ABSTRACT
This phase III clinical trial aimed to assess the safety and demonstrate the immunogenicity of a candidate freeze-dried purified Vero cell-based rabies vaccine (PVRV-WIBP) developed for human use. A cohort of 40 participants in stage 1 and 1956 subjects in stage 2 with an age range of 10–50 years were recruited for the phase III clinical trial. For safety analysis in stage 1, 20 participants received either 4-dose or 5-dose regimen of PVRV-WIBP. In stage 2, 1956 subjects were randomly divided into the 5-dose PVRV-WIBP, 5-dose PVRV-LNCD, and 4-dose PVRV-WIBP groups. The serum neutralizing antibody titer against rabies was determined on day 7 or 14 and day 35 or 42. Adverse reactions were recorded for more than 6 months. Most adverse reactions, which were mild and moderate in severity, occurred and resolved within 1 week after each injection in the PVRV-WIBP (4 and 5 doses) and PVRV-LNCD (5 doses) groups. All three groups achieved complete seroconversion 14 days after the initial dose and 14 days after completing the full vaccination schedule, the susceptible subjects in the PVRV-WIBP group (4-dose or 5-dose regimen) displayed higher neutralizing antibody titers against the rabies virus compared to those in the PVRV-LNCD group (5-dose regimen). PVRV-WIBP induced non-inferior immune responses versus PVRV-LNCD as assessed by seroconversion rate. PVRV-WIBP was well tolerated and non-inferior to PVRV-LNCD in healthy individuals aged 10–50 years. The results indicated that PVRV-WIBP (both 4- and 5-dose schedules) could be an alternative to rabies post-exposure prophylaxis.
KEYWORDS: Rabies vaccine, safety, immunogenicity
Introduction
Rabies is a fatal viral encephalomyelitis that can be prevented. Rabies continues to be a global health concern, with an impact on 150 countries and territories across the world, each displaying varying magnitudes and epidemiological patterns of the disease.1 Over 3.3 billion children and adults are vulnerable to rabies exposure, and 55,000 individuals die worldwide annually.2 The high incidence of rabies may be due to poverty, low medical level, lack of rabies awareness, timely preventive treatment post-exposure, and high cost of vaccination.3
Rabies is untreatable but can be effectively prevented by proper wound management and prompt post-exposure use of vaccines. Because anti-rabies antibodies could be induced to neutralize the virus, the demand for rabies vaccines is high.4 China has been in second place in the number of rabies cases worldwide since the late 1990s.5 In an effort to decrease the incidence of rabies infection, the government has been actively promoting educational awareness regarding post-exposure prophylaxis (PEP) to the general public. Nonetheless, the utilization of vaccines for rabies prevention remains restricted, potentially resulting in higher mortality rates. PEP is administered to more than 15 million people worldwide, preventing approximately 327,000 deaths.6 For PEP, the World Health Organization (WHO) approved several rabies vaccination schedules via the intramuscular (IM) route (5-dose 1-1-1-1-1 Essen regimen and 4-dose 2-1-1 Zagreb regimen) or the intradermal (ID) route (Thai RedCross 2-site regimen).3 The 5-dose 1-1-1-1-1 Essen regimen for rabies vaccination was widely used in China for PEP, and the 4-dose 2-1-1 Zagreb regimen was also approved in China in 2010.7
Purified Vero cell cultured freeze-dried rabies vaccine was developed by the Wuhan Institute of Biological Products Co., Ltd (PVRV-WIBP) for human use. In a phase III clinical trial, a comparison was made between the safety and immunogenicity of PVRV-WIBP (5-dose and 4-dose regimens) and PVRV produced by Liaoning Chengda Co., Ltd. (PVRV-LNCD; batch issue number: LRA20133276) available with 5-dose regimen. This phase III clinical trial aimed to provide evidence for clinical data for using PVRV-WIBP in humans under the 5- and 4-dose regimens.
Methods
Study design and participants
The randomized, parallel-controlled, multi-center phase III clinical trial was conducted between August 2014 and July 2016 at the Centers for Disease Control and Prevention in Changge and Biyang, located in Henan province, China. In stage 1, 40 subjects aged 10–50 years were randomly divided into the 5-dose (1-1-1-1-1) or 4-dose (2-1-1) PVRV-WIBP groups for safety analysis. The 4-dose group was vaccinated with one dose on both arms on day 0 and another two doses on days 7 and 21. The 5-dose group was vaccinated on days 0, 3, 7, 14, and 28. If no serious adverse events, severe abnormal reactions were observed, or AEs of Grade 3 or higher occurred in less than 15% of subjects after receiving any dose within 7 days in stage 1 of the phase III trial, the stage 2 was implemented.
