ABSTRACT
As global supply is still inadequate to address the worldwide requirements for HPV vaccines, we assessed the safety and immunogenicity of a new bivalent HPV16/18 vaccine. In this randomized, double-blind, placebo-controlled, phase 2 trial, healthy 9–45-year-old Chinese females in three age cohorts (600 aged 9–17 years; 240 aged 18–26 years; 360 aged 27–45 years) were randomized 1:1 to receive three doses (0,2,6 months) of HPV16/18 vaccine or placebo. We measured neutralizing antibodies against HPV 16 and 18 at 7 months and monitored safety to 12 months in all age cohorts; 9–17-year-old girls were monitored for safety and immunogenicity to 48 months. In vaccinees, 99.8% seroconverted for HPV 16 and 18 types at 7 months; respective GMTs of 5827 (95% CI: 5249, 6468) and 4223 (3785, 4713) were significantly (p < .001) higher than controls for all comparisons. GMTs in the 9–17-year-olds, which were significantly higher than in older women at 7 months, gradually declined to 48 months but remained higher than placebo with seropositivity rates maintained at 98.5% and 97.6% against HPV 16 and 18, respectively. Adverse events occurred at similar rates after vaccine and placebo (69.8% vs. 72.5%, p = .308), including solicited local reactions and systemic adverse events which were mainly mild-to-moderate. The bivalent HPV16/18 vaccine was well tolerated and induced high levels of neutralizing antibodies in all age groups which persisted at high levels to 48 months in the 9–17-year-old age group which would be the target for HPV vaccination campaigns.
KEYWORDS: Human papilloma virus, HPV, vaccine, reactogenicity, immunogenicity, neutralizing antibodies
Introduction
Cervical cancer is the fourth most prevalent cancer malignancy among women, and the fourth most frequent cause of cancer death in women globally with estimated 570,000 new cases and 311,000 deaths from the disease annually.1 The main cause of cervical cancer is the human papillomavirus (HPV), the most frequent sexually transmitted viral infection globally.2 HPV types 16 and 18 are responsible for up to 70% of cervical cancers.3,4 In low-resource settings, cervical cancer due to HPV infection is a leading cause of morbidity and mortality among women accounting for approximately 84% of all cases and 80% of all deaths.5 Since the first prophylactic HPV vaccine was licensed in 2006, several have become available and as of 2022, six HPV vaccines (three bivalent, two quadrivalent, and one nonavalent) have been licensed.6 HPV vaccines have been included in national routine immunization schedules in 149 countries to prevent and mitigate the health threats of HPV-related disease.7 Many high-income countries have implemented school-based HPV vaccine programs as the maximal benefit is obtained if immunization is completed among preadolescent girls before onset of sexual activity.8–10
The introduction of routine screening and HPV vaccination has resulted in a global trend of decreasing rates of cervical cancer incidence and mortality,4 but this trend has not been observed in China, where 11.9% of the world’s reported cervical cancer deaths occurred in 2017.11 Despite the availability of routine screening and HPV vaccination, the rates of cervical cancer incidence and mortality in China have remained high. This may be attributed to the low vaccine coverage in the country, as long-term studies have shown little change in HPV prevalence rates over time, particularly in younger women (≤ 25 years) who have the highest rates of infection.12,13 Studies have consistently identified HPV 16 and HPV 52 to be the most prevalent genotypes in infected women13,14 and HPV 16 has been shown to be the predominant type associated with 46% of CIN2/3 cases in China.15 Therefore, increasing HPV vaccine coverage in China could help reduce the burden of cervical cancer in the country.
The bivalent HPV16/18 vaccine, Cervarix®, and the quadrivalent HPV 6/11/16/18 vaccine, Gardasil®, were licensed in China in 2016 and 2017 following the confirmation of the safety, high immunogenicity, and efficacy against persistent infection and disease of these vaccines.16–23 However, the high cost of these vaccines and a global shortfall in the capacity to produce sufficient volumes of these vaccines7 means that vaccination coverage among Chinese girls and women was unlikely to achieve the levels of western countries. This led the Shanghai Zerun Biotechnology Co., Ltd. to develop a more affordable bivalent HPV16/18 vaccine to complement the global supply. We initiated this randomized, double-blind, placebo-controlled phase 2 trial of three doses of this new HPV16/18 vaccine in healthy females from 9 to 45 years of age in the Guangxi Zhuang Autonomous Region in 2013, at a time when there were no approved vaccines available in China, to inform recommendations for national immunization programs with the new vaccine in Chinese females.
Methods
Study design and participants
This randomized, double-blind, placebo-controlled clinical trial was designed by the study sponsor, Shanghai Zerun Biotech Co., Ltd., in collaboration with the Center for Disease Control and Prevention of the Guangxi Zhuang Autonomous Region (Guangxi CDC) and performed at two sites in that region. The study protocol was registered with ClinicalTrials.gov (identifier NCT02740790) and approved by the institutional review board of the Guangxi Zhuang CDC, Guangxi, China (Reference number: IRB00001594). The primary immunogenicity objective was to assess seroconversion rates (SCR) and geometric mean titers (GMTs) of neutralizing antibodies against HPV types 16 and 18 among participants in three different age cohorts at month 7, 1 month after the third dose; a secondary immunogenicity objective was the durability of the response measured at 6, 18, 28 and 42 months after the third vaccination in girls aged 9–17 years (study months 12, 24, 36, and 48), who constitute the primary vaccination target population. The primary safety objective was the occurrence of solicited and unsolicited adverse events in vaccinees after each dose compared descriptively with placebo recipients.
Eligible participants, females aged 9–45 years who were in good general health, were recruited using an approved, established recruitment procedure at each study site from September 2013 through October 2015. All participants provided written informed consent; those under 18 years of age provided assent and written consent from a parent or a legal guardian. Enrollment was stratified into three age cohorts: 600 girls aged 9–17 years, 240 women aged 18–26 years, and 360 women aged 27–45 years. Randomization was performed in a 1:1 ratio in blocks of 6 to vaccine and placebo groups. The main exclusion criteria were pregnancy at enrollment or any vaccine visit, a history of genital warts or cervical intraepithelial neoplasia, prior receipt of any HPV vaccine, or poor general health and/or any severe or debilitating illness. Sexually active women committed to avoiding becoming pregnant within 7 months of the study; pregnancy testing was performed before each vaccination for those ≥ 18 years of age.
