Abstract
Being choked or strangled during partnered sex is an emerging sexual behavior, prevalent among young adult women. The goal of this study was to test whether, and to what extent, frequently being choked or strangled during sex is associated with cortical surface functioning and functional connectivity. This case-control study consisted of two groups (choking vs. choking-naïve). Women who were choked 4 or more times during sex in the past 30 days were enrolled into the choking group, whereas those without were assigned to the choking-naïve group. We collected structural and resting-state functional magnetic resonance imaging (fMRI) data and analyzed the data for amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) using cortical surface-based resting-state fMRI analysis, followed by static and dynamic resting-state fMRI connectivity analysis. Forty-one participants (choking n = 20; choking-n-aïve n = 21) contributed to the analysis. An inter-hemispheric imbalance in neuronal activation pattern was observed in the choking group. Specifically, we observed significantly lower ALFF and ReHo in the left cortical regions (e.g., angular gyrus, orbitofrontal gyrus) and higher ALFF and ReHo in the right cortical regions (e.g., pre-central/post-central gyri) in the choking group compared with the choking-naïve group. A significant group difference was found in static functional connectivity between the bilateral angular gyrus and the whole brain, in which the choking group's angular gyrus showed hyperconnectivity with, for example, the post-central gyrus, pre-central gyrus, and Rolandic operculum, relative to the choking-naïve group. The dynamic analysis revealed hyperconnectivity between the left angular gyrus and the bilateral postcentral gyrus in the choking group compared with the choking-naïve group. Taken together, our data show that multiple experiences of sexual choking/strangulation are associated with an inter-hemispheric imbalance in neural activation pattern and hyperconnectivity between the angular gyrus and brain regions related to motor control, consciousness, and emotion. A longitudinal study using multi-modal neurological assessments is needed to clarify the acute and chronic consequences of sexual choking/strangulation.
Keywords: cortical surface function, functional connectivity, functional magnetic resonance imaging, sexual asphyxiation, sexual choking, strangulation
Introduction
Emerging adulthood is a time of neurodevelopment, during which neural networks within the prefrontal cortex and limbic structures continue to mature and promote emotional regulation, self-esteem, and cognition.1,2 Also, during these years, most young adults engage in various forms of sexual exploration of sexual preferences and pleasure, emotional intimacy, and sexual identities.3 Although explorations are normative within sexual development, they are not without risks: some are well-recognized (e.g., sexually transmitted infections, unintended pregnancy), whereas others have begun to emerge, such as being choked/strangled during partnered sex.
Sexual choking is technically a form of strangulation in which a partner applies external pressure to the neck using their hands, limb, or a ligature, as opposed to internal blockage of the airways.4,5 We use the term choking at times because that is the term widely used in pornography, social media, and mainstream media by people who engage in this sexual practice.6,7 Because of easier access to online sexual content and social media, choking/strangulation during sex has become prevalent among young adults, especially women.8–10 In our recent random sample survey of 4989 college students, 58% of women reported ever having been choked/strangled during sex, and 33% of women had experienced being choked at least 5 times in the past.8
Although in most cases, choking during sex is consensual,11 there are varying perspectives. For example, in recent in-depth interviews with 24 young adult women, many of them stated that being choked/strangled during sex enhances sexual arousal and is a pleasurable part of sex, whereas others described their motivations for being choked/strangled during sex as primarily to please their sexual partner. Further, some reported that the choking/strangulation experience led to physical or emotional distress (e.g., losing consciousness, feeling unsafe).12 Herbenick and colleagues13 revealed that women who had been choked/strangled during sex more than 5 times in the last month were twice as likely to report experiencing depression, anxiety, sadness, and loneliness as their choking-naïve counterparts. These findings from survey studies warrant studies to investigate the neurophysiological responses to repetitive sexual choking events.14
To begin addressing this knowledge gap, we conducted a case-control study using a battery of resting-state functional magnetic resonance imaging (rs-fMRI) techniques to examine neurophysiological characteristics in women who had been choked during sex 4 or more times in the past 30 days (choking group) compared with women without any choking/strangulation history (choking-naïve group). The lower threshold of 4 or more times reflects weekly exposure over 30 days.
Our rs-fMRI analysis involved three steps. First, we conducted a cortical surface-based analysis, which has superior test-retest reliability and spatial specificity compared with volume-based analysis,15 to examine the amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo).16 ALFF estimates the total power or density of neural signals in each brain region, whereas ReHo estimates the coherence or synchrony of neighboring neural activity. The ALFF and ReHo methods do not require an a priori definition of the region of interest (ROI) and can provide information about regional neural activity throughout the brain.18 Second, we conducted a seed-based, static resting-state functional connectivity (rs-FC) analysis,17,18 followed by a seed-based, dynamic rs-FC analysis to validate the static analysis, given that the dynamic analysis can capture temporal variability in the fMRI signal to accurately reflect functional connectivity.21 Therefore, the current study aimed to explore neurophysiological differences between the choking group and the choking-naïve group through assessments of localized neural activity and neighboring neuronal connectivity.
Methods
Participants
This case-control study consisted of two groups (choking group vs. choking-naïve group) and was conducted from February 2021 to June 2021. Individuals were recruited from our separate campus-representative sexual health survey and from an online post on the Indiana University classifieds. Following consent to study participation, all participants completed a screening questionnaire to determine eligibility and group assignment. For general inclusion, participants were required to be female, enrolled at Indiana University, and between 18 and 30 years of age. For the choking group, additional inclusion criteria were that the women reported having been choked 4 or more times during consensual partnered sexual events in the past 30 days, whereas the women in the choking-naïve group were free of any lifetime sexual choking/strangulation experience. Participants in both groups were excluded if they were pregnant or had had a moderate-to-severe traumatic brain injury (TBI), although mild TBI was permitted. However, women with mild TBI within the past year and a history of more than two mild TBIs were excluded. Additional exclusion criteria were any MRI contraindications, neurological conditions (e.g., epilepsy, aneurysm, tumor), or psychological/psychiatric conditions (e.g., psychosis, post-traumatic stress disorder). The Indiana University Institutional Review Board approved the study, and written informed consent was obtained from all participants.
After confirming eligibility and group assignment, we scheduled the participants for data collection. In addition to completing a questionnaire about their health history and experiences with being choked during partnered sexual events, participants in both groups completed the Patient Health Questionnaire (PHQ-9) for depression-related symptoms,20,21 the Generalized Anxiety Disorder Assessment (GAD-7) for anxiety-related symptoms,22,23 and the Alcohol Use Disorders Identification Test (AUDIT) for assessment of alcohol consumption, drinking behaviors, and alcohol-related problems.24,25
MRI data acquisition
The MRI data were acquired on a 3T Siemens Prisma MRI scanner (Siemens, Erlangen, Germany) equipped with a 64-channel head/neck coil. Anatomical MRI was also obtained using a T1-weighted, three-dimensional (3D) magnetization prepared rapid gradient echo (MPRAGE) pulse sequence with the following parameters: repetition time (TR)/echo time (TE) = 2400/2.3 msec, inversion time (TI) = 1060 msec, flip angle = 8 degrees, matrix = 320 × 320, bandwidth = 210 Hz/pixel, iPAT = 2, which resulted in 0.8-mm isotropic resolution. Resting-state BOLD (blood-oxygen-level-dependent) signal was collected using a simultaneous multi-slice (SMS) single-shot echo-planar imaging (EPI) sequence with the following parameters: TR/TE = 800/30 msec, flip angle = 52 degrees, matrix = 90 × 90, field of view (FOV) = 216 mm, resolution = 2.4 mm isotropic, and multi-band acceleration factor = 6, with 1000 total volumes acquired over 12 min. This sequence was acquired while the participant was asked to relax with their eyes open while passively viewing a crosshair.
