ABSTRACT
Genome-scale analyses have revealed many transcription factor binding sites within, rather than upstream of, genes, raising questions as to the function of these binding sites. Here, we use complementary approaches to map the regulon of the Escherichia coli transcription factor PhoB, a response regulator that controls transcription of genes involved in phosphate homeostasis. Strikingly, the majority of PhoB binding sites are located within genes, but these intragenic sites are not associated with detectable transcription regulation and are not evolutionarily conserved. Many intragenic PhoB sites are located in regions bound by H-NS, likely due to shared sequence preferences of PhoB and H-NS. However, these PhoB binding sites are not associated with transcription regulation even in the absence of H-NS. We propose that for many transcription factors, including PhoB, binding sites not associated with promoter sequences are transcriptionally inert and hence are tolerated as genomic “noise.”
KEYWORDS: ChIP-seq, PhoB, pho regulon, transcription factors
INTRODUCTION
Bacteria encode numerous transcription factors (TFs) that regulate transcription initiation by binding DNA near promoters and modulating the ability of RNA polymerase (RNAP) holoenzyme to bind promoter DNA or to isomerize to an actively transcribing conformation (1). TF function has been studied almost exclusively in the context of TF binding sites in intergenic regions, upstream of the regulated genes. However, genome-scale analyses of TF binding have identified large numbers of intragenic binding sites, far from gene starts. The proportion of binding sites for a TF that are intragenic varies extensively between different TFs (2, 3), with some TFs having the majority of their binding sites inside genes (3–6). Despite the large number of intragenic TF binding sites, relatively little is known about their function.
Regulatory activity has been described for few intragenic TF binding sites and can be classified into the following distinct classes based on the regulatory target and mechanism of action: (i) canonical regulation of transcription initiation of the downstream gene, generating an RNA with an extended 5′ untranslated region (UTR) that overlaps a gene (5, 7–10); (ii) canonical regulation of transcription initiation of a stable noncoding RNA that initiates inside a gene or 3′ UTR (11, 12); (iii) regulation of transcription initiation of the gene that contains the TF binding site (mechanisms of regulation in almost all such cases are unknown [3], although transcription repression can occur from a site close to the promoter due to a physical interaction with a more upstream site, resulting in formation of a DNA loop [13, 14]); and (iv) regulation of transcription elongation due to the TF acting as a roadblock for RNAP (15–18). Another possible regulatory function for intragenic TF binding sites is the regulation of pervasive transcription—transcription of large numbers of short, unstable RNAs from inside genes, a process that is ubiquitous in bacteria (19, 20). Although there are no described examples of TFs that regulate unstable, intragenic transcripts, many of these RNAs are differentially expressed between growth conditions (21), consistent with regulation by TFs. Intragenic TF binding sites might also have functions that are not directly connected to gene regulation, such as facilitating short- or long-range chromosome contacts (22–25) or serving as TF-titrating decoy sites (26, 27). Lastly, it is possible that intragenic TF binding sites serve no biological function and arise as a consequence of genetic drift (28) or genome evolution that is constrained by selection for particular codons.
PhoB is a conserved transcription factor that regulates phosphate homeostasis.
PhoB is a member of the PhoB/OmpR family of response regulator TFs and is a key regulator of phosphate homeostasis in many Gram-negative bacteria (29, 30). PhoB forms a two-component system with the sensor kinase PhoR (31). When inorganic phosphate (Pi) levels are low, PhoR autophosphorylates and then phosphorylates PhoB (30, 31), triggering PhoB dimerization and DNA binding activity (30). Phosphorylated PhoB binds direct repeat sequences called pho boxes (32) and is a dual regulator, capable of both activating and repressing transcription depending on the position of the binding site.
In Escherichia coli and related species, PhoB regulates the expression of genes encoding the high-affinity phosphate transport system (pst), a phosphonate transport complex (phn), and the glycerol-3-phosphate transporter (ugp) and other genes related to phosphate homeostasis (30, 33). These genes are collectively referred to as the pho regulon. PhoB has been implicated in the regulation of a number of other cellular processes and stress responses, including motility, biofilm formation, quorum sensing, cell surface remodeling, the stringent response, and the general stress response (34, 35). Indeed, transcriptomic and proteomic studies of phosphate-depleted E. coli have suggested that the pho regulon has many additional members (36, 37). However, most of these putative regulon members have limited experimental support (30, 33).
Here, we describe a high-resolution, genome-wide mapping of the pho regulon using chromatin immunoprecipitation sequencing (ChIP-seq) and transcriptome sequencing (RNA-seq). We refine and expand the set of known pho regulon genes and identify many intragenic PhoB binding sites. We show that the large majority of intragenic PhoB binding sites are not conserved and are not associated with detectable regulatory function. Thus, our data suggest that individual intragenic PhoB sites are nonfunctional and that TFs can bind many intragenic sites with little or no impact on local transcription.
RESULTS
Genome-wide binding of PhoB under phosphate-limiting conditions.
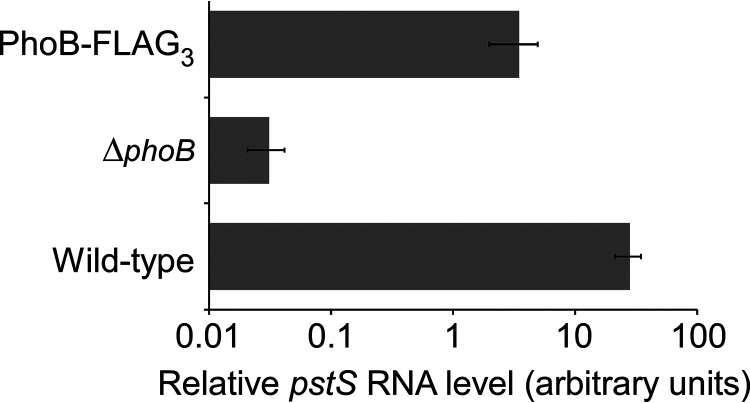
ChIP-seq is used to map the genome-wide binding of TFs. To facilitate ChIP-seq of E. coli PhoB, we introduced C-terminal FLAG tags at the native phoB locus. We used quantitative reverse transcriptase PCR (qRT-PCR) to measure the expression of pstS, a PhoB-activated gene, in wild-type cells, ΔphoB cells, and cells expressing phoB-FLAG3. Cells were grown in minimal medium with low phosphate levels to induce the kinase activity of PhoR. As expected, we observed a large decrease (~900-fold) in pstS levels in ΔphoB cells relative to those of wild-type cells (Fig. 1). In cells expressing PhoB-FLAG3, we observed a much smaller decrease (~8-fold) in pstS levels relative to those of wild-type cells (Fig. 1), indicating that the tagged PhoB derivative retains partial function.
FIG 1.
Partially reduced activity of the C-terminally FLAG3-tagged PhoB. qRT-PCR was used to measure levels of the pstS RNA relative to the minD RNA control in wild-type MG1655/pBAD24 (wild type), MG1655 ΔphoB (CDS091)/pBAD24 (ΔphoB), or MG1655 phoB-FLAG3 (DMF34)/pBAD24 (PhoB-FLAG3) for cells grown under low-phosphate conditions. Values are the average of three independent biological replicates; error bars represent ±1 standard deviation.
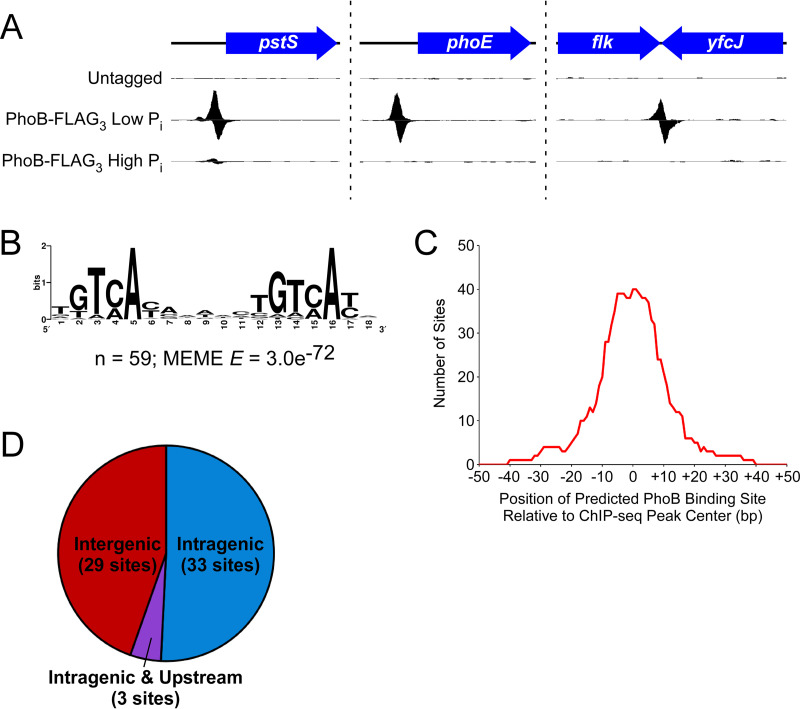
We used ChIP-seq to map the genome-wide binding of PhoB-FLAG3 during growth under low-phosphate conditions. Thus, we identified 65 enriched regions (Fig. 2A; Table 1). For a control, we performed ChIP-seq with an untagged strain grown under the same conditions; none of the regions enriched in the PhoB-FLAG3 ChIP-seq data set were enriched in the control data set (Fig. 2A). We conclude that the 65 enriched regions in the PhoB-FLAG3 ChIP-seq data set are likely to represent genuine PhoB-bound regions. Note that a single PhoB-bound region could include more than one PhoB site, as is the case for the region upstream of phoB itself, which has been reported to include two PhoB sites (38). We identified a highly enriched sequence motif, with instances of the motif found in 59 of the 65 putative PhoB-bound regions (MEME E value = 3.0e−72) (Fig. 2B). This motif contains a clearly distinguishable direct repeat and is similar to the previously reported pho box consensus sequence (39). Furthermore, the identified motif is centrally enriched relative to the calculated ChIP-seq peak centers (Fig. 2C) (CentriMo E value = 1.2e−12). The presence and central enrichment of this motif at ChIP-seq peaks further support the veracity of PhoB-bound regions and confirm the high spatial resolution of the ChIP-seq data.
FIG 2.
ChIP-seq identifies PhoB binding sites. (A) ChIP-seq data for (i) an untagged control under low-phosphate conditions, (ii) PhoB-FLAG3 under low-phosphate conditions, and (iii) PhoB-FLAG3 under high-phosphate conditions. Three genomic regions are shown, with one data set from two independent biological replicates. Values on the x axis represent genome positions. Values on the y axis represent normalized sequence read coverage, with positive values indicating sequence reads mapping to the forward strand and negative values indicating sequence reads mapping to the reverse strand. y axis scales differ between the three genomic regions but are matched for the three data sets for any given genomic region. (B) Significantly enriched DNA sequence motif derived from 100-bp regions surrounding each ChIP-seq peak. The number of sites contributing to the motif and the E value determined by MEME are indicated. (C) Analysis of the position of inferred PhoB binding sites relative to the position of ChIP-seq peak centers. For each of the binding sites contributing to the motif determined by MEME (see panel B), we determined the position of the binding site relative to the associated ChIP-seq peak center. The x axis indicates positions relative to ChIP-seq peak centers. The y axis indicates the number of binding sites that cover any given position. (D) Pie chart showing the genome context of PhoB binding sites identified by ChIP-seq. Sites designated “intragenic and upstream” are intragenic but <200 bp upstream of an annotated gene start.
TABLE 1.
