Abstract
Infections with mosquito-borne flaviviruses, such as Dengue virus, ZIKV virus, and West Nile virus, pose significant threats to public health. Flaviviruses cause up to 400 million infections each year, leading to many forms of diseases, including fatal hemorrhagic, encephalitis, congenital abnormalities, and deaths. Currently, there are no clinically approved antiviral drugs for the treatment of flavivirus infections. The non-structural protein NS4B is an emerging target for drug discovery due to its multiple roles in the flaviviral life cycle. In this review, we summarize the latest knowledge on the structure and function of flavivirus NS4B, as well as the progress on antiviral compounds that target NS4B.
Keywords: Flavivirus, nonstructural protein 4B, antivirals, drug discovery
1. Introduction
Flavivirus is one of the most severe human pathogens that have caused numerous emerging epidemics or pandemics in history. The family Flavivirudae comprises over 70 members, including Dengue virus (DENV), ZIKA virus (ZIKV), yellow fever virus (YFV), West Nile virus (WNV), Japanese encephalitis virus (JEV), and tick-borne encephalitis virus (TBEV) (Kuno et al., 1998). With four distinct serotypes, DENV alone cause 100 million people sick each year, with symptoms ranging from flu-like Dengue fever (DF) to severe, life-threatening dengue shock syndrome, or dengue hemorrhagic fever (Bhatt et al., 2013). The antibody-dependent enhancement (ADE) of DENV is caused by antibodies against viral envelope and precursor membrane proteins. These antibodies increase viral entry without inducing substantial virus neutralization. The ADE complication makes the development of DENV vaccine challenging. Thus, there is an urgent need for developing pan-serotype inhibitors against all four serotypes of DENV (Aguiar et al., 2016; Boldescu et al., 2017; Whitehead et al., 2007). WNV causes the most cases of mosquito-borne viral disease in the U.S.A.; and in severe cases, encephalitis or meningitis may occur with a risk of about 10% mortality rate (Hayes and Gubler, 2006; McDonald et al., 2021). The recent outbreak of ZIKV in the Americas and its unexpected association with severe neurological disease (i.e., microcephaly in babies born to infected pregnant mothers and Guillain-Barrie Syndrome in infected adults) has raised serious threats to public health (Mlakar et al., 2016). Only a few vaccines have been approved for clinical use, including the live-attenuated vaccine against YFV (YF17D), inactivated vaccines against JEV (IXIARO) and TBEV, (Collins and Metz, 2017; Ishikawa et al., 2014), and tetravalent DENV vaccine (Durbin et al., 2011). However, the use of the tetravalent DENV vaccine is limited because of its risk of enhanced dengue disease in naïve children.
The flavivirus genome is a single-stranded, positive-sense RNA of approximately 11,000 nucleotides in length. The viral genomic RNA contains a single open-reading-frame (ORF) flanked by the 5’ and 3’ untranslated regions (UTRs) (Chambers et al., 1990). The ORF encodes a long polyprotein that is processed into three structural proteins (capsid [C], precursor membrane [prM], and envelope [E]) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The structural proteins participate in viral attachment, entry, and fusion, virus assembly, and virion secretion (Mukhopadhyay et al., 2005). The nonstructural proteins are important for viral RNA replication (e.g., formation of the membranous replication complex [RC]), virion assembly and release, and evasion of innate immunity (Klema et al., 2015; van den Elsen et al., 2021; Xie et al., 2019b). NS1 is a glycoprotein and physically associated with the RC to regulate viral replication (Muller and Young, 2013). NS2A and NS2B are membrane proteins involving in viral replication, virion assembly, and inducing cell death (Leung et al., 2008; Xie et al., 2013; Xie et al., 2019a; Zhang et al., 2019). NS2B is an essential cofactor for NS3 protease. NS3 is a multifunctional protein with an N-terminal serine protease domain and a C-terminal RNA triphosphatase and helicase domain (Falgout et al., 1991). Both NS4A and NS4B are membrane proteins. NS4A functions in modeling cellular membrane, antagonizing host interferon response, inducing autophagy, and enabling viral replication (Klaitong and Smith, 2021). NS4B plays an important role in viral replication and host immunomodulation (Gopala Reddy et al., 2018; Xie et al., 2015). NS5 is the largest nonstructural protein, which possesses an N-terminal methyltransferase and a C-terminal RNA-dependent RNA polymerase (Egloff et al., 2002; Ray et al., 2006; Zhou et al., 2007).
Many flavivirus proteins have been investigated extensively for their structures, functions, and antiviral discovery. This is particularly true for cytosolic viral proteins, such as capsid and the two enzymatic proteins NS3 and NS5 (Bollati et al., 2010; Lim et al., 2015; Luo et al., 2015; Sampath and Padmanabhan, 2009; Troost and Smit, 2020; Xia et al., 2020). However, due to their high hydrophobic nature, the four membrane non-structural proteins (NS2A, NS2B, NS4A, and NS4B) remain poorly characterized. Recent studies indicate that NS4B is a promising target for drug discovery owing to its multifunctional properties in viral life cycle and host immunomodulation. Indeed, a number of antiviral compounds that target NS4B have been reported (Gao et al., 2022; Guo et al., 2016; Kaptein et al., 2021; Moquin et al., 2021; Xie et al., 2015). In this review, we will describe the current understanding of the structure and function of NS4B, as well as the current status and challenges of antiviral discovery against flavivirus NS4B, especially the compounds that have been developed in the past decade.
2. Biochemical and structural study of flavivirus NS4B protein
2.1. Membrane topology of flavivirus NS4B protein
In flavivirus-infected cells, NS4B is first cleaved from its precursor NS4A-2K peptide-NS4B by viral protease NS2B/NS3 on the cytosol side of the endoplasmic reticulum (ER) membrane. The resulting polypeptide 2K-NS4B (molecular weight [MW] of approximately 30 kDa) was further cleaved by host signalase (located in the ER lumen) into mature NS4B with a MW of approximately 27 kDa (Lin et al., 1993; Miller et al., 2007). NS4B from different flaviviruses share sequence homology. The sequence similarity of NS4B from the four serotype DENV is above 85%, and the sequence similarity of NS4B among different flaviviruses is above 54%. For example, DENV-2 and ZIKV NS4B proteins share 69% sequence similarity, and WNV and JEV NS4B proteins share 80% sequence similarity (Figure 1A). NS4B is the largest nonstructural membrane protein of flavivirus with 40% hydrophobic residues. In 2006, Miller and coworkers proposed a membrane topology model of DENV-2 NS4B on the ER membrane by using biochemical approaches (Miller et al., 2006).
Figure 1.
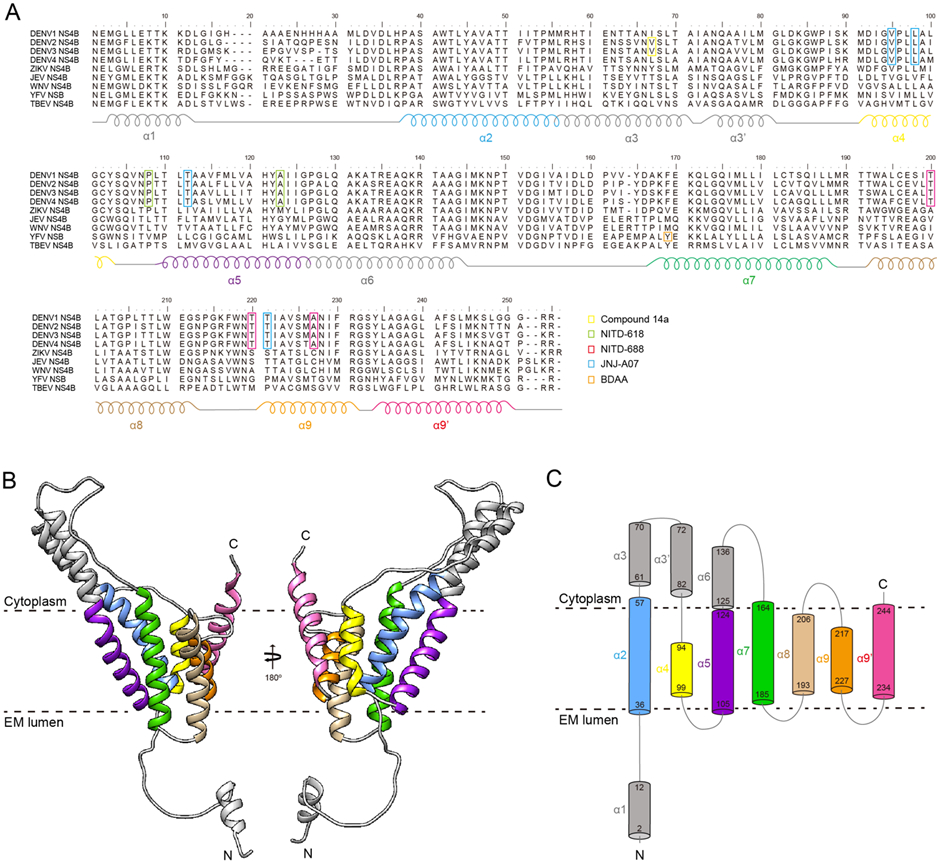
Sequence alignment, membrane topology, and structure model of flavivirus NS4B protein. (A) Sequence alignment of NS4B proteins from DENV-1 to −4, ZIKV, JEV, WNV, YFV, and TBEV. The helices α1 to α9 are labeled according to the NMR results. The resistance mutations against different NS4B inhibitors (i.e., Compound 14, NITD-618, NITD-688, JNJ-A07, and BDAA) are labeled with different colors. See details in the main text. (B) AlphaFold2 model of DENV-2 NS4B. The color is labeled as that in (A). (C) Membrane topology of DENV-2 NS4B based on the NMR probed full-length NS4B structure and AlphaFold2 model. The color is labeled as that in (A) and (B).
