treatment.
We also used phosphorylation site-specific antibodies to analyze phospho-p65 and phospho-JNK1 expression in RGC and astroglia samples. Astroglia presented high expression of phospho-p65 in support of the key role of NF-κB in transcriptional activation of astroglia-driven neuroinflammation in experimental glaucoma (Yang et al., 2020). Unlike astroglia, phospho-p65 expression was not prominent in RGCs; however, z-IETD-fmk-treated RGCs presented increased phosho-p65 expression. Although ocular hypertension induced phospho-JNK1 in RGCs, phospho-JNK1 expression was lower in z-IETD-fmk-treated RGCs than vehicle-injected ocular hypertensive samples. No prominent treatment effect was detectable in phospho-JNK1 expression of astroglia. Statistical comparison of the protein expression patterns in z-IETD-fmk-injected versus vehicle-injected samples of RGCs and astroglia are presented in Fig. 2.
3.2. Responses to cell type-targeting conditional deletion of caspase-8 in ocular hypertensive mouse eyes
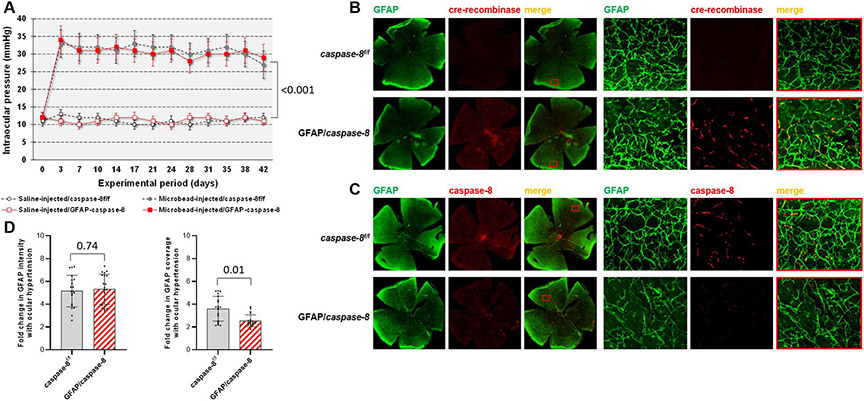
As a second approach to cell type-specific activities of caspase-8, we next studied the effects of cre/lox-based conditional deletion of astroglial caspase-8 on neuroinflammatory and neurodegenerative outcomes of ocular hypertension in mice. As presented in Fig. 3A, microbead injections into mouse eyes resulted in a moderate increase in intraocular pressure, which was similar to previous studies of the same experimental model (Chen et al., 2011; Yang et al., 2016; Yang et al., 2020). However, the intraocular pressure of physiological saline-injected eyes remained low (P < 0.001). Similar to background controls (caspase-8f/f mice wild-type for cre), microbead injections induced moderate ocular hypertension in GFAP/caspase-8 mice through the experimental period of 6 weeks (P = 0.20).
Fig. 3. Experimental modeling of ocular hypertension-induced glaucoma in mice and cre/lox-based deletion of astroglial caspase-8.
A. Intraocular pressure curves through the 6-weeks experimental period. Microbead injections resulted in a significant increase in intraocular pressure (***P < 0.001) that was similar in GFAP/caspase-8 mice and caspase-8f/f controls (P = 0.20; n > 39/group). B. Immunolabeling of retinal whole mounts demonstrated cre-recombinase (red) expression in GFAP+ astroglia (green) after tamoxifen-injection in GFAP/caspase-8 mice. C. Based on immunolabeling of retinal whole mounts, no caspase-8 (red) expression was detectable in GFAP+ astroglia (green) in GFAP/caspase-8 mice. Red boxed areas are shown in higher magnification (scale bar, 100 μm). D. Image analysis detected no alteration in the intensity of GFAP immunolabeling (reflecting the GFAP expression level of astroglia) with caspase-8 deletion. However, the coverage of GFAP immunolabeling (reflecting the size and density of individual cells) was significantly lower in GFAP/caspase-8 retinas compared with caspase-8f/f controls (n > 20/group). Data are presented as mean ± SD, P values (obtained using a one-way ANOVA) for the statistical comparison of experimental groups are given in each graph.
In order to confirm the cell type-targeting deletion of caspase-8, we comparatively analyzed cre-recombinase immunoreactivity in GFAP/caspase-8 mice and caspase-8f/f controls. Retinal whole mounts shown in Fig. 3B exemplify cre-recombinase expression in GFAP+ astroglia after tamoxifen injection in GFAP/caspase-8. In addition, there was a prominent drop in caspase-8 immunolabeling of ocular hypertensive astroglia in GFAP/caspase-8 mice compared to ocular hypertensive caspase-8f/f controls (Fig. 3C).
We also noted that the GFAP immunolabeling of astroglia presented spatial alterations in GFAP/caspase-8 retinas. Based on image analysis of GFAP immunolabeling (Fig. 3D), no alteration was detectable in the intensity of GFAP immunolabeling (reflecting the GFAP expression level of astroglia) with caspase-8 deletion (P = 0.74). However, the coverage of GFAP immunolabeling (reflecting the size and density of individual cells) was significantly lower in GFAP/caspase-8 retinas compared with caspase-8f/f controls (P = 0.01).
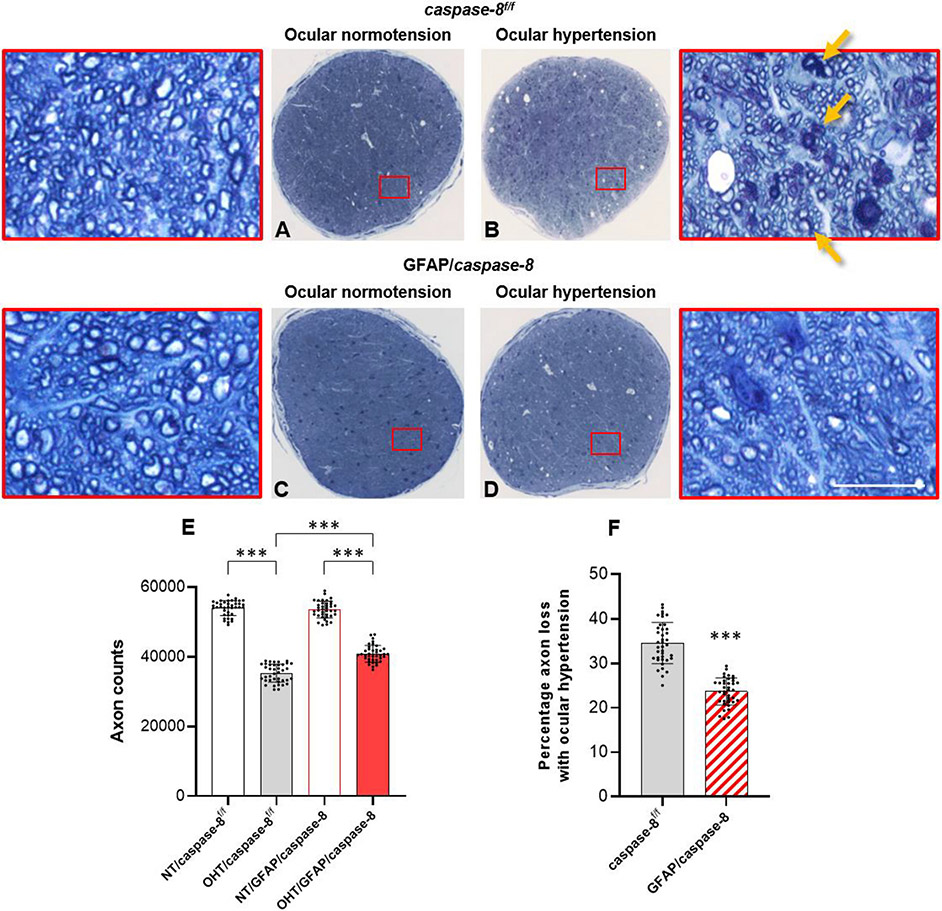
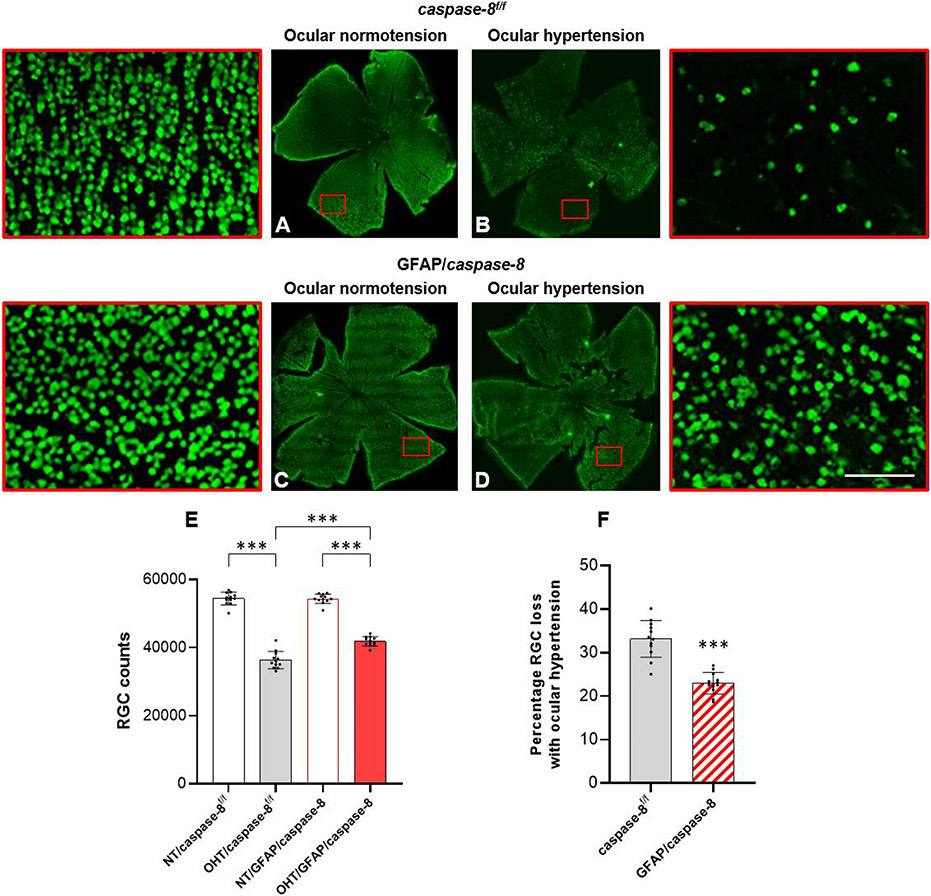