List of PhoB-bound regions identified by ChIP-seq
| Genome coordinatea | H-NS-bound?b | ChIP scorec | Associated gene(s)d | Binding site sequencee | Expression wtf | Expression ΔphoBf,g |
|---|---|---|---|---|---|---|
| 14763 | 1 | (dnaJ) | NA | (8,326) | (4,689) | |
| 22367 | 2 | ileS | TGTAATCAAACCGAAATA | 21,580 | 24,295 | |
| 201079 | 1 | (lpxD) | GGTCACATTACGTTCATG | (9,261) | (11,591) | |
| 260277 | × | 14 | phoE/proB | TGTAATAAAAGCGTAAAC | 68,155/6,306 | 69*/4,330 |
| 262258 | 1 | (proA) | TGTAATACGGTTGAAACG | (9,578) | (6,248) | |
| 332492 | × | 3 | (yahA) | TGTAACAGAAATATCACA | (2,246) | (1,084*) |
| 401676 | × | 20 | phoA | TGTCATAAAGTTGTCACG | 310,112 | 1,548* |
| 417093 | 11 | sbcD/phoB | TTTCATAAATCTGTCATA | 274/32,731 | 271/34* | |
| 417245 | 5 | (phoB) | ATTCACAGCACTGTCATA | (32,731) | (34*) | |
| 569907 | × | 6 | (ybcK) | TGTCACATCGATGTAATC | (33) | (24) |
| 595521 | 3 | cusR/cusC | TGTCATTTTTCTGTCACC | 391/208 | 239/50* | |
| 922786 | 2 | cspD/clpS | TGTCACATTCCTGTCAAT | 2,330/18,217 | 1,603/23,285 | |
| 983795 | 2 | (gloC) | TGTCAGGCCGCTGTCATC | (4,405) | (7,533*) | |
| 1015596 | 7 | rmf | ATTCACGCCACTGTCATA | 3,816 | 5,371 | |
| 1065537 | × | 2 | agp | AGTCATATTTCTGTCACA | 976 | 1,983* |
| 1084845 | 11 | phoH | TGTCATCACTCTGTCATC | 37,076 | 13,375* | |
| 1096396 | × | 1 | (ycdU) | TGTCACAAAAGAATCACT | (53) | (64) |
| 1117391 | 1 | (yceA) | GATAAAAAAATCGTCATG | (560) | (456) | |
| 1371360 | × | 5 | (ycjM) | GGTCACATTTATTTCATA | (11) | (14) |
| 1447326 | 2 | feaR/feaB | TTTCACAGAGCGAAAACG | 111/786 | 379*/1,231 | |
| 1527862 | × | 1 | rhsE | TATCAGAAAAATGTCATG | ND | ND |
| 1577158 | × | 4 | (yddB) | CGGCACAAAACTGTCATA | (94) | (99) |
| 1581949 | × | 2 | (ydeN) | TGTCAAAAATCAGTAATG | (120) | (119) |
| 1861798 | 5 | (yeaC) | NA | (1,355) | (1,480) | |
| 1874218 | 1 | yoaI/yeaL | TGTCATCAAACTGCCATT | 12,266/36 | 28*/67 | |
| 1948542 | 1 | (nudB) | CGTCACGCCGCTGCAACA | (3,654) | (3,763) | |
| 1978157 | 1 | (flhD) | TGACATCAACTTGTCATA | (71) | (57) | |
| 2055017 | 1 | amn | TTTCACATTTCTGTGACA | 42,299 | 12,429* | |
| 2137862 | × | 5 | wza/yegH | TGTCACAATTCGATCATG | 10/2,257 | 15/2,498 |
| 2240543 | 6 | mglB | TGTAACCCGTATGTAACA | 1,076 | 772 | |
| 2438961 | 8 | (yfcJ) | TGTCACGATACTGTCATT | (163) | (187) | |
| 2484480 | × | 2 | (evgS) | AGTAACAACCGTGTCACA | (1,514) | (3,343*) |
| 2743442 | 3 | (yfiN) yfiB | TGACACAAATCTTTAATC | (575) 1,293 | (656) 1,629 | |
| 2799166 | 1 | (alaE) | AGTCACGCTTGCGTCATG | (83) | (58) | |
| 2819248 | 1 | csrA | TGTAATGTGTTTGTCATT | 6,539 | 7,460 | |
| 2976292 | 4 | omrA | GGTCATCAATCTGTAACA | 1 | 3 | |
| 3031605 | 3 | (uacT) | CGTCACATTATTGCAATG | (5) | (4) | |
| 3081080 | 2 | (tktA) | AGAAATACCGTTGTCATC | (26,875) | (27,000) | |
| 3198259 | 1 | (glnE) | TGTCATCTTCCTGCAACG | (11,291) | (9,028) | |
| 3243474 | 3 | (uxaC) uxaA | TGTCATACACCCGTCACG | (608) 243 | (465) 228 | |
| 3309385 | 1 | (pnp) | CTTCACAGTACCGTCATC | (33,309) | (53,983*) | |
| 3362763 | × | 4 | (yhcA) yhcD | GGTAATAAATATGTCACT | (38) 425 | (54) 415 |
| 3458489 | 1 | (gspE) | NA | (69) | (51) | |
| 3573580 | 5 | glgB | GGTCAAAAAAATGTCACA | 21,445 | 17,405 | |
| 3592453 | 35 | ugpB | TGTCATCTTTCTGACACC | 54,461 | 2,830* | |
| 3600942 | 2 | rpoH | TTTCATCTCTATGTCACA | 7,346 | 4,943 | |
| 3648534 | 2 | (arsR) | GGTAACAGAAATGACATA | (61) | (66) | |
| 3672318 | × | 2 | yhjB/yhjC | TTTCACAATGTTGTCATG | 252/397 | 218/408 |
| 3682037 | 1 | yhjJ | GAACATGAAAATGTCACG | 1,871 | 2,721 | |
| 3709177 | 1 | (eptB) | GGTCACCGAGTTGTCATA | (585) | (1,126*) | |
| 3728905 | 1 | (xylB) | AGTAATCTTTCGGTAATA | (1,160) | (836) | |
| 3731396 | × | 1 | (xylF) | NA | (46) | (31) |
| 3767949 | × | 2 | (rhsJ) | TTACATACAAATGTAATA | (ND) | (ND) |
| 3776578 | 6 | yibT/yibL | TGTAATAGTTCTGTAACG | 5,264/1,129 | 2,601*/2,135* | |
| 3911600 | 38 | pstS | TGTCATAAAACTGTCATA | 239,097 | 310* | |
| 3937206 | 3 | rbsK | TGTCACCATCAGGTCATA | 1,452 | 1,701 | |
| 3990899 | 1 | hemC/cyaA | TTTCACGCCGTTGTAATA | 3,823/16,054 | 5,169/18,141 | |
| 4113746 | × | 1 | (fpr) | CGTAAATGTTTCGTCATC | (4,069) | (4,643) |
| 4128495 | 2 | metJ/metB | NA | 543/1,432 | 524/1,263 | |
| 4142314 | 1 | frwA/frwC | TGTAATGTAACCGTCAAT | 72/35 | 60/36 | |
| 4227508 | 1 | (metH) | ATTCACAAATCTGTCACT | (18,026) | (25,993) | |
| 4245063 | 1 | (malF) | TGTCATTAAAAAGAAACA | (486) | (853*) | |
| 4325211 | 2 | phnC | ATTAACCAAATCGTCACA | 32,470 | 13* | |
| 4459250 | 1 | (pmbA) | GGTAACGATATTGAAACA | (11,462) | (11,004) | |
| 4522523 | × | 1 | (yjhG) | NA | (478) | (444) |
Position of the center of the PhoB-bound region in the E. coli MG1655 genome (GenBank accession no. NC_000913.3). Positions in bold indicate those that overlap regions found by ChIP-chip previously (43).
An “×” indicates that the region was previously reported to be bound by H-NS (49).
A measure of relative enrichment from ChIP-seq data.
Genes in parentheses indicate an intragenic binding site. Downstream genes are listed if the PhoB ChIP-seq peak center is in an intergenic region upstream of that gene and/or if the peak center is <200 bp upstream of the gene start. Previously described pho regulon members are in bold.
Binding sites identified by MEME. Bold sequences match binding sites described previously (43). “NA” indicates ChIP-seq peaks for which no binding site sequence was found by MEME.
Relative RNA levels in MG1655/pBAD24 (wild type [wt]) or MG1655 ΔphoB (CDS091)/pBAD24 (ΔphoB) for genes indicated in the column headed “Associated genes.” (See footnote d for explanation of parentheses.) “ND” indicates genes for which RNA levels were not determined.
Asterisks indicate expression differences between the wild-type and ΔphoB strains that were determined to be statistically significant (q < 0.01).
The 65 PhoB-bound regions identified by ChIP-seq include most well-established PhoB sites, as well as many novel targets (Table 1). We identified PhoB-bound regions upstream of 7 of the 10 genes/operons described previously as being in the pho regulon (Table 1, underlined gene names) (33), with no ChIP-seq signal upstream of waaH, ytfK, or psiE. We also identified PhoB-bound regions upstream of the predicted pho regulon gene amn (Table 1) (40) and upstream of yoaI, which was described as a direct PhoB target in E. coli O157:H7 (41, 42). We identified 21 PhoB-bound regions upstream of genes/operons not previously described as part of the pho regulon and lacking a clear connection to phosphate homeostasis. The remaining 36 PhoB-bound regions, over half of the total sites identified by ChIP-seq, are located inside genes (Fig. 2D; Table 1). Strikingly, all but 3 intragenic PhoB binding sites are far from neighboring gene starts (>200 bp) and thus are unlikely to participate in promoter-proximal regulation of these genes (Fig. 2D; Table 1).
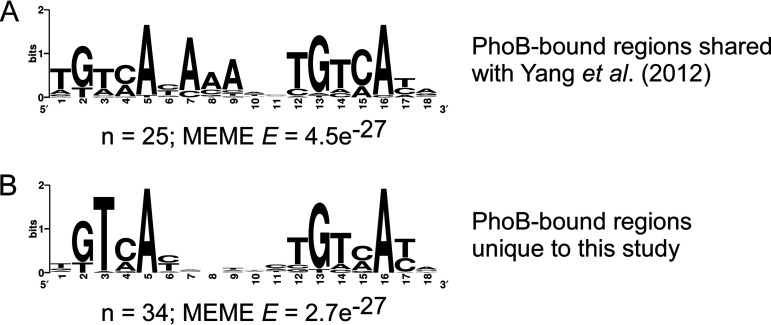
Our PhoB ChIP-seq data show only modest agreement with an earlier study that identified many putative PhoB binding sites using chromatin immunoprecipitation with microarray technology (ChIP-chip) (43), although both studies are consistent in the lack of signal upstream of waaH, ytfK, or psiE. Of the 43 ChIP-chip peaks identified by Yang et al. (43), 24 are <400 bp from a ChIP-seq peak in our data (Table 1, coordinates in bold), while the remaining 19 ChIP-chip peaks are >2,800 bp from the closest ChIP-seq peak. Even for the 24 ChIP-chip peaks close to ChIP-seq peaks identified in the current study, the peak centers calculated from the two data sets are up to 383 bp apart, and only 13 regions share a motif call between studies (Table 1, motifs in bold). These discrepancies between data sets are probably due, at least in part, to the low resolution of the ChIP-chip data and differences in peak-calling and motif-calling algorithms. It is challenging to determine whether peak calls from each of the two studies represent the same biological binding events. We performed de novo motif identification for three sets of peaks: (i) shared, (ii) unique to the current study, and (iii) unique to the study of Yang et al. (43). For shared peaks (i) and peaks unique to the current study (ii), 100 bp of sequence surrounding each ChIP-seq peak center was extracted and analyzed by MEME. In both cases, highly enriched sequence motifs that are close matches to the expected PhoB motif were found (Fig. 3A and B). For sites unique to the Yang et al. data set (iii), the same analysis was performed using both 100-bp and 500-bp windows surrounding the published peak center locations. The resulting motifs were poorly enriched (MEME E values > 1) and bear no similarity to the expected PhoB motif. We conclude that most or all of the 40 regions unique to the current study represent genuine PhoB binding sites, while those unique to the Yang et al. study largely do not.