Vaccine
The recombinant bivalent HPV (types 16 and 18) vaccine (Shanghai Zerun Biotech Co., Ltd., China) was produced using recombinant DNA technology as previously described24. HPV 16 and 18 L1 proteins produced using the yeast, Pichia pastoris, were purified to form noninfectious virus-like particles (VLPs). Each dose contained 40 μg HPV16 and 20 μg HPV18 L1 VLP protein adsorbed to 225 μg aluminum phosphate in 0.5 ml of buffered saline. Placebo was the same volume of buffered saline containing 225 μg of aluminum phosphate without antigen. GMP-compliant vaccine and placebo lots were tested by the National Institutes for Food and Drug Control before the study. Blinding was maintained using identical labels with computer-generated randomized numbers on sets of four study vials of vaccine and placebo, to allow for three injections and one spare in case of accident, prepared by non-study staff. Vaccine administrators administered the appropriate vial with the number assigned to each participant, unaware of the assignment to vaccine or placebo.
Procedures
Following a baseline blood draw, participants received their assigned dose of bivalent HPV16/18 vaccine or placebo by intramuscular injection in the deltoid. Further injections were administered during study visits at 2 and 6 months and blood was drawn at 7 and 12 months to complete the follow-up observation period in all age cohorts. In the cohort of 9–17-year-olds, there were additional annual study visits and blood draws up to 48 months. Sera were prepared immediately and stored for immunogenicity analyses.
Safety
Participants were monitored for 30 min after each vaccination for any immediate adverse events, and asked to record occurrence, outcome, and intensity on a 4-grade scale of solicited local reactions and systemic adverse events daily on standard diary cards for 7 days after each injection. Solicited local reactions included pain, redness, swelling, induration, and pruritus at the injection site, solicited systemic adverse events included fever (axillary temperature), headache, fatigue, myalgia, nausea/vomiting, diarrhea, and allergy (Tables S1 and S2). Any other adverse events occurring during a 30-day follow-up period after each injection were recorded as unsolicited. Information on any pregnancies or miscarriages in the older cohorts was collected throughout the applicable trial duration up to 12 months, with special attention to pregnancy outcomes. Serious adverse events (SAEs) were recorded throughout the entire study period, regardless of cause, and were to be reported immediately to the investigator. SAEs were defined as any untoward medical occurrence that was life-threatening, resulted in death or persistent or significant disability/incapacity, necessitated hospitalization, or prolongation of existing hospitalization, or was a congenital anomaly/birth defect in the offspring of a study subject. Investigators assessed the reported adverse events and their causality according to redefined criteria (Table S3) and determined associated secondary diagnoses and other underlying conditions of SAEs. Data were collected by the Guangxi CDC using an electronic case report form (eCRF).
Immunogenicity
Antibodies specific for neutralizing epitopes in HPV-L1 protein of HPV 16 and 18 were measured in sera at the China National Institute for Food and Drug Control using a pseudovirion-based neutralization assay. Briefly, 293 FT cells were placed in 96-well cell culture plates at 15,000 cells/well with 100 μL of growth medium and incubated for 16–24 h. The pseudovirion stocks were diluted to ~15,000 TCID50/mL, and 60 μL each of diluted pseudovirions and serially diluted sera were mixed and incubated at 25°C for 1 h. 100 μL of this pseudovirion-serum mixture was transferred into plates pre-seeded with 293 FT cells and incubated for 68–76 h following which expression of green fluorescent protein (GFP) was observed by fluorescence microscopy. Neutralization titers were defined as the reciprocal of the highest dilution that caused a 50% reduction in GFP expression compared with control cells. Seroconversion was defined as an initially seronegative participant, (i.e., < 40 at baseline), becoming seropositive or an initially seropositive participant, (i.e., ≥ 40 at baseline), displaying a four-fold or greater increase in neutralizing titer after three doses of vaccine.
Statistics
The primary hypothesis was that the seroconversion rates for HPV 16 and 18 in each of the three age cohorts one month after the final dose would be over 95% with no significant difference between them. Following regulatory guidance, the lowest sample size for the immunogenicity assessment was 300 participants. Conservatively allowing for a 20% withdrawal rate, and the assumption of high baseline seropositivity for HPV 16 or 18 among sexually active women, we calculated that a sample of 600 participants per group would give 95% power to detect a difference in the primary immunogenicity outcome between age groups, and vaccine and placebo groups, at a two-tailed alpha level of 0.05.
The safety analyses were summarized in the Safety Set (SS) comprising all participants who received at least one dose of the study vaccine or placebo. The per protocol set (PPS) included all subjects who met the inclusion/exclusion criteria and underwent vaccination and blood sampling as per protocol. Analysis for the primary immunogenicity objective was performed in the protocol defined immunogenicity Per-Protocol Set (iPPS) which included participants who were not seropositive for HPV16 or 18 at enrollment, received all three doses on schedule, adhered to all study procedures, and completed the follow-up observation 12 months after the initial injection with no protocol violations. In the long-term follow-up of the youngest cohort all participants who presented at the 24, 36, and 48-month follow-up visits were included unless they had a major protocol violation (e.g. received another HPV vaccination). Student’s t-test or the Mann-Whitney U-test (for non-normally distributed data) were used for the analysis of dimensional outcomes, and the chi-square test or Fisher’s exact test (when data were sparse) was used for the analysis of dichotomous outcomes; all tests were two-tailed. Samples with titers > 40 were assigned an arbitrary value of 20 for the calculation of GMTs. A p-value of < .05 was considered to demonstrate statistical significance, but the focus was on 95% confidence intervals (CIs) calculated by the Clopper–Pearson method for the between-group comparisons. Data analysis was performed using SAS software version 9.3 (SAS Institute).
Results
From September 26 to October 30, 2013, we screened 1307 females, 9–45 years of age, for inclusion in the study of whom 107 were excluded; 87 had an ineligibility criterion and 20 declined to give consent (Figure 1). The last participant completed the 12-month follow-up on September 29, 2015 and database lock for the persistence observations was made on November 2018. The 1200 enrolled participants randomly assigned 1:1 to receive vaccine or placebo were stratified into three age cohorts; 600 aged 9–17 years, 240 aged 18–26 years, and 360 aged 27–45 years. In the vaccine group, one participant withdrew consent before receiving any vaccine and was excluded from the Safety Set, but all 600 placebo recipients were included. There were 75 participants excluded from the per-protocol analysis in the vaccine group and 60 from the placebo group having withdrawn consent, migrated out of the study area, were non-compliant with vaccination or blood sampling schedules, were lost to follow-up or had AEs or pregnancies (Figure 1). A further 20 participants who were seropositive for both HPV 16 and 18 at baseline were excluded from the vaccine group (Table 1). In the placebo group, there were eight excluded for being seropositive for HPV 16 and 14 excluded for being seropositive for HPV 18. Thus, 504 participants in vaccine group and 532 and 526 (for HPV 16 and 18, respectively) in the placebo groups made up the immunogenicity Per-Protocol Set (iPPS) (Figure 1).