In 2015, Li and coworkers purified a truncated NS4B containing the first 125 amino acids (NS4B1-125) and determined the membrane topology using solution nuclear magnetic resonance (NMR) and paramagnetic relaxation enhancement (PRE) (Li et al., 2015b). As shown in Figure 1, NS4B1-125 was shown to contain five helices, including one soluble helix (α1: 2-16) and four transmembrane helices (α2: 36-57, α3: 61-82, α4: 94-99, and α5: 105-124). The α1 is highly flexible, making it easily recognizable by the host signalase for the cleavage between the 2K peptide and NS4B. Although α2, α3, α4, and α5 showed similar motions under LMNG micelle, helix wheel presentation indicated that the α3 is an amphipathic helix that contains a smaller hydrophilic interface. Later on, Li and coworkers further performed the backbone resonance assignments of the full-length NS4B from DENV-3 in LMPG micelles (Li et al., 2016b). The topology of the first 125 residues in full-length NS4B is similar to those in NS4B1-125. Additional 4 helices were further identified, including α6 (125-136), α7 (164-185), α8 (193-206), and α9 (217-244) (Figure 1). Among those helices, α6 is determined to be a soluble and flexible loop that belongs to the long cytoplasmic loop (125-162). Based on the PRE and Hydrogen-deuterium exchange mass spectrometry (HDX-MS) results, α7, α8, and α9 might be transmembrane helices. Both α8 and α9 comprise a kinked transmembrane helix that separated by P201 and G228/S229, respectively. It should be noted that α8 and α9 do not behave similarly to other transmembrane helices, indicating that they might be flexible and undergo conformational changes to perform different functions, such as interacting with different proteins during distinct steps of viral infection cycle.
2.2. N-Glycosylation of NS4B protein
Although several structural and nonstructural proteins have been demonstrated to be modified after translation (Roby et al., 2015), the post-translational modification of flavivirus NS4B is not well established. Zou and coworkers showed that treatment with deglycosylation enzymes did not affect DENV-2 NS4B in both human and mosquito cells (Zou et al., 2014). However, using a different NS4B-antibody, Naik et al. reported two posttranslational glycosylations of NS4B at amino acids N58 and N62 (Naik et al., 2015). The N-glycosylation is presented in both virus-infected cells and recombinant NS4B protein (expressed in the absence of viral replication). Functionally, single mutation of N58Q or N62Q had distinct effects, and double mutation of N58/62Q exhibited a more significant reduction in viral production without affecting virion secretion or infection in mammalian and mosquito cells, demonstrated by reduced viral titer and viral RNA synthesis (Naik et al., 2015). Later on, Hafirassou and coworkers showed that the N-glycosylation of NS1 and NS4B is medicated by oligosaccharyltransferase (OST) complex (Hafirassou et al., 2017). Pull-down assay indicated that OST complex directly interacts with DENV NS4B. In the OST complex-silenced cells, lower bands corresponding to non-glycosylated NS4B proteins were clearly detected (Hafirassou et al., 2017). Sequence analysis showed that N58 is only conserved in DENV NS4B, while N62 is conserved among WNV, YFV, and ZIKV NS4B. Thus, residue N62 may have more evolutionary significance. The N-glycosylation results derived from DENV NS4B remain to be verified in other flaviviruses.
2.3. Dimerization of NS4B protein
DENV and WNV NS4B protein dimerizes in cells when expressed alone, as well as in virus-infected cells, and the purified recombinant NS4B also forms homodimers (Zou et al., 2014). Zou et al. also found that the cytosolic loop (residues 125-163) and its downstream C-terminal fragment (residue 164 to the C-terminus; Figure 1C) are critical for NS4B dimerization. Recently, the formation of NS4B dimers was also observed in ZIKV (Varasteh Moradi et al., 2020). Furthermore, the DENV NS4B precursor, NS4A-2K-NS4B, was found to form dimers in virus-infected cells (Płaszczyca et al., 2019). Notably, overexpression of WNV NS4B was reported to lead to the formation of ER-derived replication-competent membrane structure (Kaufusi et al., 2014), suggesting that the NS4B dimerization may be required for the assembly of viral replication complex.
2.4. Conformational dynamics of NS4B protein
Currently, there is no experimentally determined tertiary structure for flavivirus NS4B. Bhardwaj et al. investigated the structural dynamics of a 3D structure model of ZIKV NS4B by using molecular dynamics (MD) simulations and other experimental approaches (Bhardwaj et al., 2021). The structural model of ZIKV NS4B that was constructed using AlphaFold2 is consistent with the experimental probed secondary structure, which contains nine α helices and no beta strand (Li et al., 2016b). The α3 in the predicted model is soluble, which agrees with the NMR structure that α3 is an amphipathic helix containing a smaller hydrophilic interface (Li et al., 2015b). We also calculated the atomic models of NS4B of DENV-2 and other flaviviruses using AlphaFold2 (Mirdita et al., 2021). All the NS4B models showed similar structural features (Figure 1B). Thus, according to the NMR secondary structure and AlphaFold2 calculated model, we proposed a membrane topology of DENV-2 NS4B (Figure 1C).
NS4B significantly changes its structure throughout the simulation period in the presence of a lipid bilayer (Bhardwaj et al., 2021), indicating that the protein is highly dynamic. The dynamics of the N-terminal region (1-38), cytosolic loop (131-169), and C-terminal region (194-251) were further studied with a reductionist approach. The N-terminal region of ZIKV NS4B has a tendency to form a disordered structure, while the C-terminal region remains intact in the presence of lipid bilayer but forms disordered conformation in the absence of lipid bilayer. The cytosolic loop consists of one α helix (α6) and a disordered region (Figure 1C), which was validated with a circular dichroism spectrum. However, in the presence of trimethylamine N-oxide, the disordered region gains a partial helical conformation, indicating that the cytosolic loop of NS4B might have the propensity to gain structure (Bhardwaj et al., 2021). In addition, given its sequence diversity, the structural dynamics of NS4B may vary among flavivirus.
3. Functional study of NS4B protein
3.1. NS4B-mediated immune evasion, cell dysregulation, and membrane remodeling
3.1.1. NS4B-mediated antagonism of innate immunity
In addition to viral protein-protein interactions, NS4B also interacts with diverse host proteins to evade host immune response and remodel host membrane structure. After cleavage from the precursor, amino acids 32 to 83 of DENV NS4B, including α2 and α3, were found to antagonize type-I interferon (IFN) and inflammatory cytokine signaling by blocking STAT1 phosphorylation (Munoz-Jordán et al., 2005; Muñoz-Jordán et al., 2003). Similar to DENV NS4B, WNV, JEV, YFV, and ZIKV NS4B were also shown to inhibit IFN signaling (Fanunza et al., 2021; Liu et al., 2005; Vidotto et al., 2017). The analysis of the ZIKV-human interactome also revealed that NS4B interacts with JAKI and STAT1, supporting that NS4B directly affects their phosphorylation (Esteves et al., 2017). Apart from antagonizing IFN, DENV NS4B was found to suppress RNA interference (RNAi) through its middle and C-terminal region (Kakumani et al., 2013). Besides IFN and RNAi, other cellular responses, such as stress-associated ER protein 1 (SEPR1), can also inhibit DENV replication; the SEPR1-mediated antiviral effect could be alleviated by flavivirus NS4B (Tian et al., 2019).
