Abstract
Injuries to the central nervous system (CNS) often lead to severe neurological dysfunction and even death. However, there are still no effective measures to improve functional recovery following CNS injuries. Optogenetics, an ideal method to modulate neural activity, has shown various advantages in controlling neural circuits, promoting neural remapping, and improving cell survival. In particular, the emerging technique of optogenetics has exhibited promising therapeutic methods for CNS injuries. In this review, we introduce the light-sensitive proteins and light stimulation system that are important components of optogenetic technology in detail and summarize the development trends. In addition, we construct a comprehensive picture of the current application of optogenetics in CNS injuries and highlight recent advances for the treatment and functional recovery of neurological deficits. Finally, we discuss the therapeutic challenges and prospective uses of optogenetics therapy by photostimulation/photoinhibition modalities that would be suitable for clinical applications.
Keywords: central nervous system injuries, functional recovery, optogenetics, therapeutic strategies
Introduction
The central nervous system (CNS) is sensitive to damages from external shock and internal environmental changes. Injuries to the CNS may result in permanent functional deficits in patients. The self-repair capability of the CNS is very limited and relies on effective intervention.1,2 Some therapeutic strategies have been developed to intervene in these neurological events and focus on functional rehabilitation,1 such as gene therapy,3 nanotherapy,4 and cell replacement therapy.5 However, they are still faced with a series of clinical translational barriers and innovative therapeutic methods are required.
Recently, the optogenetic technique has opened up entirely new experimental approaches for neurosciences owing to its targeting specificity and temporally precise controllability by the integration of optics, genetics, and virology.6 The technology uses genetics to express light-sensitive channel proteins (e.g., opsins) in specific cells, enabling light to activate or inhibit neuronal activity. The principles that the activation or inhibition of neuronal activity refer to changing the permeability of different channel proteins to cations and anions for regulating action potential. Through the artificial transformation of target cells, the function and behavior of cells can be regulated by external light stimulation. The development of novel opsins enables more delicate modulation of cellular signaling, and the continuous refinement of a light stimulation system allows optogenetics to incorporate more research models. The combinations of optogenetics and assistive technologies, such as two-photon fluorescence microscopy,7 genetically encoded fluorescent calcium ion indicators (GECIs),8 and genetically encoded fluorescent voltage indicators (GEVIs),9 make it possible to observe and record cell signals varying in real time.
These make optogenetics suitable for neural circuit studies, such as in epilepsy,10 psychiatric disorders,11 and cognitive impairments.12 However, the use of optogenetics for CNS injuries is still in its infancy, and current studies should be analyzed for exploring further research value. Here, we review recent advances in optogenetics and construct a comprehensive picture of the current application in CNS injuries. The potential shortcomings and future directions are also discussed.
Fundamentals of Optogenetics
After nearly 2 decades of development, optogenetics technology has evolved into a matured tool for neuroscience research. Particularly, the light-sensitive proteins and corresponding light stimulation device that are crucial for optogenetics have made rapid progress.
Opsins
Classification of opsins
According to the gene and source of opsin, they can be divided into two families: type I (microbial opsin) and type II (animal opsin).13 Compared with type II opsins, type I opsins can regulate cell activity faster and better, and they have become the most popular light-sensitive proteins.14 Based on different functions, the opsins can also be classified into excitatory and inhibitory types. Besides, light-gated ion channel types can be distinguished from light-activated ion pump types due to their differences in protein structure and mechanism of action on cell membranes.15 The commonly used opsins are summarized in Table 1.
Table 1.
Commonly Used opsins for Optogenetics
| Opsins | Mode | Features | References |
|---|---|---|---|
| ChR2 (H134R) | Excitatory | 470-nm blue light excitation. A mutant of ChR2, the protein can generate twice as much photocurrent. | Bang et al. 202216 |
| ChR2 (C128S/D156A) | Excitatory | A mutant of ChR2, an SFO ChR2 light-sensitive channel, it can open its ion channel for up to 30 min by activating the channel with 470 nm and then closing the channel with a 590-nm laser. | Bergs et al. 201817 |
| ChrimsonR | Excitatory | 590-600 nm excitation; the closing speed of the channel is increased by point mutation, which is suitable for experiments of higher stimulation frequency. | Klapoetke et al. 201418 |
| ChETA | Excitatory | Blue light excites it at around 470 nm. Mutants of ChR2, which have faster kinetics, can emit 200-Hz spikes in some neurons when stimulated by laser light. | Moshkforoush et al. 202119 |
| oChIEF | Excitatory | 450-470 nm blue light excitation. Some neurons can respond to high-frequency light stimulation, accelerate the speed of channel closure, and reduce the inactivation rate under continuous light stimulation. | López-Hernández et al. 201720 |
| Chronos | Excitatory | 500-530 nm excitation. It is the fastest native blue ChR reported so far. | Huet and Rankovic 202121 |
| ChRmine | Excitatory | Fast red-shifted opsin. Compared with other variants, it exhibits extremely large photocurrents with a 100-fold improved operational light sensitivity. | Chen et al. 202122 |
| ChRger | Excitatory | High-photocurrent ChRs with high light sensitivity can enable transcranial optogenetics. | Bedbrook et al. 201923 |
| SOUL | Excitatory | It has extremely high light sensitivity and can be photoactivated under significantly attenuated light power conditions. | Gong et al. 202024 |
| eNpHR3.0 | Inhibitory | 589-nm yellow light excitation. It pumps chloride ions into neurons and inhibits neuronal activity when illuminated by a yellow-green laser. eNpHR3.0 has good targeting to the cell membrane, the current is relatively durable, and the response is sensitive. | Ferenczi et al. 201625 |
| Arch | Inhibitory | Excitation around 566 nm. It is a yellow-laser-activated outwardly rectifying proton pump capable of moving protons from within the neuron into the extracellular environment, making the neuron hyperpolarized. | Chow et al. 201026 |
| Mac | Inhibitory | 540-nm excitation. Its mechanism of action is similar to that of NpHR. | Husson et al. 201227 |
| ST-eGtACR1 | Inhibitory | 515-nm excitation. GtACR is a Cl- channel with much higher efficiency than plasma pumps such as NpHR and Arch. | Antinucci et al. 202028 |
| Jaws | Inhibitory | 632-nm excitation. It is a red-shifted opsin that induces an influx of chloride ions under red light. | Chuong et al. 201429 |
Arch, archaerhodopsin; ChETA, channelrhodopsin-2 with E123T mutation; ChR2, channelrhodopsin-2; ChRger, channelrhodopsin Gaussian process-engineered recombinant opsin; eNpHR3.0, third-generation enhanced halorhodopsins; Mac, Leptosphaeria maculans fungal opsins; oChIEF, mammalian codon-optimized chimera E.F. with I170V mutation; SFO, step-function opsin; SOUL, step-function opsin with ultra-high light sensitivity.
Innovation of opsins types and structures
With the need for external light to successfully stimulate deep brain cells, high-precision opsins in space and time are required. To achieve this goal, researchers have searched for new opsins from different species, using genetic engineering to modify opsins, or optimizing existing ChR variants.30
Traditionally, optical fibers have to be invasively implanted into brain regions so that the light stimulation system could stimulate nerve cells transfected with light-sensitive proteins. Recently, three opsins, ChRmine, ChRger, and SOUL, were found to be photostimulated transcranially.22–24 ChRmine and SOUL can receive light stimulation at a depth of 5 mm and 6mm in the mouse brain, respectively.22,24 In addition, SOUL can even receive transcranial photostimulation in the cortex of non-human primates (NHPs) such as macaques,24 which means optical fiber implantation becomes unnecessary and optogenetics technology becomes less invasive.
Synapses are crucial for nerve cells to transmit signals. Previously, the commonly used optogenetic tools had low efficiency and off-target effects when applied to presynaptic terminals.31 As a solution, Mahn and colleagues31 screened a new optogenetic tool called targeting-enhanced mosquito homolog of the vertebrate encephalopsin (eOPN3), which can reduce the release of neurotransmitters from the presynaptic terminals through the Gi/o signaling pathway. As a result, it can be used to selectively suppress neurotransmitter release at presynaptic terminals with high spatiotemporal precision, opening new avenues for functional interrogation of long-range neuronal circuits in vivo.
Similarly, some researchers used signal tags to modify ChR2, a common class of light-sensitive proteins discovered in the algae species, to develop a new optogenetic tool—ChR2-mGluR2-PA, which could target neuronal axon terminals and induce synaptic transmission across long-distance axon terminals.32 As a signal tag of ChR2, mGluR2-PA can preferentially localize ChR2-YFP to axon terminals without interfering with normal function.32 In addition, mGluR2-PA-labeled ChR2 can effectively identify axonal projections and significantly reduce the polysynaptic excess noise in the spike collision test.32
Methods for expressing opsins
Only cells expressing opsin on the cell membrane could successfully receive light stimulation. The commonly used methods in the laboratory are: 1) breeding transgenic animal models; 2) virus transfecting targeted cells; and 3) Cre-dependent gene expression.
Constructing a corresponding transgenic animal model is a direct method.33,34 In theory, all targeted cells could stably express opsins. But this way has some drawbacks. The generation of transgenic lines is time-consuming and labor-intensive, and specific technical difficulties lead to high costs.33 In addition, the low transcriptional activity of endogenous promoters would result in insufficient expression of opsin genes in target cells.34 Nevertheless, many researchers still advocate this method because it can produce stable strains and needs no redundant operations on experimental animals.