Figure 1.
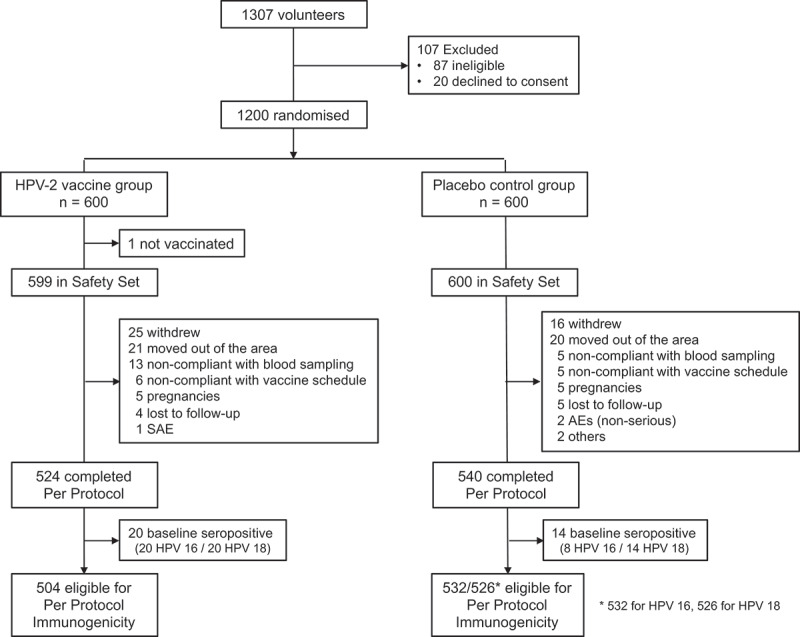
Flowchart of participants through the study.
Table 1.
Demographic characteristics of the participants included in the PPSa and baseline seropositivity rates for those excluded from the iPPSb.
| Characteristic | All participants |
9–17 years cohort |
18–26 years cohort |
27–45 years cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Vaccine | p value | Placebo | Vaccine | p value | Placebo | Vaccine | p value | Placebo | Vaccine | p value | |
| N = | 540 | 524 | 276 | 268 | 98 | 95 | 166 | 161 | ||||
| Age (y), median | 17.2 | 17.4 | .631 | 13.2 | 13.2 | .743 | 24.0 | 24.0 | .521 | 37.0 | 39.0 | .044* |
| Height (cm), median | 153 | 153 | .250 | 149 | 150 | .215 | 155 | 155 | .663 | 155 | 155 | .180 |
| Weight (kg), median | 45.9 | 46.2 | .155 | 39.3 | 40.0 | .559 | 47.1 | 47.9 | .888 | 52.6 | 55.0 | .006* |
| Ethnic group (n) | ||||||||||||
| Han | 437 | 435 | .103 | 220 | 223 | .141 | 84 | 79 | .372 | 133 | 133 | .646 |
| Zhuang | 45 | 32 | 26 | 16 | 7 | 4 | 12 | 12 | ||||
| Yao | 58 | 53 | 30 | 26 | 7 | 12 | 21 | 15 | ||||
| Other | 0 | 4 | 0 | 3 | 0 | 0 | 0 | 1 | ||||
| Baseline seropositivity rates (n, %) | ||||||||||||
| HPV16 | 8 (1.5) | 20 (3.8) | .017* | 1 (0.4) | 3 (1.1) | .366 | 3 (3.1) | 3 (3.2) | 1.000 | 4 (2.4) | 14 (8.7) | .013* |
| HPV18 | 14 (2.6) | 20 (3.8) | .256 | 5 (1.8) | 5 (1.9) | 1.000 | 3 (3.1) | 4 (4.2) | .718 | 6 (3.6) | 11 (6.8) | .190 |
| Baseline GMTs c | ||||||||||||
| HPV16 | 20.5 | 21.3 | .057 | 20.1 | 20.5 | .224 | 20.7 | 21.7 | .453 | 21.1 | 22.5 | .185 |
| HPV18 | 20.8 | 21.2 | .233 | 20.5 | 20.8 | .542 | 21.0 | 21.2 | .842 | 21.0 | 22.0 | .267 |
* indicates significant differences were observed between groups.
a. PPS = Per Protocol Set which includes those who received all doses of vaccine or placebo on schedule and provided paired blood samples.
b. iPPS = Immunogenicity Per Protocol Set which excludes those who were seropositive at baseline.
c. GMTs indicates geometric mean neutralization titers.
Demographic characteristics of participants in the vaccine and placebo groups and across age cohorts were generally similar in terms of age, height, weight, ethnicity, and seropositivity rates against HPV18 (Table 1). A significant difference in the seropositivity rate for HPV16 was noted between the vaccine and placebo groups due to some variation in the 27–45 years age cohort (Table 1). Results were consistent between the PPS and Safety Set populations (Tables S4 and S5).
Immunogenicity
Per-protocol immunogenicity results (Table 2) show there was no change in serostatus or GMTs for either HPV type in the placebo group over the first 12 months. A total of 21 placebo recipients seroconverted for HPV16 by month 7, most (13) in the oldest age cohort, and 9 seroconverted for HPV18, 5 in the oldest age cohort. In contrast, vaccinees demonstrated a marked response against both HPV16 and 18 by month 7, with seroconversion rates (SCR) of 99.6% to 100% across the age groups which were significantly higher (p < .001) in all comparisons between vaccine and placebo groups, but with no significant differences between age cohorts (p = 1.000). For the primary outcome, there was significantly higher seroconversion against HPV16 (99.8% vs. 4.0%) and HPV18 (99.8% vs. 1.7%) in vaccinees than placebo recipients.
Table 2.