Considering the importance of NS4B in counteracting host innate immune response, mutations were rationally engineered in the flavivirus NS4B gene to develop live-attenuated vaccines. The NS4B-P38G mutant WNV significantly reduced neuroinvasiveness, but induced strong innate cytokine and memory T-cell responses (Welte et al., 2011; Wicker et al., 2012). The NS4B-C100S mutant ZIKV was also attenuated through inducing potent antiviral innate and adaptive immune responses in mice. Immunization of mice with above NS4B attenuated viruses protected against wild-type ZIKV infection (Li et al., 2019).
3.1.2. NS4B-mediated cell dysregulation
Liang and coworkers reported that the cooperation of ZIKV NS4A and NS4B suppresses the Akt-mTOR pathway and leads to cellular dysregulation (Liang et al., 2016). While DENV NS4A alone is capable of inducing autophagy (McLean et al., 2011), an individual expression of ZIKV NS4A or NS4B was not sufficient to induce autophagy, indicating that ZIKV NS4A and NS4B utilize different molecular mechanism from DENV to induce autophagy. It was proposed that ZIKV NS4A and NS4B suppress the activation of Akt by inhibiting upstream PI3K signaling and modulating Akt post-translational modification, consequently leading to the impairment of proliferation and differentiation of fetal neural stem cells in vitro. The detailed mechanism of the specific means by which ZIKV NS4A and NS4B influence Akt activation are still unknown. Interestingly, a single mutation in DENV-1 NS4B (V116A or V116M) could significantly increase viral replication in human cells, but reduce viral replication in mosquito cells. The effect of the V116A or V116M on DENV-1 replication in human cells is associated with IFN-dependent response, suggesting that NS4B is important in viral replication and host adaption (Bui et al., 2018). ZIKV NS4B was found to induce the conformational activation of the proapoptotic protein Bax, leading to cell apoptosis, which is different from apoptosis induced by DENV, JEV, and WNV infection (Han et al., 2021). WNV NS4B, together with other nonstructural proteins, was found to suppress the early stage of membrane remodeling and viral RNA synthesis and therefore evade or suppress the formation of stress granules (Courtney et al., 2012; Zmurko et al., 2015). Recently, DENV and JEV NS4B proteins were reported to directly interact with host protein NPL4, promoting the proper translocation of NS4B, which could further recruit valosin-containing protein to the virus replication site; these interactions could inhibit cellular stress responses such as the formation of stress granules, and consequently facilitate viral protein synthesis (Arakawa et al., 2022).
3.1.3. ER membrane remodeling by NS4B/host protein interaction
By using genome-wide CRISPR knockout screens, the ER membrane protein complex (EMC) has been identified to be a host factor that is crucial for flaviviruses infection, including DENV, ZIKV, and YFV (Lin et al., 2017; Marceau et al., 2016; Savidis et al., 2016; Zhang et al., 2016). Lin and coworkers confirmed that the EMC is also important for efficient DENV and ZIKV replication through promoting the biogenesis of NS4A and NS4B proteins (Lin et al., 2019). When transfected to wild-type and EMC-knockout cells, only NS4A and NS4B, but not other viral transmembrane proteins, showed significantly reduced expression. The co-immunoprecipitation (co-IP) experiments revealed that DENV NS4B efficiently pulled down endogenous EMC4, while ZIKV NS4B co-precipitated endogenous EMC1 and EMC2. Mapping analysis indicated that the NS4B α2 and α3 region confers EMC dependency; substitutions of all polar and charged residues in this region of NS4B to leucine could rescue the expression of recombinant NS4B in the EMC-depleted cells (Lin et al., 2019). Ngo and coworkers further demonstrated that EMC is not only crucial for the accurate folding, but also for the post-translational stability of the NS4B protein (Ngo et al., 2019). The fluorescent-based stability assay indicates that NS4A/NS4B assumes an aberrant topology at the ER membrane in the EMC-deficient cells.
TMEM41B is another host factor that was identified as a host factor important for DENV replication. It was identified by a genome-wide CRISPR KO screen (Marceau et al., 2016). Later on, Scaturro et al. demonstrated that TMEM41B is also a ZIKV NS4B interacting partner and such TMEM41B-NS4B interaction is important for viral replication (Scaturro et al., 2018). Recently, Hoffmann and coworkers further determined that TMEM41B is a pan-flavivirus host factor that is required for viral infection, including both mosquito-borne flaviviruses and tick-borne flaviviruses (Hoffmann et al., 2021). TMEM41B is also a membrane protein that is involved in autophagy; interestingly, not the full autophagy pathway is required for flavivirus replication, suggesting that flaviviruses might hijack TMEM41B to assist the remodeling of host cell membranes (Hoffmann et al., 2021). Using tagRFP-TMEM41B, Moretti et al. observed that TMEM41B localizes to ER membranes, which is consistent with the previous imaging and proteomics results (Moretti et al., 2018; Morita et al., 2019). However, TMEM41B re-localizes from a diffuse reticular-like pattern to a large cytosolic aggregate that co-localizes with ZIKV NS4A and YFV NS4B, where replication complexes form. Based on these results, Hoffmann et al. proposed that TMEM41B is recruited to viral RNA replication complex through direct or indirect interaction with flavivirus NS4A/NS4B to promote viral replication by facilitating membrane curvature. It should be noted that TMEM41B is also required for the replication of diverse coronaviruses, including SARS-CoV-2 (Hoffmann et al., 2021; Trimarco et al., 2021). Further investigation is required for the detailed mechanism of TMEM41B as a flavivirus host factor, which could better address that whether TMEM41B is a potential host target for antiviral therapeutics. In summary, host membrane proteins might function as chaperones that interact with flaviviruses NS4B to support proper polyprotein expression, process, as well as viral replication.
3.2. Biological function of NS4B in viral replication and its interaction with other viral proteins
3.2.1. NS1/NS4B interaction
Although NS4B does not have any known enzymatic activities, it plays an essential role in viral replication by interaction with other viral proteins. The interaction between NS1 and NS4B was first found in WNV by using co-IP and mass spectrum (Youn et al., 2012). Youn and coworkers found that the F86C mutation on NS4B could rescue viral replication caused by mutations (RQ10NK) in NS1. Using forward genetic screen, Plaszczyca et al. showed an interaction of DENV NS1 with the NS4A-2K-4B cleavage intermediate, but not mature NS4A or NS4B (Płaszczyca et al., 2019). Amino acids G164 and W168, located in the Wing connector domain of NS1, were found to be critical for the observed interaction that is required for viral replication. Plaszczyca et al. also showed that the NS1 and NS4A-2K-4B interaction is not required for the formation of vesicle packets (membranous replication organelle), indicating that the observed interaction plays a critical role in viral RNA synthesis. It was also found that the binding of DENV NS1 and NS4B could be decreased by the ubiquitination of residues K182 and K189 in NS1. K189R mutation in DENV NS1, which prevents ubiquitination on K189, dramatically increased the interaction between NS1 and NS4B (Giraldo et al., 2018).
3.2.2. NS2B/NS4B interaction
Using spectroscopy, confocal microscopy, fluorescence resonance energy transfer (FRET), and biomolecular fluorescent complementation (BiFC), Yu et al. reported that WNV NS2B interacts with several other viral nonstructural proteins, including NS2A, NS4A, and NS4B (Yu et al., 2013). The NS2B/NS4B interaction was also observed in JEV with BiFC analysis (Li et al., 2016a). These results indicate that NS4B plays a critical role in bringing other transmembrane viral nonstructural proteins together, as well as recruiting NS3 and NS5 to the replication complex.
3.1.3. NS3/NS4B interaction
The NS3/NS4B interaction was initially detected with a yeast two-hybrid assay, and confirmed by biochemical assays (Umareddy et al., 2006). In vitro unwinding assay indicated that NS4B dissociates NS3 from single-stranded RNA and promotes the helicase activity of NS3. The binding interface between DENV NS4B and NS3 was mapped and confirmed by using biophysical and genetic approaches (Zou et al., 2015b). Surface plasmon resonance (SPR) assay found that the cytosolic loop of DENV-2 NS4B (amino acids 130 to 167) and subdomains 2 and 3 of NS3 helicase is required for the NS3/NS4B interaction. NMR measurements also demonstrated that the recombinant cytosolic loop of NS4B undergoes significant conformational change upon NS3 binding, and mutations targeting the four conserved amnio acids that exhibited chemical shifts in the NS3/NS4B NMR experiment significantly reduced viral replication (Zou et al., 2015b). The genetic complementation map and replication-independent expression system also demonstrated that the cytosolic loop, especially the conserved amnion acids, are critical for NS3/NS4B interaction and viral replication (Chatel-Chaix et al., 2015).