Next, we analyzed the effects of GFAP/caspase-8 on neuron injury by RGC and axon counts in GFAP/caspase-8 and caspase-8f/f eyes with or without ocular hypertension. Axons were counted in optic nerve cross-sections and RBPMS-labeled RGC somas were counted in retinal whole mounts. Despite a prominent decrease in axon counts with ocular hypertension in caspase-8f/f mice, the number of remaining axons was greater in ocular hypertensive GFAP/caspase-8 mice (P < 0.001). Ocular hypertension-induced axon loss was >30% lower in GFAP/caspase-8 mice than ocular hypertensive caspase-8f/f controls (65% axon survival with astroglial caspase-8 deletion versus 76% axon survival in controls). As presented in Fig. 4, decreased axon counts were accompanied by swollen and degenerating axons, myelin debris, and gliotic scar in ocular hypertensive optic nerves. However, compared to ocular hypertensive caspase-8f/f mice, ocular hypertensive GFAP/caspase-8 mice exhibited a preserved morphology of optic nerve axons with fewer degenerating profiles.
Fig. 4. Effects of GFAP/caspase-8 on optic nerve axon counts.
Presented are composite images of optic nerve cross-sections stained with 2% toluidine blue. Red boxed areas are shown in higher magnification (scale bar, 10 μm). Compared to normotensive caspase-8f/f control mice (A), there was a prominent axon loss and gliosis in ocular hypertensive eyes of caspase-8f/f controls (B). No alteration in optic nerve structure was detectable in normotensive mice with GFAP-targeting caspase-8 deletion (C). However, compared to ocular hypertensive caspase-8f/f controls, optic nerve structure was well preserved in ocular hypertensive eyes of GFAP/caspase-8 mice (D). E. The number of remaining axons was significantly higher in ocular hypertensive GFAP/caspase-8 mice than ocular hypertensive caspase-8f/f controls. F. Astroglial caspase deletion provided >30% protection against axon loss in experimental glaucoma. Yellow arrows show degenerating axons and myelin debris. Data (mean ± SD) represents 39 mouse eyes per group (***one-way ANOVA, P < 0.001).
Similar to axon loss, ocular hypertension-induced loss of RGC somas was decreased with astroglial caspase-8 deletion (P < 0.001). As presented in Fig. 5, the RGC soma loss with ocular hypertension was 30% lower in GFAP/caspase-8 mice than ocular hypertensive caspase-8f/f controls (66% axon survival with astroglial caspase-8 deletion versus 76% axon survival in controls).
Fig. 5. Effects of GFAP/caspase-8 on RGC soma counts.
Presented are composite images of the whole mounted retinas immunolabeled for RBPMS, an RGC marker. Red boxed areas are shown in higher magnification (scale bar, 100 μm). Compared to normotensive caspase-8f/f control mice (A), there was a visible loss of RBPMS-labeled RGC somas (green) in ocular hypertensive eyes of caspase-8f/f controls (B). No alteration in RBPMS labeling was detectable in normotensive mice with GFAP-targeting caspase-8 deletion (C). However, compared to ocular hypertensive caspase-8f/f controls, RBPMS-labeled RGCs were well protected in ocular hypertensive eyes of GFAP/caspase-8 mice (D). E. The number of RBPMS-labeled RGCs was significantly higher in ocular hypertensive GFAP/caspase-8 mice than ocular hypertensive caspase-8f/f controls. F. Astroglial caspase deletion provided an approximately 30% protection against RGC soma loss in experimental glaucoma. Data (mean ± SD) represents 12 mouse eyes per group (***one-way ANOVA, P < 0.001).
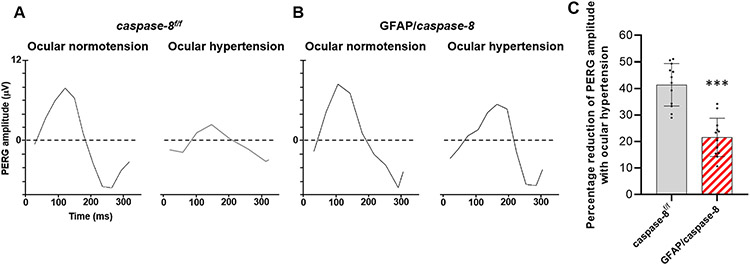
Besides structural protection in RGC somas and axons, astroglial caspase-8 deletion resulted in protection of RGC function. As shown in Fig. 6, the PERG amplitude was preserved in ocular hypertensive GFAP/caspase-8 mice compared to ocular hypertensive caspase-8f/f controls.
Fig. 6. Effects of GFAP/caspase-8 on pattern electroretinography (PERG) responses.
A. Shown are PERG responses in GFAP/caspase-8 mice and caspase-8f/f controls with or without experimentally induced ocular hypertension (A). The bar graphs show PERG amplitude in each group (B). Compared to normotensive eyes, ocular hypertensive eyes showed a reduction in PERG amplitude. However, deletion of astroglial caspase-8 resulted in preserved PERG amplitude in ocular hypertensive eyes. Data (mean ± SD) represents 12 mouse eyes per group (***one-way ANOVA, P < 0.001).