FIG 3.
Comparison of ChIP-seq and ChIP-chip data sets. (A) Significantly enriched DNA sequence motif derived from 100-bp regions surrounding each ChIP-seq peak for regions shared between the ChIP-seq data set and a published ChIP-chip data set (43). The number of sites contributing to the motif and the E value determined by MEME are indicated. (B) Significantly enriched DNA sequence motif derived from 100-bp regions surrounding each ChIP-seq peak for regions unique to the ChIP-seq data set, i.e., not found in the published ChIP-chip data set (43). The number of sites contributing to the motif and the E value determined by MEME are indicated.
Genome-wide binding of PhoB under high-phosphate conditions.
To determine whether PhoB binds any target DNA sites when PhoR is inactive, we repeated the ChIP-seq experiment but grew cells under conditions with high phosphate. We detected only a single PhoB-bound region, the intergenic region upstream of pstS (Fig. 2A). This is a well-established site of PhoB binding and was the most enriched PhoB-bound region in the low-phosphate ChIP-seq experiment. As expected, PhoB binding upstream of pstS was substantially lower under conditions of high phosphate than under conditions of low phosphate (Fig. 2A). Thus, our data suggest that under conditions of high phosphate, PhoB weakly regulates pstS but does not regulate any of its other target genes.
Reassessing the pho regulon.
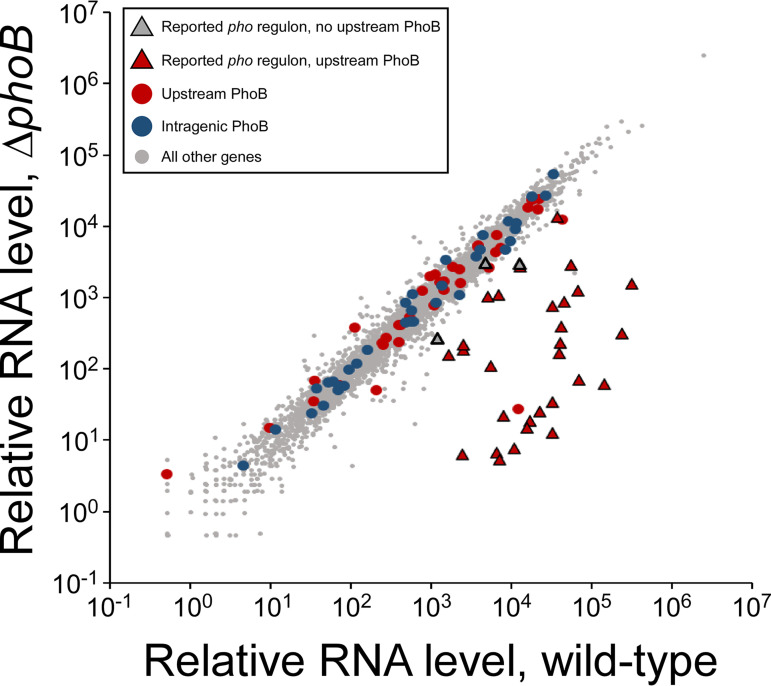
To address whether the detected PhoB sites contribute to transcription regulation, RNA-seq was performed using wild-type and ΔphoB strains grown in low-phosphate medium. In total, 181 genes were differentially expressed between the wild-type and ΔphoB strains (P value ≤ 0.01, >2-fold difference in RNA levels) (Fig. 4; see Table S1 in the supplemental material). We observed significant positive regulation of all 7 reported pho regulon operons for which we observed upstream PhoB binding by ChIP-seq, i.e., phnCDEFGHIJKLMNOP, phoH, ugpBAECQ, pstSCAB-phoU, phoA-psiF, phoE, and phoBR (Table 1; Table S1) (positive regulation was observed for all genes in all operons, except for phoB, which could not be assessed in the ΔphoB strain). We also observed significant positive regulation of amn and yoaI; ChIP-seq identified PhoB binding upstream of these genes, and although they have not generally been considered part of the pho regulon, they have been previously reported as being direct PhoB targets (41, 42).
FIG 4.
RNA-seq analysis of E. coli wild-type and ΔphoB strains. Scatter plot showing relative RNA levels for all genes in wild-type (MG1655/pBAD24) or ΔphoB (CDS091/pBAD24) cells. Each data point corresponds to a gene. Triangular data points represent genes previously reported to be in the pho regulon, with red fill indicating that the transcript has an upstream PhoB site identified by ChIP-seq and gray fill indicating no upstream site. Red circle data points represent genes not previously reported to be in the pho regulon but with upstream PhoB sites identified by ChIP-seq. Blue circle data points represent genes with internal PhoB sites identified by ChIP-seq. All other genes are represented by gray circle data points.
RNA-seq analysis. Download Table S1, XLSX file, 0.2 MB (252.2KB, xlsx) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We observed significant positive regulation of ytfK and waaH, reported pho regulon genes that lack associated PhoB binding. We conclude that ytfK and waaH are regulated indirectly by PhoB. In contrast, we did not observe significant regulation of known and predicted pho regulon genes psiE, asr, eda, argP, and pitB; none of these genes had detectable upstream PhoB binding by ChIP-seq. We conclude that psiE, asr, eda, argP, and pitB are unlikely to be regulatory targets of PhoB.
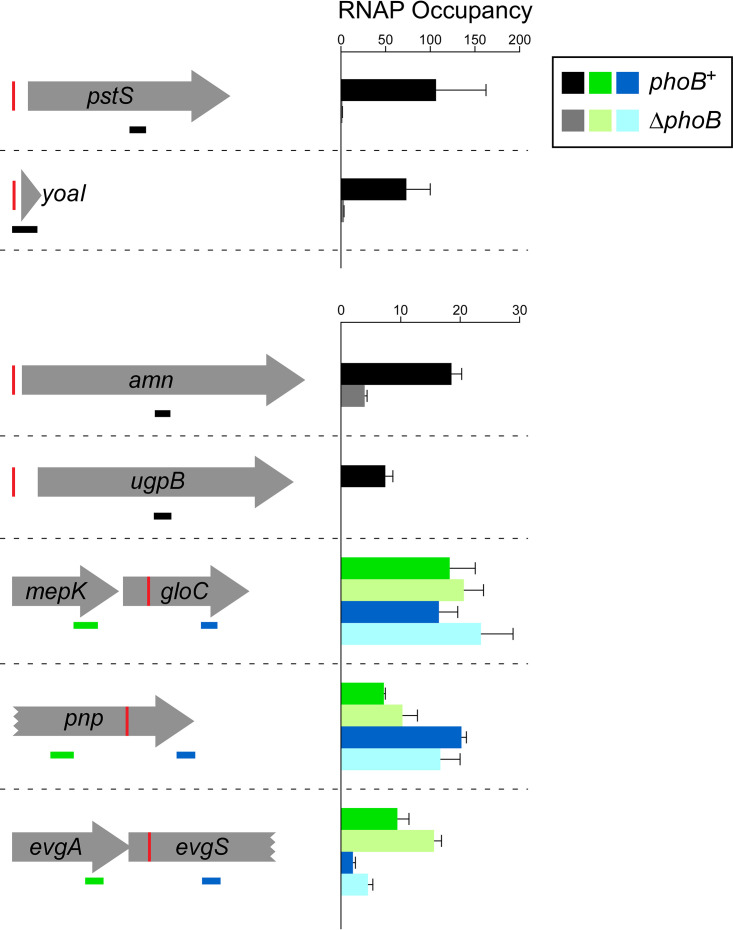
To identify novel pho regulon genes, we determined whether any additional genes with associated PhoB binding were significantly differentially expressed between wild-type and ΔphoB cells. We observed a significant, >2-fold positive regulation of cusC and yibT and a significant, >2-fold negative regulation of feaR and agp, genes with upstream PhoB binding as determined by ChIP-seq. We also observed significant regulation of 6 genes with internal PhoB sites: yahA, gloC, pnp, evgS, eptB, and malF. Although only two of these genes (yahA and evgS) were differentially expressed >2-fold between wild-type and ΔphoB cells, all but yahA were more highly expressed in ΔphoB cells than wild-type cells. We hypothesized that PhoB represses transcription of these genes by acting as a roadblock for RNAP. To test this hypothesis, we grew wild-type and ΔphoB cells under phosphate-limiting conditions and measured RNAP (β subunit) occupancy upstream and downstream of the PhoB sites within the gloC, pnp, and evgS genes by using ChIP-quantitative PCR (ChIP-qPCR). For controls, we measured RNAP occupancy within the pstS and ugpB genes, confirmed members of the pho regulon. We also measured RNAP occupancy within the yoaI and amn genes that the combined RNA-seq and ChIP-seq data suggested are members of the pho regulon. As expected, we observed substantially higher RNAP occupancy within pstS and ugpB in wild-type cells than in ΔphoB cells (Fig. 5). Moreover, we observed substantially higher RNAP occupancy within yoaI and amn in wild-type cells than in ΔphoB cells (Fig. 5), supporting the idea that these genes are part of the pho regulon. For the three genes with intragenic PhoB sites, we reasoned that if PhoB acts as a roadblock to the elongation of RNAP, RNAP occupancy downstream of PhoB sites would increase in ΔphoB cells relative to that in wild-type cells and relative to any change in RNAP occupancy upstream of the PhoB site. However, we did not observe significant increases in relative RNAP occupancy downstream of PhoB sites for any of the three genes (Fig. 5) (P > 0.2; see Materials and Methods for details of the statistical analysis), suggesting that PhoB regulation of these genes is indirect.
FIG 5.
Differences in RNAP (β subunit) occupancy in genes that are potential members of the pho regulon. RNAP (β subunit) occupancy measured by ChIP-qPCR (see Materials and Methods for details of how occupancy units are calculated) in wild-type MG1655 (dark-colored bars) and MG1655 ΔphoB (CDS091; light-colored bars) for regions within genes that are potential members of the pho regulon. Schematics on the left show genes with upstream or internal PhoB sites (red vertical lines). The horizontal bars indicate the positions of PCR amplicons used in ChIP-qPCR, with black bars indicating amplicons within genes that have upstream PhoB sites, green bars indicating amplicons upstream of intragenic PhoB sites, and blue bars indicating amplicons downstream of intragenic PhoB sites. Values are the average of three independent biological replicates; error bars represent 1 standard deviation.
PhoB-dependent recruitment of initiating RNAP.
The majority of the PhoB binding sites identified by ChIP-seq were not associated with regulation detectable by RNA-seq. We hypothesized that this could be due to three reasons: (i) the binding sites are nonregulatory, (ii) regulation is condition specific and/or requires additional factors, or (iii) PhoB regulates transcription of short, unstable, noncoding RNAs that are not detectable by conventional RNA-seq. To test the latter possibility, we used ChIP-seq to measure the association of σ70 in regions close to PhoB binding sites. σ70 is rapidly released from RNAP upon the transition from transcription initiation to elongation (44); thus, σ70 occupancy on DNA, as measured by ChIP-seq, is an indication of the level of association of initiating RNAP with DNA. Since transcription initiation occurs prior to RNA processing, σ70 occupancy can be observed even at promoters of highly unstable RNAs (45).