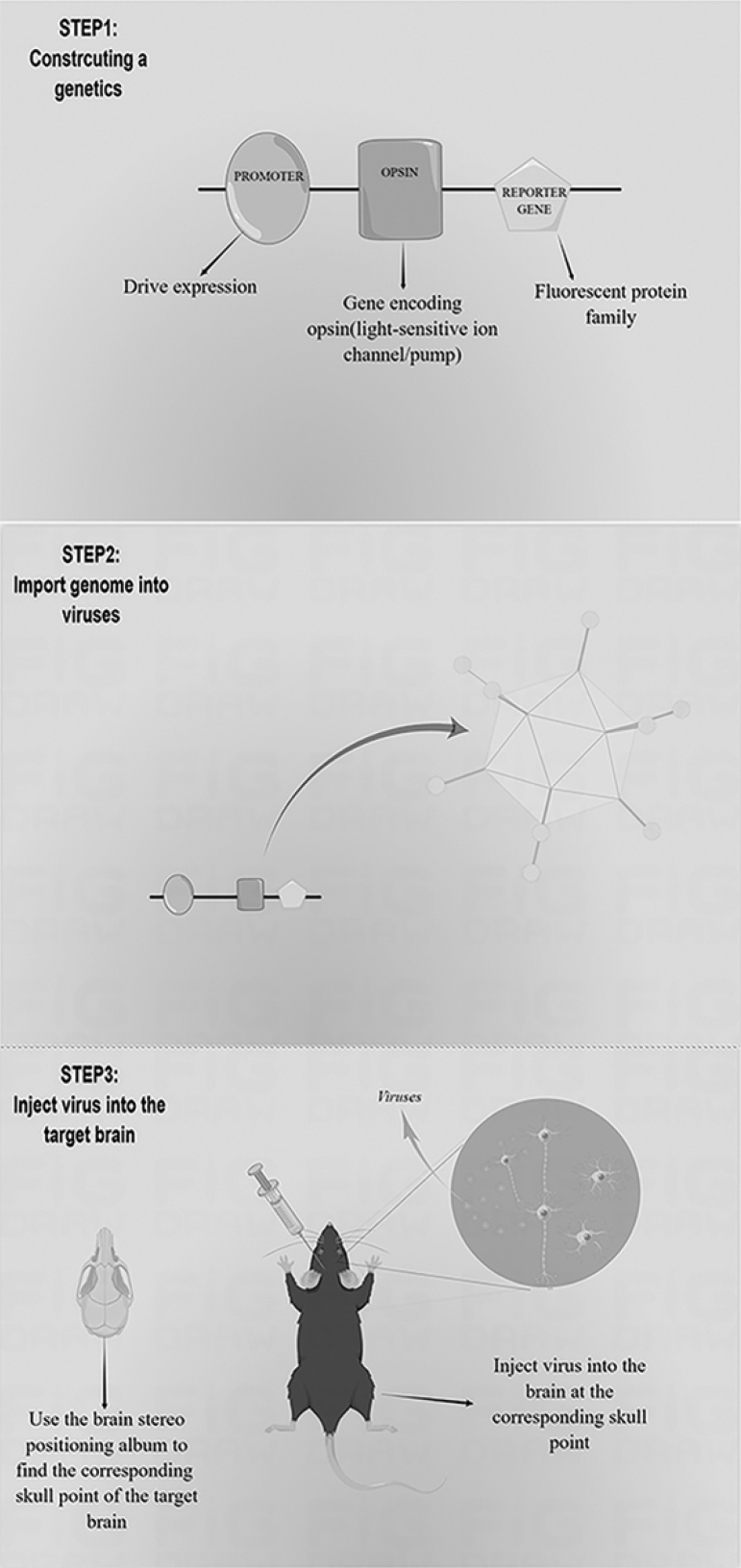
Transfecting the constructed genome into targeted cells to express specific opsins through viral vectors is also popular33 (Fig. 1). Common viral vectors include lentiviruses and recombinant adeno-associated viruses (rAAVs). Lentivirus integrates the genome into the host genome and eventually expresses foreign genes.35,36 The large size and capacity of lentiviruses are suitable for expressing genes <9 kb in length.35 The genome carried by AAV is free from the host genome and can express genes permanently in cells that are not vigorously dividing.35 The rAAVs product is high titer and suitable for expressing gene fragments not exceeding 5 kb, which make them far more diffused in the brain injection area than lentivirus.35,37–39 The properties of AAVs include extremely low immunogenicity, high safety, high stability, and a broad spectrum of infection (almost all major cells in vivo).35,37 These promote the application of AAVs in optogenetics. However, AAVs are limited by low cell infection efficiency in vitro of most AAV serotypes and the long time (1–2 weeks) of exogenous gene expression.39–41
FIG. 1.
Transfection steps by viral vectors. Step 1: constructing appropriate genetic elements is based on experimental needs, including promoters that can regulate the expression of exogenous genes in specific tissue sites or cells, genes for the expression of light-activated or light-inhibited opsins, and reporter genes to test for successful expression. Step 2: producing recombinant adeno-associated virus (AAV) particles containing the genome. Step 3: using a brain stereotaxic apparatus to inject the virus after determining the location parameters of the target brain region.
Injecting recombinase-dependent (e.g., Cre-dependent) virus or secondary virus containing a targeted recombinase in transgenic recombinase-driven animals can also make targeted cells to express specific opsins.42 This method is especially suitable for expressing opsins in cell types without defined promotor sequences, complements the way of AAVs carrying specific promters to infect targeted cells.33 Cre-dependent promoters also overcome the low transcriptional activity of some cell-type-specific promoters.33 This gene expression method combines transgenic animals with virus injection and is more convenient than only transgenic technology.
Light stimulation system
Traditional methods of light stimulation
Light-sensitive proteins require specific wavelengths of light to activate them. Thus, the light stimulation system is another vital component of optogenetics. Current light-emitting devices include lasers and light-emitting diodes (LEDs), which can be combined with single-photon (1P) or two-photon (2P) imaging to observe changes in neural activity.30 The technique of illumination is roughly divided into emitting light in the form of pulses and periods. With the development of optogenetics, the traditional devices that emit laser through optical fiber have matured considerably. Micro-LEDs enable optogenetic devices to be wireless and miniaturized, which is also the current mainstream direction of optogenetic devices. Whether the devices utilize optical fibers or micro-LEDs to provide the light source, invasive surgery on brain tissue is required.
Wireless optogenetic devices
Many researchers are exploring non-invasive light stimulation, and wireless optogenetic devices are under development. The power supply of wireless optical transmission systems mainly derives from batteries and inductive coupling methods. Batteries can provide continuous power for electronic components. However, the power attenuation and the large size of battery modules become obstacles to applying such optogenetic electronic devices. Kim and colleagues43 designed a wireless-battery-chargeable optogenetic device that can be implanted on the surface of the skull subcutaneously, with neurophilic probes placed deep in the experimental animal's brain and controlled via Bluetooth of a mobile phone. They overcame the current hurdle of battery power. But the device is still too bulky for use. The inductive coupling method only needs the power-receiving coil to supply devices, which obviously shrinks the size of wireless optogenetic devices.44
Yang and associates45 used inductive coupling to supply brain and spinal cord implants and combined near-field communication (NFC) and microprocessor technology to provide photostimulation parameters for real-time control. The small device adopts an extensible, flexible probe and offers convenient surgical implantation; it does not affect the everyday activities of experimental animals after the minimally invasive operation.45 However, a special bulky cage equipped with a radio-frequency (RF) power transfer system is one of the drawbacks of inductive coupling. In general, this implantable wireless device has evolved into a platform that integrates multiple functions, including micron-scale inorganic light-emitting diode (μ-ILED) probes for optogenetics, microfluidic channels for drug injection, and a device for recording electrical signal changes.46,47
According to recent reports, micron-scale LED (μ-LED) arrays and nanomaterials may be the future development trend of optogenetics systems. Rajalingham and co-workers48 developed a long-term implantable LED array called Opto-Array to conduct optogenetic experiments in NHPs; it can be effectively applied in studying behavioral optogenetics in complex brains such as those of macaques. Carbon nanotubes-Stretchable transparent electrode arrays made by carbon nanotubes combined nanotechnology and optogenetics for recording and monitoring cortical activity after traumatic brain injury (TBI).49 The upconverting nanomaterials' ability to absorb near-infrared light and convert it into other wavelengths allows researchers to stimulate multiple animal models transcranially.50,51 Recently, the three-color upconverting material developed by the Fudan University has been demonstrated to activate three types of neurons (responding to blue, green, and red light) with three types of near-infrared light, showing potential in neural circuit research.52
Optogenetics in CNS Injuries
CNS injuries have been recognized as one of the leading causes of death and disability worldwide, bringing heavy burdens to patients and their families.53–55 Recently, optogenetics has exhibited potentials in the pathogenesis and clinical application of CNS injuries (Table 2). Next, we discuss the roles of optogenetics in CNS injuries, including TBI, spinal cord injury (SCI), and stroke (Fig. 2).
Table 2.
The Use of Optogenetics in CNS Injuries
| Diseases | Application directions | Topics | Targets | Reference |
|---|---|---|---|---|
| TBI | An experimental tool: assessing and monitoring nerve cells | Neurogliovascular dysfunction in TBI | Pyramidal neuron | Adams et al. 201861 |
| Early activity loss of ipsilesional motor cortex after mTBI | Motor cortex | Nguyen et al. 202162 | ||
| Monitoring in critically neurologically impaired patients | Specific regions and circuits | Jones et al. 201663 | ||
| An experimental tool: altering neuronal activity | Studying or verifying the function of neural circuits in TBI models | Specific neurons | Krukowski et al. 2021; Ndode-Ekane et al. 2021; Chever et al. 2021; Mester et al. 2021; Zeng et al. 202064-68 | |
| The timing of Raf/ERK and AKT activation in protecting PC12 cells | Raf/ERK and AKT pathways | Ong et al. 201669 | ||
| Potential to be intervention methods: TBI complications | Optical depolarization promoted cognitive recovery and maturation of newborn neurons | DCX-expressing cells | Zhao et al. 201873 | |
| SCI | Reducing damage to nerve cells | Optogenetic apoptosis: light-triggered cell death | Bax | Hughes et al. 201588 |
| Optogenetic control of cell differentiation in channelrhodopsin-2-expressing OS3 | Glial progenitor cells | Ono et al. 201789 | ||
| Lentiviral IL-10 gene therapy preserves fine motor circuitry and function after a cervical SCI | Neural motor circuits | Chen et al. 202190 | ||
| Promoting functional recovery | Optogenetic modulation of neural progenitor cells improves neuroregenerative potential | Spinal-cord-derived neural precursor cells | Giraldo et al. 202092 | |
| Reducing pericyte-derived scarring promotes recovery after SCI | Cervical spinal cord rostral to a dorsal hemisection | Dias et al. 201894 | ||
| Neural stem cell grafts form extensive synaptic networks that integrate with host circuits after SCI | Corticospinal tract axons | Ceto et al. 202095 | ||
| Optical control of muscle function by transplantation of motor neurons | Motor neurons | Bryson et al. 201496 | ||
| Optogenetic control of nerve growth | Dorsal root ganglia | Park et al. 201597 | ||
| Optogenetic neuronal stimulation promotes functional recovery after SCI | Glutamatergic neurons | Deng et al. 202199 | ||
| Restoring function after SCI injury by bioluminescent-optogenetics. | Neurons | Petersen et al. 2021101 | ||
| Stroke | Neuroplastic and neuroprotective effects in the sensorimotor cortex | Optogenetic neuronal stimulation promotes functional recovery after stroke | Layer V of the ipsilateral primary motor cortex | Chen et al. 2014120 |
| Cholinergic upregulation by optogenetic stimulation | Nucleus basalis | Mirza Agha et al. 2021121 | ||
| Optogenetics stimulates nerve reorganization in the contralesional anterolateral primary motor cortex | Contralesional anterolateral primary motor cortex | Gao et al. 2022125 | ||
| Optogenetic excitation of ipsilesional sensorimotor neurons is protective | Ipsilesional sensorimotor neurons | Bo et al. 2019126 | ||
| Neurovascular coupling impairment in acute ischemic stroke by optogenetics | Ipsilesional sensorimotor cortex | Bo et al. 2020128 | ||
| Gamma frequency stimulation attenuates vascular and behavioral dysfunction | Inhibitory neurons | Balbi et al. 2021129 | ||
| Mild stimulation improves neuronal survival in an in vitro model | Penumbral neurons | Muzzi et al. 2019130 | ||
| Cellular replacement therapies for nerve repair | Optogenetic stimulation of neural grafts enhances neurotransmission and downregulates the inflammatory response | Neural stem cells | Daadi et al. 2016131 | |
| Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery | Neural progenitor cells | Yu et al. 2019132 | ||
| Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone | Glutamatergic neurons in the striatum | Song et al. 2017136 | ||
| Optogenetic inhibition of striatal neuronal activity improves the survival of transplanted neural stem cells and neurological outcomes | Striatal neurons | Lu et al. 2017137 | ||
| Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery | Striatal neurons | (He et al. 2017)138 | ||
| Optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after stroke | GABAergic neurons | Jiang et al. 2017140 | ||
| Afferent and efferent neural circuits for improved functional recovery | Optogenetic neuronal stimulation promotes persistent functional recovery after stroke | Contralesional lateral cerebellar nucleus | Shah et al. 2017142 | |
| Optogenetic stimulation reduces neuronal nitric oxide synthase expression after stroke | Contralesional lateral cerebellar nucleus | Pendharkar et al. 2021145 | ||
| Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain | Thalamocortical axons | Tennant et al. 2017147 | ||
| Closed-loop optogenetic control of thalamus interrupts seizures after cortical injury | Thalamocortical circuits | Paz et al. 2013148 | ||
| Combination with other rehabilitation therapies | Slow waves promote sleep-dependent plasticity and functional recovery after stroke | Pyramidal neurons | Facchin et al. 2020153 | |
| Environmental enrichment implies GAT-1 as a potential therapeutic target for stroke recovery | Lin et al. 2021156 | |||
| The neuronal activation is essential for environmental enrichment-induced post-stroke motor recovery | Deep cerebellar nuclei | Zhang et al. 2019157 |
GABA, gamma aminobutyric acid; IL, interleukin; iPC-NPC, induced pluripotent-stem-cell-derived neural progenitor cell; SCI, spinal cord injury; TBI traumatic brain injury.
