Abstract
Degradable materials that can support cell infiltration and remodeling are the basis of tissue engineered approaches to vascular repair. In addition, to replace or close a large area of the vasculature, a patch material or scaffold must also withstand high pressure over time. Extracellular matrix-based (ECM-based) scaffolds offer a biological substrate with environmental cues that can support the formation of appropriate vascular tissue. However, scaffolds made from pure natural materials can degrade rapidly, resulting in reduced mechanical integrity of the implant and possible chronic inflammation in the site. A hybrid biomaterial, combining the matrix-dense tissue pericardium with a layer of the degradable polymer poly(propylene fumarate) (PPF), is suited to withstand rapid enzymatic degradation and control the presentation of an unaltered natural tissue matrix for remodeling activity. In this study, we show that the polymer reinforced hybrid supports cellular infiltration, but has fewer macrophages in the vicinity of the implant after 6 weeks in vivo than an untreated tissue control in both athymic and immunocompetent rat models. This result is supported by changes seen in other inflammatory cell populations. Based on significant differences in the inflammatory response to untreated pericardium and PPF-reinforced pericardium, we conclude that the polymer reinforcement layer can be used as a tool to leverage presentation of the ECM molecules in ECM-based scaffolds.
Keywords: ECM, chronic inflammation, pericardium, vascular patch, hybrid biomaterial
INTRODUCTION
Vascular grafts and patches are essential materials for aneurysm repair, vascular reconstruction, and congenital heart dis-ease treatment.1–3 For this surgical application, material options are most commonly synthetic (Dacron, polytetrafluor-oethylene) or chemically cross-linked xenografts3,4 that can provide strength and compliance in the high-pressure environment. These options are all nonliving materials, and as such, are subject to degradation over time which can result in irregular or unfavorable healing patterns.5 As an alternative, tissue engineering strategies in vascular material development strive to create an implant that will support ingrowth and maintenance from a patient’s own tissue, leaving behind functional vasculature when the implanted scaffold slowly degrades away. To be successful in this application, tissue engineered scaffolds need to fulfill a number of broad functions, in both mechanical behavior and complex biological remodeling.6,7
To encourage biological remodeling, new designs of vascular grafts and patches attempt to utilize the inherent inflammatory response to implanted material to harness the patient’s natural wound healing capabilities.8 Extracellular matrix-based (ECM-based) scaffolds have been shown to support these natural healing capabilities through recruitment of M2 macrophages and constructive remodeling in many applications9–12 (and recently reviewed by Hussey et al.13). The constituents of ECM make the scaffold biologically active, with the potential to provide tissue-specific environmental cues for rebuilding.12,14 Specifically, for vascular tissue, unique ratios of collagen types, elastin, and other proteins can stimulate distinct cell populations such as endothelial cells and smooth muscle cells that are required in the functional development of vascular tissue.15,16
A potential risk in using an ECM-based scaffold in a large, pressurized area such as the vasculature is the possible rapid degradation of the natural components before adequately developed tissue can replace it. For this reason, most commercially available pericardium materials for high-pressure environments are chemically cross-linked with glutaraldehyde to prevent tissue degradation.4,17 In addition to detrimental mechanical failure, rapid degradation of natural components and the release of peptide fragments could induce complex inflammatory responses.18 Macrophage activity is beneficial in initial wound repair, but can become pathogenic if propagation continues,19 leading to chronic inflammation and graft failure.20 In the vascular environment in particular, several studies demonstrate a relation between excessive and sustained inflammatory response inclusive of macrophages,21 NK cells and C-reactive protein22 to stenosis in as fast as 2 weeks after implantation23 or to traits of the foreign body response.24 The temporal polarization of macrophages in response to a material could be a significant determinant of the type, density, and duration of other arriving cells.19,25–29
To help regulate macrophage proliferation and polarization in an implanted vascular graft, we suggest that a controlled presentation of ECM proteins to arriving cells and breakdown processes could result in slowed degradation and maintenance of an anti-inflammatory environment. We propose a hybrid scaffold, combining ECM together with a synthetic polymer, as a strategic approach to control the signals from ECM that would affect polarization and inflammation. We have previously reported on the biohybrid material, poly(propylene fumarate) (PPF)-reinforced pericardium, as a scaffold for vascular wall repair.30 This material combines the bioactivity of pericardium, a matrix-dense tissue found surrounding the heart muscle, with the strength of the polymer PPF. In this previous study, we report that the polymer reinforcement is as effective as the chemically crosslinked alternative (glutaraldehyde, (GA)) at preventing detrimental weakening due to enzymatic degradation upon implantation. More importantly, the polymer reinforcement results in significantly lower calcification of the pericardium compared to the GA crosslinked pericardium in a rat subdermal implant after only 3 weeks. Changes in calcification could be explained by unnatural healing in response to eliminated or masked active components of the pericardium due to chemical cross-linking.30 In addition, the PPF-reinforced tissue hosted moderate cell infiltration, indicating a “middle road” between rapid scaffold deterioration in the untreated case, and capsule formation around the GA pericardium.30 These results suggest that the PPF layer could be responsible for controlling the presentation of the potent ECM molecules to the system, allowing for the benefits of bioactive components, but eliminating the risk of rapid degradation and propagation of inflammatory cycles. This theory was developed based off of macrophage quantification (using immu-nostaining of F4/80, representing a general macrophage marker),30 and warrants a comprehensive investigation of inflammatory reaction to the material.