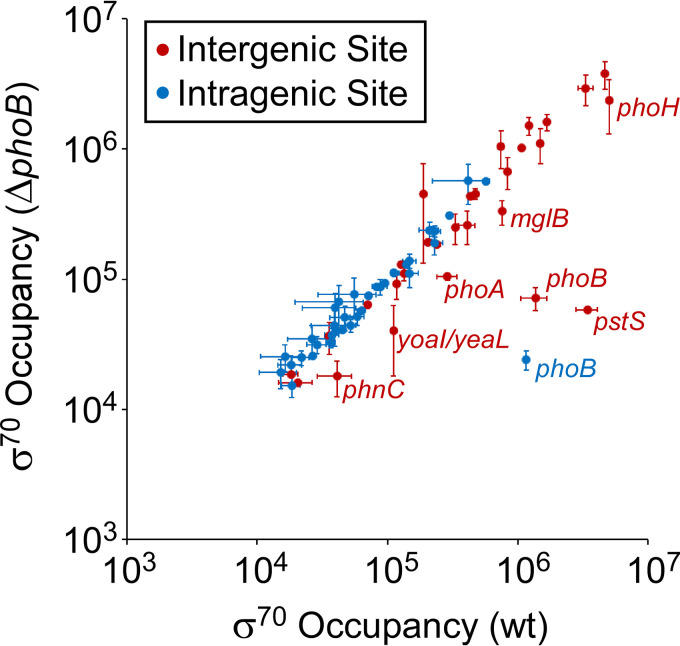
To measure the effects of PhoB on RNAP holoenzyme recruitment, we performed ChIP-seq of σ70 in wild-type and ΔphoB strains grown in low-phosphate medium. Normalized σ70 occupancy was calculated for 400-bp windows surrounding each PhoB binding site to systematically assess σ70 binding at these sites (Fig. 6). Three PhoB binding sites showed large reductions (>19-fold) in σ70 occupancy in the ΔphoB strain relative to that in the wild type. Two of these sites are associated with the phoB gene itself; σ70 occupancy measurements at these sites are impacted by the loss of associated DNA sequence resulting from deletion of phoB. The third PhoB site is the regulatory site upstream of pstS. We conclude that PhoB activates pstS transcription at the level of RNAP recruitment, as suggested by structural models of the DNA-PhoB-RNAP complex (46–48). PhoB binding sites upstream of yoaI, phoA, mglB, phnC, and phoH showed >2-fold lower σ70 occupancy in the ΔphoB strain than in the wild type, suggesting that PhoB recruits initiating RNAP to these promoters. These data are largely consistent with the RNA-seq data showing >2-fold differential expression of yoaI, phoA, phnC, and yoaI between wild-type and ΔphoB cells (Table 1). For most other PhoB sites, including almost all intragenic sites, σ70 occupancy was low in both wild-type and ΔphoB strains (Fig. 6), strongly suggesting that these sites are not associated with active promoters under the growth conditions used. The remaining sites were associated with substantial σ70 occupancy, which was similar in both wild-type and ΔphoB strains, suggesting that they are close to active promoters whose activity is independent of PhoB under the conditions tested.
FIG 6.
Differences in σ70 occupancy around PhoB binding sites between wild-type and ΔphoB cells. The scatter plot shows normalized σ70 occupancy in wild-type MG1655 and MG1655 ΔphoB (DMF84) for the 400-bp regions surrounding PhoB binding sites identified by ChIP-seq. Each data point represents a PhoB binding site. Intergenic binding sites are indicated by red data points, and intragenic binding sites are indicated by blue data points. Genes associated with PhoB binding sites are labeled with the gene name in cases where σ70 occupancy differs >2-fold between wild-type and ΔphoB cells. Values are the average of two independent biological replicates; error bars represent ±1 standard deviation.
H-NS coassociates with many intragenic PhoB sites but does not block RNAP recruitment.
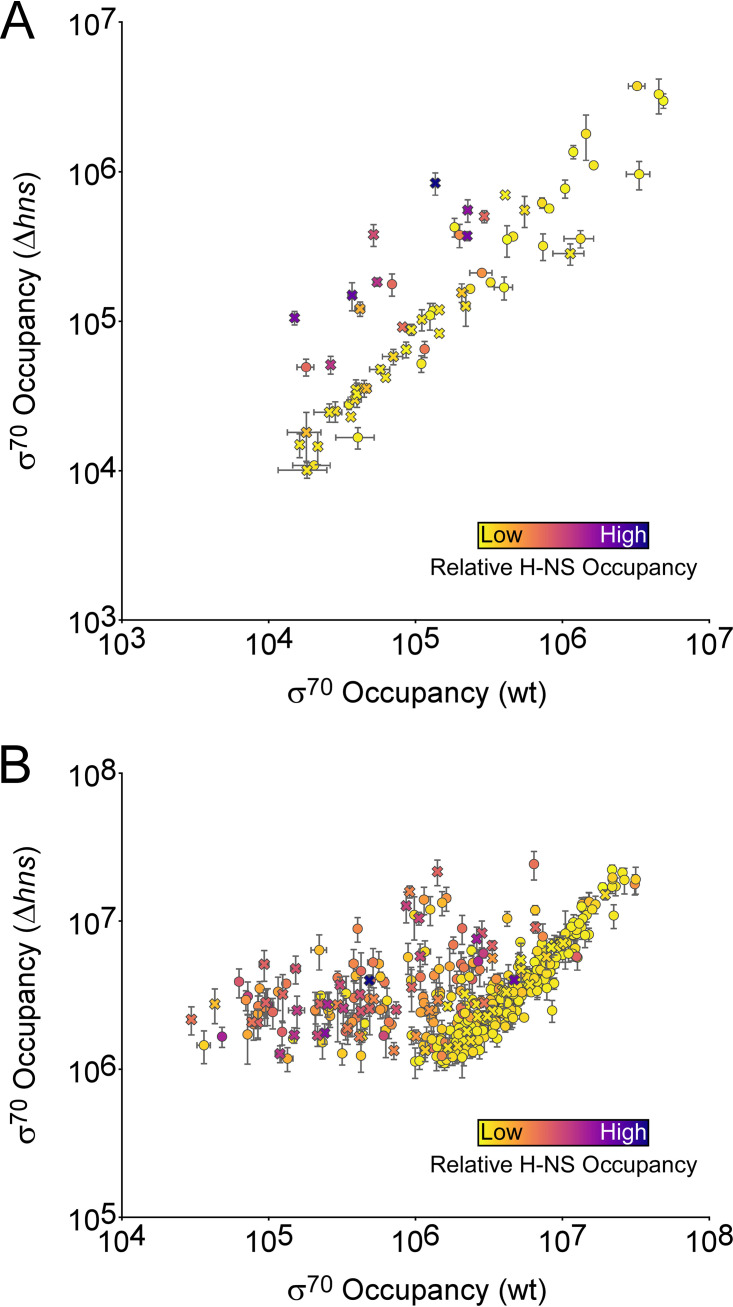
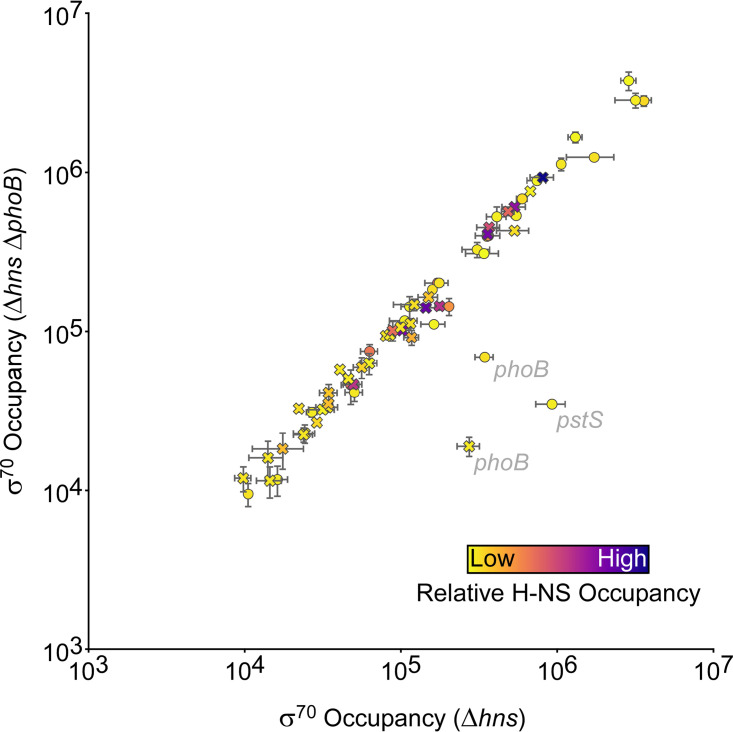
We noted that 18 PhoB sites (12 intragenic and 6 intergenic), representing 28% of all sites identified by ChIP-seq, are in regions bound by the nucleoid-associated protein H-NS (49). Thus, PhoB sites are significantly enriched in H-NS-bound regions, which represent only 17% of the genome (binomial test, P = 0.02). Since H-NS is known to silence transcription (50), we hypothesized that the lack of detectable PhoB-dependent regulation at some sites may be due to the silencing effects of H-NS. To test this hypothesis, we repeated the σ70 ChIP-seq experiment in Δhns and Δhns ΔphoB strains. Comparison of σ70 occupancy between wild-type and Δhns strains revealed substantially increased occupancy around some PhoB binding sites in the Δhns strain, with most of these sites being intragenic (Fig. 7A). Indeed, we observed widespread increases in σ70 occupancy at promoters genome-wide in the Δhns strain relative to that in the wild type; most of the promoters showing increased σ70 association are located in regions of high H-NS occupancy (Fig. 7B) (49). These data are consistent with our earlier study showing widespread transcriptional silencing by H-NS, particularly within genes (45). We next compared σ70 occupancies around PhoB binding sites between Δhns and Δhns ΔphoB strains (Fig. 8). As for hns+ cells, the only large differences (>5-fold) in σ70 occupancy were associated with the PhoB sites at phoB and pstS. Interestingly, we did not observe differences of >1.5-fold in σ70 occupancy at any other sites, including the sites upstream of yoaI, phoA, mglB, phnC, and phoH. However, the differences in σ70 occupancy that we observed for phoB and pstS sites were between 2- and 4-fold lower than the differences observed in the hns+ strains at the same sites. Hence, the more subtle differences in σ70 occupancy observed at the sites upstream of yoaI, phoA, phnC, and phoH in the hns+ strains may have escaped detection in the Δhns strains. The lower effect of PhoB on σ70 occupancy in the Δhns strain background may be due to the large-scale redeployment of RNAP that occurs in the absence of H-NS (51).
FIG 7.
H-NS suppresses transcription from many promoters. (A) Scatter plot showing normalized σ70 occupancy in wild-type MG1655 and MG1655 Δhns (AMD565a) for the 400-bp regions surrounding PhoB binding sites identified by ChIP-seq. Each data point represents a PhoB binding site. The color of each data point indicates the level of H-NS occupancy at the corresponding site (49). Intragenic PhoB sites are represented by crosses; intergenic PhoB sites are represented by circles. (B) Scatter plot showing normalized σ70 occupancy in wild-type MG1655 and MG1655 Δhns (AMD565a) for all σ70 binding sites identified by ChIP-seq from MG1655 Δhns (AMD565a) cells. The color of each data point indicates the level of H-NS occupancy at the corresponding site (49). Intragenic PhoB sites are represented by crosses; intergenic PhoB sites are represented by circles. For both panels A and B, values are the average of two independent biological replicates; error bars represent ±1 standard deviation.
