Abstract
Gastrointestinal tract breast cancer (BC) metastases represent a rare event and generally originate from the lobular subtype. Duodenal involvement was rarely described in previous case series. Abdominal symptoms are extremely unspecific and misleading. Diagnosis is challenging, and it consists of a few mandatory steps from radiological examinations to histological and immunohistochemical analyses. Here, we presented the clinical case of a 54-year-old postmenopausal woman who was hospitalized for vomiting and jaundice, presenting increased level of liver enzymes and minimal main bile duct and choledocus dilatation at abdominal ultrasonography. She underwent breast-conserving surgery and axillary lymph node dissection for stage IIIB lobular BC, 5 years before. Metastatic infiltration of the duodenal bulb originating from lobular BC was proven histologically, through fine-needle aspiration during endoscopic ultrasonography. Treatment was established after multidisciplinary team evaluation, based on the clinical status and prognosis of the patient. Pancreaticoduodenectomy was performed, and final histological examination confirmed the secondary localization of lobular BC, infiltrating the duodenal and gastric wall, pancreas parenchyma, and surrounding tissues. No metastatic lymph nodes were found. After surgery, the patient underwent first line of adjuvant systemic treatment with fulvestrant and ribociclib. After a follow-up of 21 months, the patient was in good clinical condition, without signs of locoregional or distant recurrence. This report stressed on the importance of a tailored therapeutic approach. Although systemic therapy generally represents the preferred option, surgery should not be excluded if an oncological radical resection can be performed achieving acceptable locoregional disease control.
Keywords: Breast cancer, Lobular breast cancer, Metastasis, Duodenal metastasis, Surgery
Introduction
Breast cancer (BC) most commonly metastasizes to the bones, liver, and brain [1, 2]. Gastrointestinal (GI) tract BC metastases are uncommon, and their precise incidence is hard to establish [3, 4]. The time interval between the diagnosis of primary BC and the detection of its secondary GI tract manifestations is wide. Metastatic disease can occur for up to 30 years [5, 6]. BC metastasis to the extrahepatic GI tract mainly originates from the lobular subtype due to the particular tropism of lobular cells [7, 8]. The most frequent site of BC metastasis to the GI tract is the stomach, followed by the small bowel and the colon [5, 6, 9]. However, small bowel metastases originating from BC occur in 8% of all cases of GI sites, and duodenal metastases were rarely described in previous case series [10, 11]. Here, we present the clinical case of a patient with a duodenal metastasis from lobular BC and the description of its management, with a special focus on diagnosis and surgical treatment. The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000530603).
Case Report
In May 2021, a 54-year-old postmenopausal woman with no comorbidities was hospitalized for vomiting and jaundice. Her clinical history started in February 2016, when the patient underwent breast-conserving surgery and axillary lymph node dissection for stage IIIB (pT2N2a [7/18]) carcinoma of the left breast. Definitive histopathological examination revealed an invasive lobular carcinoma with a tumor size of 4 cm, with estrogen receptor and progesterone receptor positivity, a human epidermal growth factor receptor 2 expression score of 0, a nuclear grade 1, and absence of vascular invasion. After surgery, four courses of adjuvant chemotherapy with doxorubicin and cyclophosphamide and four courses of paclitaxel were administered, followed by locoregional radiation therapy with a total dose of 50 Gy in 25 daily fractions delivered over 5 weeks. At the end of these treatments, endocrine therapy with letrozole was started. Initially, the pre-established follow-up program composed of chest radiography, abdominal ultrasonography (US), bone scintigraphy, and assays for tumor markers of BC did not reveal evidence of recurrence.