Despite the cytosolic loop of NS4B, Lu and coworkers identified another specific NS3 binding site located in the N-terminal region of NS4B (Lu et al., 2021). The interaction between the N-terminal region of NS4B and NS3 was confirmed by pull-down, microscale thermophoresis (MST), and immunoblotting. The real-time unwinding kinetics assay demonstrated that the N-terminal region of NS4B significantly accelerated the DNA unwinding activity of NS3 helicase by assisting NS3 to dissociate from single-stranded DNA, which enabled NS3 helicase to keep high activity at high ATP concentrations. Two key residues I190 and P319 in motifs I and III of NS3, and the residues 51-83 in NS4B, were identified to be involved in such interaction. These findings also indicate that NS4B would accelerate NS3 dissociation from single-stranded RNA and enhance the helicase activity of NS3, which is in agreement with the previous study (Umareddy et al., 2006). Laurent and coworkers found that DENV NS3 and NS4B are colocalized in a punctate structure that does not contain replication intermediate double-stranded RNA. The punctate structure is in physical contact with mitochondria and NS4B was found to perturb mitochondrial morphodynamics by inducing mitochondria elongation. Similar infection-induced mitochondria elongation was also observed in ZIKV, indicating that such perturbation might protect flaviviruses from innate immunity and create a favorable replicative environment (Chatel-Chaix et al., 2016). Taken together, NS4B might serve as an essential cofactor for NS3 to coordinate the ATP cycles and RNA binding during viral replication.
3.3.4. NS4A/NS4B interaction
Using a replication-defective DENV-1 containing a chimeric NS4A (with amino acids 27-34 from JEV NS4A), Tajima et al. found a single mutation in NS4B to restore viral replication (Tajima et al., 2011). Similarly, Li et al. also found that a replication-defective JEV with an NS4B mutation (Y3N) could be rescued by a single mutation at position 79 (K79R) in NS4A, suggesting a genetic interaction between NS4B and NS4A (Li et al., 2015a). The NS4A/NS4B interaction was further characterized in both virus-infected cells and in vitro (Zou et al., 2015c). DENV-2 NS4A interacts with NS4B in infected cells and in cells expressing NS4A and NS4B in the absence of other viral proteins. Purified recombinant NS4A and NS4B proteins directly bind to each other in an enzyme-linked immunosorbent assay (ELISA). The binding interfaces were mapped to residues 40-76 of NS4A and residues 84-146 of NS4B. NMR measurement further identified three amino acids in NS4A (L48, T54, and L60) that are critical for the NS4A/NS4B interaction and viral replication. As NS4A was found to oligomerize during viral replication (Lee et al., 2015; Stern et al., 2013), NS4A/NS4B interaction may modulate the formation of transmembrane complex and regulate flavivirus replication. NS4B-viral protein interactions are summarized in Table 1.
Table 1.
Host proteins and viral proteins that interact with flavivirus NS4B protein
| Host protein | Virus | Reference |
|---|---|---|
| STAT1 | DENV-2, ZIKV | (Esteves et al., 2017; Munoz-Jordán et al., 2005) |
| JAKI | ZIKV | (Esteves et al., 2017) |
| NPL4 | DENV-2, JEV | (Arakawa et al., 2022) |
| EMC | DENV-2, ZIKV, YFV | (Lin et al., 2019; Marceau et al., 2016) |
| TMEM41B | ZIKV, JEV | (Marceau et al., 2016; Scaturro et al., 2018) |
| Viral Protein | ||
| NS1 | WNV, DENV-2 | (Płaszczyca et al., 2019; Youn et al., 2012) |
| NS2B | WNV, JEV | (Li et al., 2016a; Yu et al., 2013) |
| NS3 | DENV-2, DENV-4, ZIKV | (Chatel-Chaix et al., 2016; Umareddy et al., 2006; Zou et al., 2015b) |
| NS4A | DENV-1, JEV | (Li et al., 2015a; Tajima et al., 2011) |
4. Small-molecule inhibitors that target flavivirus NS4B
4.1. NITD-618
Using a DENV-2 replicon containing a luciferase gene, Xie et al. identified an aminothiazole derivative NITD-618 capable of inhibiting all four serotypes of DENV with EC50 of 1 to 4 μM (Table 2), but not other flaviviruses or nonflaviviruses (Xie et al., 2011). The time-of-addition experiment suggests that NITD-618 blocks a late stage of viral replication. A transient replicon assay revealed that NITD-618 inhibits viral RNA synthesis rather than viral protein translation. Resistance analysis uncovered two mutations (P104L and A119T) in NS4B that could confer resistance to the compound in mammalian cells (Xie et al., 2011). It has been reported that the P104 mutant abolished NS3/NS4B interaction (Umareddy et al., 2006), indicating that NITD-618 could perturb viral protein-protein interactions, leading to a defect in viral RNA synthesis. Although unfavorable physicochemical properties, such as low solubility and high lipophilicity prevented the compound from further development, NITD-618 was the first reported NS4B inhibitors that could inhibit all four serotypes of DENV.
Table 2.
Chemical structures of representative NS4B inhibitors.
| Compound | Virus | EC50 (μM) | Structure | Reference |
|---|---|---|---|---|
| NITD-618 | DENV-1 | 1.5 |

|
(Xie et al., 2011) |
| DENV-2 | 1.0 | |||
| DENV-3 | 1.6 | |||
| DENV-4 | 4.1 | |||
| Compound 14a | DENV-1 | >1 |

|
(Wang et al., 2015) |
| DENV-2 | 0.042 | |||
| DENV-3 | 0.032 | |||
| DENV-4 | >1 | |||
| NITD-688 | DENV-1 | 0.038 |

|
(Moquin et al., 2021) |
| DENV-2 | 0.008 | |||
| DENV-3 | 0.008 | |||
| DENV-4 | 0.012 | |||
| JNJ-A07 | DENV-1 | <0.0003 |

|
(Kaptein et al., 2021) |
| DENV-2 | <0.004 | |||
| DENV-3 | <0.001 | |||
| DENV-4 | <0.006 | |||
| BDAA | YFV | 0.21 |

|
(Guo et al., 2016) |
4.2. Compound 14a
Wang et al. identified a spiropyrazolopyridone compound that inhibits DENV replication through a high-throughput phenotypic screen (Wang et al., 2015). Medicinal chemistry improved the physicochemical properties of the initial compound, leading to compound 14a (Table 2) (Zou et al., 2015a). Compound 14a selectively inhibits DENV-2 and DENV-3 viral RNA synthesis, but not the viral translation, with EC50 of 10 to 80 nM and good pharmacokinetics. Treatment of DENV-2-infected mice with compound 14a suppressed viremia, even when the treatment started 2 days after infection. Resistance mutation was mapped to amino acid V63 of NS4B, located in helix α3 of the protein. Gel filtration analysis demonstrated that 3H-labeled compound 14a directly binds to NS4B (Wang et al., 2015). Cellular assays showed that the function of NS4B in antagonizing IFN signaling was not affected by compound 14a, suggesting that the compound may inhibit protein-protein interaction during viral RNA synthesis. An NMR study suggested that resistant mutation V63I may affect its interaction with residues in helix α5 of NS4B, resulting in a decreased compound/NS4B binding (Li et al., 2015b). Further studies are required (i) to investigate the exact mechanism of how compound 14a inhibits viral RNA synthesis and (ii) to improve the antiviral spectrum against all DENV serotypes and possibly other flaviviruses.
4.3. Compound NITD-688
Using a phenotypic screening of DENV infection, Moquin and colleagues developed NITD-688 as a potent NS4B inhibitor against all four DENV serotypes, including DENV-1 (My97-10245), DENV-2 (My97-10340), DENV-3 (My05-34640), and DENV-4 (My01-22713) (Table 2) (Moquin et al., 2021). NITD-688 presented EC50 values of 8 to 38 nM against all four serotypes of DENV with a solubility of 98 μM at pH 6.8. NITD-688 does not potently inhibit other flaviviruses and CHIKV, indicating that the inhibition is specific against DENV. The target of NITD-688 is determined to be NS4B by resistance selection. Three NS4B mutations, which are highly conserved among all four serotypes of DENV, but not other flaviviruses, confer resistance to NITD-688 (Figure 1A). The NMR spectrum of wild-type DENV-2 NS4B with NITD-688 shows specific peak shifts and broadening. While the NMR spectrum of mutant NS4B with resistant mutations remained the same as the wild-type; no obvious chemical peak shifts were observed after the compound was added, further confirming that NITD-688 directly binds to the wild-type NS4B protein. Treatment of DENV-2-infected AG129 mice with 30 mg/kg of NITD-688 reduced viremia by 1.44-log and 1.16-log when the treatment started immediately and 3 days after infection, respectively. NITD-688 also has excellent oral availability and pharmacokinetic properties in mice, rats, and dogs. The preclinical safety results also showed that NITD-688 was well tolerated in preclinical species. The antiviral mechanism of NITD-688 remains to be further delineated by a high-resolution structure of the NITD-688/NS4B complex. NITD-688 has currently advanced into phase I clinical trials (https://www.novartis.com/node/72716).
