Abstract
Defects in the light-evoked responses of the retina occur early in the sequalae of diabetic retinopathy (DR). These defects, identified through the electroretinogram (ERG), represent dysfunction of retinal neurons and the retinal pigment epithelium and are commonly identifiable at the timing of, or almost immediately following, diabetes diagnosis. Recently, systemic reduction of the facilitated glucose transporter type 1, Glut1, in type 1 diabetic mice was shown to reduce retinal sorbitol accumulation, mitigate ERG defects, and prevent retinal oxidative stress and inflammation. Herein, the study investigated whether systemic reduction of Glut1 also diminished hallmarks of DR in type 2 diabetic mice. Transgenic nondiabetic Leprdb/+ and spontaneously diabetic Leprdb/db mice that expressed wild-type (Glut1+/+) or systemically reduced levels of Glut1 (Glut1+/−) were aged and subjected to standard strobe flash electroretinography and c-wave analysis before evaluation of inflammatory cytokines and oxidative stress molecules. Although Leprdb/dbGlut1+/− mice still displayed overt obesity and diabetes, no scotopic, photopic, or c-wave ERG defects were present through 16 weeks of age, and expression of inflammatory cytokines and oxidative stress molecules was also normalized. These findings suggest that systemic reduction of Glut1 is sufficient to prevent functional retinal pathophysiology in type 2 diabetes. Targeted, moderate reductions of Glut1 or inhibition of Glut1 activity in the retina of diabetic patients should be considered as a novel therapeutic strategy to prevent development and progression of DR.
Diabetes affects >537 million adults worldwide, accounting for 11.5% of the global health expenditure (International Diabetes Federation Diabetes Atlas, 10th edition, 2021). Despite differing etiology of type 1 (inability to produce insulin) and type 2 (insulin-resistant) diabetes, the characteristics of diabetic retinopathy (DR) are not distinguished between the two diseases. Nevertheless, because approximately 90% to 95% of all diabetes cases are classified as type 2 diabetes (T2D), therapeutic interventions for DR need to be tested in and specifically addressed in this group (CDC National Diabetes Statistics Report, https://www.cdc.gov/diabetes/data/statistics-report/index.html, last accessed March 1, 2023).
Inhibition of glucose transport has long been a target for the microvascular complications of diabetes. Recently, the inhibition of Sglt2, the sodium-glucose cotransporter, has been investigated for the prevention of DR, diabetic kidney disease, and diabetic neuropathy. However, the findings from these studies are mixed.1, 2, 3, 4, 5 Moreover, a recent study found reduced Sglt2 levels in peripheral blood mononuclear cells of patients with proliferative diabetic retinopathy, suggesting that although targeting of glucose is desired, inhibition of Sglt2 may not be an effective therapeutic for prevention of DR.6 Recent work demonstrated that systemic or retina-specific reduction of the primary facilitative glucose transporter in the retina and retinal pigment epithelium (RPE), Glut1 (Slc2a1), attenuated hallmarks of DR in the streptozotocin mouse model of type 1 diabetes at both acute (2 and 4 weeks) and prolonged (12 weeks) time points. This included a normalization of reductions in electroretinogram (ERG) component amplitudes, reduced accumulation of the neurotoxic polyol, sorbitol, in the retina, and a marked reduction in inflammation/oxidative stress molecules. The reversal of these outcome measures was found at both early (4 weeks) and sustained (12 weeks) durations of diabetes.7 To determine whether systemic reduction of Glut1 levels in a mouse model of T2D also prevents pathologic characteristics of DR, Glut1 expression was genetically reduced in the Leprdb/db mouse by crossing it with a Glut1+/− line. Nondiabetic Glut1+/−Leprdb/+ mice displayed 40% reduced Glut1 levels throughout the retina, compared with Glut1+/+Leprdb/+ littermates. Glut1+/+Leprdb/db mice exhibited overt obesity and hyperglycemia compared with nondiabetic Glut1+/+Leprdb/+ mice. Although Glut1+/−Leprdb/db mice remained diabetic and obese, they also exhibited a significant reduction of Glut1 in the retina. All four genotypes underwent strobe flash electroretinography and c-wave analysis at 8 and 16 weeks of age. At both time points, the reduction in Glut1 levels (Glut1+/−) in diabetic (Leprdb/db) mice normalized ERG component amplitudes, including the photoreceptor-derived a-wave, the bipolar cell-dependent b-wave, and the RPE-dependent c-wave. Retinal oscillatory potentials were also normalized at 8 and 16 weeks of age. Expression of inflammatory cytokines and markers of oxidative stress molecules was similarly attenuated in diabetic mice by the systemic reduction of Glut1.
Materials and Methods
Mice
Leprdb/db mice of the C57BLKS background were purchased from The Jackson Laboratory (Bar Harbor, ME; number 000642). Glut1+/− mice were kindly provided by Darryl De Vivo (Columbia University, New York, NY). Mice were intercrossed to generate control (Leprdb/+) and diabetic (Leprdb/db) mice with one (Glut1+/−) or two (Glut1+/+) wild-type Glut1 alleles. Both male and female mice were used. All animals were maintained on a 12-hour light/dark cycle and provided food ad libitum. All experiments were performed in accordance with the Institutional Animal Care and Use Committee at the Louis Stokes Cleveland VA Medical Center (Cleveland, OH) and followed the Association for Research in Vision and Ophthalmology Guidelines for Use of Animals in Ophthalmic and Vision Research.
Genotyping
The Glut1+/+ and Glut1+/− alleles were identified using the following primers: wild-type forward, 5′-CCATAAAGTCAGAAATGGAGGGAGGTGGTGGT-3′; wild-type reverse, 5′-GCGAGACGGAGAACGGACGCGCTGTAACTA-3′; and mutant reverse, 5′-CTACCGGTGGATGTGGAATGTGTGCGAGGC-3′.
The Leprdb/db allele was identified by high-resolution melt analysis using the following primers: forward, 5′-TGACCACTACAGATGAACCCA-3′; and reverse, 5′-GTCATTCAAACCATAGTTTAGGTTTGT-3′.