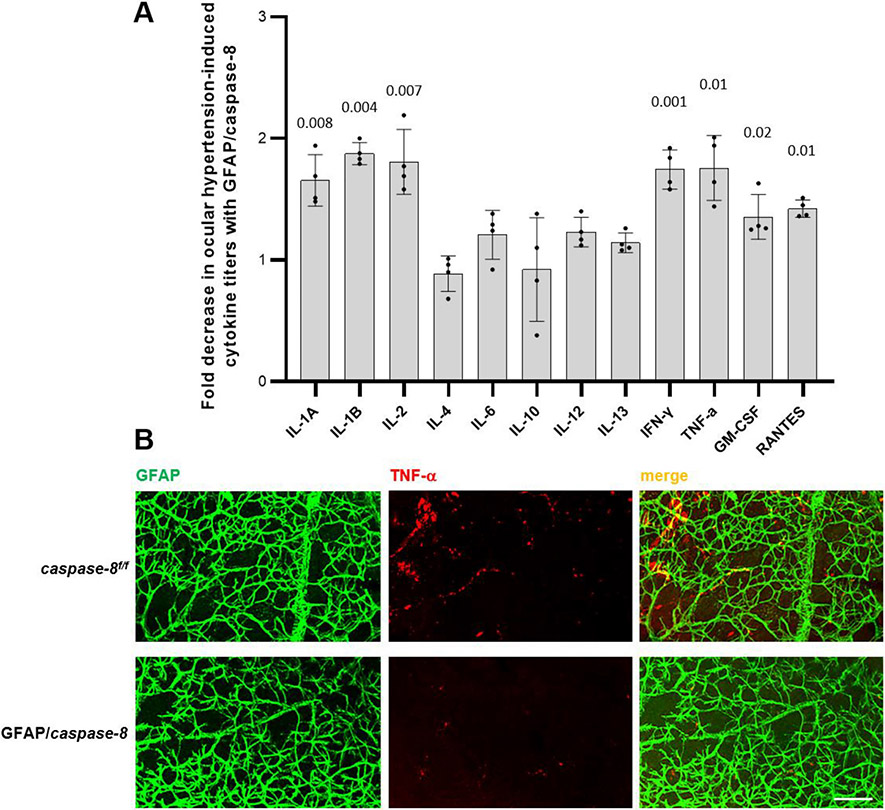
In order to determine the overall inflammatory status of the retina, we examined pro-inflammatory cytokine production by ELISA of retinal protein samples in additional mice. As presented in Fig. 7, there was ~two-fold decrease in ocular hypertension-induced titers of various cytokines (including IL1, IL-2, IFN-γ, and TNF-α) in GFAP/caspase-8 mice relative to caspase-8f/f controls (P < 0.05). Retinal whole mounts were next immunolabeled for TNF-α to further analyze the effects of astroglial caspase-8 deletion on the retinal inflammatory status with neurodegenerative potential. We considered that TNF-α would be a well representative, because this major pro-inflammatory cytokine is secreted by reactive astroglia and induces RGC apoptosis, oligodendrocyte death, and axon degeneration in experimental glaucoma (Nakazawa et al., 2006; Tezel and Wax, 2000; Tezel et al., 2004). As presented in Fig. 7, we detected a prominent decrease in retinal TNF-α immunolabeling of GFAP+ astroglia in ocular hypertensive GFAP/caspase-8 mice compared to ocular hypertensive caspase-8f/f controls.
Fig. 7. Effects of GFAP/caspase-8 on pro-inflammatory cytokine production.
A. The bar graph presents the fold decrease in ocular hypertension-induced cytokine titers with GFAP/caspase-8 relative to caspase-8f/f (statistical significance are shown by P values obtained using a one-way ANOVA). Data (mean ± SD) were obtained using a minimum of three new samples per group. B. Immunolabeling of retinal whole mounts supported decreased cytokine response (TNF-α, red) of GFAP-labeled astroglia (green) to ocular hypertension in GFAP/caspase-8 mice compared to caspase-8f/f ocular hypertensive controls (scale bar, 100 μm).
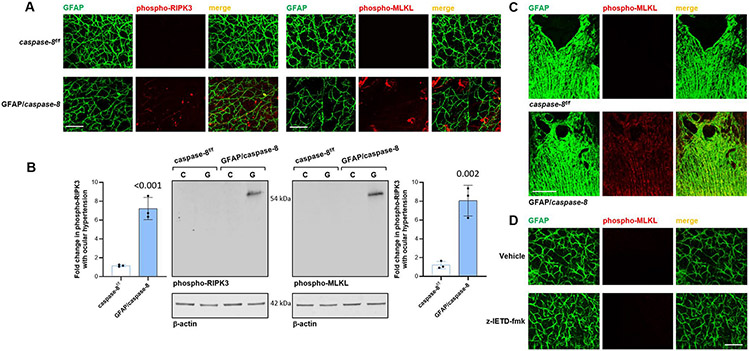
In addition, retinal whole mounts, and histological sections of the optic nerve head, from GFAP/caspase-8 mice exhibited phospho-RIPK3 and phospho-MLKL immunolabeling of the GFAP+ astroglia in some areas, which reflect the induction of necroptosis. This observation is also consistent with the decreased GFAP coverage presented in Fig. 3D. As presented in Fig. 8, immunoblots of astroglia proteins also detected a similar increase in phospho-RIPK3 (P < 0.001) and phospho-MLKL expression (P = 0.002) in ocular hypertensive GFAP/caspase-8 retinas relative to ocular hypertensive caspase-8f/f controls. As also shown in Fig. 8, however, no evidence of necroptosis was detectable in z-IETD-fmk-treated retinas.
Fig. 8. Effects of GFAP/caspase-8 on induction of astroglial necroptosis.
A. Immunolabeling of retinal whole mounts demonstrated phosho-RIPK3 and phospho-MLKL expression (red) of GFAP-labeled astroglia (green) in ocular hypertensive eyes of GFAP/caspase-8 mice compared to caspase-8f/f ocular hypertensive controls. B. Immunoblots of isolated astroglia proteins with antibodies to phosho-RIPK3 or phospho-MLKL also supported up-regulated expression of these necroptosis markers in GFAP/caspase-8 mice with experimental glaucoma (G) compared to caspase-8f/f ocular hypertensive controls (C). Data (mean ± SD) were obtained using a minimum of three new samples per group. P values (obtained using a one-way ANOVA) for the statistical comparison of experimental groups are given in each graph. C. Similar to retinal whole mounts, immunolabeling of the histological sections of optic nerve head tissue in these animals was also supportive of the induction of astroglial necroptosis in GFAP/caspase-8 mice. D. However, immunolabeling of retinal whole mounts did not detect phospho-MLKL expression in rat eyes injected with z-IETD-fmk or vehicle (scale bar, 100 μm).
No differences were detected between the outcomes in different sex groups (P > 0.05).
4. Discussion
4.1. Distinct functions of caspase-8 impact both RGC apoptosis and astroglia-driven neuroinflammation in experimental glaucoma
In order to better understand caspase-8 functions in RGCs and astroglia, this study analyzed the neuroinflammatory and neurodegenerative outcomes of experimental glaucoma using two parallel strategies for cell type-specific analysis. In the first experimental approach, we studied cell type-specific responses in freshly isolated samples of RGCs and astroglia from rat eyes that received a pharmacological treatment to inhibit caspase-8 cleavage. In the second approach, we studied the effects of cre/lox-based deletion of astroglial caspase-8 on neuroinflammatory and neurodegenerative outcomes of ocular hypertension in mice. The findings of these experiments demonstrated diverse functions of caspase-8 in RGC apoptosis and astroglia-driven neuroinflammation in experimental glaucoma. Deletion of astroglial caspase-8 (impacts both cleavage-dependent and cleavage-independent functions of caspase-8) suppressed inflammatory responses and protected RGCs against inflammatory toxicity, while the inhibition of the caspase-8 cleavage protected RGCs against apoptosis.