The current work aims to understand and capitalize on the reduction of inflammation as a result of PPF coating on an ECM-based scaffold. This may reduce the likelihood of chronic inflammation in the vascular setting. To this end, we will vary the amount of PPF coverage on pericardium as a way to regulate the exposure of ECM proteins. We hypothesize that this variation will change the inflammatory response to the implant both in terms of intensity and cell population. The hypothesis will first be tested in vivo in a macrophage-dominated environment (animal model lacking adaptive immune system) to isolate macrophage response. Using the results, the second objective is to measure the resulting inflammation intensity and cell population in response to the developed polymer pericardium hybrid in an animal with a complete immune system. A moderate inflammatory response to the subdermal implants could indicate controlled remodeling and eventual rebuilding of the tissue implant appropriate for the cardiovascular environment.
METHODS
Material synthesis
The polymer PPF was synthesized following previously published methods.31 Briefly, propylene glycol and diethyl fumarate (DEF) were combined in a 3:1 molar ratio. Zinc chloride (cata-lyst) and hydroquinone (radical inhibitor) were incorporated in a 0.01:0.002 molar ratio. Under nitrogen flow, the reaction produces ethanol as a byproduct and bis(hydroxypropyl) as the intermediate. Transesterification under vacuum of the intermediate produces PPF with propylene glycol as a byproduct. Gel permeation chromatography was used to calculate the number average molecular weight (Mn) and polydispersity index (PDI) of the purified PPF. For use in this study, PPF (Mn 1150 and PDI 1.6) was mixed with the monomer DEF in a 3:1 ratio and then mixed with the photoinitiator bis(2,4,6-trimethylbenzoyl) phenylphosphine oxide (BAPO), to create a photocurable resin. The polymer at this phase was cross-linked to a solid, thin film with UV light (3.5 mW/cm2) for 45 min. The soluble fraction of this composite in thin sheets has been calculated to be 13.3% ± 2.7%, (n = 9).30
The PPF–pericardium hybrid was created as developed in previous studies.30 Briefly, fresh bovine pericardium was obtained 2 days after harvest from Innovative Research, Inc. and cut into strips 2 × 6 cm for dehydration and decellulari-zation. A liquid layer of PPF as described above was applied either as a single layer on one surface or on both surfaces of the tissue, and the combined material was exposed to UV radiation for 45 min (3.5 mW/cm2) to cross-link the polymer layer(s). The hybrid material is then cut to 1 × 1 cm squares, washed in sterile 70% ethanol for 6 h, and then rehydrated fully in sterile PBS for 12 h. This process is illustrated in Figure 1(A).
FIGURE 1.

In vitro analysis of PPF-reinforced pericardium. Liquid PPF is applied to dehydrated pericardium tissue, cross-linked using UV light, and then the complexed material is rehydrated to form the hybrid biomaterial (A). PPF-reinforced pericardium (1× PPF and 2× PPF) is compared to GA cross-linked (GA) and untreated (UN) pericardium for overall matrix organization using Masson’s Trichrome. Each sample is compared before (B) and after in vitro degradation (C), and shows sustained organization and reduced degradation in the GA as well as the 1× PPF and 2× PPF samples. Scale bar is 50 μm. Splenocytes that positively express the macrophage marker CD68 after culture with PPF films or LPS are shown in representative histograms in (D). From the cells positive for CD68, samples were then evaluated for positive staining of CD163 and CD80 (E).
PPF-reinforced pericardium was visually evaluated using histological staining techniques and compared to GA cross-linked pericardium and untreated pericardium. PPF mass per surface area of pericardium was calculated by subtracting the mass of dehydrated tissue (taken before PPF addition) from the mass of the hybrid (PPF + dehydrated tissue) immediately after crosslinking, resulting in mass of PPF added to the tissue.
In vitro studies
Degradation resistance with polymer reinforcement was modeled in vitro using 0.4 U/mL of collagenase in PBS solution. Samples of PPF-reinforced pericardium and GA cross-linked and untreated pericardium were shaken at 60 RPM in 37°C for 4 days. Samples were preserved in 4% PFA for histological analysis.
Inflammation potential was also evaluated in vitro. Primary splenocytes were isolated from the spleens of male Sprague Dawley (IC) rats. Spleens were aseptically removed from the animal and pressed through a 60 μm nylon mesh in RPMI-1640 medium. The cells washed and resuspended in RPMI-1640 medium containing 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. PPF thin films were added to the splenocyte culture on the day of isolation (day 0). Control groups were created using 100 ng/mL lipopolysaccharide (LPS) as a stimulator. After 3 days, cells were collected via cell scraping and stained for surface marker analysis. Splenocytes were fixed with 0.1% PFA for 15 min and then permeabilized using 0.5% Tween. Cells were stained with anti-CD68 (a general macrophage marker), anti-CD163 (expressed intracellularly on M2 macrophages), and anti-CD80 (expressed intracellularly on M1 macrophages), as was seen in other studies investigating inflammatory response to ECM-based scaffolds.32 For each cell sample, 50,000 events per test were analyzed using a BD FACSCanto II flow cytometer.