Preprocessing for surface-based analysis
The workflows of the preprocessing for surface-based analysis require extensive steps, as described in fMRIPrep.26 The detailed steps can be found in Supplementary Appendix S1. Preprocessing for anatomical data included that each participant's T1-weighted image was corrected for intensity non-uniformity. The T1 image was skull-stripped, and brain tissue segmentation of cerebrospinal fluid (CSF), white matter (WM), and gray matter (GM) was performed on the brain-extracted T1 image. Brain surfaces were reconstructed using recon-all (FreeSurfer 6.0.1).27 The brief summary of preprocessing steps is as follows: 1) a reference volume and its skull-striped version were generated using fMRIPrep; 2) the BOLD reference was co-registered to the T1 image; 3) head-motion parameters with respect to the BOLD reference were estimated before any spatiotemporal filtering; 4) the BOLD time-series were resampled onto their original, native space by applying the transforms to correct for head motion; and 5) head motions that were beyond a threshold of frame-wise displacement >0.2 mm, as well as 1 volume before and 2 volumes after, were identified using Friston 24-parameter model regression and excluded to address the residual effects of motion in group analyses.
ALFF and ReHo analyses
After the preprocessing, ALFF and ReHo analyses were conducted using DPABISurf.17,30 For ALFF, the resampled functional images were spatially smoothed with a full-width-at-half-maximum (FWHM) of 6 mm. De-trend and band-pass filtering (0.01–0.1 Hz) were performed to remove the effects of low-frequency drift and high-frequency noise. The resting-state time-series for each voxel was transformed into the frequency domain using a Fourier transform. The square root of the power spectrum was calculated and averaged across 0.01–0.1 Hz within each voxel to obtain a raw ALFF map. The global mean ALFF value was calculated from all voxels across the whole brain. Finally, ALFF values for each voxel were divided by the global mean ALFF value for standardization. Group comparisons were made for both peak ALFF at specific coordinates and mean ALFF in each brain region.
ReHo analysis was conducted in a similar manner to ALFF.28 Briefly, after band-pass filtering was performed, ReHo maps were produced by calculating the concordance of the Kendall coefficient of the resting-state time-series of a given voxel with its 26 nearest neighbors. The ReHo value of each voxel was standardized by the global mean ReHo value, followed by smoothing using a 3D Gaussian kernel of 6 mm FWHM for further statistical analysis. Just as in ALFF, group comparisons were made for both peak ReHo at specific coordinates and mean ReHo in each brain region.
Preprocessing for seed-based static and dynamic rs-FC analysis
Preprocessing for both static and dynamic rs-FC was performed through the Data Processing and Analysis of Brain Imaging (DPABI) toolbox (version 6.0; http://rfmri.org/dpabi), which includes Data Processing Assistant for Resting-State fMRI advanced edition (DPARSFA V4.5, http://rfmri.org/DPARSF).29,30 First, the Digital Imaging and Communications in Medicine (DICOM) files were arranged, and the first five volumes were discarded to allow the magnetization to approach a dynamic equilibrium, followed by setting up the parameters, such as repeating time, time-points, slice number, and voxel size. Preprocessing steps included slice timing and realignment, followed by regressing out head motion parameters with Friston 24-parameter model regression.31 The spatial normalization was applied to each image based on the Montreal Neurological Institute (MNI) template (resampling voxel size of 3 × 3 × 3 mm), followed by band-pass filtering with a frequency of 0.01–0.1 Hz to reduce the effects of low-frequency drift and high-frequency physiological noise. For static rs-FC analysis, the smoothing step used the spatial Gaussian filter of 4 mm FWHM. For dynamic rs-FC analysis, we did not perform any smoothing before the group connectivity analysis because performing spatial smoothing before network construction artificially increases correlations among nearby voxels.32–35
A priori ROIs for the connectivity analysis were set to the bilateral dorsolateral prefrontal cortex (DLPFC), angular gyrus, and posterior cingulate cortex on the basis of previous research in brain trauma. Specifically, the DLPFC plays a central role in diverse cognitive functions36 and together with the cingulate cortex regulates emotions, including sensation and pleasure.37 The angular gyrus is a hub of brain networks connecting the visual, auditory, and somatosensory cortices and is involved in attention, self-processing, semantic information processing, and mentalizing.38 The effects of brain trauma and strangulation are often diffusive in nature, affecting various parts of the brain, especially the hubs of cognitive processing and emotional regulations, such as the DLPFC, cingulate gyrus, and angular gyrus. Significant cortical thinning, brain atrophy, and altered functional connectivity, and the emergence of compulsive behaviors, have been observed in patients with various severities of brain trauma.39–42 To account for multiple comparisons (n = 6: bilateral of 3 ROIs), the level of statistical significance was corrected to α = 0.008. ROIs were identified using the atlas of Automated Anatomical Labeling (AAL).
Static rs-FC analysis
After the preprocessing, the static rs-FC analysis for each participant was performed using DPABI V6.0. A Pearson correlation coefficient was determined between each ROI and the whole brain as a measure of the strength of regional functional connectivity at rest. The correlation coefficients were then transformed into Fisher z-scores for statistical analysis.
Dynamic rs-FC analysis
After the preprocessing, the dynamic rs-FC analysis for each participant was performed using the Temporal Dynamic Analysis (TDA) toolkit that was included in DPABI V6.0. Sliding window analysis, which is sensitive to time-dependent variations,43–45 was applied to examine the seed-based BOLD signal over the whole brain. In the sliding window analysis, a temporal window of a certain size and shape was chosen, and functional connectivity within that window was calculated. In the current study, a moderate-length sliding Hamming window of 32 TR (64 sec) and a shifting step size of 1 TR (2 sec) were used to maximize statistical power within the window and across levels of analyses. The linear de-trending processing was conducted to remove the linear signal drift. The dynamic rs-FC analysis with each seed region was calculated as the Fisher z-transformed Pearson correlation coefficient for each participant, and the smoothing was performed for group analysis.46 Dynamic rs-FC analysis enhances the validity of our overall findings, given that the functional networks of the brain fluctuate during resting-state data acquisition. This temporal variability in functional connectivity can be accounted for in the dynamic rs-FC analysis.47
Statistical analysis
The focus of this work was to characterize between-group differences in neurophysiological signals, as reflected in cortical surface functioning (ALFF and ReHo) and rs-FC (dynamic and static). Demographic differences between the choking and choking-naïve groups were assessed by t-tests and chi-square tests. The two-sample t-tests were performed in the DPABISurf toolkit to compare differences in mean and peak ALFF and ReHo. For ALFF and ReHo, the statistical significance threshold was set at p < 0.05. We used familywise error correction (clusters with a voxel-level p < 0.01 and cluster-level p < 0.05) to obtain a significant group difference. Because of statistically significant group differences in age, race, and AUDIT, these demographic factors were controlled as covariates. Additionally, because depression and anxiety have been shown to be associated with repetitive sexual choking, we also included scores on the PHQ-9 and GAD-7 in the model.