4.2. Inhibition of caspase-8 cleavage protects against RGC apoptosis by inhibiting extrinsic, and likely also intrinsic, apoptosis pathways
The z-IETD-fmk treatment inhibiting caspase-8 cleavage resulted in decreased apoptosis of RGC somas, and increased survival of RGC axons in ocular hypertensive rat eyes. The PERG responses reflecting the RGC function was also preserved in the ocular hypertensive eyes treated with z-IETD-fmk. Based on previous experimental studies, RGC apoptosis involves both the intrinsic pathway regulated by mitochondria and the extrinsic pathway activated after engagement of dead receptors, such as TNFR1 (Tezel et al., 2001; Tezel and Wax, 2000) or Fas (Krishnan et al., 2019). Caspase 8, the initiator caspase in the extrinsic apoptosis pathway, can also recruit mitochondrial pathway by cleaving Bid (evident in experimental glaucoma (Huang et al., 2005)). The RGC death in experimental models of glaucoma (Huang et al., 2005; McKinnon et al., 2002a; Tezel et al., 2012) involves the caspase-8/caspase-3 route of apoptosis. Thus, our findings in the present study, increased expression and apoptotic cleavage of caspase-8 in ocular hypertensive RGCs (despite faint immunoreactivity in normotensive controls), are consistent with previous observations. Our previous studies using proteomics profiling have also detected increased expression of caspase-8 (and the other molecules studied here, cFLIP, NF-κB, and JNK1) in the glaucomatous human retina (Yang et al., 2011), which also presented apoptotic cleavage. Likewise, a recent study of mouse glaucoma has pointed to caspase-8 among the genes up-regulated in ocular hypertensive eyes (Krishnan et al., 2019).
Although caspase inhibition provides some protection against RGC apoptosis in in vitro models (Tezel and Wax, 2000, 1999; Tezel and Yang, 2004), they cannot fully recover but eventually die due to mitochondrial dysfunction, similar to other neurons (Deshmukh et al., 2000). Evidently, multiple apoptosis pathways converge to a central point of Bax activation that regulates mitochondrial health, and the cell death program reaches an irreversible point once Bax is activated in early stages (Maes et al., 2017). Consistent with this, Bax deficiency provides complete protection to RGC somas in mouse glaucoma (Libby et al., 2005). Findings of the present study indicated that even though the inhibition of caspase-8 cleavage may not be sufficient to fully block RGC apoptosis itself, deletion of caspase-8 in astroglia can provide additional protection through an alternative mechanism by preventing the inflammatory outcomes of glaucoma. The functions of caspase-8 in astroglia-driven neuroinflammation are discussed later below.
Another piece of observation with the caspase-8 cleavage-inhibiting treatment may also suggest additional treatment effects on RGC survival. Besides the inhibition of apoptotic caspase-8 cleavage, the RGC response to z-IETD-fmk treatment included increased activity of NF-κB. This critical transcription factor presents pleiotropic roles in neurons and glia (Dresselhaus and Meffert, 2019). NF-κB activates inflammatory mediators and secondary injury processes; however, it also activates critical anti-apoptotic genes (Karin and Lin, 2002; Van Antwerp et al., 1996) and regulates a broad range of processes that improve neuron survival, synapse formation, and plasticity (Boersma et al., 2011; Meffert et al., 2003). Anti-apoptotic gene targets of NF-κB, which include cFLIP (also known as caspase-8 FADD-like apoptosis regulator (Kreuz et al., 2001)), inhibitor of apoptosis proteins (Stehlik et al., 1998), and trophic factors, might contribute to improved survival of RGCs after inhibition of caspase-8 cleavage. In the RGC samples isolated from z-IETD-fmk-treated ocular hypertensive rat eyes, we detected increased expression of cFLIPL that is one of the two cFLIP isoforms in rodents (55 kDa cFLIPL and 25 kDa cFLIPR). The cFLIPL has a caspase-like domain and prevents the apoptotic activity of caspase-8 in the procaspase-8/cFLIP heterodimer (Salvesen and Walsh, 2014; Ueffing et al., 2008). When considering the pro-survival functions of caspase-8 through NF-κB activation in the absence of enzymatic caspase-8 activity (Burguillos et al., 2011; Oberst et al., 2011), the improved RGC survival with z-IETD-fmk treatment might also be related to induced activation of NF-κB and up-regulation of cFLIP.
We also detected decreased activity of JNK1 in these caspase-8 cleavage-inhibited RGCs. Indeed, a critical anti-apoptotic outcome of NF-κB includes the repression of cell stress and death responses signaled by JNKs (Javelaud and Besancon, 2001). Evidently, early pathogenic insults in the glaucomatous optic nerve head (including distortion of axonal cytoskeleton, neurotrophin deprivation, energy failure, or neuroinflammation) activate JNKs. While JNK signaling contributes to distal axon degeneration in glaucoma (Fernandes et al., 2013), the DLK/JNK signaling triggered after early axon injury may also initiate a transcriptional program for RGC soma apoptosis by interacting with the Bcl2 family of genes (Fernandes et al., 2014; Syc-Mazurek et al., 2017; Watkins et al., 2013). JNKs are known to regulate the apoptosis signaling through extrinsic as well as intrinsic pathways by activating the pro-apoptotic genes or by modulating the activities of mitochondrial pro-apoptotic and anti-apoptotic proteins through phosphorylation-dependent processes (Dhanasekaran and Reddy, 2008). Interestingly, a JNK-dependent pathway was also shown to be involved in caspase-8-mediated apoptosis of RGCs after optic nerve injury (Tezel et al., 2004). JNK1 activity in RGCs (that is evident in human glaucoma and animal models (Kwong and Caprioli, 2006, Tezel et al., 2003)) may signal for caspase-8-independent cleavage of Bid, thereby amplifying the cross-talk between intrinsic and extrinsic apoptosis pathways (Deng et al., 2003). Thus, inhibition of caspase-8 cleavage can protect RGCs by inhibiting the extrinsic apoptosis pathway and by promoting the NF-κB activation that can also modulate intrinsic death signals originated at the optic nerve head and/or retina.
There also appears to be a mutual regulation between the anti-apoptotic protein cFLIP and JNK1 in cell fate decisions. The ubiquitin-mediated degradation of cFLIP requires a JNK1-dependent process during TNF-α-mediated cell death (Chang et al., 2006). In addition, cFLIP (highly expressed in astroglia) inhibits JNK pathway (Nakajima et al., 2006), while its down-regulation (as in RGCs) induces JNK activation (Nakajima et al., 2008).
Thus, signaling through dead receptors can activate caspase-8, NF-κB, and JNKs with opposing outcomes, depending on cell type-specific variations in the present signaling components (Dvoriantchikova and Ivanov, 2014). As supported by previous proteomics profiling, RGCs and astroglia present a distinct set of molecular responses in glaucoma (Tezel et al., 2010; Tezel et al., 2012; Yang et al., 2008), which typically leads to death of RGCs, but inflammatory activity of glia. The up-regulated components of inflammation signaling in glaucomatous astroglia, including TNF-α/TNFR, Fas/FasL, and TLR signaling, and inflammasome, are commonly linked to an NF-κB-regulated transcriptional program (Krishnan et al., 2019; Luo et al., 2010; Tezel et al., 2012; Yang et al., 2011). Besides TNF-α (Collart et al., 1990; Shakhov et al., 1990), various other pro-inflammatory cytokines/chemokines that present increased astroglial expression in glaucoma (Krizaj et al., 2014; Nikolskaya et al., 2009; Sapienza et al., 2016; Steele et al., 2006) are NF-κB’s transcriptional targets. In previous studies of glaucomatous human donor retinas or ocular hypertensive animal models, the cleaved caspase-8 was detected in RGCs, while NF-κB activation was prominent in astroglia (Tezel et al., 2012; Yang et al., 2011). The present study of experimental glaucoma similarly detected caspase-8 cleavage and JNK1 activity in RGCs, but not in astroglia (that exhibited prominent activation of NF-κB), and z-IETD-fmk treatment resulted in an altered pattern of caspase-8, NF-κB, and JNK1 activities in these cell types. As discussed in the following sections, inhibition of caspase-8 cleavage in RGCs might have shifted the dead receptor signaling from apoptotic caspase cascade towards NF-κB-mediated cell survival.
4.3. Deletion of caspase-8 in astroglia limits neuroinflammation and its neurodegenerative outcomes
While z-IETD-fmk treatment inhibited apoptotic caspase cascade and promoted NF-κB activation in RGCs, astroglia sustained the NF-κB-regulated inflammatory potential (Yang et al., 2020). However, deletion of caspase-8 in astroglia resulted in decreased production of NF-κB-regulated pro-inflammatory cytokines. Owing to the neurotoxicity of inflammatory mediators which can promote RGC apoptosis, oligodendrocyte death, and axon degeneration (Nakazawa et al., 2006; Tezel and Wax, 2000; Tezel et al., 2004), the inhibition of NF-κB-regulated inflammatory activity (Yang et al., 2020) might improve the survival and function of RGC somas and axons with astroglial caspase-8 deletion.