FIG 8.
H-NS does not suppress PhoB-dependent effects on recruitment of initiating RNA polymerase. The scatter plot shows normalized σ70 occupancy in wild-type MG1655 Δhns (AMD565a) and MG1655 Δhns ΔphoB (DMF85) for the 400-bp regions surrounding PhoB binding sites identified by ChIP-seq. Each data point represents a PhoB binding site. The color of each data point indicates the level of H-NS occupancy at the corresponding site (49). Intragenic PhoB sites are represented by crosses; intergenic PhoB sites are represented by circles. Genes associated with PhoB binding sites are indicated in cases where σ70 occupancy differs >2-fold between MG1655 Δhns (AMD565a) and MG1655 Δhns ΔphoB (DMF85) cells. Values are the average of two independent biological replicates; error bars represent ±1 standard deviation.
While PhoB sites are enriched within H-NS-bound regions, H-NS does not appear to modulate PhoB activity at any site. We hypothesized that the enrichment of PhoB binding within H-NS-bound regions is due simply to the nucleotide content of the PhoB binding site; like H-NS-bound regions, the PhoB binding site has a higher A/T content than the genome as a whole. To test this hypothesis, we scrambled the sequence of every PhoB binding site identified by ChIP-seq. We then derived a position weight matrix (PWM) from these scrambled sites and scored every genomic sequence for a match to this PWM. Strikingly, 36% of the top 1,000 scoring positions are within regions bound by H-NS (49). We conclude that the enrichment of PhoB binding sites within H-NS-bound regions is likely due to the A/T-rich nature of the binding motif.
Sequence conservation of PhoB binding sites.
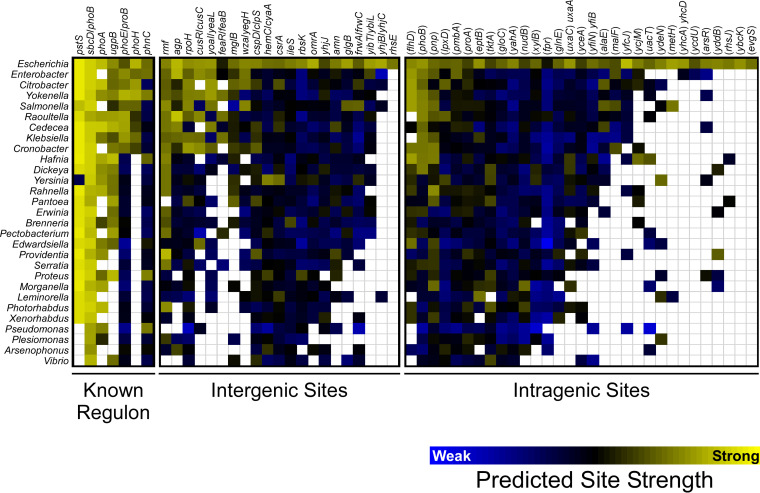
Sequence conservation of a DNA binding site is often an indication that the site is functional (52). We determined the sequence conservation of the 59 E. coli PhoB sites identified by ChIP-seq for which we could identify an instance of the PhoB binding motif. Specifically, we scored homologous regions from 29 diverse gammaproteobacterial species for matches to the PhoB binding site motif (Fig. 2B). The DNA-binding domain of PhoB is highly conserved across these species (Fig. S1). As shown in Fig. 9, the PhoB binding sites upstream of pstS, phoB, phoA, and ugpB are broadly conserved. The PhoB binding sites upstream of phoE and phoH are conserved, albeit to a lesser degree. The PhoB binding site upstream of phnC is conserved in only a few species, suggesting that phnC is not a core member of the pho regulon. Among the novel PhoB binding sites, the best conserved is the site upstream of rmf, with strong matches to the PhoB DNA binding motif found upstream of rmf in most species analyzed. We detected PhoB upstream of rmf by ChIP-seq (Table 1) but did not detect significant PhoB-dependent regulation at the level of RNA abundance or RNAP recruitment. PhoB binding sites upstream of agp, rpoH, cusR/cusC, and yoaI were also conserved, albeit to a lesser degree, similar to sites upstream of phoE and phoH. The remaining intergenic PhoB sites were not well conserved, with most having few or no strong matches to the PhoB DNA binding motif outside of E. coli. Lastly, we examined conservation of intragenic PhoB sites. In most cases, these sites had little or no conservation; however, PhoB sites within flhD, phoB, and pnp were conserved among roughly half the species examined. This conservation may reflect a conserved function for the PhoB binding site or could be due to sequence constraints on the codons.
FIG 9.
Conservation of PhoB binding sites across gammaproteobacterial species. A heat map shows conservation of PhoB binding sites across selected gammaproteobacterial species. Columns represent PhoB binding sites from E. coli, divided into known pho regulon binding sites, intergenic sites, and intragenic sites. The associated genes are indicated above each column. Rows represent species for different gammaproteobacterial genera, as indicated to the left of each row. The color of each square indicates the predicted strength of the best-scoring putative PhoB binding site in a region that is homologous to the corresponding region in E. coli. Binding site strength was predicted using a position weight matrix derived from the E. coli PhoB binding site motif (Fig. 2B). The color scale is shown below the heat map, with yellow indicating stronger predicted binding site strength and blue indicating weaker predicted binding site strength. White indicates the absence of a homologous region in the indicated species.
Alignment of PhoB protein sequences used for phylogenetic analysis of binding sites. PhoB protein sequences from 29 gammaproteobacterial species. Amino acids are color coded by chemical property; amino acids are colored only if they are identical to the equivalent position in E. coli. Asterisks under the alignment indicate amino acids reported to contact DNA (32). The percent coverage (cov) and identity (id) relative to the E. coli sequence are indicated next to the genus name. Download FIG S1, PDF file, 0.2 MB (205.1KB, pdf) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DISCUSSION
Comprehensive reassessment of the pho regulon in E. coli and beyond.
By combining ChIP-seq and RNA-seq, we were able to reassess the pho regulon, with high-resolution assignment of PhoB binding sites. As described above, the sensitivity and resolution of an earlier ChIP-chip study were substantially lower, precluding a comprehensive reassessment of the pho regulon (43). Previous studies disagree on which genes constitute the pho regulon in E. coli; however, most studies agree that the pho regulon includes the following operons: pstSCAB-phoU, phoA, phoH, phnCDEFGHIJKLMNOP, phoBR, phoE, ytfK, ugpBAECQ, and psiE (30, 33). waaH is considered in some studies to be in the pho regulon (33). Our data are largely consistent with these assignments but provide strong evidence against ytfK, psiE, and waaH being members of the pho regulon. Specifically, we did not detect PhoB binding near any of these genes (Table 1), and we did not detect PhoB-dependent regulation of psiE (see Table S1 in the supplemental material). It is formally possible that the C-terminal FLAG tags on PhoB altered its binding specificity or reduced its affinity for DNA such that we failed to detect binding to sites upstream of ytfK, psiE, and waaH. Nonetheless, in such a scenario, these binding sites would presumably have relatively low affinity for PhoB.
Our data also rule out several other putative pho regulon genes, namely, asr, eda, argP, and pitB, which were not associated with detectable PhoB binding or PhoB-dependent regulation (40, 53–57). Similarly, we did not detect binding of PhoB upstream of the small RNA (sRNA)-encoding gene esrL, despite a recent report of PhoB binding to this region in enteropathogenic E. coli, with the sequence of the reported PhoB site being identical in E. coli K-12 (58). In contrast, our ChIP-seq data support the assignment of amn and yoaI as pho regulon members, as has previously been suggested based on limited experimental evidence (40–42). Our ChIP-seq and RNA-seq data identify novel pho regulon members with confidence, namely, cusC, feaR, yibT, and agp. These genes all have PhoB binding sites upstream and were significantly differentially expressed >2-fold between wild-type and ΔphoB strains. We note that direct positive regulation of cusC and direct negative regulation of feaR by PhoB have been suggested previously (43). Our data provide no evidence to suggest that there are unannotated transcripts regulated by PhoB or transcripts whose regulation by PhoB is masked by H-NS (Fig. 6 and 8). Lastly, our data do not support direct regulation by PhoB sites located within genes (Fig. 5).
Phylogenetic analysis of PhoB binding sites highlights a highly conserved set of pho regulon genes within the gammaproteobacteria, namely, pstS, phoB, phoA, ugpB, and associated operonic genes (Fig. 9). Consistent with this, direct PhoB regulation of the pstS and phoB transcripts has been described for the more distantly related alphaproteobacterium Caulobacter crescentus (59). phoE, phoH, and yoaI represent a second set of conserved pho regulon genes, although their conservation is more phylogenetically restricted. Interestingly, while PhoB regulation of phnC and associated operonic genes does not appear to be widely conserved, we did observe evidence for strong PhoB sites upstream of phnC in a small set of species, and phnC is known to be a direct regulatory target of PhoB in C. crescentus (59), suggesting that phnC may have a niche-specific function in phosphate homeostasis.
The phylogenetic pattern of PhoB binding site conservation for sites upstream of rmf, agp, rpoH, and cusR/cusC suggests that these genes may be part of the conserved pho regulon. We observed significant differential expression of cusC and agp between wild-type and ΔphoB cells. In contrast, we did not observe significant differential expression of rmf or rpoH. We speculate that regulation of rmf and rpoH by PhoB is integrated with regulation by other TFs, such that PhoB-dependent changes in expression are detectable only under specific growth conditions. Consistent with this idea, transcription of rmf has been shown to be regulated by ppGpp (60, 61) and cyclic AMP receptor protein (CRP) (62) and possibly by additional TFs (63) and diverse stress conditions (64). PhoB binding sites upstream of agp, rpoH, and cusR/cusC are conserved in largely the same set of species as binding sites upstream of phoE, phoH, and yoaI, suggesting that these species share a set of pho regulon genes.
Most intragenic PhoB sites appear to be nonfunctional and are not under selective pressure.
Our data argue against intragenic PhoB sites having regulatory activities of the types that have been described previously for intragenic TF sites, specifically, regulation of transcription from an intragenic promoter (5, 7–12) or regulation of the overlapping gene either by roadblock repression or a novel mechanism (3, 15–18). Indeed, most intragenic PhoB sites are associated with little or no local σ70 binding (Fig. 6), indicating that PhoB binding alone is insufficient to recruit RNAP. Thus, it is likely that RNAP:σ70-interacting promoter elements are also necessary for PhoB-dependent recruitment of RNAP. Moreover, the spacing between PhoB sites and core promoter elements is likely to be important in determining whether PhoB recruits RNAP, since even intragenic PhoB sites that are close to intragenic promoters (i.e., those associated with high ChIP-seq signal for σ70) show no difference in σ70 occupancy upon deletion of phoB. Consistent with this idea, structural models of the PhoB-RNAP-DNA complex formed at PhoB-activated promoters support strict spacing requirements between the pho box and core promoter elements (46–48, 65).