Similarly, the two-sample t-tests were used to compare rs-FC strengths between groups in both static and dynamic analyses within the DPABI V6.0. Because we had 6 ROIs for rs-FC analyses, multiple comparisons were corrected by threshold-free cluster enhancement (TFCE), and the level of statistical significance was set at two-tailed p < 0.008. Demographic factors (age, race, AUDIT, PHQ-9, and GAD-7) were included in the model as covariates, and the number of permutations was set at 1000.
Results
Demographic characteristics
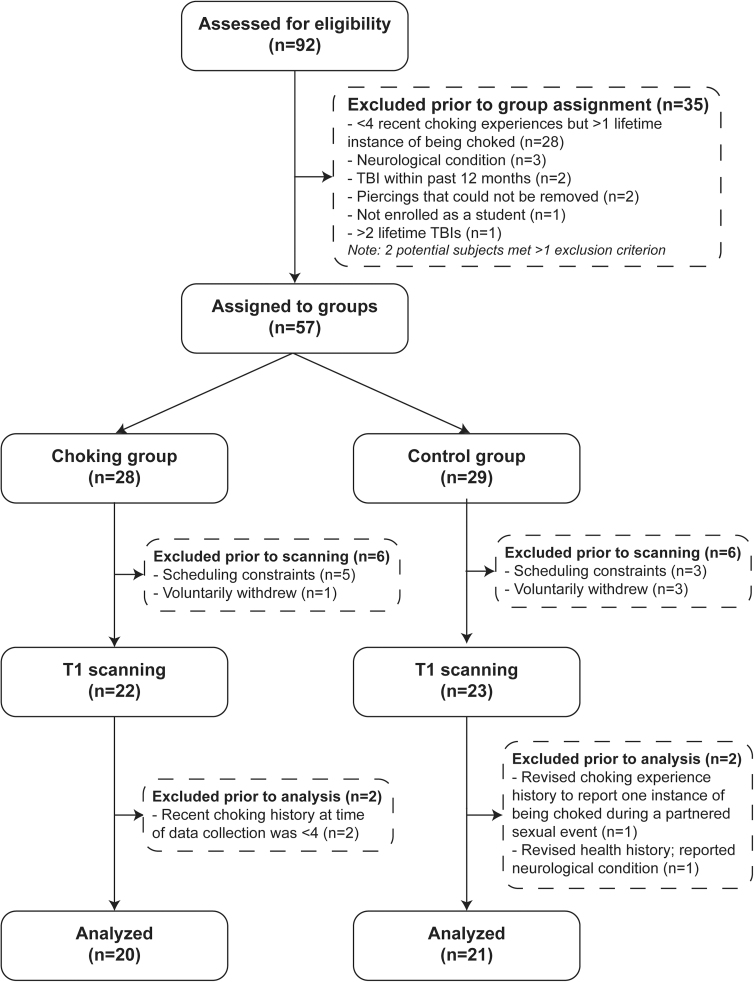
A total of 92 participants were screened for eligibility, and 57 participants who met the inclusion criteria and were free of the exclusion criteria were assigned to either the choking group (n = 28) or the choking-naïve group (n = 29), respectively. We were unable to obtain MRI data from 12 participants (choking n = 6; choking-naïve n = 6) because of claustrophobia or scheduling conflicts, and 2 participants were retroactively excluded from each group for either not being free of the exclusion criteria or not meeting all the inclusion criteria upon reexamination of their questionnaire responses. As a result, a total of 41 participants (choking n = 20; choking-naïve n = 21) contributed to the rs-FC analysis. See Figure 1 for the study flow.
FIG. 1.
Study flowchart. TBI, traumatic brain injury.
The participants in the choking group had been choked during sex on average 10, 19, and 46 times in the last 30 days, 60 days, and 12 months, respectively (Table 1). Additionally, we observed several demographic differences between groups. The choking-naïve group was significantly older than the choking group, and the choking group reported higher scores for the AUDIT questionnaire compared with scores of the choking-naïve group. The choking group included more racially diverse participants than did the choking-naïve group (Table 1). These demographic variables were included in the model as covariates.
Table 1.
Demographic Characteristics
| Variables | Choking | Choking-naïve | P-value |
|---|---|---|---|
| N | 20 | 21 | |
| Age, years, mean ± SD | 21.1 ± 1.9 | 23.3 ± 3.1 | 0.009 |
| Race, n (%)a | 0.037 | ||
| White | 14 (63) | 19 (91) | |
| Black/African American | 4 (18) | 0 (0) | |
| Asian | 3 (13) | 2 (9) | |
| American Indian/Alaskan Native | 1 (5) | 0 (0) | |
| Ethnicity, n (%) | |||
| Non-Latino/Hispanic | 18 (90) | 18 (86) | 0.999 |
| Latino/Hispanic | 2 (10) | 3 (14) | |
| Choking experiences, n, mean ± SD | |||
| Last 30 days | 10.25 ± 7.39 | 0 | |
| Last 60 days | 19.60 ± 11.86 | 0 | |
| Last 12 months | 46.10 ± 27.78 | 0 | |
| Mental health and alcohol use scales, mean ± SD | |||
| PHQ-9 | 5.75 ± 4.36 | 4.14 ± 5.29 | 0.294 |
| GAD-7 | 6.25 ± 3.92 | 4.05 ± 3.72 | 0.073 |
| AUDIT | 5.85 ± 4.44 | 2.85 ± 2.26 | 0.012 |
Several individuals in the choking group indicated that they identified as more than one race/ethnicity, so the percentages add up to more than 100%.
AUDIT, Alcohol Use Disorders Identification Test; GAD-7, the Generalized Anxiety Disorder Assessment; PHQ-9, Patient Health Questionnaire-9 depression scale; SD, standard deviation.
Cortical surface functioning assessed by ALFF and ReHo between groups
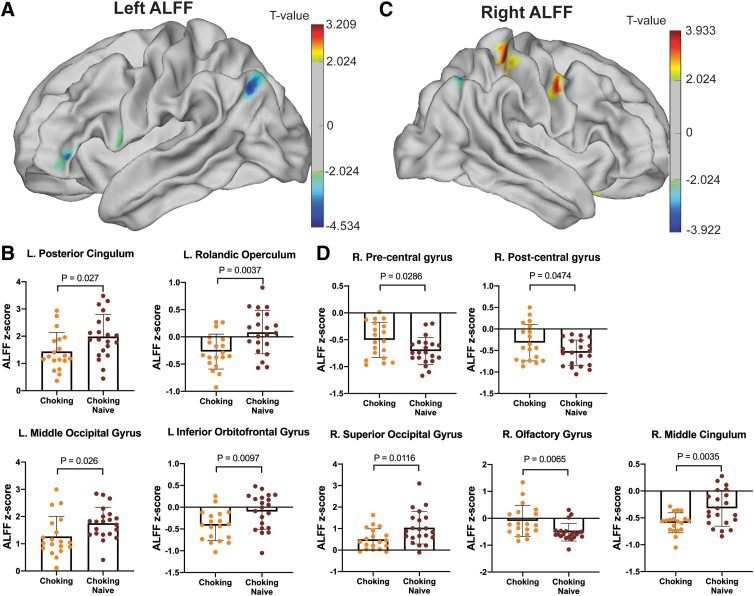
Significant group differences in ALFF intensity were detected in both hemispheres (Fig. 2A,C). For example, the choking group showed lower ALFF in the left inferior orbitofrontal gyrus, left Rolandic operculum, and right middle cingulum (choking < choking-naïve: Fig. 2B) and higher ALFF in the right olfactory gyrus (choking > choking-naïve: Fig. 2D).