Electroretinography
After overnight dark adaptation, mice were anesthetized with 65 mg/kg sodium pentobarbital. Eye drops were used to anesthetize the cornea (1% proparacaine HCl) and to dilate the pupil (2.5% phenylephrine HCl, 1% tropicamide, and 1% cyclopentolate HCl). Mice were placed on a temperature-regulated heating pad throughout the recording session, which was performed as previously described.7 In brief, ERGs were recorded in response to strobe flash stimuli presented in the dark by an Espion E3 ColorDome Full Field Ganzfeld (Diagnosys, Lowell, MA). An Ag/AgCl electrode in contact with the cornea was referenced to an Ag/AgCl pellet electrode placed in the mouth of the mouse, and a ground lead was placed in the tail. Scotopic responses were obtained in the dark with 10 steps of a white-light flash stimulus, ranging from −3.6 log cd.s/m2 to 2.1 log cd.s/m2. The duration of the inter-stimulus intervals increased from 4 seconds for low-luminance flashes to 90 seconds for the highest stimuli. Two minutes following the scotopic ERG, a 10-second white light stimulus (5 cd/m2) was presented to elicit the c-wave. After 7 minutes of light adaptation, cone ERGs were recorded with strobe-flash stimuli (−1 to 2 log cd·s/m2) superimposed on the adapting field. Amplitude of the a-wave was measured at 8.3 milliseconds following the stimulus. Amplitude of the b-wave was calculated by summing the amplitude of the a-wave at 8.3 milliseconds with the peak of the waveform after the oscillatory potentials (≥40 milliseconds). Light-adapted response amplitudes were calculated by summing the peak of the waveform with the amplitude at 8.3 milliseconds. Oscillatory potentials (OPs) were filtered from the strobe flash responses by the Diagnosys program. OP amplitude was determined by measuring the change in amplitude from the preceding trough to the peak of each potential. Amplitude of the c-wave was determined by subtracting the average baseline amplitude from the maximal response following the b-wave.
Western Blot Analysis
Retinas were isolated from enucleated eyecups, and protein was extracted using 100 μL per retina in radioimmunoprecipitation assay buffer (Cell Signaling, Danvers, MA; number 9806S) with Roche cOmplete ULTRA Protease Inhibitor (Sigma, St. Louis, MO; number 5892970001). Retinal samples were homogenized by trituration with a P200 pipette. Samples were placed on ice for 30 minutes with intermittent vortexing every 10 minutes. The samples were centrifuged at 18,626 × g for 20 minutes, and the supernatants were removed for protein quantification by BCA Protein Assay (Thermo Scientific, Rockford, IL; number 23208).
A total of 15 μg of retina protein was loaded on 4% to 15% Mini-PROTEAN TGX gels (BioRad, Hercules, CA; number 4561083) with diluted 6× Laemelli SDS reducing sample buffer. Gels were run for approximately 90 minutes at 125 V and transferred electrophoretically onto polyvinylidene difluoride transfer membranes (activated with methanol) at 100 V for 1 hour. Membranes were then incubated for 1 hour at room temperature in Odyssey blocking buffer (Li-Cor, Lincoln, NE; number 927-500) and incubated overnight with primary antibodies at 4°C in Odyssey blocking buffer. See Table 1 for sources and dilutions of all primary and secondary antibodies used for Western blot analysis. Membranes were washed 3 × 5 minutes with tris-buffered saline with 0.1% Tween 20 and incubated for 1 hour in the dark with Li-Cor IRdye secondary antibodies (1:10,000) in Odyssey blocking buffer + 0.2% Tween 20 + 0.01% SDS. Blots were washed 3 × 5 minutes in tris-buffered saline with Tween 20 at room temperature in the dark, rinsed with tris-buffered saline, and imaged with an Odyssey CLx Infrared Imaging System (Li-Cor). Densitometry was performed with Li-Cor Image Studio Software.
Table 1.
Antibodies
| Antigen | Supplier | Catalog no. | Species | Dilution: WB analysis | Dilution: IHC |
|---|---|---|---|---|---|
| Actin | Cell Signaling | 3700S | Mouse | 1:1000 | |
| Glut1 | Millipore Sigma (St. Louis, MO) | 07-1401 | Rabbit | 1:2000 | 1:500 |
| Glutamine synthetase | BD Biosciences (San Jose, CA) | 610517 | Mouse | 1:500 | |
| Alexa Fluor 488 | Invitrogen (Waltham, MA) | A11008 | Rabbit | 1:500 | |
| Alexa Fluor 594 | Invitrogen | A11032 | Mouse | 1:500 | |
| Donkey anti-mouse 800CW | Li-Cor | 926-32212 | Mouse | 1:10,000 | |
| Donkey anti-rabbit 680RD | Li-Cor | 926-68073 | Rabbit | 1:10,000 |
IHC, immunohistochemistry; WB, Western blot.
Immunohistochemistry
Enucleated eyes were fixed in 4% paraformaldehyde, and eyecups were prepared by removal of the cornea and lens. Tissue was fixed for 1 to 4 hours at room temperature before equilibration in sucrose solutions (10% for 1 hour; 20% for 1 hour; and 30% overnight). Equilibrated eyecups were embedded in Tissue Tek OCT Compound (Sakura Finetek USA, Torrence, CA), frozen on dry ice, and cryosectioned at 10 μm. Slides were stored at −20°C and brought to room temperature before rehydration with 1 × phosphate-buffered saline (PBS). Endogenous peroxidases were quenched with 50 mmol/L NH4Cl (30 minutes at room temperature), and antigen retrieval was performed by incubation of sections with hot 0.1 mol/L sodium citrate buffer (10 minutes at room temperature). Sections were blocked in 5% normal goat serum + 5% bovine serum albumin in PBS + 0.1% Triton X-100 and incubated with primary antibodies overnight at 4°C. Slides were washed 3 × 5 minutes in PBS and incubated with secondary antibodies in blocking buffer for 45 minutes at 37°C. Slides were washed 3 × 5 minutes in PBS, counterstained with DAPI, and coverslipped with 50% glycerol. All slides were imaged using a Zeiss LSM 800 confocal microscope with Airyscan superresolution (Dublin, CA). See Table 1 for dilutions of all primary and secondary antibodies.