In vivo models
In vivo analysis was first conducted in athymic adult, male Sprague-Dawley (SD) rats, followed by evaluation in immunocompetent SD adult, male rats. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Maryland, and all animals were treated in accordance with the “Guide for the Care and Use of Laboratory Animals.” The first experiment was conducted in n = 5 athymic rats (AT), as determined by a power analysis. A small incision is made in the dorsal dermal layer, and a material patch (1 × 1 cm) from each of the following three groups was sutured by the corners to the underlying tissue: untreated pericardium (UN), pericardium with PPF on both sides (2× PPF) and pericardium with PPF on one side (1× PPF). After 6 weeks animals were euthanized and the pericardium patches and surrounding tissue were explanted. Half of each explant was fixed in paraformaldehyde (4%) and embedded in paraffin for histochemical analysis. The other half of each material was homogenized and analyzed using a TNFα ELISA (Qiagen). The second study was conducted in n = 5 immunocompetent SD rats (IC). Following a similar procedure, one material patch from each of the following groups was implanted subdermally in the dorsal dermal layer: GA cross-linked pericardium (GA), untreated pericardium (UN), and PPF-reinforced pericardium (2× PPF). Material was explanted after 6 weeks, preserved in paraformaldehyde (4%), and embedded in paraffin for histological analysis.
Histological and immunohistochemical analysis
Paraffin-embedded samples were sectioned into 5 μm slides and stained using hematoxylin and eosin (H&E) to assess remodeling and total cell infiltration. To quantify the cell density near the implanted material, a midline was drawn down the center of the original material volume using Image J. Parallel lines were placed at 100 μm intervals from the center. The 100-μm-thick regions beginning at the midline are designated 1A (right of the center line) and 1B (left of the center line), moving up in number as the regions move outward from the center. A representative grid is drawn over the sample in Figure 2(A). Using standard immunohistochemistry techniques, the following cell populations were identified using specific antibodies for rat cell surface markers. Macrophages were identified using a mouse anti-CD68 antibody for general macrophages (Abcam, ab31630, 1:100), a rabbit anti-CD206 antibody for M2 phenotype (Abcam, ab64693, 1:600), and a goat anti-CD86 antibody for M1 phenotype (R&D Systems, AF1340, 1:200). Natural killer (NK) cells were identified using a combination of a mouse anti-CD8a (Abcam, ab33786, 1:100) and a rabbit anti-CD3 (Abcam, ab16669, 1:100), where T cells can be identified with positive rabbit anti-CD3 and negative CD8a. Dendritic cells (DCs) were identified using a rabbit anti-CD11b/c antibody (Abcam, ab202907, 1:200). To detect these antibodies, the following fluorophores were used to match species of primary antibody: goat anti-mouse FITC-conjugated secondary antibody (Abcam, 1:200), donkey anti-rabbit Alexa Fluor® 647 secondary (Abcam, 1:200), and donkey anti-goat Alexa Fluor® 555 secondary (Abcam, 1:200). Finally, a cell nucleus stain VectaSheild® plus 4ʹ,6-diamidino-2-phenylin-dole, dihydrochloride (DAPI) (Vector Laboratories) was used to stain cell nuclei.
FIGURE 2.


Inflammatory response to hybrid material in athymic rats. After 6 weeks of implantation, scaffolds are explanted and stained with H&E (A), showing cell infiltration (nuclei are purple) and matrix changes compared to the unimplanted images (A). Cell infiltration is represented as density of cells in each 100 μm wide lane from the center of the implant (B). Using immunohistochemical staining, cells near the scaffold were identified as macrophages using CD68, and further characterized using CD86 (an M1 marker) and CD206 (an M2 marker) (D and E). Additionally, the scaffolds and surrounding tissue were homogenized and analyzed using an ELISA for TNFα (C). Samples were also examined for DCs using CD11b/c (F and G) and NK cells using CD3 and CD8a. Scale bar is 20 μm. Statistical analysis was conducted to compare each marker between the different material groups. Cellular marker columns that do not share letters are statistically different as determined with an ANOVA followed by a post hoc Tukey’s test (p < 0.05).
Images were collected using the Zeiss LSM 710 Confocal Microscope. For each stain, 5 images from 3 independent samples per treatment were collected using a standardized method. Positive staining was quantified using ImageJ. Cells were first identified using positive DAPI staining, and the circular area surrounding the nuclei was designated as a region of interest. The maximum value for each color channel corresponding to a secondary fluorophore was recorded for each region of interest. Pooling all regions of interest together and plotting a histogram of maximum values for each color channel, two peaks are apparent, indicating a maximum value cutoff between cells with positive staining and cells with negative staining. Using this cutoff value, each region of interest or cell can be identified as positive or negative for certain markers. Positive cells are represented as a percent of the total cell population counted (DAPI staining). Statistical analysis was conducted for each marker between treatment groups.
Statistical analysis
Data were analyzed using a one way analysis of variance (ANOVA), followed by a post hoc Tukey’s test using a 95% confidence interval using Minitab statistical software. Data plotted represent the mean with standard deviation.