FIG. 2.
The applications of optogenetics in central nervous system (CNS) injuries.
The role of optogenetics in TBI
TBI pathophysiology
TBI is a frequently occurring disease with high mortality and chronic disability worldwide.56 According to U.S. Centers for Disease Control and Prevention (CDC) records,57 more than 611 TBI-related hospitalizations and 176 TBI-related deaths occurred per day. Results from the 2019 Global Burden of Disease (GBD) study indicate that the number of patients with moderate/severe TBI was up to 37,505,338.58 The process of TBI consists of primary injury and secondary injury. Unlike the acute primary injury, the secondary injury lasts from minutes to months.33 Mechanical contusion of the brain causes extracellular edema, whereas shearing and tearing of blood vessels and neural tissue results in ischemia, hemorrhage, and cell-derived edema, further damaging the nervous system.59,60 Subsequent multi-factorial biochemical cascades lead to mitochondrial dysfunction, neuronal excitotoxicity, inflammation of surrounding tissue, calcium overload, and oxidative stress.59 These cause neuronal cell death, and ultimately contribute to clinical neurological deficits. In addition, TBI-related complications may appear shortly or several years later, and include post traumatic epilepsy (PTE) and cognitive impairment such as memory loss and mood changes.
The application of optogenetics in TBI
Although optogenetic technology has been widely used in neuroscience, the application in TBI research is still in its infancy. At present, the roles of optogenetics in TBI research can be divided into two types: experimental tools and intervention methods. Compared with the former, the further discovery of the intervention effects in TBI is more needed.
Experimental tools: Monitoring and altering neuronal activity
The property of precisely modulating nerve cells enables optogenetics to monitor neuronal activities after TBI. To examine the function of pyramidal neurons in glial vascular dysfunction, Adams and colleagues61 performed photostimulation on Thy1-ChR2 mice. They conducted electrophysiological monitoring with implanted electrodes to induce neuronal responses. High and low amplitudes represented whether neurons were reduced or impaired.61 In vivo optogenetic motor maps, as a new method, were first used by Nguyen and associates62 to determine longitudinal changes in cortical motor maps in a model of mild TBI. At the same time, calcium imaging was performed in vitro using mouse brain slices to study the relationship between cortical excitability changes and motor deficits. Patients with severe TBI require reliable neurological assessment and monitoring equipment, and optogenetics may offer a probability for this clinical application. Using optogenetics to accurately monitor and record neural circuits and membrane potential at the single-cell level may have advantages over current brain monitoring techniques.63 Although current optogenetic monitoring may be limited to specific regions and circuits, it will complement existing brain monitoring methods to provide a better overall pattern of neurological damage.
Optogenetics has been used in TBI models to activate or inhibit neurons such as gamma aminobutyric acid (GABA)ergic neurons and retrosplenial cortex neurons to alter neuronal activity.64–68 Ong and associates69 used an optogenetic system to explore the precise timing of Raf/ERK and AKT pathway activation, affecting their protective effects against oxidative stress. They found that pre-conditioning activation of the Raf/ERK or AKT pathway immediately before oxidant exposure by light provided significant protection to cells. And they concluded that the optogenetic platform, with its precise temporal control and its ability to activate specific pathways, would be ideal for the mechanistic dissection of intracellular pathways in protection against oxidative stress.
Intervention methods for TBI complications
PTE is a frequent complication of TBI. Employing optogenetics to establish effective epilepsy models has been reported.70,71 But animal models of PTE are still lacking. The incidence of epilepsy after TBI varies widely, and pre-clinical models are challenging to establish. The previous method is drug-induced epilepsy in post-traumatic experimental animals without the ability of simulating the natural pathogenesis. In addition, the high mortality associated with existing models limits their feasibility in routine experimental studies.72 Optogenetics could be matched with TBI animal models to establish more natural and stable epileptic models. Besides, memory and cognitive impairment as a complex complication of TBI can also be ameliorated with the intervention of optogenetics. For example, by optical stimulation, Zhao and colleagues73 depolarized doublecortin (DCX)-expressing neonatal cells in the dentate gyrus of TBI mice. The results indicated that photostimulation enhanced the survival and maturation of these cells in the dentate gyrus and significantly improved the cognitive deficits of mice.
Surprisingly, optogenetic therapy has recently been discovered to partially recover visual function in a blind patient, through combining intraocular injection of an AAV vector encoding ChrimsonR with light stimulation via engineered goggles.74 And the psychophysical and neurophysiological evidence presented in this study suggest that the optogenetic stimulation of human retinal ganglion cells by a light-projection system linked to a camera may be a promising way to partially restore vision in blind patients. So far, many studies have used transcranial magnetic stimulation (TMS), deep brain stimulation (DBS), and other technologies to intervene in TBI.75,76 Similarly, optogenetic therapy would be a novel treatment method for TBI. And more in-depth research is required to confirm the efficiency of optogenetics in TBI.
The role of optogenetics in SCI
SCI pathophysiology
SCI is a devastating disease usually caused by traffic accidents and accidental falls. The incidence of SCI in the world is high, especially in young men. According to the National Spinal Cord Injury Statistical Center (NSCISC), nearly half (46.7%) of the 35,675 participants' injuries were caused by SCI between the ages of 16 and 30 years.77 Its recent estimate showed that the annual incidence of SCI is approximately 54 cases per one million people and approximately 18,000 new cases yearly in the United States alone.78
The pathophysiology of SCI is similar to that of TBI and could be classified into primary injury and secondary injury. After external shock, the tissue surrounding the injury site is destroyed with a series of biochemical cascade reactions.79 Changes in the local microenvironment due to lipid peroxidation, free radical production, apoptosis, and the release of numerous signaling molecules lead to cell death, neuroinflammation, and the reaction of glial cells.80,81 Migration of inflammatory cells and the release of inflammatory factors damage resident nerve cells and cause neurological dysfunction.82,83 These reactive phenotypes participate in the formation of glial scars around lesions by depositing excess neuroinhibitory chondroitin sulfate proteoglycans (CSPGs) to repair damaged spinal cord sites.82 However, the mechanism also makes axons difficult to regenerate, and neurological recovery is constrained.
The application of optogenetics in SCI
Clinically, the management of SCI is short of satisfactory therapeutic tools. And the goal of intervention for SCI comprises reducing nerve cell damage and promoting functional recovery. Optogenetics has exhibited a promising role in SCI.
Reducing damage to nerve cells
The inflammatory response after SCI plays a critical role in secondary injury. The clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system can knock down associated genes in spinal cord cells, thereby reducing the activation of inflammatory cells and the production of pro-inflammatory factors.84,85 For example, glial cell maturation factor (GMF) knockdown in microglia reduced p38 mitogen-activated protein kinase pathway signaling, thereby constricting microglia activation.86 In addition, the knockdown of caspase 3 (C3) in astrocytes also relieved the inflammatory response.85,87 Interestingly, optogenetic techniques would also achieve the results by triggering cell death. Optogenetic Bax (Cry2-mCh-BaxS184E) could be activated by blue light irradiation and specifically expressed. Similar to the endogenous mitochondrial pathway of apoptosis, Bax induced mitochondrial protein release, downstream C3 cleavage, and cell morphological changes, ultimately contributing to cell death.88 By inserting a CD86-specific promoter, the M1 phenotype of microglia can be reduced.85 Optogenetics could also reduce microglia generation by interfering with the differentiation direction of glial progenitors. Under blue light irradiation, the intracellular free Ca+ concentration of ChR2-expressing glial progenitor cells changes made them differentiate into oligodendrocytes and promoted the repair of damaged axons.89
Recently, Chen et al.90 demonstrated that the anti-inflammatory effect of interleukin (IL)-10 could alleviate the secondary injury of SCI by implanting lentivirus encoding the anti-inflammatory factor IL-10 into a cervical cord injury. Moreover, the researchers used optogenetic techniques to stimulate the injured cervical cord and performed electrophysiological recordings, successfully verifying the protective effect of IL-10 on neural motor circuits. The results show optogenetic therapy could reduce damage to nerve cells by modulating inflammatory response in SCI.
Promoting functional recovery
SCI can cause neuronal cell death, demyelination, and axonal rupture, leading to motor and sensory dysfunction in patients. Stem cell therapy works as an alternative strategy by tissue repair, replacement, regeneration, neurotrophy, promotion of angiogenesis, anti-apoptosis, and anti-inflammation.91 Here, we focus on the progress of neural stem/progenitor cells (NSPCs) combined with optogenetics to promote functional recovery of SCI.87
NSPC-based therapeutic regimens have some challenges, including low cell viability, limited differentiation, and reduced functional engraftment. The severe side effects are the tumorigenicity and instability of NSPCs in vivo.91,92 Now, optogenetics provides refreshing solutions. Giraldo and colleagues92 reported that photostimulation of spinal-cord-derived neural precursor cells (ChR2-NPCs) ectopically overexpressing ChR2 promoted a proliferation rate increase and functional neural cell in vitro maturation. Specifically, light stimulation allowed cations to enter cells and induce NPCs to differentiate into oligodendrocytes and neurons. Although not affecting astrocyte differentiation, cation promoted its polarization from a pro-inflammatory phenotype to a pro-regenerative/anti-inflammatory phenotype.92 In neuron replacement therapy, new neurons following induction of NSPC differentiation need to regenerate new neurites to establish connections with other neurons for further activation and integration into functional neuronal networks.85,92,93 ChR2-expressing NSPCs and their derived neurons have exhibited neurite outgrowth, axonal elongation, and branching under blue light stimulation.92 NSPCs and new neurons implanted in SCI are difficult to activate and regenerate because they cannot receive signal stimulation from descending fibers.93 Optogenetics is equivalent to giving these cells an external stimulus instead of the CNS. NSPCs proliferate and activate locally at the injury site, but the scar tissue formed after injuries and its inhibitors physically and biochemically prevent these cells from repairing damaged nerve tissue.82,93
Dias and associates94demonstrated that reduced scar tissue formation after SCI increased corticospinal tract axonal regeneration and improved sensorimotor function, which was proved by optogenetics. This study further showed that scar tissue removal was significant for SCI prognosis with stem cell therapy. NSPCs are implanted at the SCI site and generate neuronal relays between lesions, providing functional benefits. However, little has been done to determine whether these grafts locally form synaptic networks and join existing neural networks with spatial precision. Optogenetics combined with calcium imaging technology allows for the quantification and evaluation of stem cell treatment effects based on natural physiological conditions rather than crude assessment of neurological recovery through simple motor and sensory stimulation.95
Optogenetics combined with stem cell therapy can promote the motor circuit of spinal peripheral nervous system reconstruction and functional recovery.96 Further, it could also improve functional prognosis in SCI when used alone.96 The CNS has a limited ability to regenerate. Various physical and biochemical methods to increase nerve growth have been investigated, including electrical stimulation and neurotrophic factors.97 However, electrical stimulation could be replaced by optogenetics due to its lacking cell specificity and quickly fatiguing neurons.98 One study97 found that ChR2-expressing dorsal root ganglia (DRG) had enhanced neurite outgrowth under light stimulation. Additionally, the investigators observed increased expression of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). Photostimulation of light-sensitive DRG also induced directional growth of neurites in nearby wild-type DRG (WT-DRG).97
Similarly, Deng and co-workers99 found that activation of glutamatergic neurons in SCI rats' primary motor cortex (M1) could effectively promote tissue recovery and neurofilament growth at the SCI site. Additionally, the content of some growth-related proteins (BDNF, NGF) at the injury site also increased.99 In that study, the researchers considered the relationship between the brain and the spinal cord. Also, they pioneered selectively photostimulating glutamatergic neurons based on previous studies about the connection of M1 neurons with motor function after SCI, suggesting that M1 glutamatergic neurons may play a vital role in motor recovery of SCI.99
Another way to recover neurological function after SCI is to directly replace the signal of descending fibers with external stimulation to activate the remaining neurons. Traditionally, the method refers to functional electrical stimulation (FES). FES has been successfully used to help patients restore breathing, lower and upper extremities, limb function, and bladder and bowel control. Nevertheless, this electrical stimulation system has technical shortcomings and practical limitations.98,100 As a rapidly developing technology, optogenetics is expected to overcome the above limitations in SCI gradually. The cell-selective activation with optogenetics is suitable for controlling the delicate movements of muscles, contributing to the restoration of nerve function more physiologically.100 For muscle fatigue, optogenetics can delay it by activating anti-fatigue fibers before activating any fast-fatiguing fibers.100 Petersen and colleagues101 used a combination of chemogenetics and optogenetics to restore motor function in rats with spinal cord contusion by light stimulation.