DHE Staining
Reactive oxygen species were measured in retinal cryosections by dihydroethidium (DHE) staining. Enucleated eyes were dissected on ice-cold PBS, frozen in Tissue Tek OCT Compound within 15 minutes of enucleation, and cryosectioned at 10 μm. Slides were stored at −20°C and brought to room temperature before rehydration with 1 × PBS. Fresh frozen retinal cryosections spanning the optic nerve were incubated with DHE (Thermo Fisher, Waltham, MA; 1:5000) for 20 minutes, followed by counterstaining with DAPI (1:10,000).
Quantitative PCR
Gene expression of glucose transporters, inflammatory cytokines, and oxidative stress molecules was measured by quantitative PCR on dissected retinal tissue. RNA was extracted using the RNAeasy Mini Kit (Qiagen, Hilden, Germany; number 74104), and RT-PCR was performed using the Verso cDNA synthesis kit (Thermo Scientific; number AB1453A). Radiant Green 2X qPCR Lo-ROX enzyme (Alkali Scientific Inc., Fort Lauderdale, FL) was used for all real-time quantitative PCRs. Relative fold changes in gene expression were determined using the comparative threshold cycle (CT) method (2ΔΔCT method). For all analyses, β-actin was used as the reference gene. Hypoxia-inducible factor (HIF)-1α (number QT01039542) and vascular endothelial growth factor (VEGF; number QT00160769) were analyzed using primers from Qiagen. Primers for all other genes investigated are listed in Table 2.
Table 2.
Primers
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Tnf | 5′-CATCTTCTCAAAATTCGAGTGACAA-3′ | 5′-TGGGAGTAGACAAGGTACAACCC-3′ |
| Il1b | 5′-GATCCACACTCTCCAGCTGCA-3′ | 5′-CAACCAACAAGTGATATTCTCCATG-3′ |
| Ptgs2 (Cox2) | 5′-CACAGCCTACCAAAACAGCCA-3′ | 5′-GCTCAGTTGAACGCCTTTTGA-3′ |
| Nos2 | 5′-GACTCTTGGTGAAAGTGGTGTTC-3′ | 5′-GCAGACAACCTTGGTGTTGA-3′ |
| Actb | 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ | 5′-CCTAGAAGCATTTGCGGTGCACGATG-3′ |
Statistical Analysis
For all experiments, data are presented as average ± SEM unless otherwise indicated. At least three mice per genotype/time point were utilized for all experiments. Statistical significance was determined by one- or two-way analysis of variance (ANOVA) with Tukey multiple comparisons test using GraphPad Prism 6 Software (Boston, MA). Western blot and real-time quantitative PCR analysis was performed using one-way ANOVA (genotype). Analysis of a-waves, b-waves, and the light-adapted response was performed using two-way ANOVA (genotype and flash intensity) for each time point. The c-wave and each OP was analyzed by one-way ANOVA (genotype) for each time point. No repeated-measures tests were performed.
Results
Glut1+/+Leprdb/db mice on the C57BLKS background exhibit elevated body weight and blood glucose levels as early as 4 weeks of age.8 When crossed with the Glut1+/− line to generate a Glut1+/−Leprdb/db mouse, there was no change in the development of obesity (Figure 1A) or hyperglycemia (Figure 1B), despite the presence of only one Glut1 (Slc2a1) allele. Glut1+/− mice displayed a significant reduction in Glut1 levels, as depicted by Western blot analysis (Figure 1C) [F(3,8) = 15.73, P = 0.0010] and immunohistochemistry (Figure 1D). As previously shown, Glut1 was expressed throughout the retina, with high expression in the RPE and exclusion from the outer segments.9 Notably, as observed in the streptozotocin-induced type 1 diabetic mouse retina,7 Glut1 appeared to be elevated in the Leprdb/db retina.
Figure 1.
Leprdb/db mice exhibit obesity and hyperglycemia despite expression of one Glut1 allele. A: Body weight of nondiabetic [Lepr control (cntl)] and diabetic (Leprdb/db), Glut1 wild-type (Glut1+/+) or heterozygous (Glut1+/−) mice from 8 to 16 weeks of age. B: Nonfasting blood glucose. Measurements >250 mg/dL were considered hyperglycemic. Red line indicates threshold for hyperglycemia (250 mg/dL). C: Protein levels of Glut1 from dissected retinas of 16-week–old mice. Retinas were dissected, and total Glut1 levels were normalized to β-actin for quantitative analysis. D: Confocal images of Glut1 immunoreactivity (green) and DAPI counterstaining (blue) in cryosections from retinas at 16 weeks of age. n ≥ 3 in each group (C). ∗P ≤ 0.05, ∗∗P ≤ 0.01. Scale bar = 50 μm (D).
Leprdb/db mice on the C57BLKS background initially display reductions to ERG b- and c-wave amplitudes at 8 weeks of age.10 Herein, significant reductions were found in all ERG component amplitudes (including the a-wave) at 16 weeks. Therefore, to determine whether the reduction of Glut1 affected the onset and progression of ERG defects found in these mice, strobe flash electroretinography was conducted at both 8 and 16 weeks of age. A 10-step ERG protocol was performed on control (Leprdb/+) and diabetic (Leprdb/db) mice with wild type (Glut1+/+) or reduced levels of Glut1 (Glut1+/−). Figure 2A (8 weeks) and Figure 2C (16 weeks) depict representative waveform traces from step 9 of the protocol (1.4 log cd.s/m2). As expected, no differences were observed between Glut1+/+Leprdb/+ and Glut1+/−Leprdb/+ mice, and Glut1+/+Leprdb/db mice exhibited significant reductions in a- and b-wave amplitudes compared with controls (Figure 2, B and D) [ANOVAs by genotype: a-wave, F(3, 229) = 12.53, P < 0.0001; b-wave F(3,229) = 14.02, P < 0.0001). However, both components were fully normalized in Glut1+/−Leprdb/db mice, at both ages (Figure 2, A–D). The average amplitude of each mouse cohort in response to all flash intensities is shown in the intensity response curves in Figure 2B (8 weeks) and Figure 2D (16 weeks). Importantly, no difference between Glut1+/+Leprdb/+ and Glut1+/−Leprdb/+ mice was observed in the scotopic light-evoked response to any flash luminance, at either age.
Figure 2.