RESULTS
The hybrid material was built by marrying a layer of the polymer PPF to a pericardium tissue sheet by dehydration of the tissue followed by UV cross-linking of the polymer, and then rehydration of the tissue (Fig. 1(A)). The resulting materials were evaluated qualitatively using histological staining techniques before and after degradation modeling. In Figure 1(B), sections of pericardium tissue with PPF reinforcement on both sides of the tissue (2× PPF), on one side of the tissue (1× PPF), untreated pericardium (UN), and GA cross-linked pericardium (GA) are stained with Masson’s Trichrome. In this stain, collagen structures are stained pink by the Biebrich scarlet-acid fuchsin solution (all acidophilic tissue elements), but are then decolorized application of phos-photungstic acid, and stained blue with aniline blue. This process is inhibited where PPF is incorporated with the tissue, so the area of overlap between the PPF and pericardium remains pink. The amount of polymer applied to these tissues was calculated to 0.21 mg/mm2 of pericardium surface area and 0.07 mg/mm2 for the 2× PPF and 1× PPF samples, respectively. The 2× PPF application is observed to form a more complete envelope around the tissue (covering edges and sides), which may explain the more than double mass increase. After in vitro degradation, UN pericardium appears to have lost organization of the collagen fibers (Fig. 1(C)). This result is also found on the PPF-lacking side of 1× PPF pericardium, even though the PPF-reinforced side of the tissue remains ordered. Pericardium with both sides reinforced with PPF (2× PPF) has retained most of its original organization, indicating little to no degradation of the tissue. These results support the theory that PPF reinforcement acts as a physical block to enzymatic degradation, and allows for the use of this approach to evaluate the hypothesis.
In degradable scaffolds, macrophage recruitment and polarization are important determiners of the overall response to the implant, as has been seen in other ECM-based implants. Before evaluation of the hybrid material, PPF was investigated independently of pericardium to assess what effect, if any, PPF has on the activation of splenocytes, as is seen in other biomaterial applications.33–35 Splenocytes represent a diverse population of leukocytes and were har-vested from the spleen of untreated IC rats. After 24 h of culture with PPF, there is mild splenocyte proliferation, stained with the CD68 marker (Fig. 1(D)), although much less is observed than is seen in the LPS-treated positive control (an established inflammatory stimulator for these proteins in macrophages). Further classification of the CD68+ population in Figure 1(E) shows a percentage of the CD68+ population that is positive for both CD80 and CD163, noted markers of the M1 or M2 phenotype.32 This result suggests that PPF, if added in the concentrations tested, may act as a relatively inert polymer, with slight differences based on the mass of foreign material in the cell environment.
The hybrid material was next evaluated in vivo in the subdermal space of 6 athymic rats to isolate the role of the innate immune system. To distinguish these results from those found in the immune competent model, these groups will be noted with an AT (athymic). This study compared pericardium with either PPF on both sides of the tissue (2× PPFAT) and pericardium with PPF on one side (1× PPFAT) to untreated pericardium (UNAT) as a control. Cell infiltration into the tissues is presented with H&E staining in Figure 2(A), and quantified by region in the graph in Figure 2(B). A distinct, one-sided infiltration of cells is observed into the untreated edge of 1× PPFAT. However, this density is less than a single side of the untreated control. With either 1 or 2 sides reinforced with PPFAT, less cells are able to infiltrate to the center regions than are observed in the untreated control. Immunohistochemical staining was used to further identify the cell population in terms of specific immune cells. Macrophages are identified using the CD68 surface marker (general macrophage), and can be classified further as M1 (CD86) and M2 (CD206). CD68+ cells are identified with highest density in the UNAT (52% of cells), which is significantly higher than the 33% in the 1× PPFAT or 6.2% in the 2× PPFAT samples (p < 0.00). A similar trend is seen in the M1 indicator CD86, with significantly more positive staining (37.9%, p < 0.00) in UNAT compared to the 1× PPFAT and 2× PPFAT groups. Interestingly, CD206, the M2 marker, is low in all samples, with no significant difference between the groups. The cytokine TNFα, which is involved in macrophage recruitment, is quantified in Figure 2(C) from homogenized samples of the implants. The sample 2× PPFAT has significantly less TNFα than the UNAT sample (significance is found between groups that do not share letters). The PPFAT sample TNFα concentration is also less than UNAT, but greater than 2× PPFAT.
Additional innate immune system cells were also successfully identified at the implant site. DCs, indicated by the CD11b/c surface marker, are shown together with CD68 (macrophage staining) for reference. As indicated in the graph in Figure 2(G), the most CD11b/c staining (46.8% of cells) is seen in UNAT. This density is significantly greater than the 11.2% of cells observed in the 1xPPFAT samples. In Figure 2(H), cells that are positive for both CD3 (red) and CD8a (green) represent NK cells, while cells positive for CD3 but negative CD8a could represent T cells. NK cells are not observed in 1× PPFAT, and although there are positive cells in 2× PPFAT and UNAT (5.1% and 2.7%, respectively) these are not significantly distinct from the 1× PPFAT samples. T cells are not expected in athymic rats, although slight positive signals are seen in the 2× PPF (0.8%) and UN (2.6%) samples, which could indicate nonspecific binding or other cells presenting the CD3 marker.