FIG. 2.
Regional differences in ALFF between groups. (A) ALFF levels in the left hemisphere showed group differences in four cortical regions (B) including the posterior cingulum, Rolandic operculum, middle occipital gyrus, and inferior orbitofrontal gyrus. (C) ALFF levels in the right hemisphere, in which five cortical regions showed group differences, such as the pre/post-central gyri, superior occipital gyrus, olfactory gyrus, and middle cingulum (D). ALFF, amplitude of low-frequency fluctuation.
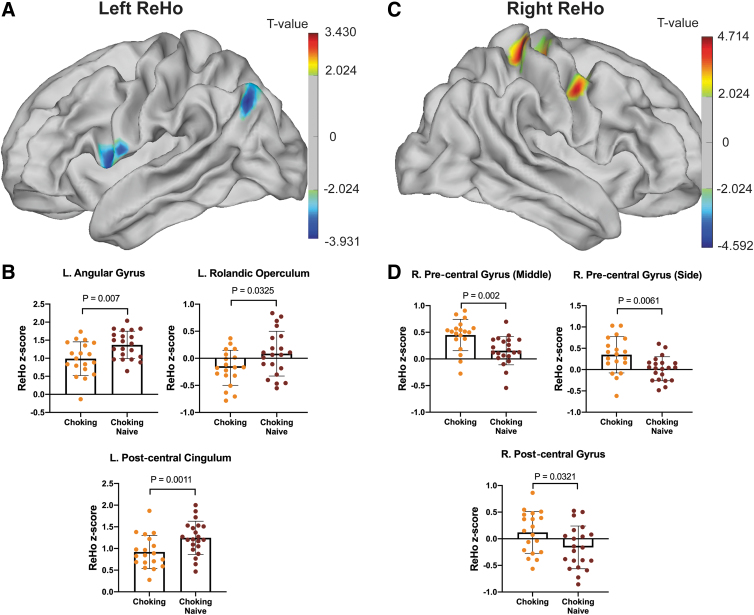
Similar to the ALFF data, in general, the choking group showed lower ReHo in the left hemisphere but higher ReHo in the right hemisphere (Fig. 3A,C). Specifically, the choking group exhibited significantly lower ReHo in the left angular gyrus, postcentral cingulum, and Rolandic operculum compared with the choking-naïve group (Fig. 3B). Conversely, significantly higher ReHo was detected in the right pre-central and post-central gyri of the choking group compared with the choking-naïve group (Fig. 3D). See Table 2 for peak ALFF and ReHo intensity and coordinates.
FIG. 3.
Regional differences in ReHo between groups. (A) ReHo levels in the left hemisphere showed group differences in three cortical regions (B) including the angular gyrus, Rolandic operculum, and post-central cingulum. (C) ReHo levels in the right hemisphere, in which two cortical regions showed group differences, such as the pre/post-central gyri (D). ReHo, regional homogeneity.
Table 2.
Differences of ALFF and ReHo Between Choking and Choking-Naïve Participants
| Cluster | Brain regions | Peak (MNI) |
Voxel size | Peak t | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ALFF | ||||||
| Left hemisphere | ||||||
| Cluster 1 | Middle occipital gyrus | -40 | -67 | 36 | 163 | -4.522 |
| Cluster 2 | Posterior cingulum | -9 | -46 | 30 | 114 | -3.206 |
| Cluster 3 | Rolandic operculum | -50 | 5 | 10 | 122 | -2.601 |
| Cluster 4 | Inferior orbitofrontal gyrus | -49 | 35 | -3 | 123 | -3.922 |
| Right hemisphere | ||||||
| Cluster 1 | Post-central gyrus | 33 | -35 | 62 | 299 | 3.831 |
| Cluster 2 | Pre-central gyrus | 22 | -24 | 57 | 201 | 3.410 |
| Cluster 3 | Olfactory gyrus | 13 | 14 | -14 | 133 | 3.881 |
| Cluster 4 | Middle cingulum | 14 | 24 | 33 | 138 | -3.452 |
| Cluster 5 | Superior occipital gyrus | 32 | -64 | 41 | 140 | -3.445 |
| ReHo | ||||||
| Left hemisphere | ||||||
| Cluster 1 | Post-central cingulum | -9 | -44 | 31 | 306 | -3.122 |
| Cluster 2 | Rolandic operculum | -51 | 7 | 3 | 232 | -3.412 |
| Cluster 3 | Angular gyrus | -40 | -68 | 37 | 173 | -3.890 |
| Right hemisphere | ||||||
| Cluster 1 | Post-central gyrus | 29 | -34 | 65 | 356 | 4.021 |
| Cluster 2 | Pre-central gyrus | 22 | -24 | 57 | 361 | 3.482 |
| Cluster 3 | Pre-central gyrus | 49 | -6 | 41 | 242 | 4.095 |
The multiple comparison correction for ALFF was used by the FWE correction with Monte Carlo simulation p < 0.05 and cluster size >107. The multiple comparison correction for ReHo was used by the FWE correction with Monte Carlo simulation p < 0.05 and cluster size >171.
ALFF, amplitude of low-frequency fluctuation; FEW, family-wise error; MNI, Montreal Neurological Institute; ReHo, regional homogeneity.
