Abstract
Case series
Patients: Male, 67-year-old • Male, 69-year-old
Final Diagnosis: Adenocarcinoma of the esophagogastric junction
Symptoms: Tachycardia and low blood pressure
Clinical Procedure: —
Specialty: Gastroenterology and Hepatology
Objective:
Unusual clinical course
Background:
Siewert type II adenocarcinoma of the esophagogastric junction is located at the boundary of the distal esophagus and gastric cardia, and surgical resection is currently performed using open or laparoscopic methods. This report presents 2 cases of laparoscopic resection of Siewert type II adenocarcinoma of the esophagogastric junction using a transhiatal approach, complicated by hemopericardium.
Case Reports:
We present 2 patients diagnosed with Siewert type II esophagogastric junction cancer. A 67-year-old man had intermittent dull pain in the epigastrium without apparent cause for 10 months. A 69-year-old man had persistent dull pain in the middle and upper abdomen for more than 3 months and acid reflux after eating. Gastroscopy with pathological examination confirmed the diagnoses. The patients underwent laparoscopic transhiatal total gastrectomy according to the Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition). Pathological analysis classified the cancers as T3N1M0 and T2N0M0, respectively. The patients’ cases were complicated with hemopericardium 18 h and 23 h after surgery, respectively. The shared clinical symptoms of the patients included tachycardia and low blood pressure. Cardiovascular color Doppler ultrasound and computed tomography (CT) were used to identify the hemopericardium. Following emergent ultrasound-guided pericardiocentesis and drainage, the vital signs of the patients improved. Both patients recovered well, and no other complications occurred.
Conclusions:
Hemopericardium is a life-threatening complication for patients with esophageal-gastric junction cancer who undergo transhiatal laparoscopic surgery. Quick detection and intervention for postoperative hemopericardium following laparoscopic transhiatal total gastrectomy are important. Ultrasound-guided pericardiocentesis and drainage is effective for the treatment of postoperative hemopericardium.
Keywords: Pericardial Effusion, Laparoscopy, Esophagogastric Junction, Gastrectomy, Adenocarcinoma
Background
The incidence of adenocarcinoma of the esophagogastric junction (AEG) is rising. According to the Siewert classification, AEG was defined as a tumor with an epicenter located within 5 cm of the esophagogastric junction. Distal esophageal adenocarcinoma with an epicenter located 1 to 5 cm above the gastroesophageal junction is classified as Siewert type I; true cardia cancer with a center within 1 cm above and 2 cm below the gastroesophageal junction is classified as Siewert type II; and gastric cardia tumors with a center located 2 to 5 cm below the gastroesophageal junction are classified as Siewert type III [1].
Curative treatment is frequently multidisciplinary, but surgery is still required for resectable AEG. There is currently no agreement on the best surgical strategy for treating Siewert type II AEG. A successful therapy for type II AEG involves surgical removal of the distal esophagus during prolonged complete gastrectomy [2,3]. Compared with the transthoracic or left thoracoabdominal surgical approach, the transhiatal method might be more suitable for Siewert type II AEG in terms of short- and long-term surgical outcomes [4,5].
Hemopericardium is a serious complication of esophagogastric junction cancer surgery and can develop into life-threatening pericardial tamponade [6]. Two patients with postoperative hemopericardium were reported by Ito et al and Kats et al [7,8]. The salvage treatment of the reported patients was pericardial resection with open drainage and sternotomy, and 1 patient died on postoperative day 4. We report 2 cases of laparoscopic resection of Siewert type II AEG using a transhiatal approach complicated by hemopericardium, which were treated successfully with ultrasound-guided pericardiocentesis.