Based on this baseline interaction with the innate immune system, cell populations suspected to interact with the pericardium PPF hybrid were next evaluated in the immune competent model. To distinguish these results from those found in the previous model, these groups will be noted with an IC (immune competent). In Figure 3(A), H&E stained explants after 6 weeks show distinct differences in number of cell infiltrations, organization of that population, and cell mediated remodeling of the pericardium. Cell density by region (Fig. 3(B)) indicates that a high number of cells were able to infiltrate to the center of the UN samples, while a similarly dense band of cells congregated near the edge of the GA samples. Although there is cellular infiltration into 2× PPFIC, density is lower and more spread out than GAIC and UNIC controls. Very densely packed cells per-sisting after 6 weeks in implantation could indicate a chronic inflammation and has characteristics of a foreign body response.32,36
FIGURE 3.
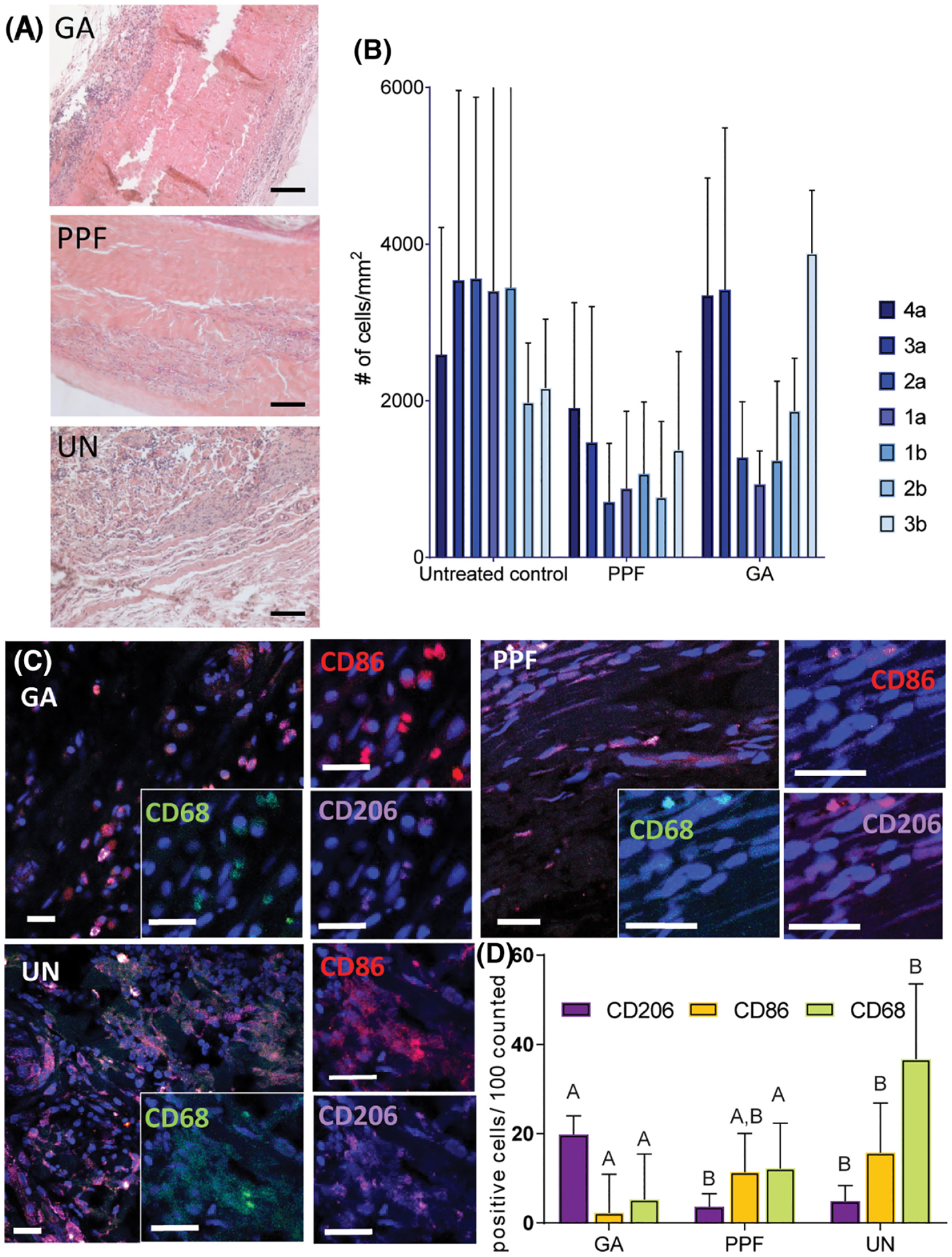

Inflammatory response to hybrid material in immunocompetent rats. After 6 weeks of implantation, scaffolds are explanted and stained with H&E (A), showing cell infiltration, which is also represented as density of cells in each 100 μm wide lane from the center of the implant (B). Cells near the scaffold were identified as macrophages using CD68, and further characterized using CD86 (an M1 marker) and CD206 (an M2 marker) (D and E). Samples were also examined for DCs using CD11b/c (F and G) and NK cells using CD3 and CD8a. Scale bar is 20 μm. Statistical analysis was conducted to compare each marker between the different material groups. Cellular marker columns that do not share letters are statistically different as determined with an ANOVA followed by a post hoc Tukey’s test (p < 0.05).