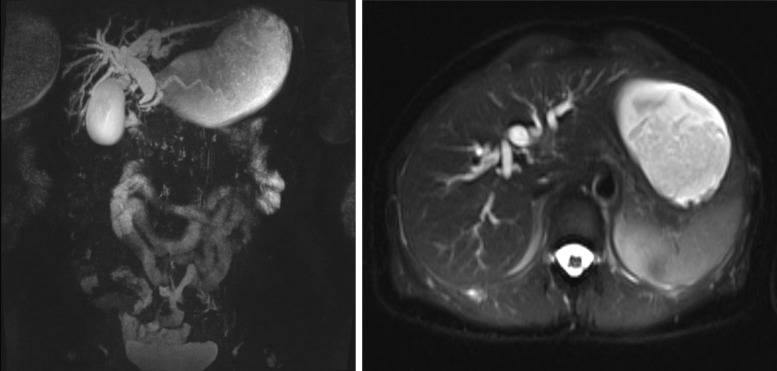
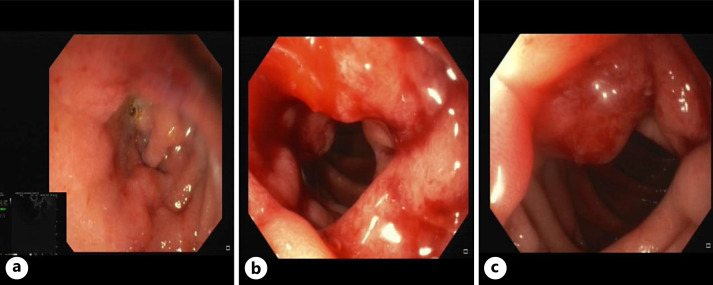
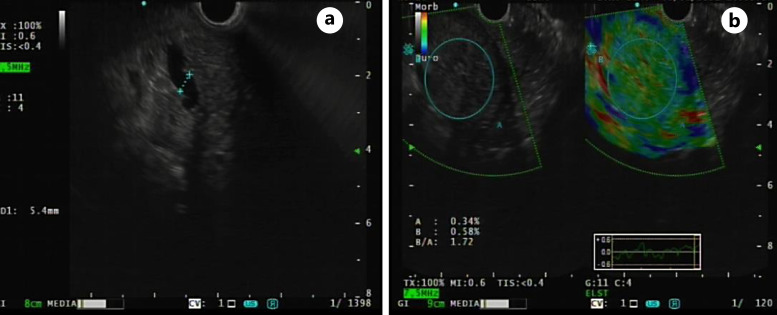
In December 2020, laboratory blood tests revealed elevated levels of liver enzymes and abdominal US showed only minimal choledochal dilatation. Liver enzymes remained elevated at the following laboratory blood tests; therefore, the patient underwent further investigations with magnetic resonance cholangiography that revealed dilatation of the choledocus, intrahepatic, and common bile ducts, which smoothly entered into the duodenum, with no distinct signs of malignancy (Fig. 1). After multidisciplinary team evaluation, an endoscopic ultrasonography (EUS) was performed showing a stricture of the second portion of the duodenum with erythematous mucosa and protrusion of the ampulla of Vater (Fig. 2). A biopsy was performed, revealing only a chronic inflammation of the mucosal layer of the duodenum. Additionally, a dilatation of the main pancreatic duct in all of its portions was described (Fig. 3a), with preserved pancreatic parenchyma (Fig. 3b). The patient underwent an abdominal contrast-enhanced computed tomography (CT) scan that confirmed the left system bile duct dilatation. A 18fluoro-deoxyglucose-positron emission tomography/CT scan was performed, showing no uptake. After 5 months, the patient presented at the emergency department with vomiting, jaundice in the skin and bulbar conjunctivae, and mild tenderness in the upper abdomen. An upper GI endoscopy was performed, showing an impassable stricture of the pylorus. An EUS with fine-needle aspiration of the thickened wall of the pylorus/first part of the duodenum was performed. Histopathological examination showed proliferation of atypical cells and immunohistochemical analysis demonstrated tissue positivity for estrogen receptor and GATA-binding protein 3, absence of progesterone receptor, and only mild reactivity for human epidermal growth factor receptor 2. Other GI tumor markers were negative. A diagnosis of duodenal localization of BC was made. After a multidisciplinary team evaluation between breast surgeons, pancreatic surgeons, medical oncologist, and radiologists, the patient underwent surgical treatment with pancreaticoduodenectomy. The postoperative course was characterized by delayed gastric emptying and fever; however, both complications were managed conservatively with nasogastric tube placement, total parenteral nutrition, and intravenous antibiotic therapy. The patient was discharged on postoperative day 12 in good general condition, eating a light diet, with normal bowel functions. Final histological examination confirmed a secondary localization of lobular BC, infiltrating the duodenal and gastric wall, pancreas, and transverse mesocolon. No metastatic locoregional lymph nodes were found. After surgery, the patient underwent the first line of adjuvant systemic treatment with fulvestrant and ribociclib. After a follow-up of 21 months, the patient was in good clinical condition, without signs of locoregional or distant recurrence.