Case Reports
Case 1
A 67-year-old man was admitted to the hospital on January 12, 2021, for “intermittent dull pain in the epigastrium without apparent cause for 10 months”. He had a history of gastric ulcer for 7 years. Gastroscopy revealed esophageal lesions, fundus neoplasia, and chronic atrophic gastritis. Computed tomography (CT) showed that the wall of the stomach was thickened, with soft tissue nodules at the junction of the esophagus and stomach, which was consistent with gastric cancer. The CT diagnosis was Siewert type II carcinoma of the esophagogastric junction (Figure 1). Laparoscopic radical gastrectomy (total gastrectomy) was performed on January 15, 2021. The patient reported concerns of palpitation, dizziness, shortness of breath, and profuse sweating 18 h after surgery. His blood pressure was 50/29 mmHg. Bilateral breath sounds were low, with scattered rales. The abdomen was flat. There was no tenderness around the incision and no rebound pain or muscle tension. CT (Figure 2) and pericardial color Doppler ultrasound (Figure 3) showed hemopericardium. At 21 h after surgery, the patient was transferred to the Intensive Care Unit. After ultrasound-guided pericardiocentesis and drainage, his blood pressure, heart rate, and respiratory rate returned to normal, and his symptoms were significantly relieved. The pathological staging was T3N1M0 adenocarcinoma (Figure 4). After the intervention, the patient recovered and was discharged un-eventfully. Upper gastrointestinal radiography revealed that the anastomosis was unobstructed, and no leakage of contrast medium was found (Figure 5).
Figure 1.
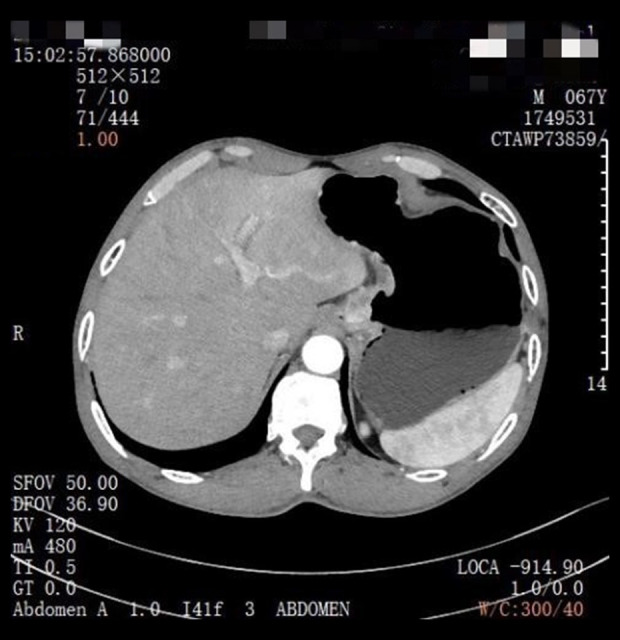
Case 1: Computed tomography for the diagnosis of esophageal and gastric junction carcinoma.
Figure 2.
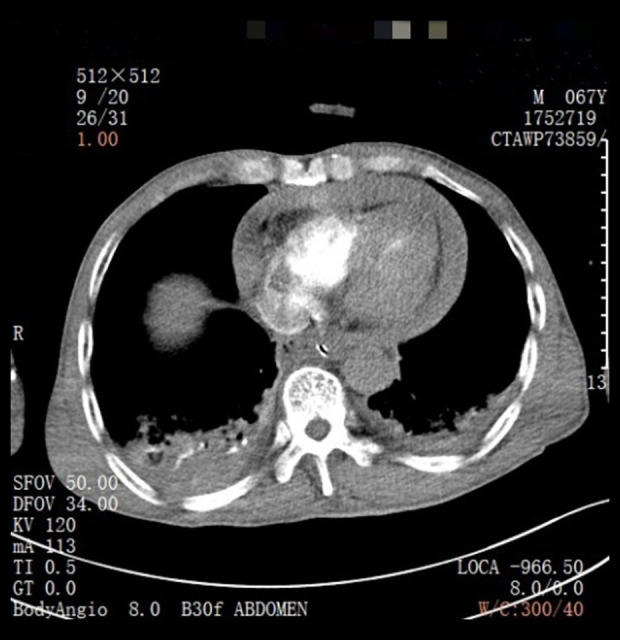
Case 1: Computed tomography indicates pericardial effusion.
Figure 3.