4.4. Compound JNJ-A07
Using a large-scale cell-based anti-DENV-2 screen, Kaptein and coworkers identified and optimized a small molecular inhibitor, JNJ-A07, with antiviral EC50 values in the nanomolar to picomolar range against 21 clinical isolates that covered all known genotypes and serotypes for DENV (Table 2) (Kaptein et al., 2021). JNJ-A07 is less potent against other flaviviruses, including ZIKV, WNV, and JEV. Resistance selection and reverse genetic studies identified several amino acids in NS4B conferring compound resistance (Figure 1A), indicating the compound targets NS4B. Interestingly, viruses containing the resistance mutations appeared to be unable to replicate in mosquito cells, suggesting that such mutant viruses may not transmit from human to human through mosquitos. JNJ-A07 could inhibit NS4B/NS3 interaction by preventing the formation of the NS3/NS4B complex or blocking the cleavage processing of the NS4 precursor at the NS4A-2K cleavage site. Notably, the antiviral effect of JNJ-A07 was reduced after the onset of viral RNA synthesis, suggesting that the compound inhibits de novo formation of the NS3/NS4B complex, but not the disruption of the already formed complex. JNJ-A07 showed favorable safety and pharmacokinetics in mice and rats. No adverse effects were observed in rats when the animals were dosed up to 300 mg/kg for 15 consecutive days by oral administration. Most importantly, JNJ-A07 was highly effective in reducing viral loads and virus-induced disease, even when the treatment was started 3 days after infection (Kaptein et al., 2021). Behnam et al. suggested that JNJ-A07 probably targets NS4A-2K-NS4B processing by disturbing the structure of cleavage events instead of direct inhibition of NS3 (Behnam and Klein, 2022). A derivative of JNJ-A07 is currently in clinical development (clinical trial ID: NCT04906980) (Behnam and Klein, 2021). Further elucidation of the mechanistic details and the combination of JNJ-A07 with other promising DENV inhibitors remains to be investigated.
4.5. Compound BDAA
There are estimated more than 400 million people live in areas at risk of YFV infection. Despite the availability of the YF17D vaccine, the YF17D vaccination rates are low, underlining the medical need for antiviral therapy for YFV (Douam and Ploss, 2018; Faria et al., 2018). An acetic acid benzodiazepine (BDAA; Table 2) compound that specifically inhibits YFV (not other flaviviruses) was identified through a high-throughput screening campaign (Guo et al., 2016). The viral production in YFV-infected cells was significantly reduced when treated with 2 μM of BDAA, and the virus-induced cytopathic effect was reduced by 50%. In a hamster model, oral administration of BDAA reduced the mortality rate by 90% and YFV infection-mediated liver damage. In a mouse model, BDAA showed a good safety profile exhibiting a maximum effective dose of 100 mg/kg without causing any neurological symptoms. Selection and genetic analysis of resistant viruses revealed that mutation in NS4B conferred YFV resistance to BDAA, suggesting that BDAA might directly interact with NS4B (Gao et al., 2020; Guo et al., 2016). A recent study reported that BDAA could dramatically impair the structure of YFV replication organelles and lead to the exposure of viral RNAs in infected cells, which consequently activates RIG-I and MDA5-like innate immune response (Gao et al., 2022). Thus, BDAA and its derivatives have the potential to be a new option for anti-YFV treatment by targeting NS4B via two mechanisms: (i) suppression of viral replication and (ii) enhancement of innate immune response. Using BDAA as a leading compound, more benzodiazepine derivatives have been synthesized to improve the solubility and to reduce plasma protein binding, leading to improved in vivo antiviral efficacy and safety profile for future preclinical and clinical development.
5. Conclusions and future perspectives
Flaviviruses are a group of viral pathogens with pandemic potential because of global urbanization, globalization, climate warming, and the expansion of competent vectors, especially mosquitos. In addition to the well-known flaviviruses, some lesser-known flaviviruses are also circulating in restricted areas and have the potential to emerge worldwide, such as Spondweni virus, Ustutu virus, and Wesselsbron virus (Pierson and Diamond, 2020). The development of antiviral treatment is urgently required for pandemic preparedness. Besides the extensive efforts on the enzymatic NS3 and NS5 targets, NS4B has emerged as a promising antiviral target. NS4B plays an important role in flaviviral replication by: (i) recruiting host protein to alter the membrane topology of ER; (ii) facilitating the formation of viral replication complex by interacting with other viral proteins, including NS1, NS2B, NS3, and NS4A. In addition, NS4B functions in counteracting the host innate immune responses and inducing cell dysregulation by: (i) blocking type I interferon signaling; (ii) inhibiting RNAi pathway; (iii) suppressing the formation of stress granules; and (iv) inducing autophagy and apoptosis.
Since the first NS4B inhibitor was reported for YFV in 2009 (Patkar et al., 2009), great progress has been made toward identifying and developing flavivirus NS4B inhibitors in clinics. Some of the DENV NS4B inhibitors, including NITD-688 and JNJ-A07 analog, have already advanced to clinical trials. As NS4B from different flaviviruses share an overall similar structure and function, a continued emphasis on the fundamental understanding of NS4B will facilitate drug discovery. The following research will further expedite the discovery and development of flavivirus NS4B inhibitors. (i) Investigation of the detailed mechanism of DENV NS4B in viral replication. Compounds that inhibit viral protein-protein interactions or viral/host protein interactions could directly block viral replication. (ii) Understanding the molecular basis of the host immune evasion and cell dysregulation governed by NS4B. Compounds that block NS4B-mediated immune evasion could boost host innate antiviral activity for patient treatment. (iii) High-resolution structure determination of NS4B alone and in complex with other viral or host proteins. The high-resolution structure of NS4B will not only deepen our understanding of the detailed mechanism of NS4B function, but also provide a solid foundation for the rationale design of drug discovery. The knowledge derived from the above studies will also help uncover the mystery why NS4B inhibitors are the most frequent “hits” that have been identified through flavivirus phenotypic screening.
Figure 2.
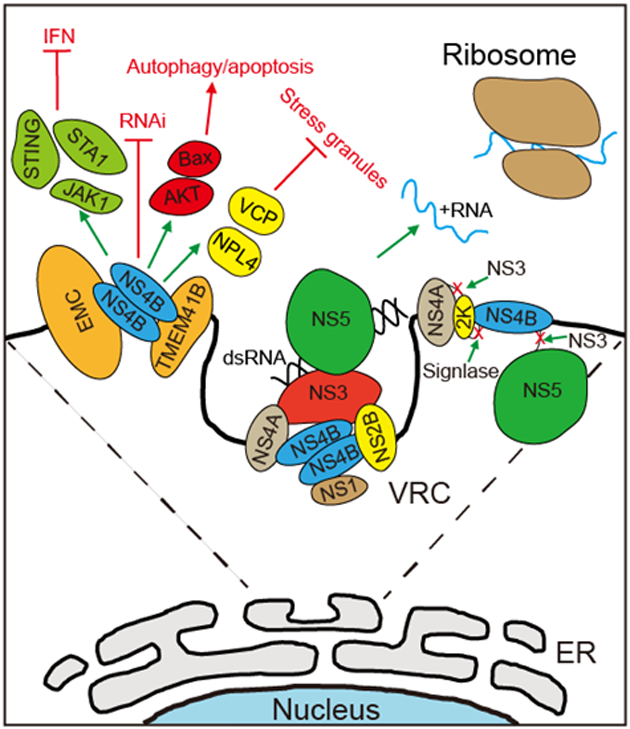
The functions of flavivirus NS4B protein in the viral life cycle. After release from the polypeptide by NS3 and host signalase, NS4B recruits host protein to alter the membrane topology of ER and facilitates the formation of viral replication complex by interacting with other viral proteins. Besides viral replication, NS4B also functions in counteracting the host innate immune responses and inducing cell dysregulation by blocking type I IFN signaling, inhibiting RNAi pathway, suppressing the formation of stress granules, and induing autophagy and apoptosis.