Similar to dead receptor signaling, inflammatory functions of caspase-8 in astroglia may also be signaled through TLRs. Glial TLRs are up-regulated in human glaucoma and experimental models, and the activation of astroglial TLRs by glaucoma-related intrinsic ligands stimulates the proliferation and cytokine secretion of T-cells through MyD88-dependent processes (Luo et al., 2010). Caspase-8 is required for NF-κB activation and mature cytokine production after TLR ligation (Lemmers et al., 2007; Shenderov et al., 2014; Su et al., 2005), and TLR-activated glia undergo RIPK-dependent necroptosis after caspase blockage (Fricker et al., 2013; Kim and Li, 2013).
Inflammasome activation, also evident in glaucoma (Albalawi et al., 2017; Krizaj et al., 2014; Pronin et al., 2019; Tezel et al., 2012; Yang et al., 2011) has similarly been linked to non-apoptotic, inflammatory functions of caspase-8. The caspase-8 functions in canonical and non-canonical inflammasome activation include proteolytic cleavage of interleukins (such as IL-1β) to their mature bioactive forms, either directly or via NLRP3 inflammasome activation (Feltham et al., 2017; Gurung and Kanneganti, 2015; Monie and Bryant, 2015). Recent studies of a retinal ischemia model that was induced by substantial acute increase in intraocular pressure have also supported the role of caspase-8 in TLR4-mediated activation of inflammasomes (Chi et al., 2015; Chi et al., 2014).
4.4. cFLIP plays a key role in the regulation cell type-specific functions of caspase-8 in RGCs and astroglia
Our findings in this study indicated cell type-specific roles of caspase-8 in cell fate regulations during glaucomatous neurodegeneration, in which cFLIP appears to be a key regulator molecule. Although we detected cleaved caspase-8 in the glaucomatous RGCs that undergo apoptosis, no apoptotic caspase-8 cleavage was detectable in the glaucomatous astroglia, in which caspase-8 exerted its pro-survival and pro-inflammatory activities through NF-κB activation.
After engagement of dead receptors, such as TNFR1, the initial membrane-bound complex (complex I), including TNFR1, RIPK1, TNFR1-associated dead domain protein (TRADD), and adaptor proteins (such as TRAF2 or TRAF5), rapidly signals for activation of NF-κB that promotes cell survival and pro-inflammatory signaling. In a second step, TRADD and RIPK1 associate with FAS-associated death domain protein (FADD) and caspase-8, forming a cytoplasmic complex (complex II) that can promote apoptosis. Recruitment of procaspase-8 to the signaling complex (death-inducing signaling complex, DISC) leads to homo-oligomerization and autocatalytic cleavage via a multi-step process producing the active enzyme. While procaspase-8 molecules process one another, autoactivated mature caspase-8 only cleaves effector caspases in the downstream apoptosis cascade (Chang et al., 2003; Martin et al., 1998).
The rapid activation of NF-κB by complex I enables anti-apoptotic NF-κB targets that interfere with the apoptotic caspase-8 in complex II. One of these molecules include cFLIP that functions as a molecular switch between cell death and survival signals. This protease-deficient caspase-8 homolog makes a cytosolic complex with caspase-8 and inhibits apoptosis signaling by competing the caspase-8 for FADD recruitment and preventing its autocleavage-mediated activation (Chang et al., 2002; Irmler et al., 1997; Silke and Strasser, 2013; Vandenabeele and Melino, 2012). Thus, transcriptional activity of NF-κB and expression of cFLIP constitute a checkpoint in dead receptor-mediated signal transduction that results in cell death (via complex II) when the initial signal (via complex I, NF-κB) leading to cell survival fails (Micheau and Tschopp, 2003).
Besides functioning as a protease for apoptosis, procaspase-8 zymogen also has a protease-independent catalytical function. By functioning as a scaffold for RIPK1 recruitment to the signaling complex, caspase-8 plays an essential role for stimulation of pro-inflammatory signaling through NF-κB activation (Henry and Martin, 2017). When caspase-8 autoprocessing is ablated by Casp8DA/DA, the caspase-8 within the complex with cFLIP continues to induce inflammatory cytokine production (Philip et al., 2016). Consistent with these observations, present study detected high activity of NF-κB in astroglia that also highly expressed cFLIP and possessed caspase-8 with no apoptotic cleavage, suggesting the signaling for cell survival and inflammation through NF-κB activation. Consequently, deletion of astroglial caspase-8 (but not the inhibition of caspase-8 cleavage as discussed below) resulted in decreased cytokine production of these glia.
In contrast, we detected no prominent cFLIP expression but apoptotic caspase-8 cleavage and JNK1 activity in the glaucomatous RGCs. Since the cFLIP concentration is a critical parameter for cell fate decisions (Tsuchiya et al., 2015), the absence of prominent cFLIP expression, or NF-κB activity, should be critical for the apoptotic function of caspase-8 in glaucomatous RGCs. Consequently, pharmacological inhibition of caspase-8 cleavage resulted in decreased apoptosis and improved survival of these neurons.
4.5. Caspase-8 deletion in astroglia induces RIPK3/MLKL-driven necroptosis
As supported by increased expression of phospho-RIPK3 and phospho-MLKL, deletion of caspase-8 shifted the signaling in glaucomatous astroglia towards necroptosis. While the formation of RIPK1-FADD-caspase 8 complex (complex IIa, ripoptosome) triggers apoptotic caspase activation, when caspase activity is deficient, RIPK1 activation leads to necroptosis through the formation of a RIPK1-RIPK3-MLKL complex (complex IIb, necrosome). By cleaving the RIPK1 after death receptor engagement, caspase-8 restrains the formation of RIPK1/RIPK3 complex and phosphorylation of RIPK3 and MLKL for MLKL-driven necroptosis (Degterev et al., 2008).
The cFLIP function that is controlled in a concentration-specific (and isoform-specific) manner appears to set a threshold on caspase-8 activity for both apoptosis and necroptosis. While the apoptosis induced after homo-oligomerization of procaspase-8 depends on proteolytic cleavage, heterodimerization of procaspase-8 with cFLIP prevents cleavage-dependent apoptosis but fulfills pro-survival and pro-inflammatory functions through NF-κB activation. However, the procaspase-8-cFLIP interaction also prevents RIPK-dependent necroptosis (Chang et al., 2002; Irmler et al., 1997). This is because the high expression of cFLIP can promote the cleavage of procaspase-8 to yield the p43 subunit (as we detected in astroglia), even though it prevents the full processing of caspase-8 to generate the apoptotic p18 subunit (Krueger et al., 2001). The cFLIP-activated procaspase-8 remains bound to the receptor signaling complex and cleaves only limited substrates, including RIPK1 and RIPK3, thereby preventing their necroptotic function. In both the canonical (TNFR-induced) and non-canonical (TLR-induced) ripoptosomes, the procaspase-8/cFLIP heterodimer can promote the catalytical activity of caspase-8 in the absence of active subunits and also blocks necroptosis through RIPK cleavage (Oberst et al., 2011; van Raam and Salvesen, 2012).
We detected high expression level of cFLIP with no apoptotic caspase-8 cleavage in astroglia, thereby suggesting that the procaspase-8-cFLIP complex prevented RIPK-dependent necroptosis without inducing apoptosis in these cells. Since the active site-directed caspase-8 inhibitors, such as z-IETD-fmk that we used in this study, target both homodimers and heterodimers, they are expected to allow for activation of necroptosis. However, necroptosis was detectable only in the caspase-8-deleted glaucomatous astroglia. One explanation for this observation may be incomplete inhibition of caspase-8 cleavage with our z-IETD-fmk injections, which should be further explored. Alternatively, individual variability and/or asynchrony of cellular responses might have resulted in limited representation of the necroptotic astroglia (beyond detection limits). It should also be clarified that although caspase-8 inhibition is a prerequisite for susceptibility to necroptosis in most cell types, caspase-independent mechanisms also control necroptosis on multiple levels (transcription, post-translational modification, stability, localization of necrosome components and interaction partners). For example, differences in expression/activity of signaling components, such TRADD and FADD, may change the balance between apoptotic, necroptotic, and pro-inflammatory signals (Fullsack et al., 2019; Hsu et al., 1996; Tummers et al., 2020). While cFLIP limits RIPK recruitment for necroptosis, by altering ubiquitination, IAPs (another gene target of NF-κB) may inhibit the necroptotic interaction of RIPK1 and RIPK3 (Dondelinger et al., 2016; Geserick et al., 2009).