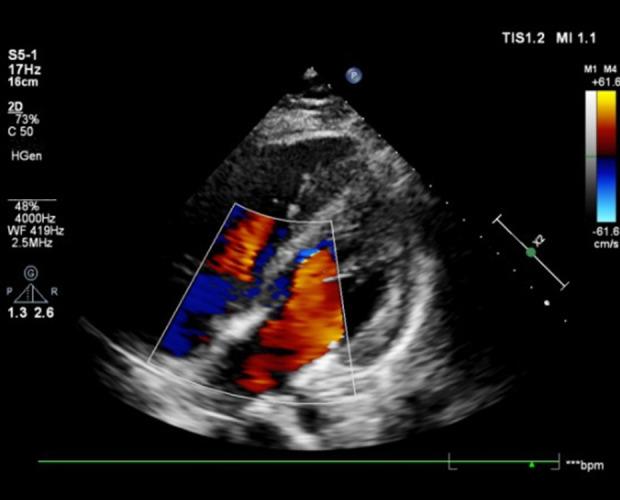
Case 1: Pericardial color ultrasound indicates pericardial effusion.
Figure 4.
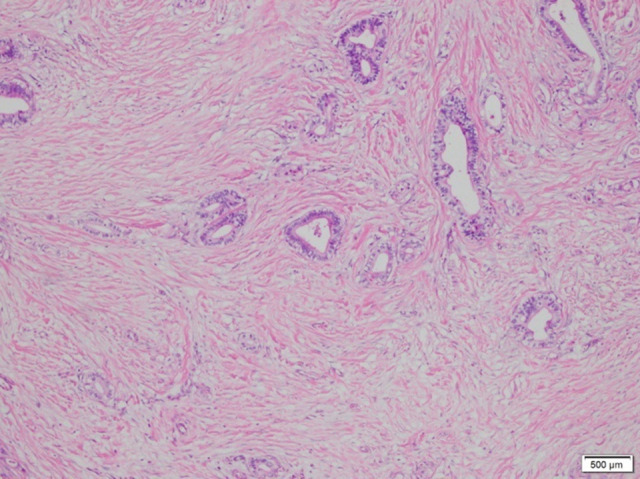
Case 1: A photomicrograph of the diagnostic histopathology shows a moderately differentiated adenocarcinoma of the gastroesophageal junction. Adenocarcinoma cells show cytological atypia and mitoses. Malignant cells are seen to form small atypical glands and cell nests that invade fibrotic stroma. Hematoxylin and eosin stain. Magnification ×40.
Figure 5.
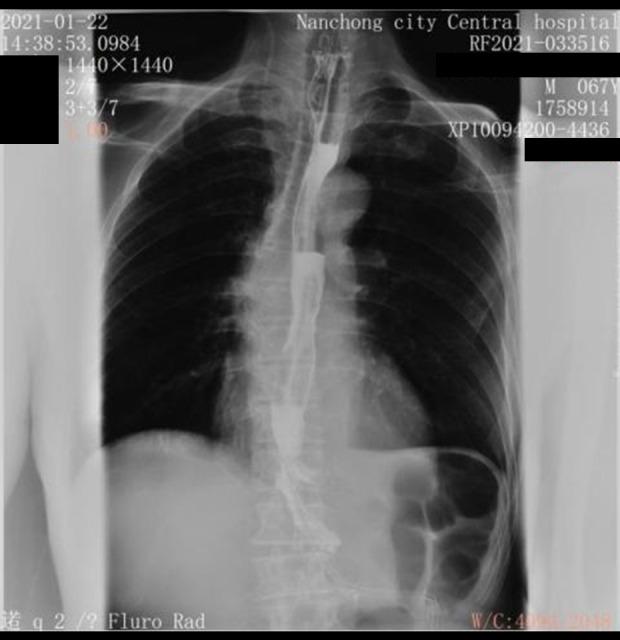
Case 1: Upper gastrointestinal radiography follow-up 1 week after pericardiocentesis and drainage.