In stage 2, the subjects were 1:1:1 randomly assign into 4-dose PVRV-WIBP, 5-dose PVRV_WIBP and 5-dose PVRV-LNCD group with randomized block design. As per to 4-dose (0-7-21 vaccination schedule), 5-dose (0-3-7-14-28 vaccination schedule), 0-7-42 and 0-14-42 blood collection schedule, the random number in the block group is randomly allocated at one time by PASS. The subjects were divided into 4-dose PVRV-WIBP (4-dose, 0-7-35) (4 doses, of 0, 7, 35 days of blood collection), 4-dose PVRV-WIBP (4-dose, 0-14-35) (4 doses, 0, 14, 35 days of blood collection), 5-dose PVRV-WIBP (5-dose, 0-7-42) (5 doses 0, 7, 42 days of blood collection), 5-dose PVRV-WIBP (5-dose, 0-14-42) (5 doses 0, 14, 42 days of blood collection), 5-dose PVRV-LNCD (5-dose, 0-7-42) (5 doses 0, 7, 42 days of blood collection) and 5-dose PVRV-LNCD (5-dose, 0-14-42) (5 doses 0, 14, 42 days of blood collection). The safety, immunogenicity data were analyzed.
The inclusion criteria were the willingness of healthy volunteers to participate in the trial with a body temperature of ≤37.0°C and without previous rabies vaccine immunization. The exclusion criteria were the use of antisera, human immunoglobulins within the past month, immunocompromised patients, or allergy to vaccine components.
Due to different immunization and blood collection schedule, the investigators and the recruited participants were not blind for the 4-dose PVRV-WIBP, but were blind for the 5-dose PVRV-WIBP and the 5-dose PVRV-LNCD group throughout the trial.
Vaccines
The PVRV-WIBP (0.5 mL/dose; lot no: 20131101) used in this study was inoculated and cultured into Vero cells with a rabies virus fixed strain (CTN-1 V strain). Subsequently, the virus was harvested, inactivated, concentrated, and purified. The vaccine was freeze-dried and packed into prefilled syringes in 0.5 mL. The production of the vaccine utilizes molecular sieve chromatography and ion chromatography in a single step, with the chromatographic column being expanded to 450 mm, thus, allowing for a greater production capacity. Moreover, the finished product displays low levels of miscellaneous protein. The positive control vaccine was the commercially used PVRV-LNCD (0.5 mL/dose; lot no: 201209271).
Safety endpoints
The safety analysis were based on the safety set (SS), which consisted of all subjects who had received at least one dose of the vaccine. In stage 1, the occurrence of solicited injection site adverse effects (AEs) (e.g., tenderness, itching, redness, swelling, induration) and system AEs (e.g., fever, fatigue, headache, allergies, nausea, vomiting, arthrodynia, myalgia) within 7 days of each vaccination has been recorded on the “diary card.” In stage 2, the primary outcome for safety is the solicited injection site and system AEs recorded on the “diary card” for 7 days of each vaccination, the secondary safety endpoint was the occurrence of AEs from first dose to 30 days after the full course of vaccinations in stage 2, which was recorded on contact cards for 8–30 days AEs. Unsolicited AEs (e.g., keratitis, dizziness, bronchitis, and discomfort of limbs) were also collected by combining self-reports of the subjects and regular follow-up. Any serious AEs (SAEs) were also recorded during the 6-month follow-up period after the first injection.
The classification of systemic and local reactions following vaccination was determined according to the “Guidelines for Adverse Reaction Classification Standards for Clinical Trials of Preventive Vaccines” by the National Medical Products Administration.8
Serological analysis
The serological analysis based on the full analysis set (FAS) in the stage 2. The subjects were randomly divided into the 4-dose PVRV-WIBP (4-dose, 0-7-35), 4-dose PVRV-WIBP (4-dose, 0-14-35), 5-dose PVRV-WIBP (5-dose, 0-7-42), 5-dose PVRV-WIBP (5-dose, 0-14-42), 5-dose PVRV-LNCD (5-dose, 0-7-42) 5-dose PVRV-LNCD (5-dose, 0-14-42) for immunogenicity analysis. Approximately 4.0 mL of serum sample was collected on days 0, 7, or 14, and 35 or 42 for detecting rabies-neutralizing antibody titers under masked conditions by using the rapid fluorescent focus inhibition test (RFFIT) based on China National Institutes for Food and Drug Control (NIFDC). Seroconversion was considered in individuals with RFFIT titer ≥0.5 IU/mL (pre-immunization antibody titer, <0.5 IU/mL) or with >4-fold antibody increase post-immunization (pre-immunization antibody titer ≥0.5 IU/mL). Non-inferiority immunogenicity was defined as the lower limit of seroconversion difference of not less than−5% after 14 days from the first dose between the PVRV-WIBP and PVRV-LNCD groups.
Sample size and statistical analysis
To prioritize the interests and safety of the subjects, only 40 individuals were recruited for stage 1 of the phase III clinical study. The sample size for stage 2 of the trial was determined based on the non-inferiority hypothesis, with the assumption that the antibody positive rate for both the 5-dose and 4-dose regimens of PVRV-WIBP would not be less than 97% 14 days after the initial dose. Additionally, it was hypothesized that both the 5-dose and 4-dose regimens of PVRV-WIBP would be non-inferior to 5-dose regimen of PVRV-LNCD. The probability of type I error was set at one-sided 0.025, and the statistical power was 0.90. SAS 9.3 was used to perform statistical analysis. Fisher’s exact test was used for the difference analysis of safety outcomes. The t-test, t’-test, and χ2 tests were used for the immunogenicity analysis.