Month 7 and 12 neutralizing antibody responses as geometric mean titers (GMTs) and seroconversion rates (SCR) in the iPPS.a.
| Antibody | Population | Placebo group |
Vaccine group |
p values# |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Seroconverted n (%) |
GMT (95%CI) | N | Seroconverted n (%) |
GMT (95%CI) | SCR* | GMTs | ||||
| Month 7 | |||||||||||
| HPV16 | All participants | 532 | 21 (4.0) | 20.7 | (20.4, 21.1) | 504 | 503 (99.8) | 5827 | (5249, 6468) | < .001 | < .001 |
| 9–17 years | 275 | 4 (1.5) | 20.2 | (20.0, 20.4) | 265 | 264 (99.6) | 8571 | (7437, 9879) | < .001 | < .001 | |
| 18–26 years | 95 | 4 (4.2) | 20.6 | (20.0, 21.2) | 92 | 92 (100) | 7296 | (5939, 8962) | < .001 | < .001 | |
| 27–45 years | 162 | 13 (8.0) | 21.7 | (20.6, 22.8) | 147 | 147 (100) | 2524 | (2183, 2918) | < .001 | < .001 | |
| HPV18 | All participants | 526 | 9 (1.7) | 20.3 | (20.1, 20.5) | 504 | 503 (99.8) | 4223 | (3785, 4713) | < .001 | < .001 |
| 9–17 years | 271 | 3 (1.1) | 20.2 | (20.0, 20.3) | 263 | 262 (99.6) | 5826 | (5087, 6672) | < .001 | < .001 | |
| 18–26 years | 95 | 1 (1.1) | 20.2 | (19.9, 20.4) | 91 | 91 (100) | 3892 | (3103, 4882) | < .001 | < .001 | |
| 27–45 years | 160 | 5 (3.1) | 20.6 | (20.1, 21.2) | 150 | 150 (100) | 2525 | (2016, 3162) | < .001 | < .001 | |
| Month 12 | |||||||||||
| HPV16 | All participants | 532 | 10 (1.9) | 20.5 | (20.1, 21.0) | 504 | 503 (99.8) | 1128 | (1030, 1236) | < .001 | < .001 |
| 9–17 years | 275 | 2 (0.7) | 20.5 | (19.7, 21.4) | 265 | 265 (100) | 1578 | (1408, 1768) | < .001 | < .001 | |
| 18–26 years | 95 | 1 (1.1) | 20.2 | (19.9, 20.4) | 92 | 92 (100) | 954 | (798, 1140) | < .001 | < .001 | |
| 27–45 years | 162 | 7 (4.3) | 20.8 | (20.2, 21.4) | 147 | 146 (99.3) | 684 | (572, 817) | < .001 | < .001 | |
| HPV18 | All participants | 526 | 6 (1.1) | 20.5 | (19.9, 21.1) | 504 | 489 (97.0) | 1835 | (1581, 2130) | < .001 | < .001 |
| 9–17 years | 271 | 3 (1.1) | 20.8 | (19.7, 22.0) | 263 | 262 (99.6) | 3111 | (2687, 3603) | < .001 | < .001 | |
| 18–26 years | 95 | 0 (0.0) | 20.0 | (20.0, 20.0) | 91 | 90 (98.9) | 1360 | (963, 1922) | < .001 | < .001 | |
| 27–45 years | 160 | 3 (1.9) | 20.3 | (20.0, 20.6) | 150 | 137 (91.3) | 872 | (620, 1226) | < .001 | < .001 | |
*SCR: seroconversion rate (% of group who seroconverted); #p values for differences between respective placebo and vaccine groups.
a. iPPS = Immunogenicity Per Protocol Set.
Responses persisted in all age cohorts until month 12, with little or no change in SCR which remained at 99.8% from months 7 to 12 for HPV16 but decreased from 99.8% to 97.0% for HPV18. The largest group difference was in the 27–45-year-old cohort in which the SCR for HPV18 decreased from 100% at month 7 to 91.3% at month 12.
When measured in terms of GMTs the response was significantly higher (p < .001) in all vaccine vs. placebo comparisons but also, with significant differences between the three age cohorts (Table 2). There were also statistically significant differences in GMTs against both HPV 16 and 18 between the three age cohorts, with higher GMTs in the younger group of all group-to-group comparisons (9–17 years vs. 18–26 years, 9–17 years vs. 27–45 years, and 18–26 years vs. 27–45 years, p < .001 for all comparisons). Consistent with the SCR, GMTs were highest in the 9–17-year-old cohort − 8571 (95% CI: 7437, 9879) and 5826 (95% CI: 5087, 6672) for HPV types 16 and 18, respectively, and lowest in the 27–45-year-olds − 2524 (95% CI: 2183, 2918) and 2525 (95% CI: 2016, 3162), respectively. The 18–26-year-old cohort had intermediate values − 7296 (95% CI: 5939, 8962) and 3892 (95% CI: 3103, 4882) for HPV 16 and 18, respectively. Antibody titers against both HPV 16 and 18 waned in all age groups by month 12, but the significant differences in GMTs between age groups persisted. The overall responses at months 7 and 12 are illustrated as reverse cumulative distribution curves in Figure 2.
Figure 2.
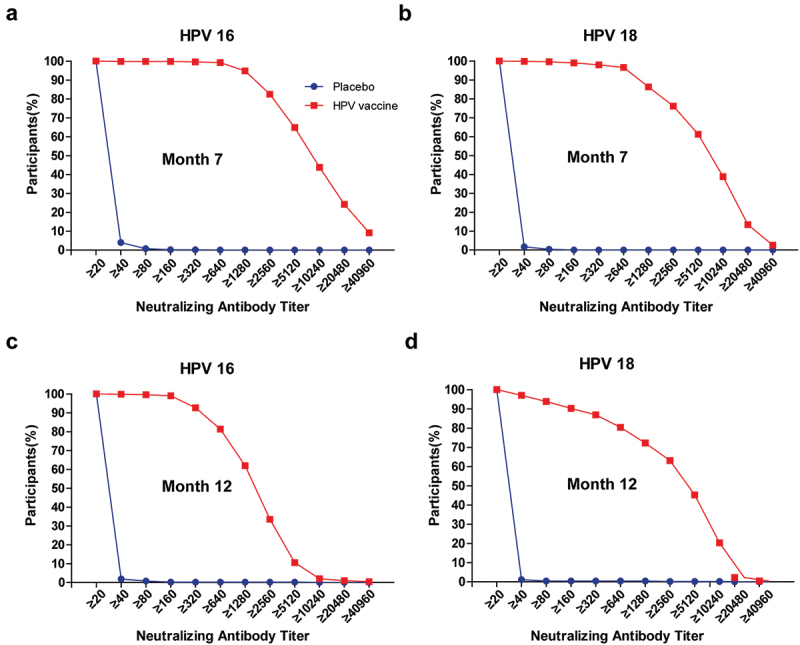
Reverse cumulative distribution curves for neutralizing antibody titers in placebo (blue) and vaccine (red) groups. Panels a and c show titers against HPV 16 at months 7 and 12, panels b and d show titers against HPV 18 at months 7 and 12.