Case 2
A 69-year-old man was admitted to the hospital on April 22, 2021. The patient’s chief concerns were persistent dull pain in the middle and upper abdomen for more than 3 months and acid reflux after eating. He had a history of gallbladder polyps for 5 years. Combined with his clinical history, gastroscopy, and CT (Figure 6), Siewert type II esophagogastric junction cancer was diagnosed. On April 25, 2021, laparoscopic radical esophagogastrostomy (total gastrectomy) was performed. Physical examination revealed tachycardia and low blood pressure 23 h after surgery. Electrocardiographic monitoring indicated sinus tachycardia with suspected pericardial effusion. Pericardial hemopericardium was identified on ultrasound (Figure 7). After ultrasound-guided pericardiocentesis and drainage, the patient’s vital signs recovered. After the intervention, the patient recovered and was discharged uneventfully. The pathological staging was T2N0M0 adenocarcinoma (Figure 8).
Figure 6.
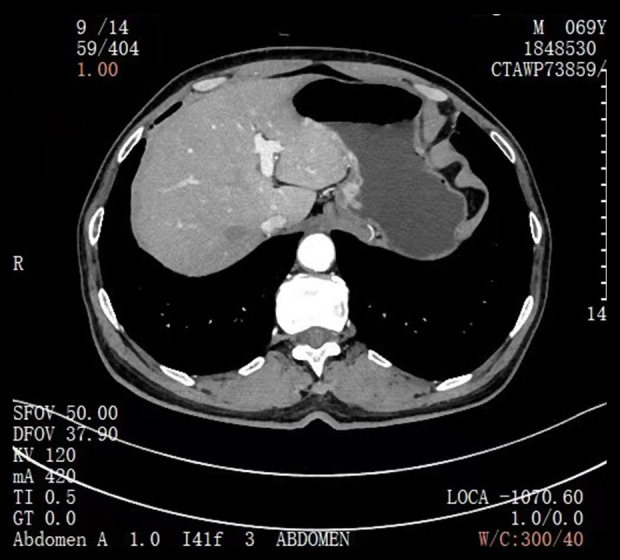
Case 2: Computed tomography for the diagnosis of esophageal and gastric junction carcinoma.
Figure 7.
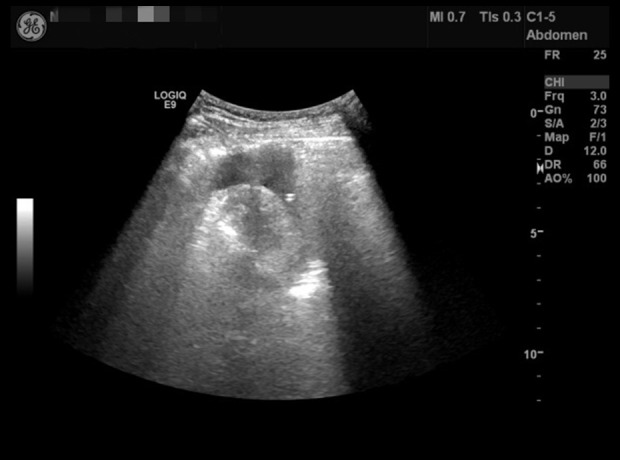
Case 2: Pericardial ultrasound indicates pericardial effusion.
Figure 8.
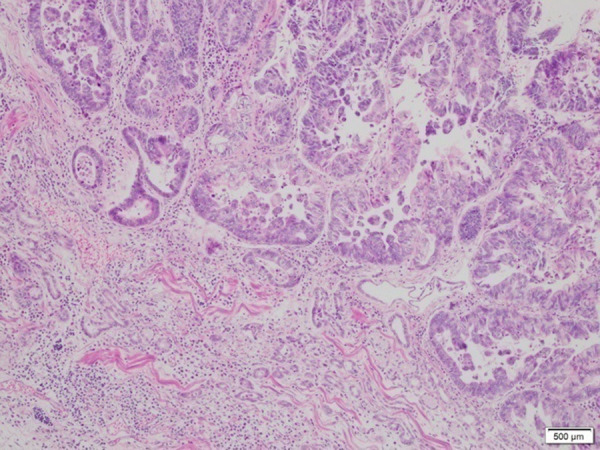
Case 2: A photomicrograph of the diagnostic histopathology shows a moderately differentiated adenocarcinoma of the gastroesophageal junction. Adenocarcinoma cells form irregular and cohesive glands with invasive single cells and cell nests. Cell and tissue necrosis is also shown. Hematoxylin and eosin stain. Magnification ×40.