Bioluminescence-optogenetics (BL-OG) is a recently developed optogenetic approach that uses bioluminescence to activate opsins and open ion channels. In one study, the optogenetic elements used in BL-OG included bioluminescent luciferase, luminopsin (LMO), and bioluminescence was generated by the breakdown of a specific enzymatic substrate (coelenterazine [CTZ]).101 Light stimulation of BL-OG was controlled by injecting CTZ. Most SCIs are contusions, leaving an intact area of nerve tissue beneath the injury site.101 However, due to the lack of stimulation of descending fibers, these fully connected neurons enter a quiescent state, resulting in motor deficits in patients. Finally, the investigators found that activation of either neuronal population below the level of injury significantly improved motor recovery. They suggested that BL-OG-mediated post-SCI recovery mainly depends on optogenetically induced neuronal plasticity, the underlying maintenance of neural networks, and improvement in inflammatory status.101 Conclusively, optogenetics has excellent value in SCI, especially combined with other treatment strategies.
Device support
Given the different anatomical structures among the brain, spinal cord, and peripheral nervous system, the optogenetic devices for the brain would not be suitable for spinal cord and the peripheral nervous system. But the implantable wireless optogenetic device developed by Montgomery and associates44 requires no additional modifications and is suitable for the spinal cord and peripheral circuits. Their study showed the feasibility of an optogenetic device suitable for both the central and peripheral nervous systems.
Recently, Mondello and colleagues102 designed a micro-LED implant for the rat spinal cord optogenetic stimulation. Although it is a wired device, it has the advantages of simple operation and low cost. In addition, it was able to induce robust movement for at least 6 weeks post-implantation without causing physical or thermal damage to the underlying spinal cord.102 Kathe et al. reported a wireless closed-loop optogenetic operating system that enables ultrafast, wireless, closed-loop manipulation of target neurons and pathways throughout the dorsoventral spinal cord of mice.103 This system is similar to the multi-lateral wireless device reported by Yang and colleagues.45 It consists of a wireless power supply platform, stretchable flexible materials, and a micro-LED array.45,103 Micro-LEDs are integrated on a soft stretchable carrier and conform to the spinal dura mater. Silicone-phosphor matrix coatings on micro-LEDs provide mechanical protection and light conversion and are compatible with an extensive library of opsin proteins.103 The wirelessly powered platform can also sense physiological signals and process them to control light stimulation in a closed loop.103 With the device, the researchers revealed the roles of various neuronal subtypes, sensory pathways, and supraspinal projections in controlling locomotion in healthy and SCI mice.103
The appropriate optogenetic equipment is essential for application in SCI, especially for in vivo experiments of long-term functional recovery after SCI. In addition, the clinical prospect of optogenetics in SCI also relies on a stable and safe operating system. A well-established optogenetic device that can be implanted long-term and affects no regular spinal activity is necessary for future studies of SCI.
The role of optogenetics in stroke
Stroke pathophysiology
Stroke is the second leading cause of death globally, affecting 13.7 million people worldwide each year.104 It is estimated that 3.4 million U.S. adults will have a stroke, accounting for 3.9% of the U.S. adult population by 2030.105
The lack of blood flow following ischemic stroke can result in various complex pathophysiological changes. Brain tissue ischemia leads to cell necrosis stemming from a lack of oxygen and glucose, failure of cellular energy metabolism to produce ATP, and rapid disintegration of the cell membrane and cytoskeleton.106 Neuronal cells cannot maintain a normal ion gradient, and neuronal depolarization caused by Ca+/Na+ influx leads to the release of many excitatory amino acids (mainly glutamate) that bind to intracellular glutamate receptors, further precipitating Ca+ influx and the initiation of apoptosis and necrosis pathways.106,107 Both excitotoxicity and calcium overload are vital factors in the early stages of ischemic cell death.108 Increased intracellular calcium ions trigger mitochondrial dysfunction and the release of free radicals, resulting in cellular damage or apoptosis.108,109
Moreover, immune cells also respond to ischemic injury. From the initial rising of microglia to the increase of dendritic cells, macrophages, and lymphocytes, the blood–brain barrier is further damaged, and neutrophils gradually enter the infarct and surrounding areas.110 Immune cells release pro-inflammatory cytokines, free radicals, and inducible nitric oxide synthases (iNOS), further enhancing the inflammatory response and cell damage.104,111 However, these inflammatory cells are not always destructive. They help clear cellular debris and damaged tissues, and later phenotypic changes (e.g., macrophages) reduce their phagocytosis and production of inflammatory factors.107,112 Therefore, the balance of the inflammatory response after stroke is also critical for treatment and recovery.107 Aiming at the complex injury pathways, the current clinical treatment for acute stroke mainly focuses on blood flow restoration and neuroprotection.108
The application of optogenetics in stroke
Increasing research has been devoted to neural remodeling and functional improvement in patients suffering from stroke.113 Studies have shown that post-stroke neuronal networks could be rewired for functional recovery.113,114 Optogenetics has demonstrated advantages in the study of neural circuit reorganization and its relationship with functional recovery. The technology has dramatically facilitated researchers' ability to discover more targets for intervention.
Neuroplastic and neuroprotective effects
Although brain networks can be reorganized by forming new connections between remaining neurons, spontaneous reorganization is often insufficient to restore function to pre-injury levels.115 The relative timing of neuronal activity around synapses is vital in driving plasticity.116 Through neural stimulation, it is possible to induce causal timing between the firing of neurons.117 All three repetitive, paired, and closed-loop stimulations are designed to induce consistent activity in presynaptic and post-synaptic neurons.115 Ultimately, these stimuli induce Hebrew-peak timing-dependent plasticity.118 Therefore, neurostimulation could enhance post-stroke neuroplasticity and promote functional recovery.
Inspiringly, the neurostimulation by optogenetics can selectively activate or inhibit specific subtypes of neurons with higher precision, which has more potential.119 For examples, Cheng and colleagues120 transiently embolized the middle cerebral artery in mice to produce infarcts in the striatum (Str) and somatosensory cortex (S1) while using optogenetics to selectively stimulate neurons in layer V of the ipsilateral primary motor cortex (iM1). They found that stimulation of iM1 neurons activated the peri-infarct region and contralateral M1, and repeated stimulation of iM1 neurons enhanced cerebral blood flow/neurovascular coupling (NVC) in stroke mice. The results suggested that selective stimulation of iM1 neurons may contribute to neural remodeling. By quantitative polymerase chain reaction (qPCR) and western blotting, the researchers found that repeated stimulation increased the expression of neurotrophins (BDNF, NGF, and NTF3) in stroke mice, and the significant expression of growth-associated protein 43 (GAP43) indicated that post-stroke neuronal stimulation could enhance synaptic plasticity. Finally, they demonstrated that stimulating neurons in the stroke hemisphere could promote functional behavioral recovery through sensorimotor behavioral experiments (rotating beam test) and monitoring weight changes in stroke mice.
Based on this study, Mirza Agha and co-workers121 examined the role of nucleus basalis cholinergic projections on functional recovery after stroke in the forelimb regions of the somatosensory cortex. Studies have shown that the basal forebrain cholinergic system played a role in synaptic plasticity, behavioral state control, and cognitive function as an essential part of the neuromodulatory system.121 Acetylcholine (ACh) is associated with cortical plasticity122; the researchers further investigated the role of ACh in cortical recovery/plasticity after stroke, daily stimulating the cholinergic nucleus basalis (NB) neurons during the acute recovery phase. Based on local field potential (LFP) records and more comprehensive behavioral tests, they found that stimulating the nucleus basalis caused upregulation of ACh release and promoted motor recovery in stroke mice, but there was no improvement in sensorimotor integration impairment. This intriguing result suggests that improving the forelimb area of the post-stroke mouse somatosensory cortex contributed to the recovery of forelimb motor behavior and that upregulating ACh might be a new therapeutic strategy in the acute recovery phase of stroke.
For the motor auxiliary area, studies have indicated that regulating the motor auxiliary area of the cerebral hemisphere on the opposite side of the brain injury might have positive significance for the functional recovery of patients with stroke.123,124 The anterolateral motor cortex of rodents is an important auxiliary motor area, and the function is similar to that of the human premotor cortex. Activation and inhibition of the contralateral anterolateral motor cortex (cALM) have been proven to affect motor behavior directly. The Gao et al. team125 further investigated the significance of cALM in stroke treatment. They used ChR2 and NpHR light-sensitive proteins to activate or inhibit neurons in cALM and found that both photoinhibition and photoactivation of cALM reduced neurological deficit scores. Photoinhibition increased dendritic length, dendritic spines, and perforated synapses in M1 on the ischemic side. At the same time, photoactivation raised the number of synaptic buttons and dendritic intersections in M1 on the ischemic side. The results showed that optogenetic stimulation of cALM could promote the reorganization of primary motor neurons on the ischemic side after stroke and reduce the neurological deficits. The activation and inhibition of cALM have different effects on neuroplasticity.
The positive effect of optogenetics on acute stroke provides a new option for treating acute stroke in the future. Bo and associates126 demonstrated that specific neuronal modulation in acute stroke is neuroprotective and reduces infarct size. They utilized optogenetics to excite ipsilateral sensorimotor neurons in a parietal cortical stroke mouse model. Further, they measured cerebral blood flow (CBF) image changes at baseline, 0, 2, and 24 h after stroke by laser speckle contrast imaging (LSCI). Moreover, neurovascular responses were measured 24 h after stroke to examine the effect of neuron-specific excitation. As a result, neuron-specific excitation in the ipsilateral sensorimotor cortex during the acute phase could reduce the expansion of the ischemic area and improve neurovascular responses. Histological and behavioral results also consistently confirmed the neuroprotection of neuronal excitability in acute stroke. NVC is impaired after ischemic stroke, and the improvement of NVC function is closely related to stroke recovery.127
In another study,128 Bo and associates delved into the NVC function in the acute phase of ischemic stroke at the cellular level. They found that upon excitatory photostimulation of sensorimotor neurons following stroke, regional cerebral blood flow (rCBF) responses were immediately decreased. In addition, the responses were associated with distance from the ischemic core rather than changes in resting CBF. The results also stated clearly that excitatory stimulation of neurons around the ischemic penumbra promoted NVC recovery, whereas neuronal stimulation around infarcts did not restore neurovascular function 24 h after ischemia. It implied that the complete area at the ischemic penumbra's edge might be the excitatory stimulation target. Similar studies also have indicated increased neuronal survival, enhanced neurovascular responses, reduced lesion size, and improved motor function following optogenetic excitatory stimulation of neurons in both in vivo and in vitro acute stroke models.129,130
Optogenetics has also demonstrated positive therapeutic effects as an intervention in the chronic recovery phase. Although selective stimulation of sensorimotor cortex neurons has neuroprotective and neuron remodeling effects in stroke models, the precise molecular mechanisms remain unknown. In the above studies, the researchers tried to put forward some hypotheses, but none were verified. More mechanistic studies may provide more affluent and precise intervention targets. Further, the optimal time to intervene in cortical neurons after stroke remains to be explored, and perhaps hemodynamic changes are one of the factors to be considered.