Ethics
This study complied with the requirements of the State Food and Drug Administration’s Technical Guidelines for Vaccine Clinical Trials and the World Medical Association’s Declaration of Helsinki’s Ethical Principles and Good Clinical Practice for medical research in humans and all applicable regulations. The clinical research protocol and informed consent form were both approved by the Ethics Committee of the Henan Provincial Center for Disease Control and Prevention. The subjects recruited for the clinical trial voluntarily signed the informed consent form.
Clinical Trial Approval Document No of CFDA: 2011L01486
Results
Study participant enrollment and characteristics
The safety population included 40 volunteers aged 10–50 years who received the first dose of vaccine in stage 1, 20 participants received 5-dose PVRV-WIBP, with two participants who withdrew, and another 20 participants received 4-dose PVRV-WIBP in the deltoid. In stage 2 trial, following the calculation by PASS software for the non-inferiority study, 290 individuals were required for each subgroup. Considering the different blood collection procedures (blood collected on day 7 or day 14 for 4-dose and 5-dose PVRV-WIBP after the first dose vaccination), and possible loss of follow-up, 652 subjects were randomly assigned to receive intramuscular injections in the 4-dose PVRV-WIBP group (0-7-35, and 0-14-35, each 326 individuals), 5-dose PVRV-WIBP group (0-7-42,and 0-14-42, each 326 individuals) and PVRV-LNCD group (0-7-42,and 0-14-42, each 326 individuals), with a total of 1956 people. Figure 1 provides a detailed summary of the elimination process of individuals in stage 2.
Figure 1.
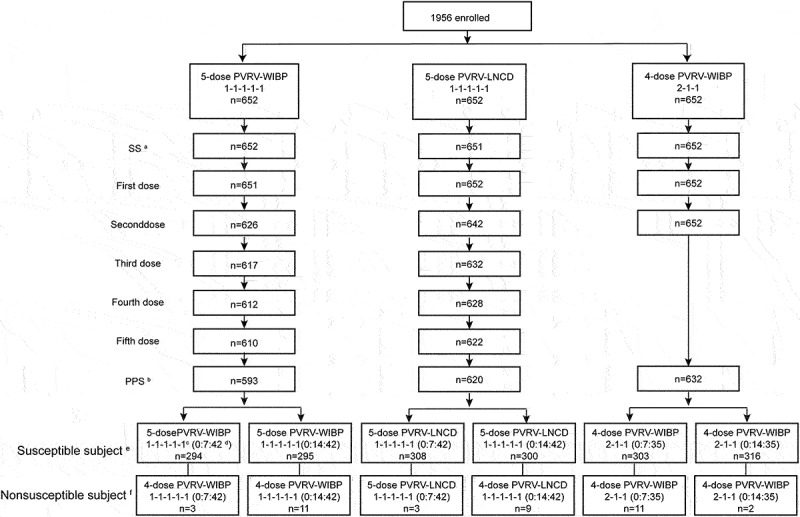
Flow diagram of the stage 2.(a) SS, safety analysis set. All individuals received the first vaccination after randomization. (b) PPS, per-protocol analysis set. The subjects with better compliance and who completed the vaccination schedule with the safety and immunogenicity indexes were recruited. Individuals excluded from the PPS had incomplete inoculation/no pre-immunization test results or violated/deviated from the protocol. (c) The 1-1-1-1-1 and 2-1-1 regimens indicate the vaccination schedule. (d) The 0:7:42 and 0:14:42 indicate the blood collection on days 0, 7, and 42 or days 0, 14, and 42 in the 5-dose PVRV-WIBP or PVRV-LNCD groups. The 0:7:35 and 0:14:35 indicate blood collection on days 0, 7, and 35 or days 0, 14, and 35 in the 4-dose PVRV-WIBP group. (e) In susceptible subjects, the pre-immunization RFFIT antibody titer of the individual is <0.5 IU/mL. (f) Nonsusceptible subjects, pre-immunization RFFIT antibody titer of the individual is ≥0.5 IU/mL.
The demographic characteristics of the participants were similar in terms of mean age and sex among groups for safety and immunogenicity analysis. In stage 1, the mean age of the participants was 33.55 and 35.60 years in the 5- and 4-dose PVRV-WIBP groups, respectively. Moreover, In stage 2, the mean age of the participants was 31.24, 31.26, and 31.22 years in the 5-dose PVRV-WIBP, 5-dose PVRV-LNCD, and 4-dose PVRV-WIBP groups, respectively (Table 1).
Table 1.