In the 20 vaccinated participants excluded from the per-protocol analyses, due to being seropositive for HPV16 or 18 at baseline, the seroconversion rates for HPV 16 and HPV 18 were 100% at 7 months in all three age cohorts (Table S6). As in the initially seronegative per-protocol cohorts, the largest increases in GMTs in these participants were observed in the 9–17-year-olds. Only one placebo recipients who was initially seropositive for HPV 16) displayed seroconversion due to a four-fold increase in titer.
Long-term persistence of the immune response was monitored in the 9–17-year-olds up to 4 years after the first vaccination (Figure 3). The peak immune responses against both HPV16 and 18 were observed at month 7, 1 month after the third dose, after which there was a gradual waning of titers to month 48 when GMTs against both HPV types were essentially the same in the 206 eligible participants: 762 (95% CI: 650, 894) against HPV16 and 742 (95% CI: 625, 881) against HPV18. Seropositivity rates at this 4-year timepoint were maintained at 98.5% (95% CI: 95.8, 99.7) and 97.6% (95% CI: 94.4, 99.2) for HPV types 16 and 18, respectively.
Figure 3.
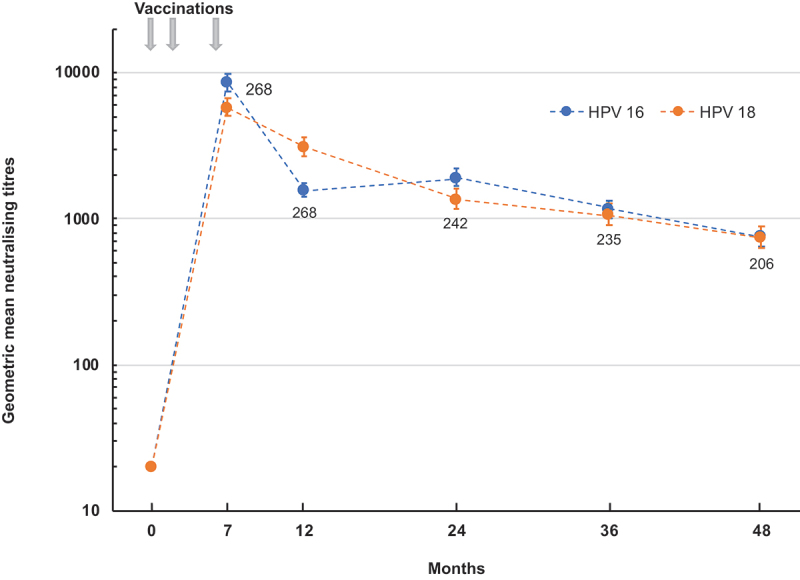
Persistence over 48 months of the neutralizing antibody responses against HPV 16 and HPV 18 after three doses of vaccine administered at 0, 2 and 6 months to 9–17-year-old girls. Values shown are geometric mean titers (with 95% CI bars) of 201–268 girls at each timepoint (indicated as numbers by each point).
Safety and reactogenicity
As shown in Table 3 the overall distribution of clinical adverse events reported during the 12-month observation period was similar in placebo and vaccine groups, in which 72.5% and 69.8%, respectively, reported one or more adverse events (p = .308). Most of these were mild or moderate (grades 1 and 2) in severity with few severe (grade 3) AEs reported, and there was a consistent trend for fewer reports after subsequent doses (Table S7). A total of 11 SAEs were experienced by 10 participants, 5 each in placebo and vaccine groups, but none of these events was considered to be attributable to the vaccine.
Table 3.
Participants reporting solicited local reactions and systemic adverse events after any dose with 7 days of vaccine or placebo, or any adverse events, unsolicited adverse events or serious adverse events in the 12-month reporting period in the safety seta.
| All participants |
9–17 years cohort |
18–26 years cohort |
27–45 years cohort |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo | Vaccine | p value | Placebo | Vaccine | p value | Placebo | Vaccine | p value | Placebo | Vaccine | p value | |
| N = | 600 | 599 | 300 | 300 | 120 | 120 | 180 | 179 | ||||
| Any adverse event up to 12 months, n (%) | ||||||||||||
| 435 (72.5) | 418 (69.8) | .308 | 241 (80.3) | 227 (75.7) | .200 | 94 (78.3) | 91 (75.8) | .759 | 100 (55.6) | 100 (55.9) | 1.000 | |
| Local reactions, n (%)b | ||||||||||||
| Any | 80 (13.3) | 104 (17.4) | .055 | 51 (17.0) | 69 (23.0) | .083 | 16 (13.3) | 23 (19.2) | .294 | 13 (7.2) | 12 (6.7) | 1.000 |
| After dose 1 | 59 (9.8) | 61 (10.2) | 38 (12.7) | 40 (13.3) | 12 (10.0) | 14 (11.7) | 9 (5.0) | 7 (3.9) | ||||
| After dose 2 | 15 (2.6) | 36 (6.2) | 13 (4.4) | 24 (8.3) | 1 (0.9) | 8 (7.1) | 1 (0.6) | 4 (2.3) | ||||
| After dose 3 | 21 (3.7) | 41 (7.3) | 13 (4.5) | 31 (10.8) | 5 (4.9) | 8 (7.7) | 3 (1.8) | 2 (1.2) | ||||
| Systemic adverse events, n (%)b | ||||||||||||
| Any | 267 (44.5) | 248 (41.4) | .294 | 163 (54.3) | 148 (49.3) | .253 | 60 (50.0) | 49 (40.8) | .195 | 44 (24.4) | 51 (28.5) | .404 |
| After dose 1 | 161 (26.8) | 152 (25.4) | 99 (33.0) | 82 (27.3) | 39 (32.5) | 32 (26.7) | 23 (12.8) | 38 (21.2) | ||||
| After dose 2 | 117 (20.3) | 88 (15.2) | 78 (26.5) | 59 (20.3) | 24 (21.6) | 19 (17.0) | 15 (8.7) | 10 (5.7) | ||||
| After dose 3 | 97 (17.3) | 99 (17.7) | 64 (22.2) | 70 (24.5) | 17 (16.5) | 16 (15.4) | 16 (9.4) | 13 (7.6) | ||||
| Unsolicited adverse events up to 12 months, n (%) | ||||||||||||
| Any | 329 (54.8) | 314 (52.4) | .418 | 174 (58.0) | 169 (56.3) | .741 | 75 (62.5) | 68 (56.7) | .430 | 80 (44.4) | 77 (43.0) | .832 |
| Serious adverse events up to 12 months, n (%) | ||||||||||||
| Any | 5 (0.8) | 5 (0.8) | 1.000 | 2 (0.7) | 1 (0.3) | 1.000 | 1 (0.8) | 2 (1.7) | 1.000 | 2 (1.1) | 2 (1.1) | 1.000 |
aSafety Set includes all participants who received at least one dose of vaccine or placebo.
bSolicited local reactions and systemic adverse events were recorded during the 7 days after each vaccination.