Widespread intragenic TF binding is emerging as a common phenomenon as more TFs are mapped using ChIP-seq (2–6, 66). Similarly, many σ factors have been shown to bind and initiate transcription from large numbers of intragenic promoters (8, 19, 20, 67–70). In the majority of cases tested, these intragenic binding sites are poorly conserved (69, 71), as is the case for intragenic PhoB sites (Fig. 9). Based on the lack of detectable transcriptional activity, and the limited conservation of intragenic PhoB sites, we speculate that intragenic TF sites often arise due to genetic drift or selective pressures on overlapping sequences such as codons. Consistent with this idea, intragenic PhoB sites tend to be weaker (lower ChIP-seq enrichment) than intergenic sites (Mann-Whitney U test, P = 0.005). A previous study showed that the predicted number of intragenic binding sites for many bacterial TFs is the same in actual genome sequences as it is in randomized genome sequences, suggesting that intragenic TF binding sites are common and arise largely due to genetic drift (28). We further speculate that the fitness cost of intragenic PhoB sites is low. Intragenic TF binding sites can therefore be considered genomic “noise.” Interestingly, the vast majority of PhoB binding events detected in C. crescentus are intergenic (59), suggesting that intragenic PhoB binding in C. crescentus may be associated with a fitness cost. Finally, we cannot rule out the possibility that intragenic PhoB sites in E. coli are functional. For example, they could contribute, en masse, to titration of PhoB, they could facilitate DNA looping that impacts chromosome structure, as has been suggested for some TFs (22–27), or they could regulate transcription by unknown mechanisms.
We have comprehensively mapped the PhoB regulon by assessing PhoB binding, PhoB-dependent transcriptome changes, and PhoB-dependent RNAP recruitment. We identified novel pho regulon members, some of which are modestly conserved across other genera, and identified many seemingly nonfunctional PhoB binding sites inside genes. We conclude that a combination of binding site information (e.g., ChIP-seq) and regulatory information (e.g., RNA-seq) is required to accurately define the regulons of most TFs.
MATERIALS AND METHODS
Strains and plasmids.
E. coli MG1655 and its derivatives were used for this study. The strains and plasmid used are listed in Table 2. All oligonucleotides used are listed in Table S2 in the supplemental material. For ChIP-seq, PhoB was C-terminally epitope tagged with a 3×FLAG tag. The tag was inserted at the native phoB locus by use of FRUIT recombineering (72) using oligonucleotides JW2973 and JW2974. MG1655 ΔphoB (DMF84) and Δhns (AMD565a) strains were constructed by P1 transduction from Keio collection strains (73) into MG1655. The Kanr genes were removed by FLP-recombinase expressed from pCP20, as previously described (74). The MG1655 Δhns ΔphoB strain was made in the same manner, with deletions introduced sequentially. MG1655 ΔphoB (CDS091) was constructed by use of FRUIT (72) using oligonucleotides JW6280, JW6281, JW6294, and JW6295. Note that there are two MG1655 ΔphoB strains used in this study; DMF34 was used for σ70 ChIP-seq experiments, and CDS091 was used for qRT-PCR, RNA-seq, and RNAP ChIP-qPCR experiments.
TABLE 2.
List of strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | Escherichia coli MG1655 | 89 |
| DMF34 | MG1655 phoB-FLAG3 | This study |
| DMF84 | MG1655 ΔphoB | This study |
| AMD565a | MG1655 Δhns | This study |
| DMF85 | MG1655 Δhns ΔphoB | This study |
| CDS091 | MG1655 ΔphoB | This study |
| Plasmid | ||
| pBAD24 | Empty vector for arabinose-inducible expression | 90 |
| pCP20 | Expression of FLP-recombinase | 74 |
List of oligonucleotides used in this study. Download Table S2, XLSX file, 0.01 MB (10.7KB, xlsx) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
qRT-PCR.
qRT-PCR was performed based on a previous study (69). MG1655/pBAD24, CDS091 (MG1655 ΔphoB)/pBAD24, or DMF34/pBAD24 cells were grown at 37°C with aeration in MOPS (morpholinepropanesulfonic acid) minimal medium with 0.2 mM K2PO4, 0.4% glucose, and 100 μg/mL ampicillin to an optical density at 600 nm (OD600) of 0.5 to 0.6. Arabinose was added to a final concentration of 0.2% for 7 min or 20 min, with one or two of the three replicate samples for each strain receiving arabinose for 7 min. Thus, the replicate samples were not always consistent with respect to the extent of growth after arabinose addition. However, since arabinose is not expected to affect pstS expression, all samples for a single strain were treated as replicates regardless of whether the cells were grown for 7 min or 20 min after arabinose addition. RNA was prepared as described for RNA-seq. RNA was reverse transcribed using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. A control reaction omitting reverse transcriptase was performed. One percent of the cDNA (or negative control) was used as a template in a quantitative real-time PCR using an Applied Biosystems 7500 fast real-time PCR machine, with primers JW156 and JW157 for amplifying the minD control gene and primers JW7802 and JW7803 for amplifying pstS. Relative expression of pstS was determined by the ΔCT method, with normalization to minD expression.
ChIP-seq.
For low-phosphate growth experiments, cells were grown at 37°C with aeration in MOPS minimal medium with 0.2 mM K2HPO4 and 0.4% glucose, as previously described (37, 75). For high-phosphate growth experiments, cells were grown in MOPS minimal medium with 1.32 mM K2HPO4 and 0.4% glucose. Subcultures were inoculated 1:100 and grown at 37°C with aeration to an OD600 of 0.5 to 0.7. ChIP-seq libraries were prepared as previously described (76). Libraries were prepared from two biological replicate cultures for each experimental group. For DMF34 (MG1655 phoB-FLAG3) or MG1655, PhoB-FLAG3 (or the untagged control) was immunoprecipitated with 2 μL of anti-FLAG M2 monoclonal antibody (Sigma). For MG1655, DMF84 (ΔphoB), AMD565a (MG1655 Δhns), and DMF85 (MG1655 ΔphoB Δhns), σ70 was immunoprecipitated with 1 μL of anti-σ70 antibody (Neoclone). Libraries were sequenced on a HiSeq 2000 (Illumina) by the University at Buffalo Next-Generation Sequencing Core Facility or on a NextSeq (Illumina) by the Wadsworth Center Applied Genomic Technologies Core Facility.
Analysis of PhoB-FLAG3 ChIP-seq data.
Duplicate ChIP-seq data for (i) MG1655 phoB-FLAG3 (DMF34) grown in low-phosphate conditions, (ii) untagged MG1655 grown in low-phosphate conditions, and (iii) MG1655 phoB-FLAG3 (DMF34) grown in high-phosphate conditions were aligned to the E. coli MG1655 genome (GenBank accession no. NC_000913.3) using CLC Genomics Workbench (version 8). ChIP-seq peaks were called using a previously described analysis pipeline (8).
Analysis of RNAP occupancy around PhoB binding sites.
Wild-type MG1655 and MG1655 ΔphoB (CDS091) cells were grown at 37°C with aeration to an OD600 of 0.6 to 0.7 in MOPS minimal medium with 0.2 mM K2HPO4 and 0.4% glucose, as previously described (37, 75). ChIP was performed as previously described (76) using 1 μL anti-β (RNA polymerase subunit) antibody (BioLegend catalog no. 663903). ChIP and input samples were analyzed using an ABI 7500 fast real-time PCR machine. Enrichment of ChIP samples was calculated relative to a control region within bglB, which is transcriptionally silent. RNAP occupancy units represent background-subtracted fold enrichment over the control region. Oligonucleotides used for qPCR were JW125 + JW126 (bglB), JW10937 + JW10938 (yoaI), JW10939 + JW10940 (amn), JW10941 + JW10942 (pstS), JW10943 + JW10944 (ugpB), JW10945 + JW10946 (mepK), JW10947 + JW10948 (gloC), JW10949 + JW10950 (evgA), JW10951 + JW10952 (evgS), JW10953 + JW10954 (pnp upstream), and JW10955 + JW10956 (pnp downstream). For statistical analysis of relative changes in RNAP occupancy upstream and downstream of intragenic PhoB sites, we used the mean and standard deviation values for ChIP-qPCR occupancy to generate 1,000 simulations for occupancy at each region tested. We then determined how frequently the ratio of predicted RNAP occupancy downstream to predicted RNAP occupancy upstream of an intragenic PhoB site was higher in ΔphoB cells than wild-type cells. We repeated this simulation 10 times to estimate a P value for each of the three intragenic PhoB sites tested.
Analysis of σ70 occupancy around PhoB binding sites.
Duplicate ChIP-seq data for σ70 from wild-type MG1655, MG1655 ΔphoB (DMF84), MG1655 Δhns (AMD565a), and MG1655 Δhns ΔphoB (DMF85) were aligned to the E. coli MG1655 genome (GenBank accession no. NC_000913.3) using Rockhopper (version 2.03; default parameters) (77), which also calculated the depth of sequence coverage at all genomic positions on each strand, normalized for total sequence read count. A custom Python script was used to determine the relative sequence read coverage from each σ70 ChIP-seq data set in 400-bp windows centered on each PhoB ChIP-seq peak (coordinates listed in Table 1).
Analysis of σ70 occupancy at promoters in wild-type and Δhns strains.
Duplicate ChIP-seq data for σ70 for wild-type MG1655 or MG1655 Δhns (AMD565a) were aligned to the E. coli MG1655 genome (GenBank accession no. NC_000913.3) using CLC Genomics Workbench (version 8). ChIP-seq peaks were called using a previously described analysis pipeline (8). A custom Python script was used to determine the relative sequence read coverage from each σ70 ChIP-seq data set in 50-bp windows centered on each σ70 ChIP-seq peak from MG1655 Δhns (AMD565a).
Determining H-NS occupancy from published ChIP-seq data.
H-NS ChIP-seq occupancy was determined from published data (49). Specifically, genome coordinates for σ70 ChIP-seq peaks were converted from NCBI genome sequence version U00096.3 to U00096.2 at https://biocyc.org/ECOLI/map-seq-coords-form?chromosome=COLI-K12. H-NS occupancy was determined as the average from four normalized sequence read coverage files available from EBI ArrayExpress under accession number E-MTAB-332.
RNA-seq.
MG1655/pBAD24 or CDS091 (MG1655 ΔphoB)/pBAD24 cells were grown at 37°C with aeration in MOPS minimal medium with 0.2 mM K2PO4, 0.4% glucose, and 100 μg/mL ampicillin to an OD600 of 0.5 to 0.6. Arabinose was added to a final concentration of 0.2% for 7 min. Note that addition of arabinose is not expected to impact the expression of PhoB-regulated genes. RNA was isolated using a modified hot-phenol method, as previously described (76). Samples were treated with Turbo DNase (Ambion) to remove genomic DNA, rRNA was removed using the Ribo-Zero rRNA removal kit for Gram-negative bacteria (Epicentre/Illumina), and libraries were prepared with the ScriptSeq Complete kit for bacteria (Epicentre/Illumina) (76). Libraries were sequenced on a HiSeq 2000 (Illumina) by the University at Buffalo Next-Generation Sequencing Core Facility. RNA-seq data were aligned to the E. coli MG1655 genome (GenBank accession no. NC_000913.3) using BWA for Illumina (v0.5.9-r16) (78) on Galaxy (https://usegalaxy.org) (79). Read counting, normalization, and differential expression analysis were performed in R using GenomicAlignments (v1.28) summarizeOverlaps (80) and DEseq2 (v1.32; betaPrior = FALSE) (81).
PhoB motif discovery and analysis.
Sequences of 100 bp surrounding PhoB ChIP-seq peaks were extracted and analyzed using MEME (version 5.1.0; default parameters) (82, 83). The position of the inferred motif relative to ChIP-seq peak centers was analyzed using CentriMo (version 5.1.0; default parameters) (84) through the MEME-ChIP tool (85).
To determine whether the nucleotide content of the PhoB binding site motif contributes to the association of PhoB binding sites with H-NS-bound regions, we first scrambled each PhoB binding site individually using a custom Python script. We then compiled the scrambled sites into a PWM and searched the E. coli MG1655 genome (GenBank accession no. NC_000913.3) for the top 1,000 matches to this PWM using FIMO (version 5.1.0; default parameters) (86).
Analysis of PhoB binding site conservation.