Cellular replacement therapies for nerve repair
Optogenetic stimulation of NSPCs induces differentiation, proliferation, and regeneration of NSPCs in SCI models. But whether optogenetic stimulation of neural stem cells produces similar effects in stroke models needs to be demonstrated. Daadi and colleagues131 made transplanted neural stem cells express ChR2 (NSC-ChR2), transferred them into the peripheral area of rat Str ischemia, and gave them regular light stimulation. Through differential gene expression analysis, the investigators discovered that light stimulation of NSC-ChR2 upregulated transcripts for neurotransmission, neuronal differentiation, regeneration, axon guidance, and synaptic plasticity. In contrast, genes involved in inflammatory responses were significantly downregulated. The behavioral analysis also showed improved motor function in stroke rats. They proved that excitatory stimulation of exogenous NSCs promotes neural network recovery in a stroke model through two modes of bystander effect and cell replacement. Yu and associates132 used optochemogenetics to stimulate induced pluripotent-stem-cell-derived neural progenitor cells (iPS-NPCs) in in vivo and in vitro experiments. Similarly, they found that LMO3-expressing iPS-NPCs (LMO3-iPS-NPCs) under activation by light or CTZ constructed an activity-enriched tissue microenvironment and improved neural connectivity.
In addition, endogenous strategies that focus on the mobilization and production of existing neural stem cells are also one of the main lines of stem cell therapy.108 The subventricular zone (SVZ)133 and the dentate gyrus134,135 are thought to be the main niches that produce neural stem cells. Using neuromodulators (e.g., BDNF, insulin-like growth factor-1) can stimulate the proliferation and migration of NSPCs to the injury area.108 Song and co-workers136 used optogenetics to stimulate the glutamatergic neurons in the mouse Str to release glutamate to the SVZ. They observed that neuroblastoma cells in the SVZ proliferated and migrated to the mouse peri-infarct cortex, with increased neuronal differentiation and improved long-term functional recovery. In the SVZ, glutamate released from glutamatergic neurons activates neuroblasts mediated by α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs), thereby promoting Ca+ influx and cell proliferation.136
Through in vivo and in vitro experiments, Lu and colleagues137 found that inhibition of Str neurons improved the survival of exogenous neural stem cells located in the peri-infarct region of the Str by reducing GABAergic neuron paracrine. Moreover, optogenetic inhibition of Str neurons increased the number of Nestin+, BrdU+/Doublecortin+, and BrdU+/NeuN+ cells in the SVZ and peri-ischemic regions, promoting proliferation, migration, and differentiation of endogenous neural stem cells.138 However, the researchers did not continue to explore the effects of the selective intervention of GABAergic neurons on endogenous neural stem cells. Although GABAergic neurons comprise the majority of medium spiny neurons (MSNs) in the Str,139 the outcome of selective photoactivation or photoinhibition in GABAergic neurons differs from non-selective ones. Selective photoinhibition of Str GABAergic neurons resulted in a marked increase in NSCs within the SVZ, but the results for neural progenitors/neural precursors were the opposite.140 The selective intervention of GABAergic neurons in the subacute phase of stroke did not improve neurogenesis,140 which could be related to the imbalance of excitatory and inhibitory signals in the Str. But the specific reasons and detailed mechanisms still need to be further demonstrated.
The bystander effect promotes the spontaneous regeneration of neural tissue by regulating the tissue microenvironment. However, this effect may not be practical due to the decreased cellular plasticity and self-repair ability in many stroke patients (older adults).141 Cell replacement may better support the sustainable recovery of damaged brain areas141 and could be a more effective treatment method.
Afferent and efferent neural circuits for improved functional recovery
The lateral cerebellar nucleus (LCN) in the deep cerebellar nucleus sends excitatory output to the motor, premotor, somatosensory, and non-motor areas of the cortex via the dentato-thalamo-cortical pathway.142 Previous studies have shown that stimulation (e.g., electrical stimulation) of the dentato-thalamo-cortical pathway could enhance the excitability of the contralateral cortex and promote functional recovery after stroke.143,144 Optogenetic stimulation of neurons in the contralesional LCN (cLCN) has been shown to send excitatory signals to the forebrain's motor and sensory areas via efferent pathways.119,142 After receiving optogenetic stimulation, mice with the cortical stroke showed significant functional improvement on behavioral tests. This pro-restorative effect was durable, with the improvement remaining after cessation of stimulation for 2 weeks.142 Further, GAP43 in the ipsilateral somatosensory cortex and neuronal nitric oxide synthase (nNOS) in the contralesional M1 significantly increased and decreased,142,145 together contributing to the recovery of the stroke cortex.
The thalamo-cortical pathway acts as an afferent circuit where sensory-evoked activity signals reach the cortex through afferent fibers in the intact thalamus. Although the thalamus is critical in upstream circuits for processing and transmitting sensory information, most studies on sensorimotor recovery after stroke have focused on cortical circuits.119,120,125,126,129,146 Little attention has been paid to the role of thalamo-cortical circuits in recovery after stroke. Through calcium imaging in stroke mice models, the Tennant team147 confirmed that strokes in the sensory cortex of the anterior limb cause loss of axonal synaptic connections in the peri-infarct cortex and long-term suppression of excitability in the surviving thalamo-cortical circuits. Subsequently, the investigators performed chronic optogenetic stimulation of thalamo-cortical axons. Their study illustrates that excitatory stimulation promotes the formation of new thalamo-cortical synaptic tracts. These synaptic tracts remained stable—the improved sensorimotor function in the mouse forelimb persisted for a long time after losing stimulation. Further, it is interesting that closed-loop optogenetic control of thalamo-cortical circuits (photoinhibition) also controls epileptic seizures caused by cortical stroke, providing a new idea for refractory epilepsy after stroke.148
Combination with other rehabilitation therapies
There is a strong bidirectional link between sleep and stroke. Changes of brain structure and function after stroke can impact sleep negatively. And sleep may also play a key role in promoting functional recovery.149 Experimental evidence suggests that slow waves (SWs) in non-rapid eye movement (NREM) sleep may enhance brain plasticity during spontaneous sleep and improve functional recovery after stroke.150–152
Facchin and colleagues153 investigated the effect of optogenetically induced sleep SWs in an animal model of ischemic stroke. First, they noticed that the amplitude and slope of ipsilateral NREM sleep SWs were decreased after stroke in stroke mice models. Next, they transfected pyramidal neurons with ChR2 and Arch, resulting in an upregulated (membrane depolarization) or downregulated (membrane hyperpolarization) state in the local cortex and mimicking spontaneous NREM SWs in mice. Ultimately, the outcomes showed that a single optogenetically induced SW (SWopto) in the peri-infarct area during sleep promoted axonal sprouting locally and on the contralesional side, significantly improving delicate motor movements correlated with the stroke site. However, SWopto in awake mice had no effect.153 The achievements demonstrated the role of sleep SWs in cortical circuit plasticity and sensorimotor recovery after stroke. They showed good prospects for the rehabilitation strategy of sleep-improving recovery after stroke by means of optogenetics.
Patients' cognitive and motor functions can be improved if they are provided with environmental enrichment (EE) in some neurodegenerative diseases and CNS injuries.154,155 It has been proven that EE can promote stroke recovery by enhancing neural network excitability.156 Although the process of utilizing EE is complicated,156 it has been an alternative rehabilitation strategy for stroke sufferers. The combination of optogenetics, electrophysiology, chemical genetics, and other methods with EE has deepened the understanding of EE's mechanism in rehabilitation.156,157
Future Perspective and Conclusions
Currently, optogenetics is flourishing in both light-sensitive proteins and light-stimulated systems. The emerging opsins increase light sensitivity and spatial precision. The advances of optical stimulation systems have transitioned wired devices to wireless ones, and invasive implants have become minimally invasive or noninvasive. These developments provide more possibilities for clinical translation.
Invasiveness is an unavoidable topic for this technology. Although the current optogenetic devices could be minimally invasive and reduce damage to the brain tissue caused by the probe, the primary injury to nerve cells or the secondary injury after virus injection sometimes would be unavoidable. In fact, there is a lack of evidence of long-term stable work about the minimally invasive implantation of optogenetic devices. And non-invasive optogenetics rely too heavily on opsins. Perhaps transcortical stimulation with near-infrared light combined with high-sensitivity opsins would be more suitable for practical applications (Fig. 3).
FIG. 3.
Transcortical stimulation. After injecting viral vectors carrying highly sensitive opsin genes and upconverting nanoparticles that can convert infrared light to visible light in a targeted brain region, a tiny device that emits near-infrared light is immobilized in the skull. Near-infrared light travels through the meninges and other brain tissue, ultimately activating or inhibiting opsin-expressing nerve cells in targeted brain regions via upconverting nanoparticles.
Near-infrared light can reach deeper brain tissue and reduce phototoxicity to cells.158 The near-infrared-light-based imaging system and light modulation strategy have also confirmed its feasibility for in vivo applications.159,160 However, even near-infrared light has to deal with the variability of human skull thickness, which seems to be the biggest obstacle to clinical application. Although non-invasively activated opsins have been successfully achieved on NHPs, more cases and data are required to demonstrate the practical feasibility. Therefore, transcortical photostimulation can avoid the influence of the skull on the optical power density and the physical damage to brain tissue caused by the photostimulation device. In addition, understanding the optical properties of different tissue structures in the brain and studying the light distribution and thermal effects can help improve the spatial resolution of illumination and reduce damage to cells.161
In the future, constructing a reference map of the optical properties of human brain regions and corresponding illumination parameters may significantly promote the use of optogenetics. The future application of optogenetics would be inseparable from materials science and nanotechnology support.
The multi-disciplinary integration of optogenetics with other emerging therapies increases its chances for clinical translation. For instance, with the use of optogenetics in stem cell therapy, neural stem cells increase the survival rate and enhance the ability of proliferation and differentiation. As a new optogenetic approach technology associated with chemogenetics, BL-OG has been considered to have great value for regulating cellular pathways and neural circuits.101,132 In consideration of the clinical application of optogenetics, autologous stem cells may partially replace AAVs as the carrier of opsin genes. The participation of optogenetics in the recovery of CNS injuries as an adjunct should be its initial form.162,163 In addition, possible tumorigenicity should be considered when AAVs are replaced with autologous stem cells.91
In conclusion, optogenetics has made rapid progress and has shown great potential in CNS injuries. Combined with other technologies, optogenetics could accelerate nerve regeneration, synaptic plasticity enhancement, and functional recovery by controlling neural circuits, promoting neural remapping, and improving cell survival. The cross-integration with electrophysiology, hylology, computer science, and other disciplines may lead to more progress. More research is needed to translate this technology into clinical application.
Authors' Contributions
Yuanming Geng, Zhenxing Li, Junhao Zhu, Chaonan Du, Feng Yuan, Xiangming Cai, Alleyar Alia, Jin Yang, Chao Tang, Zixiang Cong, and Chiyuan Ma all contributed to the review of relevant literature and the writing, editing, and/or revising of the manuscript. The authors declare that the manuscript has not been submitted for publication elsewhere, in whole or in part, in any language.