Group differences in static rs-FC
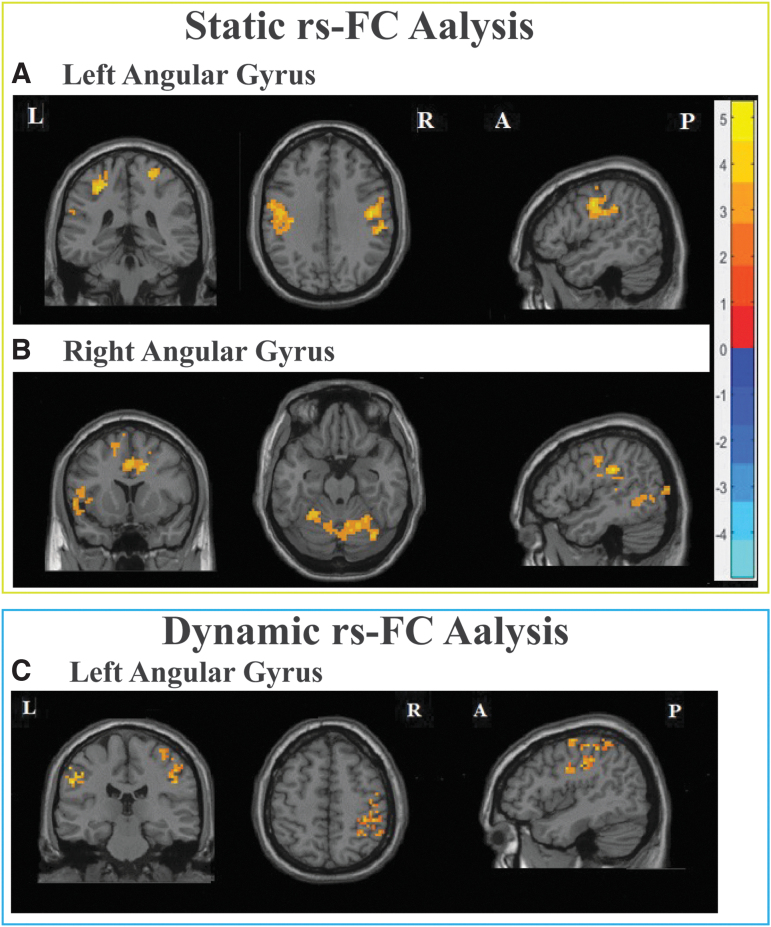
Our analysis revealed a significant group difference in the functional connectivity between the angular gyrus and various parts of the brain. Specifically, compared with the choking-naïve group, the choking group showed hyperconnectivity (higher connectivity): 1) between the left angular gyrus and bilateral postcentral gyrus, left Rolandic operculum, and right superior frontal gyrus; and 2) between the right angular gyrus and the left angular gyrus, post-central gyrus, and insula, as well as the right Rolandic operculum, lingual, and superior temporal gyrus. The visual output is shown in Figure 4A and 4B, and the detailed results are shown in Table 3. Other ROIs, including bilateral DLPFC and posterior cingulate cortex, did not show any statistically significant group differences in rs-FC.
FIG. 4.
The static rs-FC analysis revealed an increased functional connectivity in the choking group compared with the choking-naïve group between the bilateral angular gyrus (A,B) and various parts of the brain, such as the post-central gyrus and lingual gyrus. The dynamic rs-FC analysis also identified increased connectivity between the left angular gyrus and the post-central gyrus in the choking group compared with the choking-naïve group (C). Multiple comparisons were corrected by threshold-free cluster enhancement (TFCE), and the level of statistical significance was set at two-tailed p < 0.008. The number of permutations was set at 1000. A, anterior; L, left; P, posterior; R, right; rs-FC, resting-state functional connectivity; TFCE, threshold-free cluster enhancement.
Table 3.
Static and Dynamic rs-FC Differences Between Choking and Choking-Naïve Participants
| Analysis method | ROI seeds | Cluster location | BA | Peak (MNI) |
Cluster size | t | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Static rs-FC | Left angular gyrus | Left Rolandic operculum | 48 | -48 | -6 | 9 | 125 | 4.962 |
| Left postcentral gyrus | 3 | -51 | -18 | 39 | 315 | 5.386 | ||
| Left postcentral gyrus | 40 | -33 | -36 | 48 | 48 | 4.976 | ||
| Left precentral gyrus | 6 | -33 | -9 | 51 | 32 | 3.984 | ||
| Right superior frontal gyrus | 6 | 21 | -12 | 63 | 12 | 5.197 | ||
| Right postcentral gyrus | 4 | 48 | -12 | 39 | 321 | 5.111 | ||
| Right postcentral gyrus | 2 | 24 | -39 | 66 | 58 | 4.536 | ||
| Right angular gyrus | Left angular gyrus | 39 | -39 | -51 | 21 | 45 | 4.107 | |
| Left postcentral gyrus | 40 | -33 | -36 | 51 | 248 | 4.097 | ||
| Left insula | 48 | -48 | 6 | 6 | 221 | 4.469 | ||
| Left middle temporal gyrus | 21 | -51 | -33 | -6 | 52 | 3.739 | ||
| Left fusiform gyrus | 37 | -60 | -63 | -3 | 20 | 3.698 | ||
| Right superior temporal gyrus | 41 | 42 | -33 | 12 | 21 | 4.015 | ||
| Right superior temporal gyrus | 42 | 54 | -30 | 18 | 31 | 3.558 | ||
| Right Rolandic operculum | 48 | 48 | -27 | 30 | 248 | 4.328 | ||
| Right precuneus | 5 | 12 | -54 | 66 | 109 | 3.835 | ||
| Right lingual gyrus | 19 | 21 | -57 | -6 | 2096 | 4.463 | ||
| Dynamic rs-FC | Left angular gyrus | Left postcentral gyrus | 4 | -60 | -21 | 39 | 95 | 5.471 |
| Right postcentral gyrus | 3 | 36 | -36 | 57 | 363 | 4.852 | ||
| Right postcentral gyrus | 1 | 30 | -42 | 69 | 11 | 3.637 | ||
The multiple comparison correction was used with the threshold-free cluster enhancement (TFCE), which was tested at two-tailed p < .05; the number of permutations was set at 1,000. BA, Brodmann area; MNI, Montreal Neurological Institute; ROI, region of interest; rs-FC, resting-state functional connectivity.
Group differences in dynamic rs-FC
The results of the static rs-FC analysis were corroborated by the dynamic rs-FC analysis. Compared with the choking-naïve group, the choking group showed hyperconnectivity between the left angular gyrus and the bilateral post-central gyrus (Table 3 and Fig. 4C). However, no significant group differences in connectivity between the right angular gyrus and whole brain were observed.
Discussion
The current study presents a potential interaction between repetitive sexual choking and neurophysiological alterations. There were two major findings. First, we noted significant differences in neural activation patterns between groups, in which the choking group exhibited significantly lower ALFF and ReHo in the left hemisphere (e.g., Rolandic operculum, angular gyrus) and higher ALFF and ReHo in the right hemispheres (e.g., pre/post-central gyri) compared with the choking-naïve group. Second, relative to the choking-naïve group, the choking group showed hyperconnectivity between the angular gyrus and widespread brain regions. The dynamic rs-FC analysis further validated the findings from the static analysis, by demonstrating a significant hyperconnectivity between the left angular gyrus and the bilateral post-central gyrus in the choking group compared with the choking-naïve group. These data suggest that frequent choking/strangulation during sex may associate with a neuronal activation pattern distinct from that for women without sexual choking exposure.
To interpret the data, it is important to revisit the theoretical basis of our fMRI metrics. ALFF and ReHo gauge neuronal activity within brain regions, whereas functional connectivity reflects the strengths of the relationship in neuronal activities between brain regions.18 ALFF reveals the density of the BOLD signal, whereas ReHo measures the synchrony of the BOLD signal between adjacent regions, reflecting the coherence of regional brain activity.16 Intriguingly, we observed hemisphere-dependent differences in ALFF and ReHo, such that the choking group showed lower neuronal activity (↓ALFF) and coherence (↓ReHo) in the left brain regions relative to those of the choking-naïve group. Contrarily, in the right brain regions that are important for motor control and somatosensory reception (pre/post-central gyri), the choking group showed higher neuronal activity (↑ALFF) and coherence (↑ReHo) compared with the choking-naïve group, possibly as the result of receiving high volumes of incoming signals.