Acknowledgments
We thank colleagues at the University of Texas Medical Branch and the Novartis Institute for Tropical Diseases for helpful discussions. P.-Y.S. was supported by NIH grants U19AI171413, U19AI142759, and U01AI151801, and awards from the Sealy & Smith Foundation, the Kleberg Foundation, the John S. Dunn Foundation, the Amon G. Carter Foundation, the Gilson Longenbaugh Foundation, and the Summerfield Robert Foundation.
Reference
- Aguiar M, Stollenwerk N, and Halstead SB (2016). The impact of the newly licensed dengue vaccine in endemic countries. PLoS neglected tropical diseases 10, e0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Tabata K, Ishida K, Kobayashi M, Arai A, Ishikawa T, Suzuki R, Takeuchi H, Tripathi LP, and Mizuguchi K (2022). Flavivirus recruits the valosin-containing protein–NPL4 complex to induce stress granule disassembly for efficient viral genome replication. Journal of Biological Chemistry 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnam MAM, and Klein CD (2021). On track to tackle dengue: History and future of NS4B ligands. Cell Host & Microbe 29, 1735–1737. [DOI] [PubMed] [Google Scholar]
- Behnam MAM, and Klein CD (2022). Corona versus Dengue: Distinct Mechanisms for Inhibition of Polyprotein Processing by Antiviral Drugs. ACS Pharmacology & Translational Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj T, Kumar P, and Giri R (2021). Investigating the conformational dynamics of Zika virus NS4B protein. bioRxiv, 2021.2012.2008.471718. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. (2013). The global distribution and burden of dengue. Nature 496, 504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldescu V, Behnam MAM, Vasilakis N, and Klein CD (2017). Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nature Reviews Drug Discovery 16, 565–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati M, Alvarez K, Assenberg R, Baronti C, Canard B, Cook S, Coutard B, Decroly E, de Lamballerie X, and Gould EA (2010). Structure and functionality in flavivirus NS-proteins: perspectives for drug design. Antiviral research 87, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TT, Moi ML, Nabeshima T, Takemura T, Nguyen TT, Nguyen LN, Pham HTT, Nguyen TTT, Manh DH, Dumre SP, et al. (2018). A single amino acid substitution in the NS4B protein of Dengue virus confers enhanced virus growth and fitness in human cells in vitro through IFN-dependent host response. J Gen Virol 99, 1044–1057. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Hahn CS, Galler R, and Rice CM (1990). Flavivirus genome organization, expression, and replication. Annual review of microbiology 44, 649–688. [DOI] [PubMed] [Google Scholar]
- Chatel-Chaix L, Cortese M, Romero-Brey I, Bender S, Neufeldt CJ, Fischl W, Scaturro P, Schieber N, Schwab Y, Fischer B, et al. (2016). Dengue Virus Perturbs Mitochondrial Morphodynamics to Dampen Innate Immune Responses. Cell Host & Microbe 20, 342–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Chaix L, Fischl W, Scaturro P, Cortese M, Kallis S, Bartenschlager M, Fischer B, and Bartenschlager R (2015). A combined genetic-proteomic approach identifies residues within Dengue virus NS4B critical for interaction with NS3 and viral replication. Journal of virology 89, 7170–7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MH, and Metz SW (2017). Progress and works in progress: update on flavivirus vaccine development. Clinical therapeutics 39, 1519–1536. [DOI] [PubMed] [Google Scholar]
- Courtney SC, Scherbik SV, Stockman BM, and Brinton MA (2012). West Nile Virus Infections Suppress Early Viral RNA Synthesis and Avoid Inducing the Cell Stress Granule Response. Journal of Virology 86, 3647–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douam F, and Ploss A (2018). Yellow Fever Virus: Knowledge Gaps Impeding the Fight Against an Old Foe. Trends in Microbiology 26, 913–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, and Whitehead SS (2011). Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine 29, 7242–7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M-P, Benarroch D, Selisko B, Romette J-L, and Canard B (2002). An RNA cap (nucleoside-2′ -O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. The EMBO journal 21, 2757–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves E, Rosa N, Correia MJ, Arrais JP, and Barros M (2017). New Targets for Zika Virus Determined by Human-Viral Interactomic: A Bioinformatics Approach. Biomed Res Int 2017, 1734151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgout B, Pethel M, Zhang YM, and Lai CJ (1991). Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. Journal of Virology 65, 2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanunza E, Grandi N, Quartu M, Carletti F, Ermellino L, Milia J, Corona A, Capobianchi MR, Ippolito G, and Tramontano E (2021). INMI1 Zika Virus NS4B Antagonizes the Interferon Signaling by Suppressing STAT1 Phosphorylation. Viruses 13, 2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria NR, Kraemer MUG, Hill SC, Jesus J.G.d., Aguiar RS, Iani FCM, Xavier J, Quick J, Plessis L.d., Dellicour S, et al. (2018). Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 361, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang L, Ma J, Jurado A, Hong S-H, Guo J-T, Rice CM, MacDonald MR, and Chang J (2020). Development of antibody-based assays for high throughput discovery and mechanistic study of antiviral agents against yellow fever virus. Antiviral Research 182, 104907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Zhang X, Zhang L, Wu S, Ma J, Wang F, Zhou Y, Dai X, Bullitt E, and Du Y (2022). A yellow fever virus NS4B inhibitor not only suppresses viral replication, but also enhances the virus activation of RIG-I-like receptor-mediated innate immune response. PLoS Pathogens 18, e1010271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo MI, Vargas-Cuartas O, Gallego-Gomez JC, Shi PY, Padilla-Sanabria L, Castano-Osorio JC, and Rajsbaum R (2018). K48-linked polyubiquitination of dengue virus NS1 protein inhibits its interaction with the viral partner NS4B. Virus Res 246, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopala Reddy SB, Chin W-X, and Shivananju NS (2018). Dengue virus NS2 and NS4: Minor proteins, mammoth roles. Biochemical Pharmacology 154, 54–63. [DOI] [PubMed] [Google Scholar]
- Guo F, Wu S, Julander J, Ma J, Zhang X, Kulp J, Cuconati A, Block TM, Du Y, Guo J-T, et al. (2016). A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein. Journal of Virology 90, 10774–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafirassou ML, Meertens L, Umaña-Diaz C, Labeau A, Dejarnac O, Bonnet-Madin L, Kümmerer BM, Delaugerre C, Roingeard P, Vidalain P-O, et al. (2017). A Global Interactome Map of the Dengue Virus NS1 Identifies Virus Restriction and Dependency Host Factors. Cell Reports 21, 3900–3913. [DOI] [PubMed] [Google Scholar]
- Han X, Wang J, Yang Y, Qu S, Wan F, Zhang Z, Wang R, Li G, Cong H, and Dutch RE (2021). Zika Virus Infection Induced Apoptosis by Modulating the Recruitment and Activation of Proapoptotic Protein Bax. Journal of Virology 95, e01445–01420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, and Gubler DJ (2006). West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57, 181–194. [DOI] [PubMed] [Google Scholar]
- Hoffmann H-H, Schneider WM, Rozen-Gagnon K, Miles LA, Schuster F, Razooky B, Jacobson E, Wu X, Yi S, and Rudin CM (2021). TMEM41B is a pan-flavivirus host factor. Cell 184, 133–148. e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Yamanaka A, and Konishi E (2014). A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 32, 1326–1337. [DOI] [PubMed] [Google Scholar]
- Kakumani PK, Ponia SS, S RK, Sood V, Chinnappan M, Banerjea AC, Medigeshi GR, Malhotra P, Mukherjee SK, and Bhatnagar RK (2013). Role of RNA Interference (RNAi) in Dengue Virus Replication and Identification of NS4B as an RNAi Suppressor. Journal of Virology 87, 8870–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptein SJ, Goethals O, Kiemel D, Marchand A, Kesteleyn B, Bonfanti J-F, Bardiot D, Stoops B, Jonckers TH, and Dallmeier K (2021). A pan-serotype dengue virus inhibitor targeting the NS3–NS4B interaction. Nature 598, 504–509. [DOI] [PubMed] [Google Scholar]