Although necroptosis is considered a pro-inflammatory mode of cell death, we detected a prominent decrease in ocular hypertension-induced pro-inflammatory activity in GFAP/caspase-8 retinas. Our observations are concurred with the argument that necroptosis may be anti-inflammatory, or less inflammatory, because of the rapid cell lysis and early termination of the pro-inflammatory transcriptional response. Since the pro-necroptotic stimuli are highly pro-inflammatory, termination of biological processes may actually suppress excessive cytokine production through TNF or TLR signaling (Kearney et al., 2015; Kearney and Martin, 2017; Tummers et al., 2020). Even though our observations were supportive of this view, potential inflammatory consequences of astroglia necroptosis should be further investigated through a longer experimental period. Our observation of astroglial necroptosis in caspase-8-deleted glaucomatous eyes (but not in caspase-8-deleted non-glaucomatous eyes) may also open up another discussion as to whether caspase-8 inhibition may selectively affect the inflammatory glia to induce necroptosis, which was previously detected in in vitro studies of brain microglia (Fricker et al., 2013). This is because inflammatory stimuli prime glial cells for caspase-8 functions through TNF-α/TNFR, Fas/FasL, and TLR signaling, or inflammasome activation, which are up-regulated in glaucomatous eyes (Krishnan et al., 2019; Luo et al., 2010; Tezel et al., 2012; Yang et al., 2011). The microglia-initiated conversion of astrocytes to inflammatory and neurotoxic phenotype well exemplifies this priming (Liddelow et al., 2017). Our ongoing studies explore this interesting aspect.
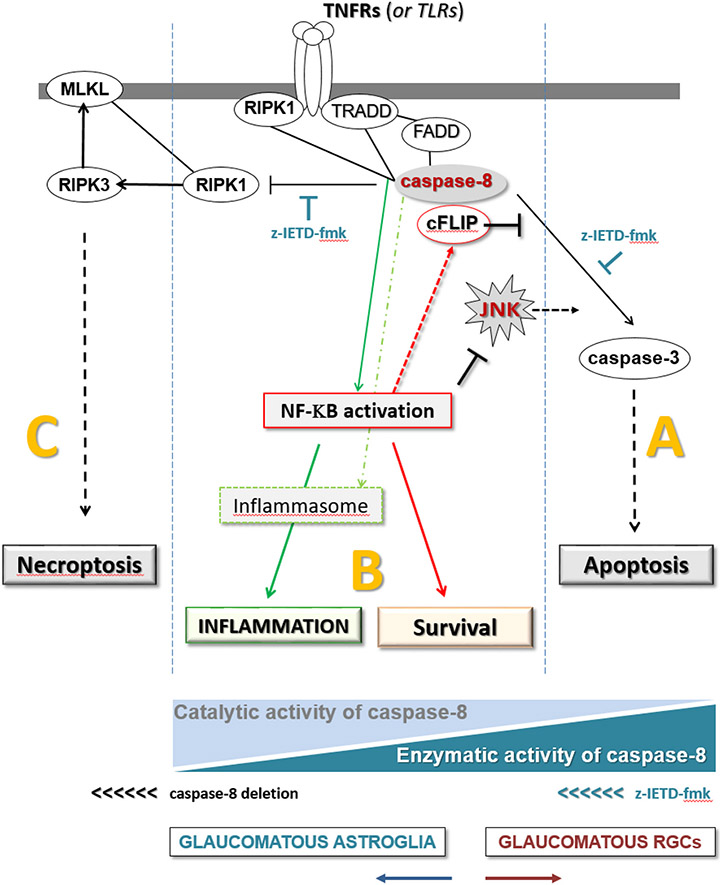
In summary, findings of this study support distinct functions of caspase-8 in RGCs and astroglia in experimental glaucoma as modeled in Fig. 9. Presented findings stimulate additional studies to test the multi-target potential of caspase-8 regulation to provide neuroprotection and immunomodulation in glaucoma.
Fig. 9. Distinct roles of caspase-8 in cell fate regulation.
As illustrated by the blue triangles at the bottom, different states of the enzymatic or catalytic activities of caspase-8 are critical for the outcome: (A) Enzymatic activity of caspase-8 induces apoptosis (as in RGCs), (B) catalytic activity (in the absence of full proteolytic cleavage) signals towards cell survival (as in astroglia, or z-IETD-fmk-treated RGCs) and (C) no caspase-8 activity induces necroptosis (as in caspase-8-deleted astroglia). Thus, caspase-8 deletion in astroglia changes the state from “B to C”, while the inhibition of caspase-8 cleavage shifts the signal from “A to B” in RGCs.
Acknowledgements
The authors are thankful to Drs. Stan Krajewski (Sanford-Burnham Medical Research Institute) and Razqallah Hakem (University of Toronto) for kindly providing the caspase-8f/f mice.
Funding sources
This study was supported in part by a research grant from National Eye Institute, Bethesda, MD (R01 EY028153, GT), the Homer McK. Rees Scholarship in Glaucoma Research (GT), and the AR and JR Peacock Trusts Research Grant (GT). This study was also supported in part by a National Eye Institute grant for Core Facilities for Vision Research (P30 EY0190070), and an unrestricted grant to the Department of Ophthalmology of Columbia University from Research to Prevent Blindness Inc. (New York, NY). Images were collected in the Confocal and Specialized Microscopy Shared Resource of the Herbert Irving Comprehensive Cancer Center at Columbia University, which is supported by a grant from National Institute of Health (P30 CA013696).
Abbreviations:
- cFLIP
cellular FLICE-like inhibitory protein
- FADD
FAS-associated death domain protein
- GFAP
glial fibrillary acidic protein
- ELISA
enzyme-linked immunosorbent assay
- JNK
c-Jun N-terminal kinase
- IFN-γ
interferon-gamma
- IL
interleukin
- MLKL
mixed lineage kinase domain like pseudokinase
- NF-κB
nuclear factor-kappaB
- PERG
pattern electroretinography
- RIPK
receptor-interacting protein kinase
- RGC
retinal ganglion cell
- RPBMS
RNA-binding protein with multiple splicing
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-alpha
- TNFR
tumor necrosis factor receptor
- TRADD
TNFR1-associated dead domain protein
Footnotes
Declaration of Competing Interest
None.