This type of inter-hemispheric imbalance has been shown to associate with declines in mental health. For example, patients with depressive disorder exhibit hemispheric asymmetries, characterized by a hypoactive left hemisphere and a hyperactive right hemisphere.48,49 Unilateral brain lesion studies substantiate this notion: patients with tumors, ischemic injury, or an epileptogenic zone in the left hemisphere also frequently have depressed mood, whereas similar lesions in the right hemisphere cause euphoria.50–53 In our study, there was no significant group difference in depression or anxiety symptom scores, which may have been because the COVID-19 pandemic non-discriminately affected the mental health of all participants. However, our pre-pandemic survey data showed that women who had been choked more than 5 times in their lifetime during sex were twice as likely to report symptoms related to depression, anxiety, sadness, and loneliness as were their choking-naïve counterparts.13
ALFF and ReHo data are useful for explaining the rs-FC findings. Lower ReHo in the left angular gyrus of the choking group indicates less coherent or irregular neural activation. Hence, the angular gyrus requires more than normal energy to operate. The angular gyrus is a hub of brain networks because of its location at the junction of the visual, auditory, and somatosensory cortices. Thus, the angular gyrus is known to be involved in attention, self-processing, semantic information processing, emotional regulation, and mentalizing.38 Partly because of this anatomical uniqueness and extensive association network, the choking group exhibited elevated functional connectivity between the angular gyrus and brain regions that are important for sound processing, object recognition, consciousness and emotion, and somatosensory and motor control. The dynamic rs-FC analysis also validated the hyperconnective relationship between the left angular gyrus and post-central gyrus in the choking group. This rs-FC result is consistent with the ReHo data showing that the angular gyrus (seed region) is experiencing reduced neural coherence (↓ReHo) and thus sends abnormal/imbalanced signals to target regions, particularly the post-central gyrus. To sort out a high volume of input, the post-central gyrus became more coherent, as reflected in higher ReHo.
Another point to consider is that activation of the angular gyrus has been shown to be closely related to one's mental state. This is exemplified in patients with progressive mild cognitive impairment54 and coronary artery disease55 who exhibit significant hyperactivation of the angular gyrus when they are subjected to mentally stressful tasks (e.g., serial arithmetic subtraction, recall tasks). Further, research on strangulation occurring in other contexts, such as intimate partner violence (IPV), may also aid in the interpretation of the widespread alterations in connectivity stemming from the angular gyrus. A recent study by Valera and colleagues56 evaluated cognitive function and psychological well-being in women with a history of IPV-related strangulation compared with women with IPV but no strangulation. The data indicated that the women with IPV-related strangulation exhibited significantly worse memory function and higher symptoms of depression and post-traumatic stress disorder than did their counterparts without strangulation. Smith and associates59 also found that physical, neurological, and psychological symptoms increase in relation to the frequency of strangulation history in women who have experienced IPV. Further exploration is warranted to examine whether alterations in consciousness or other physical responses due to sexual choking are predictive of neurophysiological and psychological alterations. Nonetheless, these data underscore the clinically observable effects of strangulation and that our ALFF/ReHo and rs-FC data provide the potential neurophysiological underpinning of the effects of sexual choking/strangulation.
It is important to mention that angular gyrus syndrome is a constellation of neuropsychological deficits including agraphia, aphasia, manifestations of depression, poor memory, and irritability.58,59 The most common cause of angular gyrus syndrome is cerebrovascular disease, especially occlusion of the angular branch of the middle cerebral artery.58 Although the duration of being choked during sex varied between participants and such data are difficult to validate, it is plausible that the angular gyrus may have a reduced resiliency to ischemic/reperfusion stress as the result of frequent exposure to strangulation. Further exploration is warranted to examine whether alterations in consciousness or other physical responses due to sexual choking/strangulation are predictive of neurophysiological and psychological alterations.
Despite research in brain trauma indicating that the DLPFC and cingulate cortex are impacted by head injury,39,41,60 our data showed no significant group differences in any of the fMRI metrics in either of the regions. The reason for this observation is difficult to articulate with our data derived from a cross-sectional design. One possibility may be that the mechanism of strangulation during sex is vastly different from blunt trauma, such as TBI, the effects of which are dispersed in diffusive areas of the brain. That may be why the angular gyrus, which is a hub of the brain network and the structural intersection between several cortical regions, showed group differences in neurophysiological outputs. Additionally, many other factors may play a role in exacerbating the effects of sexual choking/strangulation, such as the intensity and duration of strangulation, the method of strangulation (e.g., one or both hands, limb, ligature), the presence of IPV in a relationship, whether consent was given prior to being strangled during sex, and the time intervals between each strangulation event. A longitudinal investigation using multi-modal assessments is needed to delineate whether this hyperconnectivity in the angular network is negligible or pathological and to what extent it is related to elevated mental illness symptoms in young adult women with frequent exposure to sexual choking/strangulation.13 Future study is also warranted to test what aspects of motor control are excited or inhibited in these women using, for example, transcranial magnetic stimulation.
Limitations
There are several limitations to this study. Despite our best efforts to conduct a demographically matched case-control recruitment, the groups differed in age. We were unable to recruit age-matched control participants, due in part to the COVID-19 pandemic, which presented significant difficulty in recruiting enough participants to match ages between groups. Instead, we focused on ensuring that the control group consisted of individuals with no lifetime sexual strangulation experience, and we controlled our analyses for age, race, and AUDIT scores. Self-reported choking/strangulation behaviors vary in frequency, intensity, and duration, which are subject to recall bias in survey responses. Given the infancy stage of this research topic, our inclusion criterion of 4 or more choking/strangulation experiences in the past 30 days was established non-empirically. Instead, our criterion reflects weekly exposure in the past 30 days. Nevertheless, only 1 of 20 participants in the choking group experienced being choked/strangled 4 times; the rest experienced greater frequency. A study with a larger sample size is needed to explore the potential dose-dependent effects of sexual choking/strangulation.
Further, given the nature of our non-interventional design, the time since the last strangulation event was not controlled; therefore, the potential that acute strangulation effects might have contaminated the observed results cannot be eliminated. Lastly, comprehensive neuropsychiatric and behavioral evaluations, including measures of yearning and loneliness, should be incorporated into future studies to assess the clinical implications of the neurophysiological impact of sexual choking/strangulation. To study both acute and chronic effects of strangulation during sex, a longitudinal study is needed to closely monitor the incidence of strangulation events, followed by prompt assessments.
Conclusions
Our data suggest that repetitive sexual choking/strangulation may be associated with neurophysiological alterations. Our unbiased, rigorous analysis approach revealed that women who were frequently choked during sex exhibited inter-hemispheric imbalance in neural activity and hyperconnectivity between the angular gyrus and various brain regions related to motor control, consciousness, emotion, and somatosensory function. A longitudinal investigation using multi-modal assessments is needed to clarify the acute and chronic neurological consequences of strangulation during sex.