Baseline characteristics.
| Stage 1 |
Stage 2 |
||||
|---|---|---|---|---|---|
| 5-dose PVRV-WIBP | 4-dose PVRV-WIBP | 5-dose PVRV-WIBP | dose PVRV-LNCD | 4-dose PVRV-WIBP | |
| Age Median (Mean,SDa) | |||||
| SS | 35 (33.55 ± 11.38) | 41 (35.60 ± 12.26) | 34 (31.24 ± 13.13) | 34 (31.26 ± 13.50) | 34 (31.22 ± 12.88) |
| 0:7:42/35a (PPS) | - | - | 39 (35.31 ± 11.98) | 38 (34.74 ± 12.42) | 36 (34.13 ± 11.96) |
| 0:14:42/35b(PPS) | - | - | 28 (27.34 ± 13.19) | 29 (28.06 ± 13.77) | 31 (28.52 ± 13.18) |
| Sex | |||||
| Male n(%) (SS) | 13 (65%) | 13 (65%) | 307 (47.16%) | 280 (42.94%) | 314 (48.16%) |
| Female n(%) (SS) | 7 (35%) | 7 (35%) | 344 (52.84%) | 372 (57.06%) | 338 (51.84%) |
| Male n(%) (0:7:42/35b, PPS) | - | - | 120 (40.4%) | 126 (40.51%) | 143 (45.54%) |
| Female n(%) (0:7:42/35, PPS) | - | - | 177 (59.6%) | 185 (59.49%) | 171 (54.46%) |
| Male n(%) (0:14:42/35c, PPS) | - | - | 158 (51.63%) | 137 (44.34%) | 161 (50.63%) |
| Female n(%) (0:14:42/35, PPS) | - | - | 148 (48.37%) | 172 (55.66%) | 157 (49.37%) |
aSD, standard deviation.
b0:7:42/35, blood collection schedule was on days 0, 7, and 42 after the first dose in the 5-dose PVRV-WIBP and 5-dose PVRV-LNCD groups and days 0, 7, and 35 in the 4-dose PVRV-WIBP group. The serological indicator was collected 7 days after the first dose was present.
c0:14:42/35, blood collection schedule was on days 0, 1, 4, and 42 after the first dose in the 5-dose PVRV-WIBP and 5-dose PVRV-LNCD groups and days 0, 14, and 35 in the 4-dose PVRV-WIBP group. The serological indicator was collected 14 days after the first dose was present. PPS,per-protocol analysis. SS, safety analysis.
Safety outcome
In stage 1, the subjects tolerated the 5- and 4-dose PVRV-WIBP vaccination schedules well (Table 2). The incidence of both local and systemic adverse events was higher in the 4-dose PVRV-WIBP group compared to the 5-dose PVRV-WIBP group, local pain (25.00%) and systemic fever (45.00%) were the most frequent occurrences. In the stage 2 study, AEs most likely occurring after vaccination were total local pain (26.11%) and systemic fever (23.96%), followed by fatigue (9.22%) and headache (7.99%) (Table 3). The incidence of fever, headache, fatigue, and myalgia in the 5-dose PVRV-WIBP group was lower than in 4-dose PVRV-WIBP group but was higher than that in the 5-dose PVRV-LNCD group (p < .0001). The incidence of other AEs (including unsolicited AEs) was not significantly different among the groups (Tables 2 and 3). Most AEs were mild (grade 1) and moderate (grade 2) in severity and could resolve within the first week in stage 1 and 2. No severe AEs were related to vaccination during stage 1 and 2 follow-ups.
Table 2.
AEs within a week after vaccination in stage 1.
|
5-dose PVRV-WIBP group n = 20 |
|||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Total | Grade1 | Grade 2 | Total | |
| Local AEs | 3 (15.00%) | 2 (10.00%) | 5 (25.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Pain | 3 (15.00%) | 2 (10.00%) | 5 (25.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Swelling | 0 (0.00%) | 1 (5.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| System AEs | 7 (35.00%) | 4 (20.00%) | 11 (55.00%) | 2 (10.00%) | 1 (5.00%) | 3 (15.00%) |
| Fever | 5 (25.00%) | 4 (20.00%) | 9 (45.00%) | 1 (5.00%) | 1 (5.00%) | 2 (10.00%) |
| Fatigue | 3 (15.00%) | 1 (5.00%) | 4 (20.00%) | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) |
| Headache | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Nausea and Vomiting | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Myalgia | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Allergies | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (5.00%) | 1 (5.00%) |
| Unsolicited AEs | 1 (5.00%) | 2 (10.00%) | 3 (15.00%) | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) |
| Dizziness | 0 (0.00%) | 1 (5.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Bronchitis | 0 (0.00%) | 1 (5.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Limb discomfort | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Keratitis | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | 1 (5.00%) | 0 (0.00%) | 1 (5.00%) |
AE,adverse effects.
Table 3.