Fig. 1.
Magnetic resonance cholangiography showing dilatation of the choledocus, intrahepatic, and common bile ducts.
Fig. 2.
EUS showing a structure of the second portion of the duodenum (a) with erythematous mucosa (b) and protrusion of the ampulla of Vater (c).
Fig. 3.
EUS showing dilatation of main pancreatic duct (a) with preserved pancreatic parenchyma (b).
Discussion
Due to the clonal evolution and immune-phenotype shift of cancer cells, metastatic tumors show an intense immunosuppressive microenvironment. However, the biological mechanisms promoting metastatic BC remain elusive [12]. The clinical presentation of metastatic infiltration of the duodenum from lobular BC is extremely unspecific, with general GI symptoms including abdominal pain, nausea, vomiting, and (rarely) bowel obstruction or GI bleeding [13]. The first manifestation of duodenal involvement from BC metastasis is generally represented by an obstructive pattern composed of elevation of blood transaminases and mild bile duct dilatation, occasionally revealed by laboratory tests and abdominal US as mimicking other GI malignancies. Frequently, the initial diagnosis is inaccurate because a defined diagnostic path has not been established [14]. Generally, an abdominal contrast-enhanced CT scan represents the first step in the diagnostic process; however, it is not always accurate in the identification of the duodenal secondary neoplasm. The second step in the diagnostic path of duodenal metastasis from BC is an EUS, which clearly describes the mucosal layer of the duodenum, especially in cases of strictures. Moreover, due to the nature of invasive lobular carcinoma and its pleomorphic cells infiltrating tissue, a fine-needle aspiration is fundamental to reach a correct histopathological diagnosis [15]. In our clinical case, diagnosis was hard to achieve due to unspecific abdominal complaints and no radiological evidence of neoplastic mass occupying the duodenum or pancreas. We repeated EUS until histological examination and immunohistochemistry could confirm diagnosis of duodenal metastasis from BC. The histologic type of primary tumor, the interval from its first manifestation, and the presence of solitary duodenal metastasis drove the diagnostic and surgical management of this patient.
Conclusion
We described a rare case of metachronous duodenal metastasis of lobular BC treated with pancreaticoduodenectomy. The final therapeutic decision was adopted after multidisciplinary team evaluation. Nowadays, a tailored therapeutic approach represents the fundamental strategy to manage abdominal metastasis from BC, and surgical treatment has to be regarded as a powerful weapon in this battle.
