Abstract
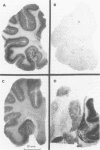
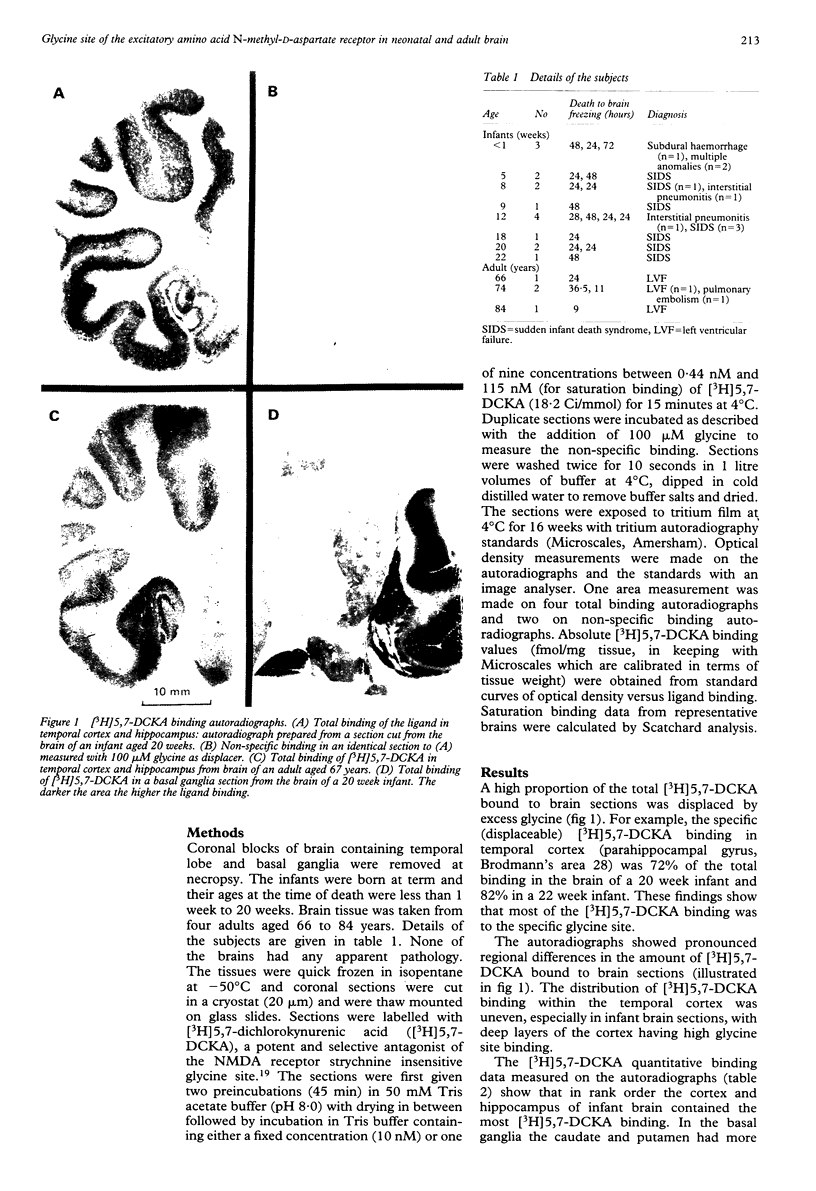
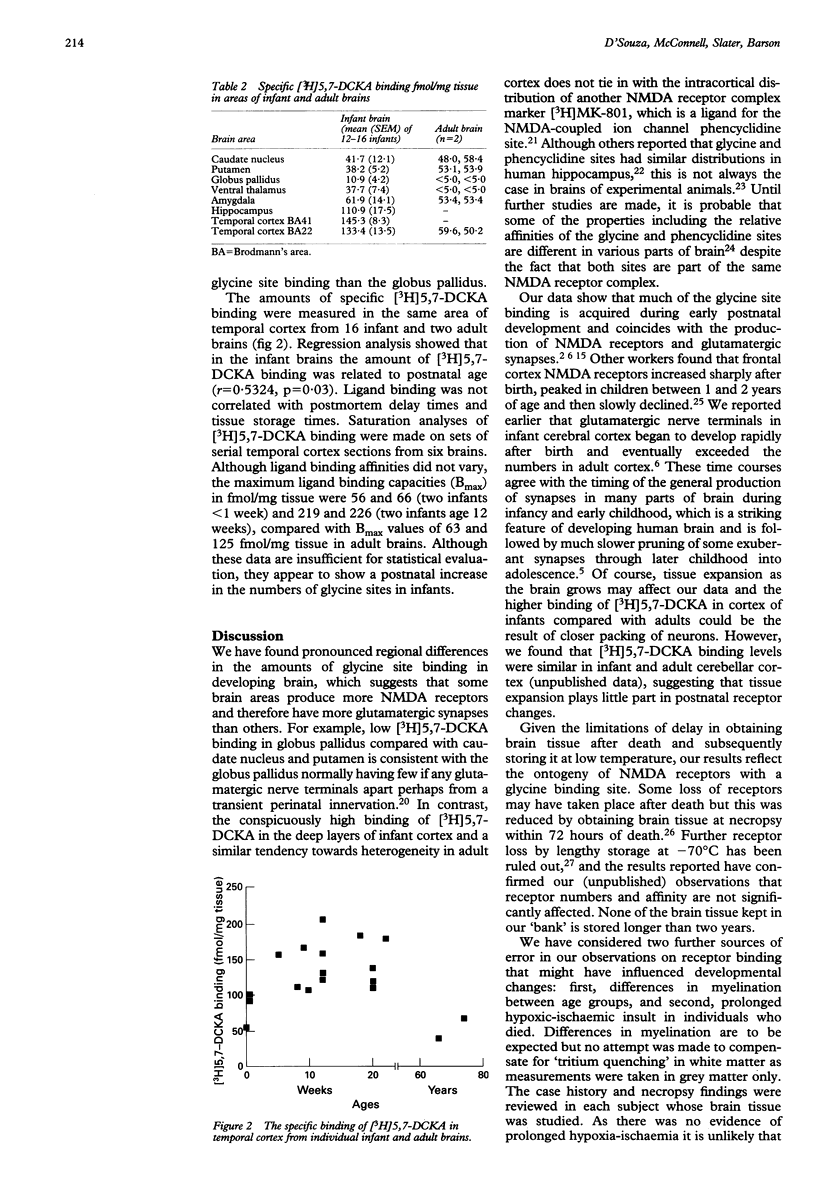
The N-methyl-D-aspartate (NMDA) receptor complex in brain is a glutamate receptor subtype with several recognition sites including a glycine site that is able to modulate and activate allosterically the receptor. This receptor may be important in the regulation of developmental synaptic plasticity. The release of glutamate and consequent overstimulation of NMDA receptors that follows hypoxia-ischaemia leads to brain damage. Brain tissue obtained at necropsy was studied in a total of 16 term infants aged less than 1 week to 22 weeks and in four adults aged from 66 to 84 years. Glycine sites were determined in brain sections by the binding of the selective ligand [3H]5,7-dichloro-kynurenic acid and measured by autoradiography. In infant brains the amount of binding to the glycine site was higher in temporal cortex and hippocampus than in basal ganglia and was also higher than in comparable areas of adult brain. The amount of glycine site binding in infant cortex increased with postnatal age. The data suggest that infant brain acquires a relatively high density of NMDA receptors in temporal lobe due to postnatal proliferation of glutamatergic synapses. These findings have therapeutic implications as drugs that reduce NMDA receptor function by blocking the glycine modulatory site would be pertinent to preventing brain damage after hypoxia-ischaemia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers G. W., Goldberg M. P., Choi D. W. N-methyl-D-aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989 Apr;25(4):398–403. doi: 10.1002/ana.410250412. [DOI] [PubMed] [Google Scholar]
- Albers G. W. Potential therapeutic uses of N-methyl-D-aspartate antagonists in cerebral ischemia. Clin Neuropharmacol. 1990 Jun;13(3):177–197. doi: 10.1097/00002826-199006000-00001. [DOI] [PubMed] [Google Scholar]
- Balázs R., Jørgensen O. S., Hack N. N-methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988 Nov;27(2):437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Bonhaus D. W., Yeh G. C., Skaryak L., McNamara J. O. Glycine regulation of the N-methyl-D-aspartate receptor-gated ion channel in hippocampal membranes. Mol Pharmacol. 1989 Aug;36(2):273–279. [PubMed] [Google Scholar]
- Choi D. W., Rothman S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Lester R. A. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989 Jun;41(2):143–210. [PubMed] [Google Scholar]