Discussion
The main results of this report are as follows. First, our cases emphasize the importance of quick detection and intervention in postoperative hemopericardium following laparoscopic transhiatal total gastrectomy. Second, we summarize the clinical presentation, diagnosis, and treatment of hemopericardium. Third, cardiovascular color Doppler ultrasound and CT are essential for the diagnosis of postoperative hemopericardium. Ultrasound-guided pericardiocentesis and drainage is effective for the treatment of hemopericardium.
Gastric cancer can generally be classified into 2 topographical subsites, the cardia (upper stomach) and noncardia (lower stomach) [9]. For gastric cancer invading less than 3 cm of the distal esophagus, a transhiatal abdominal approach is recommended [10]. Laparoscopic total gastrectomy is worthy of further promotion because it has the advantages of reduced bleeding and postoperative pain, rapid recovery of gastrointestinal function, and a short hospital stay [11].
Hemopericardium can be due to the rupture of coronary aneurysms, tumor invasion of the pericardium, or other causes that result in bleeding into the pericardium. In our 2 cases, the laparoscopic surgical field of the esophagogastric junction cancer was next to the pericardium, and the operation may have resulted in postoperative hemopericardium in the 2 patients. Other causes of postoperative hemopericardium are as follows. First, the most common malignancies with cardiac metastases include melanoma and primary mediastinal malignancies [12], which did not include the tumors in our cases. Additionally, metastatic tumors of the heart must involve the pericardium to give rise to hemopericardium. However, in our study, the 2 patient’s tumors were classified as T3N1M0 and T2N0M0, respectively, which did not invade the pericardium. Second, hemopericardium can be due to interventional procedures with cardiac perforation, idiopathic pericarditis, potential underlying diseases, and oral medication [13]. Neither of our patients had any of the above conditions.
To avoid hemopericardium after esophagogastric junction cancer surgery, intraoperative manipulation when performing laparoscopic transhiatal esophagogastric junction cancer surgery should be avoided. In addition, the depth of tumor infiltration should be identified by preoperative CT scan in patients with esophagogastric junction cancer. Finally, patient comorbidities, such as underlying diseases and oral medication, should be identified.
Regarding clinical manifestations, our pericardial hemopericardium patients both had increased heart rate and decreased blood pressure. One patient presented with panic, shortness of breath, and sweating. Both surgeries were performed by an experienced surgeon who had performed more than 200 laparoscopic total gastrectomy procedures. Both patients underwent laparoscopic total gastrectomy for esophagogastric junction cancer, which was uneventful. The surgical techniques were performed according to the Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition) [14]. Nevertheless, it cannot be determined that the hemopericardium was a technical complication. According to the Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition), patients with cT2-4 can directly undergo surgery without preoperative chemoradiotherapy [14]. In our study, the patient’s cancers were classified as T3N1M0 and T2N0M0. Therefore, neither patient received neoadjuvant chemotherapy or radiation. Hemopericardium was diagnosed in both patients by CT or cardiac color Doppler ultrasound.
Ultrasound-guided pericardiocentesis and drainage was used as a safe, effective, and rapid method for treatment. The 2 patients recovered uneventfully.
Two similar cases were reported by Ito et al and Kats et al [7,8]. Both of the patients’ cases were complicated with postoperative hemopericardium. The salvage treatment for the 2 patients was pericardial resection with open drainage and sternotomy, and 1 patient died on postoperative day 4. Ito et al reported a 76-year-old man who underwent subtotal esophagectomy via a cervico-right thoracoabdominal approach for esophageal cancer. His vital signs declined on postoperative day 4. His blood pressure was 54/42 mmHg, and his pulse was 102 beats per min. Cardiac tamponade was diagnosed by chest CT), and he was rescued by pericardial resection (3.5×3.5 cm) and open drainage. Kats et al reported a 50-year-old man who underwent transhiatal esophagectomy and emergency sternotomy for adenocarcinoma of the distal esophagus. Adenocarcinoma of the distal esophagus was diagnosed, and the patient had a history of cystectomy for bladder carcinoma, alcohol abuse, and gastroesophageal reflux disease. He developed signs of bleeding with a decrease in systolic pressure and hemoglobin level just a few hours after transhiatal esophagectomy. A relaparotomy was performed, and the hiatus was reopened. His systolic pressure declined, and his central venous pressure increased, while the posterior mediastinum was explored through the enlarged hiatus. In contrast to our patients, the cardiac tamponade was diagnosed without computed tomography (CT), and this patient was rescued by exploratory thoracotomy rather than ultrasound-guided pericardiocentesis. Furthermore, the patient died on postoperative day 4, without signs of recurrent cardiac tamponade. Relaparotomy was performed again, but no explanation of the unstable condition of the patient was found.