AEs within a month after vaccination in stage 2.
| 5-dose PVRV-WIBP (n = 651) |
5-dose PVRV-LNCD (n = 652) |
4-dose PVRV-WIBP (n = 652) |
p-value (Fisher) |
|
|---|---|---|---|---|
| Total | ||||
| Grade 1 | 282 (43.32%) | 256 (39.26%) | 408(62.58%) | <.0001 |
| Grade 2 | 75 (11.52%) | 41 (6.29%) | 163(25.00%) | |
| Grade 3 | 2 (0.31%) | 2 (0.31%) | 6(0.92%) | |
| Local AEs | ||||
| Grade 1 | 175 (26.88%) | 154 (23.62%) | 243 (37.27%) | <.0001 |
| Grade 2 | 25 (3.84%) | 15 (2.30%) | 56 (8.59%) | |
| Grade 3 | 1 (0.15%) | 2 (0.31%) | 1 (0.15%) | |
| Pain | 170 (26.11%) | 152 (23.31%) | 264 (40.03) | <.0001 |
| Swelling | 19 (2.92%) | 20 (3.07%) | 25 (3.83%) | .6215 |
| Redness | 19 (2.92%) | 16 (2.45%) | 14 (2.15%) | .6515 |
| Itching | 12 (1.84%) | 4 (0.61%) | 17 (2.61%) | .0119 |
| Induration | 3 (0.46) | 1 (0.15%) | 1 (0.15%) | .4640 |
| System AEs | ||||
| Grade 1 | 192 (29.49%) | 150 (23.01%) | 283 (43.40%) | <.0001 |
| Grade 2 | 55 (8.45%) | 29 (4.45%) | 132 (20.25%) | |
| Grade 3 | 1 (0.15%) | 0 (0.00%) | 5 (0.77%) | |
| Fever | 156 (23.96%) | 110 (16.87%) | 261 (40.03%) | <.0001 |
| Fatigue | 60 (9.22%) | 38 (5.83%) | 113 (17.33%) | <.0001 |
| Headache | 52 (7.99%) | 30 (4.60%) | 93 (14.26%) | <.0001 |
| Myalgia | 16 (2.46%) | 9 (1.38%) | 42 (6.44%) | <.0001 |
| Nausea and vomiting | 7 (1.08%) | 17 (2.61%) | 20 (3.07%) | .0301 |
| Arthrodynia | 6 (0.92%) | 5 (0.77%) | 14 (2.15%) | .0723 |
| Allergies | 5 (0.77%) | 5 (0.77%) | 9 (1.38%) | .4644 |
| Unsolicited AEs | 15 (2.30%) | 17 (2.61%) | 18 (2.76%) | .7987 |
AE, adverse effects.
The incidence of AEs after each vaccination in the 5-dose PVRV-WIBP, 5-dose PVRV-LNCD, and 4-dose PVRV-WIBP groups is shown in Table 4. The prevalence of AEs after the first dose was higher in the 4-dose PVRV-WIBP group than in the 5-dose PVRV-WIBP and 5-dose PVRV-LNCD groups, which showed a significant statistical difference (p < .0001). The incidence of AEs after the second dose significantly differed in the 4-dose PVRV-WIBP group compared with the 5-dose PVRV-WIBP (p = .0012). The rate of similar AEs after the third dose in the 4-dose PVRV-WIBP, 5-dose PVRV-WIBP, and 5-dose PVRV-LNCD groups and that of the fourth and fifth doses in the 5-dose PVRV-WIBP and 5-dose PVRV-LNCD groups was much lower compared with the AEs occurring after the first dose, indicating that occurrence of AEs did not increase with the number of doses.
Table 4.
AEs after each vaccination in the 5- and 4-dose regimens.
| 5-dose PVRV-WIBP | 5-dose PVRV-LNCD | 4-dose PVRV-WIBP |
p-value (5-dose PVRV-WIBP vs 5-dose PVRV-LNCD) |
p-value (4-dose PVRV-WIBP vs 5-dose PVRV-WIBP) |
|
|---|---|---|---|---|---|
| First dose | 204 (31.34%) | 139 (21.32%) | 427 (65.49%) | <.0001 | <.0001 |
| Second dose | 96 (15.34%) | 78 (12.15%) | 131 (20.60%) | .1031 | .0012 |
| Third dose | 84 (13.61%) | 76 (12.03%) | 45 (7.11%) | .4461 | .0930 |
| Fourth dose | 53 (8.66%) | 47 (7.48%) | – | .4667 | – |
| Fifth dose | 29 (4.75%) | 21 (3.38%) | – | .2491 | – |
The 4-dose regimen was defined as the first dose (two doses) vaccination on both arms at day 0 and another three doses on days 7, 14, and 21. The 5-dose regimen was defined as vaccination on days 0, 3, 7, 14, and 28. AE, adverse effects.
Immunogenicity analysis
Immunogenicity analysis was based on the antibody titer detected by RFFIT. As the baseline antibody titer before immunization was statistically different if participants with antibody titer >0.5 IU/mL were included (Supplementary Table S1), susceptible subjects with antibody titer <0.5 IU/mL pre-vaccination were selected for the main target population for immunogenicity analysis. On day 7 after the first dose, the geometric mean titer (GMT) of neutralizing antibodies was observed to be higher in the 4-dose PVRV-WIBP group as compared to the 5-dose PVRV-WIBP and 5-dose PVRV-LNCD groups. This suggests that the 4-dose schedule could trigger a faster antibody response. On day 14 following the initial dose, the antibody titer in the 5-dose PVRV-WIBP, 5-dose PVRV-LNCD, and 4-dose PVRV-WIBP groups was 35.36 (32.77–38.15), 29.32 (26.98–31.84), and 36.55 (33.88–39.43), respectively, with a significant difference. The GMT of antibody levels was also significantly different among the three groups 14 days after the whole schedule, and antibody level induced by 4-dose PVRV-WIBP comparable tothat of 5-dose PVRV-WIBP and better than to that of 5-dose PVRV-LNCD on 14 days after the first dose or the whole schedule (p < .0001) (Table 5, Table S2).