References
- Albalawi F, Lu W, Beckel JM, Lim JC, McCaughey SA, Mitchell CH, 2017. The P2X7 receptor primes IL-1beta and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front. Cell. Neurosci 11, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baris M, Tezel G, 2019. Immunomodulation as a neuroprotective strategy for glaucoma treatment. Curr. Ophthalmol. Rep 7, 160–169. [PMC free article] [PubMed] [Google Scholar]
- Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK, 2011. A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis. J. Neurosci 31, 5414–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burguillos MA, Deierborg T, Kavanagh E, et al. , 2011. Caspase signalling controls microglia activation and neurotoxicity. Nature. 472, 319–324. [DOI] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X, 2002. C-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 21, 3704–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DW, Xing Z, Capacio VL, Peter ME, Yang X, 2003. Interdimer processing mechanism of procaspase-8 activation. EMBO J. 22, 4132–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kamata H, Solinas G, Luo JL, Maeda S, Venuprasad K, Liu YC, Karin M, 2006. The E3 ubiquitin ligase itch couples JNK activation to TNFalpha-induced cell death by inducing c-FLIP(L) turnover. Cell. 124, 601–613. [DOI] [PubMed] [Google Scholar]
- Chen H, Wei X, Cho KS, Chen G, Sappington R, Calkins DJ, Chen DF, 2011. Optic neuropathy due to microbead-induced elevated intraocular pressure in the mouse. Invest. Ophthalmol. Vis. Sci 52, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Li F, Chen H, et al. , 2014. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1beta production in acute glaucoma. Proc. Natl. Acad. Sci. U. S. A 111, 11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi W, Chen H, Li F, Zhu Y, Yin W, Zhuo Y, 2015. HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J. Neuroinflammation 12, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TH, Bohorquez J, Toft-Nielsen J, Ozdamar O, Porciatti V, 2014. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Invest. Ophthalmol. Vis. Sci 55, 2469–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, Baeuerle P, Vassalli P, 1990. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol. Cell. Biol 10, 1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone FE, Gelman SE, Son JL, Pease ME, Quigley HA, 2010. Differential susceptibility to experimental glaucoma among 3 mouse strains using bead and viscoelastic injection. Exp. Eye Res 91, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degterev A, Hitomi J, Germscheid M, et al. , 2008. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol 4, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Ren X, Yang L, Lin Y, Wu X, 2003. A JNK-dependent pathway is required for TNFalpha-induced apoptosis. Cell. 115, 61–70. [DOI] [PubMed] [Google Scholar]
- Deshmukh M, Kuida K, Johnson EM Jr., 2000. Caspase inhibition extends the commitment to neuronal death beyond cytochrome c release to the point of mitochondrial depolarization. J. Cell Biol 150, 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP, 2008. JNK signaling in apoptosis. Oncogene. 27, 6245–6251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondelinger Y, Darding M, Bertrand MJ, Walczak H, 2016. Poly-ubiquitination in TNFR1-mediated necroptosis. Cell. Mol. Life Sci 73, 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus EC, Meffert MK, 2019. Cellular specificity of NF-kappaB function in the nervous system. Front. Immunol 10, 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureau P, Bonnel S, Menasche M, Dufier JL, Abitbol M, 2001. Quantitative analysis of intravitreal injections in the rat. Curr. Eye Res 22, 74–77. [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, 2014. Tumor necrosis factor-alpha mediates activation of NF-kappaB and JNK signaling cascades in retinal ganglion cells and astrocytes in opposite ways. Eur. J. Neurosci 40, 3171–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltham R, Vince JE, Lawlor KE, 2017. Caspase-8: not so silently deadly. Clin. Transl. Immunol 6, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, Kim J, Libby RT, 2013. JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells. Exp. Eye Res 112, 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes KA, Harder JM, John SW, Shrager P, Libby RT, 2014. DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol. Dis 69, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Vilalta A, Tolkovsky AM, Brown GC, 2013. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J. Biol. Chem 288, 9145–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullsack S, Rosenthal A, Wajant H, Siegmund D, 2019. Redundant and receptor-specific activities of TRADD, RIPK1 and FADD in death receptor signaling. Cell Death Dis. 10, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geserick P, Hupe M, Moulin M, et al. , 2009. Cellular IAPs inhibit a cryptic CD95-induced cell death by limiting RIP1 kinase recruitment. J. Cell Biol 187, 1037–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurung P, Kanneganti TD, 2015. Novel roles for caspase-8 in IL-1beta and inflammasome regulation. Am. J. Pathol 185, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X, 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 137, 1100–1111. [DOI] [PubMed] [Google Scholar]
- Henry CM, Martin SJ, 2017. Caspase-8 acts in a non-enzymatic role as a scaffold for assembly of a pro-inflammatory “FADDosome” complex upon TRAILs stimulation. Mol. Cell 65 (715–729), e715. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu HB, Pan MG, Goeddel DV, 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 84, 299–308. [DOI] [PubMed] [Google Scholar]
- Huang W, Dobberfuhl A, Filippopoulos T, Ingelsson M, Fileta JB, Poulin NR, Grosskreutz CL, 2005. Transcriptional up-regulation and activation of initiating caspases in experimental glaucoma. Am. J. Pathol 167, 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmler M, Thome M, Hahne M, et al. , 1997. Inhibition of death receptor signals by cellular FLIP. Nature. 388, 190–195. [DOI] [PubMed] [Google Scholar]
- Javelaud D, Besancon F, 2001. NF-kappa B activation results in rapid inactivation of JNK in TNF alpha-treated Ewing sarcoma cells: a mechanism for the anti-apoptotic effect of NF-kappa B. Oncogene. 20, 4365–4372. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A, 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol 3, 221–227. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, Martin SJ, 2017. An inflammatory perspective on necroptosis. Mol. Cell 65, 965–973. [DOI] [PubMed] [Google Scholar]
- Kearney CJ, Cullen SP, Tynan GA, Henry CM, Clancy D, Lavelle EC, Martin SJ, 2015. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ. 22, 1313–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Li J, 2013. Caspase blockade induces RIP3-mediated programmed necrosis in toll-like receptor-activated microglia. Cell Death Dis. 4, e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HJ, Cheng L, Bessho K, Jones TR, Davidson MC, Freeman WR, 2004. Intraocular properties of urokinase-derived antiangiogenic A6 peptide in rabbits. J. Ocul. Pharmacol. Ther 20, 439–449. [DOI] [PubMed] [Google Scholar]
- Krajewska M, You Z, Rong J, et al. , 2011. Neuronal deletion of caspase 8 protects against brain injury in mouse models of controlled cortical impact and kainic acid-induced excitotoxicity. PLoS One 6, e24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuz S, Siegmund D, Scheurich P, Wajant H, 2001. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol. Cell. Biol 21, 3964–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, Gregory-Ksander M, 2019. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflammation 16, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, Shestopalov VI, 2014. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr. Eye Res 39, 105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S, 2001. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem 276, 20633–20640. [DOI] [PubMed] [Google Scholar]
- Kwong JM, Caprioli J, 2006. Expression of phosphorylated c-Jun N-terminal protein kinase (JNK) in experimental glaucoma in rats. Exp. Eye Res 82, 576–582. [DOI] [PubMed] [Google Scholar]
- Lemmers B, Salmena L, Bidere N, et al. , 2007. Essential role for caspase-8 in toll-like receptors and NFkappaB signaling. J. Biol. Chem 282, 7416–7423. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, Nickells RW, John SW, 2005. Susceptibility to neurodegeneration in a glaucoma is modified by bax gene dosage. PLoS Genet. 1, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, et al. , 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G, 2010. Glaucomatous tissue stress and the regulation of immune response through glial toll-like receptor signaling. Invest. Ophthalmol. Vis. Sci 51, 5697–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye-Barthel M, Sun D, Jakobs TC, 2013. Morphology of astrocytes in a glaucomatous optic nerve. Invest. Ophthalmol. Vis. Sci 54, 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Nair CE, Nickells RW, 2015. Neuroinflammation in glaucoma and optic nerve damage. Prog. Mol. Biol. Transl. Sci 134, 343–363. [DOI] [PubMed] [Google Scholar]
- Maes ME, Schlamp CL, Nickells RW, 2017. BAX to basics: how the BCL2 gene family controls the death of retinal ganglion cells. Prog. Retin. Eye Res 57, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DA, Siegel RM, Zheng L, Lenardo MJ, 1998. Membrane oligomerization and cleavage activates the caspase-8 (FLICE/MACHalpha1) death signal. J. Biol. Chem 273, 4345–4349. [DOI] [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, et al. , 2002a. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest. Ophthalmol. Vis. Sci 43, 1077–1087. [PubMed] [Google Scholar]