Conclusions
Hemopericardium is a life-threatening complication for patients with esophageal-gastric junction cancer who undergo transhiatal laparoscopic surgery. We report 2 patients whose cases were complicated by hemopericardium after transhiatal laparoscopic excision of Siewert type II AEG. Early physical examinations with cardiovascular color Doppler ultrasound and CT are essential for diagnosis. Ultrasound-guided pericardiocentesis and drainage is effective for the treatment of hemopericardium.
Acknowledgments
We want to thank Dr. Michael Hillel Kleinman from Surgical Associates of Houston, Texas. Dr. Michael Hillel Kleinman helped Dr. Yunhong Tian dedicate work to the surgical practice and research in Gastrointestinal & Hernia Surgery.
Abbreviations
- AEG
adenocarcinoma of the esophagogastric junction;
- CT
computed tomography.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Siewert JR, Stein HJ. Carcinoma of the gastroesophageal junction – classification, pathology and extent of resection. Dis Esophagus. 1996;9:173–82. [Google Scholar]
- 2.Jenkins TD, Friedman LS. Adenocarcinoma of the esophagogastric junction. Dig Dis. 1999;17(3):153–62. doi: 10.1159/000016920. [DOI] [PubMed] [Google Scholar]
- 3.Rüdiger Siewert J, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: Results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353–61. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu H, Shang L, Du F, et al. Transhiatal versus transthoracic surgical approach for Siewert type II adenocarcinoma of the esophagogastric junction: A meta-analysis. Expert Rev Gastroenterol Hepatol. 2020;14(11):1107–17. doi: 10.1080/17474124.2020.1806710. [DOI] [PubMed] [Google Scholar]
- 5.Kurokawa Y, Sasako M, Sano T, et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br J Surg. 2015;102(4):341–48. doi: 10.1002/bjs.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lazaros G, Vlachopoulos C, Lazarou E, et al. New approaches to management of pericardial effusions. Curr Cardiol Rep. 2021;23(8):106. doi: 10.1007/s11886-021-01539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S, Morita M, Nanbara S, et al. Cardiac tamponade due to bleeding as a potential lethal complication after surgery for esophageal cancer. Anticancer Res. 2015;35(1):407–11. [PubMed] [Google Scholar]
- 8.Kats S, Nieuwenhuijzen GA, van Straten BH, et al. Cardiac tamponade: An unusual, lifethreatening complication after transhiatal resection of the esophagus. Interact Cardiovasc Thorac Surg. 2007;6(2):238–39. doi: 10.1510/icvts.2006.145631. [DOI] [PubMed] [Google Scholar]
- 9.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 10.Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: A randomised controlled trial. Lancet Oncol. 2006;7(8):644–51. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 11.Małczak P, Torbicz G, Rubinkiewicz M, et al. Comparison of totally laparoscopic and open approach in total gastrectomy with D2 lymphadenectomy – systematic review and meta-analysis. Cancer Manag Res. 2018;10:6705–14. doi: 10.2147/CMAR.S182557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perek B, Tomaszewska I, Stefaniak S, et al. Cardiac tamponade – unusual clinical manifestation of undiagnosed malignant neoplasm. Neoplasma. 2016;63(4):601–6. doi: 10.4149/neo_2016_414. [DOI] [PubMed] [Google Scholar]
- 13.Appleton C, Gillam L, Koulogiannis K. Cardiac tamponade. Cardiol Clin. 2017;35(4):525–37. doi: 10.1016/j.ccl.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Gastric Cancer Association Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24(1):1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