To further investigate and identify the responding cell groups, tissue sections were stained for surface markers to indicate macrophage phenotype at or near each implant (Fig. 3(C)). There is a high prevalence of the M1 stain among many macrophages in the dense cell band surrounding the GAIC sample. We also observe significantly high quantities of macrophages (identified with CD68) in the UNIC (36.7%), compared with 2× PPFIC (12.2%) and GAIC (5.5%) (p < 0.00). Although the macrophage density is greater in 2× PPFIC than GAIC, this difference is not significant. Interestingly, a higher density of CD206+ cells was counted near the GAIC samples than UNIC and 2× PPFIC. CD206+ is an indicator of an M2 phenotype, but has been found to describe a population of remodeling macrophages that promote fibroblast growth and capsule formation.37,38 This process is probable in response to chemically cross-linked materials, and may describe the high density of CD206+ macrophages.
DCs (CD11b/c marker positive) are positively identified in all samples, with no significant difference between groups (p = 0.43). Finally, both the NK and T cell profiles are shown in Figure 3(G). Possible T cells are found near these implants, which are expected in response to xenographic material implanted in an immunocompetent model. Cells that are positive for CD8a appear more dense in the UN samples (12%) compared to GA (9.9%) and 2× PPF (5.8%), although this difference is not significant (p = 0.08). However, the opposite trend is observed in NK cells, where the most NKs are counted in GA (8.25%) and the least are counted in UN samples (1.9%) (p < 0.02).
DISCUSSION
In vitro analysis of PPF indicated that it may act as a relatively inert polymer, activating less splenocytes than the LPS control. Further classification of the active population in Figure 1(E) shows a percentage that is positive for both CD80 and CD163.32 Although CD163 is a marker found on tissue resident macrophages in conjunction with other wound resolving M2 markers such as CD206,39,40 macrophage phenotype exists on a spectrum, and thus the positive staining of one surface marker is not a definitive indicator of an absolute phenotype. In the presence of high PPF, the population of activated macrophages shifts to higher positive staining of all three markers. Interestingly, a large portion of this increase is positive for CD163. As mentioned above, although CD163 is one indicator of an M2 phenotype, it is not restricted to M2 macrophages.41 It is interesting to note this increase in response to additional polymer concentration, even though it is less than the LPS control activation. Furthermore, although this result may not be predictive of in vivo activity, it allows for the comparison of PPF in the context of other common biomaterials tested in similar fashion, such as biomedical grade alginate34 and electrospun polydioxanone–elastin,35 which stimulate splenocytes less than inflammatory controls.
Subsequently, inflammatory activation in response to different concentrations of PPF as pericardium reinforcement was evaluated. In our previous work, we have reported lower density of macrophages (identified by immunohistochemical stain of the F4/80 surface marker) in response to PPF-reinforced pericardium compared to untreated and totally exposed pericardium.30 This was hypothesized to be a result of physical blocking of the ECM by the polymer, as no chemical changes were made to the protein components. To understand the circumstances surrounding that initial observation, the polymer coverage was varied and the responding cell populations from both immune compromised and immune competent models were identified. The hybrid material was initially implanted into the subdermal space of 6 athymic rats to isolate the role of the innate immune system. The objective is to improve upon pericardium alone as a vascular graft material by adding PPF to prolong degradation time and slow inflammatory response. PPF alone is not a comparable material option for biologic-based vascular grafts as it has no protein components, and so PPF alone was not included as a control. However, based on the lack of inflammatory stimulation from PPF alone in vitro and the lack of inflammatory stimulation seen in other in vivo assessments, a major contribution to inflammation is not expected.42–44
Untreated pericardium samples had the most inflammatory cells present, with significantly higher macrophage density and DC cells than the PPF-reinforced samples. Increasing PPF coating to two sides of the pericardium appears to reduce macrophage density and overall cell infiltration. The persistence of inflammatory cells and the density of infiltration may indicate a chronically inflamed environment. We therefore suggest that untreated pericardium may initiate too great of an inflammatory pathway, which may result in chronic inflammation of the implant. The significantly reduced macrophage density and reduced TNFα in response to the 2× PPFAT-reinforced samples could indicate a more controlled degradation of the material. Although TNFα is a relevant molecule in tissue regrowth, high levels at this stage (6 weeks) can recruit and stimulate more M1 macrophages, leading to over production of matrix, proliferation of fibroblasts, and maintenance of the inflamed state. TNFα concentrations, together with macrophage density, suggest that with increased PPF, less ECM is presented to the host, resulting in less inflammatory stimulation.
The host response to ECM-based biomaterials without endogenous cells will be directed by host innate immune cells participating in inflammation and/or remodeling.45 This process may be dominated by macrophages, but can be better described by surveying the whole population of responding cells, especially in light of the complex determination of macrophage phenotype. Athymic rats do not have mature T cell populations, and as such are typically used to study xenograft material implants.46 However, it is possible that in nude rats, other leukocytes contribute to the response in the absence of T cells.46 The particular cell populations could change in the immune competent rats, and can be compared to infer the role of innate immunity in responding to pericardium materials. Based on the results from the first objective, pericardium with two sides of PPF reinforcement was implanted into the subdermal space in Sprague-Dawley (immunocompetent) rats.