Funding Information
This study was supported by the National Natural Science Foundation of China (grant no. 82101461) and the Postdoctoral Sustentation Fund of Jinling Hospital (grant no. 49008).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Krucoff MO, Miller JP, Saxena T, et al. toward functional restoration of the central nervous system: a review of translational neuroscience principles. Neurosurgery 2019;84:30–40; doi: 10.1093/neuros/nyy128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambert C, Cisternas P, Inestrosa NC. Role of Wnt signaling in central nervous system injury. Mol Neurobiol 2016; 53:2297–2311; doi: 10.1007/s12035-015-9138-x [DOI] [PubMed] [Google Scholar]
- 3. Sun P, Liu DZ, Jickling GC, et al. MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab 2018;38:1125–1148; doi: 10.1177/0271678x18773871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho Y, Borgens RB. Polymer and nano-technology applications for repair and reconstruction of the central nervous system. Exp Neurol 2012;233:126–144; doi: 10.1016/j.expneurol.2011.09.028 [DOI] [PubMed] [Google Scholar]
- 5. Forraz N, Wright KE, Jurga M, et al. Experimental therapies for repair of the central nervous system: stem cells and tissue engineering. J Tissue Eng Regen Med 2013;7:523–536; doi: 10.1002/term.552 [DOI] [PubMed] [Google Scholar]
- 6. Rost BR, Wietek J, Yizhar O, et al. Optogenetics at the presynapse. Nat Neurosci 2022;25:984–998; doi: 10.1038/s41593-022-01113-6 [DOI] [PubMed] [Google Scholar]
- 7. Adesnik H, Abdeladim L. Probing neural codes with two-photon holographic optogenetics. Nat Neurosci 2021;24:1356–1366; doi: 10.1038/s41593-021-00902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rose T, Goltstein PM, Portugues R, et al. Putting a finishing touch on GECIs. Front Mol Neurosci 2014;7:88; doi: 10.3389/fnmol.2014.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villette V, Chavarha M, Dimov IK, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell 2019;179:1590–1608.e1523; doi: 10.1016/j.cell.2019.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bentley JN, Chestek C, Stacey WC, et al. Optogenetics in epilepsy. Neurosurg Focus 2013;34:E4; doi: 10.3171/2013.3.Focus1364 [DOI] [PubMed] [Google Scholar]
- 11. Touriño C, Eban-Rothschild A, de Lecea L. Optogenetics in psychiatric diseases. Curr Opin Neurobiol 2013;23:430–435; doi: 10.1016/j.conb.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Q, Song D, Xie Z, et al. Optogenetic stimulation of CA3 pyramidal neurons restores synaptic deficits to improve spatial short-term memory in APP/PS1 mice. Prog Neurobiol 2022;209:102209; doi: 10.1016/j.pneurobio.2021.102209 [DOI] [PubMed] [Google Scholar]
- 13. Nagata T, Inoue K. Rhodopsins at a glance. J Cell Sci 2021;134; doi: 10.1242/jcs.258989 [DOI] [PubMed] [Google Scholar]
- 14. Zhang F, Vierock J, Yizhar O, et al. The microbial opsin family of optogenetic tools. Cell 2011;147:1446–1457; doi: 10.1016/j.cell.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan P, He L, Huang Y, et al. Optophysiology: illuminating cell physiology with optogenetics. Physiol Rev 2022;102(3):1263–1325; doi: 10.1152/physrev.00021.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bang E, Fincher AS, Nader S, et al. Late-onset, short-term intermittent fasting reverses age-related changes in calcium buffering and inhibitory synaptic transmission in mouse basal forebrain neurons. J Neurosci 2022;42:1020–1034; doi: 10.1523/jneurosci.1442-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergs A, Schultheis C, Fischer E, et al. Rhodopsin optogenetic toolbox v2.0 for light-sensitive excitation and inhibition in Caenorhabditis elegans. PLoS One 2018;13:e0191802; doi: 10.1371/journal.pone.0191802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Klapoetke NC, Murata Y, Kim SS, et al. Independent optical excitation of distinct neural populations. Nat Methods 2014;11:338–346; doi: 10.1038/nmeth.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moshkforoush A, Balachandar L, Moncion C, et al. Unraveling ChR2-driven stochastic Ca2+ dynamics in astrocytes: a call for new interventional paradigms. PLoS Comput Biol 2021;17:e1008648; doi: 10.1371/journal.pcbi.1008648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. López-Hernández GY, Ananth M, Jiang L, et al. Electrophysiological properties of basal forebrain cholinergic neurons identified by genetic and optogenetic tagging. J Neurochem 2017;142(Suppl 2):103–110; doi: 10.1111/jnc.14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huet AT, Rankovic V. Application of targeting-optimized chronos for stimulation of the auditory pathway. Methods Mol Biol 2021;2191:261–285; doi: 10.1007/978-1-0716-0830-2_16 [DOI] [PubMed] [Google Scholar]
- 22. Chen R, Gore F, Nguyen QA, et al. Deep brain optogenetics without intracranial surgery. Nat Biotechnol 2021;39:161–164; doi: 10.1038/s41587-020-0679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bedbrook CN, Yang KK, Robinson JE, et al. Machine learning-guided channelrhodopsin engineering enables minimally invasive optogenetics. Nat Methods 2019;16:1176–1184; doi: 10.1038/s41592-019-0583-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong X, Mendoza-Halliday D, Ting JT, et al. An ultra-sensitive step-function opsin for minimally invasive optogenetic stimulation in mice and macaques. Neuron 2020;107:38–51.e38; doi: 10.1016/j.neuron.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferenczi EA, Vierock J, Atsuta-Tsunoda K, et al. Optogenetic approaches addressing extracellular modulation of neural excitability. Sci Rep 2016;6:23947; doi: 10.1038/srep23947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chow BY, Han X, Dobry AS, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 2010;463:98–102; doi: 10.1038/nature08652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Husson SJ, Liewald JF, Schultheis C, et al. Microbial light-activatable proton pumps as neuronal inhibitors to functionally dissect neuronal networks in C. elegans. PLoS One 2012;7:e40937; doi: 10.1371/journal.pone.0040937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antinucci P, Dumitrescu A, Deleuze C, et al. A calibrated optogenetic toolbox of stable zebrafish opsin lines. Elife 2020;9:353947; doi: 10.7554/eLife.54937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chuong AS, Miri ML, Busskamp V, et al. Noninvasive optical inhibition with a red–shifted microbial rhodopsin. Nat Neurosci 2014;17:1123–1129; doi: 10.1038/nn.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun Y, Li M, Cao S, et al. Optogenetics for understanding and treating brain injury: advances in the field and future prospects. Int J Mol Sci 2022;23(3):1800; doi: 10.3390/ijms23031800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahn M, Saraf-Sinik I, Patil P, et al. Efficient optogenetic silencing of neurotransmitter release with a mosquito rhodopsin. Neuron 2021;109:1621–1635.e1628; doi: 10.1016/j.neuron.2021.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamada S, Nagase M, Yoshizawa T, et al. An engineered channelrhodopsin optimized for axon terminal activation and circuit mapping. Commun Biol 2021;4:461; doi: 10.1038/s42003-021-01977-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Delaney SL, Gendreau JL, D'Souza M, et al. Optogenetic modulation for the treatment of traumatic brain injury. Stem Cells Dev 2020;29:187–197; doi: 10.1089/scd.2019.0187 [DOI] [PubMed] [Google Scholar]
- 34. Zhang F, Gradinaru V, Adamantidis AR, et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 2010;5:439–456; doi: 10.1038/nprot.2009.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen YH, Keiser MS, Davidson BL. Viral vectors for gene transfer. Curr Protoc Mouse Biol 2018;8:e58; doi: 10.1002/cpmo.58 [DOI] [PubMed] [Google Scholar]
- 36. Poletti V, Mavilio F. Interactions between retroviruses and the host cell genome. Mol Ther Methods Clin Dev 2018;8:31–41; doi: 10.1016/j.omtm.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amar AP, Zlokovic BV, Apuzzo ML. Endovascular restorative neurosurgery: a novel concept for molecular and cellular therapy of the nervous system. Neurosurgery 2003;52:402–412; discussion 412–403; doi: 10.1227/01.neu.0000043698.86548.a0 [DOI] [PubMed] [Google Scholar]
- 38. Rutka JT, Taylor M, Mainprize T, et al. Molecular biology and neurosurgery in the third millennium. Neurosurgery 2000;46 1034–1051; doi: 10.1097/00006123-200005000-00002 [DOI] [PubMed] [Google Scholar]
- 39. Warnock JN, Daigre C and Al-Rubeai M. Introduction to viral vectors. Methods Mol Biol 2011; 737:1–25; doi: 10.1007/978-1-61779-095-9_1 [DOI] [PubMed] [Google Scholar]
- 40. Grimm D, Lee JS, Wang L, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol 2008;82:5887–5911; doi: 10.1128/jvi.00254-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kimura T, Ferran B, Tsukahara Y, et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci Rep 2019;9:13601; doi: 10.1038/s41598-019-49624-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 2017;18:222–235; doi: 10.1038/nrn.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim CY, Ku MJ, Qazi R, et al. Soft subdermal implant capable of wireless battery charging and programmable controls for applications in optogenetics. Nat Commun 2021;12:535; doi: 10.1038/s41467-020-20803-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Montgomery KL, Yeh AJ, Ho JS, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods 2015;12:969–974; doi: 10.1038/nmeth.3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang Y, Wu M, Vázquez-Guardado A, et al. Wireless multilateral devices for optogenetic studies of individual and social behaviors. Nat Neurosci 2021;24:1035–1045; doi: 10.1038/s41593-021-00849-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gutruf P and Rogers JA. Implantable, wireless device platforms for neuroscience research. Curr Opin Neurobiol 2018;50:42–49; doi: 10.1016/j.conb.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Castro DC, Han Y, et al. Battery-free, lightweight, injectable microsystem for in vivo wireless pharmacology and optogenetics. Proc Natl Acad Sci U S A 2019;116:21427–21437; doi: 10.1073/pnas.1909850116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rajalingham R, Sorenson M, Azadi R, et al. Chronically implantable LED arrays for behavioral optogenetics in primates. Nat Methods 2021;18:1112–1116; doi: 10.1038/s41592-021-01238-9 [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, Liu X, Xu W, et al. Stretchable transparent electrode arrays for simultaneous electrical and optical interrogation of neural circuits in vivo. Nano Lett 2018;18:2903–2911; doi: 10.1021/acs.nanolett.8b00087 [DOI] [PubMed] [Google Scholar]
- 50. Ai X, Lyu L, Zhang Y, et al. remote regulation of membrane channel activity by site-specific localization of lanthanide-doped upconversion nanocrystals. Angew Chem Int Ed Engl 2017;56:3031–3035; doi: 10.1002/anie.201612142 [DOI] [PubMed] [Google Scholar]
- 51. Bansal A, Liu H, Jayakumar MK, et al. Quasi-continuous wave near-infrared excitation of upconversion nanoparticles for optogenetic manipulation of C. elegans. Small 2016;12:1732–1743; doi: 10.1002/smll.201503792 [DOI] [PubMed] [Google Scholar]
- 52. Liu X, Chen H, Wang Y, et al. Near-infrared manipulation of multiple neuronal populations via trichromatic upconversion. Nat Commun 2021;12:5662; doi: 10.1038/s41467-021-25993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383:245–254; doi: 10.1016/s0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil 2006;21:375–378; doi: 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- 55. Lee BB, Cripps RA, Fitzharris M, et al. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord 2014;52:110–116; doi: 10.1038/sc.2012.158 [DOI] [PubMed] [Google Scholar]
- 56. Cheng P, Yin P, Ning P, et al. Trends in traumatic brain injury mortality in China, 2006-2013: a population-based longitudinal study. PLoS Med 2017;14:e1002332; doi: 10.1371/journal.pmed.1002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Centers for Disease Control and Prevention. Traumatic Brain Injury & Contusion. TBI Data. Available from: https://www.cdc.gov/traumaticbraininjury/data/index.html [last accessed March 21, 2022].