Diabetes-induced electroretinogram (ERG) defects are normalized in mice with reduced Glut1. A–F: Strobe flash ERGs were performed on nondiabetic [Lepr control (cntl)] and diabetic (Leprdb/db), Glut1 wild-type (Glut1+/+) or heterozygous (Glut1+/−) mice at both 8 and 16 weeks of age. A and C: Representative strobe flash ERG waveform traces evoked in response to a 1.4 log cd.s/m2 light stimulus are depicted from testing at 8 (A) and 16 (C) weeks. B and D: Luminance-response functions for the a- and b-wave. E: Representative traces of filtered oscillatory potentials (OPs) from strobe flash ERGs evoked by a 1.4 log cd.s/m2 flash stimulus at 8 (left side) and 16 (right side) weeks of age. F: Average amplitude of OP1, OP2, and OP3 at 8 (left side) and 16 (right side) weeks of age. Amplitude was measured from the minimum of the preceding trough to the peak of the potential. ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, and ∗∗∗∗P ≤ 0.0001.
As reduced OP amplitudes and increased latency of these filtered wavelets are also characteristic of early DR, the OPs from each cohort of mice were analyzed at both ages. Figure 2, E and F, depicts the normalization of the OP amplitudes in the Glut1+/−Leprdb/db mice compared with Glut1+/+Leprdb/db mice (Figure 2, E and F) [ANOVAs by genotype: OP1 8 weeks, F(3,15) = 6.673, P = 0.0044; OP2 8 weeks, F(3,16) = 5.656, P = 0.0077; OP3 8 weeks, F(3,16) = 8.653, P = 0.0012; OP1 16 weeks, F(3,11) = 7.846, P = 0.0045; OP2 16 weeks, F(3,11) = 24.58, P < 0.0001; OP3 16 weeks, F(3,11) = 11.40, P = 0.0011]. Table 3 further demonstrates the significant increase in latency of OP2 and OP3 at 16 weeks in the Glut1+/+Leprdb/db mice, which was normalized by the reduction of Glut1 in the Glut1+/−Leprdb/db strain (Table 3) [ANOVA: OP2 16 weeks, F(3,11) = 13.47, P = 0.0005; OP3 16 weeks, F(3,11) = 12.10, P = 0.0008].
Table 3.
Diabetes Does Not Alter OP Latency with or without Reduction in Glut1
| Oscillatory potential latency, average ± SEM, milliseconds (n) |
||||
|---|---|---|---|---|
| Age, weeks | Genotype | OP1 | OP2 | OP3 |
| 8 | Glut1+/+Lepr cntl | 11.37 ± 0.277 (6) | 23.30 ± 0.372 (6) | 34.25 ± 0.397 (6) |
| 8 | Glut1+/+Lepr db/db | 11.81 ± 0.485 (5) | 23.80 ± 0.424 (5) | 34.61 ± 0.564 (5) |
| 8 | Glut1+/–Lepr cntl | 11.09 ± 0.277 (6) | 23.57 ± 0.411 (6) | 33.83 ± 0.668 (6) |
| 8 | Glut1+/–Lepr db/db | 11.09 ± 0.734 (3) | 23.02 ± 0.999 (3) | 34.39 ± 1.544 (3) |
| 16 | Glut1+/+Lepr cntl | 12.06 ± 0.277 (4) | 24.13 ± 0.555 (4) | 35.36 ± 0.734 (4) |
| 16 | Glut1+/+Lepr db/db | 11.23 ± 0.537 (4) | 27.04 ± 0.240 (4)∗,† | 38.27 ± 0.588 (4)†,‡ |
| 16 | Glut1+/–Lepr cntl | 10.82 ± 0.215 (4) | 23.30 ± 0.588 (4) | 33.70 ± 0.712 (4) |
| 16 | Glut1+/–Lepr db/db | 11.09 ± 0.277 (3) | 23.30 ± 0.480 (3)‡ | 33.02 ± 0.548 (3)§ |
Cntl, control; OP, oscillatory potential.
P < 0.01, Glut1+/+Lepr cntl versus Glut1+/+Lepr db/db.
P < 0.01, Glut1+/−Lepr cntl versus Glut1+/+Lepr db/db.
P < 0.05, Glut1+/+Lepr cntl versus Glut1+/+Lepr db/db.
P < 0.01, Glut1+/+Lepr db/db versus Glut1+/−Lepr db/db.
Dysfunction of the RPE is one of the earliest pathophysiological hallmarks of DR. To assess whether reduction of the Glut1 similarly prevents RPE dysfunction in T2D, c-wave amplitudes evoked in response to a 5-cd.s/m2 light stimulus (Figure 3) were analyzed. Representative waveforms are depicted in Figure 3, A and C. As observed in response to the strobe flash stimulus, i) no differences in response were identified in c-wave amplitudes between Glut1+/+Leprdb/+ and Glut1+/−Leprdb/+ mice, ii) Glut1+/+Leprdb/db mice exhibited a significant reduction in c-wave amplitude compared with both nondiabetic controls, and iii) c-wave amplitudes of Glut1+/−Leprdb/db mice were fully normalized (Figure 3, B and D) [ANOVA by genotype: F(3, 208) = 13.98, P < 0.0001].
Figure 3.
Retinal pigment epithelium defects are ameliorated in diabetic Glut1+/− mice. A and C: Representative waveforms induced by a 5-cd/m2 white stimulus for 10 seconds. Red line indicates duration of light stimulus. B and D: Average amplitude of the c-wave at 8 and 16 weeks of age. Amplitude of the c-wave was determined by subtracting the average baseline amplitude from the maximal response following the b-wave. ∗P ≤ 0.05, ∗∗P ≤ 0.01, and ∗∗∗∗P ≤ 0.0001. Cntl, control.
Cone photoreceptor function can be evaluated by the photopic light-adapted response. Following the c-wave, mice were light adapted for 7 minutes before another strobe flash stimulus (Figure 4). At 8 weeks of age, the light-adapted responses of the Glut1+/+Leprdb/db mice were reduced (Figure 4A), but not to the same extent as the dark-adapted responses, or the c-wave. However, at both 8 and 16 weeks of age (Figure 4C; when a significant reduction in the light-adapted response of Glut1+/+Leprdb/db mice was identified), Glut1+/−Leprdb/db responses were restored (Figure 4, B and D) [ANOVA by genotype: F(3, 227) = 4.570, P = 0.004].
Figure 4.