- D'Souza S. W., McConnell S. E., Slater P., Barson A. J. N-methyl-D-aspartate binding sites in neonatal and adult brain. Lancet. 1992 May 16;339(8803):1240–1240. doi: 10.1016/0140-6736(92)91188-e. [DOI] [PubMed] [Google Scholar]
- Drian M. J., Kamenka J. M., Pirat J. L., Privat A. Non-competitive antagonists of N-methyl-D-aspartate prevent spontaneous neuronal death in primary cultures of embryonic rat cortex. J Neurosci Res. 1991 May;29(1):133–138. doi: 10.1002/jnr.490290116. [DOI] [PubMed] [Google Scholar]
- Dupont J. L., Gardette R., Crepel F. Postnatal development of the chemosensitivity of rat cerebellar Purkinje cells to excitatory amino acids. An in vitro study. Brain Res. 1987 Jul;431(1):59–68. doi: 10.1016/0165-3806(87)90195-7. [DOI] [PubMed] [Google Scholar]
- Greenamyre T., Penney J. B., Young A. B., Hudson C., Silverstein F. S., Johnston M. V. Evidence for transient perinatal glutamatergic innervation of globus pallidus. J Neurosci. 1987 Apr;7(4):1022–1030. doi: 10.1523/JNEUROSCI.07-04-01022.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamosh A., McDonald J. W., Valle D., Francomano C. A., Niedermeyer E., Johnston M. V. Dextromethorphan and high-dose benzoate therapy for nonketotic hyperglycinemia in an infant. J Pediatr. 1992 Jul;121(1):131–135. doi: 10.1016/s0022-3476(05)82559-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28(6):517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- Jansen K. L., Dragunow M., Faull R. L. [3H]glycine binding sites, NMDA and PCP receptors have similar distributions in the human hippocampus: an autoradiographic study. Brain Res. 1989 Mar 13;482(1):174–178. doi: 10.1016/0006-8993(89)90557-x. [DOI] [PubMed] [Google Scholar]
- Johnson J. W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987 Feb 5;325(6104):529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Kleckner N. W., Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988 Aug 12;241(4867):835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kornhuber J., Retz W., Riederer P., Heinsen H., Fritze J. Effect of antemortem and postmortem factors on [3H]glutamate binding in the human brain. Neurosci Lett. 1988 Nov 11;93(2-3):312–317. doi: 10.1016/0304-3940(88)90101-2. [DOI] [PubMed] [Google Scholar]
- Maragos W. F., Penney J. B., Young A. B. Anatomic correlation of NMDA and 3H-TCP-labeled receptors in rat brain. J Neurosci. 1988 Feb;8(2):493–501. doi: 10.1523/JNEUROSCI.08-02-00493.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. W., Johnston M. V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- Monahan J. B., Corpus V. M., Hood W. F., Thomas J. W., Compton R. P. Characterization of a [3H]glycine recognition site as a modulatory site of the N-methyl-D-aspartate receptor complex. J Neurochem. 1989 Aug;53(2):370–375. doi: 10.1111/j.1471-4159.1989.tb07344.x. [DOI] [PubMed] [Google Scholar]