Transparency, Rigor, and Reproducibility Statement
This cross-sectional study included college-aged women with frequent (4+) sexual choking in the past month and choking-naïve control women. A sample size of 20 per group was planned to yield 80% power to detect a statistically significant group effect in ALFF/ReHo with a p-value <0.05. Ninety-two potential participants were screened, imaging data were obtained from 55, and imaging data were successfully analyzed in 41. Participants were blinded regarding any study information, including the final outcome, and will be referred to the publication of the article when it becomes available. Imaging acquisition and analyses were performed by team members blinded to relevant characteristics of the participants and group assignment. All equipment and software used to perform imaging and preprocessing are widely available from commercial sources. De-identified data from this study and analytic code are available upon reasonable request to the corresponding author (KK).
Supplementary Material
Acknowledgments
The authors would like to thank the participants who contributed their time and effort. The authors would also like to thank Jennifer Holmes, ELS, for language editing. A series of research articles from this study has been and will be presented in scientific conferences and journal publications. A portion of this article was previously submitted as a preprint (Chronic elevation of serum S100B but not neurofilament-light due to frequent choking/strangulation during sex in young adult women. Isabella L. Alexander, Megan E. Huibregtse, Tsung-Chieh Fu, Lillian M. Klemsz, J. Dennis Fortenberry, Debby Herbenick, and Keisuke Kawata. medRxiv 2021.11.01.21265760; doi: https://doi.org/10.1101/2021.11.01.21265760).
Authors' Contributions
KK, DH, JDF, and MEH conceptualized and designed the study; ILA, MEH, and LMK collected the data; JH and KK analyzed the data; JH, ILA, MEH, LMK, TCF, JDF, DH, and KK interpreted the data; JH and KK drafted the manuscript; JH, MEH, KK, LMK, TCF, JDF, and DH critically revised the manuscript for important intellectual content; all authors contributed to the final manuscript and interpretation of the final results.
Funding Information
This publication was made possible with support from the Indiana University School of Public Health (D. Herbenick), the Indiana Clinical and Translational Sciences Institute TL1 Pre-Doctoral Training Award (M.E. Huibregtse; grant # UL1TR002529 [S. Moe and S. Wiehe, co-PIs], 5/18/2018–4/30/2023 from the National Institutes of Health/National Center for Advancing Translational Sciences [NIH/NCATS], Clinical and Translational Sciences Award). This work was also partly supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS; 1R01NS113950; K. Kawata). The funding sources had no role in the design or execution of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Koolschijn PC, Crone EA. Sex differences and structural brain maturation from childhood to early adulthood. Dev Cogn Neurosci 2013;5:106–118; doi: 10.1016/j.dcn.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health 2009;45(3):216–221; doi: 10.1016/j.jadohealth.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hensel DJ, Fortenberry JD. Life-Span Sexuality through a Sexual Health Perspective. In: APA Handbook of Sexuality and Psychology. (Tolman LM, Diamond JA, Bauermeister WH, et al. eds.) American Psychological Association: 2014; pp. 385–413. [Google Scholar]
- 4. Herbenick D, Fu TC, Valdivia DS, et al. What is rough sex, who does it, and who likes it? findings from a probability sample of U.S. undergraduate students. Arch Sex Behav 2021; doi: 10.1007/s10508-021-01917-w [DOI] [PubMed] [Google Scholar]
- 5. Sauvageau A, Racette S. Autoerotic deaths in the literature from 1954 to 2004: a review. J Forensic Sci 2006;51(1):140–146; doi: 10.1111/j.1556-4029.2005.00032.x [DOI] [PubMed] [Google Scholar]
- 6. Wright PJ, Herbenick D, Tokunaga RS. Pornography consumption and sexual choking: an evaluation of theoretical mechanisms. Health Commun 2021;1–12; doi: 10.1080/10410236.2021.1991641. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 7. Wright PJ, Herbenick D, Tokunaga RS. Pornography and women's experience of mixed-gender sexual choking/strangulation: eroticization mediates, perceived similarity moderates. J Health Commun 2022;27(3):173–182; doi: 10.1080/10810730.2022.2073406 [DOI] [PubMed] [Google Scholar]
- 8. Herbenick D, Patterson C, Beckmeyer J, et al. Diverse sexual behaviors in undergraduate students: findings from a campus probability survey. J Sex Med 2021;18(6):1024–1041; doi: 10.1016/j.jsxm.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 9. Wright PJ, Sun C, Steffen NJ, et al. Pornography, alcohol, and male sexual dominance. Commun Monographs 2015;82(2):252–270. [Google Scholar]
- 10. Sun CF, Wright P, Steffen N. German heterosexual women's pornography consumption and sexual behavior. Sex Media Soc 2017;1–12; doi: oi: 10.1177/2374623817698113 [DOI] [Google Scholar]
- 11. Herbenick D, Guerra-Reyes L, Patterson C, et al. “It was scary, but then it was kind of exciting”: young women's experiences with choking during sex. Arch Sex Behav 2021;51(2):1103–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herbenick D, Bartelt E, Fu TJ, et al. Feeling scared during sex: findings from a U.S. probability sample of women and men ages 14 to 60. J Sex Marital Ther 2019;45(5):424–439; doi: 10.1080/0092623X.2018.1549634 [DOI] [PubMed] [Google Scholar]
- 13. Herbenick D, Fu TC, Kawata K, et al. Non-fatal strangulation/choking during sex and its associations with mental health: findings from an undergraduate probability survey. J Sex Marital Ther 2021;1–13; doi: 10.1080/0092623X.2021.1985025 [DOI] [PubMed] [Google Scholar]
- 14. De Boos J. Review article: non-fatal strangulation: hidden injuries, hidden risks. Emerg Med Australas 2019;31(3):302–308; doi: 10.1111/1742-6723.13243 [DOI] [PubMed] [Google Scholar]
- 15. Yan CG, Wang XD, Lu B. DPABISurf: data processing & analysis for brain imaging on surface. Sci Bull 2021;66(24):2453–2455. [DOI] [PubMed] [Google Scholar]
- 16. Lv H, Wang Z, Tong E, et al. Resting-state functional MRI: everything that nonexperts have always wanted to know. AJNR Am J Neuroradiol 2018;39(8):1390–1399; doi: 10.3174/ajnr.A5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 2006;103(37):13848–13853; doi: 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007;8(9):700–711; doi: 10.1038/nrn2201 [DOI] [PubMed] [Google Scholar]
- 19. Liegeois R, Li J, Kong R, et al. Resting brain dynamics at different timescales capture distinct aspects of human behavior. Nat Commun 2019;10(1):2317; doi: 10.1038/s41467-019-10317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32(9):509–5015; doi: 10.3928/0048-5713-20020901-06 [DOI] [Google Scholar]
- 21. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282(18):1737–1744; doi: 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry 2010;32(4):345–359; doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 23. Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166(10):1092–1097; doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 24. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998;158(16):1789–1795; doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- 25. Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol 1995;56(4):423–432; doi: 10.15288/jsa.1995.56.423 [DOI] [PubMed] [Google Scholar]
- 26. The fMRIPrep developers. Processing pipeline details. 2021. Available from: https://fmriprep.org/en/latest/workflows.html [Last accessed October 2].