Photopic electroretinogram (ERG) defects are normalized in diabetic Glut1+/− mice. A and C: Representative light-adapted ERG waveform traces evoked by a 1.4 log cd.s/m2 light stimulus superimposed over the adapting field. B and D: Luminance-response function for the light-adapted responses at 8 (B) and 16 (D) weeks of age. Amplitudes were calculated by summing the peak of the waveform with the amplitude at 8.3 milliseconds. Cntl, control.
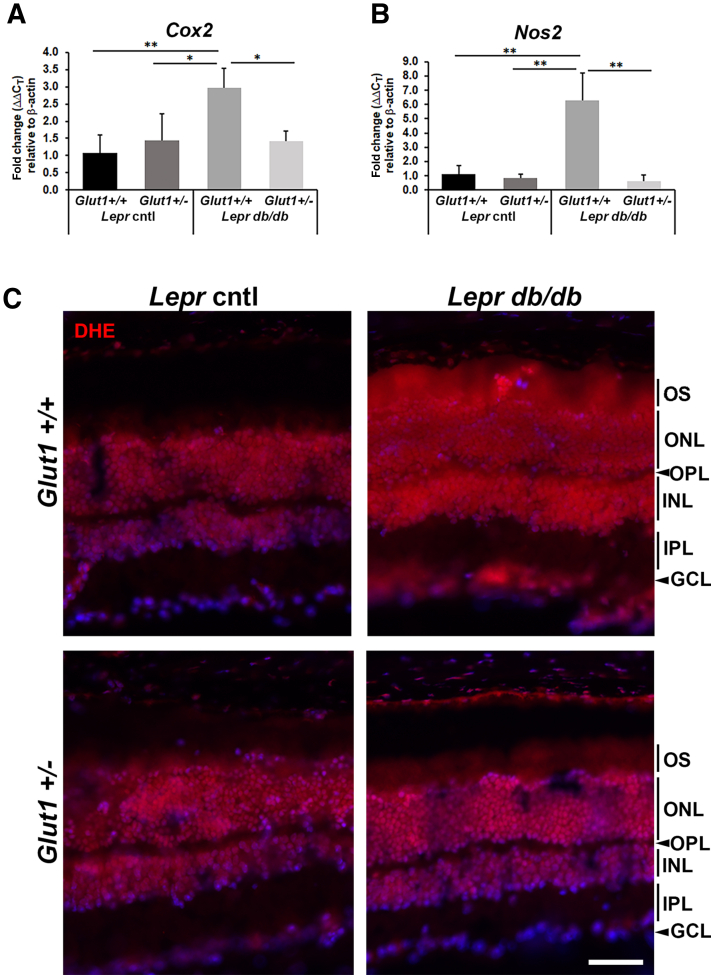
To determine whether systemic reduction of Glut1 also affected presence of oxidative stress and inflammation in the retina of Leprdb/db mice, expression of oxidative stress molecules cyclooxygenase-2 (Cox2) and nitric oxide synthase 2 (Nos2) (Figure 5, A and B) was analyzed. Expression of each gene was significantly elevated in Glut1+/+Leprdb/db retinas compared with nondiabetic controls, and levels were normalized in retinas dissected from Glut1+/−Leprdb/db mice [Cox2, F(3, 8) = 8.723, P = 0.0067;Nos2, F(3, 8) = 17.59, P = 0.0007] (Figure 5, A and B). To further evaluate the presence of superoxide production in the retina, fresh frozen sections incubated with DHE were evaluated. Figure 5C depicts elevated levels of DHE throughout the inner nuclear layer, outer nuclear layer, and outer segments of Glut1+/+Leprdb/db retinas compared with nondiabetic controls and Glut1+/−Leprdb/db mice.
Figure 5.
Oxidative stress is reduced in diabetic Glut1+/− mice. A and B: Quantification of oxidative stress mediators [Cox2 (A) and Nos2 (B)] at 16 weeks of age. Relative fold changes in gene expression were determined using the 2ΔΔCT method. Both genes were normalized to expression of β-actin and compared with expression in Leprdb/+Glut1+/+ mice. C: Confocal microscopy of fresh frozen retinal cryosections from 24-week–old mice stained with dihydroethidium (DHE; red). ∗P ≤ 0.05, ∗∗P ≤ 0.01. Scale bar = 50 μm (C). Cntl, control; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segments.
The expression of classic inflammatory cytokines, IL-1β and tumor necrosis factor (TNF)-α (Figure 6, A and B), and pro-angiogenic factors, HIF-1α and VEGF (Figure 6, C and D) was analyzed next. Expression of each gene was significantly increased in Glut1+/+Leprdb/db retinas compared with nondiabetic controls, and levels were normalized in retinas dissected from Glut1+/−Leprdb/db mice [ IL-1β, F(3, 10) = 56.23, P < 0.0001;TNF-α, F(3, 8) = 11.24, P = 0.0031;HIF-1α, F(3, 8) = 33.60, P < 0.0001;VEGF, F(3, 9) = 11.60, P = 0.0019] (Figure 6, A–D). To assess the activation status of Müller glia in the Glut1+/+Leprdb/db retina, immunohistochemistry was performed on retinal cryosections using an anti–glutamine synthetase antibody (Figure 6E). As expected, in the Glut1+/+Leprdb/db retina, staining was observed from the external limiting membrane to the ganglion cell layer, spanning the length of the Müller glia. However, immunoreactivity was primarily limited to Müller glia end feet (and autofluorescent blood vessels) in all other cohorts. These data demonstrate that reduction of Glut1 prevents both functional defects of the diabetic retina as well as the development of inflammation and oxidative stress.
Figure 6.
Inflammatory cytokines are not elevated in diabetic Glut1+/− mice. A–D: Quantification of inflammatory cytokines [IL-1β (A) and TNF-α (B)] and pro-angiogenic factors [HIF-1α (C) and VEGF (D)] at 16 weeks of age. Relative fold changes in gene expression were determined using the 2ΔΔCT method. All genes were normalized to expression of β-actin and compared with expression in Leprdb/+Glut1+/+ mice. E: Confocal microscopy of retinal cryosections from 16-week–old mice stained with anti–glutamine synthetase (red). ∗P ≤ 0.05, ∗∗P ≤ 0.01, ∗∗∗P ≤ 0.001, and ∗∗∗∗P ≤ 0.0001. Scale bar = 50 μm (E). Cntl, control; GCL, ganglion cell layer; INL, inner nuclear layer, IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer.