Statement of Ethics
All procedures were conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 (in its most recent version). Patient anonymity and all confidential information have been preserved. Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images. The Institutional Review Board of our hospital approved this study (H22-09-DMLBC, September 01, 2022).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Study conception and design: Erika Barbieri, Damiano Gentile, Giulia Caraceni, and Corrado Tinterri. Drafting of the manuscript: Erika Barbieri, Damiano Gentile, Giulia Caraceni, Francesca Gavazzi, and Alessandro Zerbi. Critical revision and final approval: all authors.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–69. 10.1016/S0140-6736(20)32381-3. [DOI] [PubMed] [Google Scholar]
- 2. Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 3. Nikkar-Esfahani A, Kumar BG, Aitken D, Wilson RG. Metastatic breast carcinoma presenting as a sigmoid stricture: report of a case and review of the literature. Case Rep Gastroenterol. 2013;7(1):106–11. 10.1159/000348760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schwarz RE, Klimstra DS, Turnbull ADM. Metastatic breast cancer masquerading as gastrointestinal primary. Am J Gastroenterol. 1998;93(1):111–4. 10.1111/j.1572-0241.1998.111_c.x. [DOI] [PubMed] [Google Scholar]
- 5. Benfiguig A, Anciaux ML, Eugène CI, Benkémoun G, Etienne JC. Gastric metastasis of breast cancer occurring after a cancer-free interval of 30 years. Ann Gastroenterol Hepatol. 1992;28(4):175–7. [PubMed] [Google Scholar]
- 6. Fu JX, Zou YN, Long-Li, Wang XJ. Widespread metastasis to the stomach 10 Years after primary breast cancer: a case report and review of the literature. Medicine. 2020;99(48):e22527. 10.1097/MD.0000000000022527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels IR, Layer GT, Chisholm EM. Lessons to be learned: a case study approach bowel obstruction due to extrinsic compression by metastatic lobular carcinoma of the breast. J R Soc Promot Health. 2002;122(1):61–2. 10.1177/146642400212200118. [DOI] [PubMed] [Google Scholar]
- 8. Bratthauer GL, Miettinen M, Tavassoli FA. Cytokeratin immunoreactivity in lobular intraepithelial neoplasia. J Histochem Cytochem. 2003;51(11):1527–31. 10.1177/002215540305101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saranovic D, Kovac JD, Knezevic S, Susnjar S, Stefanovic AD, Saranovic DS, et al. Invasive lobular breast cancer presenting an unusual metastatic pattern in the form of peritoneal and rectal metastases: a case report. J Breast Cancer. 2011;14(3):247–50. 10.4048/jbc.2011.14.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ambroggi M, Stroppa EM, Mordenti P, Biasini C, Zangrandi A, Michieletti E, et al. Metastatic breast cancer to the gastrointestinal tract: report of five cases and review of the literature. Int J Breast Cancer. 2012;2012:1–8. 10.1155/2012/439023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nihon-Yanagi Y, Park Y, Ooshiro M, Aoki H, Suzuki Y, Hiruta N, et al. A case of recurrent invasive lobular carcinoma of the breast found as metastasis to the duodenum. Breast Cancer. 2009;16(1):83–7. 10.1007/s12282-008-0045-0. [DOI] [PubMed] [Google Scholar]
- 12. Zou Y, Ye F, Kong Y, Hu X, Deng X, Xie J, et al. The single-cell landscape of intratumoral heterogeneity and the immunosuppressive microenvironment in liver and brain metastases of breast cancer. Adv Sci. 2023;10(5):e2203699. 10.1002/advs.202203699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang B, Copur-Dahi N, Kalmaz D, Boland BS. Gastrointestinal manifestations of breast cancer metastasis. Dig Dis Sci. 2014;59(9):2344–6. 10.1007/s10620-014-3155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sobinsky JD, Willson TD, Podbielski FJ, Connolly MM. Unusual metastatic patterns of invasive lobular carcinoma of the breast. Case Rep Oncol Med. 2013;2013:986517–3. 10.1155/2013/986517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romanini SG, Serrano JPR, de Castro JSL, Torres IT, Ingold A, Borini AL, et al. EUS-FNA diagnosis with core biopsy of pancreatic metastases from primary breast cancer. Case Rep Gastrointest Med. 2020;2020:7136897–4. 10.1155/2020/7136897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.