Table 5.
Immunogenicity analysis in susceptible subjects.
| 5-dose PVRV-WIBP | 5-dose PVRV-LNCD | 4-dose PVRV-WIBP | p-value | |
|---|---|---|---|---|
| Pre-vaccination | 0 | 0 | 0 | / |
| Antibody titer, GMT (95%) | ||||
| 0:7:42/35a | 1.05 (0.90–1.22) | 0.7 (0.61–0.80) | 1.5 (1.30–1.72) | <.0001 |
| 0:14:42/35b | 35.36 (32.77–38.15) | 29.32 (26.98–31.84) | 36.55 (33.88–39.43) | .0001 |
| 14-day PWVc | 19.97 (18.73–21.30) | 15.28 (14.24–16.39) | 21.55 (20.30–22.89) | <.0001 |
| Seroconversion rate, % (95%) | ||||
| 0:7:42/35a | 71.43 (65.90–76.52) | 59.42 (53.70–64.95) | 83.5 (78.83–87.5) | <.0001 |
| 0:14:42/35b | 100 (98.76–100.00) | 100.0 (98.78–100.0) | 100 (98.84–100.0) | 1.0000 |
| 14-day PWVc | 100 (99.38–100.00) | 100.0 (99.40–100.0) | 100 (99.41–100.0) | 1.0000 |
a0:7:42/35, the GMT and seroconversion rate was based on the 7 days serological indicator after the first dose.
b0:14:42/35, the GMT and seroconversion rate was based on the 14 days serological indicator after the first dose was present. GMT,geometric mean titer.
c14-day PWV, the GMT and seroconversion rate was based 14 days after the whole vaccine schedule.
On day 7 after the first dose, the seroconversion rate of the 4-dose PVRV-WIBP group (83.50%) was higher than that of the 5-dose PVRV-WIBP group (71.43%) (p = .0004) and 5-dose PVRV-LNCD group (59.42%) (p = 0.0020) (Table 5). The seroconversion rate was 100.0% on day 14 after the first dose and 14 days after the whole schedule for the three groups without significantly different among the three groups (p = 1.0000).
Non-inferiority immunogenicity was also based on serological data from susceptible subjects 14 days after the first dose. The difference of seroconversion rate between 5-dose PVRV-WIBP and 5-dose PVRV-LNCD, between 4-dose PVRV-WIBP and 5-dose PVRV-LNCD was 0.00% (−1.41%, 1.45%) and 0.00% (−1.38%, 1.45%) (p = 1.0000) (Table 6). Furthermore, the difference of antibody levels between 5-dose PVRV-WIBP and 5-dose PVRV-LNCD, between 4-dose PVRV-WIBP and 5-dose PVRV-LNCD on day 14 after the first dose and 14 days after the whole schedule demonstrated no less than 0.91, suggesting that the immunogenicity of both the 4-dose and 5-dose regimens of PVRV-WIBP was not inferior to that of PVRV-LNCD.
Table 6.
Non-inferiority immunogenicity in susceptible subjects.
| group | 5-dose PVRV-WIBP vs 5-dose PVRV-LNCD | p-value | 4-dose PVRV-WIBP vs 5-dose PVRV-LNCD | p-value |
|---|---|---|---|---|
| GMT ratio (95%) 0:14:42/35 | 1.21 (1.06,1.37) | .0011 | 1.03 (0.91,1.17) | .5452 |
| GMT ratio (95%) 14 day PWVa | 1.31 (1.17,1.46) | <.0001 | 1.08 (0.98,1.19) | .0892 |
| Seroconversion rate difference (95%) | 0.00 (−1.47,1.45) | 1.0000 | 0.00 (−1.38,1.45) | 1.0000 |
a14-day PWV, 14 days after the whole vaccine schedule. GMT,geometric mean titer.
Discussion
In China, the administration of the Essen regimen or Zagreb regimen for rabies vaccination after post-exposure prophylaxis (PEP) has demonstrated relatively good immune efficacy in preventing human rabies and a better safety profile.9 This study found that PVRV-WIBP (4- and 5-dose regimens) was safe and tolerated well among adolescents and adults aged 10–50 years. The severity of AEs in different vaccination schedules was mild and moderate. The neutralizing antibody titers and seroconversion rate in PVRV-WIBP (4- and 5-dose schedules) were comparable to those in PVRV-LNCD.