Binding site conservation analysis was performed as described previously (71). Protein sequences were aligned using Clustal Omega (87) and visualized using MView (88). The genomes analyzed were those of Arsenophonus nasoniae DSM 15247, Brenneria sp. EniD312, Cedecea davisae DSM 4568, Citrobacter rodentium ICC168, Cronobacter sakazakii ATCC BAA-894, Dickeya dadantii 3937, Edwardsiella tarda EIB202, Enterobacter cloacae subsp. cloacae ATCC 13047, Erwinia amylovora ATCC 49946, Escherichia coli strain K-12 substrain MG1655, Hafnia alvei ATCC 51873, Klebsiella pneumoniae KCTC 2242, Leminorella grimontii ATCC 33999 = DSM 5078, Morganella morganii subsp. morganii KT, Pantoea agglomerans 299R, Pectobacterium atrosepticum SCRI1043, Photorhabdus asymbiotica, Plesiomonas shigelloides 302-73, Proteus mirabilis HI4320, Providencia stuartii MRSN 2154, Pseudomonas aeruginosa PAO1, Rahnella sp. Y9602, Raoultella ornithinolytica B6, Salmonella enterica strain P125109, Serratia marcescens FGI94, Vibrio cholerae M66-2, Xenorhabdus bovienii SS-2004, Yersinia pestis KIM 10, and Yokenella regensburgei ATCC 43003.
Data availability.
ChIP-seq data are available at EBI ArrayExpress under accession number E-MTAB-9293. RNA-seq data are available at EBI ArrayExpress under accession number E-MTAB-9591.
ACKNOWLEDGMENTS
We thank the University at Buffalo Next-Generation Sequencing Core Facility and the Wadsworth Center Applied Genomic Technologies Core Facility for DNA sequencing. We thank the Wadsworth Center Tissue Culture and Media Core Facility and Glassware Facility for technical support.
This material is based on work supported by the National Science Foundation Graduate Research Fellowship under grant no. DGE-1060277 (D.M.F.). D.M.F. was also supported by National Institutes of Health training grant no. T32AI055429. This work was also supported by National Institutes of Health Director's New Innovator Award no. DP2OD007188 (J.T.W.) and National Institutes of Health grant no. R01GM114812 and R35GM144328 (J.T.W.).
Contributor Information
Joseph T. Wade, Email: joseph.wade@health.ny.gov.
Michael T. Laub, Massachusetts Institute of Technology
REFERENCES
- 1.Browning DF, Busby SJW. 2016. Local and global regulation of transcription initiation in bacteria. Nat Rev Microbiol 14:638–650. doi: 10.1038/nrmicro.2016.103. [DOI] [PubMed] [Google Scholar]
- 2.Galagan J, Lyubetskaya A, Gomes A. 2013. ChIP-Seq and the complexity of bacterial transcriptional regulation. Curr Top Microbiol Immunol 363:43–68. doi: 10.1007/82_2012_257. [DOI] [PubMed] [Google Scholar]
- 3.Galagan JE, Minch K, Peterson M, Lyubetskaya A, Azizi E, Sweet L, Gomes A, Rustad T, Dolganov G, Glotova I, Abeel T, Mahwinney C, Kennedy AD, Allard R, Brabant W, Krueger A, Jaini S, Honda B, Yu W-H, Hickey MJ, Zucker J, Garay C, Weiner B, Sisk P, Stolte C, Winkler JK, Van de Peer Y, Iazzetti P, Camacho D, Dreyfuss J, Liu Y, Dorhoi A, Mollenkopf H-J, Drogaris P, Lamontagne J, Zhou Y, Piquenot J, Park ST, Raman S, Kaufmann SHE, Mohney RP, Chelsky D, Moody DB, Sherman DR, Schoolnik GK. 2013. The Mycobacterium tuberculosis regulatory network and hypoxia. Nature 499:178–183. doi: 10.1038/nature12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada T, Ishihama A, Busby SJW, Grainger DC. 2008. The Escherichia coli RutR transcription factor binds at targets within genes as well as intergenic regions. Nucleic Acids Res 36:3950–3955. doi: 10.1093/nar/gkn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knapp GS, Lyubetskaya A, Peterson MW, Gomes ALC, Ma Z, Galagan JE, McDonough KA. 2015. Role of intragenic binding of cAMP responsive protein (CRP) in regulation of the succinate dehydrogenase genes Rv0249c-Rv0247c in TB complex mycobacteria. Nucleic Acids Res 43:5377–5393. doi: 10.1093/nar/gkv420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranganathan S, Bai G, Lyubetskaya A, Knapp GS, Peterson MW, Gazdik M, C Gomes AL, Galagan JE, McDonough KA. 2016. Characterization of a cAMP responsive transcription factor, Cmr (Rv1675c), in TB complex mycobacteria reveals overlap with the DosR (DevR) dormancy regulon. Nucleic Acids Res 44:134–151. doi: 10.1093/nar/gkv889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown DR, Barton G, Pan Z, Buck M, Wigneshweraraj S. 2014. Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat Commun 5:4115. doi: 10.1038/ncomms5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald DM, Bonocora RP, Wade JT. 2014. Comprehensive mapping of the Escherichia coli flagellar regulatory network. PLoS Genet 10:e1004649. doi: 10.1371/journal.pgen.1004649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonocora RP, Wade JT. 2015. ChIP-seq for genome-scale analysis of bacterial DNA-binding proteins. Methods Mol Biol 1276:327–340. doi: 10.1007/978-1-4939-2392-2_20. [DOI] [PubMed] [Google Scholar]
- 10.Haycocks JRJ, Grainger DC. 2016. Unusually situated binding sites for bacterial transcription factors can have hidden functionality. PLoS One 11:e0157016. doi: 10.1371/journal.pone.0157016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao Y, Papenfort K, Reinhardt R, Sharma CM, Vogel J. 2012. An atlas of Hfq-bound transcripts reveals 3’ UTRs as a genomic reservoir of regulatory small RNAs. EMBO J 31:4005–4019. doi: 10.1038/emboj.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith C, Stringer AM, Mao C, Palumbo MJ, Wade JT. 2016. Mapping the regulatory network for Salmonella enterica serovar Typhimurium invasion. mBio 7:e01024-16. doi: 10.1128/mBio.01024-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani MH, Orosz L, Adhya S. 1983. A control element within a structural gene: the gal operon of Escherichia coli. Cell 32:783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- 14.Choy HE, Park SW, Parrack P, Adhya S. 1995. Transcription regulation by inflexibility of promoter DNA in a looped complex. Proc Natl Acad Sci USA 92:7327–7331. doi: 10.1073/pnas.92.16.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielow A, Kücherer C, Kölling R, Messer W. 1988. Transcription in the region of the replication origin, oriC, of Escherichia coli: termination of asnC transcripts. Mol Gen Genet 214:474–481. doi: 10.1007/BF00330483. [DOI] [PubMed] [Google Scholar]
- 16.He B, Zalkin H. 1992. Repression of Escherichia coli purB is by a transcriptional roadblock mechanism. J Bacteriol 174:7121–7127. doi: 10.1128/jb.174.22.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belitsky BR, Sonenshein AL. 2011. Roadblock repression of transcription by Bacillus subtilis CodY. J Mol Biol 411:729–743. doi: 10.1016/j.jmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belitsky BR, Sonenshein AL. 2013. Genome-wide identification of Bacillus subtilis CodY-binding sites at single-nucleotide resolution. Proc Natl Acad Sci USA 110:7026–7031. doi: 10.1073/pnas.1300428110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lybecker M, Bilusic I, Raghavan R. 2014. Pervasive transcription: detecting functional RNAs in bacteria. Transcription 5:e944039. doi: 10.4161/21541272.2014.944039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade JT, Grainger DC. 2014. Pervasive transcription: illuminating the dark matter of bacterial transcriptomes. Nat Rev Microbiol 12:647–653. doi: 10.1038/nrmicro3316. [DOI] [PubMed] [Google Scholar]
- 21.Thomason MK, Bischler T, Eisenbart SK, Förstner KU, Zhang A, Herbig A, Nieselt K, Sharma CM, Storz G. 2015. Global transcriptional start site mapping using differential RNA sequencing reveals novel antisense RNAs in Escherichia coli. J Bacteriol 197:18–28. doi: 10.1128/JB.02096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reitzer LJ, Magasanik B. 1986. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell 45:785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- 23.Schleif R. 1992. DNA looping. Annu Rev Biochem 61:199–223. doi: 10.1146/annurev.bi.61.070192.001215. [DOI] [PubMed] [Google Scholar]
- 24.Qian Z, Dimitriadis EK, Edgar R, Eswaramoorthy P, Adhya S. 2012. Galactose repressor mediated intersegmental chromosomal connections in Escherichia coli. Proc Natl Acad Sci USA 109:11336–11341. doi: 10.1073/pnas.1208595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian Z, Trostel A, Lewis DEA, Lee SJ, He X, Stringer AM, Wade JT, Schneider TD, Durfee T, Adhya S. 2016. Genome-wide transcriptional regulation and chromosome structural arrangement by GalR in E. coli. Front Mol Biosci 3:74. doi: 10.3389/fmolb.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewster RC, Weinert FM, Garcia HG, Song D, Rydenfelt M, Phillips R. 2014. The transcription factor titration effect dictates level of gene expression. Cell 156:1312–1323. doi: 10.1016/j.cell.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Göpel Y, Görke B. 2014. Lies and deception in bacterial gene regulation: the roles of nucleic acid decoys. Mol Microbiol 92:641–647. doi: 10.1111/mmi.12604. [DOI] [PubMed] [Google Scholar]
- 28.Mrázek J, Karls AC. 2019. In silico simulations of occurrence of transcription factor binding sites in bacterial genomes. BMC Evol Biol 19:67. doi: 10.1186/s12862-019-1381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martínez-Hackert E, Stock AM. 1997. Structural relationships in the OmpR family of winged-helix transcription factors. J Mol Biol 269:301–312. doi: 10.1006/jmbi.1997.1065. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh Y-J, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13:198–203. doi: 10.1016/j.mib.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makino K, Shinagawa H, Amemura M, Kawamoto T, Yamada M, Nakata A. 1989. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol 210:551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- 32.Blanco AG, Sola M, Gomis-Rüth FX, Coll M. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701–713. doi: 10.1016/S0969-2126(02)00761-X. [DOI] [PubMed] [Google Scholar]
- 33.Gardner SG, McCleary WR. 2019. Control of the phoBR regulon in Escherichia coli. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0006-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 35.Blus-Kadosh I, Zilka A, Yerushalmi G, Banin E. 2013. The effect of pstS and phoB on quorum sensing and swarming motility in Pseudomonas aeruginosa. PLoS One 8:e74444. doi: 10.1371/journal.pone.0074444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanBogelen RA, Olson ER, Wanner BL, Neidhardt FC. 1996. Global analysis of proteins synthesized during phosphorus restriction in Escherichia coli. J Bacteriol 178:4344–4366. doi: 10.1128/jb.178.15.4344-4366.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baek JH, Lee SY. 2007. Transcriptome analysis of phosphate starvation response in Escherichia coli. J Microbiol Biotechnol 17:244–252. [PubMed] [Google Scholar]
- 38.Gao R, Stock AM. 2018. Overcoming the cost of positive autoregulation by accelerating the response with a coupled negative feedback. Cell Rep 24:3061–3071.e6. doi: 10.1016/j.celrep.2018.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makino K, Shinagawa H, Amemura M, Nakata A. 1986. Nucleotide sequence of the phoB gene, the positive regulatory gene for the phosphate regulon of Escherichia coli K-12. J Mol Biol 190:37–44. doi: 10.1016/0022-2836(86)90073-2. [DOI] [PubMed] [Google Scholar]