As previously described, some inflammatory and macrophage activity can be an important step in the vascular healing process. A carefully balanced population of macrophages between M1 and M2 could direct appropriate healing. However, sustained presence after 6 weeks of the inflammatory phenotype M1 in the GAIC and UNIC samples supports the observation of chronic inflammation and foreign body response in vascular grafts.20,23 Less cells involved in the 2× PPFIC-reinforced sample could mean fewer MMPs and matrix breakdown pathways, slowing the process of remodeling.
These data provide a basis to continue to explore the relationship between ECM-based scaffolds and the host immune response to vascular patches. Using information gained here regarding cell populations present at 3 and 6 weeks, we can begin to elucidate some theories about the signaling pathways initiated by fragments of the ECM-based scaffold. There are other cell types that are relevant to host–material interactions that are not fully considered in this study, as indicated by the difference in macrophage presence between athymic and immunocompetent rats.8 Nevertheless, the results from this study indicate that PPF presence does not increase inflammatory metrics significantly in vivo, and in fact may reduce the propagation of inflammatory pathways resulting from a substantial amount of natural and degradable material. Increasing the presentation of pericardium and therefore recognizable cues as the scaffolds move from low polymer ratio to high polymer ratio and finally reach untreated pericardium, the expected result is that cell infiltration and resulting matrix remodeling should increase. This relationship can be leveraged to direct the appropriate timing, governed by the relationship of macrophages and endothelial cells, of scaffold degradation and new tissue formation.
CONCLUSIONS
We describe here a hybrid material scaffold, combining a natural material, pericardium, with a synthetic polymer coating, PPF. The addition of the polymer layer is demonstrated as an effective tool to leverage the presentation of the ECM molecules in the pericardium to the host material. The result is a biologically active scaffold that could exhibit extended remodeling times in vivo, and therefore reduced inflammation. Reduced inflammation and sustained elastic strength will lead to better healing, and insure the graft can sustain the functions (in terms of patency and pressure) of large diameter vessels. This platform is especially useful in vascular graft applications, and other implant sites that require compliant materials to sustain function. We think the approach presented here provides a promising direction for future vascular graft development.
Acknowledgments
Contract grant sponsor: Maryland Stem Cell Research Fund; contract grant number: Grant # 4300811
Contract grant sponsor: National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH); contract grant number: Award Number F31HL132541
REFERENCES
- 1.Pashneh-Tala S, MacNeil S, Claeyssens F. The tissue-engineered vascular graft-past, present, and future. Tissue Eng Part B Rev 2015; 22(1):68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sugiura T, Hibino N, Breuer CK, Shinoka T. Tissue-engineered cardiac patch seeded with human induced pluripotent stem cell derived cardio-myocytes promoted the regeneration of host cardiomyocytes in a rat model. J Cardiothorac Surg 2016;11(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bond R, Rerkasem K, Naylor AR, AbuRahma AF, Rothwell PM. Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg 2004;40(6):1126–1135. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Guo Y, Ziegler KR, Model LS, Eghbalieh SDD, Brenes RA, Kim ST, Shu C, Dardik A. Current usage and future directions for the bovine pericardial patch. Ann Vasc Surg 2011;25(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryugo M, Yasugi T, Nagashima M, Izutani H, Okamura T, Shikata F, Kawamura M, Kawachi K. Pseudoaneurysm in the left groin due to ruptured knitted Dacron graft. Ann Vasc Dis 2011;4(2):154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hibino N et al. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 2010;139(2):431–436. [DOI] [PubMed] [Google Scholar]
- 7.Muto A, Nishibe T, Dardik H, Dardik A. Patches for carotid artery endarterectomy: Current materials and prospects. J Vasc Surg 2009;50(1):206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol 2008;20(2):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials 2007;28(25):3587–3593. [DOI] [PubMed] [Google Scholar]
- 10.Bracaglia LG, Messina M, Winston S, Kuo CY, Lerman M, Fisher JP. 3D printed pericardium hydrogels to promote wound healing in vascular applications. Biomacromolecules 2017;18(11):3802–3811. [DOI] [PubMed] [Google Scholar]
- 11.Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation 2001;71(11):1631–1640. [DOI] [PubMed] [Google Scholar]
- 12.Balestrini JL, Niklason LE. Extracellular matrix as a driver for lung regeneration. Ann Biomed Eng 2015;43(3):568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 2018;3(7):159–173. [Google Scholar]
- 14.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Printing three-dimensional tissue analogues with decellu-larized extracellular matrix bioink. Nat Commun 2014;5:3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 2009;30(29):5409–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Li W, Johnson SA, Ingram DA, Yoder MC, Badylak SF. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium 2004; 11(3–4):199–206. [DOI] [PubMed] [Google Scholar]