Discussion
This study demonstrates, for the first time, that moderate, systemic reduction of Glut1 is effective in preventing retinal pathophysiology in a mouse model of T2D. All previous work to date investigating the therapeutic potential for Glut1 in DR utilized mouse models of type 1 diabetes.7,11, 12, 13 Therefore, the findings presented here are critically important as, of the one-third of diabetic patients who will develop DR, 90% to 95% experience T2D.14,15
Functional retinal defects, neurodegeneration, and inflammation were first identified in the Leprdb/db spontaneous T2D mouse model by Bogdanov et al16 in 2014, establishing early neuronal involvement as a hallmark of DR in T2D. This study also demonstrated that caloric restriction, which reduced hyperglycemia in the mice, led to a normalization of ERG OP latency and amplitude, reduced retinal inflammation, and prevented ganglion cell death.16 Recently, in vivo ERGs from 3- and 6-month–old Leprdb/db mice demonstrated reductions in a- and b-wave amplitudes only at 6 months.17 When compared with ex vivo ERGs performed on retinas dissected at the same age, normal light-evoked responses were recorded from retinas cultured in a normoglycemic environment. These findings revealed that extrinsic factors induced the functional defects in Leprdb/db diabetic mice, indicating that the defects are reversible.
Indeed, the current findings demonstrated that reducing Glut1 normalized ERG defects seen at 8 and 16 weeks in Leprdb/db mice. However, the mice remained severely obese despite the reduction in Glut1 and were difficult to maintain through 24 weeks. Of the mice that did survive, electroretinograms were similarly restored to normal amplitudes (data not shown). These findings suggest that obesity (and presumably hyperlipidemia) does not overtly contribute to retinal dysfunction associated with DR and that glucose transport into the retina or retinal glucose metabolism can be utilized as a primary target for intervention.
It has been suggested that oxidative stress both contributes to and is a consequence of metabolic dysfunction associated with DR.18 Evidence of oxidative stress in Leprdb/db mice has been observed as early as 8 weeks of age,16 and glial activation was increased in diabetic mice at all ages/time points examined. Herein, elevated levels of inflammatory cytokines, oxidative stress molecules, and pro-angiogenic factors were found at 16 weeks of age. Moreover, glutamine synthetase elevation in Müller glia demonstrated a neuroprotective effect by these cells. Bogdanov et al16 found elevated levels of Slc38a1, the glutamine transporter. These increases in both glutamine synthetase and the glutamine transporter demonstrate that the diabetic retina is attempting to combat elevations in glutamate to prevent neural toxicity and cell death. More importantly, in addition to the prevention of ERG defects, Glut1+/−Leprdb/db mice displayed low levels of glutamine synthetase, superoxide production (DHE), as well as control expression levels of IL-1β, TNF-α, VEGF, HIF-1α, Cox2, and Nos2 (Figure 5), indicating that both functional defects as well as molecular signatures of diabetic retinas (reactive oxygen species and inflammation) are normalized by reducing Glut1.
An increase in Glut1 has been demonstrated in the streptozotocin-induced diabetic retina.7 Here, the study shows a similar trend for increased Glut1 throughout the Glut1+/+Leprdb/db retina (Figure 1, C and D), which was not observed in the Glut1+/−Leprdb/db mice. These findings indicate that a glucose-induced increase in Glut1 may drive the elevation. When glucose transport to the retina is limited by the systemic reduction in Glut1 caused by loss of one Slc2a1 allele, Glut1 is not increased. This follows the premise of glucose-induced glucose uptake more commonly observed occurring via Glut4 in muscle, fat, and heart, where glucose is insulin-regulated.19 Future investigations will determine whether Glut1 levels are altered by insulin signaling in the retina and whether Glut1 activity, rather than expression, can also be modulated to prevent DR.
Notably, the use of a mouse strain that expresses only one Glut1 allele to induce reduction of Glut1 provides a means to extrapolate our findings to hypothesize how a moderate, systemic inhibition of Glut1 achieved via pharmacology could be utilized for prevention of DR. Cancer therapies already utilize Glut1-specific and pan-Glut inhibitors to combat the increased proliferation of neoplastic cells.20,21 Cancer cells utilize the same metabolic paradigm as the retina, relying primarily on glycolysis (also known as the Warburg effect), and a promising new field of chemotherapy for cancer is the inhibition of Glut-mediated transport of glucose to starve tumors.
Interestingly, many therapeutics identified to reduce or prevent characteristics of DR are known Glut inhibitors also used in anti-neoplastic cancer studies.20 Among these include curcumin,22,23 genistein,24, 25, 26 quercetin,27, 28, 29 and resveratrol.30, 31, 32, 33 Some inhibit Glut1 at the gene/protein level (genistein and resveratrol), whereas others also noncompetitively (resveratrol) or competitively inhibit Glut1 (quercetin).20 Many of these compounds are also polyphenols and may exert their actions through multiple mechanisms, including targeting of cell signaling, hormonal regulation, and anti-oxidation. The lipid/cholesterol drug, fenofibrate, which has also emerged as a promising treatment for DR among patients with T2D,34 similarly reduces Glut1 levels in cancer cells.35 Much like the current findings of normalized retinal pathophysiology by reducing Glut1, it has been shown to be effective for DR despite sustained hyperlipidemia.
Whether by use of a naturally occurring compound, or synthetically generated small-molecule inhibitor, moderated targeting of glucose transport is essential as the reduction of glucose levels in the brain beyond normoglycemia is detrimental. Systemically, loss of a single Glut1 allele in humans leads to Glut1 deficiency syndrome, characterized by seizures, reduced locomotion, abnormal brain microvasculature, micrencephaly, neuroinflammation, developmental delay, and speech/language impairments. This sequelae of symptoms arises from insufficient glucose in the brain for neuronal metabolism.36,37 However, in a mouse model of Glut1 deficiency syndrome, when Glut1 reduction is induced in adult mice rather than at birth, the pathologic phenotypes of Glut1 deficiency syndrome are prevented.38 Therefore, as reduction of Glut1 in diabetic patients would be initiated after development is complete, targeting of Glut1 is a viable treatment option.