- 40.Baek JH, Lee SY. 2006. Novel gene members in the Pho regulon of Escherichia coli. FEMS Microbiol Lett 264:104–109. doi: 10.1111/j.1574-6968.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Makino K. 2012. Novel members of the phosphate regulon in Escherichia coli O157:H7 identified using a whole-genome shotgun approach. Gene 502:27–35. doi: 10.1016/j.gene.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 42.Chekabab SM, Jubelin G, Dozois CM, Harel J. 2014. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS One 9:e94285. doi: 10.1371/journal.pone.0094285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang C, Huang T-W, Wen S-Y, Chang C-Y, Tsai S-F, Wu W-F, Chang C-H. 2012. Genome-wide PhoB binding and gene expression profiles reveal the hierarchical gene regulatory network of phosphate starvation in Escherichia coli. PLoS One 7:e47314. doi: 10.1371/journal.pone.0047314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reppas NB, Wade JT, Church GM, Struhl K. 2006. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol Cell 24:747–757. doi: 10.1016/j.molcel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 45.Singh SS, Singh N, Bonocora RP, Fitzgerald DM, Wade JT, Grainger DC. 2014. Widespread suppression of intragenic transcription initiation by H-NS. Genes Dev 28:214–219. doi: 10.1101/gad.234336.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blanco AG, Canals A, Bernués J, Solà M, Coll M. 2011. The structure of a transcription activation subcomplex reveals how σ(70) is recruited to PhoB promoters. EMBO J 30:3776–3785. doi: 10.1038/emboj.2011.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Canals A, Blanco AG, Coll M. 2012. σ70 and PhoB activator: getting a better grip. Transcription 3:160–164. doi: 10.4161/trns.20444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung C-S, McMahon BH. 2012. A structural model of the E. coli PhoB dimer in the transcription initiation complex. BMC Struct Biol 12:3. doi: 10.1186/1472-6807-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahramanoglou C, Seshasayee ASN, Prieto AI, Ibberson D, Schmidt S, Zimmermann J, Benes V, Fraser GM, Luscombe NM. 2011. Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res 39:2073–2091. doi: 10.1093/nar/gkq934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grainger DC. 2016. Structure and function of bacterial H-NS protein. Biochem Soc Trans 44:1561–1569. doi: 10.1042/BST20160190. [DOI] [PubMed] [Google Scholar]
- 51.Lamberte LE, Baniulyte G, Singh SS, Stringer AM, Bonocora RP, Stracy M, Kapanidis AN, Wade JT, Grainger DC. 2017. Horizontally acquired AT-rich genes in Escherichia coli cause toxicity by sequestering RNA polymerase. Nat Microbiol 2:16249–16249. doi: 10.1038/nmicrobiol.2016.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Gerstein M. 2003. Of mice and men: phylogenetic footprinting aids the discovery of regulatory elements. J Biol 2:11. doi: 10.1186/1475-4924-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suziedeliené E, Suziedélis K, Garbenciūté V, Normark S. 1999. The acid-inducible asr gene in Escherichia coli: transcriptional control by the phoBR operon. J Bacteriol 181:2084–2093. doi: 10.1128/JB.181.7.2084-2093.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han JS, Park JY, Lee YS, Thöny B, Hwang DS. 1999. PhoB-dependent transcriptional activation of the iciA gene during starvation for phosphate in Escherichia coli. Mol Gen Genet 262:448–452. doi: 10.1007/s004380051104. [DOI] [PubMed] [Google Scholar]
- 55.Harris RM, Webb DC, Howitt SM, Cox GB. 2001. Characterization of PitA and PitB from Escherichia coli. J Bacteriol 183:5008–5014. doi: 10.1128/JB.183.17.5008-5014.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murray EL, Conway T. 2005. Multiple regulators control expression of the Entner-Doudoroff aldolase (Eda) of Escherichia coli. J Bacteriol 187:991–1000. doi: 10.1128/JB.187.3.991-1000.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida Y, Sugiyama S, Oyamada T, Yokoyama K, Kim S-K, Makino K. 2011. Identification of PhoB binding sites of the yibD and ytfK promoter regions in Escherichia coli. J Microbiol 49:285–289. doi: 10.1007/s12275-011-0360-6. [DOI] [PubMed] [Google Scholar]
- 58.Jia T, Wu P, Liu B, Liu M, Mu H, Liu D, Huang M, Li L, Wei Y, Wang L, Yang Q, Liu Y, Yang B, Huang D, Yang L, Liu B. 2023. The phosphate-induced small RNA EsrL promotes E. coli virulence, biofilm formation, and intestinal colonization. Sci Signal 16:eabm0488. doi: 10.1126/scisignal.abm0488. [DOI] [PubMed] [Google Scholar]
- 59.Lubin EA, Henry JT, Fiebig A, Crosson S, Laub MT. 2016. Identification of the PhoB regulon and role of PhoU in the phosphate starvation response of Caulobacter crescentus. J Bacteriol 198:187–200. doi: 10.1128/JB.00658-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izutsu K, Wada A, Wada C. 2001. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 6:665–676. doi: 10.1046/j.1365-2443.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez-Vazquez P, Dewey CN, Kitten N, Ross W, Gourse RL. 2019. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc Natl Acad Sci USA 116:8310–8319. doi: 10.1073/pnas.1819682116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimada T, Yoshida H, Ishihama A. 2013. Involvement of cyclic AMP receptor protein in regulation of the rmf gene encoding the ribosome modulation factor in Escherichia coli. J Bacteriol 195:2212–2219. doi: 10.1128/JB.02279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida H, Shimada T, Ishihama A. 2018. Coordinated hibernation of transcriptional and translational apparatus during growth transition of Escherichia coli to stationary phase. mSystems 3:e00057-18. doi: 10.1128/mSystems.00057-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moen B, Janbu AO, Langsrud S, Langsrud O, Hobman JL, Constantinidou C, Kohler A, Rudi K. 2009. Global responses of Escherichia coli to adverse conditions determined by microarrays and FT-IR spectroscopy. Can J Microbiol 55:714–728. doi: 10.1139/W09-016. [DOI] [PubMed] [Google Scholar]
- 65.Makino K, Amemura M, Kim SK, Nakata A, Shinagawa H. 1993. Role of the sigma 70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev 7:149–160. doi: 10.1101/gad.7.1.149. [DOI] [PubMed] [Google Scholar]
- 66.Grainger DC. 2016. The unexpected complexity of bacterial genomes. Microbiology (Reading) 162:1167–1172. doi: 10.1099/mic.0.000309. [DOI] [PubMed] [Google Scholar]
- 67.Wade JT, Castro Roa D, Grainger DC, Hurd D, Busby SJW, Struhl K, Nudler E. 2006. Extensive functional overlap between sigma factors in Escherichia coli. Nat Struct Mol Biol 13:806–814. doi: 10.1038/nsmb1130. [DOI] [PubMed] [Google Scholar]
- 68.Bonocora RP, Fitzgerald DM, Stringer AM, Wade JT. 2013. Non-canonical protein-DNA interactions identified by ChIP are not artifacts. BMC Genomics 14:254. doi: 10.1186/1471-2164-14-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bonocora RP, Smith C, Lapierre P, Wade JT. 2015. Genome-scale mapping of Escherichia coli σ54 reveals widespread, conserved intragenic binding. PLoS Genet 11:e1005552. doi: 10.1371/journal.pgen.1005552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wong GT, Bonocora RP, Schep AN, Beeler SM, Lee Fong AJ, Shull LM, Batachari LE, Dillon M, Evans C, Becker CJ, Bush EC, Hardin J, Wade JT, Stoebel DM. 2017. Genome-wide transcriptional response to varying RpoS levels in Escherichia coli K-12. J Bacteriol 199:e00755-16. doi: 10.1128/JB.00755-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitzgerald DM, Smith C, Lapierre P, Wade JT. 2018. The evolutionary impact of intragenic FliA promoters in proteobacteria. Mol Microbiol 108:361–378. doi: 10.1111/mmi.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stringer AM, Singh N, Yermakova A, Petrone BL, Amarasinghe JJ, Reyes-Diaz L, Mantis NJ, Wade JT. 2012. FRUIT, a scar-free system for targeted chromosomal mutagenesis, epitope tagging, and promoter replacement in Escherichia coli and Salmonella enterica. PLoS One 7:e44841. doi: 10.1371/journal.pone.0044841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neidhardt FC, Bloch PL, Smith DF. 1974. Culture medium for enterobacteria. J Bacteriol 119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stringer AM, Currenti SA, Bonocora RP, Baranowski C, Petrone BL, Palumbo MJ, Reilly AAE, Zhang Z, Erill I, Wade JT. 2014. Genome-scale analyses of Escherichia coli and Salmonella enterica AraC reveal noncanonical targets and an expanded core regulon. J Bacteriol 196:660–671. doi: 10.1128/JB.01007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:e140. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Čech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. 2018. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544. doi: 10.1093/nar/gky379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lawrence M, Huber W, Pagès H, Aboyoun P, Carlson M, Gentleman R, Morgan MT, Carey VJ. 2013. Software for computing and annotating genomic ranges. PLoS Comput Biol 9:e1003118. doi: 10.1371/journal.pcbi.1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550–550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bailey TL, Elkan C. 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc Int Conf Intell Syst Mol Biol 2:28–36. [PubMed] [Google Scholar]
- 83.Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. 2009. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bailey TL, Machanick P. 2012. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res 40:e128. doi: 10.1093/nar/gks433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Machanick P, Bailey TL. 2011. MEME-ChIP: motif analysis of large DNA datasets. Bioinformatics 27:1696–1697. doi: 10.1093/bioinformatics/btr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grant CE, Bailey TL, Noble WS. 2011. FIMO: scanning for occurrences of a given motif. Bioinformatics 27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sievers F, Higgins DG. 2014. Clustal Omega, accurate alignment of very large numbers of sequences. Methods Mol Biol 1079:105–116. doi: 10.1007/978-1-62703-646-7_6. [DOI] [PubMed] [Google Scholar]
- 88.Brown NP, Leroy C, Sander C. 1998. MView: a web-compatible database search or multiple alignment viewer. Bioinformatics 14:380–381. doi: 10.1093/bioinformatics/14.4.380. [DOI] [PubMed] [Google Scholar]
- 89.Blattner FR, Plunkett G III, Bloch CA, Perna NT, Burland V, Riley M, Collado-Vides J, Glasner JD, Rode CK, Mayhew GF, Gregor J, Davis NW, Kirkpatrick HA, Goeden MA, Rose DJ, Mau B, Shao Y. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 90.Guzman LM, Belin D, Carson MJ, Beckwith J. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA-seq analysis. Download Table S1, XLSX file, 0.2 MB (252.2KB, xlsx) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Alignment of PhoB protein sequences used for phylogenetic analysis of binding sites. PhoB protein sequences from 29 gammaproteobacterial species. Amino acids are color coded by chemical property; amino acids are colored only if they are identical to the equivalent position in E. coli. Asterisks under the alignment indicate amino acids reported to contact DNA (32). The percent coverage (cov) and identity (id) relative to the E. coli sequence are indicated next to the genus name. Download FIG S1, PDF file, 0.2 MB (205.1KB, pdf) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of oligonucleotides used in this study. Download Table S2, XLSX file, 0.01 MB (10.7KB, xlsx) .
Copyright © 2023 Fitzgerald et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
ChIP-seq data are available at EBI ArrayExpress under accession number E-MTAB-9293. RNA-seq data are available at EBI ArrayExpress under accession number E-MTAB-9591.