- 17.Sinha P, Zurakowski D, Susheel Kumar TK, He D, Rossi C, Jonas RA. Effects of glutaraldehyde concentration, pretreatment time, and type of tissue (porcine versus bovine) on postimplantation calcification. J Thorac Cardiovasc Surg 2012;143(1):224–227. [DOI] [PubMed] [Google Scholar]
- 18.Morris AH, Stamer DK, Kyriakides TR. The host response to naturally-derived extracellular matrix biomaterials. Semin Immunol 2017;29:72–91. [DOI] [PubMed] [Google Scholar]
- 19.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011;32(28):6692–6709. [DOI] [PubMed] [Google Scholar]
- 20.Hibino N, Yi T, Duncan DR, Rathore A, Dean E, Naito Y, Dardik A, Kyriakides T, Madri J, Pober JS, Shinoka T, Breuer CK. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J 2011; 25(12):4253–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol 2002;22(11):1769–1776. [DOI] [PubMed] [Google Scholar]
- 22.Gaspardone A, Crea F, Versaci F, Tomai F, Pellegrino A, Chiariello L, Gioffrè PA. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol 1998;82(4):515–518. [DOI] [PubMed] [Google Scholar]
- 23.Hibino N, Mejias D, Pietris N, Dean E, Yi T, Best C, Shinoka T, Breuer C. The innate immune system contributes to tissue-engineered vascular graft performance. FASEB J 2015;29(6):2431–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon P, Kasimir MT, Seebacher G, Weigel G, Ullrich R, Salzer-Muhar U, Rieder E, Wolner E. Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 2003;23(6):1002–1006. discussion 1006. [DOI] [PubMed] [Google Scholar]
- 25.Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G. Sequential delivery of immunomodulatory cyto-kines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015;37:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014;35(15): 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon S Alternative activation of macrophages. Nat Rev Immunol 2003;3(1):23–35. [DOI] [PubMed] [Google Scholar]
- 28.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44(3):450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald SM, Matheson LA, McBane JE, Kuraitis D, Suuronen E, Santerre JP, Labow RS. Use of monocyte/endothelial cell co-cultures (in vitro) and a subcutaneous implant mouse model (in vivo) to evaluate a degradable polar hydrophobic ionic polyure-thane. J Cell Biochem 2011;112(12):3762–3772. [DOI] [PubMed] [Google Scholar]
- 30.Bracaglia LG, Yu L, Hibino N, Fisher JP. Reinforced pericardium as a hybrid material for cardiovascular applications. Tissue Eng Part A 2014;20(21–22):2807–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kasper FK, Tanahashi K, Fisher JP, Mikos AG. Synthesis of poly(propylene fumarate). Nat Protoc 2009;4(4):518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 2008;14(11):1835–1842. [DOI] [PubMed] [Google Scholar]
- 33.Jones KS. Effects of biomaterial-induced inflammation on fibrosis and rejection. Semin Immunol 2008;20(2):130–136. [DOI] [PubMed] [Google Scholar]
- 34.Orive G, Carcaboso AM, Hernández RM, Gascón AR, Pedraz JL. Bio-compatibility evaluation of different alginates and alginate-based microcapsules. Biomacromolecules 2005;6(2):927–931. [DOI] [PubMed] [Google Scholar]
- 35.Smith MJ, White KL Jr, Smith DC, Bowlin GL. In vitro evaluations of innate and acquired immune responses to electrospun polydioxanone-elastin blends. Biomaterials 2009;30(2):149–159. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol 2008;20(2):86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medbury HJ, James V, Ngo J, Hitos K, Wang Y, Harris DC, Fletcher JP. Differing association of macrophage subsets with atherosclerotic plaque stability. Int Angiol 2013;32(1):74–84. [PubMed] [Google Scholar]
- 38.Bellón T, Martínez V, Lucendo B, del Peso G, Castro MJ, Aroeira LS, Rodríguez-Sanz A, Ossorio M, Sánchez-Villanueva R, Selgas R, Bajo MA. Alternative activation of macrophages in human peritoneum: Implications for peritoneal fibrosis. Nephrol Dial Transplant 2011;26(9):2995–3005. [DOI] [PubMed] [Google Scholar]
- 39.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R⊠szer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm 2015; 2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barros MH et al. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One 2013;8(11):e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fisher JP, Vehof JWM, Dean D, van der Waerden JPCM, Holland TA, Mikos AG, Jansen JA. Soft and hard tissue response to photocrosslinked poly(propylene fumarate) scaffolds in a rabbit model. J Biomed Mater Res 2002;59(3):547–556. [DOI] [PubMed] [Google Scholar]
- 43.Melchiorri AJ, Hibino N, Best CA, Yi T, Lee YU, Kraynak CA, Kimerer LK, Krieger A, Kim P, Breuer CK, Fisher JP. 3D-printed bio-degradable polymeric vascular grafts. Adv Healthc Mater 2016;5(3): 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vehof JW et al. Bone formation in transforming growth factor beta-1-coated porous poly(propylene fumarate) scaffolds. J Biomed Mater Res 2002;60(2):241–251. [DOI] [PubMed] [Google Scholar]
- 45.Delgado LM, Bayon Y, Pandit A, Zeugolis DI. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng Part B Rev 2015;21(3): 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rolstad B The athymic nude rat: An animal experimental model to reveal novel aspects of innate immune responses? Immunol Rev 2001;184:136–144. [DOI] [PubMed] [Google Scholar]