In the vaccine group, 17.4% of the participants reported a solicited local reaction within 7 days of injection compared with 13.3% of those in the placebo group (p = .055). The most frequently reported reaction was injection site pain, which was reported by significantly more recipients of the vaccine than of placebo (16.5% vs. 11.8%, p = .021). This overall difference was mainly due to a significant difference in 9–17-years-olds in whom 21.7% of the vaccine group and 15.0% of the placebo group, (p = .045) reported pain, mainly grade 1 in severity (Figure 4). Other common but much less frequent local reactions, including pruritus, redness, swelling, and induration at the injection site, were also reported more often by girls in the younger cohort than the women in the two older cohorts. Proportions reporting solicited systemic adverse events were similar in vaccine and placebo groups (41.4% vs. 44.5%, p = .294), and were also reported more frequently by the younger participants. The most common systemic AE was fever, in 35.2% and 35.7% of participants in vaccine and placebo groups, respectively (p = .904), but few participants (1.0% and 0.3% in vaccine and placebo groups, respectively) reported grade 3 fever (axillary temperature ≥ 39.0°C). Other common systemic AEs included headache, fatigue, allergy, nausea or vomiting, myalgia and diarrhea.
Figure 4.
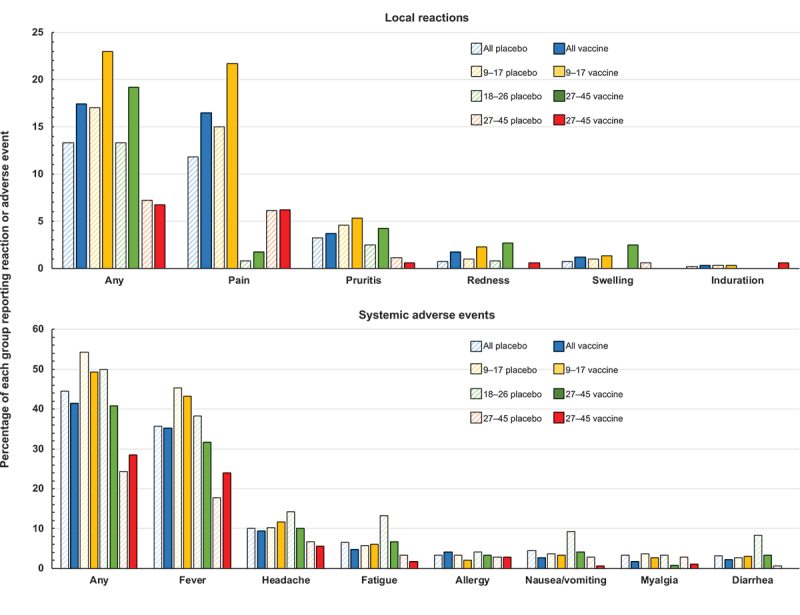
Solicited local and systemic reactogenicity within 7 days of each vaccination or placebo injection.
Unsolicited AEs within 12 months were mainly infectious diseases and respiratory system diseases, reported by 52.4% of the vaccine group and 54.8% of the placebo group (p = .418), and were mainly infectious diseases and respiratory system diseases considered to be unrelated to study treatments. Three AEs led to withdrawal from the study, none of which were considered to be related to study treatments (Table S8). During the first 12-month period of study, a total of 24 pregnancies were reported, in 15 vaccinees and 9 placebo recipients (Table S9). There were 13 normal deliveries from 8 vaccinees and 5 placebo recipients and all neonates were healthy. Among the remaining 11 pregnancies, 1 placebo participant had a spontaneous abortion due to premature membrane rupture, and 10 other participants (3 placebo recipients and 7 vaccinees) had elective abortions. No obvious congenital anomalies were found.
Discussion
This randomized, double-blind, placebo-controlled trial in 1200 healthy Chinese girls and women from 9 to 45 years of age, demonstrated the safety, good tolerability, and high immunogenicity of the bivalent HPV vaccine when administered in a three-dose schedule at 0, 2, and 6 months. There were no SAEs associated with vaccination and the high immune responses observed in the target cohort of girls from 9 to 17 years of age persisted at high levels up to 4 years after the beginning of the vaccination series, 42 months after the last vaccination. All but one of the 504 (99.8%) initially seronegative participants in the vaccine group immunogenicity per protocol set (iPPS) seroconverted for both HPV16 and 18 antibodies by month 7, one month after their last vaccination, and over 97% remained seropositive for both HPV types at month 12. These results are consistent with our primary hypothesis as more than 95% of vaccinated participants in all age cohorts seroconverted for both HPV 16 and 18 antibodies at 7 months, respectively. Nevertheless, we did observe an age-related increase in antibody titers, with HPV 16 and HPV 18 GMTs in 9–17-year-old vaccinated girls being 3.4- and 2.3-fold higher than those in the 27–45-year-old cohort at 7 and 2.3- and 3.6-fold higher at 12 months, respectively. These results are consistent with other clinical studies based on the corresponding age of female vaccination with Cervarix® or Gardasil® vaccines.16–18
As in other reported studies evaluated in different ethnic groups,25–27 the bivalent vaccine used in our trial induced high titers of neutralizing antibodies against HPV 16 and 18, which may transude from serum into cervicovaginal secretions where they may provide first-line defense against HPV. As the transmission of HPV is mainly through skin-to-skin sexual contact, the maximal prophylactic benefit of vaccination will be in preadolescent and adolescent girls who have low frequencies of premarital sexual activity and hence low rates of HPV infection.28 Maximal effectiveness will be obtained by raising immunity through vaccination of young girls prior to sexual debut to prevent cervical lesions. Previous studies have confirmed that the immune response after natural infection appears to be weak and inconsistent and does not provide adequate protection against re-infection with the same high-risk, oncogenic HPV type.29,30 As initially seropositive participants also displayed strong immune responses to vaccination, showing preexisting antibodies has little or no effect on the vaccine-induced response, our results suggest that HPV16/18 vaccination may also be beneficial to women who have previously been exposed to infection, but this needs to be explored in an efficacy study.