Compared with the 5-dose group, the occurrence of AEs seems to be higher in 4-dose PVRV-WIBP group, which may be due to the first dose of PVRV-WIBP (double antigen) being administered on both arms on day 0, similar to that in previous studies.7,10 The occurrence of systemic fever and local pain was more frequent, and most reactions were mild to moderate in severity and transient, similar to another clinical study with Zagreb and Essen regimens.11,12 No SAEs seemed to be related to vaccination, such as severe allergic reaction, acute disseminated encephalomyelitis, or anaphylaxis.13–15 Most reactions occurred at early doses, especially the first dose, and the incidence of AEs after each dose decreased as the number of immunization doses increased, indicating no dose-aggravation effect on safety, which was similar to other approved vaccines.11,16 Despite a higher incidence of AEs in the 4-dose PVRV-WIBP group, both the 4-dose and 5-dose regimens of PVRV-WIBP exhibited acceptable safety profiles, with only a few AEs of grade 3 being observed. Furthermore, no statistically significant differences were found among the 5-dose PVRV-WIBP, 4-dose PVRV-WIBP, and control PVRV-WIBP groups
The seroconversion rate and antibody levels of the 5- and 4-dose PVRV-WIBP groups were satisfactory. Additionally, the antibody levels of all subjects achieved the requirement of the WHO and the demand of Chinese Pharmacopoeia that human rabies vaccine production potency should be > 4.0 IU/mL.17 The 4-dose PVRV-WIBP schedule presents better immunogenicity 7 days after the first dose than the 5-dose PVRV-WIBP schedule and comparable neutralizing antibody titers and seroconversion 14 days after the whole schedule, which is essential for rapid antibody production to prevent rabies. To the best of our knowledge, the 4-dose PVRV-WIBP regimen with fewer injections could shorten the time of vaccine schedule required to produce adequate, long-lasting antibody response to neutralize rabies virus, indicating dose, time, and cost savings and better patient compliance to complete vaccination.18 While a 100% seroconversion rate was achieved 14 days after both the first dose and the complete vaccination schedule, the GMT of the antibody observed 14 days after the first dose was found to be higher than that observed 14 days after the entire schedule. This finding is in contrast to previous reports but is consistent with other studies.11,12,19,20 This may be primarily due to the different vaccine production technology and physical characteristics of subjects. The seroconversion rate 14 days after the first dose of the 5- and 4-dose PVRV-WIBP groups was non-inferior to the 5-dose PVRV-LNCD group, indicating that the Zagreb (2-1-1) and Essen (1-1-1-1-1) regimens could be used as candidate rabies (PVRV-WIBP) vaccination schedule.
This study has several advantages. It is a large sample phase III clinical trial with a randomized controlled design and good follow-up, and all subjects were rigorously screened, qualified volunteers. The study’s experimental design is elaborate because it is a two-stages trial, with stage 1 conducted for the safety assessment of 40 volunteers to ensure the safety of the test vaccine and stage 2 for immunogenicity and safety of a sample size of 1569. Limitations include the inability to track long-term immunogenicity during the follow-up period, and young children and older adults were excluded from the studies.
This phase III clinical trial confirmed that PVRV-WIBP was safe and tolerated well after each injection and during the follow-up, which was non-inferior to the positive control PVRV-LNCD, offering a new alternative for rabies prophylaxis.
Supplementary Material
Acknowledgments
The authors thank the investigators at the Henan Provincial Center for Disease Control and Prevention for their help with the trial implementation.
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
X.M.Y. reported being an employee of the China National Biotec Group Co Ltd. X.Y.H., J.L., X.X.N., W.C., J.Y.Z., X.X., X.L., Y.W., J.R.S., L.X.J., Q.L.L., Q.X.W., K.D., X.G.L., F.X.P., and Y.Q.J are employees of Wuhan Institute of Biological Products Co Ltd. L.L.H., W.Z., Z.Q.X., and Y.X.W. are employees of Henan Provincial Center for Disease Control and Prevention.
Author contributions
X.M.Y., X.Y.H, J.L. and Y.X.W. were responsible for the study concept design and statistical analysis. L.L.H. and X.X.N. were responsible for the manuscript drafting and revision, W.C., J.Y.Z., Q.L.L., Q.X.W., W.Z., and Z.Q.X. were responsible for the clinical trial follow-up, K.D., X.G.L., X.X., X.L., Y.W., J.R.S., L.X.J., F.X.F and Y.Q.J were responsible for the antibody examination.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2211896.
References
- 1.RABIES . [accessed 2022 Nov 13]. https://www.who.int/news-room/fact-sheets/detail/rabies#:~:text=Key%20facts,and%20prevention%20of%20dog%20bites.