- O'Shea R. D., Manallack D. T., Conway E. L., Mercer L. D., Beart P. M. Evidence for heterogenous glycine domains but conserved multiple states of the excitatory amino acid recognition site of the NMDA receptor: regional binding studies with [3H]glycine and [3H]L-glutamate. Exp Brain Res. 1991;86(3):652–662. doi: 10.1007/BF00230539. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Ochi N., Mizutani N., Hayakawa C., Watanabe K. Nonketotic hyperglycinemia: treatment with NMDA antagonist and consideration of neuropathogenesis. Pediatr Neurol. 1991 Jan-Feb;7(1):65–68. doi: 10.1016/0887-8994(91)90110-7. [DOI] [PubMed] [Google Scholar]
- Piggott M. A., Perry E. K., Perry R. H., Court J. A. [3H]MK-801 binding to the NMDA receptor complex, and its modulation in human frontal cortex during development and aging. Brain Res. 1992 Aug 21;588(2):277–286. doi: 10.1016/0006-8993(92)91586-4. [DOI] [PubMed] [Google Scholar]
- Pulsinelli W. Pathophysiology of acute ischaemic stroke. Lancet. 1992 Feb 29;339(8792):533–536. doi: 10.1016/0140-6736(92)90347-6. [DOI] [PubMed] [Google Scholar]
- Slater P., McConnell S., D'Souza S. W., Barson A. J. Age-related changes in binding to excitatory amino acid uptake sites in human cerebellum. Brain Res. 1992 May 8;579(2):219–226. doi: 10.1016/0006-8993(92)90054-d. [DOI] [PubMed] [Google Scholar]
- Slater P., McConnell S., D'Souza S. W., Barson A. J., Simpson M. D., Gilchrist A. C. Age-related changes in binding to excitatory amino acid uptake site in temporal cortex of human brain. Brain Res Dev Brain Res. 1992 Feb 21;65(2):157–160. doi: 10.1016/0165-3806(92)90174-u. [DOI] [PubMed] [Google Scholar]
- Uckele J. E., McDonald J. W., Johnston M. V., Silverstein F. S. Effect of glycine and glycine receptor antagonists on NMDA-induced brain injury. Neurosci Lett. 1989 Dec 15;107(1-3):279–283. doi: 10.1016/0304-3940(89)90831-8. [DOI] [PubMed] [Google Scholar]
- Whitehouse P. J., Lynch D., Kuhar M. J. Effects of postmortem delay and temperature on neurotransmitter receptor binding in a rat model of the human autopsy process. J Neurochem. 1984 Aug;43(2):553–559. doi: 10.1111/j.1471-4159.1984.tb00934.x. [DOI] [PubMed] [Google Scholar]
- Wood P. L., Emmett M. R., Rao T. S., Mick S., Cler J., Iyengar S. In vivo modulation of the N-methyl-D-aspartate receptor complex by D-serine: potentiation of ongoing neuronal activity as evidenced by increased cerebellar cyclic GMP. J Neurochem. 1989 Sep;53(3):979–981. doi: 10.1111/j.1471-4159.1989.tb11803.x. [DOI] [PubMed] [Google Scholar]