With specific regard to retinal function, although sustained hypoglycemia within the retina leads to age-related vision loss, characterized by reduced visual acuity, ERG amplitudes, and death of inner retinal neurons,39 acute hypoglycemia in individuals with or without diabetes induced reversible reductions in ERG amplitudes, appearance of scotomas, and reduced contrast sensitivity. All perturbations in vision and retinal function were stabilized on euglycemia.40 The use of precision pharmacology and nanoconcentrations of Glut1 inhibitors for the treatment of DR can ensure glucose concentrations are titrated to normal endogenous levels. Alternatively, topical ocular treatment with Glut1 inhibitors may be considered. Future studies will utilize data from anti-cancer drug trials to design a treatment paradigm using Glut1 inhibitors for the determination of whether systemic, pharmacologic inhibition of Glut1 can also be harnessed to prevent or treat DR. Looking toward whole-body treatment of Glut1 inhibition for T2D, which may also be relevant for the other microvascular complications of diabetes (neuropathy and nephropathy) as well as the macrovascular complications associated with diabetes (eg, coronary heart disease and cerebrovascular disease), it will be important in translation to clinical research to i) begin Glut1 inhibition early, ii) concomitantly treat the mice for comorbidities, such as obesity, and iii) determine how a multipharmacologic approach to treating T2D affects Slc2a1 expression, Glut1 levels, and Glut1 activity in the retina (and other tissues).
In sum, the current findings demonstrate that a moderate, systemic reduction of Glut1 inhibits both functional retinal defects observed by electroretinography and the molecular signatures of retinal oxidative stress and inflammation characteristic of the Leprdb/db mouse model of type 2 diabetes. These data are significant as 95% of patients with diabetes experience type 2 diabetes, and preclinical investigations for therapies must address this group.
Acknowledgments
We thank Dr. Neal Peachey, Dr. Lin Mei, and Dr. Wen-Cheng Xiong for supporting these experiments.
Footnotes
Supported by the US Department of Veterans Affairs Biomedical Laboratory Research and Development Service VA Merit Award number BX005844-01 (I.S.S.), NIHP30 EY025585, Research to Prevent Blindness Challenge grant, Cleveland Eye Bank Foundation grant, and Cleveland Clinic Foundation funds.
Disclosures: None declared. All co-authors have seen and agree with the contents of the manuscript, and there is no financial interest to report.
Portions of this work were presented as an abstract at the 2019 Association for Research in Vision and Ophthalmology Annual Meeting, April 28 to May 2, 2019, Vancouver, BC, Canada.
References
- 1.Eid S.A., O'Brien P.D., Hinder L.M., Hayes J.M., Mendelson F.E., Zhang H., Zeng L., Kretzler K., Narayanan S., Abcouwer S.F., Brosius III F.C., 3rd, Pennathur S., Savelieff M.G., Feldman E.L. Differential effects of empagliflozin on microvascular complications in murine models of type 1 and type 2 diabetes. Biology. 2020;9:347. doi: 10.3390/biology9110347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Mouhayyar C., Riachy R., Khalil A.B., Eid A., Azar S. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. 2020;2020 doi: 10.1155/2020/1762164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gong Q., Zhang R., Wei F., Fang J., Zhang J., Sun J., Sun Q., Wang H. SGLT2 inhibitor-empagliflozin treatment ameliorates diabetic retinopathy manifestations and exerts protective effects associated with augmenting branched chain amino acids catabolism and transportation in db/db mice. Biomed Pharmacother. 2022;152 doi: 10.1016/j.biopha.2022.113222. [DOI] [PubMed] [Google Scholar]
- 4.Mima A. Renal protection by sodium-glucose cotransporter 2 inhibitors and its underlying mechanisms in diabetic kidney disease. J Diabetes Complications. 2018;32:720–725. doi: 10.1016/j.jdiacomp.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Sha W., Wen S., Chen L., Xu B., Lei T., Zhou L. The role of SGLT2 inhibitor on the treatment of diabetic retinopathy. J Diabetes Res. 2020;2020 doi: 10.1155/2020/8867875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Zhang X., Liao N., Ji Y., Mi L., Gan Y., Su Y., Wen F. Decreased expression of glucagon-like peptide-1 receptor and sodium-glucose co-transporter 2 in patients with proliferative diabetic retinopathy. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holoman N.C., Aiello J.J., Trobenter T.D., Tarchick M.J., Kozlowski M.R., Makowski E.R., De Vivo D.C., Singh C., Sears J.E., Samuels I.S. Reduction of Glut1 in the neural retina but not the RPE alleviates polyol accumulation and normalizes early characteristics of diabetic retinopathy. J Neurosci. 2021;41:3275–3299. doi: 10.1523/JNEUROSCI.2010-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hummel K.P., Dickie M.M., Coleman D.L. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 9.Gospe S.M., 3rd, Baker S.A., Arshavsky V.Y. Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci. 2010;123:3639–3644. doi: 10.1242/jcs.072389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samuels I.S., Bell B.A., Pereira A., Saxon J., Peachey N.S. Early retinal pigment epithelium dysfunction is concomitant with hyperglycemia in mouse models of type 1 and type 2 diabetes. J Neurophysiol. 2015;113:1085–1099. doi: 10.1152/jn.00761.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L., Seidel C.P., Iwase T., Stevens R.K., Gong Y.Y., Wang X., Hackett S.F., Campochiaro P.A. Suppression of GLUT1; a new strategy to prevent diabetic complications. J Cell Physiol. 2013;228:251–257. doi: 10.1002/jcp.24133. [DOI] [PubMed] [Google Scholar]