The new HPV16/18 vaccine we used was generally well tolerated in females of all ages and consistent with licensed prophylactic vaccines.17,20,26,27 The safety profile for the follow-up period of 12 months, 6 months after the third dose, was similar to that of control groups and no SAEs were attributable to the vaccine. In a recent study of quadrivalent and nonavalent versions of Gardasil performed in China,31 approximately 66% and 83% of Chinese women recipients of those vaccines reported adverse events.31 In common with that study, the most common solicited local and systemic adverse events we observed were injection-site pain and fever, most of which were transient and mild to moderate in severity. Importantly, the frequency and severity of such AEs did not increase with subsequent doses. Further, although practicing contraception was a study requirement, there were 24 pregnancies reported which allowed us to observe that no spontaneous miscarriages occurred in the vaccine group and the eight normal births in the vaccine group all resulted in normal healthy babies.
Our trial has some limitations. First, the immunogenicity follow-up to 48 months was only observed in 9–17-year-old participants and we do not have persistence data on older women. However, this age group of girls represents the primary target of HPV vaccination before they become old enough for sexual activity and potential HPV infection. Second, the protective threshold of HPV antibodies is unknown, so we are unable to correlate the magnitude of the observed antibody response with vaccine efficacy, nor with similar vaccines in other clinical trials as the antibody measurements were not correlated with the accepted international standard which was not available at that time. However, the presence of HPV-specific neutralizing antibodies which we have demonstrated is believed to be extremely important for protection against HPV infection. Third, the licensed HPV vaccines have demonstrated cross-protection in some clinical trials against infection or CIN-associated with non-vaccine HPV types, in particular HPV 31, 33, and 45, due to cross-neutralization of virions of related types.32,33 This has been investigated with the new bivalent vaccine in a large-scale, multicentre phase 3 efficacy trial in women aged 18–30 years (ClinicalTrial.gov, identifier NCT02733068) which will be reported soon.
Conclusions
When administered in a three-dose schedule at 0, 2, and 6 months, the bivalent HPV16/18 vaccine was generally well tolerated and elicited a high age-dependent humoral immune response in 9–45-year-old Chinese females, with persistence of high levels of seropositivity and neutralizing antibody titers for at least four years in 9–17-year-old girls.
Supplementary Material
Acknowledgments
We thank the sponsor, Shanghai Zerun Biotech Co., Ltd., China, for providing the vaccines, assistance with the study protocol, and generous financial support. We are grateful to the district health authorities, medical practitioners, the Centers for Disease Control and Prevention in the districts of Guangxi, Hezhou, Mengshan, and the independent ethics committees at the study sites for their cooperation, facilitation, and assistance in implementing the study. We are especially grateful to the study participants and their families for their understanding, co-operation, and participation in follow-up procedures. This study would not have been possible without the tremendous effort and dedication of the study scientists, technicians, statisticians, and our field staff at our project sites. Keith Veitch (keithveitch communications, Amsterdam, the Netherlands) provided editorial support for the development of the manuscript.
Funding Statement
This study was supported by National Major Scientific and Technological Special Project for Significant New Drugs Development [2015ZX09101035], and Shanghai Science and Technology Support Project [12431901900].
Author contributions
R-C Li, J-L Xia, Z-J Mo, and Y-P Li designed the study protocol. R-C Li was Principal Investigator. J-L Xia, Z-W Jiang, and L Wang were responsible for data processing and statistical analysis. C-G Li and J Li were responsible for serological tests of neutralizing antibodies. All authors had access to data and L-W Shi and L-R Huang verified the data. Interpretation, writing, and revision of the manuscript was done by all authors, leading by L-W Shi, B-W Yu with the assistance of a medical writer. All authors reviewed and approved the final version for submission for publication.
Disclosure statement
The authors declare the following competing interests: B-W Yu, K Li, M Ji, and L-Y Zhou are full-time employees of the manufacturer. Other authors declare no conflicts of interest.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be available three months from an initial request made by researchers who provide a methodologically sound proposal with a signed data access agreement. These proposals will be reviewed and approved by the sponsor, investigator, and collaborator based on scientific merit. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2209001.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):1–10. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJF, Peto J, Meijer CJLM, Muñoz N.. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi:. [DOI] [PubMed] [Google Scholar]
- 3.de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141(4):664–70. doi: 10.1002/ijc.30716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Sanjose S, Quint WG, Alemany L, Geraets, D T., Klaustermeier, J E., Lloveras, B., Tous, S., Felix, A., Bravo, L E., Shin, H-R., et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . Human papillomavirus vaccines: WHO position paper (2022 update). Wkly Epidemiol Rec. 2022;97:645–72. [Google Scholar]
- 7.Global HPV vaccine introduction overview: projected and current national introductions, demonstration/pilot projects, gender-neutral vaccination programs, and global HPV vaccine introduction maps (2006-2023). 2022. Mar 17. https://media.path.org/documents/Global_Vaccine_Intro_Overview_Slides_Final_PATHwebsite_MAR_2022_qT92Wwh.pdf.