- Kaufusi PH, Kelley JF, Yanagihara R, and Nerurkar VR (2014). Induction of endoplasmic reticulum-derived replication-competent membrane structures by West Nile virus non-structural protein 4B. PLoS One 9, e84040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaitong P, and Smith DR (2021). Roles of Non-Structural Protein 4A in Flavivirus Infection. Viruses 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klema VJ, Padmanabhan R, and Choi KH (2015). Flaviviral replication complex: coordination between RNA synthesis and 5′-RNA capping. Viruses 7, 4640–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno G, Chang G-JJ, Tsuchiya KR, Karabatsos N, and Cropp CB (1998). Phylogeny of the genus Flavivirus. Journal of virology 72, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Xie X, Zou J, Li S-H, Lee MYQ, Dong H, Qin C-F, Kang C, and Shi P-Y (2015). Determinants of dengue virus NS4A protein oligomerization. Journal of virology 89, 6171–6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, and Khromykh AA (2008). Role of nonstructural protein NS2A in flavivirus assembly. Journal of virology 82, 4731–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Adam A, Luo H, Shan C, Cao Z, Fontes-Garfias CR, Sarathy VV, Teleki C, Winkelmann ER, Liang Y, et al. (2019). An attenuated Zika virus NS4B protein mutant is a potent inducer of antiviral immune responses. npj Vaccines 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-D, Deng C-L, Ye H-Q, Zhang H-L, Zhang Q-Y, Chen D-D, Zhang P-T, Shi P-Y, Yuan Z-M, Zhang B, et al. (2016a). Transmembrane Domains of NS2B Contribute to both Viral RNA Replication and Particle Formation in Japanese Encephalitis Virus. Journal of Virology 90, 5735–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X-D, Ye H-Q, Deng C-L, Liu S-Q, Zhang H-L, Shang B-D, Shi P-Y, Yuan Z-M, and Zhang B (2015a). Genetic interaction between NS4A and NS4B for replication of Japanese encephalitis virus. Journal of General Virology 96, 1264–1275. [DOI] [PubMed] [Google Scholar]
- Li Y, Kim YM, Zou J, Wang Q-Y, Gayen S, Wong YL, Lee LT, Xie X, Huang Q, Lescar J, et al. (2015b). Secondary structure and membrane topology of dengue virus NS4B N-terminal 125 amino acids. Biochimica et Biophysica Acta (BBA) - Biomembranes 1848, 3150–3157. [DOI] [PubMed] [Google Scholar]
- Li Y, Wong YL, Lee MY, Li Q, Wang QY, Lescar J, Shi PY, and Kang C (2016b). Secondary structure and membrane topology of the full-length dengue virus NS4B in micelles. Angewandte Chemie 128, 12247–12251. [DOI] [PubMed] [Google Scholar]
- Liang Q, Luo Z, Zeng J, Chen W, Foo SS, Lee SA, Ge J, Wang S, Goldman SA, Zlokovic BV, et al. (2016). Zika Virus NS4A and NS4B Proteins Deregulate Akt-mTOR Signaling in Human Fetal Neural Stem Cells to Inhibit Neurogenesis and Induce Autophagy. Cell Stem Cell 19, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SP, Noble CG, and Shi P-Y (2015). The dengue virus NS5 protein as a target for drug discovery. Antiviral research 119, 57–67. [DOI] [PubMed] [Google Scholar]
- Lin C, Amberg SM, Chambers TJ, and Rice CM (1993). Cleavage at a novel site in the NS4A region by the yellow fever virus NS2B-3 proteinase is a prerequisite for processing at the downstream 4A/4B signalase site. Journal of virology 67, 2327–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DL, Cherepanova NA, Bozzacco L, MacDonald MR, Gilmore R, and Tai AW (2017). Dengue virus hijacks a noncanonical oxidoreductase function of a cellular oligosaccharyltransferase complex. MBio 8, e00939–00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DL, Inoue T, Chen Y-J, Chang A, Tsai B, and Tai AW (2019). The ER Membrane Protein Complex Promotes Biogenesis of Dengue and Zika Virus Non-structural Multi-pass Transmembrane Proteins to Support Infection. Cell Reports 27, 1666–1674.e1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WJ, Wang XJ, Mokhonov VV, Shi P-Y, Randall R, and Khromykh AA (2005). Inhibition of Interferon Signaling by the New York 99 Strain and Kunjin Subtype of West Nile Virus Involves Blockage of STAT1 and STAT2 Activation by Nonstructural Proteins. Journal of Virology 79, 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zhan Y, Li X, Bai X, Yuan F, Ma L, Wang X, Xie M, Wu W, and Chen Z (2021). Novel insights into the function of an N-terminal region of DENV2 NS4B for the optimal helicase activity of NS3. Virus Res 295, 198318. [DOI] [PubMed] [Google Scholar]
- Luo D, Vasudevan SG, and Lescar J (2015). The flavivirus NS2B–NS3 protease–helicase as a target for antiviral drug development. Antiviral research 118, 148–158. [DOI] [PubMed] [Google Scholar]
- Marceau CD, Puschnik AS, Majzoub K, Ooi YS, Brewer SM, Fuchs G, Swaminathan K, Mata MA, Elias JE, Sarnow P, et al. (2016). Genetic dissection of Flaviviridae host factors through genome-scale CRISPR screens. Nature 535, 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald E, Mathis S, Martin SW, Erin Staples J, Fischer M, and Lindsey NP (2021). Surveillance for West Nile Virus Disease—United States, 2009–2018 (Wiley Online Library; ), pp. 1959–1974. [DOI] [PubMed] [Google Scholar]
- McLean JE, Wudzinska A, Datan E, Quaglino D, and Zakeri Z (2011). Flavivirus NS4A-induced Autophagy Protects Cells against Death and Enhances Virus Replication*. Journal of Biological Chemistry 286, 22147–22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Kastner S, Krijnse-Locker J, Bühler S, and Bartenschlager R (2007). The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. Journal of Biological Chemistry 282, 8873–8882. [DOI] [PubMed] [Google Scholar]
- Miller S, Sparacio S, and Bartenschlager R (2006). Subcellular localization and membrane topology of the dengue virus type 2 non-structural protein 4B. Journal of Biological Chemistry 281, 8854–8863. [DOI] [PubMed] [Google Scholar]
- Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, and Steinegger M (2021). ColabFold - Making protein folding accessible to all. bioRxiv, 2021.2008.2015.456425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, and Fabjan Vodušek V (2016). Zika virus associated with microcephaly. New England Journal of Medicine 374, 951–958. [DOI] [PubMed] [Google Scholar]
- Moquin SA, Simon O, Karuna R, Lakshminarayana SB, Yokokawa F, Wang F, Saravanan C, Zhang J, Day CW, and Chan K (2021). NITD-688, a pan-serotype inhibitor of the dengue virus NS4B protein, shows favorable pharmacokinetics and efficacy in preclinical animal models. Science Translational Medicine 13, eabb2181. [DOI] [PubMed] [Google Scholar]
- Moretti F, Bergman P, Dodgson S, Marcellin D, Claerr I, Goodwin JM, DeJesus R, Kang Z, Antczak C, Begue D, et al. (2018). TMEM41B is a novel regulator of autophagy and lipid mobilization. EMBO Rep 19, e45889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Hama Y, and Mizushima N (2019). TMEM41B functions with VMP1 in autophagosome formation. Autophagy 15, 922–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Kuhn RJ, and Rossmann MG (2005). A structural perspective of the flavivirus life cycle. Nature Reviews Microbiology 3, 13–22. [DOI] [PubMed] [Google Scholar]
- Muller DA, and Young PR (2013). The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral research 98, 192–208. [DOI] [PubMed] [Google Scholar]
- Munoz-Jordán JL, Laurent-Rolle M, Ashour J, Martínez-Sobrido L, Ashok M, Lipkin WI, and García-Sastre A (2005). Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. Journal of virology 79, 8004–8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Jordán JL, Sánchez-Burgos GG, Laurent-Rolle M, and García-Sastre A (2003). Inhibition of interferon signaling by dengue virus. Proceedings of the National Academy of Sciences 100, 14333–14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik NG, Wu H-N, and Kirkegaard K (2015). Mutation of Putative N-Glycosylation Sites on Dengue Virus NS4B Decreases RNA Replication. Journal of Virology 89, 6746–6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo AM, Shurtleff MJ, Popova KD, Kulsuptrakul J, Weissman JS, and Puschnik AS (2019). The ER membrane protein complex is required to ensure correct topology and stable expression of flavivirus polyproteins. eLife 8, e48469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patkar CG, Larsen M, Owston M, Smith JL, and Kuhn RJ (2009). Identification of inhibitors of yellow fever virus replication using a replicon-based high-throughput assay. Antimicrob Agents Chemother 53, 4103–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TC, and Diamond MS (2020). The continued threat of emerging flaviviruses. Nature Microbiology 5, 796–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Płaszczyca A, Scaturro P, Neufeldt CJ, Cortese M, Cerikan B, Ferla S, Brancale A, Pichlmair A, and Bartenschlager R (2019). A novel interaction between dengue virus nonstructural protein 1 and the NS4A-2K-4B precursor is required for viral RNA replication but not for formation of the membranous replication organelle. PLoS Pathog 15, e1007736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, and Shi PY (2006). West Nile virus 5'-cap structure is formed by sequential guanine N-7 and ribose 2'-O methylations by nonstructural protein 5. J Virol 80, 8362–8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roby JA, Setoh YX, Hall RA, and Khromykh AA (2015). Post-translational regulation and modifications of flavivirus structural proteins. Journal of General Virology 96, 1551–1569. [DOI] [PubMed] [Google Scholar]