- 2.Edukugho AA, Umoh JU, Diem M, Ajani O, Uba B, Okeke L, Adedire E, Adefisoye A, Edukugho C, Nguku P.. Knowledge, attitudes and practices towards rabies prevention among residents of Abuja municipal area council, Federal Capital Territory, Nigeria. Pan Afr Med J. 2018;31(21). doi: 10.11604/pamj.2018.31.21.15120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother. 2015;11(2):1–7. doi: 10.4161/21645515.2014.995059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li R, Huang L, Li J, Mo Z, He B, Wang Y, Wu X, Minutello M, Guinet-Morlot F, Pichon S.. A next-generation, serum-free, highly purified Vero cell rabies vaccine is safe and as immunogenic as the reference vaccine Verorab® when administered according to a post-exposure regimen in healthy children and adults in China. Vaccine. 2013;31(50):5940–7. doi: 10.1016/j.vaccine.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 5.Tu C, Feng Y, Wang Y. Animal rabies in the People’s Republic of China. Rev Sci Tech. 2018;37(2):519–28. doi: 10.20506/rst.37.2.2820. [DOI] [PubMed] [Google Scholar]
- 6.Knobel DL, Cleaveland S, Coleman PG, Fevre EM, Meltzer MI, Miranda ME, Shaw A, Zinsstag J, Meslin F-X. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ. 2005;83:360–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Shi N, Zhang Y, Zheng H, Zhu Z, Wang D, Li S, Li Y, Yang L, Zhang J, Bai Y, et al. Immunogenicity, safety and antibody persistence of a purified Vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vaccin Immunother. 2017;13(6):1–8. doi: 10.1080/21645515.2017.1279770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Medical Products Administration . Guidelines for adverse reaction classification standards for clinical trials of preventive vaccines. https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20191231111901460.html.
- 9.Wang L, Zhang J, Meng S, Ge L, You Y, Xu Q, Wang H, Yang J, Wang S, Wu H. Safety and immunogenicity of human rabies vaccine for the Chinese population after PEP: a systematic review and meta-analysis. Vaccine. 2022;40(32):4371–9. doi: 10.1016/j.vaccine.2022.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Zhao Y, Claudius M. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the Zagreb regimen (2-1-1) compared with the Essen regimen in Chinese adults. Hum Vaccin Immunother. 2014;10(10):2805–12. doi: 10.4161/21645515.2014.972773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Huang S, Cai L, Zhu Z, Chen J, Lu S, Zhu Z, Zhang M, Fang Y, Hu Q. Safety, immunogenicity of lyophilized purified Vero cell cultured rabies vaccine administered in Zagreb and Essen regimen in post-exposure subjects: a post-marketing, parallel control clinical trial. Hum Vaccin Immunother. 2021;17(8):2547–53. doi: 10.1080/21645515.2021.1880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother. 2014;10(6):1645–9. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Wang S, Zhou R, Liu H, Gan X, Wei M, Zhu F, Meng F, Hou W. Long-term immunity and the effect of one or two booster doses with a lyophilized human rabies vaccine (human diploid cells) at 10 years post primary vaccination in China. Hum Vaccin Immunother. 2021;17(9):3162–8. doi: 10.1080/21645515.2021.1906601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Liu MQ, Chen L, Zhu ZG, Zhu ZR, Hu Q. Rabies post-exposure prophylaxis for a child with severe allergic reaction to rabies vaccine. Hum Vaccin Immunother. 2016;12(7):1802–4. doi: 10.1080/21645515.2016.1143158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Zhu Z, Cai L, Zhu Z, Zhang M, Hu Q, Fang Y. Analysis on the risks of severe adverse events in rabies post-exposure prophylaxis and appropriate decision-making procedure. Hum Vaccin Immunother. 2019;15(9):2121–5. doi: 10.1080/21645515.2018.1533779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soentjens P, Andries P, Aerssens A, Tsoumanis A, Ravinetto R, Heuninckx W, van Loen H, Brochier B, Van Gucht S, Van Damme P, et al. Preexposure intradermal rabies vaccination: a noninferiority trial in healthy adults on shortening the vaccination schedule from 28 to 7 days. Clin Infect Dis. 2019;68(4):607–14. doi: 10.1093/cid/ciy513. [DOI] [PubMed] [Google Scholar]
- 17.Publication WHO . Rabies vaccines: WHO position paper–recommendations. Vaccine. 2010;28(44):7140–2. doi: 10.1016/j.vaccine.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Mittal MK. Revised 4-dose vaccine schedule as part of postexposure prophylaxis to prevent human rabies. Pediatr Emerg Care. 2013;29(10):1119–21;quiz 22–4. doi: 10.1097/PEC.0b013e3182a63125. [DOI] [PubMed] [Google Scholar]
- 19.Jenkin D, Ritchie AJ, Aboagye J, Fedosyuk S, Thorley L, Provstgaad-Morys S, Sanders H, Bellamy D, Makinson R, Xiang ZQ, et al. Safety and immunogenicity of a simian-adenovirus-vectored rabies vaccine: an open-label, non-randomised, dose-escalation, first-in-human, single-centre, phase 1 clinical trial. Lancet Microbe. 2022;3(9):e663–71. doi: 10.1016/S2666-5247(22)00126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashwathnarayana DH, Madhusudana SN, Sampath G, Sathpathy DM, Mankeshwar R, Ravish HH, Ullas PT, Behra TR, Sudarshan MK, Shamanna M, et al. A comparative study on the safety and immunogenicity of Purified duck embryo vaccine [corrected] (PDEV, Vaxirab) with purified chick embryo cell vaccine (PCEC, Rabipur) and purified Vero cell rabies vaccine (PVRV, Verorab). Vaccine. 2009;28(1):148–51. doi: 10.1016/j.vaccine.2009.09.090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