- 27. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 1999;9(2):179–194; doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 28. Liu CH, Kung YY, Yeh TC, et al. Differing spontaneous brain activity in healthy adults with two different body constitutions: a resting-state functional magnetic resonance imaging study. J Clin Med 2019;8(7); doi: 10.3390/jcm8070951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan CG, Wang XD, Zuo XN, et al. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016;14(3):339–351; doi: 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- 30. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010;4:13; doi: 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Friston KJ, Williams S, Howard R, et al. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35(3):346–355; doi: 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- 32. Hu J, Du J, Xu Q, et al. Dynamic network analysis reveals altered temporal variability in brain regions after stroke: a longitudinal resting-state fMRI study. Neural Plast 2018;2018:9394156; doi: 10.1155/2018/9394156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun Y, Collinson SL, Suckling J, et al. Dynamic reorganization of functional connectivity reveals abnormal temporal efficiency in schizophrenia. Schizophr Bull 2019;45(3):659–669; doi: 10.1093/schbul/sby077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xiao F, An D, Lei D, et al. Real-time effects of centrotemporal spikes on cognition in rolandic epilepsy: an EEG-fMRI study. Neurology 2016;86(6):544–551; doi: 10.1212/WNL.0000000000002358 [DOI] [PubMed] [Google Scholar]
- 35. Zhou Q, Zhang L, Feng J, et al. Tracking the main states of dynamic functional connectivity in resting state. Front Neurosci 2019;13:685; doi: 10.3389/fnins.2019.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lyoo IK, Kim JE, Yoon SJ, et al. The neurobiological role of the dorsolateral prefrontal cortex in recovery from trauma. Longitudinal brain imaging study among survivors of the South Korean subway disaster. Arch Gen Psychiatry 2011;68(7):701–713; doi: 10.1001/archgenpsychiatry.2011.70 [DOI] [PubMed] [Google Scholar]
- 37. Jumah FR, Dossani RH. Neuroanatomy, Cingulate Cortex. In: StatPearls [Internet]. StatPearls Publishing: Treasure Island,FL; 2022. [PubMed] [Google Scholar]
- 38. Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 2013;19(1):43–61; doi: 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yount R, Raschke KA, Biru M, et al. Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci 2002;14(4):416–423; doi: 10.1176/jnp.14.4.416 [DOI] [PubMed] [Google Scholar]
- 40. Fremont R, Dworkin J, Manoochehri M, et al. Damage to the dorsolateral prefrontal cortex is associated with repetitive compulsive behaviors in patients with penetrating brain injury. BMJ Neurol Open 2022;4(1):e000229; doi: 10.1136/bmjno-2021-000229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hocke LM, Duszynski CC, Debert CT, et al. Reduced functional connectivity in adults with persistent post-concussion symptoms: a functional near-infrared spectroscopy study. Journal of neurotrauma 2018;35(11):1224–1232; doi: 10.1089/neu.2017.5365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vergara VM, Mayer AR, Damaraju E, et al. Detection of mild traumatic brain injury by machine learning classification using resting state functional network connectivity and fractional anisotropy. J Neurotrauma 2017;34(5):1045–1053; doi: 10.1089/neu.2016.4526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen J, Sun D, Shi Y, et al. Dynamic alterations in spontaneous neural activity in multiple brain networks in subacute stroke patients: a resting-state fMRI study. Front Neurosci 2018;12:994; doi: 10.3389/fnins.2018.00994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hindriks R, Adhikari MH, Murayama Y, et al. Corrigendum to “Can sliding-window correlations reveal dynamic functional connectivity in resting-state fMRI?” [NeuroImage 127 (2016) 242–256]. Neuroimage 2016;132:115; doi: 10.1016/j.neuroimage.2016.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yip E, Yun J, Wachowicz K, et al. Sliding window prior data assisted compressed sensing for MRI tracking of lung tumors. Med Phys 2017;44(1):84–98; doi: 10.1002/mp.12027 [DOI] [PubMed] [Google Scholar]
- 46. Weng Y, Qi R, Chen F, et al. The temporal propagation of intrinsic brain activity associate with the occurrence of PTSD. Front Psychiatry 2018;9:218; doi: 10.3389/fpsyt.2018.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage 2013;80:360–78; doi: 10.1016/j.neuroimage.2013.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res 2010;68(2):77–87; doi: 10.1016/j.neures.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 49. Grimm S, Beck J, Schuepbach D, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry 2008;63(4):369–376; doi: 10.1016/j.biopsych.2007.05.033 [DOI] [PubMed] [Google Scholar]
- 50. Perini GI. Emotions and personality in complex partial seizures. Psychother Psychosom 1986;45(3):141–148; doi: 10.1159/000287940 [DOI] [PubMed] [Google Scholar]
- 51. Belyi BI. Mental impairment in unilateral frontal tumours: role of the laterality of the lesion. Int J Neurosci 1987;32(3–4):799–810; doi: 10.3109/00207458709043334 [DOI] [PubMed] [Google Scholar]
- 52. Vataja R, Pohjasvaara T, Leppavuori A, et al. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry 2001;58(10):925–931; doi: 10.1001/archpsyc.58.10.925 [DOI] [PubMed] [Google Scholar]
- 53. Carran MA, Kohler CG, O'Connor MJ, et al. Mania following temporal lobectomy. Neurology 2003;61(6):770–774; doi: 10.1212/01.wnl.0000086378.74539.85 [DOI] [PubMed] [Google Scholar]
- 54. Corriveau-Lecavalier N, Mellah S, Clement F, et al. Evidence of parietal hyperactivation in individuals with mild cognitive impairment who progressed to dementia: a longitudinal fMRI study. Neuroimage Clin 2019;24:101958; doi: 10.1016/j.nicl.2019.101958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Soufer R, Bremner JD, Arrighi JA, et al. Cerebral cortical hyperactivation in response to mental stress in patients with coronary artery disease. Proc Natl Acad Sci U S A 1998;95(11):6454–6459; doi: 10.1073/pnas.95.11.6454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Valera EM, Daugherty JC, Scott OC, et al. Strangulation as an acquired brain injury in intimate-partner violence and its relationship to cognitive and psychological functioning: a preliminary study. J Head Trauma Rehabil 2022;37(1):15–23; doi: 10.1097/HTR.0000000000000755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smith DJ Jr., Mills T, Taliaferro EH.. Frequency and relationship of reported symptomology in victims of intimate partner violence: the effect of multiple strangulation attacks. J Emerg Med 2001;21(3):323–329; doi: 10.1016/s0736-4679(01)00402-4 [DOI] [PubMed] [Google Scholar]
- 58. Perry DC. Angular Gyrus Syndrome. In: Encyclopedia of the Neurological Sciences (2nd ed.); 2014;192–193; doi: 10.1016/B978-0-12-385157-4.00438-3 [DOI] [Google Scholar]
- 59. Nagaratnam N, Phan TA, Barnett C, et al. Angular gyrus syndrome mimicking depressive pseudodementia. J Psychiatry Neurosci 2002;27(5):364–368. [PMC free article] [PubMed] [Google Scholar]
- 60. Terneusen A, Winkens I, van Heugten C, et al. Neural correlates of impaired self-awareness of deficits after acquired brain injury: a systematic review. Neuropsychol Rev 2022; doi: 10.1007/s11065-022-09535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.