- Sampath A, and Padmanabhan R (2009). Molecular targets for flavivirus drug discovery. Antiviral research 81, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidis G, McDougall WM, Meraner P, Perreira JM, Portmann JM, Trincucci G, John SP, Aker AM, Renzette N, Robbins DR, et al. (2016). Identification of Zika Virus and Dengue Virus Dependency Factors using Functional Genomics. Cell Reports 16, 232–246. [DOI] [PubMed] [Google Scholar]
- Scaturro P, Stukalov A, Haas DA, Cortese M, Draganova K, Płaszczyca A, Bartenschlager R, Götz M, and Pichlmair A (2018). An orthogonal proteomic survey uncovers novel Zika virus host factors. Nature 561, 253–257. [DOI] [PubMed] [Google Scholar]
- Stern O, Hung Y-F, Valdau O, Yaffe Y, Harris E, Hoffmann S, Willbold D, and Sklan EH (2013). An N-terminal amphipathic helix in dengue virus nonstructural protein 4A mediates oligomerization and is essential for replication. Journal of virology 87, 4080–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima S, Takasaki T, and Kurane I (2011). Restoration of replication-defective dengue type 1 virus bearing mutations in the N-terminal cytoplasmic portion of NS4A by additional mutations in NS4B. Archives of Virology 156, 63–69. [DOI] [PubMed] [Google Scholar]
- Tian JN, Yang CC, Chuang CK, Tsai MH, Wu RH, Chen CT, and Yueh A (2019). A Dengue Virus Type 2 (DENV-2) NS4B-Interacting Host Factor, SERP1, Reduces DENV-2 Production by Suppressing Viral RNA Replication. Viruses 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarco JD, Heaton BE, Chaparian RR, Burke KN, Binder RA, Gray GC, Smith CM, Menachery VD, and Heaton NS (2021). TMEM41B is a host factor required for the replication of diverse coronaviruses including SARS-CoV-2. PLoS pathogens 17, e1009599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost B, and Smit JM (2020). Recent advances in antiviral drug development towards dengue virus. Current Opinion in Virology 43, 9–21. [DOI] [PubMed] [Google Scholar]
- Umareddy I, Chao A, Sampath A, Gu F, and Vasudevan SG (2006). Dengue virus NS4B interacts with NS3 and dissociates it from single-stranded RNA. Journal of general virology 87, 2605–2614. [DOI] [PubMed] [Google Scholar]
- van den Elsen K, Quek JP, and Luo D (2021). Molecular insights into the flavivirus replication complex. Viruses 13, 956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varasteh Moradi S, Gagoski D, Mureev S, Walden P, McMahon K-A, Parton RG, Johnston WA, and Alexandrov K (2020). Mapping Interactions among Cell-Free Expressed Zika Virus Proteins. Journal of Proteome Research 19, 1522–1532. [DOI] [PubMed] [Google Scholar]
- Vidotto A, Morais ATS, Ribeiro MR, Pacca CC, Terzian ACB, Gil LHVG, Mohana-Borges R, Gallay P, and Nogueira ML (2017). Systems Biology Reveals NS4B-Cyclophilin A Interaction: A New Target to Inhibit YFV Replication. Journal of Proteome Research 16, 1542–1555. [DOI] [PubMed] [Google Scholar]
- Wang Q-Y, Dong H, Zou B, Karuna R, Wan KF, Zou J, Susila A, Yip A, Shan C, Yeo KL, et al. (2015). Discovery of Dengue Virus NS4B Inhibitors. Journal of Virology 89, 8233–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte T, Xie G, Wicker JA, Whiteman MC, Li L, Rachamallu A, Barrett A, and Wang T (2011). Immune responses to an attenuated West Nile virus NS4B-P38G mutant strain. Vaccine 29, 4853–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SS, Blaney JE, Durbin AP, and Murphy BR (2007). Prospects for a dengue virus vaccine. Nature Reviews Microbiology 5, 518–528. [DOI] [PubMed] [Google Scholar]
- Wicker JA, Whiteman MC, Beasley DW, Davis CT, McGee CE, Lee JC, Higgs S, Kinney RM, Huang CY-H, and Barrett AD (2012). Mutational analysis of the West Nile virus NS4B protein. Virology 426, 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Xie X, Zou J, Noble CG, Russell WK, Holthauzen LMF, Choi KH, White MA, and Shi P-Y (2020). A cocrystal structure of dengue capsid protein in complex of inhibitor. Proceedings of the National Academy of Sciences 117, 17992–18001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Gayen S, Kang C, Yuan Z, and Shi P-Y (2013). Membrane topology and function of dengue virus NS2A protein. Journal of virology 87, 4609–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang Q-Y, Xu HY, Qing M, Kramer L, Yuan Z, and Shi P-Y (2011). Inhibition of Dengue Virus by Targeting Viral NS4B Protein. Journal of Virology 85, 11183–11195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Zou J, Wang Q-Y, and Shi P-Y (2015). Targeting dengue virus NS4B protein for drug discovery. Antiviral Research 118, 39–45. [DOI] [PubMed] [Google Scholar]
- Xie X, Zou J, Zhang X, Zhou Y, Routh AL, Kang C, Popov VL, Chen X, Wang Q-Y, and Dong H (2019a). Dengue NS2A protein orchestrates virus assembly. Cell host & microbe 26, 606–622. e608. [DOI] [PubMed] [Google Scholar]
- Xie X, Zou J, Zhang X, Zhou Y, Routh AL, Kang C, Popov VL, Chen X, Wang QY, Dong H, et al. (2019b). Dengue NS2A Protein Orchestrates Virus Assembly. Cell Host Microbe 26, 606–622 e608. [DOI] [PubMed] [Google Scholar]
- Youn S, Li T, McCune BT, Edeling MA, Fremont DH, Cristea IM, and Diamond MS (2012). Evidence for a genetic and physical interaction between nonstructural proteins NS1 and NS4B that modulates replication of West Nile virus. Journal of virology 86, 7360–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Takeda K, and Markoff L (2013). Protein–protein interactions among West Nile non-structural proteins and transmembrane complex formation in mammalian cells. Virology 446, 365–377. [DOI] [PubMed] [Google Scholar]
- Zhang R, Miner JJ, Gorman MJ, Rausch K, Ramage H, White JP, Zuiani A, Zhang P, Fernandez E, Zhang Q, et al. (2016). A CRISPR screen defines a signal peptide processing pathway required by flaviviruses. Nature 535, 164–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xie X, Xia H, Zou J, Huang L, Popov VL, Chen X, Shi P-Y, and Horner SM (2019). Zika Virus NS2A-Mediated Virion Assembly. mBio 10, e02375–02319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard KA, Shi P-Y, and Li H (2007). Structure and function of flavivirus NS5 methyltransferase. Journal of virology 81, 3891–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Neyts J, and Dallmeier K (2015). Flaviviral NS4b, chameleon and jack-in-the-box roles in viral replication and pathogenesis, and a molecular target for antiviral intervention. Reviews in medical virology 25, 205–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou B, Chan WL, Ding M, Leong SY, Nilar S, Seah PG, Liu W, Karuna R, Blasco F, Yip A, et al. (2015a). Lead Optimization of Spiropyrazolopyridones: A New and Potent Class of Dengue Virus Inhibitors. ACS Medicinal Chemistry Letters 6, 344–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lee LT, Wang QY, Xie X, Lu S, Yau YH, Yuan Z, Geifman Shochat S, Kang C, and Lescar J (2015b). Mapping the interactions between the NS4B and NS3 proteins of dengue virus. Journal of virology 89, 3471–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Xie X, Lee LT, Chandrasekaran R, Reynaud A, Yap L, Wang Q-Y, Dong H, Kang C, and Yuan Z (2014). Dimerization of flavivirus NS4B protein. Journal of virology 88, 3379–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Xie X, Wang Q-Y, Dong H, Lee MY, Kang C, Yuan Z, and Shi P-Y (2015c). Characterization of dengue virus NS4A and NS4B protein interaction. Journal of virology 89, 3455–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]