- McKinnon SJ, Lehman DM, Tahzib NG, et al. , 2002b. Baculoviral IAP repeat-containing-4 protects optic nerve axons in a rat glaucoma model. Mol. Ther 5, 780–787. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D, 2003. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci 6, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J, 2003. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 114, 181–190. [DOI] [PubMed] [Google Scholar]
- Monie TP, Bryant CE, 2015. Caspase-8 functions as a key mediator of inflammation and pro-IL-1beta processing via both canonical and non-canonical pathways. Immunol. Rev 265, 181–193. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Komazawa-Sakon S, Takekawa M, Sasazuki T, Yeh WC, Yagita H, Okumura K, Nakano H, 2006. An antiapoptotic protein, c-FLIPL, directly binds to MKK7 and inhibits the JNK pathway. EMBO J. 25, 5549–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Kojima Y, Nakayama M, Yagita H, Okumura K, Nakano H, 2008. Downregulation of c-FLIP promotes caspase-dependent JNK activation and reactive oxygen species accumulation in tumor cells. Oncogene. 27, 76–84. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Nakazawa C, Matsubara A, et al. , 2006. Tumor necrosis factor-alpha mediates oligodendrocyte death and delayed retinal ganglion cell loss in a mouse model of glaucoma. J. Neurosci 26, 12633–12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolskaya T, Nikolsky Y, Serebryiskaya T, et al. , 2009. Network analysis of human glaucomatous optic nerve head astrocytes. BMC Med. Genet 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberst A, Dillon CP, Weinlich R, et al. , 2011. Catalytic activity of the caspase-8-FLIP (L) complex inhibits RIPK3-dependent necrosis. Nature. 471, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NH, DeLaney A, Peterson LW, et al. , 2016. Activity of uncleaved caspase-8 controls anti-bacterial immune defense and TLR-induced cytokine production independent of cell death. PLoS Pathog. 12, e1005910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin A, Pham D, An W, et al. , 2019. Inflammasome activation induces pyroptosis in the retina exposed to ocular hypertension injury. Front. Mol. Neurosci 12, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Nagaraju M, Porciatti V, 2007. Longitudinal evaluation of retinal ganglion cell function and IOP in the DBA/2J mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci 48, 4564–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmena L, Lemmers B, Hakem A, et al. , 2003. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 17, 883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Walsh CM, 2014. Functions of caspase 8: the identified and the mysterious. Semin. Immunol 26, 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza A, Raveu AL, Reboussin E, et al. , 2016. Bilateral neuroinflammatory processes in visual pathways induced by unilateral ocular hypertension in the rat. J. Neuroinflammation 13, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappington RM, Carlson BJ, Crish SD, Calkins DJ, 2010. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice. Invest. Ophthalmol. Vis. Sci 51, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R, Ohlmann A, Tamm ER, 2013. The role of Muller glia and microglia in glaucoma. Cell Tissue Res. 353, 339–345. [DOI] [PubMed] [Google Scholar]
- Shabanzadeh AP, D’Onofrio PM, Monnier PP, Koeberle PD, 2015. Targeting caspase-6 and caspase-8 to promote neuronal survival following ischemic stroke. Cell Death Dis. 6, e1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV, 1990. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J. Exp. Med 171, 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenderov K, Riteau N, Yip R, et al. , 2014. Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol 192, 2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silke J, Strasser A, 2013. The FLIP side of life. Sci. Signal 6 pe2. [DOI] [PubMed] [Google Scholar]
- Steele MR, Inman DM, Calkins DJ, Horner PJ, Vetter ML, 2006. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Invest. Ophthalmol. Vis. Sci 47, 977–985. [DOI] [PubMed] [Google Scholar]
- Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J, 1998. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med 188, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Bidere N, Zheng L, et al. , 2005. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 307, 1465–1468. [DOI] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Libby RT, 2019. Axon injury signaling and compartmentalized injury response in glaucoma. Prog. Retin. Eye Res 73, 100769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syc-Mazurek SB, Fernandes KA, Libby RT, 2017. JUN is important for ocular hypertension-induced retinal ganglion cell degeneration. Cell Death Dis. 8, e2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, 2013. Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr. Opin. Pharmacol 13, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Wax MB, 1999. Inhibition of caspase activity in retinal cell apoptosis induced by various stimuli in vitro. Invest. Ophthalmol. Vis. Sci 40, 2660–2667. [PubMed] [Google Scholar]
- Tezel G, Wax MB, 2000. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J. Neurosci 20, 8693–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, 2004. Caspase-independent component of retinal ganglion cell death, in vitro. Invest. Ophthalmol. Vis. Sci 45, 4049–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Li LY, Patil RV, Wax MB, 2001. Tumor necrosis factor-alpha and its receptor-1 in the retina of normal and glaucomatous eyes. Invest. Ophthalmol. Vis. Sci 42, 1787–1794. [PubMed] [Google Scholar]
- Tezel G, Chauhan BC, LeBlanc RP, Wax MB, 2003. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Invest. Ophthalmol. Vis. Sci 44, 3025–3033. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Yang J, Wax MB, 2004. Role of tumor necrosis factor receptor-1 in the death of retinal ganglion cells following optic nerve crush injury in mice. Brain Res. 996, 202–212. [DOI] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Peng Y, Sun SL, Sun D, 2007. Mechanisms of immune system activation in glaucoma: oxidative stress-stimulated antigen presentation by the retina and optic nerve head glia. Invest. Ophthalmol. Vis. Sci 48, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Kain AD, Powell DW, Kuehn MH, Pierce WM, 2010. Hemoglobin expression and regulation in glaucoma: insights into retinal ganglion cell oxygenation. Invest. Ophthalmol. Vis. Sci 51, 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G, Yang X, Luo C, Cai J, Powell DW, 2012. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Invest. Ophthalmol. Vis. Sci 53, 4220–4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Nakabayashi O, Nakano H, 2015. FLIP the switch: regulation of apoptosis and necroptosis by cFLIP. Int. J. Mol. Sci 16, 30321–30341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers B, Green DR, 2017. Caspase-8: regulating life and death. Immunol. Rev 277, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummers B, Mari L, Guy CS, et al. , 2020. Caspase-8-dependent inflammatory responses are controlled by its adaptor, FADD, and necroptosis. Immunity. 52 (994–1006), e1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueffing N, Keil E, Freund C, Kuhne R, Schulze-Osthoff K, Schmitz I, 2008. Mutational analyses of c-FLIPR, the only murine short FLIP isoform, reveal requirements for DISC recruitment. Cell Death Differ. 15, 773–782. [DOI] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM, 1996. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 274, 787–789. [DOI] [PubMed] [Google Scholar]
- van Raam BJ, Salvesen GS, 2012. Proliferative versus apoptotic functions of caspase-8 hetero or homo: the caspase-8 dimer controls cell fate. Biochim. Biophys. Acta 1824, 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenabeele P, Melino G, 2012. The flick of a switch: which death program to choose? Cell Death Differ. 19, 1093–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins TA, Wang B, Huntwork-Rodriguez S, et al. , 2013. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc. Natl. Acad. Sci. U. S. A 110, 4039–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, Marsh-Armstrong N, Howell GR, Lasker, I.I.O.A., Glaucomatous Neurodegeneration P, 2017. Neuroinflammation in glaucoma: a new opportunity. Exp. Eye Res 157, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Tezel G, Wax MB, Edward DP, 2000. Matrix metalloproteinases and tumor necrosis factor alpha in glaucomatous optic nerve head. Arch. Ophthalmol 118, 666–673. [DOI] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Pierce WM, Tezel G, 2008. Phosphorylation-dependent interaction with 14-3-3 in the regulation of bad trafficking in retinal ganglion cells. Invest. Ophthalmol. Vis. Sci 49, 2483–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Luo C, Cai J, Powell DW, Yu D, Kuehn MH, Tezel G, 2011. Neurodegenerative and inflammatory pathway components linked to TNF-alpha/TNFR1 signaling in the glaucomatous human retina. Invest. Ophthalmol. Vis. Sci 52, 8442–8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hondur G, Tezel G, 2016. Antioxidant treatment limits neuroinflammation in experimental glaucoma. Invest. Ophthalmol. Vis. Sci 57, 2344–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Zeng Q, Baris M, Tezel G, 2020. Transgenic inhibition of astroglial NF-kappaB restrains the neuroinflammatory and neurodegenerative outcomes of experimental mouse glaucoma. J. Neuroinflammation 17, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Caban S, Bodur E, Capan Y, Dalkara T, 2012. Transport of a caspase inhibitor across the blood-brain barrier by chitosan nanoparticles. Methods Enzymol. 508, 253–269. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Riesterer C, Ayrall AM, Sablitzky F, Littlewood TD, Reth M, 1996. Inducible site-directed recombination in mouse embryonic stem cells. Nucleic Acids Res. 24, 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J, 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 325, 332–336. [DOI] [PubMed] [Google Scholar]