- 8.Kane MA, Serrano B, de Sanjose S, Wittet, S.. Implementation of human papillomavirus immunization in the developing world. Vaccine. 2012;30(Suppl 5):F192–F200. doi: 10.1016/j.vaccine.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 9.Koulova A, Tsui J, Irwin J, Van Damme P, Biellik R, Aguado MT. Country recommendations on the inclusion of HPV vaccines in national immunization programmes among high-income countries, June 2006–January 2008. Vaccine. 2008;26(51):6529–41. doi: 10.1016/j.vaccine.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, Bocchini JA, Unger ER. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 11.Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and joinpoint analysis. BMC Public Health. 2021;21(1):1329. doi: 10.1186/s12889-021-11401-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Tang D, Wang K, Wang J, Zhang Z, Chen Y, Zhang X, Ma C. HPV genotype prevalence and distribution during 2009–2018 in Xinjiang, China: baseline surveys prior to mass HPV vaccination. BMC Women’s Health. 2019;19(1):90. doi: 10.1186/s12905-019-0785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L-P, He P, Liu Q-T, Jiang Y-H, Zhang Y-N, Li Q-Z, Li Q, Li S-T, Yang F, Ling H, et al. Prevalence and genotype distribution of HPV infection among 214,715 women from Southern China, 2012–2018: baseline measures prior to mass HPV vaccination. BMC Infect Dis. 2021;21(1):328. doi: 10.1186/s12879-021-06019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang L, Tiam X, Peng XD, Zhang L, Xie F, Bi C, Wang R, Wang J, Qi D. HPV prevalence and genotype distribution among women in Shandong Province, China: analysis of 94,489 HPV genotyping results from Shandong’s largest independent pathology laboratory. Plos One. 2019;14(1):e0210311. doi: 10.1371/journal.pone.0210311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch Gynecol Obstet. 2020;302(6):1329–37. doi: 10.1007/s00404-020-05787-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The GlaxoSmithKline Vaccine HPV-007 Study Group, Romanowski B, de Borba PC, Naud P, Roteli-Martins CM, De Carvalho NS, Teixeira JC, Aoki F, Ramjattan B, Shier, RM, et al. Sustained efficacy and immunogenicity of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine: analysis of a randomised placebo-controlled trial up to 6.4 years. Lancet. 2009;374:1975–85. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz TF, Spaczynski M, Schneider A, Wysocki, J., Galaj, A., Perona, P., Poncelet, S., Zahaf, T., Hardt, K., Descamps, D., et al. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15–55 years. Vaccine. 2009;27(4):581–7. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 18.Einstein MH, Baron M, Levin MJ, Chatterjee, A., Edwards, R P., Zepp, F., Carletti, I., Dessy, F J., Trofa, A F., Schuind, A., et al. Comparison of the immunogenicity and safety of Cervarix™ and Gardasil® human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18–45 years. Hum Vaccines. 2009;5(10):705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 19.Paavonen J, Naud P, Salmerón J, Wheeler, C.M, Chow, S-N., Apter, D, Kitchener, H, Castellsague, X, Teixeira, J.C, Skinner, S.R, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 20.McKeage K, Romanowski B. AS04-adjuvanted human papillomavirus (HPV) types 16 and 18 vaccine (Cervarix®): a review of its use in the prevention of premalignant cervical lesions and cervical cancer causally related to certain oncogenic HPV types. Drugs. 2011;71(4):465–88. doi: 10.2165/11206820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Klein NP, Hansen J, Chao C, Velicer, C., Emery, M., Slezak, J., Lewis, N., Deosaransingh, K., Sy, L., Ackerson, B., et al. Safety of quadrivalent human papillomavirus vaccine administered routinely to females. Arch Pediatr Adolesc Med. 2012;166(12):1140–8. doi: 10.1001/archpediatrics.2012.1451. [DOI] [PubMed] [Google Scholar]
- 22.Garland SM, Kjaer, S K., Muñoz, N., Block, S L., Brown, D R., DiNubile, M J., Lindsay, B R., Kuter, B J., Perez, G., Dominiak-Felden, G., et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis. 2016;63:519–27. doi: 10.1093/cid/ciw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol. 2017;146(1):196–204. doi: 10.1016/j.ygyno.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Shi L, Yu B, Huang L, Zhou L, Shi L.. Safety and immunogenicity of a pichia pastoris-expressed bivalent human papillomavirus (types 16 and 18) L1 virus-like particle vaccine in healthy Chinese women aged 9–45 years: A randomized, double-blind, placebo-controlled phase 1 clinical trial. Vaccine. 2023;41(19):3141–3149. doi: 10.1016/j.vaccine.2023.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Wang Y, Du J. Human papillomavirus vaccines: an updated review. Vaccines (Basel). 2020;8(3):391. doi: 10.3390/vaccines8030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobson SRM, McNeil S, Dionne M, Dawar, M., Ogilvie, G., Krajden, M., Sauvageau, C., Scheifele, D W., Kollmann, T R., Halperin, S A., et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793–802. doi: 10.1001/jama.2013.1625. [DOI] [PubMed] [Google Scholar]
- 27.Konno R, Dobbelaere KO, Godeaux OO, Tamura S, Yoshikawa H. Immunogenicity, reactogenicity, and safety of human papillomavirus 16/18 AS04-adjuvanted vaccine in Japanese women: interim analysis of a phase II, double-blind, randomized controlled trial at month 7. Int J Gynecol Cancer. 2009;19:905–11. doi: 10.1111/IGC.0b013e3181a23c0e. [DOI] [PubMed] [Google Scholar]
- 28.Bhatla N, Suri V, Basu P, Shastri S, Datta SK, Bi D, Descamps DJ, Bock HL. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted cervical cancer vaccine in healthy Indian women. J Obstet Gynaecol Res. 2010;36(1):123–32. doi: 10.1111/j.1447-0756.2009.01167.x. [DOI] [PubMed] [Google Scholar]
- 29.Hussain S, Bharadwaj M, Nasare V, Kumari M, Sharma S, Hedau S, Das BC. Human papillomavirus infection among young adolescents in India: impact of vaccination. J Med Virol. 2012;84(2):298–305. doi: 10.1002/jmv.22261. [DOI] [PubMed] [Google Scholar]
- 30.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Shu Y, Yu Y, Ji Y, Zhang, Li, Li, Y., Qin, H., Huang, Z., Ou, Z., Huang, M., Shen, Q., et al. Immunogenicity and safety of two novel human papillomavirus 4- and 9-valent vaccines in Chinese women aged 20–45 years: a randomized, blinded, controlled with Gardasil (type 6/11/16/18), phase III non- inferiority clinical trial. Vaccine. 2022;40(48):6947–55. doi: 10.1016/j.vaccine.2022.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Stanley M, Lowy DR, Frazer I. Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(Suppl 3):S106–13. doi: 10.1016/j.vaccine.2006.05.110. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins D. A review of cross-protection against oncogenic HPV by an HPV-16/18 AS04-adjuvanted cervical cancer vaccine: importance of virological and clinical endpoints and implications for mass vaccination in cervical cancer prevention. Gyneco Oncol. 2008;110(3 Suppl 1):S18–25. doi: 10.1016/j.ygyno.2008.06.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be available three months from an initial request made by researchers who provide a methodologically sound proposal with a signed data access agreement. These proposals will be reviewed and approved by the sponsor, investigator, and collaborator based on scientific merit. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.