- 12.You Z.P., Xiong B., Zhang Y.L., Shi L., Shi K. Forskolin attenuates retinal inflammation in diabetic mice. Mol Med Rep. 2018;17:2321–2326. doi: 10.3892/mmr.2017.8106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You Z.P., Zhang Y.L., Shi K., Shi L., Zhang Y.Z., Zhou Y., Wang C.Y. Suppression of diabetic retinopathy with GLUT1 siRNA. Sci Rep. 2017;7:7437. doi: 10.1038/s41598-017-07942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee R., Wong T.Y., Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng Y., He M., Congdon N. The worldwide epidemic of diabetic retinopathy. Indian J Ophthalmol. 2012;60:428–431. doi: 10.4103/0301-4738.100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogdanov P., Corraliza L., Villena J.A., Carvalho A.R., Garcia-Arumi J., Ramos D., Ruberte J., Simo R., Hernandez C. The db/db mouse: a useful model for the study of diabetic retinal neurodegeneration. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becker S., Carroll L.S., Vinberg F. Rod phototransduction and light signal transmission during type 2 diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang Q., Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L., Chiang S.H., Saltiel A.R. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10:65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Temre M.K., Kumar A., Singh S.M. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: promising potential of new pan-GLUT inhibitors. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1035510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zambrano A., Molt M., Uribe E., Salas M. Glut 1 in cancer cells and the inhibitory action of resveratrol as a potential therapeutic strategy. Int J Mol Sci. 2019;20:3374. doi: 10.3390/ijms20133374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldebasi Y.H., Aly S.M., Rahmani A.H. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5:194–202. [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J., Miao X., Yang F.J., Cao J.F., Liu X., Fu J.L., Su G.F. Therapeutic potential of curcumin in diabetic retinopathy (review) Int J Mol Med. 2021;47:75. doi: 10.3892/ijmm.2021.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elgayar S.A., Eltony S.A., Sayed A.A., Abdel-Rouf M.M. Genistein treatment confers protection against gliopathy and vasculopathy of the diabetic retina in rats. Ultrastruct Pathol. 2015;39:385–394. doi: 10.3109/01913123.2015.1045664. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim A.S., El-Shishtawy M.M., Pena A., Jr., Liou G.I. Genistein attenuates retinal inflammation associated with diabetes by targeting of microglial activation. Mol Vis. 2010;16:2033–2042. [PMC free article] [PubMed] [Google Scholar]
- 26.Yousefi H., Komaki A., Shahidi S., Habibi P., Sadeghian R., Ahmadiasl N., Daghigh F. Diabetic neovascularization defects in the retina are improved by genistein supplementation in the ovariectomized rat. Inflammopharmacology. 2021;29:1579–1586. doi: 10.1007/s10787-021-00852-5. [DOI] [PubMed] [Google Scholar]
- 27.Chai G.R., Liu S., Yang H.W., Chen X.L. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen Res. 2021;16:1344–1350. doi: 10.4103/1673-5374.301027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar B., Gupta S.K., Nag T.C., Srivastava S., Saxena R., Jha K.A., Srinivasan B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp Eye Res. 2014;125:193–202. doi: 10.1016/j.exer.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang S., Du S., Wang W., Zhang F. Therapeutic investigation of quercetin nanomedicine in a zebrafish model of diabetic retinopathy. Biomed Pharmacother. 2020;130 doi: 10.1016/j.biopha.2020.110573. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad I., Hoda M. Attenuation of diabetic retinopathy and neuropathy by resveratrol: review on its molecular mechanisms of action. Life Sci. 2020;245 doi: 10.1016/j.lfs.2020.117350. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Meng J., Li H., Wei H., Bi F., Liu S., Tang K., Guo H., Liu W. Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp Eye Res. 2019;181:356–366. doi: 10.1016/j.exer.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Soufi F.G., Mohammad-Nejad D., Ahmadieh H. Resveratrol improves diabetic retinopathy possibly through oxidative stress - nuclear factor kappaB - apoptosis pathway. Pharmacol Rep. 2012;64:1505–1514. doi: 10.1016/s1734-1140(12)70948-9. [DOI] [PubMed] [Google Scholar]
- 33.Toro M.D., Nowomiejska K., Avitabile T., Rejdak R., Tripodi S., Porta A., Reibaldi M., Figus M., Posarelli C., Fiedorowicz M. Effect of resveratrol on in vitro and in vivo models of diabetic retinophathy: a systematic review. Int J Mol Sci. 2019;20:3503. doi: 10.3390/ijms20143503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keech A.C., Mitchell P., Summanen P.A., O'Day J., Davis T.M., Moffitt M.S., Taskinen M.R., Simes R.J., Tse D., Williamson E., Merrifield A., Laatikainen L.T., d'Emden M.C., Crimet D.C., O'Connell R.L., Colman P.G., FIELD Study Investigators Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 35.Chen L., Peng J., Wang Y., Jiang H., Wang W., Dai J., Tang M., Wei Y., Kuang H., Xu G., Xu H., Zhou F. Fenofibrate-induced mitochondrial dysfunction and metabolic reprogramming reversal: the anti-tumor effects in gastric carcinoma cells mediated by the PPAR pathway. Am J Transl Res. 2020;12:428–446. [PMC free article] [PubMed] [Google Scholar]
- 36.Klepper J., Akman C., Armeno M., Auvin S., Cervenka M., Cross H.J., De Giorgis V., Della Marina A., Engelstad K., Heussinger N., Kossoff E.H., Leen W.G., Leiendecker B., Monani U.R., Oguni H., Neal E., Pascual J.M., Pearson T.S., Pons R., Scheffer I.E., Veggiotti P., Willemsen M., Zuberi S.M., De Vivo D.C. Glut1 deficiency syndrome (Glut1DS): state of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open. 2020;5:354–365. doi: 10.1002/epi4.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang M., Monani U.R. Glut1 deficiency syndrome: new and emerging insights into a prototypical brain energy failure disorder. Neurosci Insights. 2021;16 doi: 10.1177/26331055211011507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang M., Park S.H., Petri S., Yu H., Rueda C.B., Abel E.D., Kim C.Y., Hillman E.M., Li F., Lee Y., Ding L., Jagadish S., Frankel W.N., De Vivo D.C., Monani U.R. An early endothelial cell-specific requirement for Glut1 is revealed in Glut1 deficiency syndrome model mice. JCI Insight. 2021;6 doi: 10.1172/jci.insight.145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umino Y., Everhart D., Solessio E., Cusato K., Pan J.C., Nguyen T.H., Brown E.T., Hafler R., Frio B.A., Knox B.E., Engbretson G.A., Haeri M., Cui L., Glenn A.S., Charron M.J., Barlow R.B. Hypoglycemia leads to age-related loss of vision. Proc Natl Acad Sci U S A. 2006;103:19541–19545. doi: 10.1073/pnas.0604478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan M.I., Barlow R.B., Weinstock R.S. Acute hypoglycemia decreases central retinal function in the human eye. Vis Res. 2011;51:1623–1626. doi: 10.1016/j.visres.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]