- 58. Institute for Health Metrics and Evaluation. Global Burden of Disease Study 2019 (GBD2019) Data Resources. https://ghdx.healthdata.org/gbd-2019 [Last accessed September 5, 2022].
- 59. Kaur P, Sharma S. Recent advances in pathophysiology of traumatic brain injury. Curr Neuropharmacol 2018;16:1224–1238; doi: 10.2174/1570159x15666170613083606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am 2020;104:213–238; doi: 10.1016/j.mcna.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 61. Adams C, Bazzigaluppi P, Beckett TL, et al. Neurogliovascular dysfunction in a model of repeated traumatic brain injury. Theranostics 2018;8:4824–4836; doi: 10.7150/thno.24747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen T, Al-Juboori MH, Walerstein J, et al. Impaired glutamate receptor function underlies early activity loss of ipsilesional motor cortex after closed-head mild traumatic brain injury. J Neurotrauma 2021;38:2018–2029; doi: 10.1089/neu.2020.7225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jones S, Schwartzbauer G, Jia X. Brain monitoring in critically neurologically impaired patients. Int J Mol Sci 2016;18(1):43; doi: 10.3390/ijms18010043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Krukowski K, Nolan A, Becker M, et al. Novel microglia-mediated mechanisms underlying synaptic loss and cognitive impairment after traumatic brain injury. Brain Behav Immun 2021;98:122–135; doi: 10.1016/j.bbi.2021.08.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ndode-Ekane XE, Puigferrat Pérez MDM, Di Sapia R, et al. Reorganization of thalamic inputs to lesioned cortex following experimental traumatic brain injury. Int J Mol Sci 2021;22(12):6329; doi: 10.3390/ijms22126329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chever O, Zerimech S, Scalmani P, et al. Initiation of migraine-related cortical spreading depolarization by hyperactivity of GABAergic neurons and NaV1.1 channels. J Clin Invest 2021;131; doi: 10.1172/jci142203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mester JR, Bazzigaluppi P, Dorr A, et al. Attenuation of tonic inhibition prevents chronic neurovascular impairments in a Thy1-ChR2 mouse model of repeated, mild traumatic brain injury. Theranostics 2021;11:7685–7699; doi: 10.7150/thno.60190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zeng XJ, Li P, Ning YL, et al. A(2A) R inhibition in alleviating spatial recognition memory impairment after TBI is associated with improvement in autophagic flux in RSC. J Cell Mol Med 2020;24:7000–7014; doi: 10.1111/jcmm.15361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ong Q, Guo S, Duan L, et al. The timing of Raf/ERK and AKT activation in protecting PC12 cells against oxidative stress. PLoS One 2016;11:e0153487; doi: 10.1371/journal.pone.0153487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cela E, Sjöström PJ. A step-by-step protocol for optogenetic kindling. Front Neural Circuits 2020;14:3; doi: 10.3389/fncir.2020.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gao Y, Zheng J, Jiang T, et al. targeted reducing of tauopathy alleviates epileptic seizures and spatial memory impairment in an optogenetically inducible mouse model of epilepsy. Front Cell Dev Biol 2020;8:633725; doi: 10.3389/fcell.2020.633725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Brady RD, Casillas-Espinosa PM, Agoston DV, et al. Modelling traumatic brain injury and posttraumatic epilepsy in rodents. Neurobiol Dis 2019;123:8–19; doi: 10.1016/j.nbd.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhao ML, Chen SJ, Li XH, et al. Optical depolarization of DCX-expressing cells promoted cognitive recovery and maturation of newborn neurons via the Wnt/β-catenin pathway. J Alzheimers Dis 2018;63:303–318; doi: 10.3233/jad-180002 [DOI] [PubMed] [Google Scholar]
- 74. Sahel JA, Boulanger-Scemama E, Pagot C, et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 2021;27:1223–1229; doi: 10.1038/s41591-021-01351-4 [DOI] [PubMed] [Google Scholar]
- 75. Sharma M, Naik V and Deogaonkar M. Emerging applications of deep brain stimulation. J Neurosurg Sci 2016;60:242–255. [PubMed] [Google Scholar]
- 76. Shin SS, Pelled G. Novel neuromodulation techniques to assess interhemispheric communication in neural injury and neurodegenerative diseases. Front Neural Circuits 2017;11:15; doi: 10.3389/fncir.2017.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. National Spinal Cord Injury Center. Spinal Cord Injury Model Systems. 2021 Annual Report—Complete Public Version. Available from: https://www.nscisc.uab.edu/public/AR2021_public%20version.pdf [Last accessed December 2021].
- 78. National Spinal Cord Injury Center. Traumatic Spinal Cord Injury. Facts and Figures 2022-English Final. Available from: https://www.nscisc.uab.edu/public/Facts%20and%20Figures%202022%20-%20English%20Final.pdf [Last accessed November 2022].
- 79. Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ 2002;26:238–255; doi: 10.1152/advan.00039.2002 [DOI] [PubMed] [Google Scholar]
- 80. Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain 2006;129:3249–3269; doi: 10.1093/brain/awl296 [DOI] [PubMed] [Google Scholar]
- 81. Su Z, Yuan Y, Chen J, et al. Reactive astrocytes inhibit the survival and differentiation of oligodendrocyte precursor cells by secreted TNF-α. J Neurotrauma 2011;28:1089–1100; doi: 10.1089/neu.2010.1597 [DOI] [PubMed] [Google Scholar]
- 82. Song YH, Agrawal NK, Griffin JM, et al. Recent advances in nanotherapeutic strategies for spinal cord injury repair. Adv Drug Deliv Rev 2019;148:38–59; doi: 10.1016/j.addr.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Forn-Cuní G, Varela M, Pereiro P, et al. Conserved gene regulation during acute inflammation between zebrafish and mammals. Sci Rep 2017;7:41905; doi: 10.1038/srep41905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang F, Wang L, Zou X, et al. Advances in CRISPR-Cas systems for RNA targeting, tracking and editing. Biotechnol Adv 2019;37:708–729; doi: 10.1016/j.biotechadv.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 85. Paschon V, Correia FF, Morena BC, et al. CRISPR, prime editing, optogenetics, and DREADDs: new therapeutic approaches provided by emerging technologies in the treatment of spinal cord injury. Mol Neurobiol 2020;57:2085–2100. 2020/01/14; doi: 10.1007/s12035-019-01861-w [DOI] [PubMed] [Google Scholar]
- 86. Raikwar SP, Thangavel R, Dubova I, et al. Targeted gene editing of glia maturation factor in microglia: a novel Alzheimer's disease therapeutic target. Mol Neurobiol 2019;56:378–393; doi: 10.1007/s12035-018-1068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Guo Q, Li S, Liang Y, et al. Effects of C3 deficiency on inflammation and regeneration following spinal cord injury in mice. Neurosci Lett 2010;485:32–36; doi: 10.1016/j.neulet.2010.08.056 [DOI] [PubMed] [Google Scholar]
- 88. Hughes RM, Freeman DJ, Lamb KN, et al. Optogenetic apoptosis: light-triggered cell death. Angew Chem Int Ed Engl 2015;54:12064–12068; doi: 10.1002/anie.201506346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ono K, Suzuki H, Yamamoto R, et al. Optogenetic control of cell differentiation in channelrhodopsin-2-expressing OS3, a bipotential glial progenitor cell line. Neurochem Int 2017;104:49–63; doi: 10.1016/j.neuint.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 90. Chen JY, Fu EJ, Patel PR, et al. Lentiviral -nterleukin-10 gene therapy preserves fine motor circuitry and function after a cervical spinal cord injury in male and female mice. Neurotherapeutics 2021;18503–514; doi: 10.1007/s13311-020-00946-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao L, Peng Y, Xu W, et al. Progress in stem cell therapy for spinal cord injury. Stem Cells Int 2020;2020:2853650; doi: 10.1155/2020/2853650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Giraldo E, Palmero-Canton D, Martinez-Rojas B, et al. Optogenetic modulation of neural progenitor cells improves neuroregenerative potential. Int J Mol Sci 2020;22(1):365 doi: 10.3390/ijms22010365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ahmad A, Ashraf S, Komai S. Optogenetics applications for treating spinal cord injury. Asian Spine J 2015;9:299–305; doi: 10.4184/asj.2015.9.2.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dias DO, Kim H, Holl D, et al. Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 2018;173:153–165.e122; doi: 10.1016/j.cell.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ceto S, Sekiguchi KJ, Takashima Y, et al. Neural Stem Cell Grafts Form Extensive Synaptic Networks that Integrate with Host Circuits after Spinal Cord Injury. Cell Stem Cell 2020; 27: 430–440.e435; doi: 10.1016/j.stem.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bryson JB, Machado CB, Crossley M, et al. Optical control of muscle function by transplantation of stem cell-derived motor neurons in mice. Science 2014;344:94–97; doi: 10.1126/science.1248523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Park S, Koppes RA, Froriep UP, et al. Optogenetic control of nerve growth. Sci Rep 2015;5:9669; doi: 10.1038/srep09669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bryson JB, Machado CB, Lieberam I, et al. Restoring motor function using optogenetics and neural engraftment. Curr Opin Biotechnol 2016;40:75–81; doi: 10.1016/j.copbio.2016.02.016 [DOI] [PubMed] [Google Scholar]
- 99. Deng WW, Wu GY, Min LX, et al. optogenetic neuronal stimulation promotes functional recovery after spinal cord injury. Front Neurosci 2021;15:640255; doi: 10.3389/fnins.2021.640255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mallory GW, Grahn PJ, Hachmann JT, et al. Optical stimulation for restoration of motor function after spinal cord injury. Mayo Clin Proc 2015;90:300–307; doi: 10.1016/j.mayocp.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Petersen ED, Sharkey ED, Pal A, et al. Restoring function after severe spinal cord injury through BioLuminescent-OptoGenetics. Front Neurol 2021;12:792643; doi: 10.3389/fneur.2021.792643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mondello SE, Pedigo BD, Sunshine MD, et al. A micro-LED implant and technique for optogenetic stimulation of the rat spinal cord. Exp Neurol 2021;335:113480; doi: 10.1016/j.expneurol.2020.113480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kathe C, Michoud F, Schönle P, et al. Wireless closed-loop optogenetics across the entire dorsoventral spinal cord in mice. Nat Biotechnol 2022;40:198–208; doi: 10.1038/s41587-021-01019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers 2019;5:70; doi: 10.1038/s41572-019-0118-8 [DOI] [PubMed] [Google Scholar]
- 105. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020;141:e139–e596; doi: 10.1161/cir.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 106. Lipton P. Ischemic cell death in brain neurons. Physiol Rev 1999;79:1431–1568; doi: 10.1152/physrev.1999.79.4.1431 [DOI] [PubMed] [Google Scholar]
- 107. George PM, Steinberg GK. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron 2015;87:297–309; doi: 10.1016/j.neuron.2015.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Azad TD, Veeravagu A, Steinberg GK. Neurorestoration after stroke. Neurosurg Focus 2016;40:E2; doi: 10.3171/2016.2.Focus15637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Szydlowska K and Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium 2010;47:122–129; doi: 10.1016/j.ceca.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 110. Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 2009;40:1849–1857; doi: 10.1161/strokeaha.108.534503 [DOI] [PubMed] [Google Scholar]
- 111. Moro MA, Cárdenas A, Hurtado O, et al. Role of nitric oxide after brain ischaemia. Cell Calcium 2004;36:265–275; doi: 10.1016/j.ceca.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 112. Zrzavy T, Machado-Santos J, Christine S, et al. Dominant role of microglial and macrophage innate immune responses in human ischemic infarcts. Brain Pathol 2018;28:791–805; doi: 10.1111/bpa.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cheng MY, Aswendt M, Steinberg GK. Optogenetic approaches to target specific neural circuits in post-stroke recovery. Neurotherapeutics 2016;13:325–340; doi: 10.1007/s13311-015-0411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wahl AS. State-of-the-art techniques to causally link neural plasticity to functional recovery in experimental stroke research. Neural Plast 2018;2018:3846593; doi: 10.1155/2018/3846593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ting WK, Fadul FA, Fecteau S, et al. Neurostimulation for stroke rehabilitation. Front Neurosci 2021;15:649459; doi: 10.3389/fnins.2021.649459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Feldman DE. The spike-timing dependence of plasticity. Neuron 2012;75:556–571; doi: 10.1016/j.neuron.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Markram H, Lübke J, Frotscher M, et al. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 1997;275:213–215; doi: 10.1126/science.275.5297.213 [DOI] [PubMed] [Google Scholar]
- 118. Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci 1998;18:10464–10472; doi: 10.1523/jneurosci.18-24-10464.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lu C, Wu X, Ma H, et al. Optogenetic stimulation enhanced neuronal plasticities in motor recovery after ischemic stroke. Neural Plast 2019;2019:5271573; doi: 10.1155/2019/5271573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cheng MY, Wang EH, Woodson WJ, et al. Optogenetic neuronal stimulation promotes functional recovery after stroke. Proc Natl Acad Sci U S A 2014;111:12913–12918; doi: 10.1073/pnas.1404109111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Mirza Agha B, Akbary R, Ghasroddashti A, et al. Cholinergic upregulation by optogenetic stimulation of nucleus basalis after photothrombotic stroke in forelimb somatosensory cortex improves endpoint and motor but not sensory control of skilled reaching in mice. J Cereb Blood Flow Metab 2021;41:1608–1622; doi: 10.1177/0271678x20968930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Engineer ND, Kimberley TJ, Prudente CN, et al. Targeted vagus nerve stimulation for rehabilitation after stroke. Front Neurosci 2019;13:280; doi: 10.3389/fnins.2019.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kantak SS, Stinear JW, Buch ER, et al. Rewiring the brain: potential role of the premotor cortex in motor control, learning, and recovery of function following brain injury. Neurorehabil Neural Repair 2012;26:282–292; doi: 10.1177/1545968311420845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sankarasubramanian V, Machado AG, Conforto AB, et al. Inhibition versus facilitation of contralesional motor cortices in stroke: Deriving a model to tailor brain stimulation. Clin Neurophysiol 2017;128:892–902; doi: 10.1016/j.clinph.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gao BY, Cao YX, Fu PF, et al. Optogenetics stimulates nerve reorganization in the contralesional anterolateral primary motor cortex in a mouse model of ischemic stroke. Neural Regen Res 2022;17:1535–1544.; doi: 10.4103/1673-5374.330615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bo B, Li Y, Li W, et al. Optogenetic excitation of ipsilesional sensorimotor neurons is protective in acute ischemic stroke: a laser speckle imaging study. IEEE Trans Biomed Eng 2019;66:1372–1379; doi: 10.1109/tbme.2018.2872965 [DOI] [PubMed] [Google Scholar]
- 127. Pan HC, Liao LD, Lo YC, et al. Neurovascular function recovery after focal ischemic stroke by enhancing cerebral collateral circulation via peripheral stimulation-mediated interarterial anastomosis. Neurophotonics 2017;4:035003; doi: 10.1117/1.NPh.4.3.035003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bo B, Li Y, Li W, et al. neurovascular coupling impairment in acute ischemic stroke by optogenetics and optical brain imaging. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:3727–3730; doi: 10.1109/embc44109.2020.9176641 [DOI] [PubMed] [Google Scholar]
- 129. Balbi M, Xiao D, Jativa Vega M, et al. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep 2021;34:108696; doi: 10.1016/j.celrep.2021.108696 [DOI] [PubMed] [Google Scholar]
- 130. Muzzi L, Hassink G, Levers M, et al. Mild stimulation improves neuronal survival in an in vitro model of the ischemic penumbra. J Neural Eng 2019;17:016001; doi: 10.1088/1741-2552/ab51d4 [DOI] [PubMed] [Google Scholar]
- 131. Daadi MM, Klausner JQ, Bajar B, et al. optogenetic stimulation of neural grafts enhances neurotransmission and downregulates the inflammatory response in experimental stroke model. Cell Transplant 2016;25:1371–1380; doi: 10.3727/096368915x688533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yu SP, Tung JK, Wei ZZ, et al. Optochemogenetic stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J Neurosci 2019;39:6571–6594; doi: 10.1523/jneurosci.2010-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A 1983;80:2390–2394; doi: 10.1073/pnas.80.8.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron 2001;30:19–35; doi: 10.1016/s0896-6273(01)00260-4 [DOI] [PubMed] [Google Scholar]
- 135. Doetsch F, Petreanu L, Caille I, et al. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 2002;36:1021–1034; doi: 10.1016/s0896-6273(02)01133-9 [DOI] [PubMed] [Google Scholar]
- 136. Song M, Yu SP, Mohamad O, et al. Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol Dis 2017;98:9–24; doi: 10.1016/j.nbd.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lu Y, Jiang L, Li W, et al. optogenetic inhibition of striatal neuronal activity improves the survival of transplanted neural stem cells and neurological outcomes after ischemic stroke in mice. Stem Cells Int 2017;2017:4364302; doi: 10.1155/2017/4364302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. He X, Lu Y, Lin X, et al. Optical inhibition of striatal neurons promotes focal neurogenesis and neurobehavioral recovery in mice after middle cerebral artery occlusion. J Cereb Blood Flow Metab 2017;37:837–847; doi: 10.1177/0271678x16642242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chiu FL, Lin JT, Chuang CY, et al. Elucidating the role of the A2A adenosine receptor in neurodegeneration using neurons derived from Huntington's disease iPSCs. Hum Mol Genet 2015;24:6066–6079; doi: 10.1093/hmg/ddv318 [DOI] [PubMed] [Google Scholar]
- 140. Jiang L, Li W, Mamtilahun M, et al. Optogenetic inhibition of striatal GABAergic neuronal activity improves outcomes after ischemic brain injury. Stroke 2017;48:3375–3383; doi: 10.1161/strokeaha.117.019017 [DOI] [PubMed] [Google Scholar]
- 141. Palma-Tortosa S, Coll-San Martin B, Kokaia Z, et al. neuronal replacement in stem cell therapy for stroke: filling the gap. Front Cell Dev Biol 2021;9:662636; doi: 10.3389/fcell.2021.662636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shah AM, Ishizaka S, Cheng MY, et al. Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci Rep 2017;7:46612; doi: 10.1038/srep46612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Machado AG, Baker KB, Schuster D, et al. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res 2009;1280:107–116; doi: 10.1016/j.brainres.2009.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liepert J, Kucinski T, Tüscher O, et al. Motor cortex excitability after cerebellar infarction. Stroke 2004;35:2484–2488; doi: 10.1161/01.STR.0000143152.45801.ca [DOI] [PubMed] [Google Scholar]
- 145. Pendharkar AV, Smerin D, Gonzalez L, et al. optogenetic stimulation reduces neuronal nitric oxide synthase expression after stroke. Transl Stroke Res 2021;12:347–356; doi: 10.1007/s12975-020-00831-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Cheng MY, Wang EH and Steinberg GK. Optogenetic approaches to study stroke recovery. ACS Chem Neurosci 2014;5:1144–1145; doi: 10.1021/cn500216f [DOI] [PubMed] [Google Scholar]
- 147. Tennant KA, Taylor SL, White ER, et al. Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nat Commun 2017;8:15879; doi: 10.1038/ncomms15879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Paz JT, Davidson TJ, Frechette ES, et al. Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat Neurosci 2013;16:64–70; doi: 10.1038/nn.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mensen A, Pigorini A, Facchin L, et al. Sleep as a model to understand neuroplasticity and recovery after stroke: observational, perturbational and interventional approaches. J Neurosci Methods 2019;313:37–43; doi: 10.1016/j.jneumeth.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 150. Timofeev I and Chauvette S. Sleep slow oscillation and plasticity. Curr Opin Neurobiol 2017;44:116–126; doi: 10.1016/j.conb.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 151. Duss SB, Seiler A, Schmidt MH, et al. The role of sleep in recovery following ischemic stroke: a review of human and animal data. Neurobiol Sleep Circadian Rhythms 2017;2:94–105; doi: 10.1016/j.nbscr.2016.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hodor A, Palchykova S, Baracchi F, et al. Baclofen facilitates sleep, neuroplasticity, and recovery after stroke in rats. Ann Clin Transl Neurol 2014;1:765–777;doi: 10.1002/acn3.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Facchin L, Schöne C, Mensen A, et al. Slow waves promote sleep-dependent plasticity and functional recovery after stroke. J Neurosci 2020;40:8637–8651; doi: 10.1523/jneurosci.0373-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Segovia G, del Arco A, Mora F. Environmental enrichment, prefrontal cortex, stress, and aging of the brain. J Neural Transm (Vienna) 2009;116:1007–1016; doi: 10.1007/s00702-009-0214-0 [DOI] [PubMed] [Google Scholar]
- 155. He C, Tsipis CP, LaManna JC, et al. Environmental enrichment induces increased cerebral capillary density and improved cognitive function in mice. Adv Exp Med Biol 2017;977:175–181; doi: 10.1007/978-3-319-55231-6_24 [DOI] [PubMed] [Google Scholar]
- 156. Lin Y, Yao M, Wu H, et al. Environmental enrichment implies GAT-1 as a potential therapeutic target for stroke recovery. Theranostics 2021;11:3760–3780; doi: 10.7150/thno.53316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang Q, Wu JF, Shi QL, et al. The neuronal activation of deep cerebellar nuclei is essential for environmental enrichment-induced post-stroke motor recovery. Aging Dis 2019;10:530–543; doi: 10.14336/ad.2018.1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chen G, Cao Y, Tang Y, et al. Advanced near-infrared light for monitoring and modulating the spatiotemporal dynamics of cell functions in living systems. Adv Sci (Weinh) 2020;7:1903783; doi: 10.1002/advs.201903783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mostany R, Miquelajauregui A, Shtrahman M, et al. Two-photon excitation microscopy and its applications in neuroscience. Methods Mol Biol 2015;1251:25–42; doi: 10.1007/978-1-4939-2080-8_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hipskind SG, Grover FL Jr., Fort TR, et al. Pulsed transcranial red/near-infrared light therapy using light-emitting diodes improves cerebral blood flow and cognitive function in veterans with chronic traumatic brain injury: a case series. Photobiomodul Photomed Laser Surg 2019;37:77–84; doi: 10.1089/photob.2018.4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Gysbrechts B, Wang L, Trong NN, et al. Light distribution and thermal effects in the rat brain under optogenetic stimulation. J Biophotonics 2016;9:576–585; doi: 10.1002/jbio.201500106 [DOI] [PubMed] [Google Scholar]
- 162. Conti E, Scaglione A, de Vito G, et al. Combining optogenetic stimulation and motor training improves functional recovery and perilesional cortical activity. Neurorehabil Neural Repair 2022;36107–36118; doi: 10.1177/15459683211056656 [DOI] [PubMed] [Google Scholar]
- 163. Wahl AS, Büchler U, Brändli A, et al. Optogenetically stimulating intact rat corticospinal tract post-stroke restores motor control through regionalized functional circuit formation. Nat Commun 2017;8:1187; doi: 10.1038/s41467-017-01090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]